Abstract
Major focus has been placed on the identification of vulnerable plaques as a means of improving the prediction of myocardial infarction. However, this strategy has recently been questioned on the basis that the majority of these individual coronary lesions do not in fact go on to cause clinical events. Attention is therefore shifting to alternative imaging modalities that might provide a more complete pan-coronary assessment of the atherosclerotic disease process. These include markers of disease activity with the potential to discriminate between patients with stable burnt-out disease that is no longer metabolically active and those with active atheroma, faster disease progression and increased risk of infarction. This review will examine how novel molecular imaging approaches can provide such assessments, focusing on inflammation and microcalcification activity, the importance of these processes to coronary atherosclerosis and the advantages and challenges posed by these techniques.
Keywords: Non-invasive imaging, atherosclerosis, calcification, inflammation, vulnerable patient, 18F-Fluoride, 18F-Fluorodeoxyglucose
Introduction
The past 15 years has witnessed a remarkable expansion in non-invasive cardiovascular imaging technology. Indeed we now have access to a wide spectrum of modalities, each offering distinct and potentially complementary information with respect to the pathophysiology of atherosclerosis. Considerable interest has surrounded the use of this technology to improve cardiovascular risk prediction so that we can better identify high-risk patients, allowing us to intervene and avert subsequent myocardial infarction. For many years the major focus in atherosclerosis imaging research has been to identify individual coronary plaques at high-risk of rupture, the so-called vulnerable plaque. Ultimately however this strategy has failed to have a major impact on clinical care, prompting many to consider a switch of emphasis to pan-coronary and pan-vascular assessments of disease activity that may be more closely related to the vulnerable patient: those subjects at highest risk of cardiovascular events.1 This review will describe novel non-invasive imaging strategies developed to tackle this issue; in particular how measures of disease activity targeted to both inflammation and microcalcification might be used to identify patients at the highest risk of stroke and myocardial infarction.
Problems with the Vulnerable Plaque Paradigm
The majority of acute coronary events are due to atherosclerotic plaque rupture. However identifying plaques at risk of rupture, the so-called vulnerable plaque, has proved problematic. The majority of plaques causing myocardial infarction are non-obstructive on antecedent coronary angiography,2,3 whilst up to one-third of ruptured plaques demonstrate <75 percent cross-sectional vascular area narrowing at post-mortem.3 These lesions are therefore frequently missed on angiography and stress testing, prompting interest in novel imaging strategies that might better predict myocardial infarction. Histological studies have consistently associated several key adverse plaque characteristics with rupture and myocardial infarction. These include a thin fibrous cap, macrophage infiltration, a large necrotic core and plaque volume, microcalcification, angiogenesis and intraplaque hemorrhage. These features are often observed in constellation in lesions known as thin-capped fibroatheroma (TCFA), with each feature representing a potential imaging target for identifying plaque vulnerability regardless of the extent of luminal stenosis. Indeed this has been the subject of intense research over the past 10-15 years and the principle underlying the development of numerous invasive and non-invasive imaging techniques (Figure 1). However to date this approach has failed to impact clinical practice.
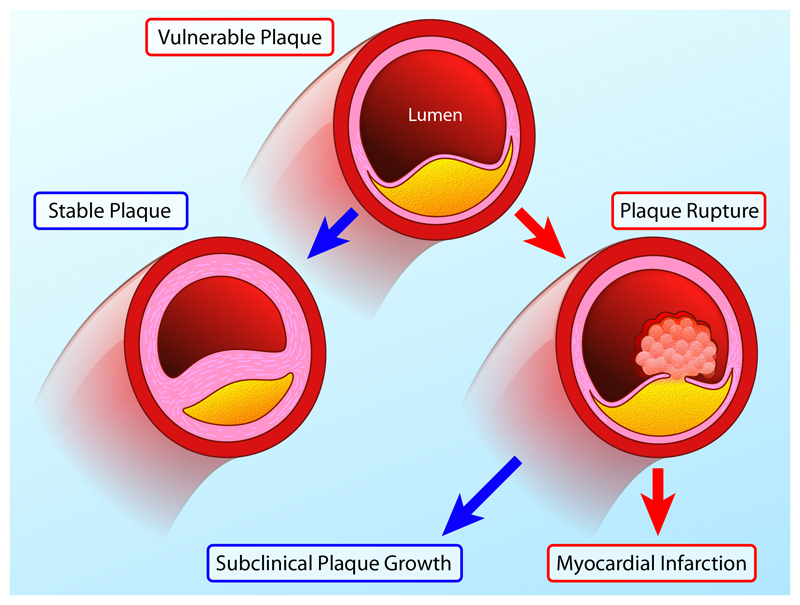
Figure 1. Natural History of the Vulnerable Plaque.
Vulnerable atherosclerotic plaques are thought to account for the majority of myocardial infarctions and are characterised by macrophage inflammation, a thin fibrous cap, positive remodelling, microcalcification and angiogenesis. Histological and imaging studies conducted post-MI have consistently associated these plaques with rupture and myocardial infarction. However, in prospective observational studies only a minority of these plaques go on to cause adverse clinical events (red arrows). This is because many vulnerable plaques will in fact heal and stabilize via multiple processes including calcification. Whilst a proportion will go on to rupture the majority of such events remain subclinical resulting in plaque growth rather than MI. As a consequence the number of vulnerable plaques appears to greatly outnumber the clinical events that ensue.
The PROSPECT trial investigated whether detection of TCFA by virtual histology intravascular ultrasound (VH-IVUS) would predict adverse clinical events.4 In this study, 595 VH-IVUS defined TCFAs were identified in 697 patients, however after a median follow up of 3.4 years only 6 lesions resulted in myocardial infarction. Similarly the VIVA study identified 550 VH-TCFAs with only 8 resulting in a major adverse cardiovascular event not related to stent restenosis.5 Indeed the low predictive value of individual plaques progressing to cause events has emerged as the major limitation with the vulnerable plaque strategy.6,7 Whilst imaging and histological studies conducted post-MI have demonstrated that the above adverse characteristics have consistently been associated with culprit and ruptured plaques, prospective observational studies have shown that such plaques are, in fact, relatively common but go on to cause myocardial infarction in only a small minority of cases.8
Why then do vulnerable plaques outnumber the cardiac events that they cause? The answer lies in the natural history of these lesions (Figure 1). First the majority of these inflamed high-risk lesions are likely to heal and stabilize with time rather than rupture. In particular progressive calcification sees the transition from the early stages of high-risk microcalcification to the stable end stages of macroscopic calcification.9 Second even in those lesions where the healing process is unsuccessful and plaque rupture occurs, the majority of these events appear to be sub-clinical resulting in silent plaque growth rather than myocardial infarction.8,10 Indeed evidence of old healed plaque rupture is seen in more than four-fifths of lesions with >50% luminal stenosis.10 As a consequence whilst retrospective studies demonstrate that vulnerable plaques are consistently responsible for myocardial infarction, prospective studies indicate that only a minority of these supposedly high-risk plaques proceed to cause clinically apparent events. The very worth of identifying vulnerable plaques has therefore been questioned indeed if the majority go on to cause no harm, how can their treatment be justified?8,11
Targeting the Vulnerable Patient
Strategies focused on broader patient-related factors have proved more effective. Cardiovascular risk scores, such as the Framingham risk score (FRS), have been used for several decades to estimate a patient’s risk of suffering a cardiovascular event based on well-established epidemiological studies and the presence of cardiovascular risk factors such as age, diabetes mellitus, hypertension and smoking. Whilst useful on a population level, the accuracy of predicting a patient’s individual risk is limited. Interest has therefore surrounded non-invasive imaging techniques that can directly image the disease process in a patient’s coronary arteries and therefore provide more personalized estimates of risk.
The atherosclerotic plaque burden can be measured using several simple and inexpensive imaging approaches. These predict adverse cardiovascular events presumably on the basis that the more plaques a patient has, the more likely one will rupture or erode and cause an event. Computed tomography (CT) calcium scoring quantifies the amount of macroscopic calcification in the coronary arteries. This provides a surrogate of the total coronary atherosclerotic plaque burden and improves risk prediction models when added to Framingham risk scores.12,13,14 More detailed plaque assessments are now available with coronary CT angiography, allowing visualization of both calcified and non-calcified plaque as well as adverse plaque characteristics.6,15 Yet despite these considerable technological advances, individual risk prediction remains limited.
Molecular Imaging of Disease Activity
A limitation of anatomical plaque assessments is that while they provide an indication as to how much plaque has accumulated over time, they give no indication as to the current activity of the disease process. They are therefore unable to distinguish those patients that have burnt-out, stable disease that is no longer metabolically active versus those with active atheroma. This is of potential importance because patients with active disease will develop multiple unstable plaques over time and whilst the majority will heal without incident, overall there will be an increased probability that one will rupture when the blood is thrombogenic and cause an event. The poor prognosis associated with an active disease process was first suggested by studies investigating disease progression. Raggi et al demonstrated the added value of examining the progression in plaque burden compared to one-off baseline measures.16 In a cohort of patients treated with statin therapy who underwent serial CT calcium scoring those who demonstrated <15% annual progression in their CT calcium score had an excellent prognosis with few events irrespective of their baseline plaque burden. By contrast subjects who demonstrated >15% change in their calcium score had an adverse prognosis, which increased progressively depending on their baseline disease burden. Whilst more than 10 years old this study was consistent with the hypothesis that subjects with increased disease activity and greater rates of progression, are more likely to have events.
Changes in the CT calcium score provide only an indirect measure of disease activity and require serial imaging. Improved clinical imaging methods are therefore required to measure disease activity directly. The emergence of molecular imaging now allows us to do exactly that. Molecular tracers targeted to pathological processes of interest are injected into the body where they accumulate at sites of increased disease activity. These tracers are labeled with an imaging reporter that provides signal on the relevant imaging platform. Potentially the activity of any disease process can be assessed dependent on the availability of a suitable imaging tracer and scanner. However in practice the strict requirements of the FDA for regulatory approval make the development of novel clinical tracers both time-consuming and expensive. Human research has therefore largely focused on tracers already approved for non-cardiac conditions but which target disease processes relevant to atherosclerosis and acute plaque rupture. In particular markers of inflammation and microcalcification activity have been evaluated. These are discussed in detail here although an array of other tracers targeting processes such as angiogenesis, hypoxia and plaque hemorrhage are also in development.17–19
USPIO Imaging of Inflammation
Macrophages play a pivotal role in the destabilization of atherosclerotic plaques, secreting fibrous cap–degrading matrix metalloproteinases, pro-inflammatory cytokines, and pro-thrombotic tissue factor.20 These then weaken the fibrous cap, and drive growth of the necrotic core increasing the propensity to plaque rupture and clinical events. Multiple molecular imaging techniques have been developed to measure inflammatory activity in atherosclerosis (Figure 2).
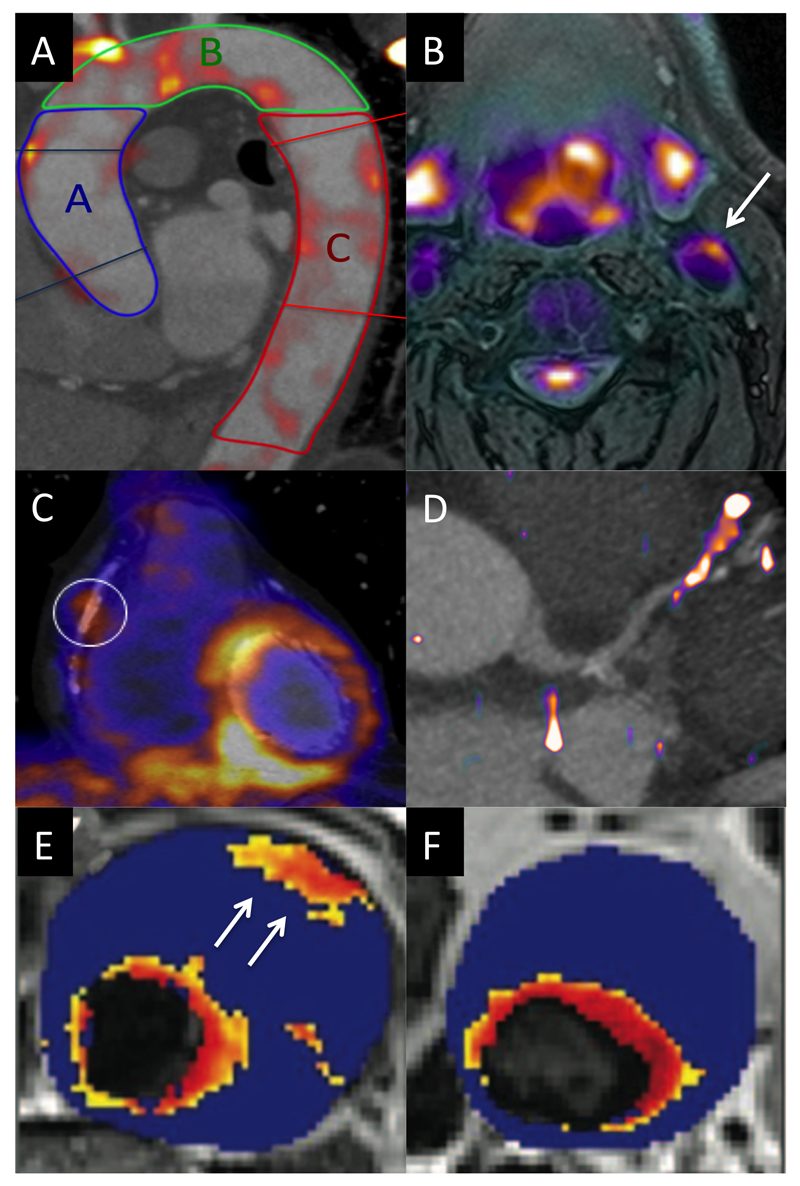
Figure 2. Molecular Imaging of Vascular Inflammation Activity.
A.18F-FDG PET image fused with contrast CT angiogram of the thoracic aorta, demonstrating regions of increased activity in the ascending aorta (blue), aortic arch (green) and descending aorta (red).
B. 18F-FDG PET/MR image of the carotid arteries demonstrating increased activity in the left carotid artery and the excellent soft tissue contrast provided by MR.
C. 18-FDG PET fused with a CT coronary angiogram (CTCA) demonstrating increased uptake in the left ventricle but also in a remote plaque in the mid right coronary artery.
D. 68Ga-DOTATATE PET/CT image with increased activity localizing to a plaque in the mid left anterior descending artery.
E. T2* Map from patients with an abdominal aortic aneurysm that had been administered ultra small particles of iron oxide (USPIO). A hot spot is observed in the anterior wall of the aneurysm (arrow).
F. In a second patient focal areas of increased USPIO uptake can be observed (the increased signal adjacent to the lumen is considered normal due to high signal in the blood pool).
Image C reproduced from JNM. Cheng VY, Slomka PJ, Le Meunier L, Tamarappoo BK, Nakazato R, Dey D, Berman DS. Coronary arterial 18F-FDG uptake by fusion of PET and coronary CT angiography at sites of percutaneous stenting for acute myocardial infarction and stable coronary artery disease. J Nucl Med. 2012;53:575-583. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.92
Images E and F reproduced from Richards et al Circ CVS Imaging 2012 22
Macrophages can be imaged directly using iron oxide contrast agents that have superparamagnetic properties on T2* weighted sequences. Iron oxide is clinically approved for the treatment of iron deficiency anemia, and although concerns have been expressed about the risk of hypersensitivity reactions leading to restricted availability in the United States, these appear less of an issue at the lower doses used in imaging. After injection of ultrasmall superparamagnetic particles of iron oxide (USPIO), these particles are removed from the circulation by the reticuloendothelial system and accumulate in macrophages present in atherosclerotic plaques. Iron oxide causes distortion in the MR signal, that can be used to localize USPIO accumulation and to estimate the macrophage burden in a range of disease processes including atherosclerosis, abdominal aortic aneurysms and myocardial infarction.21,22,23
Kooi et al studied 11 symptomatic patients scheduled for carotid endarterectomy with USPIO-enhanced MR and found a 24% decrease in signal intensity in the culprit vessel on T2*-weighted sequences, with histological evidence of USPIO uptake in 75% of these plaques post-surgery.24 The USPIO signal also appears to be modifiable with drug therapy and therefore may be of value in assessing the anti-inflammatory properties of atherosclerotic therapies. Tang and colleagues showed that the intensity of the USPIO signal could be reduced by 3-months of statin therapy, and that this reduction was increased with high- versus low-dose statin.25 Current limitations of USPIO-enhanced MR imaging with agents such as ferumoxtran are the need for two scans, one before USPIO administration and one ~36-hour following injection.26 Accurate co-registration is then required to detect differences in the T2* signal due to USPIO accumulation. This can be challenging in complex 3-dimensional vascular structures. Moreover T2* artifact and high blood-pool activity are frequently observed making the detection of true signal in atherosclerotic plaques difficult. Whilst these issues can largely be overcome in the carotids and aorta, they currently preclude imaging in the small and constantly moving coronary arteries.
PET Imaging of Inflammation Activity
Positron Emission Tomography (PET) is a nuclear imaging modality used for a variety of clinical purposes. It is ideally suited to measuring disease activity, using radiolabelled tracers targeted to specific pathological processes. PET tracers combine a positron emitter (usually 18F due to its advantageous half-life) with a molecular vehicle targeting the cellular process of interest. After injection these tracers accumulate in areas of disease activity, emitting positrons, which collide with nearby electrons. This collision results in particle annihilation and the release of a pair of photons with a specific energy. Simultaneous detection of these paired photons allows the PET scanner to localize tracer accumulation with exquisite sensitivity and a spatial resolution of ~4 mm. Two tracers in particular hold promise in assessing atherosclerosis: 18F-fluorodeoxyglucose (18F-FDG) as a marker of inflammation activity,27 and 18F-fluoride as a marker of newly forming microcalcification.28 These tracers are used for the routine clinical imaging of oncology patients. They are therefore FDA approved, commercially available, inexpensive and have established excellent safety records.
18F-Fluorodeoxyglucose PET
18F-FDG is a PET tracer and glucose analogue that is taken up by metabolically active cells. On this basis it has become widely used to image cancer but more recently has been employed as a marker of vascular inflammation given that macrophages utilize more glucose than surrounding cells, particularly in hypoxic conditions.29
18F-FDG PET has been most extensively investigated in the carotid arteries and aorta (Figure 2) with uptake correlating with macrophage burden in excised carotid plaques30 and multiple adverse imaging plaque characteristics.31,32 Importantly accumulating data demonstrate the prognostic value of 18F-FDG PET, albeit largely in the form of single-center, retrospective studies. In the Dublin Carotid Atherosclerosis Stroke Study, carotid artery inflammation detected by 18F-FDG was useful for identifying patients most at risk of early stroke recurrence.33 In another retrospective study of 513 patients without cancer, inflammatory disease or prior CVD but who underwent PET imaging for oncological evaluation, the aortic 18F-FDG PET signal again provided independent prediction of cardiovascular events after a median follow up of 4.2 years (HR 4.71, p<0.001).34 Similarly in 1,089 asymptomatic adults being screened for cancer increased carotid 18F-FDG uptake predicted future stroke with incremental value above FRS.35
Scan-rescan reproducibility of the vascular 18F-FDG signal is excellent36 and coupled with the established mechanistic and prognostic data available for this tracer, 18F-FDG PET is increasingly being used as an end-point in trials of novel atherosclerosis therapies. The excellent reproducibility means that such studies require relatively few patients (n=30 to 50) to demonstrate an anti-inflammatory treatment effect, which can be observed quickly after drug initiation (3-6 months). Vascular 18F-FDG studies are therefore relatively low-cost and time-efficient, serving as an economical introduction to larger and more expensive clinical end-point trials. The beneficial effects of statin therapy are well documented so it is reassuring that statins have demonstrated a consistent and clear reduction in the vascular 18F-FDG PET signal, proportional to the dose of statin used.37–39 In contrast, inhibitors of lipoprotein-associated phospholipase A2, failed to modify both the vascular 18F-FDG signal and hard clinical endpoints.40,41 Similarly dalcetrapib, a cholesteryl ester transfer protein (CETP) inhibitor, demonstrated no impact on either vascular 18F-FDG activity nor clinical events.42,43
Translation of 18F-FDG imaging in to the coronary arteries has proved more challenging. This is because glucose is the predominant energy source of the myocardium, such that avid uptake of 18F-FDG by the left ventricle is frequently observed. Whilst myocardial 18F-FDG uptake can be suppressed by dietary restrictions (that aim to switch myocardial metabolism from glucose to free fatty acids), this is ineffective in 20-30% of cases. Indeed in a recent study, more than 50% of the coronary territories assessed were not interpretable due to spill over of myocardial 18F-FDG uptake.44 Until improved methods of myocardial suppression are developed, this is likely to limit the assessment of 18F-FDG uptake in individual coronary plaques. It may however be possible to measure more generalized 18F-FDG uptake in the proximal coronary vessels or the ascending aorta. Rogers et al studied 10 patients post- acute coronary syndrome, demonstrating increased uptake in the left main stem and ascending aorta compared to 15 patients with stable angina.45 Similarly Joshi et al demonstrated increased 18F-FDG uptake in the thoracic aorta of 40 patients following myocardial infarction compared to 40 stable patients despite similar plaque burden.46 Whether this increased vascular inflammation activity represents the cause or effect of the acute infarct remains to be established, although a correlation was observed between infarct size and aortic 18F-FDG uptake,47 consistent with the latter interpretation and recent pre-clinical data.48
Alternative PET tracers
The fact that reliable coronary evaluation with 18F-FDG is often not possible because of high myocardial muscle uptake, coupled with its inherent lack of cellular specificity, drives the search for alternative PET tracers targeting atherosclerotic inflammation. 68Ga-DOTATATE ([1,4,7,10-tetraazacyclododecane-N,N′,N″,N″′-tetraacetic acid]-D-Phe(1),Tyr(3)-octreotate) is a PET tracer with high specific binding affinity for the somatostatin receptor subtype-2 (~0.2 nM), which is currently used clinically for neuroendocrine tumor imaging. Up-regulation of the somatostatin receptor subtype-2 appears to occur on the cell surface of activated macrophages, offering a potential novel imaging target for tracking vascular inflammation. Initial studies are encouraging, and there is some histological evidence in mice showing significant correlation between the aortic 68Ga-DOTATATE PET signal and macrophage density (%CD68 staining).49 In a retrospective study Rominger et al observed detectable 68Ga-DOTATATE uptake in the left anterior descending artery in each of the 70 patients evaluated, with increased tracer activity seen among patients with coronary calcification or past history of cardiovascular events.50 In another retrospective study, increased vascular 68Ga-DOTATATE uptake was, again, observed in the aorta, carotid and iliac arteries in patients with cardiovascular risk factors and coronary calcification.51 While low myocardial binding of 68Ga-DOTATATE is advantageous for coronary imaging, high physiological liver uptake can potentially obscure interpretation of signals originating for the distal right coronary artery. The use of this tracer for atherosclerosis imaging is being studied in more detail in the ongoing prospective Vascular Inflammation imaging using Somatostatin receptor positron emissION tomography (VISION) study (ClinicalTrial.gov NCT02021188).
11C-PK11195 is one of several PET tracers targeting the translocator protein (TSPO), a receptor that is highly expressed on the surface of macrophages. This family of tracers has therefore also been used to target vascular inflammation.52,53 Fujimura et al showed specific binding of 3H-PK11195 to macrophages in human carotid atheroma,54 whilst Pugliese et al demonstrated increased 11C-PK11195 PET activity in patients with symptomatic large-vessel vasculitis.55 Gaemperli et al demonstrated increased activity of 11C-PK11195 in culprit carotid plaques post-stroke with uptake localizing to macrophages and TSPO expression on histology.56 However using 11C in clinical studies is challenging due its half-life, so that 18F labeled TSPO tracers are keenly anticipated, whilst issues also surround genetic polymorhpisms that appear to have a major effect on tracer binding affinity.
Finally PET imaging with 18F-fluorodeoxymannose has recently been described in a pre-clinical study.57 Mannose is an isomer of glucose, and the mannose receptor is up regulated on alternatively activated or M2 macrophages in high-risk plaque. There is therefore hope that 18F-fluorodeoxymannose PET may provide improved detection of such lesions. Macrophages demonstrate a 35% increase in tracer uptake compared to 18-FDG, with a similar pattern of vascular uptake in a rabbit model of atherosclerosis.57 Further studies are awaited, although they may be limited by the difficulties in radiolabeling mannose.
The Link Between Inflammation and Calcification
The exact role of calcification in atherosclerosis remains to be defined. Whilst significant progress in our understanding has been achieved over recent years, confirmation of many of the proposed mechanisms described below requires confirmation by future studies.
Emerging evidence suggests that intimal calcification occurs as a healing response to intense inflammation and cell death within the atherosclerotic plaque, mediated by extracellular vesicles (Figure 3). It appears to be a different disease process to the medial calcification often unassociated with inflammation and more commonly observed in the larger arteries of patients with renal failure and altered calcium metabolism (e.g. patients on dialysis, osteoporosis).58 In the coronary arteries, intimal calcification predominates. Using longitudinal molecular imaging studies, Aikawa et al linked inflammation and intimal calcification in a mouse model of atherosclerosis, demonstrating a close association between the early stages of in vivo microcalcification and macrophage inflammatory activity (R2=0.93).59 The link between inflammation and calcification also appears closely related to cell death within the plaque and the release of extracellular vesicles.60,61,62 These pathological extracellular vesicles are loaded with pro-osteogenic mediators (e.g., proteins, micro-RNAs), containing high concentrations of calcium and phosphate, and appear to act as nucleation sites for the formation of hydroxyapatite crystal.63,9,64,65 Extracellular vesicles, recently demonstrated to be of exosomal origin, are released directly by inflammatory macrophages66 and by vascular smooth muscle cells, implying that atherosclerotic plaques contain a continuous source of the precursors to microcalcification.9,65 Concurrent with this process, a range of resident vascular and recruited progenitor cells differentiate into those with an osteoblastic or chondrocytic phenotypes. This differentiation process appears to be associated with inflammation, promoted by pro-inflammatory cytokines, particularly TNF-alpha.60 These pro-calcific cells then provide an alternative source of calcifying extracellular vesicles, driving the ongoing formation of microcalcifications until large stable sheets of macroscopic calcification develop.
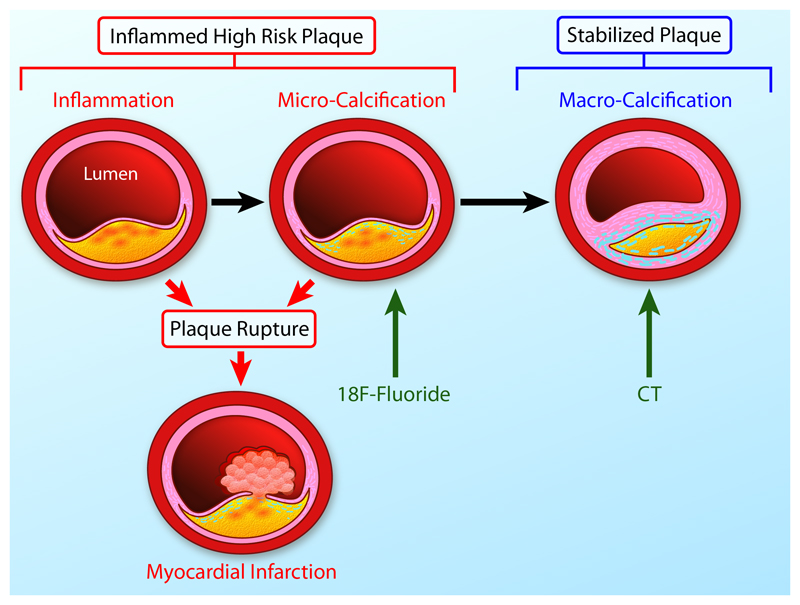
Figure 3. The Link Between Inflammation, Microcalcification and Macrocalcification.
A large necrotic core, a thin fibrous cap and intense inflammation are key precipitants of acute plaque rupture and myocardial infarction. Intimal calcification is thought to occur as a healing response to this intense necrotic inflammation. However the early stages of microcalcification (detected by 18F-fluoride PET) are conversely associated with an increased risk of rupture. In part this is because of residual plaque inflammation and in part because microcalcification itself increases mechanical stress in the fibrous cap further increasing propensity to rupture. With progressive calcification, plaque inflammation becomes pacified and the necrotic core walled off from the blood pool. The latter stages of macrocalcification (detected by CT) are therefore associated with plaque stability and a lower risk of that plaque rupturing.
Differences between microcalcification and macrocalcification
The presence of calcium in the coronary arteries can have diverse clinical implications. It is useful to consider calcification as a two-phase process: the initial stage of microcalcification versus the end stages of macroscopic calcium formation (Figure 3).67 Macroscopic calcific deposits are traditionally associated with plaque stability, supported by recent models of mechanical stress.68 Indeed, we consider them to represent the end-stage of the healing process, effectively walling off the inflamed plaque contents in a process similar to other intense inflammatory conditions such as tuberculosis. These large deposits are readily identified by CT. Indeed CT calcium scoring is a powerful predictor of coronary events.69 Of note, extensively calcified plaques themselves only rarely result in rupture and adverse events, but rather calcium scoring acts as a biomarker of overall disease and coronary plaque burden.
By comparison, microcalcification represents the very early stages of intimal calcium formation and is associated with high-risk and culprit atherosclerotic plaque in histopathological and imaging studies alike.70–74 This may in part reflect the close relationship between microcalcification and the inflammation thought to act as its trigger.59 However recent data has suggested that microcalcification might itself directly contribute to plaque rupture, greatly amplifying mechanical stresses on the surface of the fibrous plaque.75,76 In particular deposits ranging between 5 and 60µm are associated with an increase in local stresses of more than 500%.76,77 Whether directly or indirectly microcalcification therefore appears closely related to the processes driving plaque rupture and whilst it remains beyond the resolution of CT, these structures can now be detected non-invasively using molecular imaging.
PET Imaging of Microcalcification Activity
18F-Fluoride has been used safely as a PET bone tracer for over 40 years but has only recently been assessed in the vasculature. Indeed the first description was in 2010 by Derlin et al who retrospectively studied patients that had been scanned for investigation of cancer. They described increased uptake in the aorta, carotids and femoral vessels in 57 out of the 75 patients studied.78 Whilst sites of 18F-fluoride uptake were commonly observed in close association with regions of existing calcium on CT, increased uptake was also observed in their absence. Moreover the vast majority of calcific lesions on CT did not demonstrate increased 18F-fluoride uptake suggesting that the two approaches provide different information about vascular calcification. The same group went on to establish an association between femoral 18F-fluoride uptake and cardiovascular risk factors,79 and to demonstrate that this tracer provides distinct information to 18F-FDG.80
Whilst these studies provided early insight, the exact mechanisms underlying 18F-fluoride uptake in the vasculature have only recently been elucidated. Irkle et al provided comprehensive assessment of carotid endarterectomy samples using electron microscopy, autoradiography, histology and preclinical and clinical PET/CT to analyze 18F-fluoride binding.28 They demonstrated that 18F-fluoride adsorbs to calcified deposits within plaque with high affinity and is both selective and specific. Moreover 18F-fluoride was able to distinguish between areas of macro- and microcalcification, binding preferentially to the latter. It therefore provided differential information to CT, which was only able to detect large macroscopic deposits. Indeed 18F-fluoride is the only currently available clinical imaging platform that can non-invasively detect microcalcification in active unstable atheroma. The explanation for 18F-fluoride’s preferential binding to microcalcification is the very high surface area of hydroxyapatite in these nanocrystalline areas. By comparison in large macroscopic deposits much of the hydroxyapatite is internalized and not available for binding so that 18F-fluoride uptake is only observed at the periphery (Figure 4).
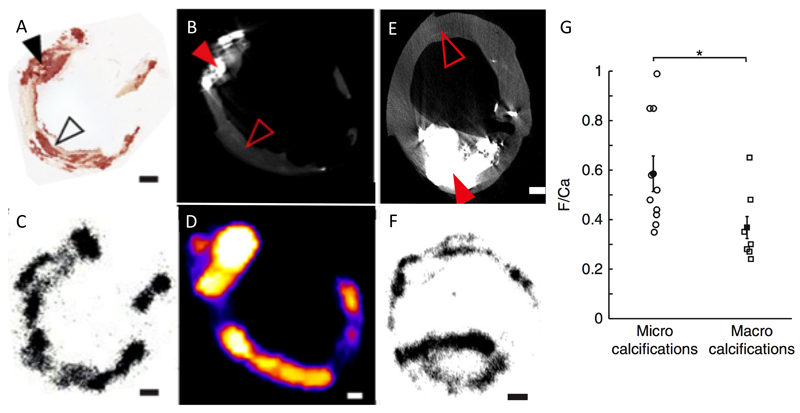
Figure 4. 18F-Fluoride Preferentially Binds Microcalcification Beyond the Resolution of CT.
Images are taken ex vivo of a carotid endarterectomy specimen excised from a patient who had suffered a recent stroke. (A) Histological section of the excised plaque stained for calcium with Alizarin red. Filled black arrow shows an area of dense macroscopic calcification that is visible on micro-CT (B). By comparison the empty black arrow head demonstrates areas of micro-calcification that are beyond the resolution of the micro-CT but by comparison demonstrate avid binding with 18F-Fluoride on both autoradiography (C) and micro-PET imaging (D). A second carotid endarterectomy sample from a patient post stroke demonstrates a large macro calcific deposit on micro CT (E). Autoradiography shows that whilst 18F-fluoride is able to bind to the surface of the plaque it is is unable to penetrate in to the center (F). As a consequence of this effect 18-fluoride binds preferentially to regions of microcalcification compared to macroscopic deposits.
Images reproduced from Irkle et al Nature Communications. 2015 28
Beheshti and colleagues first described 18F-fluoride activity in the heart,81 with further studies localizing this activity to the valves of patients with aortic stenosis82,83 and to individual coronary plaques.84 In aortic stenosis, isolated 18F-fluoride uptake predicts the location of future macroscopic calcium formation on CT, accurately predicting progression of the aortic valve CT calcium score,85,86 and correlating with alkaline phosphatase activity on histology.86 18F-Fluoride therefore acts as a marker of vascular calcification activity in aortic stenosis, appearing to bind to newly forming microcalcification within the valve leaflets.87
In the coronary arteries, 18F-fluoride is the first tracer to consistently localize to individual coronary plaques with very little background activity and excellent signal-to-noise (Figure 5).84 Those plaques demonstrating increased 18F-fluoride uptake have multiple high-risk characteristics when assessed with virtual-histology intravascular ultrasound, including microcalcification, positive remodeling and a large necrotic core: findings confirmed in pathological validation studies of the carotid arteries.44 18F-Fluoride also appears to localize to ruptured culprit plaque post-MI. In a study of 40 patients who had suffered a recent MI, 37 demonstrated increased uptake in their adjudicated culprit plaque.44 This would suggest that microcalcification is indeed implicated in atherosclerotic plaque rupture although it remains impossible to know whether increased 18F-fluoride activity was truly present before the rupture event or whether it developed in response. Moreover for the reasons discussed above, it would seem likely that in observational cohort studies the majority of 18F-fluoride positive plaques would heal rather than causing events. Nevertheless 18F-fluoride holds promise in improving our understanding of the pathophysiology of atherosclerosis and in predicting events at the patient-level. Indeed the ability of hybrid 18F-fluoride PET/CT to predict disease progression and to identify patients at risk of myocardial infarction is currently being tested in a large multi-center and prospective observational study, PREFFIR (ClinicalTrial.gov NCT02278211).
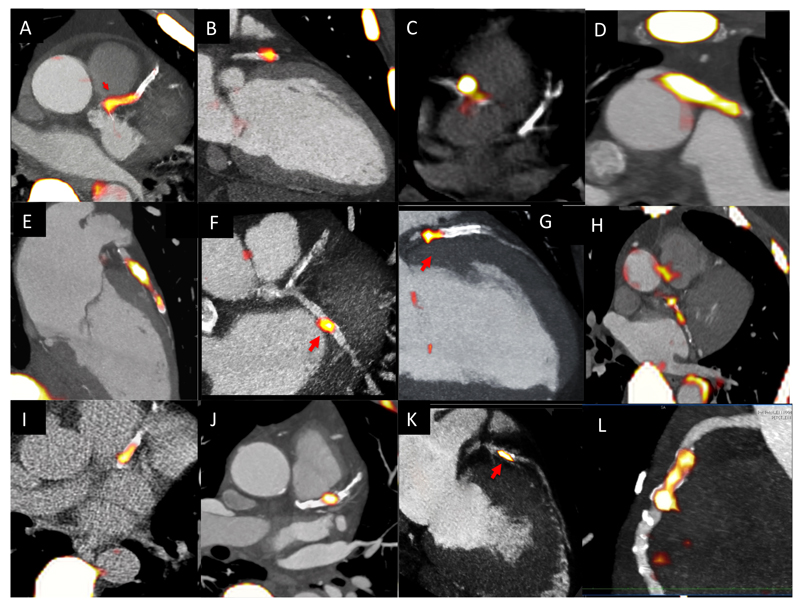
Figure 5. 18F-Fluoride PET Imaging in the Coronary Arteries.
Multiple examples of 18F-Fluoride localizing to individual coronary plaques on fused PET/CT images of the heart. This can be observed in the left anterior descending artery (A,B,E,G,I,J,K), circumflex (F,H) and right coronary arteries (C, L) and in a saphenous vein graft (D).
Hybrid Coronary Imaging
Given the highly complex, multi-faceted pathophysiology underlying atherosclerosis and its progression to plaque rupture and myocardial infarction, accurate risk prediction may well depend upon a similarly multi-faceted imaging approach, simultaneously assessing a range of pathophysiological and prognostic factors. Hybrid cardiovascular imaging has the potential to provide exactly that information. PET/CT scanners incorporate both CT and PET within the same gantry allowing sequential imaging with the patient in the same position. The two images can then be fused, and used to provide complimentary information: anatomy from the CT and disease activity from PET. Progressively more complex imaging protocols have been introduced combining CT calcium scoring, contrast CT angiography and PET motion correction so that a single scan can potentially provide an assessment of plaque burden, high-risk plaque characteristics, luminal obstruction and disease activity. The hope is that the combined predictive capability of these parameters will facilitate highly accurate prognostic models, allowing systemic therapies to be targeted to patients at highest risk, and reducing the incidence of future myocardial infarction (Figure 4). Again this combined strategy will be assessed in PREFFIR.
Optimization of Coronary PET Imaging
Further work is required to optimize 18F-fluoride imaging in the coronary arteries. This is made particularly difficult by both the effects of coronary motion and by the small caliber of these vessels causing partial voluming.88 ECG-gating improves localization of the signal to the coronary arteries but discards 75% of the data increasing noise.44 New image analysis techniques model and then correct for both cardiac and respiratory motion without discarding any data, allowing improved localization, increased signal and reduce noise.89 Background 18F-fluoride uptake in the coronary arteries appears lower than in the blood-pool of the right atrium, due to partial voluming and the influence of low uptake in the adjacent lungs and myocardium. Further work is required to account for this issue and to determine the best method for quantifying coronary 18F-fluoride uptake and whether increased activity is present. For example it remains unclear whether correcting coronary activity for background uptake in the blood pool or adjacent coronary vasculature is preferable. Moreover whilst 18F-fluoride preferentially binds newly developing areas of microcalcification, there remains some binding to the surface of large macroscopic deposits, suggesting that coronary uptake should perhaps also account for the underlying CT calcium score. The scan-rescan reproducibility of these image analysis approaches also needs to be established before studies examining how the coronary 18F-fluoride signal changes with time are performed. Finally a potential barrier to the future application of PET/CT is the associated high radiation exposure (~10mSV). This is likely to preclude serial imaging of patients and limit our ability to track disease progression and response to therapy. Whilst CT radiation doses are being reduced rapidly an alternative approach is to use PET/MRI that might not only reduce radiation by up to 70% but also improve motion correction and avoid the need for contrast administration. 90,91
Conclusion
Concerns have emerged with imaging strategies aimed at identifying individual vulnerable plaques in the coronary arteries. There is hope that newer strategies providing more global assessments of disease activity across the vasculature will lead to improved risk prediction, differentiating patients with truly stable disease compared to those with active potentially unstable atheroma. These strategies have largely focused on imaging inflammation in larger arteries and more recently microcalcification in the coronary arteries providing important pathophysiological insight and with the potential to ultimately influence patient management. However these relatively expensive techniques will need to demonstrate both added value to risk instruments based on soluble biomarkers and traditional risk factors, and ultimately that their use is clinically effective in reducing cardiovascular events within rigorous large-scale clinical trials.
This Review is in a thematic series on Cardiovascular Imaging which includes the following articles:
T1 Mapping in Characterizing Myocardial Disease: A Comprehensive Review
Non-Invasive Molecular Imaging of Disease Activity in Atherosclerosis
Editors:Jagat Narula and Y. Chandrashekhar
Funding
M.R.D and D.E.N are supported by the British Heart Foundation (CH/09/002 to D.E.N., FS/14/78/31020 to M.R.D). M.R.D is the recipient of the Sir Jules Thorn Biomedical Research Award 2015 (M.R.D.) E.A. research is supported by R01HL 114805 and R01HL 109506. JHFR is part-supported by the NIHR Cambridge Biomedical Research Centre, the British Heart Foundation and the Wellcome Trust.
Nonstandard Abbreviations and Acronyms
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CT
computed tomography
- CVD
cardiovascular disease
- ECG
electrocardiogram
- FDG
fluorodeoxyglucose
- FRS
Framingham risk score
- MI
myocardial infarction
- MR
magnetic resonance
- PCI
percutaneous coronary intervention
- PET
positron emission tomography
- TCFA
thin-cap fibroatheroma
- USPIO
ultra-small particles of iron oxide
Footnotes
Disclosures and Conflicts of Interest: none
References
- 1.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V. Angiographic progression of coronary artery disease and the development of myocardial infarction. JAC. 1988;12:56–62. doi: 10.1016/0735-1097(88)90356-7. [DOI] [PubMed] [Google Scholar]
- 3.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 4.Stone GW, Maehara A, Lansky AJ, De Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. The New England journal of medicine. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 5.Calvert PA, Obaid DR, O’Sullivan M, Shapiro LM, McNab D, Densem CG, Schofield PM, Braganza D, Clarke SC, Ray KK, West NEJ, et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) Study. JACC Cardiovascular imaging. 2011;4:894–901. doi: 10.1016/j.jcmg.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. Journal of the American College of Cardiology. 2015;66:337–346. doi: 10.1016/j.jacc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadi A, Leipsic J, Blankstein R, Taylor C, Hecht H, Stone GW, Narula J. Do plaques rapidly progress prior to myocardial infarction? The interplay between plaque vulnerability and progression. Circulation research. 2015;117:99–104. doi: 10.1161/CIRCRESAHA.117.305637. [DOI] [PubMed] [Google Scholar]
- 8.Kaul S, Narula J. In search of the vulnerable plaque: is there any light at the end of the catheter? Journal of the American College of Cardiology. 2014;64:2519–2524. doi: 10.1016/j.jacc.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, Yabusaki K, Faits T, Bouten C, Franck G, Quillard T, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016 doi: 10.1038/nmat4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann J, Davies MJ. Mechanisms of progression in native coronary artery disease: role of healed plaque disruption. Heart (British Cardiac Society) 1999;82:265–268. doi: 10.1136/hrt.82.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbab-Zadeh A, Nakano M, Virmani R, Fuster V. Acute Coronary Events. Circulation. 2012;125:1147–1156. doi: 10.1161/CIRCULATIONAHA.111.047431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondos GT, Hoff JA, Sevrukov A, Daviglus ML, Garside DB, Devries SS, Chomka EV, Liu K. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 13.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 14.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. The New England journal of medicine. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 15.Øvrehus KA, Schuhbaeck A, Marwan M, Achenbach S, Nørgaard BL, Bøtker HE, Dey D. Reproducibility of semi-automatic coronary plaque quantification in coronary CT angiography with sub-mSv radiation dose. Journal of cardiovascular computed tomography. 2015 doi: 10.1016/j.jcct.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1272–1277. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi T, Kawasaki T, Tanaka A, Yasuda S, Goto Y, Ishihara M, Nishimura K, Miyamoto Y, Node K, Koga N. High-intensity signals in coronary plaques on noncontrast T1-weighted magnetic resonance imaging as a novel determinant of coronary events. Journal of the American College of Cardiology. 2014;63:989–999. doi: 10.1016/j.jacc.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 18.Beer AJ, Pelisek J, Heider P, Saraste A, Reeps C, Metz S, Seidl S, Kessler H, Wester H-J, Eckstein HH, Schwaiger M. PET/CT imaging of integrin αvβ3 expression in human carotid atherosclerosis. JACC Cardiovascular imaging. 2014;7:178–187. doi: 10.1016/j.jcmg.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Mateo J, Izquierdo-Garcia D, Badimon JJ, Fayad ZA, Fuster V. Noninvasive assessment of hypoxia in rabbit advanced atherosclerosis using 18F-fluoromisonidazole positron emission tomographic imaging. Circulation Cardiovascular imaging. 2014;7:312–320. doi: 10.1161/CIRCIMAGING.113.001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104:365–372. doi: 10.1161/01.cir.104.3.365. [DOI] [PubMed] [Google Scholar]
- 21.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 22.Richards JMJ, Semple SI, Macgillivray TJ, Gray C, Langrish JP, Williams M, Dweck M, Wallace W, McKillop G, Chalmers RTA, Garden OJ, et al. Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of iron oxide: a pilot study. Circulation Cardiovascular imaging. 2011;4:274–281. doi: 10.1161/CIRCIMAGING.110.959866. [DOI] [PubMed] [Google Scholar]
- 23.Alam SR, Shah ASV, Richards J, Lang NN, Barnes G, Joshi N, MacGillivray T, McKillop G, Mirsadraee S, Payne J, Fox KAA, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circulation Cardiovascular imaging. 2012;5:559–565. doi: 10.1161/CIRCIMAGING.112.974907. [DOI] [PubMed] [Google Scholar]
- 24.Kooi ME, Cappendijk VC, Cleutjens KBJM, Kessels AGH, Kitslaar PJEHM, Borgers M, Frederik PM, Daemen MJAP, van Engelshoven JMA. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 25.Tang TY, Howarth SPS, Miller SR, Graves MJ, Patterson AJ, U-King-Im J-M, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, Warburton EA, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. Journal of the American College of Cardiology. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Trivedi RA, U-King-Im J-M, Graves MJ, Cross JJ, Horsley J, Goddard MJ, Skepper JN, Quartey G, Warburton E, Joubert I, Wang L, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–1635. doi: 10.1161/01.STR.0000131268.50418.b7. [DOI] [PubMed] [Google Scholar]
- 27.Rudd JHF, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, Tahara N, Fuster V, Warburton EA, Fayad ZA, Tawakol AA. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? Journal of the American College of Cardiology. 2010;55:2527–2535. doi: 10.1016/j.jacc.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 28.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JLE, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nature communications. 2015;6:1–11. doi: 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, Libby P. Hypoxia But Not Inflammation Augments Glucose Uptake in Human Macrophages. Journal of the American College of Cardiology. 2011;58:603–614. doi: 10.1016/j.jacc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. Journal of the American College of Cardiology. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 31.Silvera SS, Aidi HE, Rudd JHF, Mani V, Yang L, Farkouh M, Fuster V, Fayad ZA. Multimodality imaging of atherosclerotic plaque activity and composition using FDG-PET/CT and MRI in carotid and femoral arteries. Atherosclerosis. 2009;207:139–143. doi: 10.1016/j.atherosclerosis.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, Hoffmann U, Brady TJ, Tawakol A. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circulation Cardiovascular imaging. 2012;5:69–77. doi: 10.1161/CIRCIMAGING.110.959478. [DOI] [PubMed] [Google Scholar]
- 33.Marnane M, Merwick A, Sheehan OC, Hannon N, Foran P, Grant T, Dolan E, Moroney J, Murphy S, O’Rourke K, O’Malley K, et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol. 2012;71:709–718. doi: 10.1002/ana.23553. [DOI] [PubMed] [Google Scholar]
- 34.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, Lawler MA, Grinspoon SK, Brady TJ, Nasir K, Hoffmann U, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovascular imaging. 2013;6:1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Moon S-H, Cho YS, Noh TS, Choi JY, Kim B-T, Lee K-H. Carotid FDG Uptake Improves Prediction of Future Cardiovascular Events in Asymptomatic Individuals. JACC Cardiovascular imaging. 2015;8:949–956. doi: 10.1016/j.jcmg.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Rudd JHF, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. Journal of the American College of Cardiology. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. Journal of the American College of Cardiology. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe T, Kawasaki M, Tanaka R, Ono K, Kako N, Saeki M, Onishi N, Nagaya M, Sato N, Miwa H, Arai M, et al. Anti-inflammatory and morphologic effects of pitavastatin on carotid arteries and thoracic aorta evaluated by integrated backscatter trans-esophageal ultrasound and PET/CT: a prospective randomized comparative study with pravastatin (EPICENTRE study) Cardiovasc Ultrasound. 2015;13:17. doi: 10.1186/s12947-015-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii H, Nishio M, Takahashi H, Aoyama T, Tanaka M, Toriyama T, Tamaki T, Yoshikawa D, Hayashi M, Amano T, Matsubara T, et al. Comparison of atorvastatin 5 and 20 mg/d for reducing F-18 fluorodeoxyglucose uptake in atherosclerotic plaques on positron emission tomography/computed tomography: a randomized, investigator-blinded, open-label, 6-month study in Japanese adults scheduled for percutaneous coronary intervention. Clin Ther. 2010;32:2337–2347. doi: 10.1016/j.clinthera.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Tawakol A, Singh P, Rudd JHF, Soffer J, Cai G, Vucic E, Brannan SP, Tarka EA, Shaddinger BC, Sarov-Blat L, Matthews P, et al. Effect of treatment for 12 weeks with rilapladib, a lipoprotein-associated phospholipase A2 inhibitor, on arterial inflammation as assessed with 18F-fluorodeoxyglucose-positron emission tomography imaging. Journal of the American College of Cardiology. 2014;63:86–88. doi: 10.1016/j.jacc.2013.07.050. [DOI] [PubMed] [Google Scholar]
- 41.STABILITY Investigators. White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, et al. Darapladib for preventing ischemic events in stable coronary heart disease. The New England journal of medicine. 2014;370:1702–1711. doi: 10.1056/NEJMoa1315878. [DOI] [PubMed] [Google Scholar]
- 42.Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif J-C, Rudd JHF, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. The New England journal of medicine. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 44.Joshi NV, Vesey AT, Williams MC, Shah ASV, Calvert PA, Craighead FHM, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJR, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 45.Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, Brady TJ, Tawakol A. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovascular imaging. 2010;3:388–397. doi: 10.1016/j.jcmg.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Joshi NV, Toor I, Shah ASV, Carruthers K, Vesey AT, Alam SR, Sills A, Hoo TY, Melville AJ, Langlands SP, Jenkins WSA, et al. Systemic Atherosclerotic Inflammation Following Acute Myocardial Infarction: Myocardial Infarction Begets Myocardial Infarction. J Am Heart Assoc. 2015;4:e001956. doi: 10.1161/JAHA.115.001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joshi NV, Toor I, Shah ASV, Carruthers K, Vesey AT, Alam SR, Sills A, Hoo TY, Melville AJ, Langlands SP, Jenkins WSA, et al. Systemic Atherosclerotic Inflammation Following Acute Myocardial Infarction: Myocardial Infarction Begets Myocardial Infarction. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.115.001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Bauer W, Kreissl MC, Weirather J, Bauer E, Israel I, Richter D, Riehl G, Buck A, Samnick S. Specific somatostatin receptor II expression in arterial plaque: (68)Ga-DOTATATE autoradiographic, immunohistochemical and flow cytometric studies in apoE-deficient mice. Atherosclerosis. 2013;230:33–39. doi: 10.1016/j.atherosclerosis.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Rominger A, Saam T, Vogl E, Ubleis C, la Fougere C, Forster S, Haug A, Cumming P, Reiser MF, Nikolaou K, Bartenstein P, et al. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: correlation with coronary calcium burden and risk factors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:193–197. doi: 10.2967/jnumed.109.070672. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Samnick S, Lapa C, Israel I, Buck AK, Kreissl MC, Bauer W. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res. 2012;2:52. doi: 10.1186/2191-219X-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bird JLE, Izquierdo-Garcia D, Davies JR, Rudd JHF, Probst KC, Figg N, Clark JC, Weissberg PL, Davenport AP, Warburton EA. Evaluation of translocator protein quantification as a tool for characterising macrophage burden in human carotid atherosclerosis. Atherosclerosis. 2010;210:388–391. doi: 10.1016/j.atherosclerosis.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaemperli O, Shalhoub J, Owen DRJ, Lamare F, Johansson S, Fouladi N, Davies AH, Rimoldi OE, Camici PG. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. European heart journal. 2012;33:1902–1910. doi: 10.1093/eurheartj/ehr367. [DOI] [PubMed] [Google Scholar]
- 54.Fujimura Y, Hwang PM, Trout lii H, Kozloff L, Imaizumi M, Innis RB, Fujita M. Increased peripheral benzodiazepine receptors in arterial plaque of patients with atherosclerosis: an autoradiographic study with [(3)H]PK 11195. Atherosclerosis. 2008;201:108–111. doi: 10.1016/j.atherosclerosis.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 55.Pugliese F, Gaemperli O, Kinderlerer AR, Lamare F, Shalhoub J, Davies AH, Rimoldi OE, Mason JC, Camici PG. Imaging of vascular inflammation with [11C]-PK11195 and positron emission tomography/computed tomography angiography. Journal of the American College of Cardiology. 2010;56:653–661. doi: 10.1016/j.jacc.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 56.Gaemperli O, Shalhoub J, Owen DRJ, Lamare F, Johansson S, Fouladi N, Davies AH, Rimoldi OE, Camici PG. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. European heart journal. 2012;33:1902–1910. doi: 10.1093/eurheartj/ehr367. [DOI] [PubMed] [Google Scholar]
- 57.Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N, Tahara A, Constantinescu CC, Zhou J, Boersma HH, Imaizumi T, et al. 2-deoxy-2-[(18)F]fluoro-d-mannose positron emission tomography imaging in atherosclerosis. Nature medicine. 2014;20:215–219. doi: 10.1038/nm.3437. [DOI] [PubMed] [Google Scholar]
- 58.Shaw LJ, Narula J, Chandrashekhar Y. The never-ending story on coronary calcium: is it predictive, punitive, or protective? Journal of the American College of Cardiology. 2015;65:1283–1285. doi: 10.1016/j.jacc.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 59.Aikawa E, Nahrendorf M, Figueiredo J-L, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 60.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 61.Shanahan CM. Inflammation ushers in calcification: a cycle of damage and protection? Circulation. 2007;116:2782–2785. doi: 10.1161/CIRCULATIONAHA.107.749655. [DOI] [PubMed] [Google Scholar]
- 62.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circulation research. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 63.Hsu HH, Camacho NP. Isolation of calcifiable vesicles from human atherosclerotic aortas. Atherosclerosis. 1999;143:353–362. doi: 10.1016/s0021-9150(98)00322-0. [DOI] [PubMed] [Google Scholar]
- 64.Bobryshev YV, Killingsworth MC, Huynh TG, Lord RSA, Grabs AJ, Valenzuela SM. Are calcifying matrix vesicles in atherosclerotic lesions of cellular origin? Basic Res Cardiol. 2007;102:133–143. doi: 10.1007/s00395-006-0637-9. [DOI] [PubMed] [Google Scholar]
- 65.Kapustin AN, Chatrou MLL, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RTM, Alvarez-Hernandez D, Shroff R, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circulation research. 2015;116:1312–1323. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- 66.New SEP, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circulation research. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hutcheson JD, Maldonado N, Aikawa E. Small entities with large impact: microcalcifications and atherosclerotic plaque vulnerability. Current opinion in lipidology. 2014;25:327–332. doi: 10.1097/MOL.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin TC, Tintut Y, Lyman A, Mack W, Demer LL, Hsiai TK. Mechanical response of a calcified plaque model to fluid shear force. Ann Biomed Eng. 2006;34:1535–1541. doi: 10.1007/s10439-006-9182-9. [DOI] [PubMed] [Google Scholar]
- 69.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA : the journal of the American Medical Association. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 70.Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, Nakamura Y, Yamashita H, Yamagishi H, Takeuchi K, Naruko T, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 71.Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, Finn AV, Virmani R. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16:285–292. doi: 10.1097/00001573-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Burke AP, Weber DK, Kolodgie FD, Farb A, Taylor AJ, Virmani R. Pathophysiology of calcium deposition in coronary arteries. Herz. 2001;26:239–244. doi: 10.1007/pl00002026. [DOI] [PubMed] [Google Scholar]
- 73.Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, Tuzcu EM, Nissen SE, Nicholls SJ. Spotty calcification and plaque vulnerability in vivo: frequency-domain optical coherence tomography analysis. Cardiovasc Diagn Ther. 2014;4:460–469. doi: 10.3978/j.issn.2223-3652.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kume T, Okura H, Kawamoto T, Yamada R, Miyamoto Y, Hayashida A, Watanabe N, Neishi Y, Sadahira Y, Akasaka T, Yoshida K. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2011;6:768–772. doi: 10.4244/EIJV6I6A130. [DOI] [PubMed] [Google Scholar]
- 75.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10741–10746. doi: 10.1073/pnas.1308814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maldonado N, Kelly-Arnold A, Cardoso L, Weinbaum S. The explosive growth of small voids in vulnerable cap rupture; cavitation and interfacial debonding. Journal of biomechanics. 2013;46:396–401. doi: 10.1016/j.jbiomech.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, Klutmann S. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:862–865. doi: 10.2967/jnumed.110.076471. [DOI] [PubMed] [Google Scholar]
- 79.Janssen T, Bannas P, Herrmann J, Veldhoen S, Busch JD, Treszl A, Münster S, Mester J, Derlin T. Association of linear 18F-sodium fluoride accumulation in femoral arteries as a measure of diffuse calcification with cardiovascular risk factors: a PET/CT study. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2013;20:569–577. doi: 10.1007/s12350-013-9680-8. [DOI] [PubMed] [Google Scholar]
- 80.Derlin T, Tóth Z, Papp L, Wisotzki C, Apostolova I, Habermann CR, Mester J, Klutmann S. Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, and vascular calcification in atherosclerotic plaque: a dual-tracer PET/CT study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:1020–1027. doi: 10.2967/jnumed.111.087452. [DOI] [PubMed] [Google Scholar]
- 81.Behesti M, Alavi A. Detection and global quantifification of cardiovascular molecular calcifification by flfluoro--18--flfluoride positron emission tomography/computed tomography--A novel concept. Hell J Nucl Med. 2011:14. [PubMed] [Google Scholar]
- 82.Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA, Salter DM, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. doi: 10.1161/CIRCULATIONAHA.111.051052. [DOI] [PubMed] [Google Scholar]
- 83.Dweck MR, Khaw HJ, Sng GKZ, Luo ELC, Baird A, Williams MC, Makiello P, Mirsadraee S, Joshi NV, van Beek EJR, Boon NA, et al. Aortic stenosis, atherosclerosis, and skeletal bone: is there a common link with calcification and inflammation? European heart journal. 2013;34:1567–1574. doi: 10.1093/eurheartj/eht034. [DOI] [PubMed] [Google Scholar]
- 84.Dweck MR, Chow MWL, Joshi NV, Williams MC, Jones C, Fletcher AM, Richardson H, White A, McKillop G, van Beek EJR, Boon NA, et al. Coronary Arterial 18F-Sodium Fluoride Uptake. Journal of the American College of Cardiology. 2012;59:1539–1548. doi: 10.1016/j.jacc.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 85.Jenkins WSA, Vesey AT, Shah ASV, Pawade TA, Chin CWL, White AC, Fletcher A, Cartlidge TRG, Mitchell AJ, Pringle MAH, Brown OS, et al. Valvular (18)F-Fluoride and (18)F-Fluorodeoxyglucose Uptake Predict Disease Progression and Clinical Outcome in Patients With Aortic Stenosis. Journal of the American College of Cardiology. 2015;66:1200–1201. doi: 10.1016/j.jacc.2015.06.1325. [DOI] [PubMed] [Google Scholar]
- 86.Dweck MR, Jenkins WSA, Vesey AT, Pringle MAH, Chin CWL, Malley TS, Cowie WJA, Tsampasian V, Richardson H, Fletcher A, Wallace WA, et al. 18F-Sodium Fluoride Uptake Is a Marker of Active Calcification and Disease Progression in Patients With Aortic Stenosis. Circulation Cardiovascular imaging. 2014;7:371–378. doi: 10.1161/CIRCIMAGING.113.001508. [DOI] [PubMed] [Google Scholar]
- 87.Pawade TA, Newby DE, Dweck MR. Calcification in Aortic Stenosis: The Skeleton Key. Journal of the American College of Cardiology. 2015;66:561–577. doi: 10.1016/j.jacc.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 88.Bucerius J, Hyafil F, Verberne HJ, Slart RHJA, Lindner O, Sciagra R, Agostini D, Ubleis C, Gimelli A, Hacker M Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. European journal of nuclear medicine and molecular imaging. 2015 doi: 10.1007/s00259-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rubeaux M, Joshi N, Dweck MR, Fletcher A, Motwani M, Thomson LE, Germano G, Dey D, Li D, Berman DS, Newby DE, et al. Motion correction of 18F-sodium fluoride PET for imaging coronary atherosclerotic plaques. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015 doi: 10.2967/jnumed.115.162990. [DOI] [PubMed] [Google Scholar]
- 90.Dweck MR, Puntman V, Vesey AT, Fayad ZA, Nagel E. MR Imaging of Coronary Arteries and Plaques. JACC Cardiovascular imaging. 2016;9:306–316. doi: 10.1016/j.jcmg.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 91.Vesey AT, Dweck MR, Fayad ZA. Utility of Combining PET and MR Imaging of Carotid Plaque. Neuroimaging Clin N Am. 2016;26:55–68. doi: 10.1016/j.nic.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Cheng VY, Slomka PJ, Le Meunier L, Tamarappoo BK, Nakazato R, Dey D, Berman DS. Coronary arterial 18F-FDG uptake by fusion of PET and coronary CT angiography at sites of percutaneous stenting for acute myocardial infarction and stable coronary artery disease. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:575–583. doi: 10.2967/jnumed.111.097550. [DOI] [PubMed] [Google Scholar]