Abstract
Allelic exclusion of immunoglobulin genes ensures the expression of a single antibody molecule in B cells through mostly unknown mechanisms. Large-scale contraction of the immunoglobulin heavy-chain (Igh) locus facilitates rearrangements between Igh variable (VH) and diversity gene segments in pro–B cells. Here we show that these long-range interactions are mediated by ‘looping’ of individual Igh subdomains. The Igk locus also underwent contraction by looping in small pre–B and immature B cells, demonstrating that immunoglobulin loci are in a contracted state in rearranging cells. Successful Igh recombination induced the rapid reversal of locus contraction in response to pre–B cell receptor signaling, which physically separated the distal VH genes from the proximal Igh domain, thus preventing further rearrangements. In the absence of locus contraction, only the four most proximal VH genes escaped allelic exclusion in immature μ-transgenic B lymphocytes. Pre–B cell receptor signaling also led to rapid repositioning of one Igh allele to repressive centromeric domains in response to downregulation of interleukin 7 signaling. These data link both locus ‘decontraction’ and centromeric recruitment to the establishment of allelic exclusion at the Igh locus.
The diverse antigen receptor repertoire of lymphocytes is generated by V(D)J recombination, which assembles the variable regions of immunoglobulin and T cell receptor genes from discontinuous variable (V), diversity (D) and joining (J) gene segments during B and T cell development1,2. These gene segments are flanked by recombination signal sequences that function as recognition sites for the V(D)J recombinase consisting of recombination activating gene 1 (RAG1) and RAG2 proteins. After pairing of two compatible recombination signal sequences, the RAG1-RAG2 complex introduces double-strand DNA breaks between the recombination signal sequences and flanking gene segments, followed by processing and religation of the DNA ends by repair factors of the nonhomologous end-joining machinery1,2.
V(D)J recombination is tightly controlled in a lineage- and stage-specific way. Immunoglobulin and T cell receptor genes are rearranged only in B and T lymphocytes, respectively1,2. In the B lymphoid lineage, the immunoglobulin heavy-chain (Igh) locus undergoes rearrangements in pro–B cells before recombination of the genes encoding immunoglobulin light chains (IgL) in small pre–B and early immature B cells1,2. Moreover, DH-JH rearrangements precede VH-DJH recombination in the Igh locus, whereas, among the two IgL genes, the Igk locus rearranges before the Igl locus3. The observed temporal order of V(D)J recombination is determined mainly by the accessibility of the different gene loci and segments to the V(D)J recombinase4,5, which is controlled at multiple levels, including sub-nuclear relocation6, DNA demethylation7, chromatin remodeling8, histone acetylation9,10 and germline transcription4 of the different immunoglobulin loci.
The approximately 200 VH genes of the Igh locus are spread over a 2.4-megabase region and can be divided into 15 distal, central or proximal VH gene families according to their sequence similarity and position relative to the proximal DH segments11. In non–B lymphoid cells and lymphoid progenitors, the two Igh alleles are present in an extended conformation at the potentially repressive periphery of the nucleus6, where they are anchored via the distal VHJ558 gene region with the proximal Igh domain facing toward the center of the nucleus12. This orientation of the Igh locus is likely to facilitate activation of the proximal domain in lymphoid progenitors, thus resulting in DH-JH rearrangements10,13. Early pro–B cell development is characterized by relocation of the Igh alleles to central nuclear positions6, histone acetylation of the distal VHJ558 genes in response to interleukin 7 (IL-7) signaling10, antisense transcription along the entire VH gene cluster14 and long-range contraction of the Igh locus6,12, which ultimately results in VH-DJH recombination. The transcription factor Pax5 has an essential function in regulating contraction of the Igh locus12. The central and distal VH genes are fully accessible in active chromatin and yet fail to rearrange in Pax5−/− pro–B cells15 because of the physical separation of these genes from the proximal DJH-rearranged domain in the absence of locus contraction12.
Successful rearrangement of the Igh locus leads to cell surface expression of the μ protein as part of the pre–B cell receptor (pre-BCR), which functions as an important checkpoint to signal proliferative expansion of populations of large pre–B cells, to induce subsequent differentiation to small pre–B cells and to establish allelic exclusion at the second, DJH-rearranged Igh allele3,16,17. Feedback inhibition of Igh recombination by the membrane-bound μ protein (referred to as allelic exclusion) was initially noted in mice expressing a μ transgene, which efficiently prevents VH-DJH rearrangements at both endogenous Igh alleles during B cell development18–21. RAG protein expression is rapidly lost after pre-BCR signaling, which halts all further V(D)J recombination and ‘prepares the ground’ for the establishment of allelic exclusion in large pre–B cells22. Pre-BCR signaling also leads to histone deacetylation and thus reduced accessibility of the VH genes in small pre–B cells, which has been considered as a possible feedback mechanism underlying allelic exclusion23. These chromatin alterations, however, could be an indirect consequence of pre-BCR signaling, as they depend on the pre-BCR-induced down-regulation of IL-7 signaling23. It is therefore still unknown what changes occur on the DJH-rearranged Igh locus during the short recombinase-free ‘window’ in large pre–B cells so that this allele is unable to further rearrange after subsequent reexpression of the RAG proteins in small pre–B cells.
Here we have identified, using fluorescence in situ hybridization (FISH), two previously unknown mechanisms that are likely to establish allelic exclusion during the recombinase-free transition phase in large pre–B cells. ‘Decontraction’ of both Igh loci was initiated in large pre–B cells and was maintained at all subsequent developmental stages. The reversal of Igh locus contraction is likely to prevent VH-DJH rearrangements in small pre–B cells, in analogy to the extended Igh conformation in Pax5−/− pro–B cells12. Pre-BCR signaling simultaneously induced rapid repositioning of one Igh allele to repressive centromeric domains. This monoallelic centromeric recruitment was transiently maintained in small pre–B and early immature B cells, where it kept the nonfunctional Igh allele in an inactive state during IgL gene rearrangements. In contrast to the Igh alleles, the Igk gene underwent locus contraction specifically in small pre–B and immature B cells, thus demonstrating the general principle that immunoglobulin loci are in a contracted state only in rearranging B lymphocytes. Furthermore, ‘looping’ of individual subdomains was responsible for the contraction of the Igh and Igk loci. Finally, both endogenous Igh alleles were in an extended state without having undergone centromeric recruitment in pre–B and immature B cells of μ-transgenic mice. In the absence of locus contraction, only the four most proximal VH genes escaped allelic exclusion in μ-transgenic B lymphocytes. These data strongly suggest that both locus decontraction and centromeric recruitment establish allelic exclusion at the Igh locus in response to pre-BCR signaling.
RESULTS
Decontraction of the Igh locus in early pre–B cells
To study the contraction state of the Igh locus throughout B cell development, we used two-color three-dimensional DNA FISH analysis to localize the distal VHJ558 and proximal Cγ1 gene segments in three-dimensionally preserved nuclei using confocal laser-scanning microscopy12,24. We isolated B lymphocytes of the following developmental stages by cell sorting: c-Kit+CD19+ bone marrow pro–B cells25, which we cultured on ST2 cells in the presence of IL-7; B220+CD25+ bone marrow pre–B cells25, which we visually identified as large cycling or small resting pre–B cells after FISH analysis; and splenic IgM+ B cells, which we cultured in activating conditions24. We analyzed the sorted B lymphocyte populations using three-dimensional FISH with a labeled Cγ1 probe in combination with a VHJ558 probe (Fig. 1a) that does not cross-hybridize with members of other VH gene families12. The results of these three-dimensional FISH experiments are presented as confocal images (Fig. 1b) and as a statistical evaluation of the distances between the proximal Cγ1 and distal VHJ558 gene segments of the Igh alleles (Fig. 1c). Consistent with previous results6,12, most of the pro–B cells undergoing Igh recombination had their Igh loci in a contracted conformation (Fig. 1b), as the proximal and distal domains were separated in only 10% and 1% of the analyzed Igh alleles by a distance of 0.3–1 μm or 1–1.5 μm, respectively (Fig. 1c and Supplementary Table 1 online). In contrast, the large pre–B cells mainly contained the Igh locus in an extended state (Fig. 1b), as there was a distance of 0.3–1 μm or 1–1.5 μm separating the VHJ558 and Cγ1 genes in 33% and 30% of all Igh alleles, respectively (Fig. 1b,c). All subsequent developmental stages contained the Igh locus in a similarly extended state (Fig. 1b,c). Therefore, Igh alleles undergo decontraction after the production of a functional rearrangement at the onset of pre–B cell development.
Figure 1.
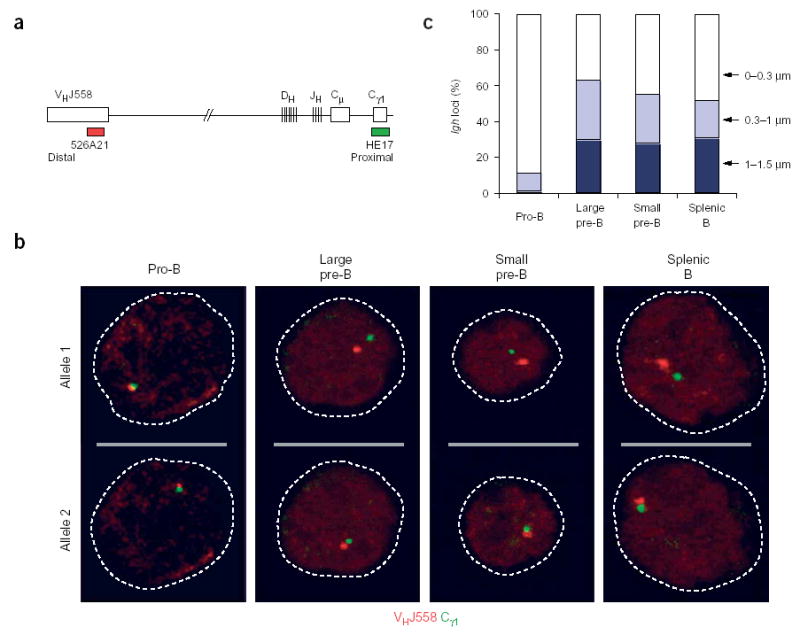
Decontraction of the Igh locus in large pre–B cells. (a) Igh locus (not drawn to scale), indicating the positions of bacterial artificial chromosome (BAC) 526A21 (ref. 12) and plasmid HE17 (ref. 24), which were used to generate the VHJ558 (red) and Cγ1 (green) probes, respectively. (b) Representative confocal sections through the nuclei of B lymphocytes at various developmental stages (above images) in which three-dimensional DNA FISH analysis of the Igh locus was done with VHJ558 (red) and Cγ1 (green) probes. The two Igh alleles of each cell are presented on separate optical sections. Broken lines outline the contours of the nuclei. Pre–B cells were identified as large or small cells under the microscope. (c) Separation of VHJ558 and Cγ1 gene segments. The distance (in μm) separating the VHJ558 and Cγ1 segments was evaluated statistically for B lymphocytes of various developmental stages (horizontal axis). Actual numbers and sample sizes are in Supplementary Table 1 online.
The VHJ558 gene family at the distal end of the Igh locus comprises about 44% of all Igh VH genes11 and should give rise to the least extension after decontraction in response to productive VH-DJH recombination, which juxtaposes the VHJ558 gene family next to the Cγ1 region within a short distance of 100 kilobases (ref. 11) that cannot be resolved by three-dimensional DNA FISH analysis12. The signals of the VHJ558 and Cγ1 genes were colocalized in 37–45% of the Igh alleles analyzed in pre–B cells. Large pre–B cells (‘preB-II’ cells)26 and small pre–B cells (fraction D)27 carry VHJ558-DJH rearrangements on 46% and 41% of their Igh loci, respectively. The close correlation between the frequencies of colocalized signals and VHJ558DJH-rearranged Igh loci strongly suggests that the distance separating the VHJ558 and Cγ1 genes reflects the recombination status of the decontracted Igh alleles in pre–B cells. Accordingly, rearrangements involving genes from the middle of the VH gene cluster are likely to fall within the category of 0.3- to 1.0-μm gene separation. Moreover, Igh alleles with proximal VHQ52, VH7183 and DJH rearrangements should give rise to the full extent of separation (1–1.5 μm), as noted before for the Igh alleles in Rag2−/−Pax5−/− pro–B cells, which are unable to undergo Igh locus contraction and V(D)J recombination12.
Thus, our data demonstrate that the contraction of the Igh locus is reversible and occurs only during V(D)J recombination in pro–B cells. Decontraction in response to pre-BCR signaling is therefore likely to prevent further distal and central VH gene recombination in pre–B cells, whereas it may still be compatible with proximal VHgene rearrangements on the extended Igh locus, analogous to the contraction-deficient Pax5−/− pro–B cells12.
Igk locus contraction coincides with recombination
The Igk locus contains approximately 140 Vκ genes spread over a 3-megabase region28,29 and is thus as large as the Igh locus. The question therefore arises as to whether transient contraction of the Igk locus also facilitates Vκ-Jκ recombination by promoting the interaction between distal Vκ and proximal Jκ gene segments. Although a few Vκ-Jκ rearrangements take place early in pro–B cells30, efficient recombination occurs in small resting pre–B cells and continues in early immature B cells31 undergoing receptor editing32. We therefore divided the immature B cell population of the bone marrow by flow cytometry into early (B220+IgMlo) and late (B220+IgMhi) immature B cells (Supplementary Fig. 1 online) and used these cells together with bone marrow pro–B and pre–B cells and activated splenic B cells to determine the contraction state of the Igk locus at various developmental stages. For this three-dimensional DNA FISH analysis, we used probes detecting the distal Vκ24 gene family and proximal Cκ gene segment at either end of the Igk locus (Fig. 2a). The results of these FISH experiments indicated that the Vκ24 and Cκ genes were separated by a large distance of 1–1.5 μm in 85% of the Igk alleles of pro–B and large cycling pre–B cells (Fig. 2b,c and Supplementary Table 1 online). In contrast, the Vκ24 and Cκ signals were colocalized in most small pre–B cells (90%) and early immature B cells (78%). Late immature B cells were characterized by a notable increase in partially separated (0.3–1.0 μm) or widely separated (1–1.5 μm) Igk alleles (24% and 32%, respectively). In splenic B cells, the Vκ24 and Cκ signals were once again separated by a large distance (1–1.5 μm) in most Igk alleles (72%). The higher proportion of fully extended Igk (72%) versus Igh (31%) alleles noted in splenic B cells (Figs. 1c and 2c) is explained by the different outcome of V(D)J recombination at the two immunoglobulin loci. As all VH genes are oriented in the same transcriptional direction as the DH and JH segments11, recombination invariably results in deletion of the intervening sequences at the Igh locus. In contrast, about two thirds of all Vκ genes are present in antisense orientation relative to the Jκ segments29, which leads to inversion and thus retention of the intervening sequence after Igk recombination. Thus, our data unequivocally demonstrate that Igk alleles are present in a contracted state only when they undergo Vκ-Jκ recombination in small pre–B and immature B cells. Hence, a general principle emerges demonstrating that the contraction of Igh and Igk loci is reversible and occurs only in cells actively undergoing V(D)J recombination.
Figure 2.

Contraction and decontraction of the Igk locus. (a) Igk locus (not drawn to scale), indicating the positions of BAC 296M13 (ref. 50) and plasmid IgkC24, which were used to generate the Vκ24 (red) and Cκ (green) probes, respectively. (b) Representative confocal sections through B cells at various stages of development (above images) in which three-dimensional DNA FISH analysis of the Igk locus was done with Vκ24 (red) and Cκ (green) probes. The two Igk alleles of each cell are presented in separate optical sections. Flow cytometry sorting of the developmental stages is in Supplementary Fig. 1 online. (c) Separation of Vκ24 and Cκ gene segments. The distance (in μm) separating the Vκ24 and Cκ segments was evaluated statistically for cells of various developmental stages (horizontal axis). Actual numbers and sample sizes are in Supplementary Table 1 online. Imm.B, immature B cell.
Locus contraction by looping
We next took advantage of three-color three-dimensional DNA FISH to further analyze the conformation of Igh and Igk loci in cells in which locus contraction occurs (Fig. 3). For these experiments, we used a third probe detecting the VH11 or Vκ21 gene, which are located between the probes used in previous FISH experiments (Fig. 3a,c). DNA FISH analysis of Rag2−/− pro–B cells with VHJ558, VH11 and Cγ1 probes identified several Igh alleles (n = 24) for which the signals of the three probes contacted each other in an order that did not reflect their linear arrangement on the Igh locus (Fig. 3b and Supplementary Fig. 2 online). For example, the VHJ558 signal was located in the middle between the VH15 and Cγ1 signals in the Igh allele in Figure 3b, demonstrating that the VHJ558 gene region looped back onto the proximal Igh domain. Likewise, DNA FISH analysis of early immature B cells identified Igk alleles (n = 20) for which the relative arrangement of the Vκ24, Vκ21 and Cκ signals was incompatible with their gene order in the Igk locus (Fig. 3c,d and Supplementary Fig. 2 online). The Igk allele in Figure 3d showed a looping configuration that brought the proximal Cκ domain in contact with both the Vκ24 and Vκ21 gene families. These data indicate that looping mediates long-range interactions between distinct domains of the Igh or Igk loci, thus resulting in locus contraction.
Figure 3.
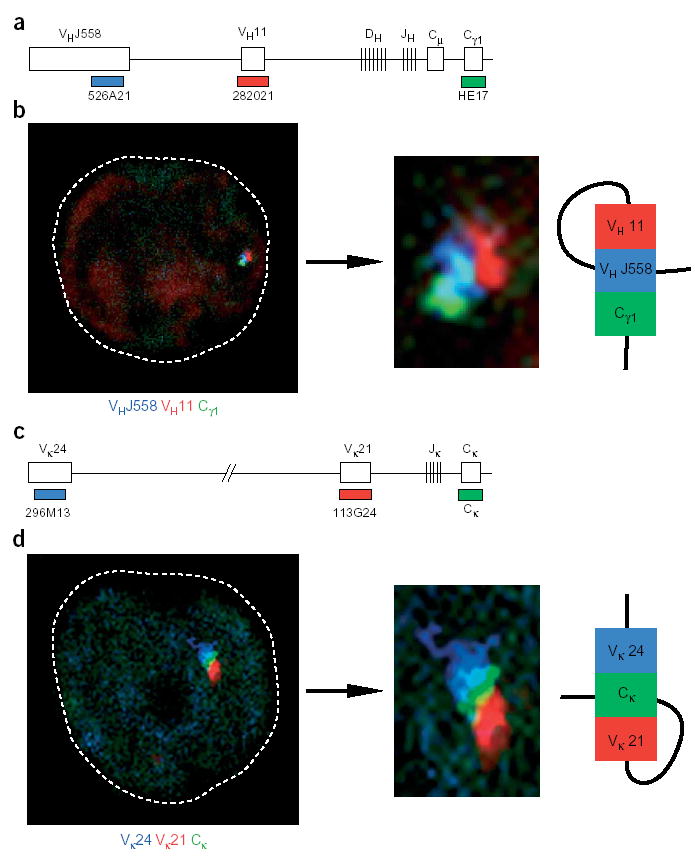
Contraction by looping of the Igh and Igk loci. (a) Igh locus, indicating the position of the third VH11 probe (BAC 282021). (b) Confocal section through the nucleus of a Rag2−/− pro–B cell, which was analyzed by three-dimensional DNA FISH with VHJ558 (blue), VH11 (red) and Cγ1 (green) probes. Middle, enlargement of the Igh allele shown at left; right, looping configuration. (c) Igk locus, indicating the position of the third Vκ21 probe (BAC 113G24; ref. 51). (d) Confocal image of an early immature B cell showing the relative positions of the Vκ24 (blue), Vκ21 (red) and Cκ (green) probe signals. Middle, higher magnification of the Igk allele shown at left; right, looping configuration. There was similar looping of Igk and Igh loci in 20 and 24 cells, respectively. Additional alleles with Igk and Igh looping are in Supplementary Fig. 2 online.
Centromeric location of one Igh allele in pre–B cells
The transcriptionally silent Igh allele is located at centromeric heterochromatin in activated splenic B cells, whereas the productively rearranged, expressed Igh allele is positioned away from centromeric clusters24. To determine whether centromeric recruitment of one Igh allele may contribute to allelic exclusion in pre–B cells, we examined the nuclear position of Igh loci relative to centromeres at various developmental stages using three-color three-dimensional DNA FISH (Fig. 4 and Supplementary Table 2 online). We used a γ-satellite probe to visualize the centromeric foci, whereas VHJ558 and Cγ1 probes localized the distal and proximal Igh domains within the nucleus. There was infrequent centromeric recruitment (16%) in pro–B cells, as reported before24 (Fig. 4c). In contrast, we detected association of one Igh allele with γ-satellite clusters in 66–75% of large and small pre–B cells and in 65% of early immature B cells (Fig. 4a,c). During subsequent development, the frequency of centromeric recruitment was decreased to 34% in late immature B cells (Fig. 4c) and to 28% in resting splenic B cells24 (Fig. 5). Hence, centromeric recruitment of one Igh allele is initiated together with allelic exclusion at the onset of pre–B cell development and is transiently maintained in B lymphocytes undergoing IgL gene rearrangements.
Figure 4.

Monoallelic centromeric recruitment of the Igh locus during B cell development. (a) Centromeric location of one Igh allele in bone marrow pre–B cells. Large and small pre–B cells and activated splenic B cells were analyzed by three-color three-dimensional DNA FISH with VHJ558 (red), Cγ1 (green) and γ-satellite (γ-sat; blue) probes. The relative positions of the three signals are shown in confocal sections through the nuclei of these cells. (b) Association of the distal VHJ558 gene domain with centromeric clusters. Enlargements show the orientation and decontraction of the Igh locus at γ-satellite clusters. Below images, distance between the VHJ558 and Cγ1 probe signals. (c) Monoallelic recruitment of the Igh locus to centromeres. Data represent the percentage of cells showing association of one Igh allele with centromeric heterochromatin in various B cell developmental stages, sorted as indicated in Supplementary Fig. 1 online. Actual numbers and sample sizes are in Supplementary Table 2 online. (d) Preferential location of widely separated Igh alleles at the centromeres. The cells showing monoallelic centromeric recruitment were subdivided into a population of cells containing an Igh allele with wide separation (1–1.5 μm) of the VHJ558 and Cγ1 genes. Data represent the percentage of centromeric recruitment of the widely separated Igh allele in this cell population for large and small pre–B cells and activated (Act.) splenic B cells.
Figure 5.

IL-7 signaling prevents centromeric recruitment of the Igh locus in splenic B cells. (a) Two-color three-dimensional DNA FISH analysis of activated splenic B cells with (+IL-7) or without (Control) IL-7, showing the proximity of the Cγ1 (Igh) and Cκ (Igk) signals (green) with γ-satellite clusters (red). Both Igh or Igk alleles are on the same optical section. (b) Statistical analysis of cells with centromeric association of one Igh or Igk allele in splenic B cells before and after in vitro activation with anti-CD40 in the presence (+IL-7) or absence of IL-7. Actual numbers and sample sizes are in Supplementary Table 4 online.
To gain insight into whether the DJH-rearranged or nonproductively VHDJH-rearranged Igh allele is recruited to centromeres in pre–B cells, we assessed individual Igh alleles for both centromeric recruitment and locus decontraction. For this, we evaluated all cells that showed centromeric recruitment and additionally contained one Igh allele with widely separated (1–1.5 μm) VHJ558 and Cγ1 genes. The widely separated Igh allele in this cell population was recruited to the centromere in 81–91% of small and large pre–B cells and in 88% of activated splenic B cells (Fig. 4d). The class of widely separated Igh loci is likely to consist of all Igh alleles with proximal VH7183-DJH, VHQ52-DJH and DH-JH rearrangements, whereas the recombination of VH genes located in the central and distal Igh domains should give rise to only partial gene separation (0–1 μm) after decontraction in pre–B cells. The rearrangement status of Igh alleles was analyzed before in large pre–B (preB-II) cells by single-cell PCR assay26. Reevaluation of those data demonstrated that DH-JH and nonfunctional VH-DJH rearrangements accounted for 81% of all rearrangements within the proximal Igh domain compared with 26% of nonfunctional VH gene rearrangements in the central and distal Igh domains (Supplementary Table 3 online). The close correlation between centromeric recruitment (81–91%) and nonproductive recombination (81%) in the proximal Igh domains strongly suggests that the DJH-rearranged or nonfunctionally VHDJH-rearranged Igh allele is recruited to the centromere at the onset of pre–B cell development.
IL-7 signaling prevents centromeric Igh recruitment
As shown by three-dimensional FISH analysis, the recruited Igh locus was oriented at the centromere in pre–B and activated B cells in such a way that the distal VHJ558 gene family was positioned closer to the γ-satellite cluster than the proximal VH7183 and Cγ1 genes in 96% (50 of 52) and 90% (102 of 113) of recruited Igh alleles, respectively (Fig. 4a,b and data not shown). Centromeric recruitment of the VHJ558 gene family thus coincides with histone deacetylation of the distal Igh domain in pre–B cells23, suggesting a link between these two processes. Deacetylation of the distal VH genes has been associated with downregulation of IL-7 receptor signaling at the pro–B cell–to–pre–B cell transition23. Moreover, the distal VHJ558 genes are hypoacetylated in resting splenic B cells, but can be acetylated during B cell activation in response to IL-7 signaling23, which mimics opening of the distal Igh domain in early pro–B cell development10. To investigate whether IL-7 signaling prevents centromeric recruitment of the Igh locus, we activated splenic B cells for 4 d with an antibody to CD40 (anti-CD40) in the presence or absence of IL-7, as described23. DNA FISH analysis with Cγ1 and γ-satellite probes showed that B cell activation in the presence of IL-7 resulted in substantially reduced centromeric recruitment of the Igh locus (38%), which approximated the basal recruitment (28%) found in resting B cells at day 0 (Fig. 5a,b and Supplementary Table 4 online). The observed inverse correlation between IL-7 signaling and centromeric Igh recruitment in splenic B cells thus suggests that the downregulation of IL-7 receptor signaling in large pre–B cells23,33 may be required for the initiation of centromeric recruitment of the Igh locus at the onset of pre–B cell development. Treatment of activated B cells with IL-7, however, had no effect on repositioning of the Igk allele24 to centromeric clusters (Fig. 5a,b), indicating that different factors regulate centromeric recruitment at the Igh and Igk loci. In activated splenic B cells, the Igk locus was recruited to the centromeres also via the distal Vκ24 region (Supplementary Fig. 3 online), demonstrating that centromeric repositioning of immunoglobulin loci via their distal domain is a more general phenomenon.
Extended Igh alleles in μ-transgenic B lymphocytes
Cell surface expression of a functional μ protein is essential for initiating of feedback inhibition of VH-DJH recombination and Igh allelic exclusion in pre–B cells3,16. Allelic exclusion of the Igh locus has been studied extensively in μ-transgenic mice, in which the endogenous Igh alleles rarely undergo VH-DJH rearrangements, in contrast to efficient DH-JH recombination18–21. To further investigate the relationship between centromeric recruitment, locus decontraction and VH-DJH recombination, we next analyzed the pre–B and B cells of two μ-transgenic strains of mice, M54 (ref. 34) and MD4 (ref. 35). Flow cytometry analysis confirmed that the splenic B cells of M54 and MD4 mice expressed transgenic IgMa and no endogenous IgMb (C57BL/6), as published36,37 (Fig. 6a and data not shown). Before three-dimensional FISH analysis, we tested the VHJ558 and Cγ1 probes on metaphase spreads of activated splenic MD4 B cells to ensure that they detected only the endogenous Igh loci at the telomeres of chromosome 12 instead of the μ transgene (Fig. 6b and data not shown). Contrary to expectations, the three-dimensional DNA FISH experiments failed to show any substantial association of the endogenous Igh loci with γ-satellite clusters in bone marrow pre–B and immature B cells and activated splenic B cells of either M54 or MD4 mice (Fig. 6c–e and Supplementary Table 2 online). In addition, three-color FISH analyses of pre–B and immature B cells showed that the Cγ1 signals were separated from the VHJ558 signals by a distance of 1–1.5 μm in more than 84% of all endogenous Igh alleles (Fig. 6d,f and Supplementary Table 4 online). Hence, this situation resembles the noncontracted state of the germline Igh alleles in Rag2−/−Pax5−/− pro–B cells12. We conclude therefore that the endogenous Igh alleles of μ-transgenic B lymphocytes are present in an extended conformation and undergo allelic exclusion in the absence of centromeric recruitment.
Figure 6.
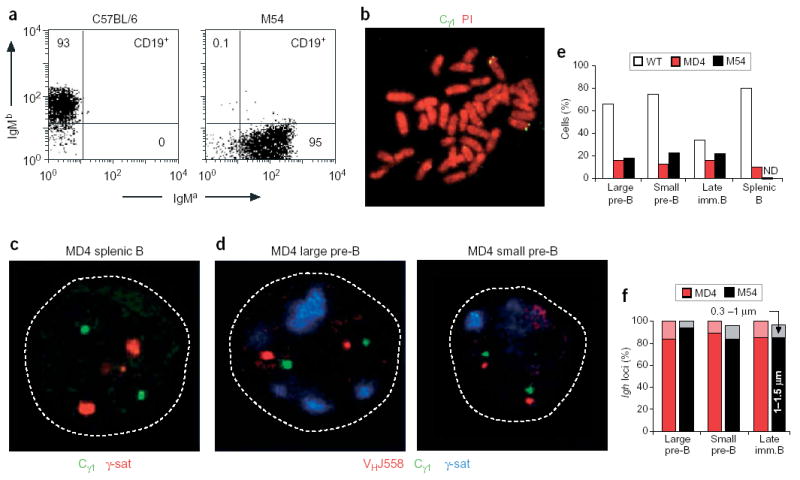
Absence of centromeric recruitment and locus contraction of endogenous Igh alleles in μ-transgenic B cells. (a) Flow cytometry of IgM expression on CD19+ B cells of C57BL/6 and M54 transgenic mice. The M54 transgene of the IgMa haplotype was derived from the BALB/c hybridoma 17.2.25 (ref. 34) and was crossed into the C57BL/6 mouse strain of the IgMb haplotype. Lymph node B cells from a 3-week-old M54 mouse express transgenic IgMa but no endogenous IgMb, as published36,37. Numbers in dot plots indicate the percentages of cells in quadrants. The same degree of allelic exclusion was noted for MD4 transgenic B cells35 (data not shown). (b) Metaphase spread of MD4 transgenic B cells. Specific hybridization of the Cγ1 probe (green) is detected at the telomeres of chromosome 12. Chromosomes were counterstained with propidium iodide (PI; red). (c) Confocal section through the nucleus of an activated splenic MD4 B cell. The Cγ1 regions (green) of both endogenous Igh alleles are not associated with γ-satellite clusters (red), as visualized by three-dimensional DNA FISH. (d) Confocal sections through the nuclei of large and small pre–B cells of the MD4 transgenic mouse. The Cγ1 (green) and VHJ558 (red) signals are widely separated and are not associated with γ-satellite clusters (blue). (e) Statistical analysis of the monoallelic centromeric association of endogenous Igh loci in B lymphocytes of wild-type (WT) and MD4 and M54 transgenic mice. ND, not determined. Actual numbers and sample sizes are in Supplementary Table 2 online. (f) Statistical analysis of the distance separating the distal VHJ558 and proximal Cγ1 regions of the endogenous Igh loci in developing MD4 (red) and M54 (black) transgenic B lymphocytes (Supplementary Table 4 online). Light or dark shading indicates VHJ558-Cγ1 gene separation of 0.3–1 μm or 1–1.5 μm, respectively.
Allelic inclusion of the most proximal VH genes
The data reported above suggest that decontraction rather than centromeric recruitment is responsible for allelic exclusion of endogenous Igh alleles in μ-transgenic pre–B cells. The absence of locus contraction furthermore suggests that proximal VH genes should be able to rearrange in μ-transgenic pre–B cells in analogy to Pax5−/− pro–B cells12. Indeed, mouse B cells expressing a human μ transgene have been shown to carry proximal VHQ52 gene rearrangements at a relatively high frequency20, although the results obtained with the mouse M54 transgene are more ambiguous37. We therefore reinvestigated the rearrangement status of endogenous Igh alleles in splenic M54 B cells, which we sorted for expression of the μa transgene (Supplementary Fig. 4 online). Rearrangements of the VHQ52 and VH7183 genes were readily identified in M54 B cells, albeit at a lower frequency than in wild-type B cells and Pax5−/− pro–B cells (Fig. 7). However, we were unable to detect VHGam3.8 and VHJ558 gene rearrangements in M54 B cells (Fig. 7), consistent with the fact that these genes depend on Igh locus contraction for efficient recombination12. Hence, our results, together with earlier work20, unequivocally demonstrate that proximal VH genes fail to be allelically excluded in B lymphocytes expressing either a mouse or a human μ transgene.
Figure 7.
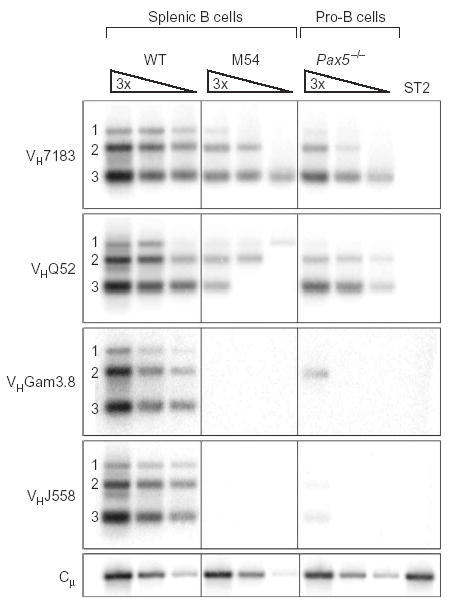
Proximal VH-DJH rearrangements in M54 transgenic B cells. IgMa CD19+ B cells expressing the μa transgene were isolated by flow cytometry sorting from the spleens of M54 transgenic mice (Supplementary Fig. 4 online). PCR was used to determine the frequency of the different VH-DJH rearrangements in these transgenic B cells versus Pax5−/− bone marrow pro–B cells and wild-type B220+ splenocytes. Threefold (3×) serial DNA dilutions were analyzed by PCR with VH family–specific forward primers and a JH3 reverse primer12, which was unable to amplify the VHDJH4-rearranged M54 transgene34. Bottom, input DNA was normalized by PCR amplification of an Igh Cμ fragment; far right lane, DNA of stromal ST2 cells (negative control). Numbers along the left margin indicate rearrangements to the JH1, JH2 and JH3 segments. The same result was obtained in two independent experiments.
As the two overlapping VHQ52 and VH7183 gene families constitute the proximal 320-kilobase region of the VH gene cluster11 (Fig. 8a), the question arises as to whether all of these genes or only a subset of them are able to undergo VH-DJH recombination in the absence of Igh locus contraction. To investigate this, we compared the spectrum of VHQ52-DJH and VH7183-DJH rearrangements in the presence of Igh locus contraction in wild-type pro–B cells12 and in the absence of Igh locus contraction in Pax5−/− pro–B cells12, M54 transgenic IgMa B cells and ‘IkPax5/+’ pro–T cells12, which ectopically express a Pax5 ‘mini-gene’ under the control of the Ikaros (Zfpn1a1) locus38. We amplified VH-DJH4 rearrangements of pro–B and pro–T cells and VH-DJH3 rearrangements of M54 (VHDJH4)34 transgenic B cells by PCR, then cloned and sequenced them and assigned the sequences to the various members of the VHQ52 and VH7183 gene families (Fig. 8b,c). In the presence of locus contraction, VH genes across the entire 320-kilobase region underwent VH-DJH recombination in wild-type pro–B cells, although with different efficiencies, as exemplified by the published high rearrangement frequency of the VH7183.b2 (VH81X) gene39,40. In contrast, only the two most proximal, functional VH genes of the VHQ52 (b1 and b2) and VH7183 (b2 and b4) gene families were able to rearrange at a high frequency in M54 B lymphocytes and IkPax5/+ pro–T cells, whereas Pax5−/− pro–B cells showed the same trend of preferentially rearranging the more proximally located VH gene (Fig. 8b,c). These data indicate that only the four most proximal VH genes of the Igh locus efficiently escape allelic exclusion in μ-transgenic B lymphocytes.
Figure 8.

The most proximal VH genes escape allelic exclusion in M54 transgenic B lymphocytes. (a) VHQ52 and VH7183 gene families of the mouse C57BL/6 strain. The family members were identified by annotation of the Igh sequences of the mouse genome database (http://mendel.imp.univie.ac.at/SEQUENCES/VH/). The VH genes are numbered according to published nomenclature11. b, C57BL/6; p, pseudogenes. Our annotation identified previously unknown family members (n). Thick horizontal black bars indicate a large sequence duplication; genes with high sequence similarity are in the same color. The VH7183.b2 gene segment of the C57BL/6 mouse corresponds to the VH81X gene segment of the BALB/c strain. (b,c) Statistical analysis of VH-DJH rearrangements involving different members of the VHQ52 (b) and VH7183 (c) gene families in wild-type and Pax5−/− pro–B cells, IkPax5/+ pro–T cells and M54 transgenic IgMa B cells. VH-DJH4 rearrangements of pro–B and pro–T cells and VH-DJH3 rearrangements of M54 B cells were amplified by PCR, cloned, sequenced and assigned to the various family members. Data represent the percentage of rearrangements involving individual genes for each cell type. Total number of distinct rearrangements analyzed: wild-type pro–B cells, 76 (VHQ52) and 123 (VH7183); Pax5−/− pro–B cells, 61 (VHQ52) and 83 (VH7183); IkPax5/+ pro–T cells, 73 (VHQ52) and 81 (VH7183); splenic M54 B cells, 71 (VHQ52) and 79 (VH7183).
DISCUSSION
Allelic exclusion is responsible for monoallelic expression of immunoglobulin genes, which ensures the single-receptor specificity of B cells, and yet the molecular mechanisms underlying this important phenomenon are still mostly ‘enigmatic’3. Here we have described centromeric recruitment and locus decontraction as two previously unknown mechanisms that contribute to allelic exclusion of immunoglobulin genes. Locus decontraction seems to be particularly important, as all VH genes, with the notable exception of the four most proximal VH genes, were allelically excluded in the absence of Igh locus contraction in μ-transgenic B lymphocytes.
Centromeric recruitment has been associated with transcriptional silencing of the nonfunctionally rearranged Igh allele in activated B cells24. Here we have shown that centromeric repositioning of one Igh allele was initiated by the onset of pre–B cell development and was transiently maintained in small pre–B and immature B cells undergoing IgL rearrangements. The close statistical correlation between fully extended Igh alleles at the centromere and alleles with DH-JH and nonfunctional VH-DJH rearrangements in the proximal Igh domain strongly indicated that centromeric recruitment contributes to the inactivation of the nonproductively rearranged Igh allele in pre–B and immature B cells. As the mechanism controlling the monoallelic repositioning to centromeres discriminates between the two Igh alleles, the question arises as to which distinguishing feature of these alleles determines the choice of the nonproductively rearranged Igh locus for centromeric recruitment. For example, 40% of all pre–B cells contain one productive and one nonproductive VH-DJH rearrangement3. These two alleles functionally differ from each other only by a single frameshift mutation resulting in transcripts with a premature termination codon, which could be discriminated in the nucleus by the ‘nonsense-mediated’ mRNA decay pathway41. As transcription, mRNA processing and monitoring of the nonsense codon take place in the proximal Igh region, it is conceivable that this information is already transmitted in the contracted state to the distal VHJ558 gene region, which subsequently is recruited to centromeric clusters in pre–B cells. The Igk locus is also associated with centromeres via the distal Vκ24 region in activated splenic B cells, indicating that the centromeric recruitment of immunoglobulin loci via their distal domain is a more general phenomenon. During the pro–B cell–to–pre–B cell transition, the distal VHJ558 genes are not only recruited to centromeres but also undergo histone deacetylation, leading to reduced chromatin accessibility23 as a consequence of the downregulation of IL-7 receptor signaling in pre–B cells23,33. The idea of a causal link between histone deacetylation and centromeric recruitment of the Igh locus is supported by the fact that treatment of activated splenic B cells with IL-7 interferes with centromeric recruitment of the Igh allele (as reported here) while simultaneously inducing histone acetylation of the distal VHJ558 genes23. These findings are in agreement with the temporal coincidence of histone deacetylation and centromeric recruitment during the establishment of heritable silencing at the terminal deoxynucleotidyltransferase (Dntt) locus42. It is unclear, however, whether histone deacetylation is the cause or consequence of subnuclear repositioning to centromeres, which constitute an environment with abundant histone deacetylase complexes43. Finally, centromeric recruitment seems to be incomplete in pre–B cells, as only 66–75% of all nonfunctional Igh alleles are positioned at centromeres. However, the Igh alleles in the remaining approximately 30% of cells were located at the nuclear periphery12 (data not shown), which may function, in addition to the centromeres, as a repressive subnuclear compartment44.
In contrast to centromeric recruitment, locus decontraction equally affects the two Igh alleles at the onset of pre–B cell development, as it disconnects the proximal region from the central-distal domains on both Igh loci. This physical separation, however, has a functional consequence only for the incompletely DJH-rearranged Igh allele, where rearrangements of central and distal VH genes are probably prevented, analogous to the situation described for Pax5−/− pro–B cells12,15. Whereas the Igh locus is in a decontracted state in pre–B cells and all subsequent developmental stages, the Igk locus specifically undergoes contraction during the phase of Igk rearrangements in small pre–B and immature B cells. Hence, distinct factors must be involved in controlling the contraction of Igh and Igk loci at different stages of B lymphopoiesis. Our FISH analyses demonstrated the new general principle that immunoglobulin loci actively undergo contraction only in rearranging cells, whereas they are in the extended default state at all other developmental stages. Our experiments furthermore showed that the looping of multiple subdomains is responsible for contraction of both the Igh and Igk loci in rearranging B lymphocytes. At the molecular level, Pax5 has been identified as a key regulator, which induces Igh locus contraction in pro–B cells in cooperation with an unknown factor ‘X’12. The inactivation of Pax5, however, is unlikely to be the cause of locus decontraction in response to pre-BCR signaling, as the function of this transcription factor is required throughout B cell development45. Identification of factor ‘X’ will be needed to test whether pre-BCR signaling induces the loss of this regulator, thereby leading to decontraction of the Igh locus.
Immunoglobulin μ transgenes have been used successfully to study allelic exclusion of the endogenous Igh alleles during B cell development18–21. Our analysis of two different transgenes (M54 and MD4) has unequivocally demonstrated that the endogenous Igh alleles are present in an extended conformation but are not recruited to centromeres in transgenic pre–B and immature B cells. The absence of centromeric recruitment raises the question of how relevant the use of immunoglobulin μ transgenes is for investigating the phenomenon of allelic exclusion. Indeed, early expression of a functional μ transgene is known to considerably shorten or even bypass pro–B cell development46, in which both Igh loci are normally made accessible at the chromatin level4,5,10 and undergo locus contraction6,12 before VH-DJH recombination. As μ-transgenic B cell precursors have only very low expression of VHJ558 germline transcripts (data not shown), the distal VH gene region may never be reorganized into accessible acetylated chromatin, which, however, seems to be a prerequisite for subsequent centromeric recruitment of the Igh locus in pre–B cells. Our experiments using B cells from μ-transgenic mice thus suggest a function for centromeric recruitment in reducing the accessibility of central-distal domains of Igh loci that have previously been opened up during proB cell development. In contrast, the proximal Igh domain is activated in both wild-type as well as μ-transgenic pre–B and immature B cells, as germline transcription of the proximal VH7183 genes is as efficient in these cells as in pro–B cells (data not shown), and DH-JH recombination of endogenous Igh loci occurs at a high rate in transgenic pre–B cells21. Unexpectedly, however, only the four most proximal VH genes were efficiently rearranged in transgenic B cells, indicating that the absence of locus contraction interferes with VH-DJH recombination of all other VH genes in Igk-rearranging pre–B and immature B cells. In wild-type mice, the rare allelically included B cells, which express two different IgM proteins on their cell surface, show a disproportionately high frequency of proximal VHQ52-DJH and VH7183-DJH rearrangements47. Given our results, it is conceivable that the most proximal VH genes escaping allelic exclusion may give rise to some of these VH-DJH rearrangements not only in pro–B cells but also in small pre–B and immature B cells.
In summary, our study has provided insight into how pre-BCR signaling controls allelic exclusion at the Igh locus. Pre-BCR signaling results in the rapid loss of RAG protein expression, thereby halting all further V(D)J recombination and ‘preparing the ground’ for the establishment of allelic exclusion22. However, it is not known which changes the Igh locus would undergo during the short recombinase-free ‘window’ so that it could no longer be rearranged after subsequent re-expression of RAG proteins. As shown here, pre-BCR signaling in large cycling pre–B cells additionally induces rapid decontraction and centromeric recruitment of the Igh locus, which are likely to prevent VH-DJH rearrangement of the second Igh allele after re-expression of the V(D)J recombinase in small pre–B cells.
METHODS
Mice
IkPax5/+, Pax5+/− and Rag2−/− mice38,48,49 and μ-transgenic M54 and MD4 mice34,35 were maintained on the C57BL/6 background and were genotyped as described34,35,38,48,49.
Flow cytometry sorting and analysis
Antibodies to the following, coupled to fluorescein isothiocyanate (FITC), phycoerythrin or allophycocyanin, were used for flow cytometry: B220 (RA3-6B2), CD4 (L3T4), CD8a (53-6.7), CD19 (1D3), CD25/IL-2Rα (PC61), CD117/c-Kit (2B8), IgMa (DS1) and IgMb (AF6-78; PharMingen), and goat polyclonal anti-mouse IgM (Southern Biotech). Wild-type bone marrow was stained with the appropriate antibody combination, and pro–B cells were isolated on a MoFlo cell sorter (Dako-Cytomation) as c-Kit+CD19+ cells; pre–B cells, as B220+CD25+ cells; early immature B cells, as B220+ IgMlo B cells; and late immature B cells, as B220+IgMhi cells. The purity of the sorted cells was verified by flow cytometry reanalysis (Supplementary Fig. 1 online).
Activation of splenic B cells
Splenic B cells of BALB/c mice were isolated by elimination of non–B cells by magnetic cell sorting with anti-CD43 MACS beads (Miltenyi Biotec) and then were activated with anti-CD40 (FGK-45) as described24. The activated B cells were grown with or without 1% conditioned supernatant of IL-7–producing J558L cells48 and were collected after 4 d for three-dimensional DNA FISH analysis.
Probes for FISH
The locus-specific DNA probes were prepared from the published bacterial artificial chromosomes CT7-526A21 (VHJ558)6, RP24-282021 (VH11), CT7-296M13 (Vκ24)50 and CT7-113G24 (Vκ21)51 and plasmids HE17 (Cγ1) and IgkC (Cκ)24 and were labeled by nick translation with dUTP-indodicarbocyanine, digoxygenin-dUTP or biotin-dUTP (Roche/Enzo Biochem). The γ-satellite probe was prepared from a plasmid containing eight copies of the γ-satellite repeat sequence24 and was labeled directly with dUTP-rhodamine or dUTP-indodicarbocyanine (Amersham Pharmacia).
Three-dimensional DNA FISH and confocal analysis
Cells sorted by flow cytometry were washed three times in PBS and then were fixed onto poly-L-lysine-coated slides for two- and three-color three-dimensional DNA FISH analysis as described in detail12,24,52. Digoxygenin-labeled DNA probes were detected with sheep rhodamine-coupled anti-digoxygenin (Roche/Enzo Biochem), followed by further signal amplification with Texas red–coupled anti-sheep (Vector). Biotinylated DNA probes were detected with FITC-avidin followed by further signal amplification with biotinylated FITC-coupled anti-avidin and FITC-avidin (Vector). Cells were analyzed by confocal microscopy on a Leica SP2 AOBS (acoustica optical beam splitter) system. Optical sections separated by 0.3 μm were collected, and only cells with signals from both alleles (typically 90%) were analyzed.
V(D)J recombination assay
B220+c-Kit+ pro–B cells were sorted from the bone marrow of 2-week-old Pax5−/− mice; B220+ splenocytes, from wild-type mice; and IgMa CD19+ splenocytes, from M54 transgenic mice at the age of 4.5 months (Supplementary Fig. 4 online). DNA was isolated and analyzed for the presence of VH-DJH rearrangements (Fig. 7) by PCR assay with published primers as described12.
PCR cloning and sequencing of VH-DJH rearrangements
Pax5+/+ and Pax5−/− bone marrow pro–B cells as well as IkPax5/+ CD4+CD8+ thymocytes were isolated by flow cytometry sorting as described12, followed by DNA preparation and PCR amplification of VHQ52-DJH4 and VH7183-DJH4 rearrangements with the following primers27:5′-CTCACAGAGCCTGTCCATCAC-3′ (forward VHQ52 VHB), 5′-GTGGAGTCTGGGGGAGGCTTA-3′ (forward VH7183 VHE) and 5′-TCTCAGCCGGCTCCCTCAGGG-3′ (reverse JH4A). IgMa CD19+ splenocytes were isolated from 4.5-month-old M54 mice as shown in Supplementary Fig. 4 online. As the μ transgene M54 carries a JH4 rearrrangement34, we amplified the rearrangements of endogenous Igh alleles from M54 transgenic B cells using the forward VH7183 VHE or VHQ52 VHB primer in combination with the JH3 primer12 5′-GTCTAGATTCTCACAAGAGTCCGATA-GACCCTGG-3′. The VH-DJH4 or VH-DJH3 PCR fragment was gel-purified and was cloned into the pGEM-T easy vector (Promega), followed by DNA sequencing of individual clones. The 250– to 300–base pair 3′ sequence of each VH gene was assigned by sequence comparison to its corresponding VHQ52 and VH7183 family member. For this purpose, we annotated the entire VHQ52 and VH7183 gene region of the mouse C57BL/6 strain (http://mendel.imp.univie.ac.at/SEQUENCES/VH/) based on the Igh sequences of the mouse genome database (www.ensembl.org; release NCBIm32). Only VH sequences with distinct VDJ joints were used for statistical analysis, with identical sequences being counted only once.
Supplementary Material
Acknowledgments
We thank F. Batista for providing MD4 mice, and A. Corcoran and N. Mitchison for critically reviewing the manuscript. Supported by a Wellcome Trust University Award, Boehringer Ingelheim and the Austrian GEN-AU initiative (financed by the Bundesministerium für Bildung, Wissenschaft und Kultur).
Footnotes
Note: Supplementary information is available on the Nature Immunology website.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
References
- 1.Hesslein DG, Schatz DG. Factors and forces controlling V(D)J recombination. Adv Immunol. 2001;78:169–232. doi: 10.1016/s0065-2776(01)78004-2. [DOI] [PubMed] [Google Scholar]
- 2.Bassing CH, Swat W, Alt FW. The mechanism and regulation of chromosomal V(D)J recombination. Cell. 2002;109:S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 3.Mostoslavsky R, Alt FW, Rajewsky K. The lingering enigma of the allelic exclusion mechanism. Cell. 2004;118:539–544. doi: 10.1016/j.cell.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Yancopoulos GD, Alt FW. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- 5.Stanhope-Baker P, Hudson KM, Shaffer AL, Constantinescu A, Schlissel MS. Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell. 1996;85:887–897. doi: 10.1016/s0092-8674(00)81272-6. [DOI] [PubMed] [Google Scholar]
- 6.Kosak ST, et al. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 7.Mostoslavsky R, et al. K chain monoallelic demethylation and the establishment of allelic exclusion. Genes Dev. 1998;12:1801–1811. doi: 10.1101/gad.12.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maes J, et al. Chromatin remodeling at the Ig loci prior to V(D)J recombination. J Immunol. 2001;167:866–874. doi: 10.4049/jimmunol.167.2.866. [DOI] [PubMed] [Google Scholar]
- 9.McBlane F, Boyes J. Stimulation of V(D)J recombination by histone acetylation. Curr Biol. 2000;10:483–486. doi: 10.1016/s0960-9822(00)00449-8. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhury D, Sen R. Stepwise activation of the immunoglobulin μ heavy chain gene locus. EMBO J. 2001;20:6394–6403. doi: 10.1093/emboj/20.22.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riblet R. Molecular Biology of B cells. In: Honjo T, Alt FW, Neuberger MS, editors. Elsevier Academic Press; London: 2004. pp. 19–26. [Google Scholar]
- 12.Fuxa M, et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghesi L, et al. B lineage-specific regulation of V(D)J recombinase activity is established in common lymphoid progenitors. J Exp Med. 2004;199:491–502. doi: 10.1084/jem.20031800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolland DJ, et al. Antisense intergenic transcription in V(D)J recombination. Nat Immunol. 2004;5:630–637. doi: 10.1038/ni1068. [DOI] [PubMed] [Google Scholar]
- 15.Hesslein DGT, et al. Pax5 is required for recombination of transcribed, acetylated, 5′ IgH V gene segments. Genes Dev. 2003;17:37–42. doi: 10.1101/gad.1031403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamura D, Rajewsky K. Targeted disruption of μ chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 17.Chowdhury D, Sen R. Mechanisms for feedback inhibition of the immunoglobulin heavy chain locus. Curr Opin Immunol. 2004;16:235–240. doi: 10.1016/j.coi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Nussenzweig MC, et al. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin μ. Science. 1987;236:816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 19.Manz J, Denis K, Witte O, Brinster R, Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane μ, but not by secreted μ heavy chains. J Exp Med. 1988;168:1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa TE, Suh H, Nussenzweig MC. Chromosomal position of rearranging gene segments influences allelic exclusion in transgenic mice. Proc Natl Acad Sci USA. 1992;89:2205–2208. doi: 10.1073/pnas.89.6.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y, Bosma MJ, Bosma GC. Extended duration of DH-JH rearrangement in immunoglobulin heavy chain transgenic mice: implications for regulation of allelic exclusion. J Exp Med. 1999;189:1295–1305. doi: 10.1084/jem.189.8.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grawunder U, et al. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury D, Sen R. Transient IL-7/IL-7R signaling provides a mechanism for feedback inhibition of immunoglobulin heavy chain gene rearrangements. Immunity. 2003;18:229–241. doi: 10.1016/s1074-7613(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 24.Skok JA, et al. Nonequivalent nuclear location of immunoglobulin alleles in B lymphocytes. Nat Immunol. 2001;2:848–854. doi: 10.1038/ni0901-848. [DOI] [PubMed] [Google Scholar]
- 25.Rolink A, Grawunder U, Winkler TH, Karasuyama H, Melchers F. IL-2 receptor α chain (CD25, TAC) expression defines a crucial stage in pre-B cell development. Int Immunol. 1994;6:1257–1264. doi: 10.1093/intimm/6.8.1257. [DOI] [PubMed] [Google Scholar]
- 26.ten Boekel E, Melchers F, Rolink AG. Changes in the VH gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7:357–368. doi: 10.1016/s1074-7613(00)80357-x. [DOI] [PubMed] [Google Scholar]
- 27.Ehlich A, Martin V, Müller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 28.Kirschbaum T, Jaenichen R, Zachau HG. The mouse immunoglobulin κ locus contains about 140 variable gene segments. Eur J Immunol. 1996;26:1613–1620. doi: 10.1002/eji.1830260731. [DOI] [PubMed] [Google Scholar]
- 29.Thiebe R, et al. The variable genes and gene families of the mouse immunoglobulin κ locus. Eur J Immunol. 1999;29:2072–2081. doi: 10.1002/(SICI)1521-4141(199907)29:07<2072::AID-IMMU2072>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 30.Novobrantseva TI, et al. Rearrangement and expression of immunoglobulin light chain genes can precede heavy chain expression during normal B cell development in mice. J Exp Med. 1999;189:75–87. doi: 10.1084/jem.189.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Constantinescu A, Schlissel MS. Changes in locus-specific V(D)J recombinase activity induced by immunoglobulin gene products during B cell development. J Exp Med. 1997;185:609–620. doi: 10.1084/jem.185.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemazee D. Receptor selection in B and T lymphocytes. Annu Rev Immunol. 2000;18:19–51. doi: 10.1146/annurev.immunol.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schebesta M, Pfeffer PL, Busslinger M. Control of pre-BCR signaling by Pax5-dependent activation of the BLNK gene. Immunity. 2002;17:473–485. doi: 10.1016/s1074-7613(02)00418-1. [DOI] [PubMed] [Google Scholar]
- 34.Grosschedl R, Weaver D, Baltimore D, Costantini F. Introduction of a μ immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibodies. Cell. 1984;38:647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- 35.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 36.Grandien A, Coutinho A, Andersson J. Selective peripheral expansion and activation of B cells expressing endogenous immunoglobulin in μ-transgenic mice. Eur J Immunol. 1990;20:991–998. doi: 10.1002/eji.1830200507. [DOI] [PubMed] [Google Scholar]
- 37.Iacomini J, Yannoutsos N, Bandyopadhay S, Imanishi-Kari T. Endogenous immunoglobulin expression in mu transgenic mice. Int Immunol. 1991;3:185–196. doi: 10.1093/intimm/3.2.185. [DOI] [PubMed] [Google Scholar]
- 38.Souabni A, Cobaleda C, Schebesta M, Busslinger M. Pax5 promotes B lympho-poiesis and blocks T cell development by repressing Notch1. Immunity. 2002;17:781–793. doi: 10.1016/s1074-7613(02)00472-7. [DOI] [PubMed] [Google Scholar]
- 39.Yancopoulos GD, et al. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984;311:727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- 40.Williams GS, et al. Unequal VH gene rearrangement frequency within the large VH7183 gene family is not due to recombination signal sequence variation, and mapping of the genes shows a bias of rearrangement based on chromosomal location. J Immunol. 2001;167:257–263. doi: 10.4049/jimmunol.167.1.257. [DOI] [PubMed] [Google Scholar]
- 41.Maquat LE. Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics. Nat Rev Mol Cell Biol. 2004;5:89–99. doi: 10.1038/nrm1310. [DOI] [PubMed] [Google Scholar]
- 42.Su RC, et al. Dynamic assembly of silent chromatin during thymocyte maturation. Nat Genet. 2004;36:502–506. doi: 10.1038/ng1351. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 44.Baxter J, Merkenschlager M, Fisher AG. Nuclear organisation and gene expression. Curr Opin Cell Biol. 2002;14:372–376. doi: 10.1016/s0955-0674(02)00339-3. [DOI] [PubMed] [Google Scholar]
- 45.Horcher M, Souabni A, Busslinger M. Pax5/BSAP maintains the identity of B cells in late B lymphopoiesis. Immunity. 2001;14:779–790. doi: 10.1016/s1074-7613(01)00153-4. [DOI] [PubMed] [Google Scholar]
- 46.Arakawa H, Takeda S. Early expression of Igμ chain from a transgene significantly reduces the duration of the pro-B stage but does not affect the small pre-B stage. Int Immunol. 1996;8:1319–1328. doi: 10.1093/intimm/8.8.1319. [DOI] [PubMed] [Google Scholar]
- 47.Barreto V, Cumano A. Frequency and characterization of phenotypic Ig heavy chain allelically included IgM-expressing B cells in mice. J Immunol. 2000;164:893–899. doi: 10.4049/jimmunol.164.2.893. [DOI] [PubMed] [Google Scholar]
- 48.Nutt SL, Urbánek P, Rolink A, Busslinger M. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 1997;11:476–491. doi: 10.1101/gad.11.4.476. [DOI] [PubMed] [Google Scholar]
- 49.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 50.Röschenthaler F, Hameister H, Zachau HG. The 5′ part of the mouse immunoglobulin κ locus as a continuously cloned structure. Eur J Immunol. 2000;30:3349–3354. doi: 10.1002/1521-4141(2000012)30:12<3349::AID-IMMU3349>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 51.Kirschbaum T, et al. The 3′ part of the immunoglobulin κ locus of the mouse. Eur J Immunol. 1998;28:1458–1466. doi: 10.1002/(SICI)1521-4141(199805)28:05<1458::AID-IMMU1458>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Brown K. Visualizing nuclear proteins together with transcribed and inactive genes in structurally preserved cells. Methods. 2002;26:10–18. doi: 10.1016/S1046-2023(02)00003-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


