Abstract
Offspring of hypertensive pregnancies are more likely to have microvascular rarefaction and increased blood pressure in later life. We tested the hypothesis that maternal angiogenic profile during a hypertensive pregnancy is associated with fetal vasculogenic capacity and abnormal postnatal microvascular remodelling. 255 infants born following either hypertensive or normotensive pregnancies were recruited for quantification of postnatal dermal microvascular structure at birth and three months of age. Vasculogenic cell potential was assessed in umbilical vein endothelial cells from 55 offspring based on in vitro microvessel tube formation and proliferation assays. Maternal angiogenic profile (sFlt-1, sENG, VEGF, PlGF) was measured from post-partum plasma samples to characterise severity of pregnancy disorder. At birth, offspring born after hypertensive pregnancy had similar microvessel density to those born after a normotensive pregnancy, but during the first three postnatal months they had an almost two-fold greater reduction in total vessel density (TVD) (-17.7%±16.4 vs -9.9%±18.7, p=0.002). This postnatal loss varied according to the vasculogenic capacity of the endothelial cells of the infant at birth (r=0.49, p=0.02). The degree of reduction in both in vitro and postnatal in vivo vascular development was proportional to levels of anti-angiogenic factors in the maternal circulation. In conclusion, our data indicate that offsping born to hypertensive pregnancies have reduced vasculogenic capacity at birth that predicts microvessel density loss over the first three postnatal months. Degree of postnatal microvessel reduction is proportional to levels of anti-angiogenic factors in the maternal circulation at birth.
Keywords: Hypertensive pregnancy, Preeclampsia, Microvascular, Angiogenesis, HUVECs, sFlt-1
Introduction
A hypertensive pregnancy identifies both a mother and offspring with an increased risk of later hypertension, cardiovascular diseases and stroke1–4. The more severe the hypertensive disorder, such as occurrence of the clinical syndrome of preeclampsia5, the greater the risk of later disease6. Indeed, one in five of those born following more severe disorders are themselves hypertensive by the age of 20 years3.
Placental dysfunction is thought to be a primary event in a large proportion of women who go on to develop more severe, new-onset hypertension during pregnancy. The ischaemic placenta triggers an adverse maternal circulatory milieu, including deranged angiogenic factors, inflammation and oxidative stress7. Pathophysiologically this leads to reduced microvascular density, increased peripheral resistance and hypertension in the mother8, 9. The fetus is dependent on this dysfunctional placenta10, 11 and, plausibly, the associated stress could have a disruptive impact on the rapidly developing fetal vascular system. Alternatively, there may be a more direct association between fetal and feto-placental vascular development with long term consequences for later risk of cardiovascular diseases. Consistent with this hypothesis, offspring born to preeclamptic mothers have evidence of altered endothelial cell function from very early in life12–14 and, in both experimental hypertensive pregnancy models and human studies, display altered microvascular structure15–18. However, the time-point when microvascular structural differences emerge and the degree to which they relate to endothelial cell dysfunction in the offspring or the severity of angiogenic markers in the mother is unclear.
Therefore we studied whether there is a correlation between fetal vascular cell potential at birth and microvascular development, in particular, during the criticial postnatal phase, as the disorderly fetal microvascular plexus remodels into the mature ex utero horizontal papillary loop structure19. We then determined whether these vascular developmental differences are predicted by angiogenic markers in the maternal circulation.
Methods
Study overview
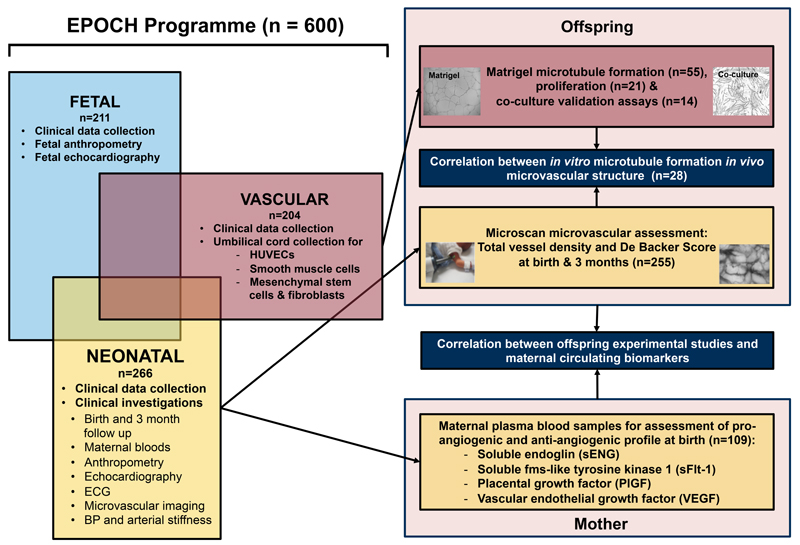
Between 2011 and 2015, 600 mothers being cared for by Oxford University Hospitals NHS Foundation Trust were identified by their clinical care team and invited to take part in one or more of a portfolio of studies coordinated by the Oxford Cardiovascular Clinical Research Facility. These studies investigated associations between pregnancy complications and cardiovascular development during fetal and neonatal life (Figure 1).
Figure 1.
Overview of EPOCH programme and study design using clinical and experimental assessments in offspring born to hypertensive pregnancies. HUVEC indicates human umbilical endothelial cell; MSC, mesenchymal stem cells.
To study microvascular development, we used maternal blood samples and longitudinal in vivo microvascular measures at birth and three months of age in the offspring recruited to the Effect of Prematurity and hypertensive disorders of pregnancy on Offspring Cardiovascular Health (EPOCH) study (Ethics ref. 11/SC/0006, Clinical trials ref. NCT01888770). A stratified recruitment strategy was employed in this study to recruit similar numbers of mother and infant dyads from hypertensive and normotensive pregnancies across a range of gestations and hypertensive pregnancy disorders, including pregnancy-induced hypertension and preeclampsia, defined according to ISSHP guidelines20 [see Supplementary Methods for definitions]. In addition, we performed in vitro assays using human umbilical vein endothelial cells (HUVECs) provided under ethical approval from the Oxford Cardiovascular Tissue Bioresource (ethical approval 09/H0606/68 & 07/H0606/148 ethics number 11/SC/0230). Matched in vivo measures and cell samples were available for a subset of offspring whose mothers had participated in both studies.
Mothers with a history of hypertension before pregnancy were excluded, as were infants with evidence of congenital cardiovascular disease, chromosomal abnormalities or genetic disorders. Those with persistent features of a fetal circulation at birth i.e. a Persistent Ductus Arteriosus (PDA) or Atrial Septal Defect (ASD), were not excluded but presence was recorded. All mothers gave written informed consent, and assent for involvement of their children, including permission to access maternal and offspring clinical records and link data between studies. Systems for collection of clinical data and characterisation of pregnancy complications based on medical records and questionnaire were standardised across studies and performed by the same data collection team (ED, CA, GY, YK, LS). Data collection details are available from https://clinicaltrials.gov (NCT0188870).
Clinical visit and maternal blood sample collection and analysis
At both the birth and three month assessments blood pressure and anthropometry were assessed using standard methodology [see Supplementary Methods]. Blood samples collected from the mothers when the infants had their postnatal assessment, were centrifuged and separated within 30 minutes for storage at -80°C pending analysis. Plasma circulating vascular endothelial growth factor A (VEGF-A), soluble fms-like tyrosine kinase-1 (sFlt-1), placental growth factor (PlGF), and soluble endoglin (sENG) concentrations were quantified with commercial enzyme-linked immunosorbent assays (ELISAs) according to standard manufacture protocols (Quantikine, R&D Systems Europe, Abingdon, UK) [see Supplymentary Methods for details]. To confirm measures at this early postnatal time point reflected levels during late pregnancy we additionally measured the angiogenic factor sFlt-1 in a group of women who had samples collected at 34 weeks gestation and 5 days postnatally. A subgroup of them also had measures at three months postnatally.
In vivo microvascular imaging
Imaging of the axillary small vessel network was performed with Side Stream Dark Field (SSDF) imaging (Microscan, Microvision Medical, Amsterdam), as previously reported for neonates21(Figure 2A). Measurements were performed after birth and again at three months, on the same side, in a temperature-controlled room, with the infant at rest, either in their mother’s arms, or in a crib [see Supplementary Methods]. Analysis was performed by one of three operators (CA, ED, CS) blinded to the clinical background of the clip. Coefficient of variation for intra and inter-observer variability based on ten sequences was 4.35% (intra-observer) and 6.54% (inter-observer) for TVD, and 4.46% (intra-observer) and 6.10% (inter-observer) for DB score.
Figure 2.
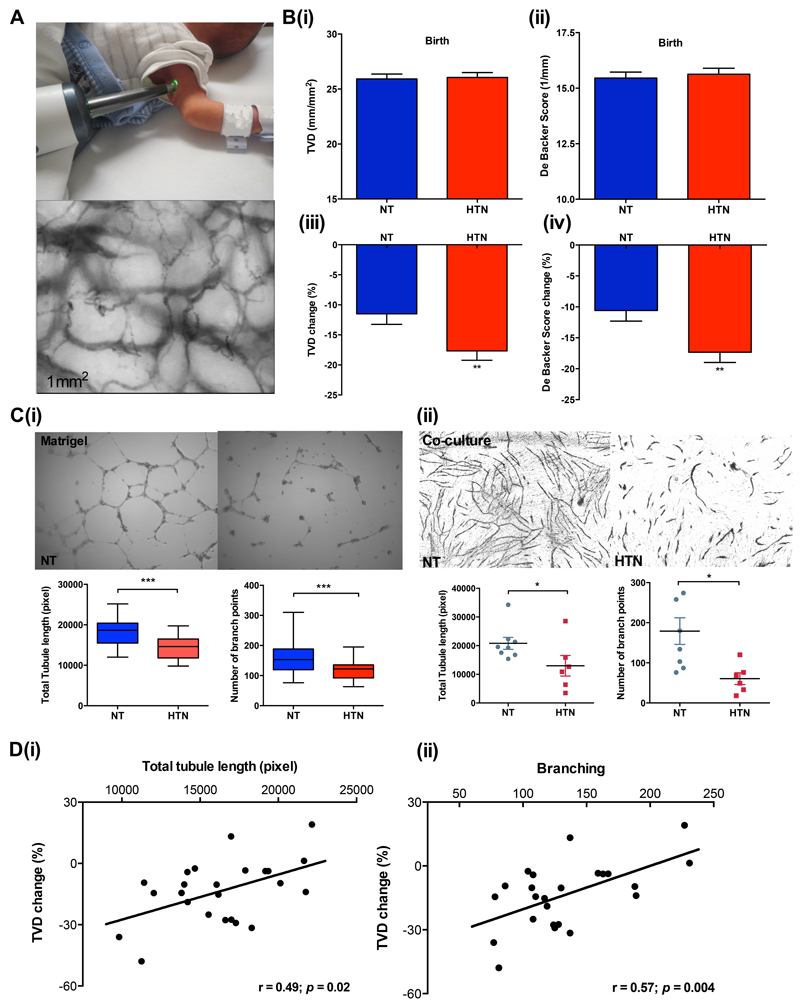
(A) Representative images of the in vivo microscan assessment. Individual image of microscan assessment is measured of size at 1mm x 1mm. (B)(i) Total vessel density (i) and De Backer scores (ii) are similar in offspring born to normotensive and hypertensive pregnancies at birth. Reduction in total vessel density (iii) and De Backer scores (iv) between birth and three months is greater in the hypertensive group. (C)(i) Representative images of umbilical derived endothelial tubules in growth factor-reduced Matrigel. Cells were placed on Matrigel at 1x104 HUVECs per well in triplicates on a 96-well plate, and cultured with EGM-2 medium for 16 hours. Images were taken at x4 magnification. Quantification of total tubule length and branching formed by normotensive and hypertensive HUVECs in Matrigel were measured using AngioSys (n=55, pixel), HUVECs were used at passage two.
(ii) Representative images of co-culture assay using normotensive and hypertensive HUVECs and BMMSCs. HUVECs and bone marrow stromal MSCs were directly co-cultured at 1:5 cell ratio for 14 days in a flat bottom collagenase coated 48-well plate (n=14). Each data point represents the average (in triplets) total tubule length or branch point of one subject. HUVECs were used at passage two. (D) In vitro tubule network measurements (total tubule length (i) and branching (ii)) correlate with in vivo microvasculature at three months using matching samples from the same infant (n=24). NT indicates offspring/HUVECs from normotensive pregnancies; HTN, offspring/HUVECs from hypertensive pregnancies; TVD, total vessel density; DB score, De Backer score. * p<0.05, **p<0.01, ***p<0.001. Bar plots are presented as Mean±SEM.
In vitro angiogenic capacity at birth
Umbilical cords were collected at birth and HUVECs were isolated and stored according to standard operating procedures [see Supplementary Methods]. Angiogenic capacity was assessed by two complementary approaches: tube formation assays, including Matrigel and co-culture with human bone marrow stromal mesenchymal stem cells (BMMSC); and CyQUANT® NF Cell Proliferation assay [see Supplymentary Methods].
Statistical analysis
Statistical analysis was performed using SPSS Version 22 and GraphPad Prism 6.0 [Full details are provided in the Supplementary Methods]. A sample size of 200 for the clinical study provided 80% power at a significance level of α=0.05 to detect a difference of 0.35 SD between equal sized groups. For the in vitro tube formation cohort, a sample size of 50 provided 80% power at a significance level of α=0.05 to detect a difference of 0.75 SD between groups.
Results
Microvascular measures at birth and three months
Cohort characteristics
Maternal and offspring demographic and anthropometric characteristics are presented in Tables 1 and 2 [for characteristics of hypertensive subgroups see Supplementary Table S1]. Hypertensive and normotensive pregnancy groups were matched for maternal age and smoking, sex ratio, birth order, gestational age and age at birth and follow-up assessments. Maternal BMI and blood pressure (booking, highest and discharge) were higher in the hypertensive group (p<0.001) and the infants had lower birthweight z-score (p=0.002) with greater incidence of iatrogenic delivery (p=0.02). Microvascular measures at both birth and three months were available for 197 infants. Of those initially recruited, one was excluded due to a subsequent diagnosis of Turner’s syndrome and, of the remainder, both sets of images were not available due to non-attendance at one visit or equipment failure (five visits), unanalysable images (six scans) or inability to obtain image (four scans). There were no differences in the demographic or clinical characteristics of those with or without microvascular images at both timepoints (data not presented).
Table 1. Characteristics of Cohort – Mothers.
| Parameters |
in vivo Vascular (microscan) |
in vivo/in vitro Comparison |
in vitro Vascular (tube formation) |
|||
|---|---|---|---|---|---|---|
|
Normotensive (n=104) |
Hypertensive (n=151) |
Normotensive (n=18) |
Hypertensive (n=10) |
Normotensive (n=31) |
Hypertensive (n=24) |
|
| Maternal Demographics & Anthropometrics | ||||||
| Maternal age, years | 32.6±4.6 | 32.7±6.0 | 32.8±3.3 | 34.2±3.8 | 33.6±4.0 | 33.1±5.4 |
| BMI at booking, kg/m2 | 23.7±4.0 | 26.9±6.9‡ | 23.1±2.7 | 29.8±8.3† | 23.3±6.2 | 28.0±6.9* |
| Smokers, n (%) | 6 (6) | 5 (3) | 0 (0) | 0 (0) | 6 (21) | 7 (32) |
| Booking sBP, mmHg | 107.6±9.8 | 118.4±20.6‡ | 108.6±10.8 | 127.9±26.7* | 110.2±11.8 | 116.1±11.2 |
| Booking dBP, mmHg | 65.2±8.5 | 72.9±14.9‡ | 64.8±8.9 | 78.0±13.9† | 67.9±11.0 | 70.8±11.1 |
| Highest sBP, mmHg | 123.2±10.5 | 162.2±16.8‡ | 123.4±11.6 | 166.5±20.2‡ | 112.3±11.8 | 148.1±19.3‡ |
| Highest dBP, mmHg | 76.4±8.5 | 100.8±10.5‡ | 75.6±6.8 | 103.2±14.9‡ | 68.7±8.2 | 92.0±10.8‡ |
| Discharge sBP, mmHg | 113.7±11.4 | 129.8±12.3‡ | 114.2±12.3 | 128.1±11.3† | 111.6±11.8 | 127.1±8.9‡ |
| Discharge dBP, mmHg | 67.3±8.2 | 79.8±9.9‡ | 67.9±8.5 | 76.5±4.9† | 68.1±9.0 | 75.8±7.0† |
| Maternal Biomarkers | ||||||
| sFlt-1, pg/mL | 971.4±1162.3 | 1297.1±1341.6 | - | - | - | - |
| sENG, ng/mL | 7.3±5.6 | 11.3±6.7‡ | - | - | - | - |
| VEGF, pg/mL | 61.2±102.4 | 54.5±80.3 | - | - | - | - |
| PlGF, pg/mL | 12.1±7.7 | 17.2±27.0 | - | - | - | - |
Values as Mean±Standard Deviation unless stated otherwise. ψMedian±Interquartile range. sBP systolic blood pressure; dBP diastolic blood pressure. Statistically significant p-values are asterisked.
p<0.05;
p<0.01;
p<0.001
Table 2. Characteristics of Cohort – Offspring.
| Parameters | in vivo Vascular (microscan) | in vivo/in vitro Comparison | in vitro Vascular (tube formation) | |||
|---|---|---|---|---|---|---|
| Normotensive (n=104) | Hypertensive (n=151) | Normotensive (n=18) | Hypertensive (n=10) | Normotensive (n=31) | Hypertensive (n=24) | |
| Offspring Demographics & Anthropometrics | ||||||
| Birth | ||||||
| Gestational age, weeks | 36.8±3.5 | 36.8±3.2 | 39.3±2.2 | 36.6±2.5† | 39.2±1.7 | 36.5±2.6‡ |
| Males, n (%) | 52 (50) | 67 (44) | 10 (56) | 5 (50) | 14 (48) | 13 (57) |
| Birth orderψ | 1±1 | 1±1 | 1±1 | 1±1 | 1±1 | 1±1 |
| Caesarean section, n(%) | 37 (36) | 76 (50)* | 13 (72) | 4 (40) | 5 (18) | 15 (71) |
| Age at birth assessment (days) | 5.74±5 | 4.85±5 | 2.3±3 | 5.2±6 | - | - |
| Birthweight, grams | 2796±854 | 2670±851 | 3321±684 | 2686±839* | 3419±650 | 2797±823† |
| Birthweight z-score | -0.07±1.1 | -0.49±1.1† | -0.07±1.1 | -0.31±1.6 | 0.39±0.8 | -0.44±1.0† |
| Head circumference, cms | 32.8±2.8 | 32.7±2.7 | 34.4±1.69 | 33.2±2.39 | 34.3±1.2 | 33.7±1.6 |
| sBP, mmHg | 78.6±14.6 | 78.3±14.5 | 78.9±17.1 | 84.3±13.9 | - | - |
| dBP, mmHg | 42.8±9.8 | 43.7±10.0 | 41.8±8.2 | 50.2±7.8* | - | - |
| Follow up | ||||||
| Age at follow up, days | 99.9±15.4 | 97.4±13.6 | 101.9±9.6 | 108.2±11.1 | - | - |
| Weight, grams | 5640±1051 | 5468±1092 | 6202±748 | 6058±823 | - | - |
| Weight z-score | -0.21±1.0 | -0.39±1.1 | -0.07±0.8 | 0.19±0.8 | - | - |
| Weight gain z-score | -0.22±1.2 | -0.13±1.1 | -0.12±1.0 | 0.42±1.0 | - | - |
| Head circumference, cms | 40.1±2.0 | 40.0±2.1 | 40.8±1.6 | 41.3±1.5 | - | - |
| sBP, mmHg | 95.6±11.7 | 95.2±12.9 | 99.9±12.2 | 92.9±11.6 | - | - |
| dBP, mmHg | 53.0±12.7 | 51.9±12.4 | 54.2±14.1 | 48.3±9.9 | - | - |
p<0.05
p<0.01
p<0.001
Microvasuclar measures
There were no differences in total vessel density (TVD) or De Backer (DB) score between the offspring born to normotensive and hypertensive pregnancies at birth (Figure 2B (i) and (ii)). However, there was a significantly greater reduction in both TVD and small vessel density (SmVD) as well as DB score between birth and three months of age in those born to hypertensive pregnancy compared to normotensive pregnancy (Figure 2B, results for SmVD not shown). The reduction in TVD and DB score between birth and three months was almost double in the hypertensive group compared to the normotensive group (TVD change -9.9%±18.7 versus -17.7%±16.4, p=0.002; DB change -9.6±17.2 versus -17.3±17.7, p=0.002) so that by three months, TVD was significantly lower in the hypertensive pregnancy group (21.2±4.1mm/mm2 vs 22.6±4.1mm/mm2, p=0.017) with a borderline difference in the semi-quantitative De Backer score (12.9±3.0mm-1 vs 13.6±2.6mm-1, p=0.08).
In vitro angiogenic capacity at birth
Cohort characteristics
Maternal and fetal characteristics of the samples used for microvessel tube Matrigel measures are presented in Table 1and 2. As in the neonatal cohort, there was a balance of genders between groups and maternal age, and smoking did not differ. Women with hypertensive pregnancy also tended to have slightly higher booking BMI (p=0.01) as well as systolic and diastolic blood pressures during pregnancy. Samples available for normotensive mothers from the Cardiovasuclar Tissue Bioresource tended to have been collected at term and so there were differences in gestational age (p=0.03) and exposure to antenatal steroids (p<0.001) as well as higher birth weight z-score (p=0.002) and lower rates of iatrogenic delivery (p<0.001). The hypertensive pregnancy history tended to be more severe in the available tissue samples with a higher incidence of abnormal maternal liver function tests (LFT) (p<0.001) and oedema (p<0.001).
Tube formation and proliferation
Hypertensive pregnancy HUVECs developed a more fragmented, shorter capillary-like microvascular network in Matrigel compared to HUVECS from normotensive pregnancies (Figure 2C (i)) with a significant reduction in total tubule length (hypertensive, 14,438.4±2,728.1; normotensive, 18,239.8±3,217.7; p<0.001; pixel), and branching (hypertensive, 118±30; normotensive, 166±49; p<0.001). These differences were validated in a subgroup (n=14) by co-culture tube formation (Figure 2C (ii)) which confirmed a reduced ability to form tube assessed by tubule length (hypertensive, 12,967.2±3,600.3; normotensive, 20,800.1±2,085.2 pixel, p=0.03), and branching (hypertensive, 61±15; normotensive 179±33, p=0.007) compared to cells from normotensive pregnancies. Furthermore, proliferation evaluated by CyQUANT assay in a subset (n=34) (Figure not shown) showed hypertensive pregnancy HUVECs had significantly slower growth, presented as fold change between day three vs day zero, than those from normotensive pregnancies (6.3±2.0 versus 8.3±2.7; p=0.03).
Correlation of in vitro tube formation and in vivo microvasculature
Umbilical-derived cell samples and in vivo microvascular measures were available in the same individuals for 28 participants (10 hypertensive and 18 normotenisve pregnancies). In vitro tubule length and branching was not associated with in vivo measures at birth but there was a graded positive association between in vitro total tubule length and the loss of in vivo microvascular density over the first three postnatal months (r=0.56, p=0.004) (Figure 2D (i)). Similarly, there was a graded positive association between in vitro branching and change in in vivo De Backer score (r=0.55, p=0.006) (Figure 2D (ii)).
Severity of hypertensive pregnancy disorder and offspring microvascular development
Clinical severity of hypertensive pregnancy disorder
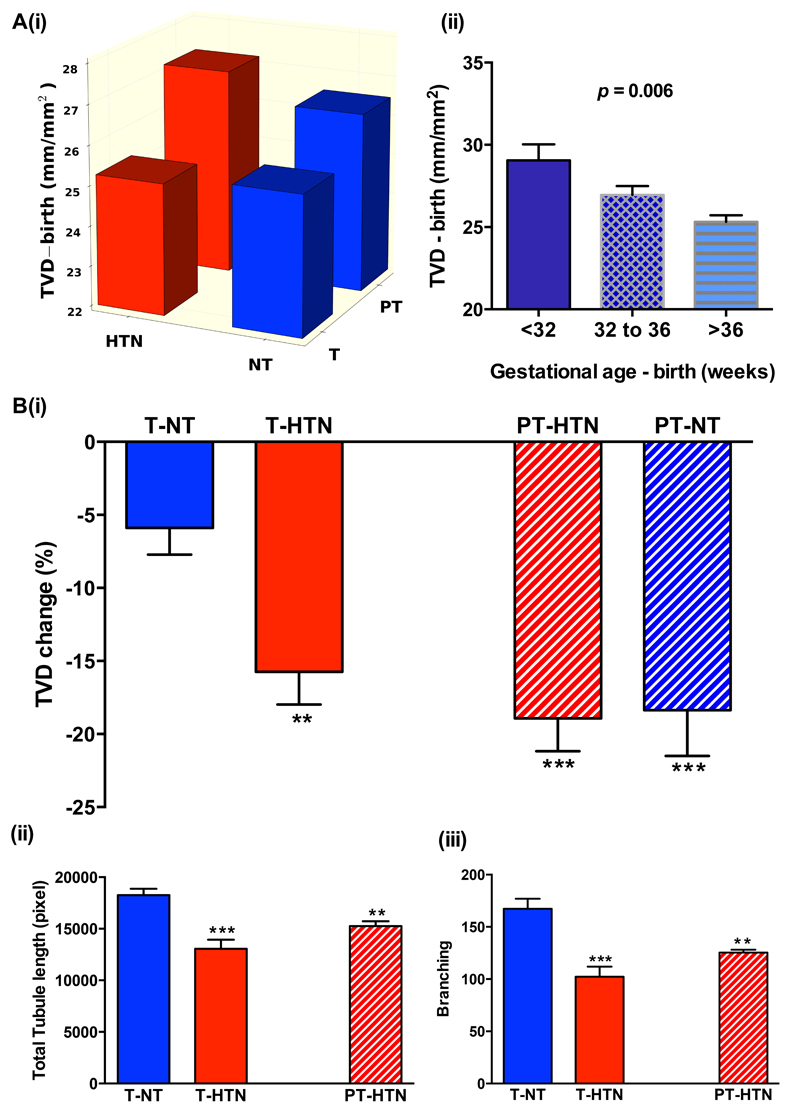
In view of the association between preterm birth and hypertensive pregnancy disorders we additionally performed analysis separately in those born term and preterm. TVD was higher in offspring born preterm to both hypertensive and normotensive pregnancies compared to term pregnancies (Figure 3A (i)), and there was a graded relationship between gestational age and microvessel density at birth (r=-0.19, p=0.005) (Figure 3A (ii)). However, significant loss of microvessel density was evident in both groups compared to those born at term to normotensive pregnancy (p<0.001 for preterm hypertensive and p=0.001 for term hypertensive pregnancies) (Figure 3B (i)). Similar patterns were observed when hypertensive pregnancy groups were divided based on diagnosis of preeclampsia or pregnancy induced hypertension, or on the basis of gestation of diagnosis (data not shown). For in vitro measures, similar reductions in total tubule length (p=0.004 and p<0.001) and branching (p=0.007 and p<0.001) were evident in both preterm and term hypertensive pregnancies compared to term normotensive pregnancies (Figure 3B (ii) and (iii)). Additionally, responses in samples from both early and late onset preeclampsia had similar changes in tubule length and branch points compared to normotensive pregnancy samples (total tubule length, p=0.004 and p=0.006; branching p=0.02 and p<0.001).
Figure 3.
(A) Total vessel density is higher in offspring born preterm to both hypertensive and normotensive pregnancies compared to term pregnancies (i) and there is a graded relationship between gestational age and microvessel density at birth (ii). p-value is presented as one-way ANOVA test between groups. (B) Both hypertensive and normotensive preterm offspring show a significant reduction in microvessel density during the first three months of life similar to that seen in term hypertensive pregnancies (i). Altered in vitro tube formation is also evident in samples from both term and preterm hypertensive pregnancy groups (ii) and (iii). TVD indicates total vessel density; T-NT term-normotensive pregnancies; T-HTN term-hypertensive pregnancies; PT-HTN preterm-hypertensive pregnancies; PT-NT preterm-normotensive pregnancies. The asterisks indicate the level of significance (*p<0.05, ** p<0.01, *** p<0.001) for the 2-tailed independent sample t-test between each pregnancy complication group with normotensive group. Bar plots are presented as Mean±SEM.
Maternal angiogenic profile
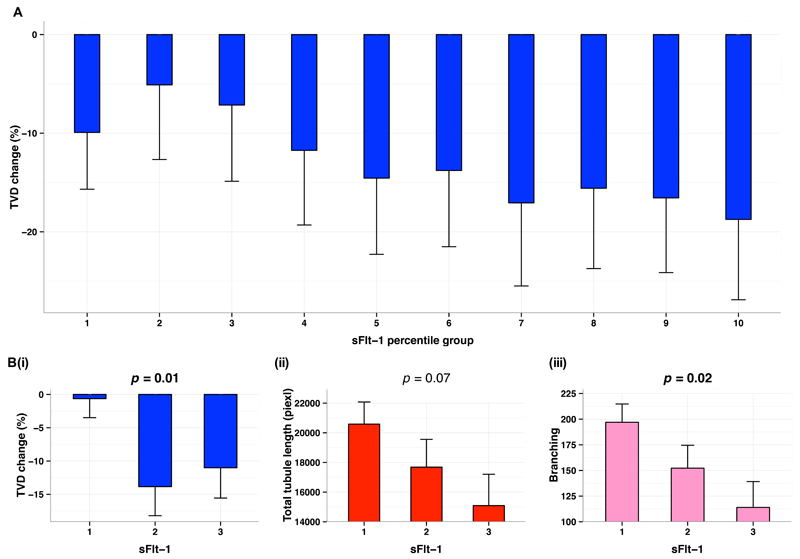
Maternal postnatal blood samples were available for 107 mother and offspring dyads. Samples were collected on average five days after birth, at which point, maternal sENG levels were still significantly higher in hypertensive mothers with a trend for higher sFlt-1 and lower VEGF (Table 1). Our study of sFlt-1 compares measures in the last trimester, to early postnatal and three month postnatal values. Measures at five days postnatally strongly correlate to late gestation values (r=0.85, p=0.007) [Supplementary Figure S1]. By three months levels had fallen to very low values and no longer reflected either late gestation or early postnatal values. Therefore associations identified with measures at 5 days are likely to relate to variation during pregnancy. There was a graded relation between maternal sFlt-1 level and offspring microvessel loss between birth and three months (Figure 4A) with higher levels predictive of greater vessel density loss (p=0.05). Similar relations were evident between maternal sFlt-1 level and in vitro offspring tube formation (total tubule length (p=0.03) and branching (p=0.02)) (Table 4). Although similar patterns were seen with sENG levels for both vessel reduction and in vitro measures, associations were not significant. VEGF levels were not related to total vessel reduction but positively related to in vitro proliferative capacity (p=0.008). To study whether these associations represented co-association with other factors linked to hypertensive pregnancy, we performed a sensitivity analysis in normotensive pregnancies. Interestingly, significant differences across tertiles of sFlt-1 in mothers who had not developed hypertension were still evident for offspring TVD change (p=0.01) (Figure 4B (i)) and in vitro tube formation (total tubule length p=0.07, branching p=0.02,) (Figure 4B (ii) and (iii)). There were also differences in proliferation across maternal VEGF levels (p=0.02) (data not shown).
Figure 4.
(A) Association between maternal sFlt-1 after delivery with total vessel density % change from birth to three months. Maternal sFlt-1 is presented in ten percentile groups. (B) Comparison of maternal sFlt-1 tertiles with total vessel density (i), total tubule length (ii), and branching (iii), in normotensive pregnancies. TVD indicates total vessel density; sFlt-1, soluble fms-like tyrosine kinase-1. p-values are presented as one-way ANOVA test between groups; p-values <0.05 are highlighted in bold; ranks are based on percentile groups with each group containing approximately the same number of cases. Bar plots are presented as Mean±SEM.
Table 4. Comparison of maternal pro-angiogenic and anti-angiogenic profile with vascular tubule formation and postnatal microvasculature change.
| TVD change (%) | Total Tubule Length | Branching | Proliferation | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | p-value | Mean | p-value | Mean | p-value | Mean | p-value | |
| sFlt-1 | -7.2 | 0.05 | 18403.9 | 0.03 | 165 | 0.02 | 8.4 | 0.25 |
| -15.8 | 17676.2 | 148 | 7.7 | |||||
| -15.3 | 14403.0 | 107 | 5.8 | |||||
| sENG | -9.8 | 0.32 | 17653.8 | 0.61 | 158 | 0.28 | 8.5 | 0.21 |
| -11.8 | 15836.0 | 124 | 5.7 | |||||
| -16.1 | 16351.3 | 131 | 7.6 | |||||
| PlGF | -11.9 | 0.04 | 15565.5 | 0.16 | 124 | 0.12 | 7.4 | 0.84 |
| -7.1 | 18787.9 | 167 | 8.0 | |||||
| -18.2 | 15978.2 | 128 | 7.1 | |||||
| VEGF | -16.7 | 0.13 | 16195.8 | 0.56 | 134 | 0.72 | 5.7 | 0.008 |
| -8.3 | 16495.5 | 137 | 7.2 | |||||
| -12.1 | 18749.3 | 158 | 10.8 | |||||
Ranks are based on percentile groups with each group containing approximately the same number of cases. p-values are calculated using one-way ANOVA. Bolded p-values are statistically significant (p<0.05). TVD, total vessel density; sFlt-1, soluble fms-like tyrosine kinase 1; sENG, soluble endoglin; PlGF, placental growth factor; VEGF, vascular endothelial growth factor.
Other predictors of tube formation and microvascular density
To study the role of other perinatal factors we performed bivariable regression analyses with in vitro and in vivo measures (Supplementary Table S2). For TVD, there were associations with blood pressure at birth, birthweight z-score, gestational age and exposure to antenatal steroids. However, there was significant co-linearity between antenatal steroid exposure and preterm birth with no differences in those born in late gestation who had received antenatal steroids compared to those who had not (data not shown). Therefore, offspring blood pressure at birth, birthweight z-score and gestational age were taken forward into a multivariate model with maternal hypertension (Table 3). In this model, maternal hypertension remained a significant independent predictor of greater TVD change along with more preterm birth and lower blood pressure at birth. For in vitro measures, clinical markers of preeclampsia including LFT abnormalities and oedema related to tube formation, with borderline significances for gestational age, birthweight z-score and preeclampsia-associated iatrogenic delivery. In multivariate modelling only maternal hypertension remained an independent predictor for total tubule length (B=-0.66; p=0.006) and branching (B=-0.55; p=0.03).
Table 3. Multivariable Regression Coefficients for Maternal and Perinatal Characteristics and Microvascular measures.
| Parameters | Change in TVD (%) | Total Tubule Length (pixel) | Branching | |||
|---|---|---|---|---|---|---|
| Unstandardised Coefficient (B) | p-value | Unstandardised Coefficient (B) | p-value | Unstandardised Coefficient (B) | p-value | |
| Maternal hypertension during pregnancy | -7.32 | 0.004 | -0.66 | 0.006 | -0.55 | 0.03 |
| Gestational age | 1.01 | 0.01 | - | - | - | - |
| Birthweight z-score | 1.22 | 0.28 | - | - | - | - |
| Birth sBP | 0.17 | 0.05 | - | - | - | - |
| LFT abnormalities | - | - | -0.18 | 0.29 | -0.05 | 0.78 |
| Significant oedema | - | - | -0.05 | 0.75 | -0.03 | 0.85 |
LFT – liver function test
Discussion
This study provides the first evidence of an almost two-fold greater postnatal reduction in microvascular density in neonates born following pregnancies complicated by new onset hypertension, compared to normotensive pregnancies. Degree of microvessel loss was predicted by the vasculogenic capacity of their endothelial cells at birth, which, in turn, related to maternal angiogenic factors. These phenomena were evident across the spectrum of hypertensive pregnancy disorders, and not attenuated by an increased vascular density in those born preterm. Intriguingly, associations were present in normotensive pregnancies if maternal anti-angiogenic factors had been higher around the time of birth. These findings identify a critical postnatal microvascular developmental window and provide a potential explanation for how pregnancy pathophysiology links with microvascular rarefaction in the offspring in later life.
A small reduction in total vessel density during the first three postnatal months22, 23 19 occurs as the disorderly fetal capillary network remodels into a horizontal subpapillary plexus and singular papillary loops structure. We hypothesised this may be a critical period for microvascular remodelling, sensitive to inherent changes in infant endothelial cell function. Our observation that, at birth, normotensive and hypertensive pregnancy offspring had similar vessel density but then there was a loss of vessel density in the hypertensive pregnancy group, highlights the apparent importance of the postnatal window. Similar changes were seen across the spectrum of hypertensive pregnancy disorders. However clinical diagnostic criteria may have lacked specificity for identification of offspring at the greatest risk and maternal biomarkers may better reflect disease severity24. Consistent with this hypothesis, maternal sFlt-1 after birth predicted offspring vascular postnatal changes. Strikingly, sFlt-1 associated with vascular phenotype when analysis was restricted to normotensive, full-term or appropriate for gestational age pregnancy groups. Hypertensive pregnancy syndromes may sit at one end of a spectrum25, with subclinical endothelial activation, low grade inflammation and cardiovascular dysfunction26 evident in a broader group of apparently clinically normal pregnancies7. Normotensive mothers who have hypertension risk factors tend to have an anti-angiogenic pregnancy profile27–29. Therefore, our findings may have relevance to a broader group of offspring than defined by hypertensive pregnancy alone.
Upon placental delivery maternal circulating profile starts to normalise. For practical purposes, our samples were collected at the postnatal infant assessment. However, in a validation study we were able to demonstrate measures at this timepoint, although lower, still clearly reflect levels in late gestation. The reduction in absolute levels may be why there was only a trend with sENG and timing may also explain why there was no association with PlGF. A recent report linked mid-pregnancy PlGF to childhood retinal vasculature17, 28 but differences in PlGF are most evident in mid-pregnancy7, 24, 27, 28, 30, 31. Biological reasons for persistent, post-partum elevation of anti-angiogenic markers could be relevant to the underlying mechanisms for postnatal offspring differences as this would identify mothers with an inherent, abnormal anti-angiogenic response or an extra-placental sources of sFlt-132. However, our longitudinal study of maternal sFlt-1 levels suggest there is a general persistent elevation for several days following delivery and that values at this time-point do not reflect baseline values measured three months postnatally. Nevertheless, adults born to hypertensive pregnancies have differences in circulating levels of sFlt-1 in later life33 and persistent elevations in anti-angiogenic factors in the infant may also be relevant. Serum from preeclamptic mothers inhibits normal HUVEC tube formation34 and angiogenic factors in umbilical cord blood, although significantly lower, reflect maternal levels10, 11. However, in our in vitro assays, with control media, tubologenesis was still reduced, suggesting a specific alteration in cellular phenotype. As endothelial colony-forming cells (ECFCs) account for a proportion of HUVECs35 our findings may indicate a common endothelial cell dysfunctional phenotype previously described for cord blood ECFCs collected after preeclamptic pregnancies. These exhibited delayed colony development13 and, after preterm birth, were fixed in an anti-angiogenic state36. If so, we have linked for the first time this altered in vitro cellular behaviour with in vivo vascular responses, and maternal measures.
Our study is associative and does not prove causality but our case control recruitment approach meant the number of pregnancies with complications that we could study was comparable to large population-based samples. The study size meant we could undertake very detailed clinical, physiological and sample data collection along with repeated measures over short time frames. Therefore we could undertake detailed analysis of potential related factors that could impact on vascular development, independent of the hypertensive pregnancy. Higher skin capillary density has previously been reported in infants born preterm after hypertensive pregnancy18 but our range of gestational ages demonstrated that increased vessel density is a feature of prematurity rather than hypertensive disorders. Interestingly, gestational age was also an independent predictor of postnatal microvessel loss, along with maternal hypertension, which may explain a previous observation of capillary rarefaction in adults born preterm to normotensive pregnancies16. In the present study, endothelial cells were not available from normotensive preterm pregnancies to explore whether prematurity associated with in vitro cellular function, but altered cord blood ECFC function has been reported in preterm offspring36. In our large sample we did not replicate the previous finding of reduced vessel density at birth in hypertensive pregnancy offspring born at term. In bivariable analysis, although maternal body mass index was not related to microvessel density change, birthweight z-score was related but, in our study, the association was not independent of a history of hypertension pregnancy and preterm birth in regression models37, 38. However, this factor may be of value to explore further as experimental models show restrictive diet pregnancies can induce epigenetic cardiovascular changes39. An association between low infant systolic blood pressure at birth and microvessel change persisted in multivariate analysis, which raises the possibility that complications that result in a low cardiac output or peripheral vasodilataion, for example hypoxia or infection, may also impact on postnatal microvascular development. It will be of interest to investigate whether the changes in dermal microvasculature reflect a generalised phenomenon involving other organ-specific vascular beds such as cardiac and pulmonary systems. Future longitudinal follow up of this cohort planned over the next few years should provide definitive information on the associations between neonatal microvasculature development and emergence of cardiovascular risk evident in early life including elevated blood pressure and metabolic abnormalities.
In conclusion, we have found offspring born to hypertensive pregnancies have reduced endothelial capacity for microtubulogenesis in vitro at birth. The degree of impairment predicts the degree of reduction in vascular density during the first three months of postnatal life, as the fetal microvasculature remodels into its postnatal ex utero structure. Intriguingly, these changes can also be predicted by the circulating biomarker profile of the mother around the time of birth. Collectively, these findings suggest a close association between the maternal biological state during pregnancy and offspring vasculogenic capacity. As a result, although the offspring maintain microvascular structure in utero, perhaps as a compensatory response to the relative hypoxia of the hypertensive pregnancy, on transition to the normal ex utero environment there is failure of appropriate remodelling.40 These changes may have long term relevance as in adulthood offspring of complicated pregnancies are known to have microvascular rarefaction and higher blood pressure2, 3, 6, 40. Blood pressures were similar at three months of age in our neonates, despite reductions in vessel density, consistent with these microvascular changes being a primary event, as seen in hypertensive models and at risk populations38, 41. Future studies will help define mechanisms underlying the altered vasculogenic capacity and determine whether these changes are tractable to reduce the long term cardiovascular and hypertensive risk.
Perspectives
This is the first demonstration that neonates born following hypertensive pregnancies have almost two fold greater postnatal reduction in microvascular density compared to offspring of normotensive pregnancies and that umbilical endothelial cell tube formation predicts the degree of this postnatal microvessel reduction. Furthermore, these vascular phenotypes are predicted by levels of angiogenic factors in the maternal circulation around the time of birth; an association that is evident if the mother did not develop hypertension during pregnancy.
Supplementary Material
Novelty and Significance.
What is New?
Blood vessel density decreases more rapidly in offspring of hypertensive, compared to normotensive, pregnancies during early postnatal life. This appears to be predicted by how well cells from their blood vessel wall grow at birth.
Factors that inhibit blood vessel growth are found in maternal blood during pregnancy and predict risk of hypertension during pregnancy. Higher levels in the mother also appear to predict offspring postnatal blood vessel development, even if the mother did not develop hypertension.
What is Relevant?
A reduced number of blood vessels in the skin increases risk for hypertension and knowledge of maternal biological changes during pregnancy may help identify offspring who may benefit from strategies to protect vascular development during early postnatal life.
Summary
Blood vessel wall cells in babies do not grow as effectively if their mother had hypertension during pregnancy, or had altered blood markers linked with hypertensive pregnancies, even if the maternal blood pressure was normal. These babies then have a more rapid loss of small vessels in the skin during early postnatal life, which could explain their increased risk for hypertension in later life.
Acknowledgments
We are grateful to all the pregnant woman and babies who participated in this study.
Sources of Funding
This work was supported by the British Heart Foundation (BHF; grants FS/06/024 and FS/11/65/28865 to PL; RG/13/1/30181 and CH/16/1/32013 to KMC), the NIHR Oxford Biomedical Research Centre, the Oxford BHF Centre for Research Excellence (RE/13/1/30181) and the Wellcome Trust Summer Studentship Programme (109264/Z/15/Z). Support was also provided by NHS blood and Transplant.
Footnotes
Conflicts of Interest/Disclosures
None.
References
- 1.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics. 2012;129:e1552–1561. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 3.Davis EF, Lewandowski AJ, Aye C, Williamson W, Boardman H, Huang RC, Mori TA, Newnham J, Beilin LJ, Leeson P. Clinical cardiovascular risk during young adulthood in offspring of hypertensive pregnancies: Insights from a 20-year prospective follow-up birth cohort. BMJ Open. 2015;5:e008136. doi: 10.1136/bmjopen-2015-008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: The helsinki birth cohort study. Stroke. 2009;40:1176–1180. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 5.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 6.Lazdam M, de la Horra A, Diesch J, Kenworthy Y, Davis E, Lewandowski AJ, Szmigielski C, Shore A, Mackillop L, Kharbanda R, Alp N, et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension. 2012;60:1338–1345. doi: 10.1161/HYPERTENSIONAHA.112.198366. [DOI] [PubMed] [Google Scholar]
- 7.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, et al. Circulating angiogenic factors and the risk of preeclampsia. The New England Journal of Medicine. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 8.Hasan KM, Manyonda IT, Ng FS, Singer DR, Antonios TF. Skin capillary density changes in normal pregnancy and pre-eclampsia. J Hypertens. 2002;20:2439–2443. doi: 10.1097/00004872-200212000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–487. doi: 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 10.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sflt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2005;122:33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (cd105) in fetal and maternal serum and in amniotic fluid in preeclampsia. American Journal of Obstetrics and Gynecology. 2007;197:176 e171–176. doi: 10.1016/j.ajog.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, Kylintireas I, Contractor H, Singhal A, Lucas A, Neubauer S, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: Is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56:159–165. doi: 10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 13.Munoz-Hernandez R, Miranda ML, Stiefel P, Lin RZ, Praena-Fernandez JM, Dominguez-Simeon MJ, Villar J, Moreno-Luna R, Melero-Martin JM. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension. 2014;64:165–171. doi: 10.1161/HYPERTENSIONAHA.113.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–494. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 15.Davis EF, Newton L, Lewandowski AJ, Lazdam M, Kelly BA, Kyriakou T, Leeson P. Pre-eclampsia and offspring cardiovascular health: Mechanistic insights from experimental studies. Clinical Science. 2012;123:53–72. doi: 10.1042/CS20110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, Leeson P. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65:607–614. doi: 10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- 17.Gishti O, Jaddoe VW, Felix JF, Reiss I, Hofman A, Ikram MK, Steegers EA, Gaillard R. Influence of maternal angiogenic factors during pregnancy on microvascular structure in school-age children. Hypertension. 2015;65:722–728. doi: 10.1161/HYPERTENSIONAHA.114.05008. [DOI] [PubMed] [Google Scholar]
- 18.Antonios TF, Raghuraman RP, D’Souza R, Nathan P, Wang D, Manyonda IT. Capillary remodeling in infants born to hypertensive pregnancy: Pilot study. American Journal of Hypertension. 2012;25:848–853. doi: 10.1038/ajh.2012.51. [DOI] [PubMed] [Google Scholar]
- 19.Perera P KA, Ryan TJ. The development of the cutaneous microvascular system in the newborn. Br J Derm. 1970;82:86–90. [Google Scholar]
- 20.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the international society for the study of hypertension in pregnancy (isshp) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 21.Genzel-Boroviczeny O, Strotgen J, Harris AG, Messmer K, Christ F. Orthogonal polarization spectral imaging (ops): A novel method to measure the microcirculation in term and preterm infants transcutaneously. Pediatr Res. 2002;51:386–391. doi: 10.1203/00006450-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Kroth J, Weidlich K, Hiedl S, Nussbaum C, Christ F, Genzel-boroviczeny O. Functional vessel density in the first month of life in preterm neonates. Pediatr Res. 2008;64:567–571. doi: 10.1203/PDR.0b013e318184134e. [DOI] [PubMed] [Google Scholar]
- 23.Top AP, van Dijk M, van Velzen JE, Ince C, Tibboel D. Functional capillary density decreases after the first week of life in term neonates. Neonatology. 2011;99:73–77. doi: 10.1159/000316945. [DOI] [PubMed] [Google Scholar]
- 24.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. The New England Journal of Medicine. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 25.Redman CW, Sargent IL. Pre-eclampsia, the placenta and the maternal systemic inflammatory response--a review. Placenta. 2003;24(Suppl A):S21–27. doi: 10.1053/plac.2002.0930. [DOI] [PubMed] [Google Scholar]
- 26.Melchiorre K, Sharma R, Thilaganathan B. Cardiac structure and function in normal pregnancy. Current Opinion in Obstetrics & Gynecology. 2012;24:413–421. doi: 10.1097/GCO.0b013e328359826f. [DOI] [PubMed] [Google Scholar]
- 27.Kim YN, Lee DS, Jeong DH, Sung MS, Kim KT. The relationship of the level of circulating antiangiogenic factors to the clinical manifestations of preeclampsia. Prenatal Diagnosis. 2009;29:464–470. doi: 10.1002/pd.2203. [DOI] [PubMed] [Google Scholar]
- 28.Staff AC, Harsem NK, Braekke K, Hyer M, Hoover RN, Troisi R. Maternal, gestational and neonatal characteristics and maternal angiogenic factors in normotensive pregnancies. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2009;143:29–33. doi: 10.1016/j.ejogrb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Troisi R, Braekke K, Harsem NK, Hyer M, Hoover RN, Staff AC. Blood pressure augmentation and maternal circulating concentrations of angiogenic factors at delivery in preeclamptic and uncomplicated pregnancies. American Journal of Obstetrics and Gynecology. 2008;199(653):e651–610. doi: 10.1016/j.ajog.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahay AS, Patil VV, Sundrani DP, Joshi AA, Wagh GN, Gupte SA, Joshi SR. A longitudinal study of circulating angiogenic and antiangiogenic factors and at1-aa levels in preeclampsia. Hypertension Research : Official Journal of the Japanese Society of Hypertension. 2014;37:753–758. doi: 10.1038/hr.2014.71. [DOI] [PubMed] [Google Scholar]
- 31.Ratsep MT, Carmeliet P, Adams MA, Croy BA. Impact of placental growth factor deficiency on early mouse implant site angiogenesis. Placenta. 2014;35:772–775. doi: 10.1016/j.placenta.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (flt-1) and soluble flt-1 (sflt-1), by peripheral blood mononuclear cells (pbmcs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, Leeson P. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65:607–614. doi: 10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- 34.Livingston JC, Chin R, Haddad B, McKinney ET, Ahokas R, Sibai BM. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. American Journal of Obstetrics and Gynecology. 2000;183:1554–1557. doi: 10.1067/mob.2000.108022. [DOI] [PubMed] [Google Scholar]
- 35.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 36.Ligi I, Simoncini S, Tellier E, Vassallo PF, Sabatier F, Guillet B, Lamy E, Sarlon G, Quemener C, Bikfalvi A, Marcelli M, et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood. 2011;118:1699–1709. doi: 10.1182/blood-2010-12-325142. [DOI] [PubMed] [Google Scholar]
- 37.Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart. 2003;89:175–178. doi: 10.1136/heart.89.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Souza R, Raghuraman RP, Nathan P, Manyonda IT, Antonios TF. Low birth weight infants do not have capillary rarefaction at birth: Implications for early life influence on microcirculation. Hypertension. 2011;58:847–851. doi: 10.1161/HYPERTENSIONAHA.111.179226. [DOI] [PubMed] [Google Scholar]
- 39.Rexhaj E, Bloch J, Jayet PY, Rimoldi SF, Dessen P, Mathieu C, Tolsa JF, Nicod P, Scherrer U, Sartori C. Fetal programming of pulmonary vascular dysfunction in mice: Role of epigenetic mechanisms. Am J Physiol Heart Circ Physiol. 2011;301:H247–252. doi: 10.1152/ajpheart.01309.2010. [DOI] [PubMed] [Google Scholar]
- 40.Tsao PN, Wei SC, Su YN, Chou HC, Chen CY, Hsieh WS. Excess soluble fms-like tyrosine kinase 1 and low platelet counts in premature neonates of preeclamptic mothers. Pediatrics. 2005;116:468–472. doi: 10.1542/peds.2004-2240. [DOI] [PubMed] [Google Scholar]
- 41.Pladys P, Sennlaub F, Brault S, Checchin D, Lahaie I, Le NL, Bibeau K, Cambonie G, Abran D, Brochu M, Thibault G, et al. Microvascular rarefaction and decreased angiogenesis in rats with fetal programming of hypertension associated with exposure to a low-protein diet in utero. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1580–1588. doi: 10.1152/ajpregu.00031.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.