Abstract
The recruitment of lymphoid progenitors to the thymus is essential to sustain T-cell production throughout life. Importantly, it also limits T-lineage regeneration following bone marrow transplantation, and so contributes to the secondary immunodeficiency that is caused by delayed immune reconstitution. Despite this significance, the mechanisms that control thymus colonisation are poorly understood. Here, we show that in both the steady-state and post-bone marrow transplant, Lymphotoxinβ Receptor controls entry of T-cell progenitors to the thymus. We show that this requirement maps to thymic stroma, further underlining the key importance of this Tumor Necrosis Receptor Superfamily member in regulation of thymic microenvironments. Importantly, analysis of the requirement for Lymphotoxinβ Receptor in relation to known regulators of thymus seeding suggests that it acts independently of its regulation of thymus-homing chemokines. Rather, we show that Lymphotoxinβ Receptor differentially regulates intrathymic expression of adhesion molecules known to play a role in T-cell progenitor entry to the thymus. Finally, antibody-mediated in vivo Lymphotoxinβ Receptor stimulation following bone marrow transplant enhances initial thymus recovery and boosts donor derived T-cell numbers, which correlates with increased adhesion molecule expression by thymic stroma. Collectively, we reveal a novel link between Lymphotoxinβ Receptor and thymic stromal cells in thymus colonisation, and highlight its potential as an immunotherapeutic target to boost T-cell reconstitution post-transplantation.
Introduction
In the thymus, immature lymphoid progenitors undergo a complex differentiation programme that biases thymocyte development towards the generation of self-tolerant MHC-restricted T-cells (1). Importantly, the haemopoietic progenitors that colonise the thymus are generated in extrathymic sites, and so T-cell development depends upon thymic colonisation by migrant progenitors (2, 3). As the thymus does not contain haemopoietic stem cells with long-term self-renewal capacity, there is an on-going requirement for this recruitment process, and this is important for several reasons. Firstly, it creates successive waves of thymopoiesis to maintain long-term T-cell production (4, 5). Secondly, it establishes competition for intrathymic niches that limits the self-renewal of intrathymic progenitors (6–8). Importantly, absence of competition manifests as T-cell acute lymphoblastic leukaemia, indicating that thymus seeding is part of an intrathymic tumour suppression mechanism that requires constant replacement of the immature thymocyte pool (9).
Although lymphoid progenitors are known to enter the adult thymus via blood vessels at the corticomedullary junction (10), their rarity means that their exact nature remains unclear (11–13). However, insight into the mechanisms that control thymus colonisation can be obtained by studying the frequency and requirements of CD4-CD8-CD44+CD25-CD117+ thymocytes that represent the earliest thymic progenitors (ETP) in the adult mouse thymus (13–16). Thus, thymus entry is recognised as a multi-step process involving chemokines, adhesion molecules and growth factors produced by thymic microenvironments. For example, thymic endothelial cells express VCAM-1, ICAM-1 and P-selectin (17–19) to enable the attachment of blood-borne lymphoid progenitors. Significantly, antibody blockade of VCAM1/ICAM1 impairs lymphoid progenitor entry to the thymus (20), while mice deficient in either P-selectin or its receptor PSGL1 have fewer ETP and an increased availability of intrathymic niches (18). ETP express the chemokine receptors CXCR4, CCR7, and CCR9 (21–24), and the chemokines CCL19, CCL21, CCL25 and CXCL12 are all products of thymic stroma (21, 25, 26). Significantly, disruption of these molecules either individually or in combination results in impaired thymus seeding (22, 23, 27, 28). Importantly, however, although these studies emphasise the importance of the thymic microenvironment in the recruitment of lymphoid progenitors to the thymus, this process is still poorly understood and relatively few of its regulators are known.
The importance of thymus seeding is further emphasised by its regulation of immune system recovery that follows ablative therapy and bone marrow transplantation (BMT), where limited thymus entry of donor progenitors slows down T-cell reconstitution in comparison to other blood cell lineages (29, 30). Indeed, intrathymic progenitor niches are not saturated until at least 10 weeks post-BMT (29), suggesting delayed T-cell reconstitution is linked to inefficient thymus seeding. Interestingly, while PSGL-1 has been identified as an important regulator of thymus seeding post-BMT (29), the cellular and molecular mechanisms that limit T-cell recovery post transplant, and how they relate to the requirements of steady-state T-cell development, remain poorly understood.
Here, we show that mice lacking Lymphotoxinβ Receptor (LTβR) demonstrate a dramatic reduction in the frequency of ETP, and that increased compensatory intrathymic progenitor proliferation accounts for their normal thymocyte numbers. Importantly, thymus transplant and bone marrow chimaera experiments show the requirement for LTβR maps to thymic stromal cells. We also show that LTβR differentially regulates thymic stromal expression of VCAM-1 and ICAM-1 but not P-selectin, that collectively represent adhesion molecules previously linked to thymus entry. Finally, we show that thymic recovery post-BMT also requires LTβR, and that agonistic anti-LTβR treatment enhances donor-derived T-cell reconstitution. Collectively, our findings identify a novel regulatory axis of T-cell progenitor entry to the thymus, and extend our understanding of the importance of LTβR in the functional control of thymic stromal microenvironments.
Materials and Methods
Mice
Adult wildtype (WT) C57BL/6 and congenic CD45.1+ C57BL/6 mice, and Ltbr-/- (31) and plt/plt (32) mice on a C57BL/6 background were used at 8-12 weeks of age. All mice were housed at the Biomedical Services Unit at the University of Birmingham in accordance with local and national Home Office regulations.
Antibodies and Flow Cytometry
For thymocyte and splenocyte analysis, tissues were enzymatically digested(33) using Collagenase D (2.5mg/ml, Roche) and DNase I (40μg/ml, Roche). Cells were stained with antibodies specific for CD44 (IM7), CD25 (PC61.5), CD117 (2B8), CD45 (30-F11), CD45.1 (A20), CD45.2 (104), CD4 (GK1.5), CD8β (53-6.7), TCRβ (597), Foxp3 (FJK-16s), conjugated to Brilliant Violet (BV) 605, BV510, Pacific Blue, eFluor450, PE, PECy7, PerCP-eFluor710, APC-eFluor780 and AlexaFluor700. Antibodies were purchased from eBioscience, BD Biosciences or BioLegend. Foxp3 staining was performed using an intracellular Foxp3 kit purchased from eBioscience. Streptavidin-BV786 was used to reveal staining with biotinylated antibodies. The following lineage markers were used: CD3ε (145-2C11), CD4 (GK1.5), CD8α (53-6.7), CD8β (H35-17.2), CD11b (M1/70), CD11c (N418), B220 (RA36B2), Ly-6G (RB6-865), NK1.1 (PK136), Ter-119 (TER-119), TCRβ (H57-597), TCRδ (GL3). Prior to surface staining, cells were stained with a fixable viability dye (Near-IR stain, Invitrogen). For the analysis of stromal cells, thymuses were enzymatically digested(34) using Collagenase Dispase (2.5mg/ml, Roche) and DNase 1 (40ug/ml, Roche). Prior to surface staining, CD45+ cells were depleted using anti-CD45 microbeads and LD columns (Miltenyi Biotech) and then stained with a fixable viability dye (Near-IR stain, Invitrogen). Cells were stained with monoclonal antibodies against CD45 (30-F11), EpCAM1 (G8.8), TER-119 (TER-119), podoplanin (8.1.1), CD31 (390), ICAM-1 (YN1/1/7/4), VCAM-1 (429). Antibodies were conjugated to APC, APCeFluor780, PE, PECy7, FITC, AlexaFluor700, BV605 or BV421. Data was acquired using a BD LSR Fortessa, and was analysed using FlowJo software (TreeStar). Forward and side scatter gates were set to exclude none viable and aggregated cells.
5-Bromo-2-Deoxyuridine Incorporation
Mice were injected IP with 1.5mg 5-bromo-2-deoxyuridine (BrdU) (Sigma) and tissue was harvested 3 hours later. Staining for BrdU was performed using the BrdU Flow Kit (BD Biosciences), according to the manufacturer’s instructions.
Generation of Bone Marrow Chimeras
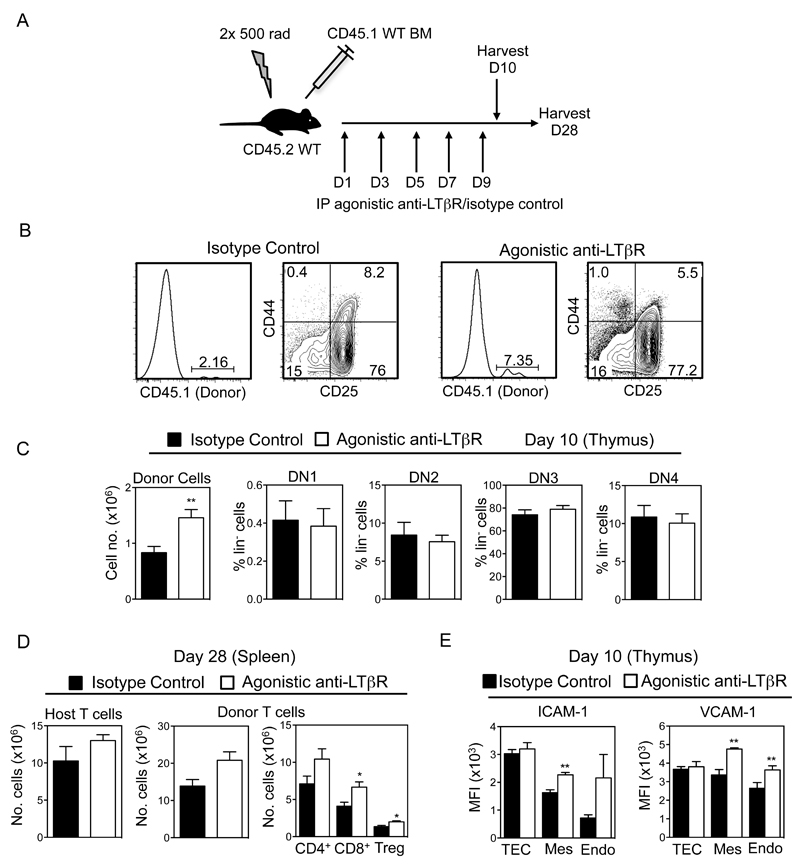
Recipient mice were lethally irradiated (2x 500 rad) and reconstituted intravenously with 5x106 T-cell depleted adult bone marrow preparations from CD45 congenically marked mice, as indicated. Depletion of T-cells was performed using anti CD3-PE and anti-PE microbeads (Miltenyi Biotech) according to manufacturer’s instructions. Mice were sacrificed at the indicated time points, and tissues were analysed by flow cytometry. In some experiments, mice received 100μg agonistic anti-LTβR (35) or isotype control on day 1, 3, 5, 7 and 9, and in these experiments tissues were harvested for analysis at day 10 or day 28.
Stromal Cell Isolation and PCR
Digested thymuses(34) were depleted of CD45+ cells using anti-CD45 microbeads (Miltenyi Biotech), in conjunction with LD columns. Cells were stained with antibodies to CD45 (30-F11), EpCAM1 (G8.8), TER-119 (TER-119) and podoplanin (8.1.1), and CD45-EpCAM1+ TEC and CD45-EpCAM1-podoplanin+ mesenchyme were FACS sorted using a MoFlo XDP (Beckman Coulter). CD31+ endothelial cells were sorted using anti-CD31 microbeads (Miltenyi Biotech) and MS columns, according to the manufacturer’s instructions. Sorted populations were analysed by qPCR for expression of the indicated genes exactly as described (36). Primer sequences are as follows:
Actb (NM_007393) QuantiTect Mm_Actb_1_SG PRIMER Assay (Qiagen QT00095242);
Ccl19 NM_(011888.2) Forward sequence GCTAATGATGCGGAAGACTG, reverse sequence ACTCACATCGACTCTCTAGG;
Ccl21a (NM_011124.4) Forward sequence ATCCCGGCAATCCTGTTCTC, Reverse sequence GGGGCTTTGTTTCCCTGGG;
Ccl25 (NM_009138.3) Forward sequence TTACCAGCACAGGATCAAATGG, Reverse sequence CGGAAGTAGAATCTCACAGCA,
Cxcl12 (NM_021704.3) Forward Sequence GCTCTGCATCAGTGACGGTA, Reverse sequence TGTCTGTTGTTGTTCTTCAGC,
Kitl (NM_013598.2) Forward sequence CCCTGAAGACTCGGGCCTA, Reverse sequence CAATTACAAGCGAAATGAGAGCC;
Selp (NM011347.2) Forward Sequence CATCTGGTTCAGTGCTTTGATCT, Reverse sequence ACCCGTGAGTTATTCCATGAGT.
Thymus Transplantation
Embryonic day 15 thymuses, organ cultured for 5 days in 1.35mM 2-deoxyguanosine, were transplanted under the kidney capsule of WT mice and harvested after 6-8 weeks(34).
Statistics
Data was analysed using an unpaired t-test. P values less than 0.05 were considered significant. Statistical analysis was performed using GraphPad Prism. Data represented as mean ± SEM.
Results
LTβR Controls ETP Frequency
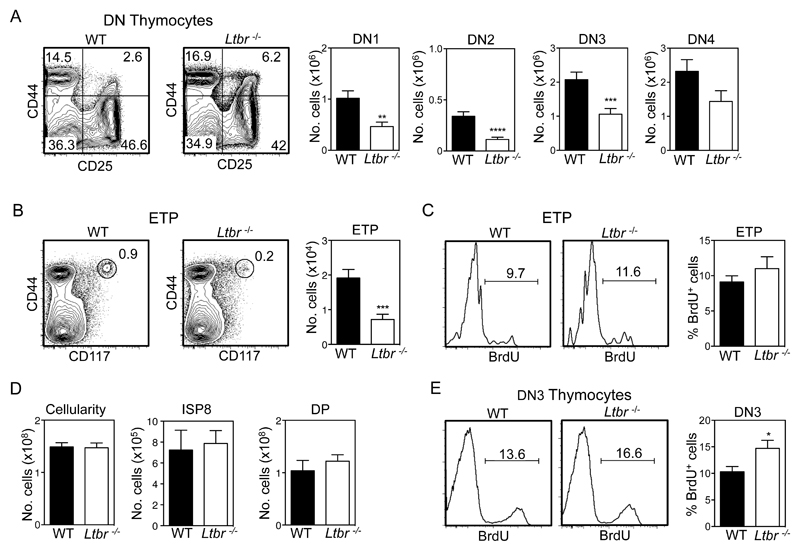
Given the importance of the Tumor Necrosis Factor Receptor Superfamily (TNFRSF) in the organisation and development of functionally competent thymic stromal microenvironments, we screened a panel of mutant mice for evidence of impaired thymus colonisation. While no obvious alterations were found in Tnfsf1-/- and Tnfrsf11b-/- mice, we found that Tnfrsf3-/- mice (described here as Ltbr-/- mice) had reduced numbers of downstream early T-cell progenitors including those at the DN1-3 stages of development (Figure 1A). Importantly, we also saw a significant reduction in both the proportion and absolute number of Lin-CD44+CD25-CD117+ ETP in Ltbr-/- mice (Figure 1B). Analysis of cellular proliferation following BrdU injection showed a similar frequency of BrdU+ ETP in WT and Ltbr-/- mice (Figure 1C), arguing against the notion that the ETP reduction in Ltbr-/- mice was due to alterations in their ability to proliferate intrathymically. Despite these changes, total thymocyte numbers in WT and Ltbr-/- mice were comparable, including numbers of CD4-CD8+ immature single positive (ISP) and CD4+CD8+ cells (Figure 1D), suggesting that increased thymocyte expansion during early stages of T-cell development may restore normal thymocyte cellularity. Indeed, analysis of BrdU incorporation showed an increased frequency of BrdU+ DN3 thymocytes in Ltbr-/- mice compared to WT (Figure 1E). Taken together, these findings show that LTβR controls the frequency of ETP in the adult, suggesting a role in the regulation of lymphoid progenitor entry to the thymus. They also indicate the ETP reduction in Ltbr-/- mice is compensated by enhanced DN3 stage thymocyte proliferation that restores thymus cellularity to normal levels.
Figure 1. Reduced Early Thymus Progenitors In LTβR-/- Mice.
CD4-CD8- (DN) thymocytes (A) and ETP (B) in WT and Ltbr-/- thymus, gated on lineage- and lineage-CD25- cells respectively. Representative FACS plots are shown, n≥23. (C) Representative FACS plots and proportions of BrdU+ ETP in WT and Ltbr-/- thymus, n=10. (D) Total cellularity and numbers of immature SP8 (ISP8) and CD4+CD8+ (DP) thymocytes in WT and Ltbr-/- thymus, n≥8. (E) BrdU incorporation and proportions of BrdU+ DN3 thymocytes from WT and Ltbr-/- thymus, n≥8. All data are from at least 3 independent experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Stromal Cell Expression of LTβR Controls Thymus Entry of T-cell Progenitors
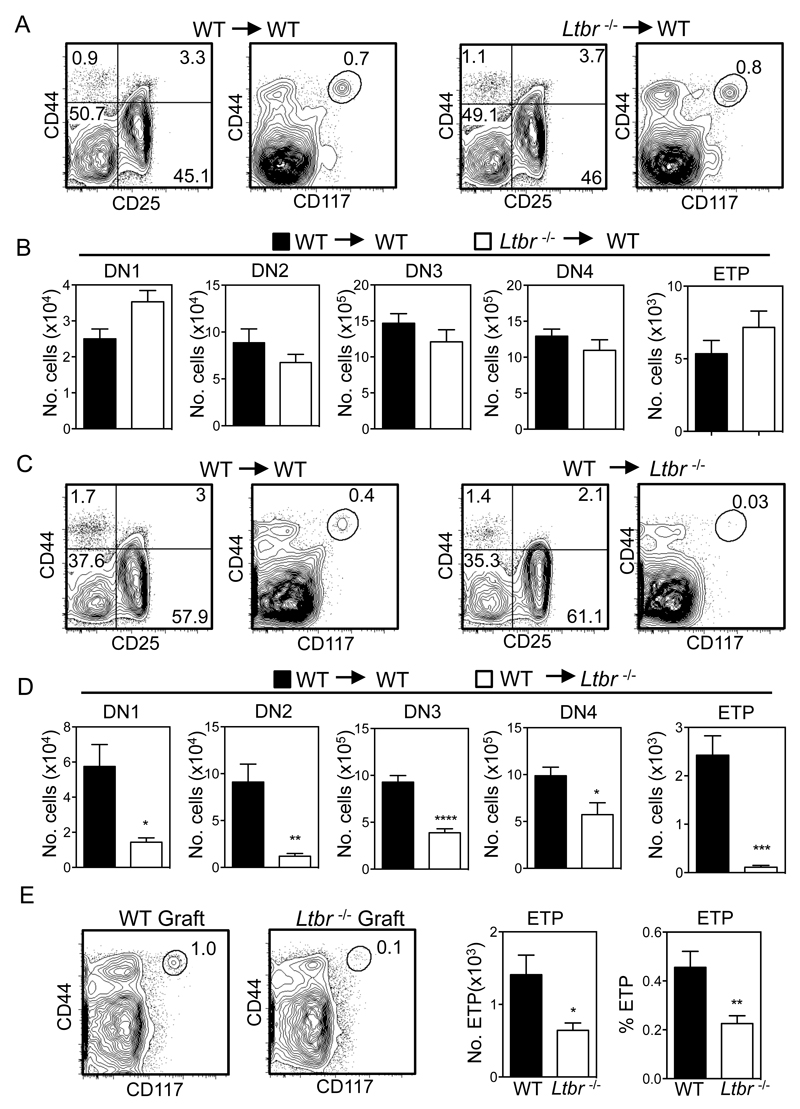
Although LTβR regulates the development and function thymic stromal microenvironments (25, 37), it can also directly influence haemopoietic cells (38). To investigate the LTβR-expressing cellular compartment that regulates progenitor entry to the thymus, we first established a series of reciprocal bone marrow chimaeras to confine LTβR expression to either stromal cells or haemopoietic cells. Initially, WT mice at 8 weeks of age were lethally irradiated and injected intravenously with congenically marked T-cell depleted bone marrow (BM) obtained from either WT or Ltbr-/- donor mice. In addition, Ltbr-/- adult recipients were reconstituted with congenic T-depleted BM preparations from either WT or Ltbr-/- donors. Mice were harvested after 8 weeks, and analysis of thymocyte development from donor-derived progenitors was performed. In chimaeras using WT hosts, similar ETP proportions and numbers were generated from both WT and Ltbr-/- BM, and no significant alterations in early stage T-cell development were observed (Figure 2A, B). In contrast, a dramatic reduction in ETP frequency and proportion was observed when Ltbr-/- hosts were reconstituted with WT donor BM (Figure 2C, D). Alterations in early thymocyte development, including a reduction in DN1-3 T-cell progenitor compartments were also noted in WT > Ltbr-/- chimaeras (Figure 2D). Next, to directly assess whether LTβR expression by thymic stroma is involved in progenitor entry to the thymus, we transplanted alymphoid WT or Ltbr-/- thymus lobes under the kidney capsule of WT mice. In this setting, LTβR is exclusively absent from stromal cells in the transplanted thymus. Importantly, flow cytometric analysis 6-8 weeks post grafting showed a significant reduction in the proportion and absolute number of ETP in Ltbr-/- grafts compared to WT (Figure 2E). Collectively, these results indicate that LTβR expression by thymic stroma is important for lymphoid progenitors to enter the thymus, a finding consistent with the cell-intrinsic requirement for LTβR in the development and function of thymic stromal microenvironments (25, 37, 39).
Figure 2. LTβR Expression By Thymic Stroma Controls Thymus Entry.
(A-D) Lethally irradiated WT/Ltbr-/- mice were reconstituted with congenically marked T-cell depleted WT/Ltbr-/- BM cells as indicated. Representative FACS plots are shown, after gating on congenically marked donor-derived thymocytes. n=10 from 3 independent experiments. *p<0.05, **p<0.01, ****p<0.0001. (E) Frequency and absolute number of ETP in WT and Ltbr-/- dGuo thymus grafts following transplant into WT hosts for 6-8 weeks, n≥8 grafts from 3 independent experiments. Representative FACS plots are shown.
LTβR Differentially Controls Known Regulators of Thymus Seeding
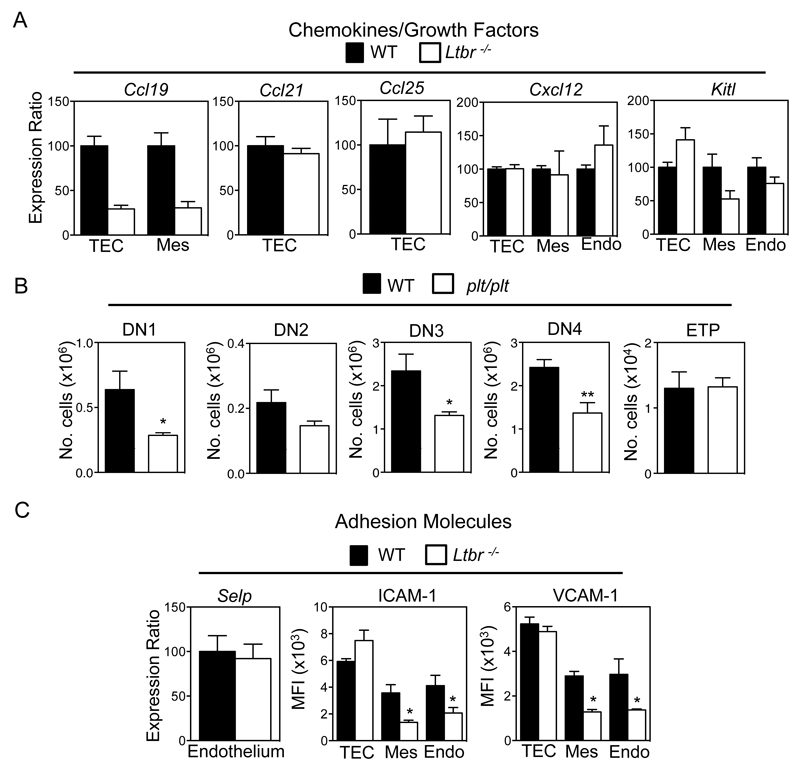
To investigate how LTβR influences T-cell progenitor entry to the thymus, we compared the expression of known regulators of thymus seeding in purified thymic stromal subsets from WT and Ltbr-/- mice. Ccl25 and Cxcl12 mRNA levels were not altered in Ltbr-/- mice (Figure 3A), suggesting the requirement for LTβR is not explained by its regulation of ligand availability for the chemokine receptors CCR9 and CXCR4. Similarly, we saw no substantial alteration in Kitl expression, an important growth factor for immature T-cell progenitors (40, 41). Interestingly, we also found no differences in Ccl21 mRNA levels in WT and Ltbr-/- TEC (Figure 3A). However, it is important to note that this observation is not incompatible with an earlier study showing that CCL21+ mTEC are present, but at a reduced frequency in Ltbr-/- mice(25), and may simply reflect differences in methods (qPCR/flow cytometry) used to measure of CCL21 expression. Interestingly however, we did see decreased expression of Ccl19 mRNA in both TEC and thymic mesenchyme from Ltbr-/- mice (Figure 3A), suggesting that LTβR could play a role in the recruitment of T-cell progenitors via regulation of intrathymic CCR7 ligand availability. To investigate this directly, we examined ETP frequency and early T-cell development in plt/plt mice that lack expression of CCL19 and CCL21 (32). Interestingly, while our analysis of plt/plt mice showed alterations in DN1-4 T-cell progenitor frequencies that are consistent with an earlier report (24), we saw no differences in ETP numbers in WT and plt/plt thymuses (Figure 3B). This agrees with other studies showing that the major impact of CCR7-deficiency on ETP requires the combined absence of CCR9 (22, 23, 27). Thus, mice lacking either LTβR or CCR7 ligands do not share alterations in ETP frequency, suggesting that control of CCL19 and CCL21 expression by LTβR does not explain its requirement during thymus seeding.
Figure 3. LTβR Differentially Regulates Known Mediators Of Thymus Seeding.
(A) Purified stromal samples from WT and Ltbr-/- mice were analysed by qPCR for the indicated genes. mRNA levels were normalized to β-actin (mean±SEM), and represent at least two independent biological experiments. (B) Frequency of DN thymocyte subsets and ETP in thymuses from adult WT and plt/plt mice, n≥9 from 3 independent experiments, *p<0.05, **p<0.01. (C) Comparison of Selp mRNA expression in WT and Ltbr-/- endothelium, and MFI analysis of VCAM-1 and ICAM-1 in the indicated stromal subsets of WT and Ltbr-/- mice.
In addition to chemokines, thymus entry requires attachment of lymphoid progenitors to stromal cells expressing the adhesion molecules P-selectin, VCAM-1 and ICAM-1 (17, 19, 20). We found that Selp mRNA levels were comparable in WT and Ltbr-/- thymic endothelium (Figure 3C). By contrast, levels of VCAM-1 and ICAM-1 were altered. Specifically, both Ltbr-/- thymic endothelium and mesenchyme had significantly reduced levels of VCAM-1/ICAM-1 (Figure 3C). Interestingly, levels of expression on WT and Ltbr-/- TEC were comparable (Figure 3C). Collectively, our findings demonstrate a differential requirement for LTβR in the control of adhesion molecule expression by TEC and non-TEC stroma, and show that within the latter, adhesion molecules known to influence thymus entry can be subdivided into LTβR-dependent (VCAM-1/ICAM-1) and LTβR-independent (P-selectin) groups.
LTβR Mediates Thymus Recovery Post-BMT
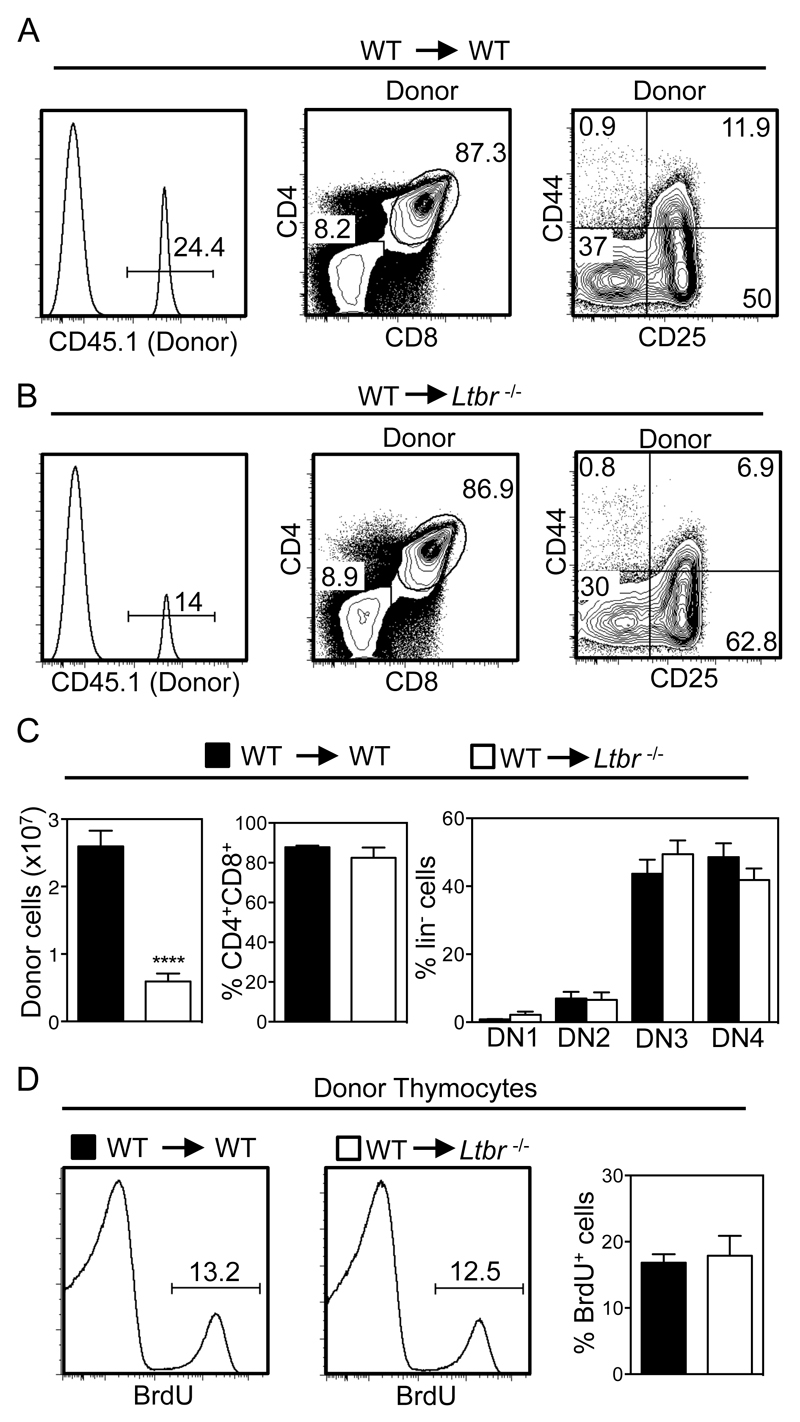
T-cell progenitor recruitment to the thymus influences T-cell reconstitution and thymus recovery following ablative therapy and BMT (29). We next examined the possible role of LTβR in this context, and focussed on early events in thymus reconstitution (42–44). Importantly, other studies have shown that donor-derived ETP are not detectable in irradiated mice at time points shortly after BMT, with a clearly defined ETP population not apparent until after 3 weeks post transplant (45). Thus, to assess the role of LTβR in early phases of thymus recovery, we determined the frequency and number of donor-derived congenically marked thymocytes 13 days after the transplantation of WT BM into lethally irradiated WT and Ltbr-/- mice. At early stages of thymus recovery, it is important to note that the thymus is dominated by thymocytes of host origin that survive and expand post irradiation (Figure 4A, B) (43, 44). Importantly, while 2-3x107 CD45.1+ donor-derived thymocytes were recovered from the thymuses of irradiated WT hosts, a frequency that is in line with other studies (46), we saw a significant 3-4 fold reduction in the number of CD45.1+ donor-derived thymocytes recovered from Ltbr-/- hosts (Figure 4A-C). Interestingly, the pattern of development (Figure 4A-C) and frequency of BrdU+ cells (Figure 4D) in donor-derived thymocytes was comparable in WT and Ltbr-/- hosts, indicating that this difference was not due to an inability of donor progenitors to undergo proliferation and differentiation in the irradiated Ltbr-/- host thymus. Rather, the reduced frequency of donor-derived thymocytes in the thymus of Ltbr-/- hosts suggests that as in the steady state, progenitor entry to the thymus post-BMT involves LTβR.
Figure 4. Initial Thymic Reconstitution Post-BMT Is Controlled by LTβR.
Lethally irradiated WT (A) and Ltbr-/- (B) mice were reconstituted with T-depleted congenically marked WT BM cells and harvested after 13 days. Thymic reconstitution was determined by calculating the intrathymic frequency of CD45.1+ donor cells, and bar charts in (C) show numbers of total donor thymocytes, and percentages of donor-derived DP and DN thymocytes. n≥13 from 5 independent experiments, representative FACS plots are shown. (D) Analysis of BrdU incorporation in WT donor-derived CD45.1+ thymocytes from WT and Ltbr-/- hosts, n=6 from 3 independent experiments. ****p<0.0001.
In Vivo Agonistic Anti-LTβR Treatment Enhances Thymus Recovery Post-BMT
Given our findings on thymus reconstitution in Ltbr-/- mice, we next investigated whether exogenous LTβR stimulation may be a potential therapeutic means to boost thymus recovery post-BMT. WT mice were lethally irradiated and reconstituted them with congenic CD45.1+ T-cell depleted BM preparations. The day after BMT, mice then received either 100μg agonistic anti-LTβR (35) or an isotype control antibody, every other day until day 9 (Figure 5A). Tissues were harvested from chimeric mice on days 10 and 28 post-BMT, and donor-derived thymocytes and T-cells were analysed by flow cytometry. Again, at this early day 10 timepoint (Figure 5B), the dominant population of cells in the thymus were host-derived thymocytes that survived irradiation. Importantly however, although ETP cannot be detected at this early time point (45), we found that a population of donor-derived thymocytes was detectable, representing initial donor engraftment of the host thymus (Figure 5B). Strikingly, 10 days post-transplant, the proportion and absolute number of donor-derived CD45.1+ thymocytes was significantly increased in mice receiving agonistic LTβR compared to isotype control (Figure 5B and C), and cells showed a normal pattern of progression through DN thymocyte stages at this early post-transplant timepoint (Figure 5B,C). Furthermore, analysis of chimaeras 28 days post-transplant showed a significant increase in donor-derived peripheral T-cell numbers in the spleens of mice receiving agonistic anti-LTβR (Figure 5D). Interestingly, this effect of anti-LTβR was specific, as residual host-derived splenic T-cell numbers were not affected by antibody treatment (Figure 5D). Thus, our data suggests that agonistic anti-LTβR treatment enhances the recovery of thymopoiesis by increasing the frequency of donor-derived progenitors in the thymus of irradiated recipient mice, and this leads to an increase in donor-derived T-cells in the periphery.
Figure 5. LTβR Stimulation Enhances Thymic Reconstitution Post-BMT.
(A) Lethally irradiated WT mice were reconstituted with T-depleted congenic WT BM cells, injected IP with 100μg agonistic anti-LTβR or isotype on days 1, 3, 5, 7 and 9, and harvested on day 10 or 28. (B, C) Thymic reconstitution was determined by calculating the intrathymic frequency of total CD45.1+ donor thymocytes and donor DN thymocyte subsets at day 10. Representative FACS plots are shown, n≥8 from 3 independent experiments. (D) Frequencies of host or donor derived splenic T-cells were determined at day 28. (E) MFI expression of VCAM-1 and ICAM-1 on CD45-EpCAM1+ TEC, CD31+podoplanin- endothelium, and CD31-podoplanin+ mesenchyme from mice treated with either anti-LTβR or control antibody control treated mice at day 10. n≥8 from 2 independent experiments, *p<0.05, **p<0.001.
Finally, given our data suggesting that LTβR may influence thymus seeding by regulating expression of VCAM-1 and ICAM-1, we analysed the impact of anti-LTβR treatment on their levels in thymic stroma post-BMT. Lethally irradiated mice were reconstituted with T-cell depleted BM and subjected to anti-LTβR/isotype control treatment, and thymuses were harvested after 10 days. Following digestion, CD45-EpCAM1+ TEC, CD45-CD31+ endothelium and CD45-podoplanin+ mesenchymal stromal cells were analysed by flow cytometry. Interestingly, in mice receiving anti-LTβR treatment, both thymic endothelium and mesenchymal cells showed increased levels of both VCAM-1 and ICAM-1 while levels on TEC were not altered (Figure 5E). Collectively, these observations show that in vivo stimulation of LTβR boosts thymic recovery post-BMT, and this correlates with enhanced expression of adhesion molecules known to facilitate thymic entry of T-cell progenitors in non-TEC stroma.
Discussion
The absence of an intrathymic haemopoietic stem cell pool means that in order to sustain T-cell production throughout life, the thymus must continuously import lymphoid progenitors from extrathymic sites. In addition, the entry of donor-derived lymphoid progenitors to the thymus represents a rate limiting step in re-establishing T-cell mediated immunity that follows ablative therapy and BMT. Despite this importance, relatively few regulators of T-cell progenitor entry to the thymus are known. Collectively, the work described here shows that the TNFR superfamily member LTβR plays a key role in the regulation of thymus seeding. We also show LTβR is involved in thymus entry in both the steady-state and during the early phases of thymus recovery that take place post-BMT. Regarding the latter, manipulation of the LTβR axis using agonistic antibodies significantly improved donor-derived thymopoiesis and boosted T-cell recovery post-BMT, suggesting that LTβR stimulation is part of a common mechanism that controls thymus entry in both the steady state and during immune reconstitution.
Our finding that the requirement for LTβR maps to thymic stromal cells is significant as it extends our understanding of its importance in the regulation of thymus function. For example, identification of a role for LTβR in thymic entry complements work demonstrating its importance for the thymic egress of mature thymocytes(37). Interestingly, another study has shown that mature thymocytes and T-cell progenitors are present within the same perivascular spaces that surround intrathymic blood vessels (47). Taken together, these findings raise the possibility that the limited entry of T-cell progenitors to the Ltbr-/- thymus is caused by an accumulation of mature thymocytes at a common site of thymic exit and entry. Further experiments are required to examine the possible relationship between these processes and the mechanisms that regulate them.
Although LTβR has been shown to influence the frequency of CCL21+ mTEC (25), our findings, including normal ETP frequency in plt/plt mice, suggest that its involvement in thymus seeding is not simply explained by its regulation of CCR7 ligands. However, whether the requirement for LTβR shown here is linked to the noted decrease in availability of CCL21-expressing mTEClo cells (25) is currently not clear. Importantly, the positive impact of LTβR stimulation on thymic reconstitution shown here is observed shortly after BMT, a time-point at which thymus seeding is transiently independent of CCR7 and CCR9 (29), further suggesting that LTβR stimulation augments thymic reconstitution via mechanisms other than chemokine availability. As indicated from studies on peripheral lymphoid tissues (48), another way in which LTβR may influence thymus entry is through its ability to regulate the expression of adhesion molecules by endothelium and/or mesenchyme. This would fit well with our demonstration of reduced levels of VCAM-1/ICAM-1 in steady state Ltbr-/- mice, and their enhanced expression in both thymic endothelium and mesenchyme following in vivo anti-LTβR treatment. It is also supported by previous studies in which antibody-mediated blockade of VCAM-1/ICAM-1 inhibited thymic entry of transferred lymphoid progenitors(20). Interestingly, unlike VCAM-1 and ICAM-1, we found LTβR did not control P-selectin expression, a finding that highlights its differential ability to influence expression of adhesion molecules linked to thymus entry. Together, these data raise the possibility that LTβR regulates thymic entry through control of integrin-mediated firm adhesion and transendothelial migration, rather then initial phases of selectin-mediated rolling and chemokine-driven activation/migration. Alternatively, the requirement for LTβR may relate to its importance in the regulation of medullary microenvironments. Thus, altered mTEC development and organisation in Ltbr-/- mice (25, 37) may limit the ability of the thymus to attract migrant lymphoid progenitors. However, it is interesting to note that while thymus medulla disorganisation is also evident in plt/plt mice(24), we found that their ETP frequency was not altered.
Finally, the identity of the LTβR ligands and the cells they are expressed by that influence thymus seeding are not known. Relevant to this, earlier studies have shown the difficulty in correlating known roles for LTβR in thymus development and function with the availability of the LTβR ligands Lymphotoxin and Light. For example, whether defects in thymus organisation caused by absence of LTβR are mirrored in mice lacking LTβR ligands, either individually or in combination, is not certain (37, 49). However, as thymic expression of LTβR-ligands has been mapped to a variety of haemopoeitic cells (37, 50), we suggest the requirement for LTβR during thymus entry represents a further example of how thymic crosstalk regulates the TNFR-mediated control of thymus function. In summary, our study identifies a new role for LTβR in the control of thymus function, where it acts as a regulator of the earliest phases of T-cell development by influencing the intrathymic availability of the earliest thymocyte progenitors. That this role extends to thymic recovery post-BMT suggests the potential of LTβR as an immunotherapeutic target to boost T-cell recovery and restore essential immune system functioning following ablative therapy.
Acknowledgements
We thank Andrea Bacon and BMSU staff for expert animal husbandry, and Prof Eric Jenkinson for critical reading of the manuscript.
This work was supported by an MRC Programme Grant to GA, a BBSRC Project Grant to WEJ, and a CRUK Cancer Immunology award to GA/WEJ. EC is supported by an ARUK RACE Centre of Excellence PhD studentship.
Footnotes
Joint senior authors.
ETP, Early Thymus Progenitors.
BMT, Bone Marrow Transplant.
LTβR, Lymphotoxinβ Receptor.
References
- 1.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SL, Bhandoola A. Trafficking to the thymus. Curr Top Microbiol Immunol. 2014;373:87–111. doi: 10.1007/82_2013_324. [DOI] [PubMed] [Google Scholar]
- 3.Boehm T, Bleul CC. Thymus-homing precursors and the thymic microenvironment. Trends Immunol. 2006;27:477–484. doi: 10.1016/j.it.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Porritt HE, Gordon K, Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–962. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 6.Martins VC, Ruggiero E, Schlenner SM, Madan V, Schmidt M, Fink PJ, von Kalle C, Rodewald HR. Thymus-autonomous T cell development in the absence of progenitor import. J Exp Med. 2012;209:1409–1417. doi: 10.1084/jem.20120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peaudecerf L, Lemos S, Galgano A, Krenn G, Vasseur F, Di Santo JP, Ezine S, Rocha B. Thymocytes may persist and differentiate without any input from bone marrow progenitors. J Exp Med. 2012;209:1401–1408. doi: 10.1084/jem.20120845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004;173:1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 9.Martins VC, Busch K, Juraeva D, Blum C, Ludwig C, Rasche V, Lasitschka F, Mastitsky SE, Brors B, Hielscher T, Fehling HJ, et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature. 2014;509:465–470. doi: 10.1038/nature13317. [DOI] [PubMed] [Google Scholar]
- 10.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlenner SM, Rodewald HR. Early T cell development and the pitfalls of potential. Trends Immunol. 2010;31:303–310. doi: 10.1016/j.it.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Zietara N, Lyszkiewicz M, Puchalka J, Witzlau K, Reinhardt A, Forster R, Pabst O, Prinz I, Krueger A. Multicongenic fate mapping quantification of dynamics of thymus colonization. J Exp Med. 2015;212:1589–1601. doi: 10.1084/jem.20142143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlenner SM, Madan V, Busch K, Tietz A, Laufle C, Costa C, Blum C, Fehling HJ, Rodewald HR. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 16.Luc S, Luis TC, Boukarabila H, Macaulay IC, Buza-Vidas N, Bouriez-Jones T, Lutteropp M, Woll PS, Loughran SJ, Mead AJ, Hultquist A, et al. The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nat Immunol. 2012;13:412–419. doi: 10.1038/ni.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lepique AP, Palencia S, Irjala H, Petrie HT. Characterization of vascular adhesion molecules that may facilitate progenitor homing in the post-natal mouse thymus. Clin Dev Immunol. 2003;10:27–33. doi: 10.1080/10446670310001598492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi FM, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, So L, Yi L, Ziltener HJ. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 19.Sultana DA, Zhang SL, Todd SP, Bhandoola A. Expression of functional P-selectin glycoprotein ligand 1 on hematopoietic progenitors is developmentally regulated. J Immunol. 2012;188:4385–4393. doi: 10.4049/jimmunol.1101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkinson WE, Rossi SW, Parnell SM, Agace WW, Takahama Y, Jenkinson EJ, Anderson G. Chemokine receptor expression defines heterogeneity in the earliest thymic migrants. Eur J Immunol. 2007;37:2090–2096. doi: 10.1002/eji.200737212. [DOI] [PubMed] [Google Scholar]
- 22.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2010;115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 23.Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Forster R. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200:481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lkhagvasuren E, Sakata M, Ohigashi I, Takahama Y. Lymphotoxin beta receptor regulates the development of CCL21-expressing subset of postnatal medullary thymic epithelial cells. J Immunol. 2013;190:5110–5117. doi: 10.4049/jimmunol.1203203. [DOI] [PubMed] [Google Scholar]
- 26.Seach N, Ueno T, Fletcher AL, Lowen T, Mattesich M, Engwerda CR, Scott HS, Ware CF, Chidgey AP, Gray DH, Boyd RL. The lymphotoxin pathway regulates Aire-independent expression of ectopic genes and chemokines in thymic stromal cells. J Immunol. 2008;180:5384–5392. doi: 10.4049/jimmunol.180.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Saito F, Liu Z, Lei Y, Uehara S, Love P, Lipp M, Kondo S, Manley N, Takahama Y. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 28.Calderon L, Boehm T. Three chemokine receptors cooperatively regulate homing of hematopoietic progenitors to the embryonic mouse thymus. Proc Natl Acad Sci U S A. 2011;108:7517–7522. doi: 10.1073/pnas.1016428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zlotoff DA, Zhang SL, De Obaldia ME, Hess PR, Todd SP, Logan TD, Bhandoola A. Delivery of progenitors to the thymus limits T-lineage reconstitution after bone marrow transplantation. Blood. 2011;118:1962–1970. doi: 10.1182/blood-2010-12-324954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velardi E, Dudakov JA, van den Brink MR. Clinical strategies to enhance thymic recovery after allogeneic hematopoietic stem cell transplantation. Immunol Lett. 2013;155:31–35. doi: 10.1016/j.imlet.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 32.Nakano H, Mori S, Yonekawa H, Nariuchi H, Matsuzawa A, Kakiuchi T. A novel mutant gene involved in T-lymphocyte-specific homing into peripheral lymphoid organs on mouse chromosome 4. Blood. 1998;91:2886–2895. [PubMed] [Google Scholar]
- 33.Lucas B, White AJ, Ulvmar MH, Nibbs RJ, Sitnik KM, Agace WW, Jenkinson WE, Anderson G, Rot A. CCRL1/ACKR4 is expressed in key thymic microenvironments but is dispensable for T lymphopoiesis at steady state in adult mice. Eur J Immunol. 2015;45:574–583. doi: 10.1002/eji.201445015. [DOI] [PubMed] [Google Scholar]
- 34.White AJ, Jenkinson WE, Cowan JE, Parnell SM, Bacon A, Jones ND, Jenkinson EJ, Anderson G. An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J Immunol. 2014;192:2659–2666. doi: 10.4049/jimmunol.1303057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banks TA, Rickert S, Benedict CA, Ma L, Ko M, Meier J, Ha W, Schneider K, Granger SW, Turovskaya O, Elewaut D, et al. A lymphotoxin-IFN-beta axis essential for lymphocyte survival revealed during cytomegalovirus infection. J Immunol. 2005;174:7217–7225. doi: 10.4049/jimmunol.174.11.7217. [DOI] [PubMed] [Google Scholar]
- 36.Cowan JE, McCarthy NI, Parnell SM, White AJ, Bacon A, Serge A, Irla M, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G. Differential requirement for CCR4 and CCR7 during the development of innate and adaptive alphabetaT cells in the adult thymus. J Immunol. 2014;193:1204–1212. doi: 10.4049/jimmunol.1400993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng D, Maitre B, Cummings D, Lin A, Ward LA, Rahbar R, Mossman KL, Ohashi PS, Gommerman JL. A Lymphotoxin/Type I IFN Axis Programs CD8+ T Cells To Infiltrate a Self-Tissue and Propagate Immunopathology. J Immunol. 2015;195:4650–4659. doi: 10.4049/jimmunol.1501053. [DOI] [PubMed] [Google Scholar]
- 39.White AJ, Nakamura K, Jenkinson WE, Saini M, Sinclair C, Seddon B, Narendran P, Pfeffer K, Nitta T, Takahama Y, Caamano JH, et al. Lymphotoxin signals from positively selected thymocytes regulate the terminal differentiation of medullary thymic epithelial cells. J Immunol. 2010;185:4769–4776. doi: 10.4049/jimmunol.1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buono M, Facchini R, Matsuoka S, Thongjuea S, Waithe D, Luis TC, Giustacchini A, Besmer P, Mead AJ, Jacobsen SE, Nerlov C. A dynamic niche provides Kit ligand in a stage-specific manner to the earliest thymocyte progenitors. Nat Cell Biol. 2016;18:157–167. doi: 10.1038/ncb3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodewald HR, Kretzschmar K, Swat W, Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3:313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 42.Kadish JL, Basch RS. Hematopoietic thymocyte precursors. I. Assay and kinetics of the appearance of progeny. J Exp Med. 1976;143:1082–1099. doi: 10.1084/jem.143.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang SL, Wang X, Manna S, Zlotoff DA, Bryson JL, Blazar R, Bhandoola A. Chemokine treatment rescues profound T-lineage progenitor homing defect after bone marrow transplant conditioning in mice. Blood. 2014;124:296–304. doi: 10.1182/blood-2014-01-552794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirokawa K, Sado T, Kubo S, Kamisaku H, Hitomi K, Utsuyama M. Intrathymic T cell differentiation in radiation bone marrow chimeras and its role in T cell emigration to the spleen. An immunohistochemical study. J Immunol. 1985;134:3615–3624. [PubMed] [Google Scholar]
- 45.Maillard I, Schwarz BA, Sambandam A, Fang T, Shestova O, Xu L, Bhandoola A, Pear WS. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–3519. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frasca D, Guidi F, Arbitrio M, Pioli C, Poccia F, Cicconi R, Doria G. Hematopoietic reconstitution after lethal irradiation and bone marrow transplantation: effects of different hematopoietic cytokines on the recovery of thymus, spleen and blood cells. Bone Marrow Transplant. 2000;25:427–433. doi: 10.1038/sj.bmt.1702169. [DOI] [PubMed] [Google Scholar]
- 47.Mori K, Itoi M, Tsukamoto N, Kubo H, Amagai T. The perivascular space as a path of hematopoietic progenitor cells and mature T cells between the blood circulation and the thymic parenchyma. Int Immunol. 2007;19:745–753. doi: 10.1093/intimm/dxm041. [DOI] [PubMed] [Google Scholar]
- 48.Onder L, Danuser R, Scandella E, Firner S, Chai Q, Hehlgans T, Stein JV, Ludewig B. Endothelial cell-specific lymphotoxin-beta receptor signaling is critical for lymph node and high endothelial venule formation. J Exp Med. 2013;210:465–473. doi: 10.1084/jem.20121462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mouri Y, Yano M, Shinzawa M, Shimo Y, Hirota F, Nishikawa Y, Nii T, Kiyonari H, Abe T, Uehara H, Izumi K, et al. Lymphotoxin signal promotes thymic organogenesis by eliciting RANK expression in the embryonic thymic stroma. J Immunol. 2011;186:5047–5057. doi: 10.4049/jimmunol.1003533. [DOI] [PubMed] [Google Scholar]
- 50.White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJ, Jenkinson EJ, Anderson G. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol. 2008;38:942–947. doi: 10.1002/eji.200738052. [DOI] [PubMed] [Google Scholar]