Abstract
Background/Objectives
The molecular chaperone αB-crystallin is expressed in estrogen receptor, progesterone receptor and human epidermal growth factor receptor-2 “triple-negative” breast carcinomas and promotes brain and lung metastasis. We examined αB-crystallin expression in primary breast carcinomas with metastatic data to evaluate its association with prognosis and site-specific metastases.
Methods
αB-crystallin gene (CRYAB) expression was examined using publically available global-gene expression data (n=855 breast tumors) with first site of distant metastasis information (“855Met”). αB-crystallin protein expression was determined by immunohistochemistry using the clinically annotated tissue microarray (n=3987 breast tumors) from British Columbia Cancer Agency (BCCA). Kaplan-Meier and multivariable Cox regression analyses were used to evaluate the prognostic value of αB-crystallin. Multivariable logistic regression analysis was used to evaluate risks of αB-crystallin and other markers for site of metastasis.
Results
In the 855Met dataset, αB-crystallin gene (CRYAB) expression was an independent predictor of brain as the first distant site of relapse (HR = 1.2, (95% CI 1.0-1.4), P = 0.021). In the BCCA series, αB-crystallin protein expression was an independent prognostic marker of poor breast cancer specific survival (HR = 1.3, (95% CI 1.1-1.6), P = 0.014). Among patients with metastases, αB-crystallin was the strongest independent predictor of brain metastasis (OR = 2.99 (95% CI 1.83-4.89), P < 0.0001) and the only independent predictor of brain as the first site of distant metastasis (OR = 3.15 (95% CI1.43-6.95), P = 0.005). αB-crystallin was also associated with worse survival (3.0 versus 4.7 months, P = 0.007).
Conclusions
αB-crystallin is a promising biomarker to identify breast cancer patients at high risk for early relapse in the brain, independent of ER and HER2 status.
Keywords: αB-crystallin, metastasis, triple-negative breast cancer, tissue microarray, biomarker, brain
Introduction
Over the past fifteen years, gene expression studies in breast cancer have led to more accurate prognostic tools and to a greater understanding of the molecular heterogeneity of breast cancer (1–5). Basal-like breast carcinomas have emerged as a distinctive molecular subtype defined by expression of genes characteristic of basal epithelial cells that overlap in large part with estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) “triple-negative” breast cancers (TNBC) (6, 7). More recent studies point to molecular heterogeneity within the triple-negative subgroup with potential prognostic and therapeutic implications (8–10). Overall, basal-like tumors are associated with a higher risk of recurrence following conventional adjuvant treatment and poor survival (4, 11, 12). Furthermore, basal-like tumors have a characteristic pattern of distant metastasis to the lungs and brain with infrequent relapse in bone (13–15). Although lung- and brain-metastasis gene signatures have been identified (16, 17), the functional contribution of individual genes to site-specific metastasis in this subtype is poorly understood. Clearly, the identification of pathogenic drivers of brain and lung metastasis in this subtype could lead to biomarkers to help identify women at high risk for relapse at these sites.
αB-crystallin is a widely expressed member of the small heat shock protein family that protects cells from stress by its dual function as a molecular chaperone to preserve proteostasis and as a cell death antagonist that inhibits caspase-3 activation and oxidative stress (18–22). αB-crystallin is commonly expressed in basal-like and TNBCs and its expression correlates with resistance to neoadjuvant chemotherapy and poor survival (23–26). More recently, αB-crystallin has been demonstrated to inhibit extracellular matrix-detachment induced apoptosis (“anoikis”), enhance penetration through an endothelial/astrocyte co-culture model of the blood-brain barrier (BBB) in vitro and promote lung and brain metastasis in orthotopic models of TNBC in immunodeficient mice (27, 28). In addition, αB-crystallin is frequently expressed in brain metastases and its expression in primary breast carcinomas is associated with poor overall survival and poor survival after brain metastasis in a cohort of 76 women with breast cancer brain metastasis (28). Collectively, these findings point to a key pathogenic role of αB-crystallin in lung and brain metastasis in TNBC.
Given the recent evidence supporting a prometastatic function for αB-crystallin in TNBC, we postulated that αB-crystallin might have utility as a prognostic biomarker to predict the risk of disease progression in TNBC patients. To this end, we examined the prognostic value of αB-crystallin (gene and protein) expression in breast cancer using (1) publically available gene expression data from multiple breast cancer cohorts, which included sites of first distant metastasis, and (2) a large breast cancer tissue microarray linked to long-term outcomes, including metastatic sites. Using these datasets, we confirmed the association between αB-crystallin protein expression and basal-like phenotype and poor survival in univariable and multivariable models. Moreover, we observed that expression of the αB-crystallin gene (CRYAB) was associated with brain as a first site of distant relapse, while αB-crystallin protein expression was associated with the development of brain metastasis (first site or any occurrence) and poor survival after diagnosis of brain metastasis. Collectively, our findings suggest that αB-crystallin (gene and/or protein) expression may be of clinical utility as a biomarker to identify women at high risk for brain metastasis.
Materials and methods
Human breast tumor gene expression analysis
Publicly available global-gene expression profiles of 855 tumors were downloaded from four studies (GSE2034, GSE12276, GSE2603, and the NKI295), and combined using Distance Weighted Discrimination to remove the systematic biases present in different microarray datasets (29). The four tumor datasets contained the sites of first distant relapses and time to relapse, and will be referred as “855Met” in this current study. The combined clinical data can be found at the University of North Carolina microarray database (30). Gene expression data were row (gene) median centered and column (sample) standardized (standard deviation = 1) respectively. Tumors were assigned into one of the intrinsic subtypes using the PAM50 classifier (2).
British Columbia Cancer Agency (BCCA) cohort
The BCCA cohort includes 3987 formalin-fixed paraffin-embedded (FFPE) specimens from patients referred to BCCA from 1986 to 1992 that were used to construct a large tissue microarray linked to detailed clinicopathological data and long-term outcomes (median follow-up of 12 years) (12, 15). Most patients were treated with adjuvant systemic therapy according to provincial cancer management guidelines. The BC Cancer Agency Research Ethics Board approved all biomarker studies on tissue specimens, and associated clinical and outcomes data correlation.
Immunohistochemistry (IHC) and tissue microarrays (TMA)
TMAs were constructed from tissue blocks as described (6, 31). αB-crystallin protein expression was evaluated by IHC using a mouse monoclonal antibody (1:200 dilution; ADI-SPA-222; Stressgen Biotechnologies/Enzo Life Sciences) as described previously and was scored using an established, previously published cut-off, whereby tumors were considered positive for αB-crystallin if there was any staining above background levels (23). The αB-crystallin protein expression was visually assessed by T.O.N. blinded to clinical outcome. Samples with less than 50 tumor cells present in the TMA core were excluded from analysis. TMAs were digitally scanned, and the images are available for public access (http://www.gpecimage.ubc.ca/tma/web/viewer.php; username: CRYAB4000; password: CRYAB4000). Breast cancer subtypes were defined using a previously validated immunopanel of six biomarkers including ER, PR, HER2, Ki67, epidermal growth factor receptor (EGFR), and cytokeratin 5/6 (CK 5/6) (32). In brief, tumors were classified as Luminal A if ER and/or PR positive, and HER2 negative, and Ki67 low (< 14%); Luminal B if ER and/or PR positive, and HER2 negative, and Ki67 high (≥ 14%); Luminal-HER2 if ER and/or PR positive, and HER2 positive; HER2-enriched if ER and PR negative and HER2 positive; Basal-like if negative for all ER, PR and HER2 but expressing EGFR and/or CK5/6; and as Triple negative-non basal if negative for ER, PR, HER2, EGFR and CK5/6.
Statistical analysis
Univariable survival estimates were calculated using the Kaplan-Meier method, and survival differences estimated using log-rank tests. The BCCA patient cohort was derived from a sequential cohort of 3987 female patients with newly diagnosed, invasive breast cancer in British Columbia, whose tumor specimens were tested by central estrogen receptor laboratory at Vancouver Hospital between 1986 and 1992. Among these 197 developed brain metastasis and represented the subset of patients to test the hypothesis. Due to the retrospective nature of the study the sample size was not pre-specified. The primary endpoint for the BCCA cohort is breast cancer specific survival (BCSS). Multivariable Cox regression analyses were used to test the independent prognostic value of αB-crystallin. Smoothed plots of weighted Schoenfeld residuals were used to test proportional hazard assumptions. The association of αB-crystallin expression with intrinsic subtypes was assessed using the Chi-square test. Multivariable logistic regression analysis was used to evaluate the association of αB-crystallin and other clinicopathological markers for site of metastasis.
Results
αB-crystallin gene (CRYAB) expression is associated with distant relapse in basal-like tumors
To investigate whether expression of the αB-crystallin gene (CRYAB) is associated with survival and site-specific metastasis, we interrogated publicly available gene expression data of 855 breast tumors from four studies with available information regarding the first site of distant metastasis (“855Met”). Within the 855Met dataset, there were 188 basal-like tumors defined by gene expression using the PAM50 classifier. Within this subset of basal-like tumors, CRYAB expression was significantly associated with an increased risk of distant relapse by multivariable cox regression analysis adjusted for ER status, age at diagnosis and nodal status (Table 1, HR = 1.1 (95% CI 1.0-1.2), P = 0.045). This suggested that CRYAB gene expression could be a potential biomarker for metastatic behavior of basal-like tumors.
Table 1.
Multivariable Cox regression analysis for the development of any distant relapse in 188 patients with basal-like breast tumors in the 855Met cohort
| Basal-like tumors* | Hazard ratio to distant relapse (95% CI) | P-value |
|---|---|---|
| CRYAB | 1.1 (1.0-1.2) | 0.045 |
| ER Positive (n = 29) vs. Negative (n = 159) |
1.0 (0.6-1.8) | 0.96 |
| Age (continuous variable) | 1.0 (0.97-1.0) | 0.43 |
| Nodal status Positive (n = 28) vs. Negative (n = 160) |
0.5 (0.3-1.0) | 0.066 |
n = 188, events = 89
CRYAB expression is associated with brain as the first site of distant relapse
Given the recent study linking αB-crystallin to breast cancer brain metastasis (28), we examined the relationship between CRYAB expression and brain metastasis as the first distant relapse. In the 855Met dataset, 376 patients developed distant relapse: 49 of these patients had brain metastasis as the first site of relapse. In the multivariable Cox regression analysis for brain as a first site of distant relapse of using all available cases in this cohort (852 tumors), CRYAB expression was significantly associated with earlier development of brain relapse, independent of standard clinicopathological variables, including the PAM50-defined intrinsic subtypes (Table 2, HR = 1.2 (95% CI 1.0-1.4), P = 0.021). Interestingly, although intrinsic subtypes were still significantly associated with distant relapse to brain, the basal-like subtype was not independently significant, when compared to Luminal A tumors, in the multivariable model including CRYAB. Analysis of the subgroup of 376 patients who developed distant recurrences revealed that CRYAB expression was significantly associated with increased likelihood of developing brain metastasis (OR: 1.2 (95% CI 1.0-1.3), P = 0.016). However, this latter association was not independent of gene expression-based intrinsic subtypes in the multivariable logistic regression analysis (P = 0.08) of this smaller subset of patients. Collectively, these gene expression analyses indicate that CRYAB expression is associated with increased risk of disease progression in TNBC and with brain metastasis as a first site of distant relapse in the entire cohort.
Table 2.
Multivariable Cox regression analysis for the development of brain as the first site of distant relapse among 852 patients with complete clinicopathological data in the 855Met cohort.
| Breast Tumors* | Hazard ratio to brain mets as first site (95% CI) | P-value |
|---|---|---|
| CRYAB | 1.2 (1.0-1.4) | 0.021 |
| ER Positive (n = 596) vs. Negative (n = 256) |
0.4 (0.2-0.9) | 0.025 |
| Nodal status Positive (n = 158) vs. Negative (n = 694) |
1.0 (0.5-2.0) | 0.9 |
| Age (continuous) | 0.98 (0.9-1.0) | 0.078 |
| PAM50 | 0.036 | |
| LumA | 1 | |
| LumB | 4.7 (1.7-13) | 0.0031 |
| HER2-Enriched | 2.9 (0.9-9.3) | 0.066 |
| Basal-like | 1.3 (0.4-4.7) | 0.66 |
| Normal-like | 1.7 (0.5-5.6) | 0.39 |
n = 852, brain metastasis events = 49
αB-crystallin protein expression is associated with the basal-like phenotype and poor survival
To validate and extend our observations from gene expression studies, we interrogated the BCCA TMA of 3987 breast tumors for the expression of αB-crystallin protein by IHC. The clinicopathological characteristics and treatment regimens for this cohort are indicated (Table 3). Scoring of αB-crystallin expression was possible for 3235 cases. Of these, 359 (11%) were positive for αB-crystallin expression. All the stained tissue microarrays were digitally scanned, and primary image data is available for public access: http://www.gpecimage.ubc.ca/tma/web/viewer.php; (username: CRYAB4000; password: CRYAB4000). Using the six biomarker immunopanel to subclassify each breast tumor (32), a molecular subtype was assigned to 3009 of the 3235 breast tumors with αB-crystallin IHC data. αB-crystallin was infrequently expressed in luminal A tumors (54 of 1312, 4.1%), luminal B tumors (33 of 766, 4.4%), luminal-HER2+ tumors (8 of 199, 4.0%) or HER2+ tumors (18 of 221, 8.1%). In contrast, αB-crystallin was highly expressed in basal-like tumors defined by triple-negative status and expression of basal markers CK5/6 or EGFR (170 of 309, 55%) and to a lesser extent in triple-negative tumors that lacked expression of these basal markers (47 of 202, 23%). The differential distribution of αB-crystallin expression among the various molecular subtypes of breast cancer was highly statistically significant (P < 0.0001 by chi-square test). These findings are consistent with prior reports specifically linking αB-crystallin expression to basal-like breast carcinomas/TNBCs (23–26, 28, 33, 34).
Table 3.
Clinicopathological characteristics and treatment regimens of the 3987 patients in the BCCA Cohort
| N | % | |
|---|---|---|
| Age (years) | ||
| < 40 | 294 | 7 |
| 40-49 | 843 | 21 |
| 50-65 | 1423 | 36 |
| > 65 | 1427 | 36 |
| Grade | ||
| 1 | 209 | 6 |
| 2 | 1562 | 41 |
| 3 | 2037 | 54 |
| N/A | 179 | |
| Menstrual status at referral | ||
| Pre-menopausal | 1178 | 30 |
| Post-menopausal | 2717 | 70 |
| N/A | 92 | |
| Nodal status | ||
| Negative | 2265 | 57 |
| Positive | 1714 | 43 |
| N/A | 8 | |
| Lymphovascular invasion | ||
| Negative | 2105 | 55 |
| Positive | 1709 | 45 |
| N/A | 173 | |
| Tumor size (cm) | ||
| ≤ 2 | 2077 | 52 |
| 2-5 | 1665 | 42 |
| >5 | 221 | 6 |
| N/A | 24 | |
| Initial Adjuvant Systemic Therapy | ||
| None | 1676 | 42 |
| Tamoxifen only | 1274 | 32 |
| Chemotherapy only (AC, FAC or CMF) | 725 | 18 |
| Tamoxifen and chemotherapy | 296 | 8 |
| Other | 16 |
Abbreviations: N/A, not available; AC, adriamycin and cyclophosphamide; FAC, 5-fluorouracil and AC; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil
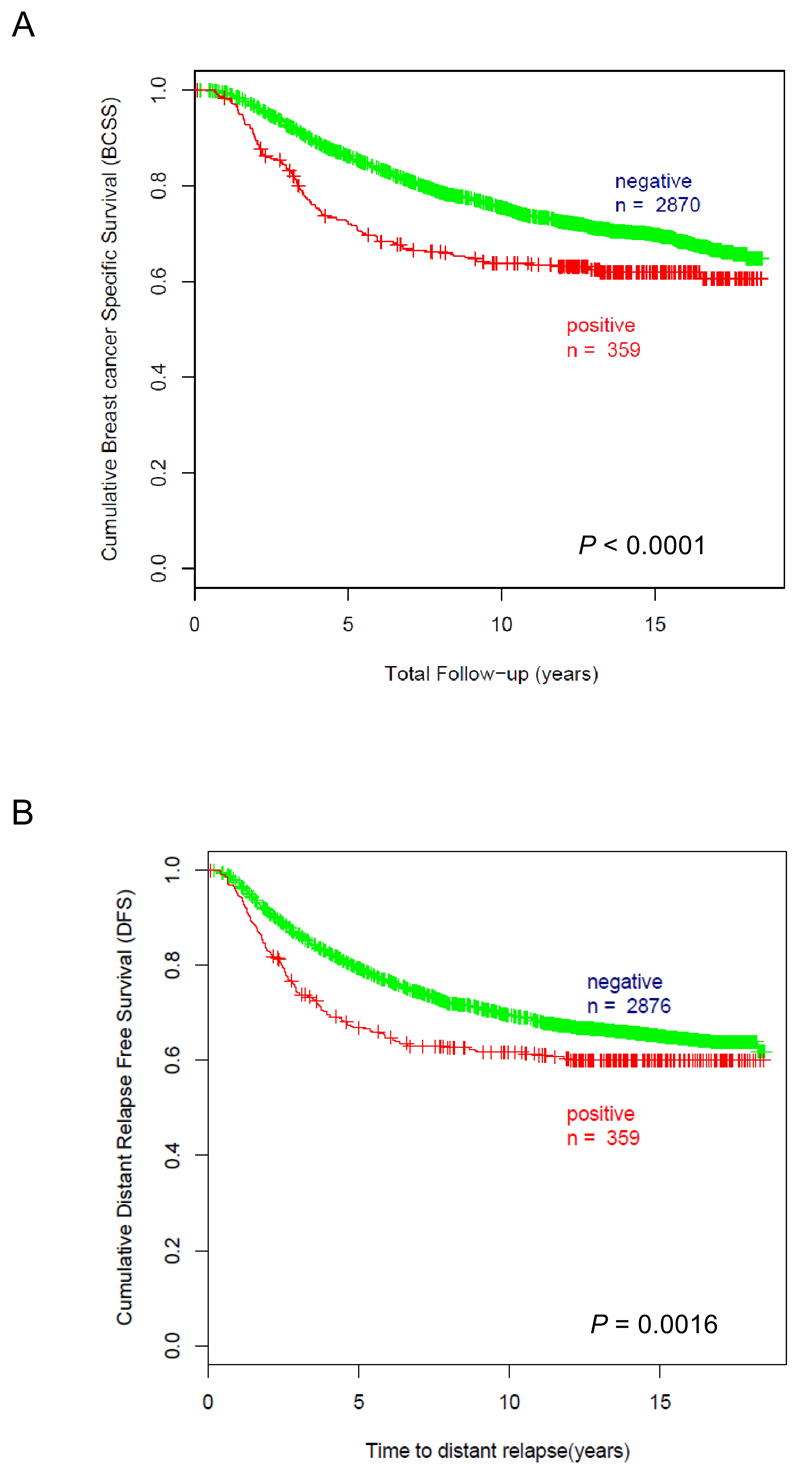
In univariable analysis among all breast cancer subtypes, αB-crystallin was a statistically significant marker of poor prognosis. Breast cancer specific survival at 10 years was 64% in αB-crystallin-positive cases versus 75% in αB-crystallin-negative cases (Fig. 1A, P < 0.0001 by log-rank test). Distant relapse free survival at 10 years was 62% in αB-crystallin-positive cases versus 70% in αB-crystallin-negative cases (Fig.1B, P = 0.0016 by log-rank test). αB-crystallin had independent prognostic significance for breast cancer specific survival (Table 3, HR = 1.3 (95% CI 1.1-1.6), P = 0.014) in a multivariable Cox model adjusting for patient age, tumor grade, systemic therapy, ER and HER2 status. In this model, patient age, tumor grade, lymph node status, tumor size, and HER2 status were also independently prognostic for breast cancer specific survival. These findings validate our prior results from a much smaller cohort of breast cancer patients (n = 438) that αB-crystallin protein expression is an independent prognostic biomarker of breast cancer specific survival (23).
Figure 1. αB-crystallin expression is associated with worse breast cancer specific survival and distant relapse free survival in univariable analyses.
(A) Kaplan-Meier analysis of breast cancer specific survival based on αB-crystallin expression. 10-year breast cancer specific survival was 64% for αB-crystallin-positive cases (n = 359, number of events = 133) versus 75% for αB-crystallin-negative cases (n = 2870, number of events = 809), P < 0.0001 by log-rank test. (B) Kaplan-Meier analysis of distant relapse free survival based on αB-crystallin expression. 10-year distant relapse free survival was 62% for αB-crystallin-positive cases (n = 359, # of events = 139) versus 70% for αB-crystallin-negative cases (n = 2876, # of events = 915), P = 0.0016 by log-rank test.
αB-crystallin expression is associated with site-specific brain metastasis
1054 (33%) of patients in the BCCA cohort with available αB-crystallin IHC staining developed metastatic breast cancer to one or more organs, including 148 (4.5%) who developed brain metastases. Among the patients with metastatic disease, αB-crystallin expression was significantly associated with brain metastasis: 12% of patients with αB-crystallin-negative metastatic breast cancer had brain metastasis, in contrast to 33% of patients with αB-crystallin-positive cancer (P < 0.0001 by Fischer exact test). In a multivariable logistic regression model including only patients with metastatic breast cancer, αB-crystallin was the strongest independent predictor of brain metastasis (OR = 2.99 (95% CI 1.83-4.89), P < 0.0001); ER and HER2 status were also independent predictors of brain metastasis (Table 4). Multivariable logistic regression, relating tumor size, nodal status, tumor grade, systemic therapy, ER, HER and αB-crystallin to the development of brain metastasis as the first site of distant relapse found that αB-crystallin status was the only independent predictor for brain metastasis as the first site of distant relapse (OR = 3.15 (95% Cl 1.43-6.95), P = 0.005). αB-crystallin was also associated with a shorter time interval between initial diagnosis of breast cancer and the subsequent diagnosis of brain metastasis (median time to brain metastasis = 2.7 years versus 4.2 years, P < 0.0001 by log-rank test). Following the diagnosis of brain metastasis, patients with αB-crystallin-positive metastatic breast cancer had worse survival (median survival = 3.0 months versus 4.7 months, P = 0.007 by log-rank test). In addition, αB-crystallin expression was associated with lower risk of liver metastasis (OR = 0.53, P = 0.009). However, there was no significant association between αB-crystallin expression and lung or bone metastases. Collectively, these results indicate that αB-crystallin protein expression is an independent predictor for the development of brain metastasis (first site or any occurrence) and is associated with poor survival after diagnosis of brain metastasis relative to metastatic αB-crystallin-negative breast cancer.
Table 4.
Cox multivariable analysis for breast cancer specific survival (BCSS) in the BCCA Cohort*
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Age at diagnosis, years | 0.022 | ||
| >55 (n = 1790) | 1 | ||
| <40 (n = 239) | 1.2 | 0.91-1.5 | 0.22 |
| 40-55 (n = 946) | 0.87 | 0.72-1.0 | 0.12 |
|
Grade 3 (n = 1643) vs. 2/1 (n = 1332) |
1.5 | 1.3-1.8 | < 0.0001 |
|
Nodal status Positive (n = 1686) vs. Negative (n = 1289) |
2.6 | 2.1-3.0 | < 0.0001 |
| Tumor size, cm | < 0.0001 | ||
| ≤ 2 (n = 1545) | 1 | ||
| 2-5 (n = 1290) | 1.6 | 1.4-1.8 | < 0.0001 |
| > 5 (n = 140) | 2.2 | 1.6-2.8 | < 0.0001 |
| Initial systemic therapy | |||
| Any hormonal (n = 1128) vs. None (n = 1847) | 0.88 | 0.74-1.1 | 0.16 |
| Any chemo (n = 773) vs. None (n = 2202) | 0.86 | 0.69-1.1 | 0.14 |
| Biomarkers | |||
| ER-positive (n = 2148) vs. neg. (n = 827) | 0.89 | 0.75-1.1 | 0.18 |
| HER2-positive (n = 414) vs. neg. (n = 2561) | 1.4 | 1.2-1.7 | < 0.0001 |
| αB-crystallin-positive (n = 332) vs. neg. (n = 2643) | 1.3 | 1.1-1.6 | 0.014 |
n = 2972 cases with complete covariate data, also excluding 3 cases censored before the first event
Discussion
HER2-positive and basal-like tumors/TNBC have a distinctive pattern of organ-specific distant metastasis that includes brain metastasis, a debilitating event that typically follows a rapidly fatal course (13–15, 35). Indeed, the prevalence of brain metastases among patients with metastatic TNBC has been reported as high as 46% with median survival after diagnosis of 4.9-6.6 months (36, 37). Moreover, the brain is the first site of distant metastasis in 4.7% of patients with early-stage TNBC (38). Although HER2 has been previously linked to the pathogenesis of brain metastasis, as discussed further below, αB-crystallin has only recently been reported to play a pathogenic role in brain metastasis in TNBC models (28, 39–41). Consistent with this pathogenic role, we have demonstrated that αB-crystallin gene (CRYAB) expression is associated with distant relapse in TNBC patients and with earlier development of brain relapse as a first distant site, independent of standard clinicopathological variables and PAM50 subtyping in the entire cohort. Moreover, αB-crystallin protein expression in the primary breast tumor was an independent prognostic biomarker of poor breast cancer specific survival and was associated with a 33% risk of brain metastasis among patients in the BCCA cohort who developed metastatic disease. This is comparable to the high incidence of brain metastasis observed in HER2-positive metastatic breast cancer (25-40%) (39). Notably, both HER2 and αB-crystallin were independent predictors of brain metastasis (any occurrence) in multivariate analyses. However, αB-crystallin expression was the strongest predictor of brain metastasis (any occurrence) and the only independent predictor of brain metastasis as the first site of distant relapse. These findings suggest that αB-crystallin (gene and/or protein) expression in primary breast cancers may be a useful biomarker to identify patients at greater risk for developing brain metastasis. Because we observed that αB-crystallin is preferentially expressed in basal-like tumors (55%) and rarely in HER2-positive tumors (8.1%), consistent with prior reports, our findings suggest that αB-crystallin expression status may be particularly informative among TNBC patients to help identify individuals at greater risk for early or first-site brain relapse (23–26, 28, 33, 34, 38).
We have also demonstrated that αB-crystallin protein expression is associated with a shorter time interval between the breast cancer diagnosis and the development of brain metastasis (2.7 years versus 4.2 years, P < 0.0001 by log-rank test) and shorter survival after diagnosis of brain metastasis (3.0 months versus 4.7 months, P = 0.007 by log-rank test). These results are particularly striking given the poor survival of patients who developed brain metastasis. Even among this subset of poor-prognosis patients, αB-crystallin expression was associated with significantly shorter survival after diagnosis of brain metastasis. Our findings are consistent with a recent report from a cohort of 76 patients with breast cancer who developed brain metastasis (28). In that cohort, αB-crystallin expression was associated with inferior survival after breast cancer diagnosis (1.4 versus 4.7 years, P = 0.0002) and survival after brain metastasis diagnosis (0.13 versus 0.91 years, P = 0.001). Collectively, these results point to αB-crystallin expression in primary breast cancers as a promising biomarker to identify patients at high risk for early relapse in the brain and poor survival after diagnosis. As such, these patients could be enrolled in clinical trials to evaluate preventive agents or modalities for brain metastasis, particularly as less toxic options become available. Indeed, with further validation, αB-crystallin expression testing could be incorporated as one of the pre-planned biomarker tests for contemporary biomarker-driven trials in patients with metastatic TNBC with extracranial disease to determine optimal surveillance and treatment approaches. The importance of validating αB-crystallin as a biomarker for brain metastasis is underscored by a recent study in which a promising 3-gene signature for the development of early brain metastasis in HER2-positive breast cancer failed subsequent validation (42).
Intriguingly, the observed association of αB-crystallin expression in primary breast carcinomas and brain metastasis is biologically plausible in light of recent data from cellular assays and murine models pointing to a pathogenic role for αB-crystallin in breast cancer brain metastasis. αB-crystallin promotes adhesion to human brain microvascular endothelial cells (HBMECs), transendothelial migration and penetration through an HBMEC/primary human astrocyte co-culture BBB model (28). Furthermore, overexpression of αB-crystallin in human TNBC cells enhances brain metastasis in an orthotopic model of metastatic TNBC characterized by widespread distant metastases, whereas silencing αB-crystallin inhibits brain metastasis in a second orthotopic TNBC model (28). It is tempting to speculate that the enhanced adhesion to HBMECs and BBB penetration in vitro, two critical steps in brain metastasis (41), could provide a biological rationale for the observed robust association between αB-crystallin expression in primary breast cancers and increased risk of developing brain metastasis, particularly aggressive brain metastases that develop rapidly after breast cancer diagnosis. Consistent with this idea, αB-crystallin is commonly expressed in brain metastases from patients with αB-crystallin-positive breast carcinomas, while some αB-crystallin-positive brain metastases develop from primary breast cancers that lack αB-crystallin expression (28). αB-crystallin also promotes resistance to matrix detachment-induced apoptosis (anoikis), a hallmark of metastatic carcinoma cells, which would be expected to promote circulating tumor cell survival (27, 43). Although αB-crystallin promotes lung metastasis in some orthotopic TNBC models (27), our immunohistochemistry data point to a specific association between αB-crystallin expression in breast tumors and brain metastasis; there was no correlation with lung metastasis in the BCCA cohort. These latter findings suggest that preclinical murine models may not accurately recapitulate all aspects of the metastatic cascade in patients. Nevertheless, our clinical data point to a robust association between αB-crystallin and early metastasis to the brain that is highly congruent with its recently delineated pathogenic role in brain metastasis.
In summary, we present data from multiple breast cancer cohorts with metastatic site data demonstrating that αB-crystallin gene (CRYAB) expression in primary breast carcinomas is an independent predictor for the development of brain as the first site of distant relapse. We have also demonstrated that αB-crystallin protein expression in primary breast tumors is an independent prognostic biomarker for poor breast cancer specific survival, early site-specific metastasis to the brain and worse survival after diagnosis of brain metastasis. We plan to examine the correlation between αB-crystallin gene and protein expression in appropriate breast cancer cohorts to determine whether one is a better biomarker than the other or whether they are equivalent. Collectively, our findings point to αB-crystallin (gene and/or protein expression) as a promising biomarker to identify patients at high risk for early relapse in the brain who could be enrolled in clinical trials to evaluate preventive strategies.
Table 5.
Multivariable logistic regression analysis for the development of any brain metastasis and brain as the first site of relapse in the BCCA cohort.
| Odds to any Brain Mets (95% CI), (Events = 141) |
P-value | Odds to Brain as first distant site (95% CI), (Events = 46) |
P-value | |
|---|---|---|---|---|
| Tumor size | ||||
| <= 2cm (n = 375) | 1 | 1 | ||
| 2-5 cm (n = 525) | 1.13 (0.76-1.69) | 0.54 | 0.70 (0.37-1.33) | 0.28 |
| >5 cm (n= 69) | 0.39 (0·13-1·18) | 0.095 | ||
| Nodal Status | ||||
| Negative (n = 374) | 1 | 1 | ||
| Positive (n = 595) | 0.53 (0·34-0·82) | 0.005 | 0.55 (0.28 – 1.08) | 0.082 |
| Grade | ||||
| 1 or 2 (n = 336) | 1 | 1 | ||
| 3 (n = 633) | 1.2 (0·76-1·86) | 0.441 | 1.19 (0.57-2.48) | 0.65 |
| Any initial chemo | ||||
| No (n = 648) | 1 | 1 | ||
| Yes (n = 321) | 1.46 (0.92-2.30) | 0.11 | 0.36 (0.19-0.71) | 0.003 |
| Any initial hormonal | ||||
| No (n = 556) | 1 | 1 | ||
| Yes ( n = 413) | 0.79 (0.50-1.24) | 0.3 | 0.91 (0.45-1.84) | 0.79 |
| ER | ||||
| negative (n = 316) | 1 | 1 | ||
| Positive (n = 653) | 0.58 (0.37-0.91) | 0.018 | 0.58 (0.28-1.24) | 0.16 |
| HER2 | ||||
| negative (n = 783) | 1 | 1 | ||
| positive (n = 186) | 1.72 (1.08-2.73) | 0.023 | 1.88 (0.89-3.96) | 0.096 |
| αB-crystallin | ||||
| negative (n = 842) | 1 | 1 | ||
| Positive (n = 127) | 2.99 (1.83-4.89) | < 0.0001 | 3.15 (1.43-6.95) | 0.005 |
Acknowledgements
We are indebted to technologists at the Genetic Pathology Evaluation Centre (University of British Columbia) for assistance with tissue microarray histology and immunohistochemistry.
Grant support/Funding
Breast Cancer Research Foundation (CMP and VLC), the NCI Breast SPORE program grant P50-CA58223-09A1 (CMP), the Cancer Research UK (CRUK) Core grant C1491/A15955 (MCUC), and the Canadian Breast Cancer Foundation (BC / Yukon Division) (KDV, TON, HK).
Footnotes
Competing interests
CMP and TON are equity stock holders, and Board of Director Member of BioClassifier LLC. CMP, TON and MCUC are also listed as inventors on a patent application on the PAM50 assay. The other authors declare they have no competing interests.
Contributions
KDV analyzed data, wrote and edited the manuscript. TON assembled the tissue microarray, generated the biomarker data, analyzed data, and edited the manuscript. CMP, JCH and CF assembled the gene expression data and edited the manuscript. HK assembled the clinical data and edited the manuscript. AM analyzed gene expression data and edited the manuscript. VLC conceptualized the project, analyzed data, wrote and edited the manuscript. MCUC conceptualized the project, assembled the tissue microarray, generated the biomarker data, analyzed the data, wrote and edited the manuscript. All authors read and approved the final manuscript.
References
- 1.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 2.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 6.Voduc D, Nielsen TO. Basal and triple-negative breast cancers: impact on clinical decision-making and novel therapeutic options. Clin Breast Cancer. 2008;8(Supp 4):S171–S178. doi: 10.3816/CBC.2008.s.014. [DOI] [PubMed] [Google Scholar]
- 7.Toft DJ, Cryns VL. Minireview: Basal-like breast cancer: from molecular profiles to targeted therapies. Mol Endocrinol. 2011;25:199–211. doi: 10.1210/me.2010-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 9.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clinical Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 12.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 13.Gaedcke J, Traub F, Milde S, Wilkens L, Stan A, Ostertag H, et al. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Modern Pathol. 2007;20:864–870. doi: 10.1038/modpathol.3800830. [DOI] [PubMed] [Google Scholar]
- 14.Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 15.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 16.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamradt MC, Chen F, Cryns VL. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J Biol Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 19.Kamradt MC, Lu M, Werner ME, Kwan T, Chen F, Strohecker A, et al. The small heat shock protein αB-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J Biol Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- 20.Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human αB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFα–induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy VS, Reddy GB. Emerging role for αB-crystallin as a therapeutic agent: pros and cons. Curr Mol Med. 2015;15:47–61. doi: 10.2174/1566524015666150114112853. [DOI] [PubMed] [Google Scholar]
- 22.Stegh AH, Kesari S, Mahoney JE, Jenq HT, Forloney KL, Protopopov A, et al. Bcl2L12-mediated inhibition of effector caspase-3 and caspase-7 via distinct mechanisms in glioblastoma. Proc Natl Acad Sci USA. 2008;105:10703–10708. doi: 10.1073/pnas.0712034105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, et al. αB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanov O, Chen F, Wiley EL, Keswani A, Diaz LK, Memmel HC, et al. αB-crystallin is a novel predictor of resistance to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2008;111:411–417. doi: 10.1007/s10549-007-9796-0. [DOI] [PubMed] [Google Scholar]
- 25.Sitterding SM, Wiseman WR, Schiller CL, Luan C, Chen F, Moyano JV, et al. αB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann Diagn Pathol. 2008;12:33–40. doi: 10.1016/j.anndiagpath.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Kim HS, Lee Y, Lim YA, Kang HJ, Kim LS. αB-Crystallin is a novel oncoprotein associated with poor prognosis in breast cancer. J Breast Cancer. 2011;14:14–19. doi: 10.4048/jbc.2011.14.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malin D, Strekalova E, Petrovic V, Rajanala H, Sharma B, Ugolkov A, et al. ERK-regulated αB-crystallin induction by matrix detachment inhibits anoikis and promotes lung metastasis in vivo. Oncogene. 2015 doi: 10.1038/onc.2015.12. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malin D, Strekalova E, Petrovic V, Deal AM, Al Ahmad A, Adamo B, et al. αB-crystallin: a novel regulator of breast cancer metastasis to the brain. Clin Cancer Res. 2014;20:56–67. doi: 10.1158/1078-0432.CCR-13-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benito M, Parker J, Du Q, Wu J, Xiang D, Perou CM, et al. Adjustment of systematic microarray data biases. Bioinformatics. 2004;20:105–114. doi: 10.1093/bioinformatics/btg385. [DOI] [PubMed] [Google Scholar]
- 30.Harrell JC, Prat A, Parker JS, Fan C, He X, Carey L, et al. Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res Treat. 2012;132:523–535. doi: 10.1007/s10549-011-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. New Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 32.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsang JY, Lai MW, Wong KH, Chan SK, Lam CC, Tsang AK, et al. αB-crystallin is a useful marker for triple negative and basal breast cancers. Histopathol. 2012;61:378–386. doi: 10.1111/j.1365-2559.2012.04234.x. [DOI] [PubMed] [Google Scholar]
- 34.Won JR, Gao D, Chow C, Cheng J, Lau SY, Ellis MJ, et al. A survey of immunohistochemical biomarkers for basal-like breast cancer against a gene expression profile gold standard. Modern Pathol. 2013;26:1438–1450. doi: 10.1038/modpathol.2013.97. [DOI] [PubMed] [Google Scholar]
- 35.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 36.Arslan UY, Oksuzoglu B, Aksoy S, Harputluoglu H, Turker I, Ozisik Y, et al. Breast cancer subtypes and outcomes of central nervous system metastases. Breast. 2011;20:562–567. doi: 10.1016/j.breast.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dawood S, Lei X, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer. 2012;118:4652–4659. doi: 10.1002/cncr.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 40.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67:4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 41.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nature Rev Cancer. 2011;11:352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duchnowska R, Jassem J, Goswami CP, Dundar M, Gokmen-Polar Y, Li L, et al. Predicting early brain metastases based on clinicopathological factors and gene expression analysis in advanced HER2-positive breast cancer patients. J Neuro-Oncol. 2015;122:205–216. doi: 10.1007/s11060-014-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guadamillas MC, Cerezo A, Del Pozo MA. Overcoming anoikis--pathways to anchorage-independent growth in cancer. J Cell Sci. 2011;124:3189–3197. doi: 10.1242/jcs.072165. [DOI] [PubMed] [Google Scholar]