Abstract
5(4H)-Oxazolones can be formed through the activation of acylated α-amino acids or of peptide C termini. They constitute potentially activated intermediates in the abiotic chemistry of peptides that preceded the origin of life or early stages of biology and are capable of yielding mixed carboxylic-phosphoric anhydrides upon reaction with phosphate esters and nucleotides. Here, we present the results of a study aimed at investigating the chemistry that can be built through this interaction. As a matter of fact, the formation of mixed anhydrides with mononucleotides and nucleic acid models is shown to take place at positions involving a mono-substituted phosphate group at the 3’- or 5’-terminus but not at the internal phosphodiester linkages. In addition to the formation of mixed anhydrides, the subsequent intramolecular acyl or phosphoryl transfers taking place at the 3’-terminus are considered to be particularly relevant to the common prebiotic chemistry of α-amino acids and nucleotides.
Keywords: anhydrides, nucleotides, peptides, phosphates, prebiotic chemistry
Introduction
The question of the origin of life is now considered as that of co-evolution of different subsystems rather than a self-organization process in a system made of a single biopolymer. It has therefore been considered as a co-evolutionary process [1–3] that overcomes the difficulties associated with past views on the origin of life starting from a single kind of biopolymer. Previous review articles have demonstrated the advantages that could be expected from system chemistry views[4, 5] associating different subsystems with each other.[6, 7] Experimental approaches have indeed shown the benefits resulting from the association of components coming from different subsystems for self-organization, as in the cases of the combination of peptides and nucleotides,[8] peptides and lipids,[9] or nucleic bases and fatty acids.[10] A strong support to these views has recently been brought about by the discovery that most of the essential building blocks of biochemistry can be formed from very similar processes possibly occurring in the same location.[11, 12] With respect to the emergence of translation, and whatever the stage at which it evolved, its development required a co-evolutionary process involving peptide and nucleic acid subsystems.[13] For the peptide and nucleic acid systems, the translation apparatus gathers several of the most conserved systems,[14] so that prebiotic chemistry studies can take advantage of information coming from molecular phylogeny with respect to the emergence of the genetic code[15] or of the ribosomal machinery.[16, 17] Covalent adducts of amino acids with nucleotides (aminoacyl adenylates or tRNA esters) are still essential in biochemistry and constitute the biochemical tools on which the translation process was built. They are actually the physical support of the code embedded in the correspondence of amino acids, with the triplet bases forming the anticodons of tRNA. The question of the non-enzymatic formation of these kind of adducts cannot be escaped when considering early life and the origin of translation, because its emergence constituted one of the most important transitions in evolution.[18] It resulted in the separation of structures capable of information storage from those expressing functions, and therefore, generated almost unlimited possibilities of evolution. Mixed anhydrides of amino acids and phosphate esters represent energy-rich species, as shown by the determination of the equilibrium constant for the formation of adenylates.[19] Formation of mixed anhydrides from adenosine triphosphate (ATP) is not thermodynamically favorable and rendered possible only through their stabilization in the active sites of a family of enzymes, the aminoacyl-tRNA-synthetases (aaRS).[20] These enzymes perform the selective aminoacylation process that is responsible for the proper linkage of tRNAs with their cognate amino acids as a result of the reaction of mixed anhydrides. α-Amino acid esters have also been classified among energy-rich biochemicals owing to the role of aminoacyl-tRNA as activated amino acids in the ribosomal translation process.[21] Early studies of these systems, in the context of the origin of life, were aimed at demonstrating a possible role of nucleotide aminoacyl esters as platforms for peptide formation.[22–25] Ribozymes capable of aminoacylating RNAs as putative components of an RNA world[26, 27] have also been isolated. The group of Yarus demonstrated that very short sequences are active in aminoacylation.[28, 29] Starting from chemical grounds, the group of Montpellier has also proposed scenarios through which the evolutionary process leading to translation could have unfolded.[2, 30]
In our view, establishing a detailed chemical landscape involving the common chemistries of α-amino acids and nucleotides is a prerequisite for understanding reaction networks set up in these chemical environments. A major question is that of the availability of reaction pathways leading to covalent adducts. An analysis has been developed on the importance of high-energy intermediates in the formation of peptides, which gave impetus to processes starting from α-amino acid N-carboxyanhydrides (NCAs) and oxazolones.[2, 6] This requirement is not only related to the need of endergonic paths of polymerization to overcome the limitation to short oligomers but also to a more profound condition. As a matter of fact, processes leading to self-organization in replicative chemical systems must be irreversible so that the growth of self-reproducing entities becomes exponential.[32–34] This gives rise to a possibility of selection of the variants that have an increased dynamic kinetic stability.[35] Consequently, an evolutionary process driven by the increase of this stability form can be set up, provided that variability in these systems is inherited. This rationale shed light on the importance of the formation of mixed anhydrides from NCA and inorganic phosphates or phosphate esters. A similar remark applies to phosphoramidates that have been proposed as prebiotic-relevant intermediates.[36, 37] However, the question of time instability of amino acid mixed anhydrides has been raised in several instances,[2, 38, 39] and has recently received particular consideration because the conversion of mixed anhydrides of free α-amino acids is fast in the presence of dissolved CO2 at equilibrium with air, even at current atmospheric levels.[40] NCAs are readily formed from adenylates and undergo polymerization so that mixtures involving oligomer products can be obtained. On the contrary, the amino acid moiety of 5(4H)-oxazolones adducts with RNA should not be capable of undergoing other processes than hydrolysis and reactions with nucleotides. Experiments can thus be designed to focus on the reactivity of the RNA moiety. Here, we investigate the reaction of 5(4H)-oxazolones with models of RNA monomers to analyze the scope of the formation of phosphate ester mixed anhydrides (PEMAs). We also look into the specific reactivity to which PEMAs give rise as a prerequisite to the analysis of possible scenarios for the development of translation.
Results and Discussion
Formation of phosphate ester mixed anhydrides (PEMAs) using simple models
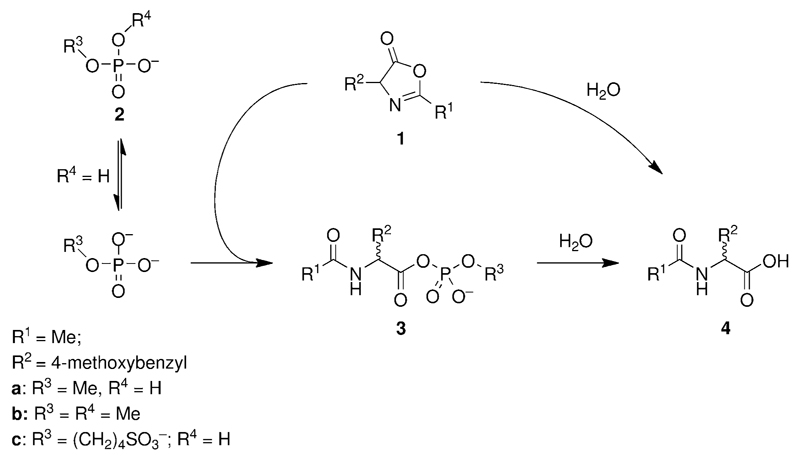
The reaction of 3-methyl-4-(4-methoxybenzyl)-5(4H)-oxazolone (1) with phosphate esters 2 was studied in aqueous buffers at pH 6.5, a value that is representative of aqueous media in equilibrium with an atmosphere containing a non-negligible amount of CO2. This starting material was selected as a model for the study owing to its ultra-violet absorption, allowing us to easily monitor the progress of hydrolysis and other chemical processes by HPLC. The regular HPLC method A is based on acidic gradients containing trifluoroacetic acid in which 1 is immediately hydrolyzed after injection. This method failed for monitoring the progress of the hydrolysis reaction because the reactant and the product cannot be differentiated. In contrast, the use of a triethylammonium acetate buffered gradient (pH 6, method C) allowed the separation of 1 (retention time 23.6 min) and its hydrolysis product Ac-Tyr(Me)-OH (4, retention time 6.06 min) due to a strongly reduced hydrolysis rate compared to usual acidic TFA-based gradients. The presence of a 50 mm methyl phosphate buffer (pH 6.5) induced a significant increase in the rate (t1/2 = 47 min at 25 °C, ca. two-fold) compared to a 100 mm 2-(N-morpholino)ethanesulfonic acid (MES) buffer at the same pH value (t1/2 = 93 min at 25 °C). This increase in rate was concomitant with the formation of a mixed anhydride intermediate (retention time 8.52 min) through the nucleophilic reaction of methyl phosphate (pKa1 = 1.54, pKa2 = 6.31)[41], confirming the earlier reported formation of this intermediate.[40] The presence of the phosphate nucleophile as a di-anion was required for reactivity (Scheme 1), as shown by an experiment in which the 1,3-dimethylimidazolium salt of dimethyl phosphate (2b, 50 mm) (pKa1 = 1.29)[41] was added to the MES buffer. Under those conditions, the reaction rate (t1/2 = 96 min at 25 °C) was not significantly different from the control in MES buffer only and no formation of adduct could be detected by HPLC. Additionally, the absence of any adduct could be confirmed by 31P NMR from a reaction of 20 mm of 2b with 20 mm of 1 in D2O. Any reaction of phosphate ester mono-anions can thus be considered as negligible compared with that of di-anions at the moderate pH values considered in this work. In regards to polynucleotides, this observation means that mixed anhydrides can be formed with phosphate monoesters potentially present as di-anions at either the 3’- or the 5’-terminus and that no reaction can be expected from the internucleotidic phosphodiester linkage.
Scheme 1.
The reaction of 3-methyl-4-(4-methoxybenzyl)-5(4H)-oxazolone (1) with phosphate esters 2 in aqueous solution. The nucleophilic reaction of phosphate ester di-anions competes with the direct hydrolysis of 1 into the acylated amino acid 4.
Effect of additional negative charges
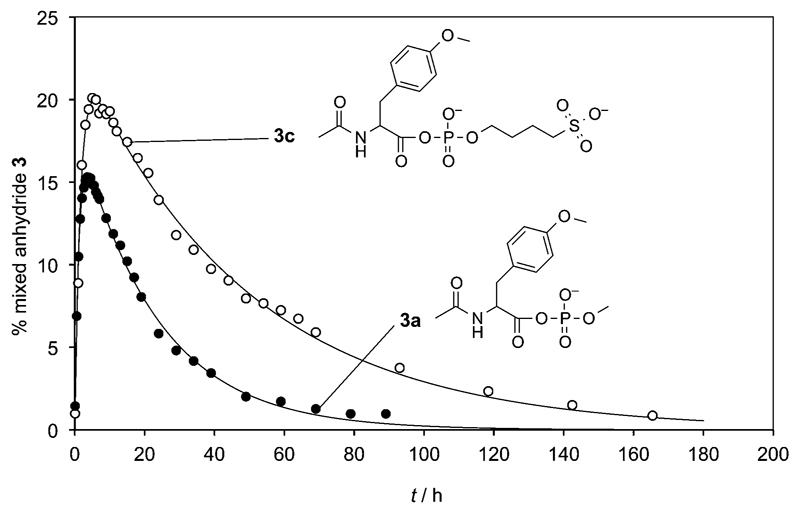
Negative charges present in the vicinity of the reacting group, for instance in polynucleotides or oligonucleotides, can be predicted to reduce the reactivity of nucleophiles with the mixed anhydrides through electrostatic interactions with the negatively charged transition state. Due to presence of other chemically reactive groups in nucleotides making the analysis of results possibly delicate, we preferred to study the behavior of a model phosphate substrate bearing a non-reactive second negative charge, 4-(phosphonooxy)butane-1-sulfonate (2c) used as a sodium salt. This phosphate ester contains only a single reactive phosphate ester moiety and a non-reactive sulfonate group that brings about the second negative charge. Additionally, its preparation from 1,4-butane sultone proved to be straightforward.[42] 31P NMR and HRMS analyses were performed to demonstrate that the mixed anhydride 3c could be formed by reaction with 1. The reaction progress (Figure 1) was monitored by HPLC (method B) illustrating the transient formation of 3c from phosphate ester 2c and 1. The reaction progress was compared with a similar reaction of methyl phosphate 2a bearing a single negatively charged group.[40] No significant difference could be observed with respect to the formation of the mixed anhydride, whereas the half-life of 3c (33 h) was significantly increased compared to that of the methyl phosphate derivative 3a (15.7 h). Negative charges present in polynucleotides are then likely to favorably influence the stability of phosphate mixed anhydrides with a relatively limited influence on their formation.
Figure 1.
Formation and hydrolysis of mixed anhydrides from oxazolone 1 and phosphate esters monitored by the evolution of HPLC peak areas (%); (a) formation and hydrolysis of mixed anhydride 3a in a 50 mm pH 6.5 methyl phosphate (2a) aqueous buffer (filled circles); (b) similar reaction of oxazolone 1 and hydrolysis of 3c in a pH 6.5 buffer prepared from 50 mm phosphate ester 2c (open circles).
Reactions of 5(4H)-oxazolone 1 with nucleotide monomers
The reaction of phosphate di-anions with 1 could be influenced by any additional groups present in the vicinity of the reacting center within mono- as well as in polynucleotides. Therefore, we studied the reactivity of 1 with the two different isomers 5’-AMP and 3’-AMP (AMP = adenosine monophosphate), as well as with the deoxyribose counterpart 5’-dAMP (d = deoxy; Scheme 2). The reaction always led to two families of products, namely mixed anhydrides with the phosphoryl group and esters with hydroxyl groups available on the ribose moiety. The two kinds of products could be differentiated owing to their different kinetic behavior.
Scheme 2.
The nucleotides selected in this work as models of reactivity of ribonucleotides and deoxyribonucleotides.
Reaction with adenosine-5’-monophosphate (5’-AMP)
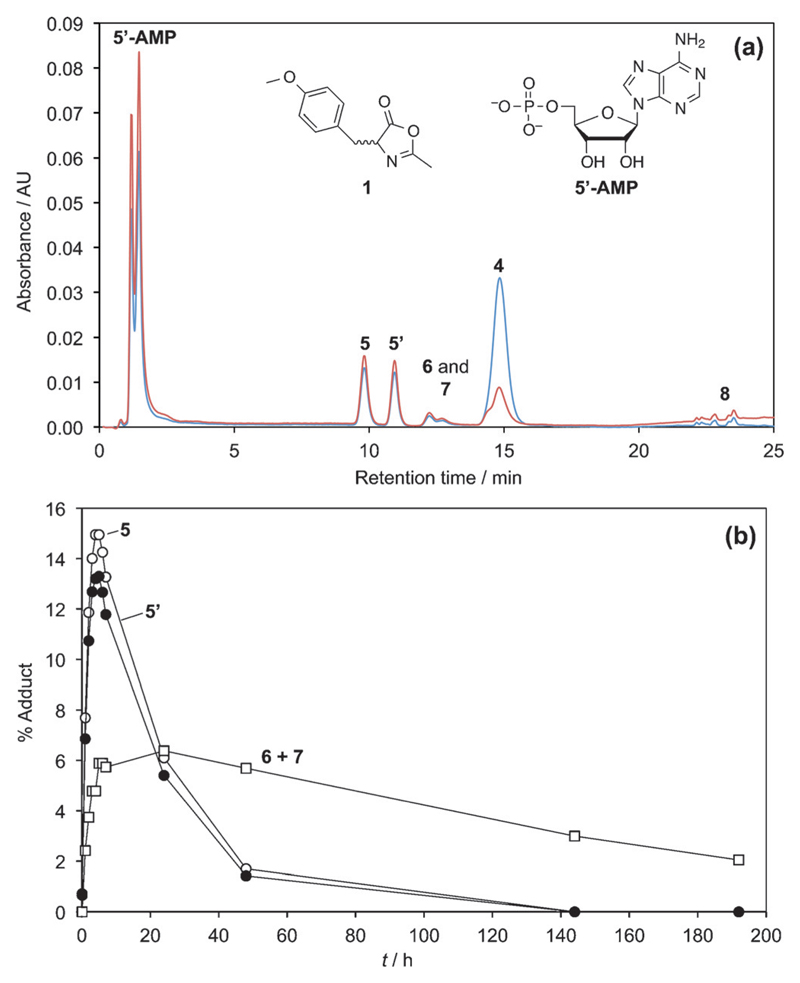
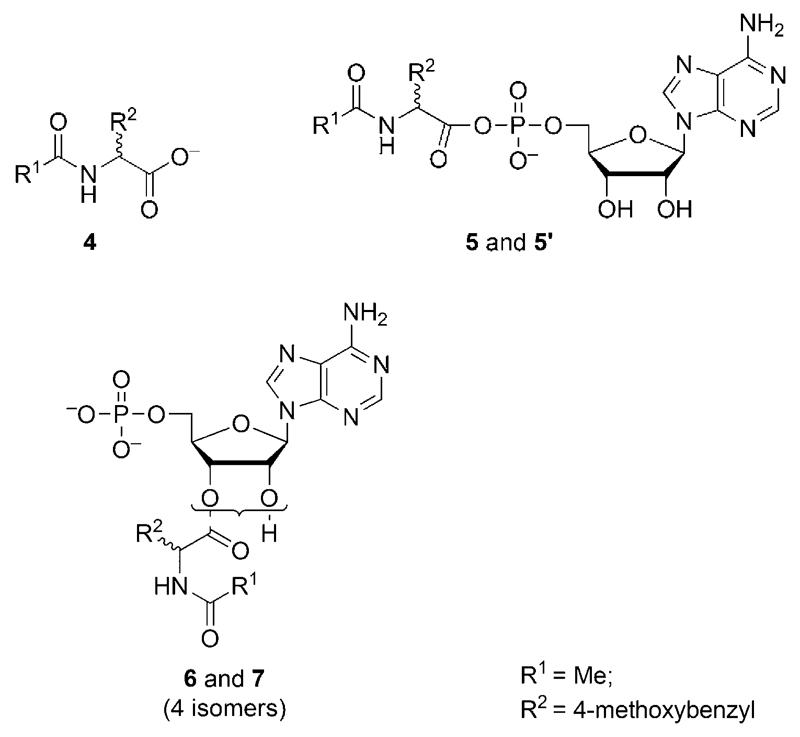
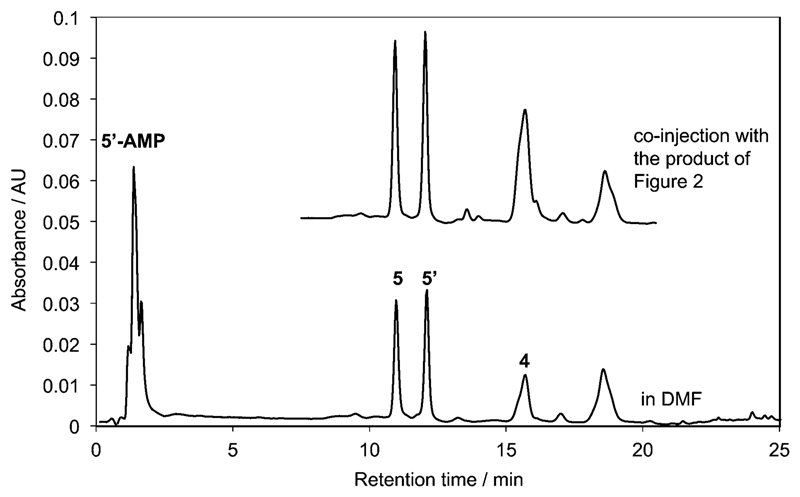
This chemical behavior is illustrated in Figure 2 in the case of 5’-AMP. In a first stage (ca. 5 h), the formation of adducts takes place rapidly and synchronically until 1 is exhausted with rates consistent with the earlier described reaction in the absence of nucleotide (t1/2 = 93 min). This observation supports the conclusion that intermediates are all formed from a direct reaction of the oxazolone 1. In a second stage, the adducts undergo hydrolysis or further evolution at different rates depending on their peculiar reactivity. The HPLC analysis of the medium after 5 h of reaction is represented in Figure 2a (HPLC method A). Peaks corresponding to the acetylated amino acid 4 (HPLC retention time 14.8 min), which is the final product of hydrolysis, the unreacted nucleotide 5’-AMP (HPLC retention time 1.47 min), and adducts (5, 5’, 6, 7; HPLC retention times 9.8, 10.9, 12.2, 12.7 min, respectively) as well as a limited amount of hydrophobic products (putatively attributed to disubstituted species) were observed. The presence of adducts having a 1:1 and 2:1 stoichiometry with respect to amino acid versus nucleotidic moieties was confirmed by HPLC-MS analysis (HPLC method D). The amount of these intermediates was monitored (Figure 2b) according to the change in HPLC peak areas.
Figure 2.
The reaction of 25 mm oxazolone 1 with 5 mm 5’-AMP in 100 mm MES buffer (pH 6.5) at room temperature; (a) HPLC analysis of the medium (after a 50-fold dilution of a 20 μL sample of the reaction medium in the buffer) after 5 h of reaction using method A and detection at 248 nm (brown) and 273 nm (blue); (b) evolution of the amount of the presumed mixed anhydride diastereomers 5 (open circles) and 5’ (filled circles) and of the presumed esters 6 and 7 at the 2’- and 3’-position (open squares).
As shown in Figure 2b, the evolution of the concentrations of the intermediates 5 and 5’ was very similar with a maximum concentration reached after 5 h followed by a decrease with a half-life of about 20 h. This half-life is fully compatible with the values determined in the first section for other phosphate ester mixed anhydrides (t1/2 = 15.7 h for the methyl phosphate derivative 3). Therefore, we considered on kinetic grounds that peaks 5 and 5’ both corresponded to 5’-PEMAs (Scheme 3) present as a diastereomeric mixture because of the fast epimerization of 1. The identification was confirmed by comparison with a similar reaction carried out in DMF that led to increased yields of the mixed anhydrides 5 and 5’ [in the presence of N,N-diisopropylethylamine (DIEA) as a base]. In the absence of water, the mixed anhydrides thus proved to be stable for long periods of time and could accumulate in higher yields (Figure 3). The identification was confirmed by performing this reaction in [D7]DMF, which allowed the observation of a 31P NMR signal at −9.03 ppm (characteristic of the presence of a mixed anhydride) in addition to that of the phosphate group of the nucleotide at −0.17 ppm. The correspondence of the species formed in DMF to intermediates 5 and 5’ formed in the reaction in aqueous MES buffers was demonstrated through HPLC spiking experiments (Figure 3). The stereochemistry difference between diastereomers 5 and 5’ certainly induces changes in both their rates of formation and hydrolysis. However, no significant difference (<10%) could be observed between the amount of mixed anhydrides 5 and 5’, probably because the chiral nucleotide moiety has a limited influence on the phosphoryl group reactivity.
Scheme 3.
The intermediates and products identified from the reaction of 5(4H)-oxazolone (1) with 5’-AMP.
Figure 3.
The reaction of 25 mm oxazolone 1 with 5 mm 5’-AMP in the presence of 5 mm DIEA in DMF for 3 days at room temperature. HPLC profile (bottom) of a sample of the reaction mixture (20 µL) diluted in 1 mL of 100 mm MES buffer pH 6.5 (method A, detection 248 nm). The identity of peaks 5 and 5’ with those observed in the experiment described in Figure 2 was demonstrated by co-injection (top).
The additional adducts 6 and 7 observed in the chromatogram of Figure 2a formed at rates similar to that of mixed anhydrides 5 and 5’, whereas the adducts disappeared much slower. They were not produced in significant yield by the reaction in DMF (Figure 3). Both their slow hydrolysis rate (t1/2~100 h) compared to that of 5 and 5’ and the different outcome of the reaction in DMF are indicative of a different structure for 6 and 7, which are therefore considered to be esters (Scheme 3) at the two available positions (2’- and 3’-hydroxyl groups of the ribose moiety of 5’-AMP). In terms of regio- and stereoselectivity, ester formation can be predicted to yield four isomers because two positions are available, and 1 is chirally unstable in comparison to the lifetime of the reaction[43] and is an equilibrium racemic mixture as previously demonstrated by performing acid hydrolysis.[43] The difference in the areas of the peaks for 6 and 7 is indicative of a significant selectivity among diastereomers or regioisomers during the reaction of 2’- and 3’-hydroxyl groups with 1. Though they are present in negligible concentration by comparison with water (a concentration ratio of 1/55 000), the 2’- and 3’-hydroxyl groups are then capable of favorably competing. Moreover, the data observed in Figure 2b support a formation through a direct reaction of 1 rather than an intramolecular acyl transfer from the mixed anhydrides as previously described for non-acylated aminoacyl adenylate at much lower concentrations.[39] Otherwise, the formation of 6 and 7 should not follow a pattern similar to that of the mixed anhydrides 5 and 5’ and should continue after the consumption of 1 until these anhydrides disappear from the reaction medium. A lower pKa, increasing the content of the reactive anion,[44] and the presence of two free hydroxyl groups[45] may explain this unexpected efficiency of esterification at the 2’- and 3’-positions,[46, 47] although these conclusions require further analysis that is beyond the scope of this present study of the mixed anhydride formation.
Reaction with 2’-deoxyadenosine-5’-monophosphate (5’-dAMP)
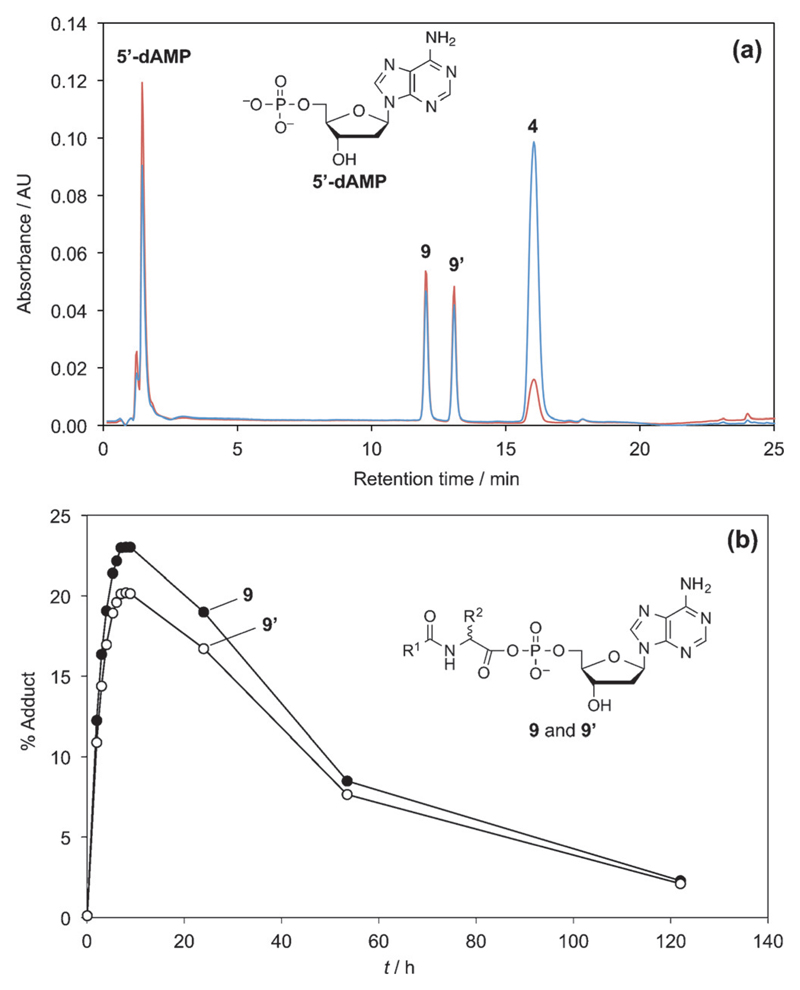
The presence of a single free hydroxyl substituent in 5’-dAMP is likely to simplify the outcome of the reaction with 1, by limiting ester formation compared with 5’-AMP, this nucleotide was therefore selected for a subsequent series of experiments. The nature of the transient species formed by reacting 1 with 5’-dAMP was confirmed by a behavior very similar to the intermediate of the reaction with 5’-AMP. This reaction was carried out in a MES buffer at pH 6.5. The HPLC analysis of the reaction medium at 7 h is presented in Figure 4a, indicating the presence of two adducts 9 and 9’, in addition to the acetylated amino acid 4 formed by hydrolysis, and unchanged 5’-dAMP. The evolution of the HPLC peak areas of adducts 9 and 9’ (Figure 4b) is compatible with the order of magnitude of the rate of hydrolysis of the simple models of PEMAs reported in the first section. Consequently, this evolution is in agreement with the presence of two diastereomers of the mixed anhydrides formed by reaction of the racemic 5(4H)-oxazolone 1 with the chiral nucleotide. As already observed from 5’-AMP, no significant diastereomeric preference could be observed. Starting from 5’-dAMP, this experimental protocol was not able to detect significant amounts of esters with the 3’-hydroxyl group of deoxyribose. This could therefore be considered as a minor pathway confirming that the presence of the 2’,3’-diols in the ribose moiety is essential to the above described direct aminoacylation process.
Figure 4.
The reaction of 25 mm oxazolone 1 with 5 mm 5’-dAMP in 100 mm MES aqueous buffer (pH 6.5) at room temperature; (a) HPLC analysis of the medium (after a 50-fold dilution of samples of the reaction medium (20 µL) in the 100 mm MES buffer pH 6.5) after 7 h of reaction using method A, detection 248 nm (brown) and 273 nm (blue); (b) evolution of the amount of the presumed mixed anhydride diastereomers 9 (filled circles) and 9’ (open circles).
Reaction with adenosine-3’-monophosphate (3’-AMP)
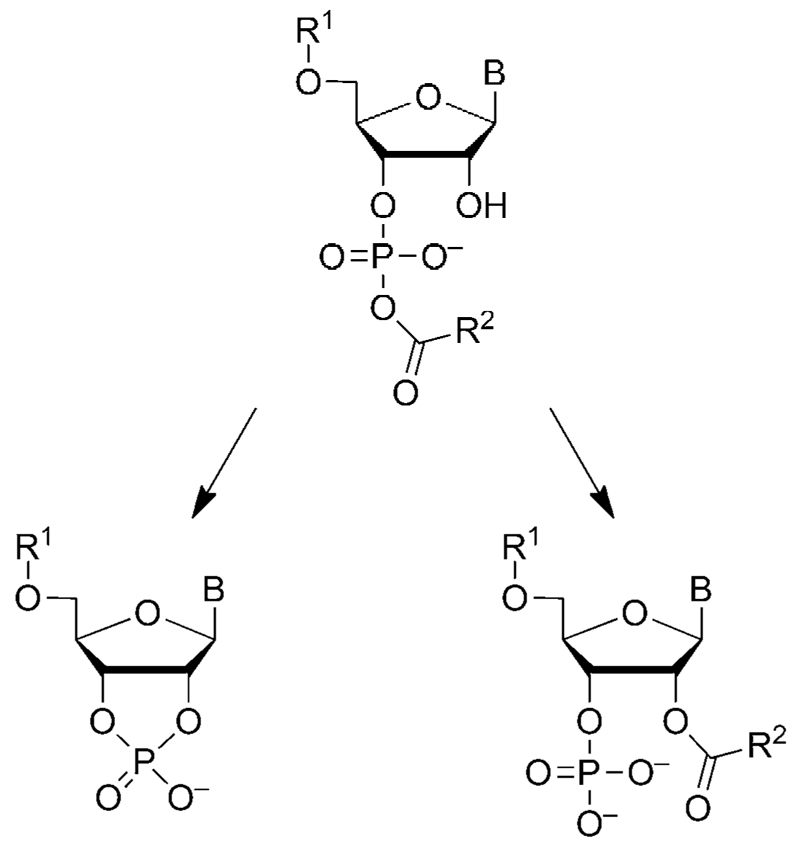
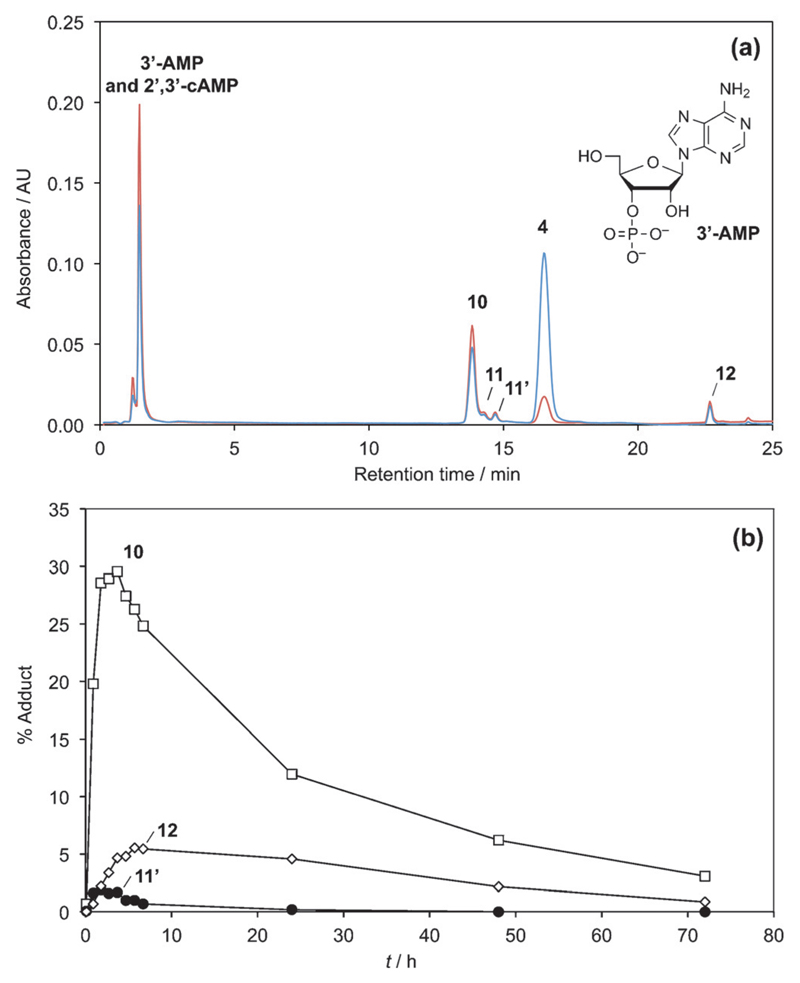
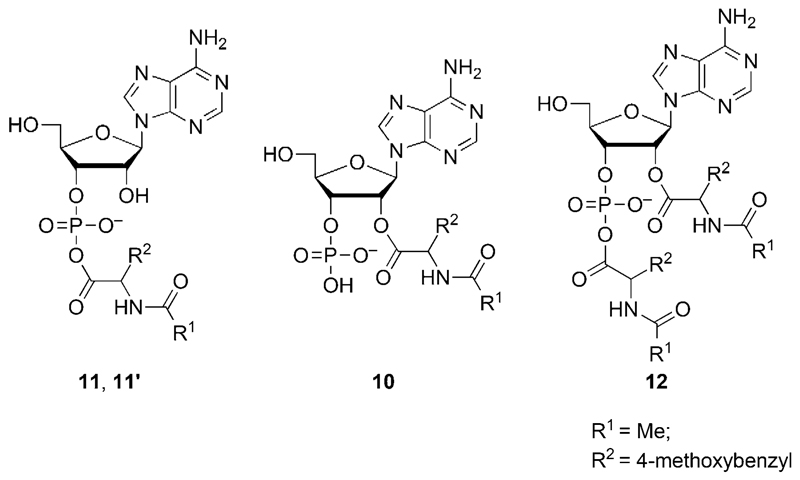
We anticipated that any mixed anhydrides formed with 3’-AMP could lead to a more complex behavior as a result of the possible intramolecular reactions of mixed anhydrides (Scheme 4).[8, 48, 49] Thus, we studied the reactivity of this nucleotide, which could additionally provide a model of the reaction of a phosphorylated 3’-end of a ribonucleotide strand. The reaction of 5(4H)-oxazolone 1 with 3’-AMP was similarly carried out in a pH 6.5 MES buffer. After 3.7 h of reaction, adducts of amino acid and 3’-AMP were found (Figure 5). The presence of several peaks (13.7, 14.1, 14.6 min, method A) corresponded to 1:1 adducts 10, 11, 11’, which was confirmed by HPLC-MS analysis (1.78 min, method D). A more hydrophobic peak (22.61 min, method A; 2.21 min, method D) corresponded to the adduct 12 having a 2:1 stoichiometry. Then, we monitored the progress of this reaction through the evolution of HPLC peak areas. The evolution of the different species was quite different from the one observed for 5’-AMP (Figure 5). Relatively short-lived intermediates 11 and 11’ (14.1, 14.6 min, method A) were observed. As shown in Figure 5b, the short-lived intermediate 11’ formed and rapidly reached a maximum at 2 h, and then its concentration decreased until it was no longer present after 25 h. This behavior, also observed for 11 but with a less accurate determination due to the overlap with the peak of intermediate 10, can be compatible with that of mixed anhydrides (the presumably less stable of the possible amino acid/ribonucleotide adducts). Thus, we assigned the structure of diastereomers of PEMA formed from the two enantiomers of the oxazolone 1 with 3’-AMP to intermediates 11 and 11’, without further attempt to assign the configuration of these intermediates. The unexpected short lifetime of this PEMA formed from 3’-AMP can be rationalized by considering the two additional intramolecular pathways of decay in Scheme 4,[8, 48, 49] pathways which are likely to increase the kinetic rates compared to the other PEMAs mentioned earlier (Scheme 3). Therefore, mixed anhydrides must undergo faster kinetics of breakdown than expected for a direct hydrolysis and should consequently be present in decreased amounts. This assumption was confirmed by peak areas never exceeding 2% of the total area of adenine-containing species for each of the peaks 11 and 11’, instead of about 15 or 20–25% for each of the isomers formed from 5’-AMP or 5’-dAMP, respectively.
Scheme 4.
The two intramolecular pathways observed earlier for the decay of mixed anhydrides formed with 3’-phosphorylated nucleotides.[8, 48, 49].
Figure 5.
The reaction of 25 mm 5(4H)-oxazolone (1) with 5 mm 3’-AMP in a pH 6.5 MES buffer. (a) HPLC analysis of the reaction medium after 3.7 h using method A, detection 248 nm (brown) and 273 nm (blue); (b) evolution of the yield of adducts 10 (open squares, presumed to be the ester at the 2’-position), 11’ (filled circles, putatively attributed to a mixed anhydride), and 12 (open diamonds, presumed bis-adduct).
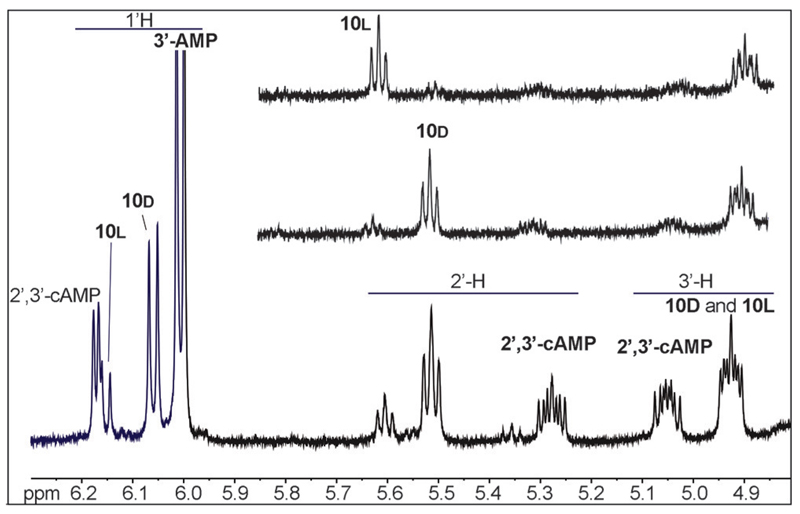
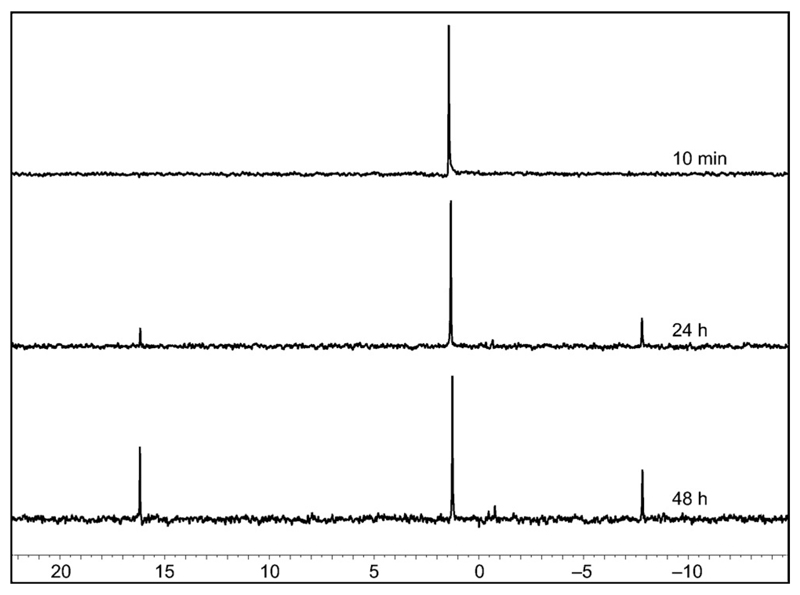
The occurrence of intramolecular processes could be confirmed by 1H NMR (Figure 6). An identical reaction was carried out in heavy water and the analysis of the reaction medium at 3 h allowed the observation of signals at 5.28 and 5.05 for the 2’-and 3’-protons of the phosphodiester 2’,3’-cAMP (c = cyclic adenosine monophosphate), which could be confirmed by HPLC-MS with the observation of a signal at 1.24 min (ESI–, m/z = 328.0455 Da). Additional signals were observed at 5.51 and 5.61 ppm, which were considered as characteristic of the 2’-protons of the ribose moiety of aminoacylated species[8, 49, 50] and therefore, correspond to the presence of esters such as 10 and 12 (Scheme 5). This assignment could be confirmed by acidifying the solution to about pH 2, a procedure known to destroy any PEMA that could be present in the medium.[51] Incidentally, acidification led to the disappearance of the HPLC signal at 23 min indicating that the bis-adduct contained a mixed anhydride moiety and could therefore be tentatively assigned to the structure of 12 in Scheme 5. The reaction of the 1 with 3’-AMP was additionally carried out in [D7]DMF, and monitored by 31P NMR (Figure 7), which confirmed the formation of a mixed anhydride (δ = −7.9 ppm) and that of 2’,3’-cAMP (δ = 16.1 ppm). However, for kinetic reasons (see discussion below), it is likely that the signal at δ = −7.9 ppm corresponds to the bis-adduct 12 rather than to the mixed anhydrides 11 and 11’ with a 1:1 stoichiometry rapidly exhausted by the intramolecular processes of Scheme 4.
Figure 6.
1H NMR analysis of the products of the reaction of 25 mm oxazolone 1 with 5 mm 3’-AMP in H2O (bottom). The reaction medium was freeze-dried and the residue dissolved in D2O. Formation of 2’,3’-cAMP and of the diastereoisomers of the esters at the 2’-position (identified by the comparison with similar reactions of the imidazolides obtained from the l-form (top) and d-form (middle) of Ac-Tyr(Me)-OH 4).
Scheme 5.
The putative products formed from the reaction of oxazolone 1 with 3’-AMP.
Figure 7.
1H NMR spectra of the formation of a mixed anhydride (δ = −7.9 ppm) and of 2’,3’-cAMP (δ = 16.1 ppm) in [D7]DMF (From top to bottom, 10 min, 24 h, 48 h) as a result of the reaction of 25 mm oxazolone 1 with 5 mm 3’-AMP in the presence of 5 mm DIEA at room temperature.
Both the kinetic behavior of species in Figure 5b and the 1H NMR data suggest that the adduct 10, representing up to about 30% of the area of adenine-containing species, corresponds to the ester at the 2’-position of the ribose moiety resulting from an intramolecular transfer from short-lived mixed anhydride diastereomers 11 and/or 11’. The lifetime of 10 (ca. 25 h) is compatible with the structure of an ester though its cleavage is faster than that of esters 6 and 7 formed from 5’-AMP (Figure 2b) that are characterized by longer half-life (ca. 100 h). This faster decay suggests a neighboring assistance of the phosphate group to the hydrolysis of the ester at the 2’-position as a result of intramolecular base catalysis (the hydrolysis of the ester became tedious by acidifying the medium to pH~2). A nucleophilic catalysis pathway reverting the ester 10 to its mixed anhydride precursor with a low kinetic barrier could constitute an alternative. The bis-adduct 12 has a lifetime of approximately 30 h, which is compatible with that of mixed anhydrides formed with other phosphate esters. Thus, we considered that behavior of 12 is compatible with the structure suggested in Scheme 5 that accumulates in higher concentration than PEMAs 11 and 11’ because of the impossibility of further intramolecular reaction. This analysis confirms that the presence of a phosphate substituent at the 3’-terminus of an RNA strand induces a very specific behavior as a consequence of the kinetic instability of the mixed anhydride that undergoes intramolecular processes, rendering its decay faster by more than one order of magnitude.
We considered the possibility of stereoselectivity in the intramolecular transfer leading to the ester 10, which is possibly present as two diastereomers but was only observed as a single HPLC signal. The analogous reaction of the Ac-l-Tyr(Me)- and Ac-d-Tyr(Me)-imidazolides, considered as efficient aminoacylation reagents[52] but also described as mixed anhydrides precursors,[51] was carried out to efficiently yield mixtures of ester diastereomer at the 2’-position. A substantial degree of retention of configuration was present and the NMR signals at 5.51 and 5.60 ppm could be assigned to the 2’-hydroxyl protons of 3’-AMP esterified by an amino acid with the d- and l-configuration, respectively (Figure 6). The formation of the 2’-aminoacylated species 10 from 1 therefore takes place with a diastereomeric excess of about 45% in favor of the incorporation of the non-natural d-amino acid configuration. This observation is in agreement with the fact that a preference for the d-configuration is generally observed for aminoacylation at the 2’-position,[53] which is not modified by the intramolecular path occurring in the present reaction. The reaction of 1 with 3’-AMP exhibits a very specific behavior that should be considered as relevant to origin of life processes because 3’-phosphorylated nucleosides can be formed through convincing pathways consistent with the conditions of early Earth.[54] This statement may be strengthened by the fact that acylation at the 2’-hydroxyl group of ribose[49] could facilitate elongation of RNA strands possessing a phosphate at the 3’-terminus. This behavior is confirmed by the time stability profile of adduct 12 (Figure 5), in which the aminoacyl substituent plays the role of protecting group.
Conclusion
The reaction of 5(4H)-oxazolones with phosphate esters turned out to constitute a useful method for studying mixed anhydride formation. This method is more practical than the corresponding reactions of α-amino acid N-carboxyanhydrides,[8, 55] which are accompanied by polymerization of the starting material into oligopeptides rendering analyses more delicate. The formation of mixed anhydrides is a consequence of the high degree of activation that 5(4H)-oxazolones share with NCAs, independently considered as a requirement for the development of self-organization in α-amino acid/peptide systems.[6] Though significant differences are probable, the conclusions of the present study of the reaction of 5(4H)-oxazolones may, however, be extended to that of other strongly activated α-amino acid derivatives. This was confirmed here by the comparable behavior of imidazolides prepared by the method of Gottikh et al.[52] The formation of mixed anhydrides from phosphate monoesters was observed in all the examples studied but not detected in the case of phosphodiesters. No inhibition of the formation of mixed anhydrides was observed as a result of the presence of additional negative charges in the vicinity of the phosphate reagent. Even a certain degree of protection of the anhydride against hydrolysis was observed. Consequently, mixed anhydrides can be formed from relatively low concentrations of reactants at two positions in RNA; the 5’-end and the 3’-end with the notable exception of the internucleotidic phosphodiester linkages that are not reactive (Scheme 6). However, phosphorylation at the 3’-terminus induces a very specific reactivity regarding the mixed anhydride that undergoes intramolecular processes yielding the cyclic phosphodiester 2’,3’-cAMP, and the 2’-ester. We also observed the direct formation of esters, which seems especially easy at the 2’- and 3’-positions. In contrast to earlier observations,[39] starting from diluted solutions of preformed aminoacyl adenylates, we had no indication that the reaction proceeds intramolecularly from 5’-AMP. There is indeed a possibility that acylation of the amino acid reagent induces a different behavior compared to α-amino acids having a protonated free amino group at the pH of the experiment. These results also illustrate that aminoacylation by 5(4H)-oxazolones could also play a role through the temporary protection of the 2’-hydroxyl group[49] that could facilitate 3’→5’ ligation. The systems chemistry view of the emergence of translation through the association of peptide and nucleic acid subsystems is supported by the experimental results described in this work. It also strengthens additional advantages in terms of function that can be obtained by associating amino acids with membranes or nucleic acids that have already been observed in our group as well as by others.[9–12] Diversity in prebiotic “clutter”[56] can also present advantages in terms of a self-organization process. The emergence of translation may therefore lie in the intimate chemistry of RNA and the specific behavior of the intramolecular transfer in 3’-phosphorylated RNA as well as in the very specific behavior of the ribose 2’- and 3’-hydroxyl groups. It confirms the view that intramolecular pathways were essential in the absence of enzyme catalysts because they could divert chemical processes from the regular decay of reactants through kinetically efficient routes.[57, 58]
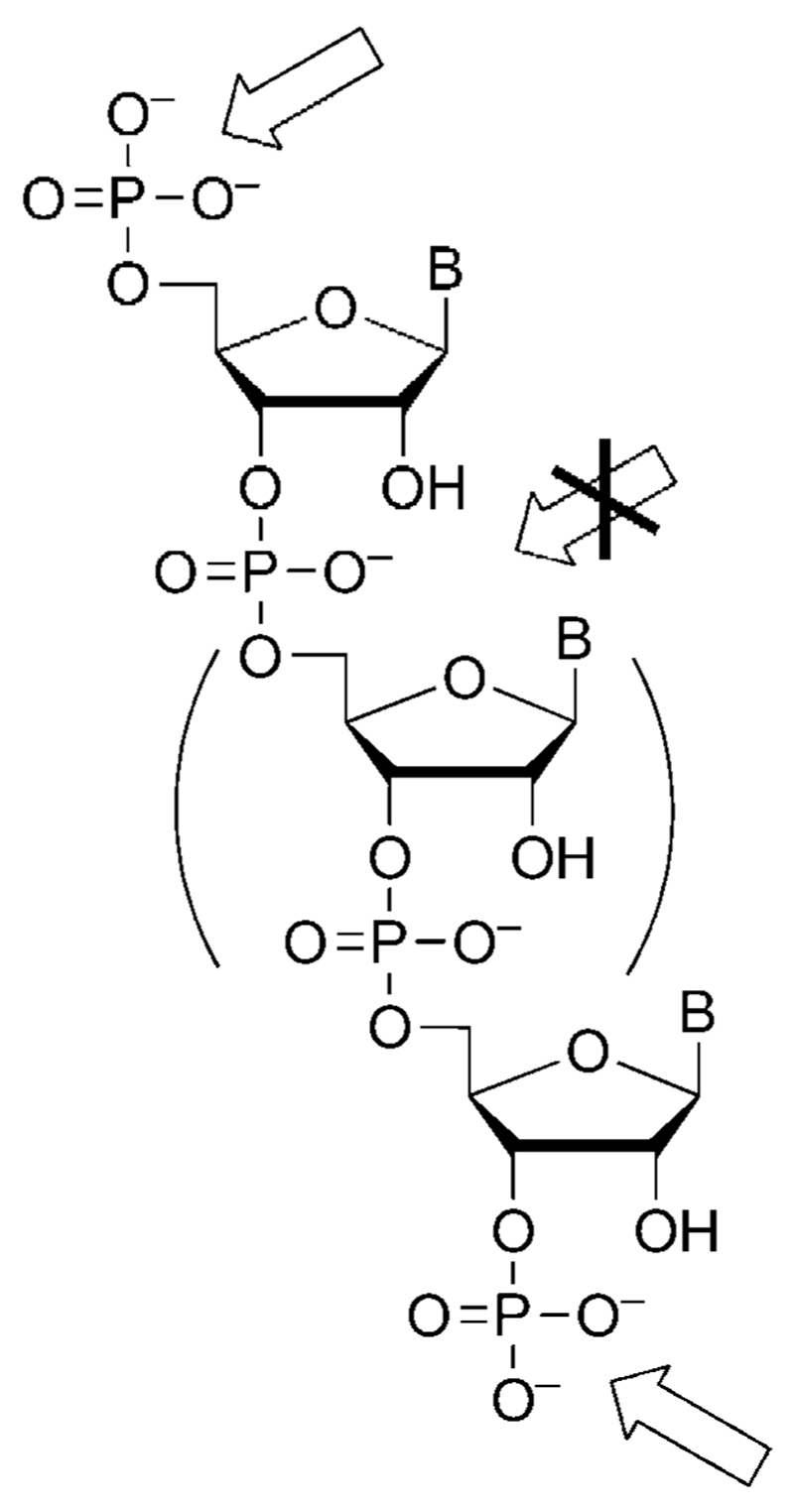
Scheme 6.
Mixed anhydrides can be formed specifically at the 5’-and 3’-terminus of RNA strands, though the presence of the 2’-hydroxyl group in ribonucleotides induces efficient intramolecular pathways of decay.
Experimental Section
General procedures
Amino acids and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) were purchased from Bachem, deuterated solvents were purchased from Euriso-Top. All other reagents and solvents were purchased from Sigma–Aldrich. All compounds were used without further purification. In all the experiments, the pH was monitored using a Thermo Orion 3-STAR pH-meter with a VWR electrode. NMR spectra were recorded on a Bruker Avance 300 apparatus at 300 MHz for 1H, 121 MHz for 31P, and 75 MHz for 13C, and on a Bruker Avance III HD apparatus at 400 MHz. HPLC analyses were performed on a Waters Alliance 2690 system with a photodiode array detector 996 using a Thermo Scientific BDS Hypersil C18 5 μm 2.1 × 50 mm column; method A: mobile phase: A: H2O+0.1% TFA, B:CH3CN+0.1 % TFA; flow rate: 0.2 mL min−1; 0 min (5% B), to 15 min (15% B), 25 min (60% B) and 26 min (100% B); method B: mobile phase: A: H2O+0.1% TFA, B:CH3CN+0.1% TFA; flow rate: 0.2 mL min−1; 0 min (5% B), to 10 min (20 % B) and 11 min (100 % B); method C: mobile phase: A: 5 mm triethylammonium acetate pH 6 in water, B: CH3CN; flow rate: 0.2 mL min−1; 0 min (5% B), to 15 min (15% B), 25 min (60% B) and 26 min (100% B); HPLC-ESI-MS analyses were carried out on a Waters Synapt G2-S system connected to a Waters Acquity UPLC H-Class apparatus equipped with a Acquity UPLC BEH C18, 1.7 mm 2.1 × 50 mm column; method D: A: H2O+0.01% formic acid, B: CH3CN+0.01% formic acid; flow rate: 0.5 mL min−1; linear gradient 0% to 100% B over 3 min.
4-(Phosphonooxy)butane-1-sulphonic acid, sodium salt (2 c)
Pure anhydrous phosphoric acid (0.862 g, 8.8 mmol) was mixed with DIEA (1.54 mL, 8.8 mmol). 1,4-Butane sultone (1.02 mL, 10 mmol) was added to the mixture. The reaction medium was heated to 110 °C for 14 h. Then the mixture was dissolved in ethanol/water (95:5 v/v, 10 mL). Cyclohexylamine was added to obtain a precipitate. The unreacted phosphoric acid cyclohexylamine salt was removed by filtration. The solvent was evaporated, and the residue was re-dissolved in a minimum amount of ethanol/water (95:5). Acetone was poured into the solution to get a precipitate. The precipitate was separated by filtration. The product was converted into a sodium salt after dissolution in water (10 mL), using a Dowex 50 (Na+ form) ion exchange resin. The solution was introduced and the column was extensively eluted with water. The eluate was collected in a flask and then freeze-dried. Yield: 1.4 g, 45%. 1H NMR (300 MHz, D2O, 25 °C) δ = 3.68 (q, 3J(H,H) = 6.3 Hz, 2H; CH2), 2.92–2.84 (m, 2H; CH2), 1.79–1.56 ppm (m, 4H; CH2CH2); 13C NMR (75 MHz, D2O, 25 °C) δ = 63.65, 50.84, 29.20, 20.79 ppm; 31P NMR (121 MHz, D2O, 25 °C) δ = 4.05 ppm. HRMS (ESI): 232.9886[C4H10O7SP–].
Acetyl-O-methyl-tyrosine (Ac-Tyr(Me)-OH, 4)
A mixture of H-Tyr(Me)-OH (0.976 g, 5 mmol) and Na2CO3(0.795 g, 7.5 mmol) was suspended in dioxane (5 mL) and water (10 mL), and then vigorously stirred at 0 °C. Then, 1 mL of a 5 mL solution of acetic anhydride (0.52 mL, 5.5 mmol) in dioxane and 2 mL of a 10 mL solution of NaOH (260 mg, 6.5 mmol) in water were added to the mixture. The remaining solution was added in four equivalent portions, one every 10 min. The reaction was allowed to reach completion at RT for 2 h. The solution was acidified to pH 2 with 1 N HCl and extracted with ethyl acetate (2 × 50 mL). The organic layer was recovered, washed with brine, dried over Na2SO4 and concentrated under reduced pressure. The oily residue was allowed to crystallize for 16 h at 4 °C in a minimum volume of ethyl acetate and hexane. A white solid was collected, washed with hexane and dried. Yield: 980 mg, 83%. 1H NMR (300 MHz, [D6]DMSO, 25°C), δ = 8.14 (d, 3J(H,H) = 8.04 Hz, 1H; NH); 7.14 (d, 3J(H,H) = 8.46 Hz, 2H; CH2C(CH)2); 6.84 (d, 3J(H,H) = 8.48 Hz, 2H; CH3OC(CH)2); 4.34 (m, 3J(H,H) = 8.96 Hz, 3J(H,H) = 5.02 Hz, 1H; NHCH); 3.72 (s, 3H; OCH3); 2.70–3.00 (m, 2H; CHCH2); 1.78 ppm (s, 3H; CCH3); HRMS (ESI): 260.0896 [C12H15NNaO4+].
2-Methyl-4-(4-methoxybenzyl)-5(4H)-oxazolone (1)
A solution of Ac-Tyr(Me)-OH (500 mg, 2.11 mmol) in CH2Cl2 (20 mL) was cooled using an ice bath and treated with EDC (445 mg, 2.87 mmol). The mixture was maintained at 0 °C for 1 h with stirring. Then, CH2Cl2 (20 mL) was added. The solution was washed with H2O (2 × 20 mL), a saturated aqueous NaHCO3 (1 × 20 mL), brine (1 × 20 mL), and dried with Na2SO4. The solvent was removed under vacuum to afford a colorless oily residue. Yield 440 mg, 95.2%. The product was allowed to crystallize at 4 °C. As determined earlier,[43] this procedure results in a complete loss of chirality and 1 is obtained as a racemic mixture. 1H NMR (300 MHz, [D6]DMSO, 25°C) δ = 7.20–7.03 (m, 2H; CH2C(CH)2), 6.91–6.78 (m, 2H; CH3OC(CH)2), 4.63 (tq, 3J(H,H) = 4.16 Hz, 3J(H,H) = 2.04 Hz, 1H; NCH), 3.72 (s, 3H; CH3O), 2.97 (m, 2H; CHCH2C), 2.06 ppm (d, 3J(H,H) = 2.08 Hz, 3H; CH3C); 13C NMR (75 MHz, [D6]DMSO, 25°C) δ = 178.83, 162.12, 158.58, 130.85, 128.33, 113.98, 65.97, 55.41, 35.66, 15.05 ppm. HRMS (ESI): 218.0796 [C12H12NO3–]
2’-N-Acetyl-O-methyl-l(or d)-tyrosyl-3’-AMP (10)
1,1’-Carbonyldiimidazole (CDI, 4.5 mg, 27.8 μmol) was added to a suspension of N-acetyl-O-methyl-l-(or d)-tyrosine (6 mg, 25.3 μmol) in CH3CN (50 μL), and mixed by vortexing for 5 min at room temperature. The resulting solution of the imidazolide tyrosine derivative was added to a solution of 3’-AMP in H2O (0.3 mL), and allowed to react for 1 h at room temperature. Aliquots of the solution (20 μL) were withdrawn and diluted in 100 mm pH 6.5 MES buffer (1 mL), then analyzed by HPLC. The solution was adjusted to pH 3.3 by adding a KHSO4 saturated solution, and then lyophilized. The residue was dissolved in D2O and analyzed by 1H NMR. 2’-N-Acetyl-O-methyl-l-tyrosyl-3’-AMP: 1H NMR (400 MHz, D2O, 25°C) δ = 8.38 (s, 1H; H-C(2)), 8.30 (s, 1H, H-C(8)), 7.00 (d, 3J(H,H) = 8.7 Hz, 2H; H-C(2 and 6 on methyl phenol)), 6.68 (d, 3J(H,H) = 8.7 Hz, 2H; H-C(3 and 5 on methyl phenol)), 6.22 (d, 3J(H,H) = 5.7 Hz, 1H; H-C(1’)), 5.68 (t, 3J(H,H) = 5.6 Hz, 1H; H-C(2’)), 4.96 ppm (m, 1H; H-C(3’)). Other signals for this compound were mainly obscured. 2’-N-Acetyl-O-methyl-d-tyrosyl-3’-AMP: 1H NMR (400 MHz, D2O, 25 °C) δ = 8.34 (s, 1H; H-C(2)), 8.27 (s, 1H; H-C(8)), 6.90 (d, 3J(H,H) = 8.7 Hz, 2H; H-C(2 and 6 on methyl phenol)), 6.57 (d, 3J(H,H) = 8.7 Hz, 2H; H-C(3 and 5 on methyl phenol)), 5.56 (t, 3J(H,H) = 5.4 Hz, 1H; H-C(2’)), 4.98–4.91 ppm (m, 1H; H-C(3’)). Other signals for this compound were mainly obscured.
General protocol for the reactions of 5(4H)-oxazolone (1)
O-methylated tyrosine was used as a model of usual amino acid derivatives. Phenol methylation was introduced to simplify analyses by avoiding any side reaction of this group. The UV-absorption of the O-methylated tyrosine side chain (λmax = 273 nm) was selected to monitor reactions by HPLC at a reasonably low (0.05–1 mm) concentration range. Tyrosine oxazolone was stable at −20°C as a solid or in solution in CH3CN for several months. Reactions were carried out in non-nucleophilic MES buffers at pH values of 6.5, whereas a 50 mm sodium salt buffer of 4-(phosphonooxy)butane-1-sulphonic (2c) was used for studying the transient formation of mixed anhydrides from this reagent. Analyses were performed to monitor the reaction progress of samples stored in the HPLC systems located in a room maintained at 20°C.
Hydrolysis of 5-(4H)-oxazolone (1) in the presence of dimethyl phosphate (2 b)
The 1,3-dimethylimidazolium salt of dimethyl phosphate 2b (13 μL, 75 μmol) was dissolved in a 100 mm MES buffer (pH 6.5, 1.5 mL). A solution of 1 (20 μL of 0.625 m CH3CN solution, 12.5 mmol) was added and the resulting solution was then mixed by vortexing. The reaction medium was stored in the HPLC system and analyzed by HPLC (Method B). For 31P NMR analysis, MES buffer (0.75 mL) was lyophilized then re-dissolved in D2O (0.75 mL). 2b (26 μL, 150 μmol) was dissolved in the buffer. 1 (20 μL of CH3CN solution, 12.5 mmol) was added to the medium, mixed by vortexing, and the reaction monitored by 31P NMR.
Preparation of methyl phosphate 2a or 4-(phosphonooxy)butane-1-sulphonate (2 c) buffers
The corresponding salts (0.5 mmol) were dissolved in a small volume of water and the pH was adjusted to 6.5 with 1 N HCl, and the solution was then diluted to 10 mL to reach a 50 mm concentration.
Reaction between 4-(phosphonooxy)butane-1-sulphonate (2c) and 5(4H)-oxazolone (1)
The reaction of 1 (20 μL of 0.625 m CH3CN solution, 12.5 mmol) was performed in the buffer prepared as described above (1 mL) in the HPLC system located in a room maintained at 20°C. The progress was monitored by the HPLC integration of peaks at 273 nm corresponding to the maximum absorbance of the 4-methoxyphenyl moiety. The assumption was made that reactions provoked no change in the absorbance of the chromophore group, which was reasonable with respect to the limited precision required for the present study. The formation of mixed anhydride was confirmed by LC-ESI-HRMS and 31P NMR. 31P NMR (121 MHz, D2O, 25°C) δ = −7.15 ppm (s); HRMS (ESI): 452.0779 [C16H23NO10PS–]
Reactions between 5-(4H)-oxazolone (1) and 5’-AMP or 5’-dAMP
The reaction was performed in 100 mm MES buffer (pH 6.5, containing 10% CH3CN) or in DMF (0.5 mL). The nucleotide (2.5 μmol) was dissolved in the medium, 1 (20 μL of 0.625 m CH3CN solution, 12.5 mmol) was added and the mixture was stirred by vortexing. After a period of time, aliquots were withdrawn (20 μL) and dissolved in 100 mm pH 6.5 MES buffer (1 mL), then analyzed by HPLC and LC-HRMS. For 31P NMR analysis, the nucleotide (2.5 μmol) was dissolved in [D7]DMF, 1 (20 μL of 0.625 m CH3CN solution, 12.5 mmol) was added, the resulting reaction mixture was stirred by vortexing and analyzed by 31P NMR. 5’-AMP PEMA: 31P NMR (121 MHz, [D7]DMF, 25 °C) δ = −0.17 (s; 5’-AMP), −9.03 ppm (s; 5’-PEMA); HRMS (ESI): 565.1459 [C22H26N6O10P–]. 5’-dAMP PEMA: 31P NMR (121 MHz, [D7]DMF, 25 °C) δ = −0.41 (s; 5’-dAMP), −8.98 ppm (s; 5’-dPEMA); HRMS (ESI): 549.1495 [C22H26N6O9P–].
Reaction between 5-(4H)-oxazolone (1) and 3’-AMP
The reaction was performed in 100 mm pH 6.5 MES buffer (10% CH3CN in the aqueous buffer) or in [D7]DMF (0.5 mL). 3’-AMP (2.5 μmol) and 1 (20 μL of a 0.625 m CH3CN solution, 12.5 mmol) were added to the solution and the mixture was vigorously stirred by vortexing. After a period of time, samples were withdrawn (20 μL) and dissolved in a 100 mm pH 6.5 MES buffer (1 mL), then analyzed by HPLC, LC-HRMS and 31P NMR. 3’-AMP 2’-amino acyl ester: HRMS (ESI): 565.1450 [C22H26N6O10P–]. 2’,3’-cAMP: HRMS (ESI): 328.0455 [C10H11N5O6P–]. The products of the reaction in [D7]DMF were monitored by 31P NMR. 31P NMR (121 MHz, [D7]DMF, 25°C) δ = 16.18 ppm (s). For 1H NMR analysis, 3’-AMP (7 mg, 20 μmol) was dissolved in H2O (4 mL), the pH was adjusted to 6.5 by NaHCO3. 1 (20 μL of 0.625 m CH3CN solution, 12.5 mmol) was added and the mixture was stirred. During the reaction, the pH was kept at 6.5 by adding NaHCO3 saturated solution. After 4 h, the pH was adjusted to 3.3 with a KHSO4 saturated solution, then the solution was lyophilized. The residue was dissolved in D2O and analyzed by 1H NMR.
Supplementary Material
Supporting information for this article can be found under http://dx.doi.org/10.1002/chem.201602697.
Acknowledgements
This work was supported by grants from the Simons Foundation (Grant Number 293065 to Z.L.) and from the Agence Nationale de la Recherche (ANR-14-CE33–0020–01 to the Pepti-Systems project). The authors thank the COST action CM1304 Emergence and Evolution of Complex Chemical Systems for facilitating the collaboration between our groups with a Short Term Scientific Mission for Z.L.
References
- [1].Borsenberger V, Crowe MA, Lehbauer J, Raftery J, Helliwell M, Bhutia K, Cox T, Sutherland JD. Chem Biodiversity. 2004;1:203–246. doi: 10.1002/cbdv.200490020. [DOI] [PubMed] [Google Scholar]
- [2].Pascal R, Boiteau L, Commeyras A. Top Curr Chem. 2005;259:69–122. [Google Scholar]
- [3].Ruiz-Mirazo K, Briones C, de La Escosura A. Chem Rev. 2014;114:285–366. doi: 10.1021/cr2004844. [DOI] [PubMed] [Google Scholar]
- [4].Ludlow RF, Otto S. Chem Soc Rev. 2008;37:101–108. doi: 10.1039/b611921m. [DOI] [PubMed] [Google Scholar]
- [5].von Kiedrowski G, Otto S, Herdewijn P. J Syst Chem. 2010;1:1. [Google Scholar]
- [6].Danger G, Plasson R, Pascal R. Chem Soc Rev. 2012;41:5416–5429. doi: 10.1039/c2cs35064e. [DOI] [PubMed] [Google Scholar]
- [7].Wilson MA, Wei C, Pohorille A. Orig Life Evol Biosph. 2014;44:357–361. doi: 10.1007/s11084-014-9393-2. [DOI] [PubMed] [Google Scholar]
- [8].Biron J-P, Parkes AL, Pascal R, Sutherland JD. Angew Chem Int Ed. 2005;44:6731–6734. doi: 10.1002/anie.200501591. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2005;117:6889–6892. [Google Scholar]
- [9].Murillo-Sánchez S, Beaufils D, González Mañas JM, Pascal R, Ruiz-Mirazo K. Chem Sci. 2016;7:3406–3413. doi: 10.1039/c5sc04796j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Black RA, Blosser MC, Stottrup BL, Tavakley R, Deamer DW, Keller SL. Proc Natl Acad Sci USA. 2013;110:13272–13276. doi: 10.1073/pnas.1300963110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ritson D, Sutherland JD. Nat Chem. 2012;4:895–899. doi: 10.1038/nchem.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sutherland JD. Angew Chem Int Ed. 2016;55:104–121. doi: 10.1002/anie.201506585. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2016;128:108–126. [Google Scholar]
- [13].Szathmáry E. Trends Genet. 1999;15:223–229. doi: 10.1016/s0168-9525(99)01730-8. [DOI] [PubMed] [Google Scholar]
- [14].Delaye L, Becerra A, Lazcano A. Orig Life Evol Biosph. 2005;35:537–554. doi: 10.1007/s11084-005-5760-3. [DOI] [PubMed] [Google Scholar]
- [15].Davidovich C, Belousoff M, Wekselman I, Shapira T, Krupkin M, Zimmerman E, Bashan A, Yonath A. Isr J Chem. 2010;50:29–35. doi: 10.1002/ijch.201000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Di Giulio M. J Mol Evol. 2013;77:131–133. doi: 10.1007/s00239-013-9593-9. [DOI] [PubMed] [Google Scholar]
- [17].Petrov AS, Gulen B, Norris AM, Kovacs NA, Bernier CR, Lanier KA, Fox GE, Harvey SC, Wartell RM, Hud NV, Williams LD. Proc Natl Acad Sci USA. 2015;112:15396–15401. doi: 10.1073/pnas.1509761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maynard-Smith J, Szathmáry E. The Major Transitions in Evolution. Freeman Press; Oxford: 1995. [Google Scholar]
- [19].Wells TNC, Fersht AR. Biochemistry. 1986;25:1881–1886. doi: 10.1021/bi00356a007. [DOI] [PubMed] [Google Scholar]
- [20].Ribas de Pouplana L, Schimmel P. Trends Biochem Sci. 2001;26:591–596. doi: 10.1016/s0968-0004(01)01932-6. [DOI] [PubMed] [Google Scholar]
- [21].Jencks WP. In: Handbook of Biochemistry and Molecular Biology. 3rd ed. Fasman GD, editor. Vol. 1. CRC; Cleveland: 1976. pp. 296–304. [Google Scholar]
- [22].Weber AL, Orgel LE. J Mol Evol. 1978;11:189–198. doi: 10.1007/BF01734480. [DOI] [PubMed] [Google Scholar]
- [23].Profy AT, Usher DA. J Am Chem Soc. 1984;106:5030–5031. doi: 10.1021/ja00329a080. [DOI] [PubMed] [Google Scholar]
- [24].Orgel LE. J Mol Evol. 1989;29:465–474. doi: 10.1007/BF02602917. [DOI] [PubMed] [Google Scholar]
- [25].Lacey JC, Wickramasinghe NS, Cook GW. Orig Life Evol Biosph. 1992;22:243–275. doi: 10.1007/BF01810856. [DOI] [PubMed] [Google Scholar]
- [26].Illangasekare M, Sanchez G, Nickles T, Yarus M. Science. 1995;267:643–647. doi: 10.1126/science.7530860. [DOI] [PubMed] [Google Scholar]
- [27].Kumar RK, Yarus M. Biochemistry. 2001;40:6998–7004. doi: 10.1021/bi010710x. [DOI] [PubMed] [Google Scholar]
- [28].Chumachenko NV, Novikov Y, Yarus M. J Am Chem Soc. 2009;131:5257–5263. doi: 10.1021/ja809419f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Turk RM, Chumachenko NV, Yarus M. Proc Natl Acad Sci USA. 2010;107:4585–4589. doi: 10.1073/pnas.0912895107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pascal R, Boiteau L. In: Origin and Evolution of Life: An Astrobiology Perspective. Gargaud M, Lopez-Garcia P, Martin H, editors. Cambridge University Press; Cambridge: 2011. pp. 247–258. [Google Scholar]
- [31].Pascal R. J Syst Chem. 2012;3:3. [Google Scholar]
- [32].Pascal R. In: Astrochemistry and Astrobiology: Physical Chemistry in Action. Smith IWM, Cockell CS, Leach S, editors. Springer; Heidelberg: 2013. pp. 243–269. [Google Scholar]
- [33].Pross A, Pascal R. Open Biol. 2013;3:120190. doi: 10.1098/rsob.120190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pascal R, Pross A, Sutherland JD. Open Biol. 2013;3:130156. doi: 10.1098/rsob.130156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pross A. Isr J Chem. 2016;56:83–88. [Google Scholar]
- [36].Jauker M, Griesser H, Richert C. Angew Chem Int Ed. 2015;54:14564–14569. doi: 10.1002/anie.201506593. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2015;127:14772–14777. [Google Scholar]
- [37].Ni F, Gao X, Zhao Z-X, Huang C, Zhao Y-F. Eur J Org Chem. 2009:3026–3035. [Google Scholar]
- [38].Brack A. Origins Life. 1987;17:367–379. doi: 10.1007/BF02386475. [DOI] [PubMed] [Google Scholar]
- [39].Wickramasinghe NSMD, Staves MP, Lacey JC. Biochemistry. 1991;30:2768–2772. doi: 10.1021/bi00225a005. [DOI] [PubMed] [Google Scholar]
- [40].Liu Z, Beaufils D, Rossi J-C, Pascal R. Sci Rep. 2014;4:7440. doi: 10.1038/srep07440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kumler WD, Eiler JJ. J Am Chem Soc. 1943;65:2355–2361. [Google Scholar]
- [42].Matsui H, Miki T, Igarashi N, (Teijin Ltd., Japan),Jpn JP 49031621A. Kokai Tokkyo Koho. 1974 [Chem. Abstr. 1974, 84, 48884]
- [43].Beaufils D, Danger G, Boiteau L, Rossi J-C, Pascal R. Chem Commun. 2014;50:3100–3102. doi: 10.1039/c3cc49580a. [DOI] [PubMed] [Google Scholar]
- [44].Velikyan I, Acharya S, Trifonova A, Földesi A, Chattopadhyaya J. J Am Chem Soc. 2001;123:2893–2894. doi: 10.1021/ja0036312. [DOI] [PubMed] [Google Scholar]
- [45].Izatt RM, Rytting JH, Hansen LD, Christensen JJ. J Am Chem Soc. 1966;88:2641–2645. doi: 10.1021/ja00964a003. [DOI] [PubMed] [Google Scholar]
- [46].Weinger JS, Strobel SA. Biochemistry. 2006;45:5939–5948. doi: 10.1021/bi060183n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cameron LL, Wang SC, Kluger R. J Am Chem Soc. 2004;126:10721–10726. doi: 10.1021/ja049538l. [DOI] [PubMed] [Google Scholar]
- [48].Rammler DH, Lapidot J, Khorana HG. J Am Chem Soc. 1963;85:1989–1997. [Google Scholar]
- [49].Bowler FR, Chan CK, Duffy CD, Gerland B, Islam S, Powner MW, Sutherland JD, Xu J. Nat Chem. 2013;5:383–389. doi: 10.1038/nchem.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lacey JC, Thomas RD, Staves MP, Watkins CL. Biochim Biophys Acta. 1991;1076:395–400. doi: 10.1016/0167-4838(91)90482-f. [DOI] [PubMed] [Google Scholar]
- [51].Wickramasinghe NSMD, Lacey JC. Orig Life Evol Biosph. 1992;22:361–368. doi: 10.1007/BF01809372. [DOI] [PubMed] [Google Scholar]
- [52].Gottikh BP, Krayevskyn AA, Tarussovap B, Purygin P, Tsilevic TL. Tetrahedron. 1970;26:4419–4433. doi: 10.1016/s0040-4020(01)93090-x. [DOI] [PubMed] [Google Scholar]
- [53].Lacey JC, Jr, Hawkins AF, Thomas RD, Watkins CL. Proc Natl Acad Sci USA. 1988;85:4996–5000. doi: 10.1073/pnas.85.14.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Powner MW, Gerland B, Sutherland JD. Nature. 2009;459:239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- [55].Leman LJ, Orgel LE, Ghadiri MR. J Am Chem Soc. 2006;128:20–21. doi: 10.1021/ja056036e. [DOI] [PubMed] [Google Scholar]
- [56].Krishnamurthy R. Isr J Chem. 2015;55:837–850. [Google Scholar]
- [57].Pascal R. Eur J Org Chem. 2003:1813–1824. [Google Scholar]
- [58].Pascal R. Isr J Chem. 2015;55:865–874. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.