Abstract
The role of smooth muscle endothelinB receptors in regulating vascular function, blood pressure and neointimal remodelling has not been established. Selective knockout mice were generated to address the hypothesis that loss of smooth muscle endothelinB receptors would reduce blood pressure, alter vascular contractility, and inhibit neointimal remodelling.
EndothelinB receptors were selectively deleted from smooth muscle by crossing floxed endothelinB mice with those expressing cre-recombinase controlled by the transgelin promoter. Functional consequences of endothelinB deletion were assessed using myography. Blood pressure was measured by telemetry, and neointimal lesion formation induced by femoral artery injury. Lesion size and composition (day 28) were analysed using optical projection tomography, histology and immunohistochemistry.
Selective deletion of endothelinB was confirmed by genotyping, autoradiography, PCR and immunohistochemistry. EndothelinB-mediated contraction was reduced in trachea, but abolished from mesenteric veins, of knockout mice. Induction of endothelinB-mediated contraction in mesenteric arteries was also abolished in these mice. Femoral artery function was unaltered and baseline blood pressure modestly elevated in smooth muscle endothelinB knockout compared to controls (+4.2±0.2mmHg; P<0.0001) but salt-induced and endothelinB blockade-mediated hypertension were unaltered. Circulating endothelin-1 was not altered in knockout mice. EndothelinB-mediated contraction was not induced in femoral arteries by incubation in culture medium or lesion formation, and lesion size was not altered in smooth muscle endothelinB knockout mice.
In the absence of other pathology, endothelinB receptors in vascular smooth muscle make a small but significant contribution to endothelinB-dependent regulation of blood pressure. These endothelinB receptors have no effect on vascular contraction or neointimal remodelling.
Keywords: Endothelin-1, endothelin B receptors, vascular smooth muscle, hypertension, vasoconstriction, neointima formation
Introduction
Endothelin-1 (ET-1), released by vascular endothelial (EC) and inner medullary collecting duct cells (and other cells under pathological conditions), stimulates endothelinA (ETA) and endothelinB (ETB) receptor subtypes1,2. ETA are present on vascular smooth muscle cells (VSMC), predominantly mediating contraction3 and regulating blood pressure (BP)4. They also influence mitogenesis5, generation of reactive oxygen species and adhesion molecule expression6,7. ETA receptors on leucocytes mediate cytokine release and cellular chemotaxis8. Many of these processes contribute to vascular remodelling, and ET-1 clearly drives arterial lesion formation (including neointimal proliferation after injury)7. This can be inhibited by selective ETA antagonism9,10.
Regulation of arterial function, BP and arterial lesion formation by ETB receptors is likely to be more complex since they are expressed in EC, VSMC and the kidney where they mediate physiologically antagonistic responses. ECETB receptors mediate production of vasodilator, anti-proliferative and anti-inflammatory molecules (e.g. nitric oxide; NO)11,12, clearance of ET-1 from the circulation13,14 and regrowth of damaged EC15. VSMC ETB can mediate vascular contraction, similar to the ETA subtype16, and may compensate for ETA receptor dysfunction17. ETB upregulation in VSMC may mediate vasoconstriction and proliferation in cardiovascular disease18,19.
ETB-dependent regulation of BP is demonstrated by the sustained hypertension caused by ETB receptor antagonism in mice20. The importance of receptor distribution in this response is indicated by increased BP following deletion of ETB receptors in the renal collecting duct21 but not after deletion of EC ETB22. The influence of VSMC ETB on BP has not been established but, given their potential to mediate vasoconstriction, deletion or antagonism of VSMC ETB would be predicted to reduce BP.
Despite the influence of ET-1 in vascular remodelling23, the role of ETB is less clear. ETB activation in EC (NO release) and kidney (reduced BP) would be predicted to inhibit arterial remodelling, thus favouring selective ETA antagonism for reducing neointimal proliferation9. Certainly, global deletion of ETB receptors increases vascular lesion size.10,24. However, selective ECETB deletion did not influence lesion formation, suggesting the protective role was mediated by ETB receptors in other tissues9. If ETB receptors in VSMC contribute to lesion formation, mixed ETA/B antagonists might have advantages over ETA selective compounds, although recent investigations9,10,24 favour the latter.
We generated novel smooth muscle ETB receptor knockout (SMETB KO) mice to address the hypothesis that loss of these receptors would impair arterial contraction, lower BP and reduce neointimal lesion formation in response to vascular injury.
Methods
Mice with VSMC-selective ETB receptor deletion were generated by crossing homozygous floxed ETB mice with SM22-Cre transgenic mice, which express cre-recombinase in the heart and smooth muscle, (then backcrossed to a C57Bl/6J background for 4-6 generations), as described for ECETB KO22. Controls were Cre-negative littermates (ETBf/f). Genotyping was performed using ear clips22,25. Wild Type C57Bl/6J mice were from Charles River (U.K.). Mice were housed according to United Kingdom Home Office recommendations (22°C; 12-hour light/dark cycles) with free access to water and chow. Procedures were performed under the provisions of the Animals Scientific Procedures Act (1986) and approved by the local ethics committee.
Selective SMETB deletion was demonstrated in organs and in isolated aortic smooth muscle cells using PCR, autoradiography14,26, immunohistochemistry27, and functional (myographic) investigation of isolated trachea, arteries and veins28,29.
The impact of SMETB KO on BP was assessed using radiotelemetry22 in conscious, unrestrained male SMETB KO mice and age-matched controls (n=8/ group), fed on chow (7 days), high (7.6%) salt diet (7 days), then high salt plus ETB antagonist (SB192621; 30/mg/kg/day in drinking water, 7 days). ET-1 concentrations in plasma from wild type C57Bl/6J, controls and SMETB KO were measured after exposure to chow or to high salt diet plus ETB antagonist, by enzyme-linked immunosorbent assay (Endothelin-1 Quantikine ELISA kit; R&D Systems, Oxford, UK).
Intraluminal (left) or non-denuding (right) femoral artery injury were achieved by insertion of an angioplasty guidewire or ligation, respectively, as described9. After 28 days, arteries were retrieved (following perfusion fixation) and analysed using optical projection tomography (OPT), histology and immunohistochemistry9,30.
Statistics
Results are mean±SEM, for n mice. Group sizes were chosen to detect 5%, 20% and 20% differences in BP (n=7), lesion size (n=7), and maximum responses to vasoactive agents (n=6) with >90% power. Investigations were performed by operators blinded to treatment. Components of lesions were expressed as a percentage of the neointimal area. Analyses were performed with GraphPad Prism using Student’s t-test, one-way or two-way analysis of variance with a Tukey post hoc test, as indicated. Significance was assumed for P<0.05.
Detailed methods are in the online supplement.
Results
Identification of SMETB KO
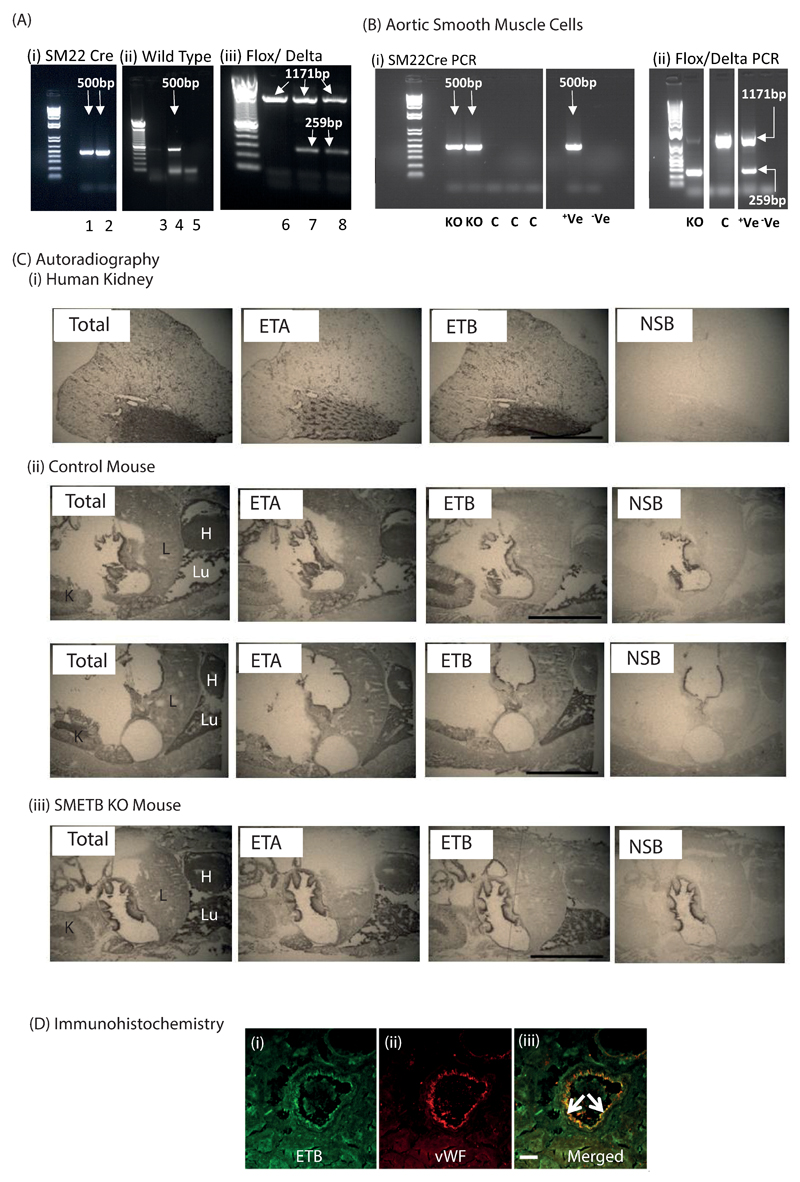
Genotyping for SM22cre, wild type (WT) and delta band alleles (Figure 1A) identified SMETB KO (positive for SM22cre, floxed and delta band, negative for WT allele) and controls (SM ETBf/f cre-negative littermates; negative for WT allele, positive for floxed allele and negative for SM22 cre and delta band). SMC isolated from the aorta of SMETB KO mice expressed the cre-, delta and flox bands, whereas controls did not express the cre and the delta bands (Figure 1B).
Figure 1. Selective ETB receptor deletion from smooth muscle.
(A) Mice were genotyped for (i) SM22Cre (band at 500bp), (ii) Wild type (band a 500bp) and (iii) Flox (band at 1171bp)/Delta (band at 259bp) alleles in ear clip DNA. (i) Samples 1 and 2 are cre-positive, (ii) sample 4 is positive for the wild type allele; samples 3 and 5 are not, (iii) samples 7 and 8 are positive for both the flox and the delta band; sample 6 has only the flox band. (B) PCR for cre and flox/delta bands in murine aortic smooth muscle cells isolated from SMETBKO and control (C) mice. Control mice lacked cre and delta alleles whereas SMETBKO expressed all three. +Ve – positive control; -Ve – negative control. Standard DNA ladders have band sizes 1500-100bp. (C) Autoradiography showing maintained ETB ligand binding in SMETB KO lung and kidney (representative of n=3 mice/genotype). H, heart; K, kidney; Li, liver; Lu, lung; NSB, non-specific binding. (D) Confocal images of a coronary artery from an SMETB KO mouse stained for (i) ETB receptor (green) or (ii) the endothelial cell marker von Willebrand factor (vWF; red). Merged images (iii) show clear co-localisation of ETB with the endothelium (arrows). There is no ETB staining in medial smooth muscle. Scale bar = 50 µm.
Autoradiography (Figure 1C) identified ETB receptors in the gut lining, lung and kidney. This signal was not diminished after SMETB deletion. ETB expression (real time PCR) was not altered in the colon, heart or gastrocnemius muscle of SMETB KO mice (Supplementary Figure S1). Confocal imaging of immunofluorescence (Figure 1D) clearly showed ETB receptors localising to the endothelium (von Willebrand factor (vWF) positive) in SMETB KO coronary artery. ETB staining in medial SM remained at background levels. This confirms maintained ETB receptor expression in the endothelium of SMETB KO mice.
Functional confirmation of SMETB KO
SMETB KO mice were healthy with normal body and organ weights (Supplementary Table S1).
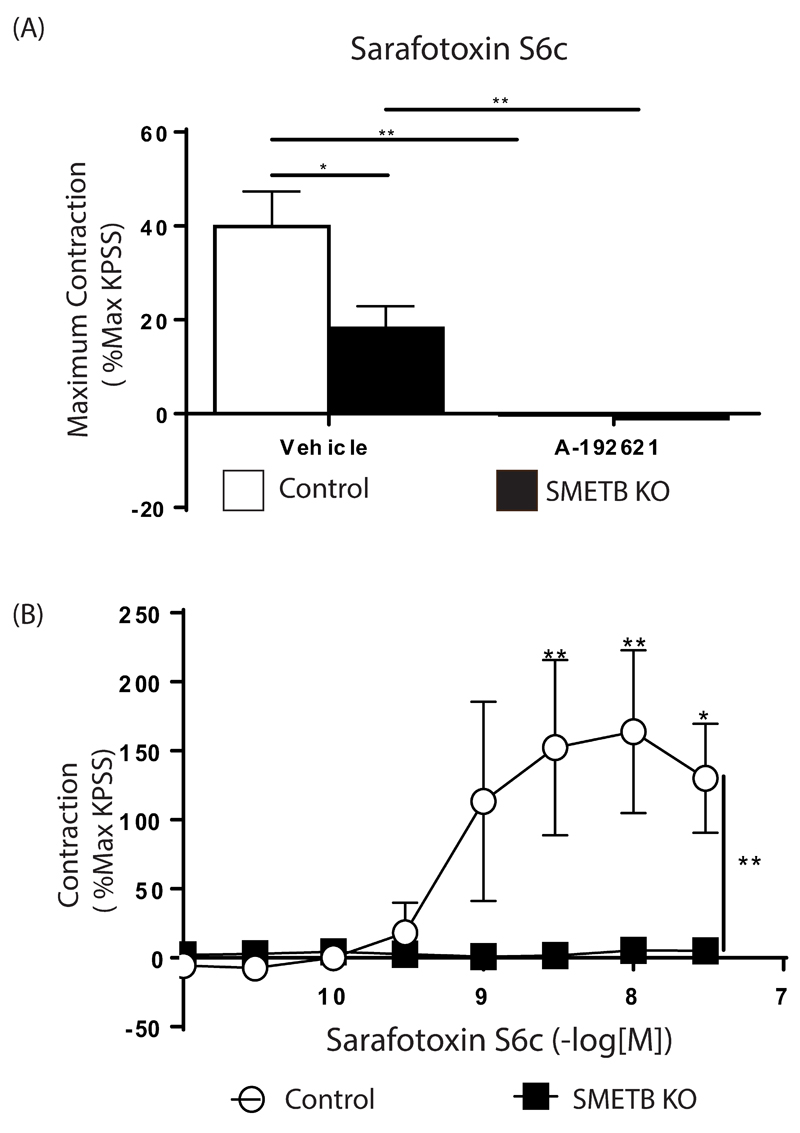
Sarafotoxin S6c (S6c)-mediated contraction in tracheas (which express ETB receptors on SM)22 from controls was abolished by incubation with the selective ETB antagonist A192621 (Figure 2A)22. In SMETB KO mice S6c-mediated contraction was reduced (~30%), but not abolished. The residual contraction was blocked by ETB antagonism. S6c-mediated contraction of mesenteric veins was abolished by selective deletion of SMETB (Figure 2B).
Figure 2. Functional consequences of selective ETB deletion from smooth muscle (SM).
(A) Sarafotoxin s6C (S6c)-induced contraction of isolated trachea was abolished by ETB receptor antagonism (A192621; 100nM) but only reduced by selective SMETB deletion (residual contraction was blocked by A192621). Columns are mean ± s.e.mean (n=4). *P<0.02; **P<0.005. (B) S6c-induced contraction in murine mesenteric veins was abolished by SMETB deletion. Symbols represent mean ± s.e.mean (n=4). *P<0.05, **P<0.01.
SMETB KO and BP
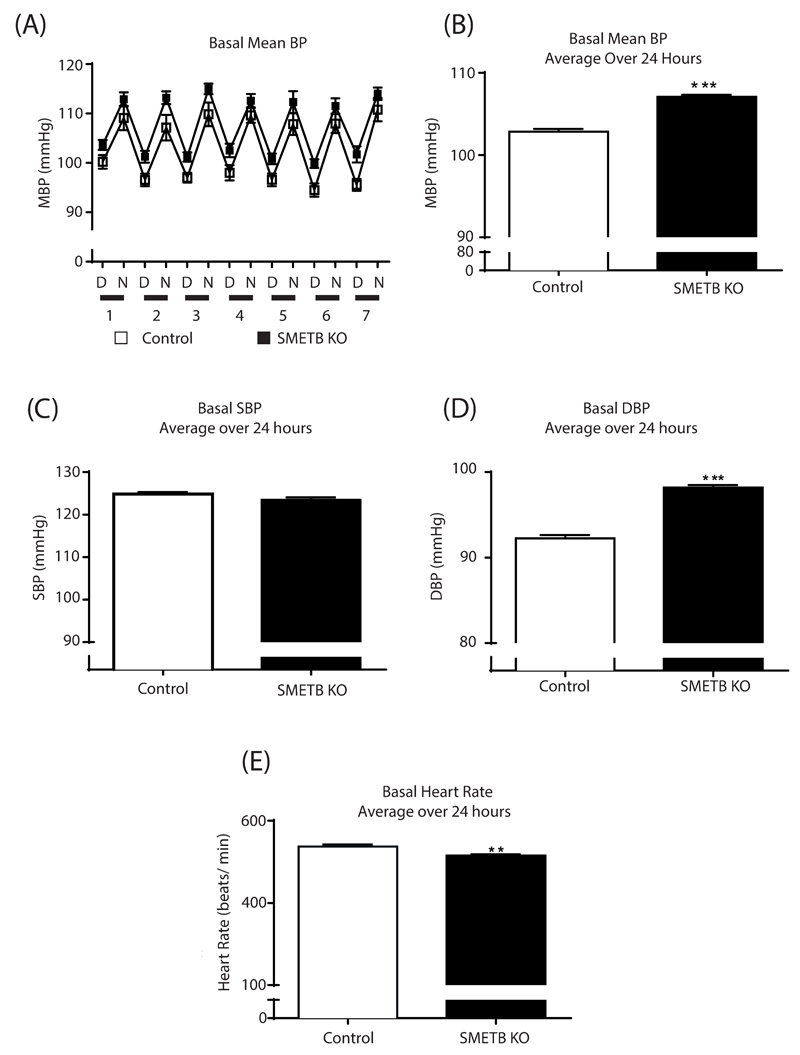
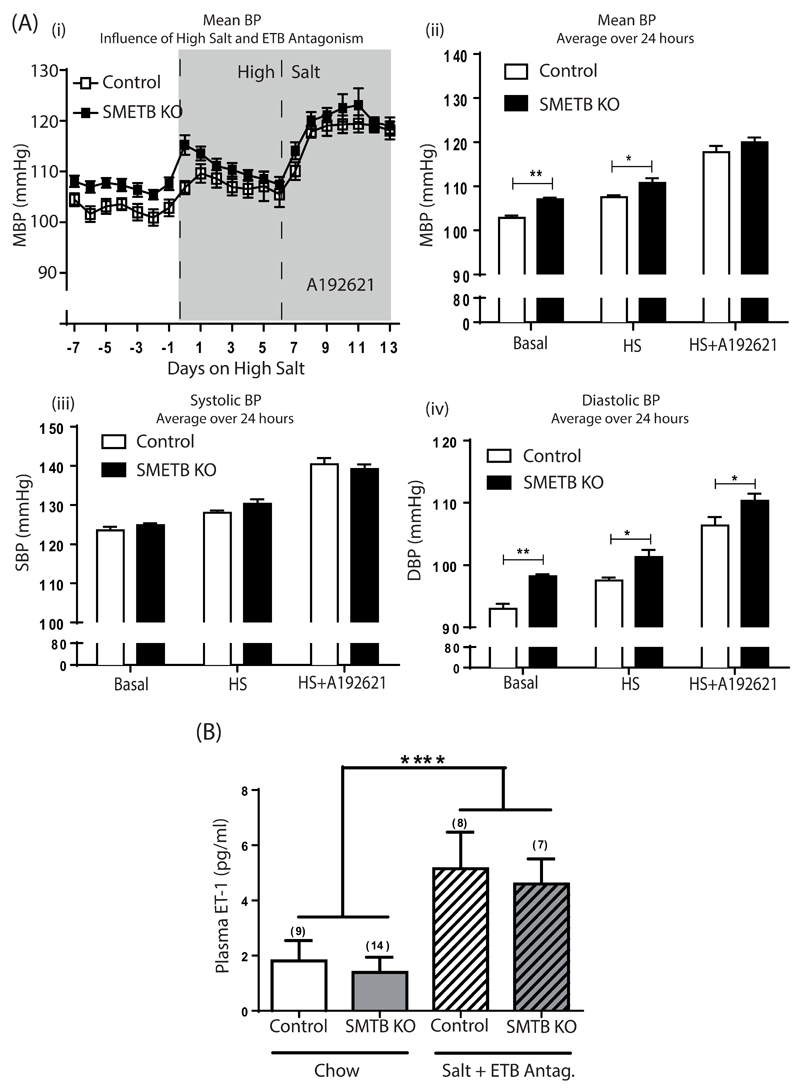
Control and SMETB KO mice demonstrated a clear diurnal rhythm in BP (Figure 3A). Mean 24 hour BP was higher in SMETB KO mice than in controls (107.1±0.3 vs. 102.8±0.5mmHg; n=7, P<0.0001; Figure 3B). Systolic BP was not different between groups (123.5±0.6 vs. 124.8±0.5mmHg; P=0.09; Figure 3C) but SMETB KO mice had an increased diastolic BP (98.2±0.3 vs 92.2±0.4mmHg; P<0.0001; Figure 3D). BP elevation occurred despite reduced heart rate (515±3 vs. 538±5 bpm; P=0.004; Figure 3E). High salt increased blood pressure in controls with a further increase induced by ETB antagonism (Figure 4A). These responses were similar in SMETB KO.
Figure 3. Selective deletion of ETB receptors from smooth muscle increases baseline blood pressure (BP).
(A) BP, assessed in conscious, unrestrained male SMETB KO mice and controls (n=8/ group) using radiotelemetry, demonstrated a clear diurnal rhythm. Mean blood pressure (MBP) in SMETB KO (red) mice was consistently higher than controls (blue). (B) Data averaged over 24 hours confirmed elevated MBP in SMETB KO, with no difference in (C) systolic blood pressure (SBP) but (D) elevated diastolic blood pressure (DBP). (E) Increased MBP was accompanied by reduced heart rate. D, day; N, night. Data are mean±s.e.mean (n=8/ group). **P<0.005, ***P<0.0001.
Figure 4. Selective deletion of ETB receptors from smooth muscle does not alter blood pressure responses.
(A) BP, assessed in conscious, unrestrained male SMETB KO mice and controls (n=8/ group) using radiotelemetry (i) was elevated by high salt diet (7 days) and by ETB antagonism (A192621; 30/mg/kg/day; 7 days) in both groups. (ii) Comparison of BP (averaged over 24 hours) demonstrates the elevation in mean blood pressure (MBP) in response to high salt diet and high salt diet plus A192621. (iii) There was no difference in systolic blood pressure (SBP) in control compared with SMETB KO mice but (iv) diastolic blood pressure (DBP) was higher in SMETB KO for all treatment groups. (B) Plasma ET-1 concentrations were similar in SMETB KO and controls and consistent with wild type C57Bl/6J mice (1.14±0.08pg/ml; n=6). ET-1 concentrations were elevated in control and SMETB KO mice after exposure to a high salt diet plus A192621. Data (mean ± s.e.mean) were analysed using 2 way ANOVA with Tukey or Bonferroni post-hoc test, as appropriate. (A) *P<0.05, **P<0.01 compared with controls. (B) ****P<0.00001 (effect of diet).
SMETB KO and circulating ET-1
Plasma ET-1 concentrations were similar in SMETB KO and control mice (Figure 4B), and consistent with levels in wild type C57Bl/6J (1.14±0.08, n=6). The combination of high salt diet and ETB antagonism increased plasma ET-1 to a similar extent in control type and SMETB KO mice (Figure 4C).
SMETB KO and neointimal remodelling
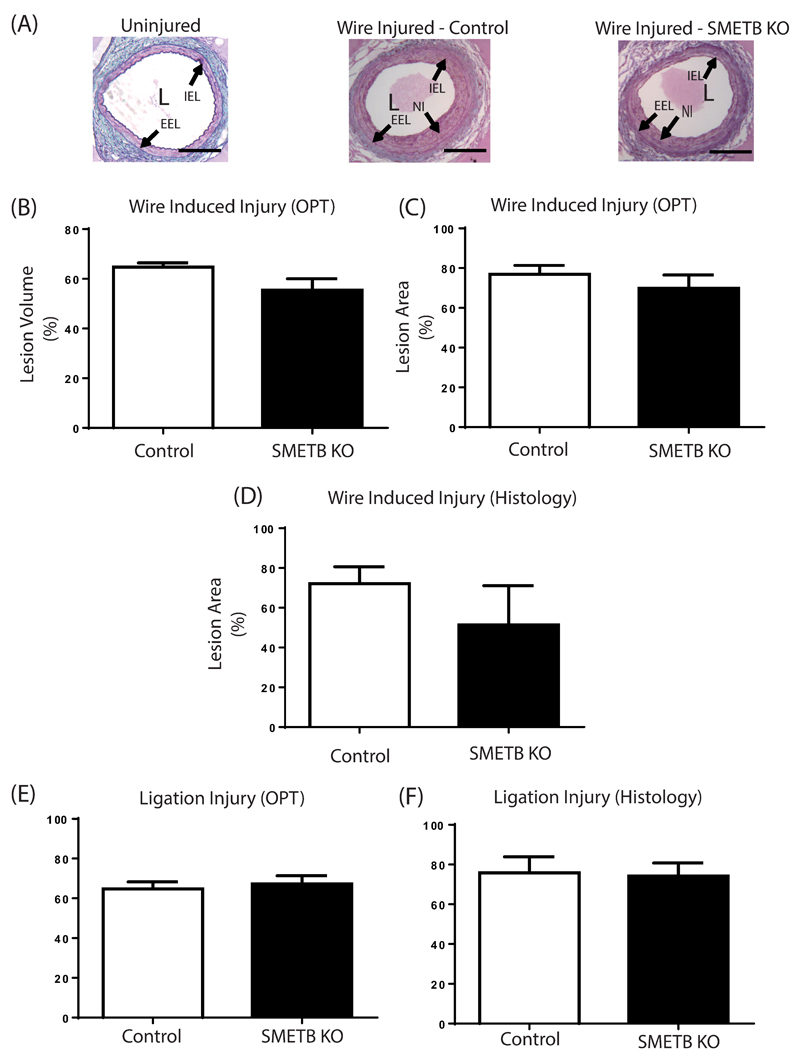
Wire injury of the left femoral artery generated neointimal lesions (Figure 5A)9. OPT demonstrated that SMETB KO altered neither the lesion volume (Figure 5B) nor cross-sectional narrowing (Figure 5C). Histological analysis showed a trend towards reduced cross-sectional narrowing in SMETB KO (Figure 5D). Ligation of the right femoral artery generated lesions9 with similar volume (Figure 5E) and maximal cross sectional area (Figure 5F) in SMETB KO mice and control mice.
Figure 5. Selective smooth muscle ETB deletion does not alter neointimal lesion formation.
(A) Wire injury-induced lesion formation in femoral arteries from control and SMETB KO mice. Neointimal lesion volume (B) and maximal cross-sectional area (C) were similar in control and SMETB KO mice when measured by optical projection tomography. Similar results were obtained when maximal cross-sectional area was measured histologically (D). Volume (E) and maximal cross-sectional area (F) of lesions induced by ligation were similar in control and SMETB KO mice (optical projection tomography). Data are mean ±s.e.mean; n=7.
Immunohistochemistry (Supplementary Figure S2) showed that SMETB KO did not differ from controls in the amount of macrophage (Mac-2) (SMETB KO 2.7±0.9% vs. Control 2.6±0.7 % lesion area), α-smooth muscle actin (SM ETB KO 14.8±4.1% vs. Control 19.9±3.8% lesion area), or collagen (SM ETB KO 9.7±3.1% vs. Control 14.9±3.2% lesion area) staining in the neointimal lesions.
SMETB KO and vascular reactivity
In wild type C57Bl/6J mice, EC removal from aortic rings abolished acetylcholine (ACh)-mediated relaxation and enhanced the contractile response to phenylephrine (PE) but not to ET-1. EC removal from femoral arteries also abolished ACh-mediated relaxation, but had no effect on PE or ET-1 (Supplementary Figure S3; Supplementary Table S2). SMETB KO had no effect on contractile responses to PE or ET-1, or ACh-mediated relaxation in femoral arteries (Supplementary Figure S4; Supplementary Table S3).
Induction of ETB-mediated contraction in isolated mesenteric arteries
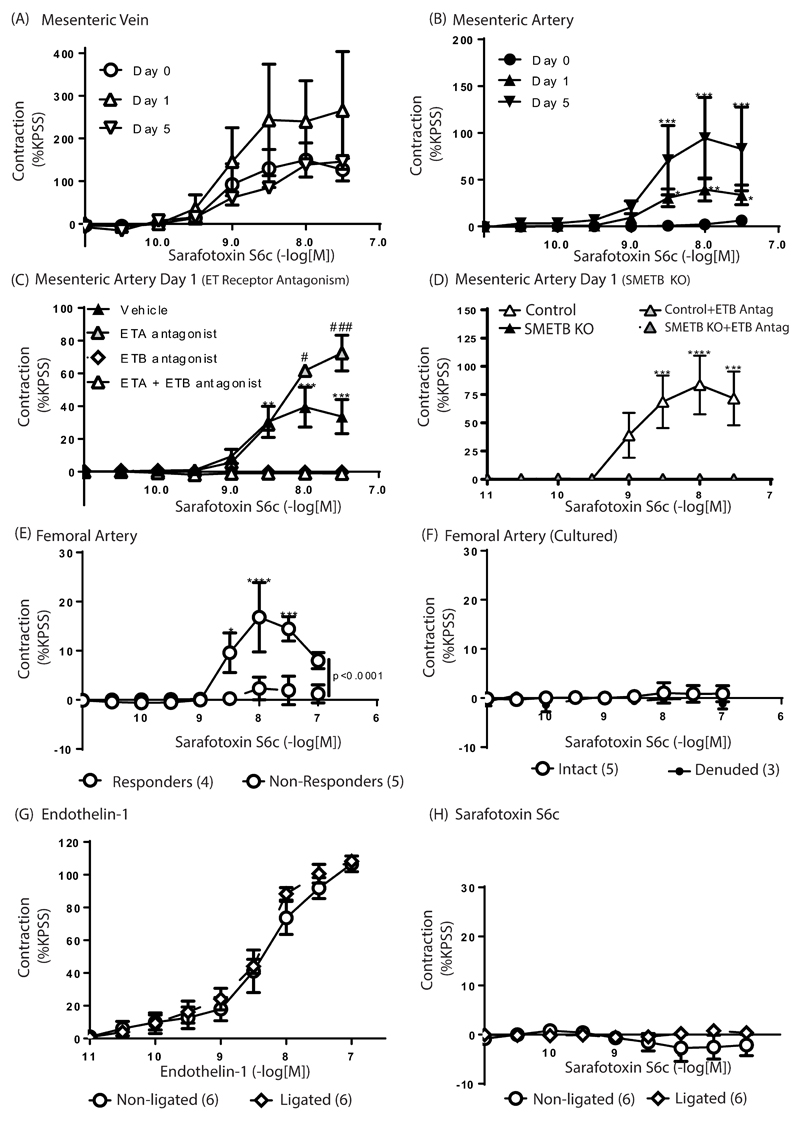
ET-1-mediated contraction in mesenteric arteries from wild type C57Bl/6J mice was shifted to the right by mixed ETA/B, or selective ETA, antagonism, but not by ETB selective antagonism (Supplementary Figure S5; Supplementary Table S4). Unlike mesenteric veins (Figure 6A), mesenteric arteries freshly isolated from wild type C57Bl/6J mice did not contract in response to S6c (Figure 6B).
Figure 6. Impact of SM ETB receptors on vascular function.
(A) Sarafotoxin S6c (S6c)-induced contraction in mesenteric veins (n=6) was not increased by incubation for 1 (n=3) or 5 (n=1) days in culture. (B) Freshly isolated mesenteric arteries (n=6) did not respond to S6c but contractions were induced by incubation in culture medium for 1 (n=7) or 5 (n=3) *P<0.05, **P<0.01, ***P<0.005 compared with Day 0. (C) S6c-mediated contraction of mesenteric arteries after 24 in culture (n=7) was abolished by ETB selective (A192621; 100nM; n=3) or mixed ETA/B (BQ-123 +A192621; n=3) antagonism, but not by ETA receptor antagonism (BQ123; 100nM; n=3); **P<0.01, ***P<0.005 compared with ETB or ETA/B antagonism; #P<0.05, ###P<0.005 compared with vehicle. (D) In contrast to controls (n=4), S6c-mediated, A192621 (100nM)-sensitive contraction was not induced in mesenteric arteries from SMETB KO mice (n=4) by incubation in culture medium (24 h); ***P<0.005, ****P<0.001 compared with antagonists. (E) Contractile responses to S6c were unreliable in femoral arteries – some failed to contract whereas others produced small contractions. *P<0.05, ***P<0.005, ****P<0.001 compared with non-responders. (F) Incubation in culture did not induce S6c-mediated contraction in these arteries. Femoral arteries after ligation (28 days) contracted in response to endothelin-1 (G) but not to S6c (H). Data are mean±s.e.mean, n=3-6.
Incubation in culture medium (≤5 days) can induce ETB-mediated contraction in rat arteries29. Incubation of C57Bl/6J mesenteric veins in culture medium had no effect on S6c-mediated contraction (Figure 6A). In mesenteric arteries, incubation in culture medium selectively increased the contractile response to ET-1 (Supplementary Table S5). Strikingly, S6c-mediated contraction was induced in isolated mesenteric arteries after incubation in culture medium (Figure 6B; Supplementary Table S5), a response abolished by selective ETB, or mixed ETA/B, antagonism, but not by selective ETA antagonism (Figure 6C; Supplementary Table S6). Incubation of mesenteric arteries from SMETB KO mice in culture medium did not induce S6c-mediated contraction (Figure 6D).
No induction of ETB-mediated contraction in femoral arteries
S6c-mediated contraction was variable in femoral arteries from wild type C57Bl/6J mice: some contracted but others did not (Figure 6E). Neither incubation of femoral arteries in culture medium (24 hours; Figure 6F) nor lesion formation induced S6c-mediated contraction; femoral arteries isolated 28 days following ligation contracted in response to ET-1 (Figure 6G) but not to S6c (Figure 6H). Responses to ACh, sodium nitroprusside (SNP) and PE were unaltered by lesion formation (Supplementary Figure S6).
Discussion
Tissue-specific knockout mice were generated to address the hypothesis that selective deletion of ETB receptors from VSMC would impair arterial contraction, lower BP and reduce neointimal lesion size. SMETB KO attenuated S6c-mediated vascular and tracheal contraction, without altering other functional responses, but produced a modest (~4mmHg) increase in BP. ETB-mediated contraction was not induced in femoral arteries following ligation, while injury-induced intimal lesion formation was unaffected by SMETB KO. Key findings are summarised (Supplementary Figure S7) and compared with the ECETB KO (Supplementary Table S7).
SMETB KO was based on our generation of ECETB KO22, crossing mice expressing Cre-recombinase controlled by the SM-specific SM22 promoter25 with those bearing a floxed ETB gene22. This strategy was used to produce mice with SM-selective ETA deletion4, and renal collecting duct-selective ETB deletion21. It has also been used within our group to produce mice with SM-selective deletion of glucocorticoid receptor31 or 11β-hydroxysteroid dehydrogenase 132 (with LacZ staining in Rosa26 reporter mice showing SM22-cre expression in the blood vessels and heart but not in the brain, kidney or adrenal gland). As with ECETB KO22, SMETB KO mice were healthy. This contrasts with global ETB deletion, which causes coat spotting and death from megacolon33, requiring transgenic ETB “rescue” in the enteric nervous system34. Autoradiographic detection of ETB receptors in lungs of SMETB KO mice indicates maintained expression in EC (which was lost in ECETB KO)14. This was supported by co-localisation of immunoreactivity for ETB with an EC marker (vWF) in coronary arteries; absence of medial ETB staining was consistent with deletion from SMCs. PCR confirmed that ETB had been deleted from aortic smooth muscle but not from heart, colon or skeletal muscle (although direct evidence of ETB deletion from tracheal, mesenteric vein, mesenteric or femoral artery smooth muscle was not obtained using this technique). Functional investigations confirmed that SMETB-dependent responses were lost in the knockout, with the abolition of S6c-mediated contraction in mesenteric veins. Furthermore, induction of S6c-mediated contraction in mesenteric arteries incubated in culture medium (as in rat arteries35), was abolished by SMETB KO (although these functional changes do not necessarily confirm selective SMETB deletion). The failure to abolish S6c-induced contraction in trachea was unexpected and suggests either incomplete penetrance of SM22cre-mediated recombination or a role for ETB receptors in other cells (e.g. epithelium) in mediating tracheal contraction. Detection of the delta band in some ear clip samples may suggest deletion of the floxed gene in germ cells which is a possible limitation with these mice. However, our F+/Cre0 x F+/Cre0 crosses did not produce piebald mice (which inevitably would occur if germ line recombination takes place). Therefore, the delta band during genotyping can only be explained by the presence of SMC in the ear clip preparations.
Selective deletion of ETB from EC increased plasma ET-122 due to impaired clearance14. In contrast, SMETB KO did not alter circulating ET-1, consistent with the proposal that ECETB predominantly mediate ET-1 clearance.
Transgenic and pharmacological approaches suggest ETB receptors regulate BP. Selective ETB receptor antagonism20, global ETB deletion10 and selective ETB deletion from the collecting duct21 all increased (~10-13mmHg) BP. Furthermore, ETB receptors in peripheral ganglia can influence BP36 suggesting that sympathetic activation accounts for ETB-induced hypertension37. In contrast, BP was not elevated by ECETB KO22. The small (~4mmHg) increase in BP, which persisted in SMETB KO mice despite reduced heart rate, suggests that loss of SMETB contributes to the increased BP induced by systemic ETB antagonism20 or global ETB deletion10. However, it requires rejection of our hypothesis that ETB-mediated vascular contraction contributes to BP elevation. Indeed, our data support a role for extra-vascular ETB (e.g.in the kidney or peripheral ganglia) in regulating BP. This is supported by the demonstration that, as in ECETB KO22, salt- and ETB antagonist-induced elevations of BP are unaltered by SMETB KO. The mechanism underlying increased BP following SMETB KO is not apparent but is unlikely to be a consequence of cre over-expression in SM as this did not alter baseline BP in SMETA KO mice4. Several possible explanations can be proposed. First, ETB in VSMC may contribute to the clearance of ET-1 from tissue where it is preferentially secreted by EC, and where it acts. Therefore, SMETB KO may cause ET-1 accumulation in the vascular wall, thus increasing ET-1-mediated vasoconstriction. Second, loss of SMETB may up-regulate ETA-mediated contraction. Third, SMETB in the kidney may influence sodium homeostasis. Since SM22 may be expressed in perivascular fat precursors36, loss of ETB from perivascular fat may have caused developmental changes in vascular function that also contribute to elevated BP, but this has not been established. It is also not clear why basal DBP is selectively increased in the SMETB KO but this would be worthy of future investigation.
Increased BP in SMETB KO mice could not be attributed to vascular dysfunction as, with the exception of responses to S6c, we found no evidence of impaired arterial relaxation or contraction. Weak ETB-mediated contraction in arteries is consistent with studies in rats35. Preliminary investigations (unpublished data) indicated that sarafotoxin S6c-induced contraction of freshly-isolated murine arteries (femoral, mesenteric, carotid) was not increased by nitric oxide synthase inhibition or by removal of the endothelium. These results indicate that we are not missing an ETB-mediated contraction that has been obscured by ETB-mediated relaxation. Induction of ETB-mediated contraction following incubation has been attributed to transcriptional regulation and MEK-ERK1/2 signalling22,38. Abolition of this response in mesenteric arteries from SMETB KO mice indicated that they lack both functional arterial ETB receptors and the means to generate new receptors in this tissue.
ETB upregulation in SMC, mediating vasoconstriction and proliferation in cardiovascular disease,18,19 might explain studies reporting similar benefit from mixed ETA/B and selective ETA antagonism in reducing lesion formation23,39,40 (despite the protective roles of ETB in several tissues; e.g. EC, kidney). However, the effectiveness of mixed ETA/B and selective ETA antagonism is likely to depend on the balance of ETB receptor activity in EC and VSMC of an affected artery. Transient up-regulation of ETA and ETB receptors has been demonstrated in arterial lesions41. If these ETB receptors contribute to lesion formation, then ETB antagonism would be desirable. There was, however, no evidence of induced ETB-mediated contraction in mouse femoral arteries after ligation. Similar investigations could not be performed following wire injury as these vessels fail to contract ex vivo. It remains possible that ETB up-regulation occurs in other (e.g. carotid) arteries.
Neointimal lesion formation is increased in “rescued” global ETB knockout mice10 and in (spotted-lethal) rats with global deletion of ETB 24, consistent an anti-proliferative role for ETB receptors. This is supported by demonstrations that ETB receptor antagonism increases lesion size9,24, with the suggestion that this is due to impaired ETB–mediated release of NO from EC. Indeed, increased lesion formation in mice with global ETB deletion was partly attributed to impaired EC-derived NO release9. In contrast, selective ECETB deletion inhibited ETB-mediated relaxation22 but had no effect on arterial lesion formation9. These results suggest, therefore, that the protective role of ETB receptors is played by non-EC ETB receptors. The demonstration here that deletion of ETB from the SMC does not alter lesion size indicates that, as with the receptors in EC9, ETB in SMC do not influence neointimal remodelling. This implicates non vascular ETB receptors, for example in monocyte-derived macrophages, in the regulation of neointimal proliferation and atherosclerosis42.
In conclusion, we have demonstrated that selective ETB receptors in SMC may contribute modestly to regulation of BP but have little influence on vascular contraction or neointimal proliferation. These data suggest that any detrimental role of SMETB is minor (at least during normal physiology) and, therefore, that selective ETA receptor antagonists (which preserve protective EC/renal ETB signalling) should be preferred to mixed ETA/B antagonists for treatment of vascular disease.
Perspectives
Generation of mice with selective deletion of ETB from SMC indicate that these receptors contribute to the increased BP induced by ETB receptor antagonism, but do not regulate arterial function or the fibro-proliferative response to acute arterial injury. It would be interesting to determine whether ETB in SMCs influence other cardiovascular diseases (e.g. diabetic complications). Whether the data generated in these animals are replicated in mice with cardiovascular disease (e.g. atherosclerosis), or in man, remains to be established. However, these results support the proposal that selective ETA receptor antagonists may have advantages over mixed ETA/B antagonists for combatting elevated BP or restenosis following revascularisation.
Supplementary Material
Novelty and Significance.
(1) What is new?
This study describes newly generated mice with selective ETB receptor deletion from smooth muscle. This was used to clarify the influence of smooth muscle ETB receptors on: (i) blood pressure, (ii) arterial and venous contraction, and (iii) arterial remodelling following injury.
(2) What is relevant?
Generation of the knockout was necessary as ETB receptors in vascular endothelial and smooth muscle cells cannot be distinguished pharmacologically. This work shows that ETB receptors in smooth muscle have little influence on arterial function or neointimal remodelling, but have a small suppressive effect on diastolic blood pressure. This is consistent with the proposal that selective ETA antagonism would be preferable to mixed ETA/ETB antagonism for inhibiting arterial remodelling.
Summary
Selective smooth muscle ETB deletion indicated that these receptors play a minor role in regulation of BP but do not affect vascular function or remodelling. This suggests that, beyond ECETB, ETB-dependent regulation of these processes is mediated by receptors in extravascular cells (e.g. renal collecting ducts).
Acknowledgements
A192621 was a gift from AbbVie, USA.
Sources of Funding: Funded by the British Heart Foundation (Project Grant PG/08/068/25461, PWFH, DJW; Intermediate Clinical Research Fellowship FS/13/30/29994, ND; Centre of Research Excellence Award) and the Wellcome Trust (107715/Z/15/Z, APD, REK).
Abbreviations
- BP
blood pressure
- ET-1
endothelin-1
- ETA
endothelin A receptor
- ETB
endothelin B receptor
- PSS
physiological salt solution
- PSS
physiological salt solution
- KPSS
high (125mM) potassium physiological salt solution
- NO
nitric oxide
- vWF
von Willebrand factor
- WT
wild type
Footnotes
Conflicts of Interest/ Disclosures:
DJW has provided advice to Abbott, AbbVie, AstraZeneca, Encysive, Pfizer, Retrophin and Roche in relation to clinical development of ET receptor antagonists. KMD received a Pfizer Young Investigator Award. ND has received research grants from Pfizer.
References
- 1.Kirkby NS, Hadoke PWF, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: great expectations or bleak house? Brit J Pharmacol. 2008;153:1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharm Rev. 2016;68:366–427. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanagisawa M, Kurihari H, Kimura S, Tomobe Y, Obayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;32:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 4.Donato AJ, Lesniewski LA, Stuart D, Walker AE, Henson G, Sorensen L, Li D, Kohan DE. Smooth muscle specific disruption of the endothelin-A receptor in mice reduces arterial pressure, and vascular reactivity and affects vascular development. Life Sciences. 2014;118:238–243. doi: 10.1016/j.lfs.2013.12.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komuro I, Kurihara H, Sugiyama T, Yoshizumi M, Takaku F, Yazaki Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS Lett. 1988;238:249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Chu Y, Fin GD, Engelhardt JF, Heistad DD, Chen AF. Endothelin-1 stimulates arterial VCAM-1 expression via NADPH oxidase-derived superoxide in mineralocorticoid hypertension. Hypertension. 2003;42:997–1003. doi: 10.1161/01.HYP.0000095980.43859.59. [DOI] [PubMed] [Google Scholar]
- 7.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TM, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodelling and endothelial dysfunction. Circulation. 2004;110:2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 8.Helset E, Sildnes T, Seljelid R, Konopski ZS. Endothelin-1 stimulates human monocytes in vitro to release TNF-alpha, IL-1beta and IL-6. Mediators Inflamm. 1993;2:417–422. doi: 10.1155/S0962935193000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkby NS, Duthie KM, Miller E, Kotelevtsev YV, Bagnall AJ, Webb DJ, Hadoke PWF. Non-endothelial cell endothelin-B receptors limit neointima formation following vascular injury. Cardiovasc Res. 2012;95:19–28. doi: 10.1093/cvr/cvs137. [DOI] [PubMed] [Google Scholar]
- 10.Murakoshi N, Miyauchi T, Kakinuma Y, Ohuchi T, Goto K, Yanagisawa M, Yamaguchi I. Vascular endothelin-B receptor system in vivo plays a favorable inhibitory role in vascular remodelling after injury revealed by endothelin-B receptor knockout mice. Circulation. 2002;106:1991–1998. doi: 10.1161/01.cir.0000032004.56585.2a. [DOI] [PubMed] [Google Scholar]
- 11.de Nucci G, Thomas R, D’Orleans-Juste P, Antunes E, Walder C, Warner TD, Vane JR. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta Km, Marumo F. Endothelin receptor B mediates synthesis of nitric oxide by cultured bovine aortic endothelial cells. J Clin Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 14.Kelland NF, Kuc RE, McLean DL, Azfer A, Bagnall AJ, Gray GA, Gulliver-Sloan FH, Maguire JJ, Davenport AP, Kotelevtsev YV, Webb DJ. Endothelial cell specific ETB receptor knockout: autoradiographic and histological characterisation and crucial role in the clearance of endothelin-1. Can J Physiol Pharmacol. 2010;88:644–651. doi: 10.1139/Y10-041. [DOI] [PubMed] [Google Scholar]
- 15.Goligorsky MS, Budzikowski AS, Tsukahara H, Noiri E. Co-operation between endothelin and nitric oxide in promoting endothelial cell migration and angiogenesis. Clin Exp Pharmacol Physiol. 1999;26:269–271. doi: 10.1046/j.1440-1681.1999.03029.x. [DOI] [PubMed] [Google Scholar]
- 16.McCulloch KM, Docherty CC, Morecroft I, MacLean MR. Endothelin B receptor-mediated contraction in human pulmonary resistance arteries. Br J Pharmacol. 1996;119:1125–1130. doi: 10.1111/j.1476-5381.1996.tb16013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mickley EJ, Gray GA, Webb DJ. Br J Pharmacol. 1997;120:1376–1382. doi: 10.1038/sj.bjp.0701036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janakidevi K, Fisher MA, Del Vecchio PJ, Tiruppathi C, Figge J, Malik AB. Endothelin-1 stimulates DNA synthesis and proliferation of pulmonary artery smooth muscle cells. Am J Physiol. 1992;263:C1295–C1301. doi: 10.1152/ajpcell.1992.263.6.C1295. [DOI] [PubMed] [Google Scholar]
- 19.Dimitrijevic I, Edvinsson ML, Chen Q, Malmsjo M, Kimblad PO, Edvinsson L. Increased expression of vascular endothelin type B and angiotensin type 1 receptors in patients with ischemic heart disease. BMC Cardiovasc Disord. 2009;9:40–51. doi: 10.1186/1471-2261-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fryer RM, Rakestraw PA, Banfor PN, Cox BF, Opgenorth TJ, Reinhart GA. Blood pressure regulation by ETA and ETB receptors in conscious, telemetry-instrumented mice and role of ETA in hypertension produced by selective ETB blockade. Am J Physiol Heart Circ Physiol. 2006;290(6):H2554–2559. doi: 10.1152/ajpheart.01221.2005. [DOI] [PubMed] [Google Scholar]
- 21.Ge Y, Bagnall A, Stricklett P, Strait K, Webb D, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–F1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 22.Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, Webb DJ, Kotelevtsev YV. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48:286–293. doi: 10.1161/01.HYP.0000229907.58470.4c. [DOI] [PubMed] [Google Scholar]
- 23.Douglas SA, Louden C, Vickery-Clark LM, Storer BL, Hart T, Feuerstein GZ, Elliott JD, Ohlstein EH. A role for endogenous endothelin-1 in neointima formation after rat carotid artery balloon angioplasty: protective effects of the non-peptide endothelin receptor antagonist SB209670. Circ Res. 1994;75:190–197. doi: 10.1161/01.res.75.1.190. [DOI] [PubMed] [Google Scholar]
- 24.Kitada K, Yui N, Matsumoto C, Mori T, Ohkita M, Matsumura Y. Inhibition of endothelin ETB receptor system aggravates neointimal hyperplasia after balloon injury of rat carotid artery. J Pharmacol Exp Ther. 2009;331:998–1004. doi: 10.1124/jpet.109.157065. [DOI] [PubMed] [Google Scholar]
- 25.Holtwick R, Gotthardt M, Skryabin B, Steinmetz M, Potthast R, Zetsche B, Hammer RE, Herz J, Kuhn M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc Natl Acad Sci USA. 2002;99:7142–147. doi: 10.1073/pnas.102650499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davenport AP, Kuc RJ. Radioligand binding assays and quantitative autoradiography of endothelin receptors. Methods Mol Cell Biol. 2002;206:45–70. doi: 10.1385/1-59259-289-9:045. [DOI] [PubMed] [Google Scholar]
- 27.Ling L, Kuc RE, Maguire JJ, Davie NJ, Webb DJ, Gibbs P, Alexander GJM, Davenport AP. Comparison of endothelin receptors in normal versus cirrhotic human liver and in the liver from endothelial cell-specific ETB knockout mice. Life Sciences. 2012;91:716–722. doi: 10.1016/j.lfs.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Opgenorth TJ, Adler AL, Calzadilla SV, Chiou WJ, Dayton BD, Dixon DB, Gehrke LJ, Hernandez L, Magnusson SR, Marsh KC, Novosad EI, et al. Pharmacological characterisation of A-127722: an orally active and highly potent ET(A)-selective receptor antagonist. J Pharmacol Exp Ther. 1996;276:473–481. [PubMed] [Google Scholar]
- 29.Adner M, Uddmann E, Cardell LO, Edvinsson L. Regional variation in appearance of vascular contractile endothelin-B receptors following organ culture. Cardiovascular Res. 1998;37:254–262. doi: 10.1016/s0008-6363(97)00206-x. [DOI] [PubMed] [Google Scholar]
- 30.Kirkby NS, Low L, Riemersma RA, Seckl JR, Walker BR, Webb DJ, Hadoke PWF. Quantitative 3-dimensional imaging of murine neointimal and atherosclerotic lesions. PLoS ONE. 2011;6:e16906. doi: 10.1371/journal.pone.0016906. 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rog-Zielinska EA, Thomson A, Kenyon CJ, Brownstein DG, Moran C, Szumska D, Michailidou Z, Richardson J, Watt A, Morrison H, Forrester L, et al. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet. 2013;22:3269–3282. doi: 10.1093/hmg/ddt182. [DOI] [PubMed] [Google Scholar]
- 32.White CI, Jansen MA, McGregor K, Mylonas KJ, Richardson RV, Thomson A, Moran CM, Seckl JR, Walker BR, Chapman KE, Gray GA. Cardiomyocyte and vascular smooth muscle independent 11β-hydroxysteroid dehydrogenase 1 amplifies infarct expansion, hypertrophy and the development of heart failure following myocardial infarction in male mice. Endocrinol. 2015;157:346–357. doi: 10.1210/en.2015-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giad A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 34.Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa M. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest. 1998;102:1092–1101. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adner M, Geary GG, Edvinsson L. Appearance of contractile endothelin-B receptors in rat mesenteric arterial segments following organ culture. Acta Physiol Scand. 1998;163:121–129. doi: 10.1046/j.1365-201X.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- 36.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator–activated receptor-deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fink G, Li M, Lau Y, Osborn J, Watts S. Chronic activation of endothelin B receptors: new model of experimental hypertension. Hypertens. 2007;50:512–518. doi: 10.1161/HYPERTENSIONAHA.107.094821. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Li X-J, Zeng X, Shen D-Y, Liu C-Q, Zhang H-J, Xu C-B, Li X-Y. Activation of nuclear factor-B pathway is responsible for tumor necrosis factor-induced up-regulation of endothelin B2 receptor expression in vascular smooth muscle cells in vitro. Toxicol Lett. 2012;209:107–112. doi: 10.1016/j.toxlet.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Azuma H, Sato J, Masuda H, Goto M, Tamaoki S, Sugimoto A, Hamasaki H, Yamashita H. ATZ1993, an orally active and novel nonpeptide antagonist for endothelin receptors and inhibition of intimal hyperplasia after balloon denudation of the rabbit carotid artery. Jpn J Pharmacol. 1999;81:21–28. doi: 10.1254/jjp.81.21. [DOI] [PubMed] [Google Scholar]
- 40.Sanmartin M, Fernandez-Ortiz A, Fantidis P, Aragoncillo P, Fernandez-Durango R, Rollin R, Alfonso F, Hernandez R, Escaned J, Macaya C. Effects of bosentan on neointimal response following coronary angioplasty. Eur J Clin Invest. 2003;33:762–768. doi: 10.1046/j.1365-2362.2003.01217.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Douglas SA, Louden C, Vickery-Clark LM, Feuerstein GZ, Ohlstein EH. Expression of endothelin-1, endothelin-3, endothelin-converting enzyme-1, and endothelin-A and endothelin-B receptor mRNA after angioplasty-induced neointimal formation in the rat. Circ Res. 1996;78:322–328. doi: 10.1161/01.res.78.2.322. [DOI] [PubMed] [Google Scholar]
- 42.Haug C, Schmid-Kotsas A, Zorn U, Schuett S, Gross HJ, Gruenert A, Bachem MD. Endothelin-1 synthesis and endothelin B receptor expression in human coronary artery smooth muscle cells and monocyte-derived macrophages is up-regulated by low density lipoproteins. J Mol Cell Cardiol. 2001;33:1701–1712. doi: 10.1006/jmcc.2001.1421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.