Abstract
Background
Age-specific retention challenges make ART initiation in adolescents difficult, often requiring a lengthy preparation process. This needs to be balanced against the benefits of starting treatment quickly. The optimal time to initiation duration in adolescents is currently unknown.
Objective
To assess the effect of time to ART initiation on mortality and loss to follow-up (LTFU) among treatment eligible adolescents.
Methods
We conducted a retrospective cohort analysis among 1,499 ART eligible adolescents aged ≥10 to <19 years registered in a public sector HIV programme in Bulawayo, Zimbabwe between 2004 and 2011. Hazard ratios (HR) for mortality and LTFU were calculated for different time to ART durations using multivariate Cox regression models.
Results
Median follow-up duration was 1.6 years. Mortality HRs of patients who initiated at 0-≤7 days, >14 days to ≤1 month, >1-≤2 months, >2 months, and prior to initiation were 1.59, 1.19, 1.56, 1.08, and 0.94, respectively, compared to the reference group of 7-14 days. LTFU HRs were 1.02, 1.07, 0.85, 0.97, and 3.96, respectively. Among patients not on ART, 88% of deaths and 85% of LTFU occurred during the first three months after becoming ART eligible, but only 37% and 29% among adolescents on ART, respectively.
Conclusions
Neither mortality or LTFU was associated with varying time to ART. The initiation process can be tailored to the adolescents´ needs and individual life situations without risking to increase poor treatment outcomes. Early mortality was high despite rapid ART initiation, calling for earlier rather than faster initiation through HIV testing scale-up.
Keywords: ART initiation, adolescents, eligibility, mortality, loss to follow-up, Zimbabwe
Introduction
Antiretroviral therapy (ART) scale-up has reduced human immunodeficiency virus (HIV)-related deaths substantially.1 Adolescents remain the only age group where HIV-associated mortality is still rising, mainly due to delayed diagnosis and high attrition, and in particular around the time of ART initiation.2–7 Large cohorts of vertically infected infants born during the peak of the epidemic are now growing into adulthood without ever having been diagnosed or treated.8–10 This adolescent HIV epidemic of “slow progressors”11–13 has been unfolding largely unnoticed for many years.14–16 Only recently have adolescents been receiving more attention on the global HIV agenda.17–20
Currently, multiple preparatory counselling and education sessions stretching over several weeks are considered necessary prior to treatment start to ensure long-term retention and adherence.21–24 Adolescents living with HIV face particularly complex issues such as emerging sexuality, peer influence, stigma, delayed disclosure of diagnosis, and consent legislation, all of which complicates retention and adherence to ART.2,19,25–28 Addressing these issues takes time and can result in delaying ART initiation even if clinical treatment eligibility criteria are met.29 This needs to be balanced against the well-established life-saving effects of rapid treatment initiation.30 Also, a lengthy pre-initiation preparatory phase can have negative effects on access to and linkage of care.24,31–37
Existing research on ART initiation in adolescents revolves mostly around comparing clinical outcomes before and after ART initiation,6,38 or between adolescents and adults,3,5,7,39,40 or around determining the best clinical time point to start treatment.30,41 The period between becoming ART eligible and starting treatment, the so-called “third stage” of pre-ART care,42 is often overlooked,43 which is particularly concerning as guidelines now suggest treatment of all HIV-infected individuals regardless of age or immune status.44 For the ART initiation process in adolescents, it is currently not known how fast is “fast enough but not too fast” in order to keep both mortality and loss to follow-up (LTFU) before and after ART initiation at a minimum.20 A better understanding of the effect of varying time to ART initiation in long-term infected adolescents would help clinicians decide as to how much the initiation process can be adapted to individual patient needs without compromising clinical outcomes.
We investigated treatment initiation patterns and related patient outcomes, and in particular the effect of time to ART initiation on mortality and LTFU among treatment eligible adolescents aged ≥10 to <19 years registered in a public sector HIV care service in Bulawayo, Zimbabwe.
Methods
Setting
Bulawayo is the second biggest city in Zimbabwe, a country that has been experiencing an early-onset severe generalised HIV epidemic.45,46 HIV prevalence in Zimbabwe peaked in 1997, reaching 27% in the general population,47 and 15% and 8% among females and males aged 15-24 years, respectively.48 During the late 1990s, HIV-related mortality increased five-fold in Zimbabwe, peaking to 11 per 1,000 persons per year in 2002, has been declining gradually since then.45
Mpilo Central Hospital, the biggest public health facility in Bulawayo, opened an HIV clinic in 2004 in collaboration with the non-governmental organization Doctors Without Borders (Médecins Sans Frontières (MSF)), and was one of the first facilities nationwide to provide ART. Patients entered the HIV programme of the Mpilo ART clinic usually after testing positive at one of the outpatient or inpatient departments of the hospital, in the private sector, or at stand-alone voluntary counselling and testing facilities. ART was initiated by doctors with follow-up care being provided by nurses trained in HIV/AIDS management. ART eligibility criteria during the study period were a cluster of differentiation type 4 (CD4) cell count of ≤200 cells/µL, and/or WHO Stage 3 or 4 HIV disease.49,50 ART eligibility was routinely assessed at each patient visit. In Zimbabwe, as in many other SSA countries, initiation of ART in eligible patients is recommended following two to three visits for treatment preparedness counselling.39,51,52 Systematic tracing of defaulting ART patients, defined as having missed a scheduled appointment by more than two months, was done by community volunteers through home visits and telephone calls. Mpilo hospital remained the only provider of ART initiation services for adolescents in the wider Bulawayo area throughout the study period.
Study design and population
We conducted a cohort analysis using routinely collected data from the HIV programme at Mpilo Hospital in Bulawayo, Zimbabwe. All data collection had ended before this research idea was conceived. Records from all patients aged ≥10 to <19 years with confirmed ART eligibility between February 2004 and September 2011 was considered eligible for this analysis.
Data management and analysis
Clinical data were entered from paper-based hospital charts into an electronic database (FUCHIA, Epicentre, Paris), and updated after each patient visit as part of routine programme activities. Patient outcomes were obtained on an ongoing basis from defaulter tracing activities, community reporting, and death register reviews, and entered into the FUCHIA database.
Patient outcomes were defined as died, retained (being alive and in care with ≤3 months since last recorded visit), LTFU (outcome unknown with >3 months since last recorded visit; time of LTFU was set at 30 days after last recorded visit), and transferred out (TO). Time to ART initiation was defined as time elapsed between becoming ART eligible and initiating ART, and was grouped into five categories (0-7 days, >7-≤14 days, >14 days to 1 month, 1-2 months, and >2 months). Person-time accruing prior to initiation was categorized separately. Available covariates were sex, age (≥10-<15 or ≥15-<19 years), WHO staging (clinical stage 0-2 or 3-4), CD4 count (0-≤200 or >200 cells/µL) and calendar year of ART eligibility.
The database was censored at 24 months after becoming ART eligible. Patients who had their first visit <30 days prior to the end of the study period (1 September 2011) were excluded. ART initiation and incidence of death, LTFU, and TO by treatment status were calculated for different follow-up intervals. Cumulative hazards were plotted for mortality and LTFU to assess differences by time to ART initiation. Crude and adjusted rates and hazard ratios (HR) using Cox regression analysis, including 95% confidence intervals (95%CI) and p-values from likelihood ratio tests (LRT), were calculated. Departure from the proportional hazard assumption was evaluated graphically using logarithmic plots, as well as formally assessed by testing for changes in hazard ratios over time using LRT. Time-dependent exposure assignment based on the Mantel-Byar approach was used to account for immortal time bias.53 Sex, age, WHO staging, and calendar year of ART eligibility were considered a priori confounding factors and adjusted for in the multivariate models. CD4 count was not included due to the high proportion of missing values. Effect modification for calendar year was explored for both mortality and LTFU. Statistical analyses were conducted using the STATA v.14 software (StatCorp, College Station, Texas, USA).
Ethics
Data collection was covered by a memorandum of understanding between MSF and the Zimbabwean Ministry of Health. Permissions to use these data for analysis and publication were obtained from both entities. As only anonymized data were used, and no intervention or patient contact was made for research purposes, the issue of informed consent did not apply. The requirement for ethical approval was waived the Mpilo Hospital Institutional Review Board, Bulawayo, and by the National Medical Research Council of Zimbabwe, Harare.
Results
Of the 2,184 adolescents enrolled into the HIV programme, 1,506 (69%) met ART eligibility criteria during the study period. Of these, 20% (307/1,506) met CD4 as well as WHO criteria, 13% (195/1,506) met CD4 but not WHO criteria, 17% (256/1,506) met WHO but not CD4 criteria, and 50% (748/1,506) met WHO criteria with CD4 results missing. Due to missing CD4 results, ART eligibility could not be confirmed for 17% (364/2,184) of all enrolled adolescents who did not meet WHO criteria (see Table, Supplemental Digital Content 1, showing patient characteristics by ART eligibility status). Seven (0.5%) of the 1,506 ART eligible patients had to be excluded from analysis due to insufficient follow-up time or missing data (see Figure, Supplemental Digital Content 2, showing patient record selection for analysis). The remaining 1,499 patients provided a total of 3,097 person-years (py) of follow-up (median 1.6 years; IQR 0.7-3.2). Median time to ART initiation was 17 days (IQR 9-42). 10% (150/1,499) of patients did not start ART. Median age was 13.2 years (IQR 11.3-15.2), and 52% (773/1,499) of the adolescents were female. The majority (87%; 1,305/1,499) had WHO Stage 3 or 4 HIV disease. The median CD4 cell count was 145 cells/µL (IQR 44-267) for the 754 (50%) of patients with available test result (Table 1).
Table 1. Patient characteristics at baseline by time to ART initiation.
| ≤7d (N=319) |
>7-≤14d (N=291) |
>14d-≤1mo (N=324) |
>1mo-≤2mo (N=170) |
>2mo (N=245) |
Prior to initiation (N=150) |
Total (N=1,499) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | PY/100 | n | PY/100 | n | PY/100 | n | PY/100 | n | PY/100 | n | PY/100 | n | PY/100 | |
| Sex | ||||||||||||||
| male | 164 | 3.17 | 133 | 2.51 | 160 | 3.36 | 88 | 2.36 | 107 | 3.46 | 74 | 0.23 | 726 | 15.09 |
| female | 155 | 2.68 | 158 | 2.88 | 164 | 3.83 | 82 | 2.01 | 138 | 4.13 | 76 | 0.34 | 773 | 15.88 |
| Age (years) | ||||||||||||||
| ≥10-<15 | 227 | 4.72 | 188 | 4.00 | 234 | 5.58 | 126 | 3.63 | 190 | 6.19 | 111 | 0.42 | 1,076 | 24.53 |
| ≥15-<19 | 92 | 1.13 | 103 | 1.40 | 90 | 1.62 | 44 | 0.74 | 55 | 1.40 | 39 | 0.15 | 423 | 6.44 |
| Median (IQR) | 13.27 | 11.82-15.40 | 14.00 | 11.78-15.95 | 13.09 | 11.27-15.26 | 13.33 | 11.50-15.09 | 12.70 | 11.00-14.78 | 12.74 | 11.24-15.18 | 13.20 | 11.31-15.24 |
| WHO stage | ||||||||||||||
| ≤2 | 34 | 0.71 | 27 | 0.51 | 67 | 1.67 | 35 | 0.98 | 25 | 0.87 | 6 | 0.01 | 194 | 4.74 |
| >2 | 285 | 5.14 | 264 | 4.88 | 257 | 5.53 | 135 | 3.40 | 220 | 6.72 | 144 | 0.56 | 1,305 | 26.23 |
| CD4 count(cells/µL) | ||||||||||||||
| >200 | 50 | 0.89 | 48 | 0.89 | 41 | 0.89 | 20 | 0.62 | 66 | 2.66 | 30 | 0.22 | 255 | 6.16 |
| ≤200 | 88 | 1.63 | 99 | 2.12 | 148 | 3.78 | 81 | 2.27 | 64 | 2.22 | 19 | 0.02 | 499 | 12.03 |
| missing | 181 | 3.33 | 144 | 2.39 | 135 | 2.52 | 69 | 1.49 | 115 | 2.71 | 101 | 0.33 | 745 | 12.78 |
| Median (IQR) | 149.5 | 48-280 | 141 | 33-256 | 111 | 30-188 | 111 | 44-173 | 220 | 90-419 | 276 | 43-565 | 145 | 44-267 |
| Year of establishing ART eligibility | ||||||||||||||
| >31DEC2009 | 119 | 0.92 | 95 | 0.71 | 98 | 0.78 | 29 | 0.26 | 24 | 0.29 | 32 | 0.10 | 397 | 3.05 |
| 2009 | 61 | 0.89 | 64 | 0.95 | 46 | 0.70 | 34 | 0.47 | 42 | 0.78 | 18 | 0.02 | 265 | 3.81 |
| 2008 | 46 | 0.96 | 59 | 1.25 | 45 | 0.97 | 31 | 0.82 | 48 | 1.28 | 31 | 0.10 | 260 | 5.39 |
| 2007 | 39 | 1.17 | 38 | 1.10 | 59 | 1.80 | 22 | 0.62 | 38 | 1.29 | 29 | 0.18 | 225 | 6.15 |
| 2006 | 16 | 0.44 | 29 | 1.13 | 45 | 1.67 | 22 | 0.92 | 37 | 1.62 | 18 | 0.11 | 167 | 5.90 |
| ≤31DEC2005 | 38 | 1.47 | 6 | 0.24 | 31 | 1.28 | 32 | 1.30 | 56 | 2.33 | 22 | 0.05 | 185 | 6.66 |
ART: Antiretroviral therapy. CD4: Cluster of differentiation type 4. d: Days . DEC: December. IQR: Inter-quartile range. mo: Month. N: Total. n: Sub-total. PY: Person-year. WHO: World Health Organization.
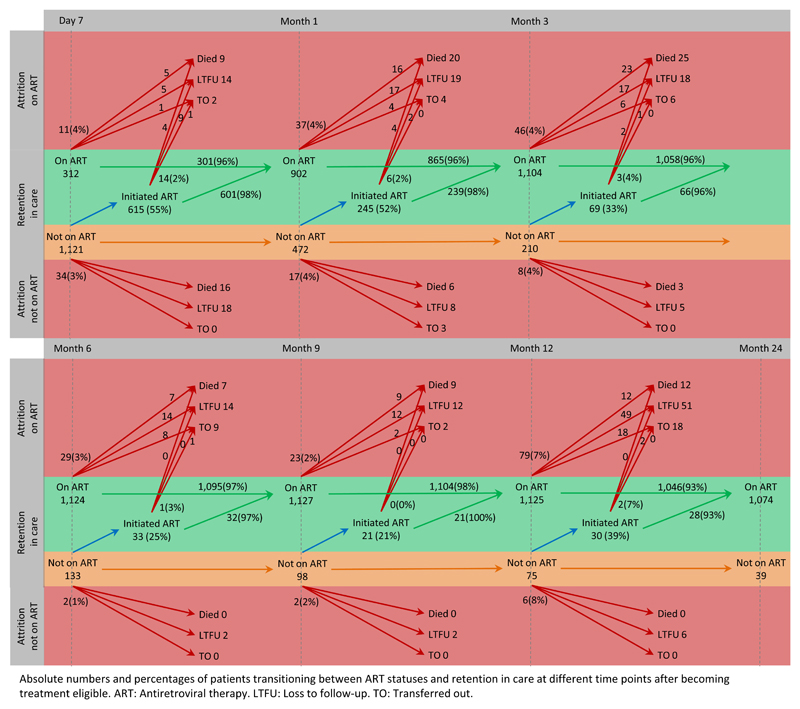
At 24 months after becoming ART eligible, 72% (1,074/1,499) of patients were retained and on ART. Mortality and LTFU was 6% (84/1,332) and 10% (133/1,332) among patients on ART, and 15% (25/167) and 59% (98/167) among patients not on ART, respectively. Half of all deaths (49%; 53/109) and LTFU (52%; 121/231) occurred within the first three months after becoming ART eligible. Among patients not on ART, the vast majority of deaths (88%; 22/25) and LTFU (85%; 83/98) occurred during this period, but only 37% (31/84) and 29% (38/133) among adolescents on ART, respectively (Table 2). New ART initiations decreased with prolonged time elapsing after becoming ART eligible. However, those who initiated ART slower had no worse outcomes than those initiating fast, or than those being on ART already for longer time (Figure 1).
Table 2. Patient outcomes after becoming ART eligible by ART status.
| Day 7 | Month 1 | Month 3 | Month 6 | Month 9 | Month 12 | Month 24 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ART status | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) |
| On ART | N=319 | N=934 | N=1,179 | N=1,248 | N=1,281 | N=1,302 | N=1,332 | |||||||
| Retained | 312 | 97.81 | 902 | 96.57 | 1,104 | 93.64 | 1,124 | 90.06 | 1,127 | 87.98 | 1,125 | 86.41 | 1,074 | 80.63 |
| Dead | 2 | 0.63 | 11 | 1.18 | 31 | 2.63 | 56 | 4.49 | 63 | 4.92 | 72 | 5.53 | 84 | 6.31 |
| LTFU | 5 | 1.57 | 19 | 2.03 | 38 | 3.22 | 56 | 4.49 | 70 | 5.46 | 82 | 6.30 | 133 | 9.98 |
| Transferred out | 0 | 0.00 | 2 | 0.21 | 6 | 0.51 | 12 | 0.96 | 21 | 1.64 | 23 | 1.77 | 41 | 3.08 |
| Not on ART | N=1,180 | N=565 | N=320 | N=251 | N=218 | N=197 | N=167 | |||||||
| Retained | 1,121 | 95.0 | 472 | 83.54 | 210 | 65.63 | 133 | 52.99 | 98 | 44.95 | 75 | 38.07 | 39 | 23.35 |
| Dead | 0 | 0.00 | 16 | 2.83 | 22 | 6.88 | 25 | 9.96 | 25 | 11.47 | 25 | 12.69 | 25 | 14.97 |
| LTFU | 57 | 4.83 | 75 | 13.27 | 83 | 25.94 | 88 | 35.06 | 90 | 41.28 | 92 | 46.70 | 98 | 58.68 |
| Transferred out | 2 | 0.17 | 2 | 0.35 | 5 | 1.56 | 5 | 1.99 | 5 | 2.29 | 5 | 2.54 | 5 | 2.99 |
ART: Antiretroviral therapy. LTFU: Loss to follow-up. N: Total. n: Sub-total. %: Column percentages.
Figure 1. ART status transitions and patient outcomes after becoming ART eligible.
Absolute numbers and percentages of patients transitioning between ART statuses and retention in care at different time points after becoming treatment eligible. ART: Antiretroviral therapy. LTFU: Loss to follow-up. TO: Transferred out.
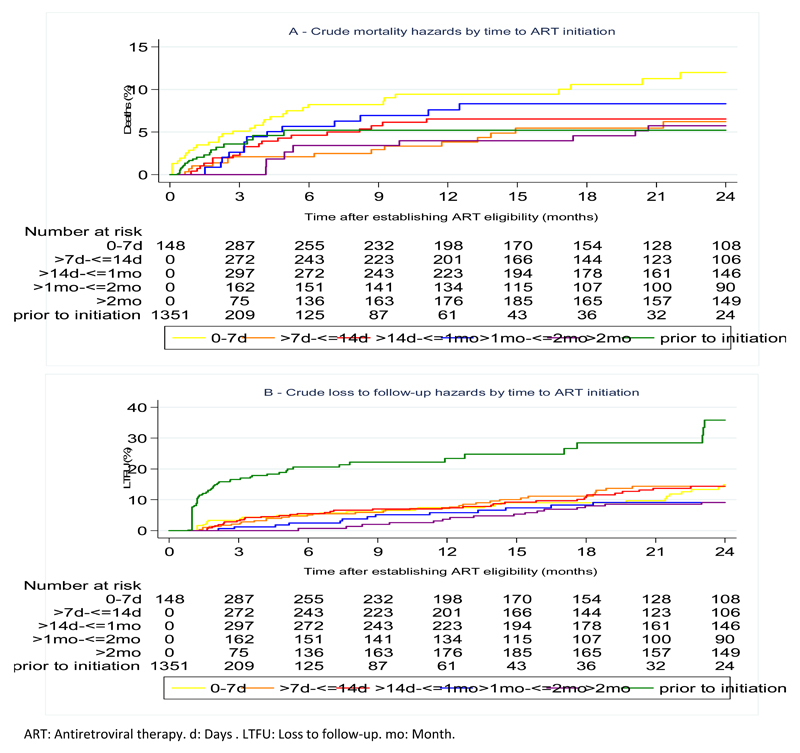
The crude mortality rate during the 24 months after becoming ART eligible was 5.46/100py (95%CI 4.53-6.59). The corresponding LTFU rate was 11.32/100py (95%CI 9.94-12.90). Stratified by time to ART initiation, cumulative hazards for mortality were highest in patients with fastest initiation (0-7 days). LTFU was highest in patients prior to ART initiation. However, no clear pattern emerged for mortality or LTFU with increasing time to ART duration (Figure 2). After adjusting for sex, age, WHO stage, and calendar year, hazard ratios for mortality were 1.59 (95%CI 0.83-3.04), 1.19 (95%CI 0.59-2.40), 1.56 (95%CI 0.72-3.41), 1.08 (95%CI 0.44-2.71), and 0.94 (95%CI 0.46-1.92) for patients with ART initiation at 0-≤7 days, >14 days to ≤1 month, >1-≤2 months, >2 months, and prior to initiation, respectively, using patients with ART initiation at 7-14 days as reference group. The corresponding adjusted hazard ratios for LTFU were 1.02 (95%CI 0.62-1.67), 1.07 (95%CI 0.66-1.73), 0.85 (95%CI 0.44-1.64), 0.97 (95%CI 0.52-1.81), and 3.96 (95%CI 2.60-6.04) (Table 3). There was no evidence of effect modification by calendar year for either mortality or LTFU.
Figure 2. Cumulative hazards for mortality (A) and for loss to follow-up (B) by time to ART initiation.
ART: Antiretroviral therapy. d: Days . LTFU: Loss to follow-up. mo: Month.
Table 3. Crude and adjusted rates and hazard ratios for mortality and for loss to follow-up by time to ART initiation.
| Time to ART initiation | Outcome | PY/100 | Rate/100py | 95% CI | cHRa | 95%CI | p-valueb | aHRc | 95%CI | p-valuec | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths (N=109) | ||||||||||||||
| 0-≤7d | 30 | 4.04 | 7.43 | 5.20 | 10.63 | 1.89 | 1.00 | 3.57 | 1.59 | 0.83 | 3.04 | |||
| >7-≤14d | 14 | 3.83 | 3.65 | 2.16 | 6.17 | 1 | 0.316 | 1 | <0.398 | |||||
| >14d-≤1mo | 19 | 4.32 | 4.39 | 2.80 | 6.88 | 1.37 | 0.69 | 2.74 | 1.19 | 0.59 | 2.40 | |||
| >1mo-≤2mo | 13 | 2.40 | 5.39 | 3.13 | 9.29 | 1.96 | 0.92 | 4.26 | 1.56 | 0.72 | 3.41 | |||
| >2mo | 8 | 2.80 | 2.85 | 1.43 | 5.70 | 1.59 | 0.65 | 3.90 | 1.08 | 0.44 | 2.71 | |||
| Prior to initiation | 25 | 2.55 | 9.81 | 6.63 | 14.53 | 1.23 | 0.61 | 2.47 | 0.94 | 0.46 | 1.92 | |||
| LTFU (N=226) | ||||||||||||||
| 0-≤7d | 32 | 4.04 | 7.93 | 5.61 | 11.21 | 0.96 | 0.59 | 1.57 | 1.02 | 0.62 | 1.67 | |||
| >7-≤14d | 32 | 3.83 | 8.35 | 5.91 | 11.81 | 1 | <0.04 | 1 | <0.04 | |||||
| >14d-≤1mo | 36 | 4.33 | 8.31 | 6.00 | 11.53 | 0.99 | 0.61 | 1.60 | 1.07 | 0.66 | 1.73 | |||
| >1mo-≤2mo | 13 | 2.41 | 5.40 | 3.13 | 9.30 | 0.78 | 0.41 | 1.48 | 0.85 | 0.44 | 1.64 | |||
| >2mo | 16 | 2.80 | 5.71 | 3.50 | 9.32 | 0.92 | 0.50 | 1.71 | 0.97 | 0.52 | 1.81 | |||
| Prior to initiation | 97 | 2.55 | 38.08 | 31.21 | 46.47 | 3.77 | 2.49 | 5.69 | 3.96 | 2.60 | 6.04 | |||
From Cox regression.
From LRT.
From Cox regression adjusted for sex, age, WHO stage, and calendar year of establishing ART eligibility.
aHR: Adjusted hazard ratio. ART: Antiretroviral therapy. cHR: Crude hazard ratio. d: Days. LTFU: Loss to follow-up. LRT: Likelihood ratio test mo: Month. PY: Person-year. WHO: World Health Organization. 95%CI: 95% confidence interval.
Discussion
To our knowledge, this is the first assessment of ART initiation dynamics and related patient outcomes, and the effect of varying time to ART initiation on mortality and LTFU among treatment eligible adolescents. Retention was high overall, and most patients started ART rapidly. We found high mortality and LTFU shortly after becoming eligible, especially among adolescents not on ART, but there was no evidence for differences in mortality or LTFU among adolescents with varying time to ART duration.
High mortality and LTFU around the time of becoming ART eligible has also been found elsewhere across different settings and patient groups, in particular among patients not initiated on ART.6,33,54–57 Considering that many of those coded as LTFU are like to have actually died,59,60 mortality among our patients is probably even higher than reported in the data. We attribute the slightly increased mortality among patients initiated at 0-7 days in our analysis to selection bias caused by health care workers prioritizing sicker patients, who had a higher probability of dying despite fast ART initiation, over less sick patients. This effect probably outweighed the benefits of rapid ART initiation on survival in these patients. The fact that we did not find elevated LTFU in patients who initiated rapidly suggests that, contrary to current understanding,21–24 a long preparatory process is not needed to keep post-initiation LTFU low.
Our findings are supported by another routine programme evaluation from South Africa, which found high ART uptake, good adherence, and no evidence for increased post-initiation LTFU after introducing a comprised preparation schedule for accelerated treatment start within one week.58 Similarly encouraging evidence is emerging from a randomized trial in Haiti, where preliminary results suggest better retention and lower mortality among patients initiated at the same day of establishing ART eligibility compared to patients who initiated after three weekly preparatory visits.59 Another recently completed cluster-randomized trial in Uganda using a multi-component intervention primarily targeting health care workers to accelerate ART initiation, including the option to start ART at the same day of HIV diagnosis, found better adherence among rapidly initiated patients, and no difference in LTFU and mortality compared to standard of care.60 However, some evidence for increased post-initiation loss to care was found in a small randomized trial from South Africa, in which patients in the intervention arm were offered same-day initiation compared to three to four preparation visits over up to four weeks in standard of care.61 Interestingly, considerable flexibility was granted in both arms in this trial, which allowed patients in the intervention arm to delay up to 30 days, and patients in the comparison group to initiate after only one week if considered necessary. Notably, all mentioned studies excluded patients below the age of 18 years, and findings can hence not be readily applied to adolescents. There is still no evidence that patients actually benefit from the currently prevailing initiation model with multiple obligatory preparation visits.32 Ultimately, much still needs to be learnt about how to best initiate ART in adolescents, in particular regarding the number and timing of preparatory sessions once ART eligibility criteria are met.43 Patients´ individual readiness rather than adherence to rigid counselling schedules should remain the guiding principal.62 However, providing individualized care will become increasingly difficult in SSA as the number of eligible patients continues to rise with eligibility criteria being steadily broadened.
ART initiation is a crucial step in the continuum of HIV care to reduce mortality.63–66 In our study, early mortality on ART was high despite the relatively short median time to ART initiation of 17 days. Also, CD4 levels at time of becoming ART eligible were relatively low in our cohort. This makes it unlikely that shortening the time to ART duration further will have substantial effects on mortality. Instead, scale-up of HIV testing coupled with effective pre-ART follow-up, broad eligibility criteria and better access to care, thereby initiating ART earlier in a larger number of infected adolescents, is needed to reduce mortality. While eligibility continues to be widened in Zimbabwe and globally,44,52 provision and uptake of testing services remains challenging.67
Our analysis demonstrates important pitfalls in ART outcomes research. Status transitions between not yet being ART eligible, being eligible but not yet on ART, and being on ART, and how these dynamics relate to patient outcomes are difficult to disentangle. Mere pre- vs post-initiation outcome comparisons to assess the effect of ART, as commonly done, are misleading since the varying durations between eligibility and ART initiation cannot be accounted for. However, existing research rarely considers the transient nature of care during this phase, with eligible patients starting and stopping treatment at varying time points, thereby leading to a skewed understanding of the effect of ART initiation on treatment outcomes.20 Also, risk comparisons based on survival analyses are inevitably hampered by bias and competing risks of LTFU and death. Allowing patients to switch treatment status, and to present percentages of cumulative and incident outcomes accordingly, as done in this analysis (see Table 2 and Figure 1), provides a more realistic picture of this crucial phase of HIV care.
Other strengths of this study are its big sample size and standardized data collection by trained encoders into a dedicated database with regular systematic data quality checks. By using programmatic data originating from a routine care setting in a public sector facility, our findings are more realistic of real-life conditions in HIV programmes from resource-limited settings than evidence from controlled trial environments.
This research is subject to the usual limitations inherent to analyses using existing programme data, most notably missing data. First, ART eligibility could not be confirmed for 364/2,184 (17%) of all enrolled patients. While their age and sex distribution was similar to included patients, fewer deaths occurred in this group (see Table, Supplemental Digital Content 1, showing patient characteristics by ART eligibility status), which might have biased our results. Second, CD4 results could not be included in the adjusted model due to missing data. Third, since follow-up and documentation of patients not yet ART eligible was patchy, only data after becoming ART eligible could be used. Information about the time point and mode of HIV infection, and regular eligibility status updates of patients prior to becoming ART eligible would have allowed to assess the evolution of the immune response and hence the clinical matureness of our cohort at time of becoming ART eligible. Fourth, the number of preparatory appointments each patient actually attended prior to ART initiation was not known. Fifth, reasons for deliberate delays in ART initiation were not captured during programme implementation. For example, we could not account for clinical conditions such as tuberculosis or cryptococcal infection, or particular psycho-social circumstances that demanded deferred ART initiation. Lastly, many SSA countries have now changed ART initiation thresholds from 200 to 350 or 500 CD4 cells/µL,44,52 or are moving towards a general test and treat approach. However, a substantial number of HIV-infected adolescents today are perinatally-infected long-term survivors,8–10 thus having a delayed diagnosis by definition, and start ART with low CD4 levels due to the persisting testing gap. Therefore, the question of how fast to initiated such patients upon diagnosis remains pertinent. Also the complexities of ART initiation in this age group have not changed. The upcoming test-all-treat-all era will increase the need for preparatory counselling massively, and hence make it even more crucial to find efficient yet sustainable ways to prepare patients for ART. However, our results should be treated with caution when applied to patients with high CD4 levels who were not eligible for ART during the study period. Numbers of patients with high CD4 counts are likely to increasein the future through the treat-all approach.
In summary, we found high mortality during the first three months following ART eligibility despite fast treatment initiation. Starting ART earlier through broader eligibility criteria and scale-up of HIV testing in adolescents, rather than faster initiation, is needed to reduce these deaths. We found no association between rapid treatment initiation and increased post-initiation LTFU. This adds to the mounting evidence that health care providers and care givers should be given considerable flexibility to tailor the initiation process to adolescents´ individual needs and life situations during this period.
Supplemental Digital Content
Acknowledgements
This research includes data provided by Médecins Sans Frontières. Médecins Sans Frontières is not otherwise research partner or party to this research. The study was supported by the Wellcome Trust through an Intermediate Fellowship to RAF (Grant 095878/Z/11/Z).
Sources of support:
None
Footnotes
Address for reprints:
Same as corresponding author
References
- 1.UNAIDS. Global Report - UNAIDS report on the global AIDS epidemic. Geneva: UNAIDS; 2013. [Google Scholar]
- 2.Adejumo OA, Malee KM, Ryscavage P, et al. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: a narrative review. J Int AIDS Soc. 2015;18:20049. doi: 10.7448/IAS.18.1.20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shroufi A, Gunguwo H, Dixon M, et al. HIV-infected adolescents in southern Africa can achieve good treatment outcomes: results from a retrospective cohort study. AIDS. 2013;27(12):1971–1978. doi: 10.1097/QAD.0b013e32836149ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryscavage P, Anderson EJ, Sutton SH, et al. Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr. 2011;58(2):193–197. doi: 10.1097/QAI.0b013e31822d7564. [DOI] [PubMed] [Google Scholar]
- 5.Auld AF, Agolory SG, Shiraishi RW, et al. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults - seven African countries, 2004-2013. MMWR. 2014;63(47):1097–1103. [PMC free article] [PubMed] [Google Scholar]
- 6.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth (15-24 years of age) enrolled in HIV care. AIDS. 2014;28(4):559–568. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shroufi A, Ndebele W, Nyathi M, et al. Risk of death among those awaiting treatment for HIV infection in Zimbabwe: adolescents are at particular risk. J Int AIDS Soc. 2015;18:19247. doi: 10.7448/IAS.18.1.19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowenthal ED, Bakeera-Kitaka S, Marukutira T, et al. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis. 2014;14(7):627–639. doi: 10.1016/S1473-3099(13)70363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton JW, Garnett GP, Takavarasha FR, et al. Increasing adolescent HIV prevalence in Eastern Zimbabwe - evidence of long-term survivors of mother-to-child transmission? PloS One. 2013;8(8):e70447. doi: 10.1371/journal.pone.0070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51(7):844–851. doi: 10.1086/656361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrand RA, Corbett EL, Wood R, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23(15):2039–2046. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–185. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 13.Bastard M, Poulet E, Nicolay N, et al. Pediatric access and continuity of HIV care before the start of antiretroviral therapy in sub-Saharan Africa. Pediatr Infect Dis J. 2016;35(9):981–6. doi: 10.1097/INF.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 14.Kasedde S, Luo C, McClure C, et al. Reducing HIV and AIDS in adolescents: opportunities and challenges. Curr HIV/AIDS Rep. 2013;10(2):159–168. doi: 10.1007/s11904-013-0159-7. [DOI] [PubMed] [Google Scholar]
- 15.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S144–153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 16.Jaspan H, Li R, Johnson L, Bekker L. The emerging need for adolescent-focused HIV care in South Africa. South Afr J HIV Med. 2009;10(4):9. [Google Scholar]
- 17.Sohn AH, Hazra R. The changing epidemiology of the global paediatric HIV epidemic: keeping track of perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16:18555. doi: 10.7448/IAS.16.1.18555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lake A, Sidibé M. To end the AIDS epidemic, start focusing on adolescents. 2015 Feb 17; Available at: http://www.unaids.org/en/resources/presscentre/featurestories/2015/february/20150217_oped_all-in.
- 19.Mavedzenge SN, Luecke E, Ross DA. Effective approaches for programming to reduce adolescent vulnerability to HIV infection, HIV risk, and HIV-related morbidity and mortality: a systematic review of systematic reviews. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S154–169. doi: 10.1097/QAI.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 20.Kapogiannis BG, Legins KE, Chandan U, et al. Evidence-based programming for adolescent HIV prevention and care: operational research to inform best practices. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S228–235. doi: 10.1097/QAI.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 21.Myer L, Zulliger R, Pienaar D. Diversity of patient preparation activities before initiation of antiretroviral therapy in Cape Town, South Africa. Trop Med Int Health. 2012;17(8):972–977. doi: 10.1111/j.1365-3156.2012.03033.x. [DOI] [PubMed] [Google Scholar]
- 22.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaiyachati KH, Ogbuoji O, Price M, et al. Interventions to improve adherence to antiretroviral therapy: a rapid systematic review. AIDS. 2014;28(Suppl 2):S187–204. doi: 10.1097/QAD.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 24.Plazy M, Dray-Spira R, Orne-Gliemann J, Dabis F, Newell ML. Continuum in HIV care from entry to ART initiation in rural KwaZulu-Natal, South Africa. Trop Med Int Health. 2014;19(6):680–689. doi: 10.1111/tmi.12301. [DOI] [PubMed] [Google Scholar]
- 25.Bernays S, Jarrett P, Kranzer K, et al. Children growing up with HIV infection: the responsibility of success. Lancet. 2014;383(9925):1355–1357. doi: 10.1016/S0140-6736(13)62328-4. [DOI] [PubMed] [Google Scholar]
- 26.WHO. HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV: recommendations for a public health approach and considerations for policy-makers and managers. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 27.WHO. HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV: Annex 4: Disclosure, adherence and retention in care. Geneva: WHO; 2013. [Google Scholar]
- 28.Busza J, Strode A, Dauya E, et al. Falling through the gaps: how should HIV programmes respond to families that persistently deny treatment to children? J Int AIDS Soc. 2016;19(1):20789. doi: 10.7448/IAS.19.1.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowshen N, D'Angelo L. Health care transition for youth living with HIV/AIDS. Pediatrics. 2011;128(4):762–771. doi: 10.1542/peds.2011-0068. [DOI] [PubMed] [Google Scholar]
- 30.Anglemyer A, Rutherford GW, Easterbrook PJ, et al. Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. AIDS. 2014;28(Suppl 2):S105–118. doi: 10.1097/QAD.0000000000000232. [DOI] [PubMed] [Google Scholar]
- 31.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–2067. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 32.Siedner MJ, Lankowski A, Haberer JE, et al. Rethinking the “pre” in pre-therapy counseling: no benefit of additional visits prior to therapy on adherence or viremia in Ugandans initiating ARVs. PloS One. 2012;7(6):e39894. doi: 10.1371/journal.pone.0039894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Micek MA, Gimbel-Sherr K, Baptista AJ, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Syndr. 2009;52(3):397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott V, Zweigenthal V, Jennings K. Between HIV diagnosis and initiation of antiretroviral therapy: assessing the effectiveness of care for people living with HIV in the public primary care service in Cape Town, South Africa. Trop Med Int Health. 2011;16(11):1384–1391. doi: 10.1111/j.1365-3156.2011.02842.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann CJ, Lewis JJ, Dowdy DW, et al. Mortality associated with delays between clinic entry and ART initiation in resource-limited settings: results of a transition-state model. J Acquir Immune Defic Syndr. 2013;63(1):105–111. doi: 10.1097/QAI.0b013e3182893fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahuerta M, Ue F, Hoffman S, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24(1):359–383. doi: 10.1353/hpu.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koech E, Teasdale CA, Wang C, et al. Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. AIDS. 2014;28(18):2729–2738. doi: 10.1097/QAD.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bygrave H, Mtangirwa J, Ncube K, et al. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PloS One. 2012;7(12):e52856. doi: 10.1371/journal.pone.0052856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matyanga CM, Takarinda KC, Owiti P, et al. Outcomes of antiretroviral therapy among younger versus older adolescents and adults in an urban clinic, Zimbabwe. Public Health Action. 2016;6(2):97–104. doi: 10.5588/pha.15.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schomaker M, Leroy V, Wolfs T, et al. Optimal timing of antiretroviral treatment initiation in HIV-positive children and adolescents: a multiregional analysis from Southern Africa, West Africa and Europe. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw097. pii:dyw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox MP, Larson B, Rosen S. Defining retention and attrition in pre-antiretroviral HIV care: proposals based on experience in Africa. Trop Med Int Health. 2012;17(10):1235–1244. doi: 10.1111/j.1365-3156.2012.03055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosen S, Fox MP, Larson BA, et al. Accelerating the Uptake and Timing of Antiretroviral Therapy Initiation in Sub-Saharan Africa: An Operations Research Agenda. PLoS Med. 2016;13(8):e1002106. doi: 10.1371/journal.pmed.1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO. Guidelines on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: WHO; 2015. [PubMed] [Google Scholar]
- 45.Dlodlo RA, Fujiwara PI, Hwalima ZE, et al. Adult mortality in the cities of Bulawayo and Harare, Zimbabwe: 1979-2008. J Int AIDS Soc. 2011;14(Suppl 1):S2. doi: 10.1186/1758-2652-14-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Statistics Agency. Zimbabwe national population census 2012. Harare: National Statistics Agency; 2012. [Google Scholar]
- 47.National AIDS Council Zimbabwe. Zimbabwe Country Report (January 2008-December 2009) Harare: National AIDS Council Zimbabwe; 2010. United Nations General Assembly Special Session Report on HIV and AIDS - Follow up to the declaration of commitment on HIV and AIDS. [Google Scholar]
- 48.UNAIDS. HIV estimates with uncertainty bounds 1990-2015. Available at: http://www.unaids.org/en/resources/documents/2016/HIV_estimates_with_uncertainty_bounds_1990-2015.
- 49.Ministry of Health and Child Care of Zimbabwe. Guidelines for Antiretroviral Therapy in Zimbabwe. Harare: Ministry of Health and Child Care of Zimbabwe; 2003. [Google Scholar]
- 50.Ministry of Health and Child Care of Zimbabwe. Guidelines for Antiretroviral Therapy in Zimbabwe. Harare: Ministry of Health and Child Care of Zimbabwe; 2007. [Google Scholar]
- 51.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 52.Ministry of Health and Child Welfare of Zimbabwe. Guidelines for Antiretroviral Therapy for the Prevention and Treatment of HIV in Zimbabwe. Harare: Ministry of Health and Child Care of Zimbabwe; 2013. [Google Scholar]
- 53.Mantel N, Byar DP. Evaluation of response-time data involving transient states: an illustration using heart-transplant data. J Am Stat Assoc. 1974;69(345):81–86. [Google Scholar]
- 54.Brown JP, Ngwira B, Tafatatha T, et al. Determinants of time to antiretroviral treatment initiation and subsequent mortality on treatment in a cohort in rural northern Malawi. AIDS Res Ther. 2016;13:24. doi: 10.1186/s12981-016-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abuogi LL, Smith C, McFarland EJ. Retention of HIV-Infected Children in the First 12 Months of Anti-Retroviral Therapy and Predictors of Attrition in Resource Limited Settings: A Systematic Review. PloS One. 2016;11(6):e0156506. doi: 10.1371/journal.pone.0156506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brennan AT, Long L, Useem J, et al. Mortality in the First 3 Months on Antiretroviral Therapy Among HIV-Positive Adults in Low- and Middle-income Countries: A Meta-analysis. J Acquir Immune Defic Syndr. 2016;73(1):1–10. doi: 10.1097/QAI.0000000000001112. [DOI] [PubMed] [Google Scholar]
- 57.Gupta A, Nadkarni G, Yang WT, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PloS One. 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkinson L, Duvivier H, Patten G, et al. Outcomes from the implementation of a counselling model supporting rapid antiretroviral treatment initiation in a primary healthcare clinic in Khayelitsha, South Africa. South Afr J HIV Med. 2015;16(1):1–7. doi: 10.4102/sajhivmed.v16i1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koenig S, Dorvil N, Severe P, et al. Same-day HIV testing and antiretroviral therapy initiation results in higher rates of treatment initiation and retention in care. J Int AIDS Soc. 2016;19(6 Suppl 5):21264. WEAE0202. [Google Scholar]
- 60.Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV. 2016 doi: 10.1016/S2352-3018(16)30090-X. pii: S2352-3018(16)30090-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosen S, Maskew M, Fox MP, et al. Initiating Antiretroviral Therapy for HIV at a Patient's First Clinic Visit: The RapIT Randomized Controlled Trial. PLoS Med. 2016;13(5):e1002015. doi: 10.1371/journal.pmed.1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ford N, Nsanzimana S. Accelerating initiation of antiretroviral therapy. Lancet HIV. 2016 doi: 10.1016/S2352-3018(16)30152-7. pii: S2352-3018(16)30152-7. [DOI] [PubMed] [Google Scholar]
- 63.MacPherson P, Munthali C, Ferguson J, et al. Service delivery interventions to improve adolescents' linkage, retention and adherence to antiretroviral therapy and HIV care. Trop Med Int Health. 2015;20(8):1015–1032. doi: 10.1111/tmi.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Govindasamy D, Meghij J, Kebede Negussi E, et al. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings--a systematic review. J Int AIDS Soc. 2014;17:19032. doi: 10.7448/IAS.17.1.19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kranzer K, Govindasamy D, Ford N, et al. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15(2):17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plazy M, Orne-Gliemann J, Dabis F, et al. Retention in care prior to antiretroviral treatment eligibility in sub-Saharan Africa: a systematic review of the literature. BMJ Open. 2015;5(6):e006927. doi: 10.1136/bmjopen-2014-006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.UNAIDS. The Gap Report. Geneva: UNAIDS; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.