Abstract
We sought to assess the ratio of soluble fms-like tyrosine kinase 1 (sFlt-1) to placental growth factor (PlGF) in maternal serum as a screening test for preeclampsia in unselected nulliparous women with a singleton pregnancy.
We studied 4,099 women recruited to the Pregnancy Outcome Prediction study (Cambridge, UK). The sFlt-1:PlGF ratio was measured using the Roche cobas e411 platform at ˜20, ˜28 and ˜36 weeks of gestational age (wkGA). Screen positive was defined as an sFlt-1:PlGF ratio >38, but higher thresholds were also studied.
At 28wkGA, an sFlt-1:PlGF ratio >38 had a positive predictive value (PPV) of 32% for preeclampsia and preterm birth, and the PPV was similar comparing women with low and high prior risk of disease. At 36wkGA, an sFlt-1:PlGF ratio >38 had a PPV for severe preeclampsia of 20% in high risk women and 6.4% in low risk women. At 36wkGA, an sFlt-1:PlGF ratio >110 had a PPV of 30% for severe preeclampsia, and the PPV was similar comparing low and high risk women. Overall, at 36wkGA 195 (5.2%) women either had an sFlt-1:PlGF ratio of >110 or an sFlt-1:PlGF ratio >38 plus maternal risk factors: 43% of these women developed preeclampsia, about half with severe features. Among low risk women at 36wkGA, an sFlt-1:PlGF ratio ≤38 had a negative predictive value for severe preeclampsia of 99.2%.
The sFlt-1:PlGF ratio provided clinically useful prediction of the risk of the most important manifestations of preeclampsia in a cohort of unselected nulliparous women.
Keywords: preeclampsia, placenta growth factor, sFlt-1 protein, angiogenesis, cohort studies, pregnancy, circulating biomarkers
Introduction
Preeclampsia is one of the most common adverse outcomes of pregnancy.1 The condition consists of new onset hypertension and proteinuria in the second half of pregnancy, but can also be "super-imposed" on pre-existing hypertension or renal disease. It is associated with increased risks of maternal and perinatal morbidity and mortality. A substantial proportion of severe adverse perinatal outcomes occurs as a consequence of preterm birth due to preeclampsia, and a substantial proportion of adverse maternal outcomes occurs in severe preeclampsia.1
Preeclampsia is associated with an altered maternal pattern of circulating, placentally-derived proteins regulating angiogenesis,2, 3 such as soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF). One of the simplest methods to quantify the pattern is to calculate the ratio between these two angiogenic factors in maternal serum. A recent study of women with clinically suspected disease demonstrated that a sFlt-1:PlGF ratio cut-off of 38 provided clinically useful prediction of the risk of preeclampsia.4 Higher sFlt-1:PlGF ratios, namely >85 at 28wkGA and >110 at 36wkGA, have been shown to be more strongly associated with the risk of preeclampsia.5 However, evidence for the diagnostic effectiveness of the ratio in screening women without clinical suspicion of the disease is poor. A meta-analysis published in 2015 concluded that further studies were required.6
The aim of the present analysis was to evaluate the effectiveness of the sFlt-1:PlGF ratio as a screening test for preeclampsia in unselected nulliparous women recruited to the Pregnancy Outcome Prediction (POP) study.7, 8 Most of the participants were healthy, as the cohort selection was solely based on nulliparity, singleton pregnancy and the study catchment area. We analyzed the sFlt-1:PlGF ratio measured repeatedly at 20, 28 and 36 weeks of gestational age (wkGA) using the Roche Cobas e411 Elecsys immunoassay system, which has been certified by the Conformité Européenne (CE) mark for use as an in vitro medical device. Screen positive was defined on the basis of the previously described and validated cut off of >38.4 We studied the most clinically important manifestations of preeclampsia, namely any severity of the disease leading to preterm birth or preeclampsia with severe features.
Methods
Study design
The POP study was conducted at the Rosie Hospital, Cambridge (UK), as previously described.7, 8 In brief, it was a prospective cohort study of nulliparous women attending the hospital for their dating ultrasound scan between January 14, 2008, and July 31, 2012 with a viable singleton pregnancy. The only clinical exclusion criterion was multiple pregnancy. The population was drawn from Cambridge and surrounding areas, with low rates of severe socio-economic deprivation. Therefore, the cohort can be considered a population with low prior risk of disease and homogeneous from a socio-economic perspective. Blood was obtained at the time of recruitment (not analyzed in the present study). Study participants attended the NIHR Cambridge Clinical Research Facility at ˜20wkGA, ˜28wkGA and ˜36wkGA for blood sampling and ultrasound scans. Ethical approval was given by the Cambridgeshire 2 Research Ethics Committee (reference number 07/H0308/163) and all participants provided written informed consent.
Outcome data
Outcome data were ascertained by review of each woman's paper case record by research midwives and by record linkage to clinical electronic databases of ultrasonography (Astraia, Munchen, Germany), delivery (Protos, iSoft, Banbury, UK), biochemical tests (Meditech, Westwood MA, USA) and neonatal intensive care (Badgernet, Clevermed Ltd, Edinburgh, UK). Where preeclampsia was suspected on the basis of these data, there was a second review of the clinical case record to confirm the diagnosis and classification (i.e. with or without severe features) on the basis of the objective criteria of the 2013 ACOG Guideline (Supplemental Material).9 Super-imposed preeclampsia was defined as preeclampsia in women with pre-existing renal disease or hypertension. Socio-economic status was quantified using the Index of Multiple Deprivation10 and birth weight percentiles were calculated using a population-based UK reference.11
Analysis plan and reporting
The definition of exposures, primary outcomes, secondary outcomes and sensitivity analyses were agreed in an analysis plan (Supplemental Material) prior to performing any analysis of the sFlt-1 and PlGF data. Analyses which were not pre-defined are identified as such. The primary outcome for the 20wkGA measurement was a composite of (i) preeclampsia with delivery prior to 28wkGA or (ii) preeclampsia with delivery prior to 37wkGA where the onset of hypertension was prior to 28wkGA. The primary outcome for the 28wkGA measurement was preeclampsia with delivery prior to 37wkGA. The primary outcome for the 36wkGA measurement was preeclampsia with severe features (i.e. preeclampsia with either severe hypertension or evidence of hepatic, renal, hematological, cerebral or pulmonary complications: see Supplemental Material). Secondary outcomes are defined in the Supplemental Material. High risk of preeclampsia was defined as either (i) maternal characteristics, using the UK's National Institute for Health and Care Excellence (NICE) Guideline (Supplemental Material)12 or (ii) elevated 20wkGA uterine artery Doppler, defined as a mean pulsatility index in the highest decile, as previously described.8 Family history of preeclampsia was not included in the definition of risk status as this information was not available. The reporting of this study conforms to the STROBE (The Strengthening the Reporting of Observational Studies in Epidemiology) statement.
Samples and Immunoassays
Serum samples were collected as previously reported6 and stored at -80°C. All samples used in the current analysis had not previously been thawed prior to the day of analysis. Researchers performing the assays were blinded to the patients’ clinical information and pregnancy outcome. Maternal serum levels of sFlt-1 and PlGF were measured using Roche Elecsys assays on the electro-chemiluminescence immunoassay platform, Cobas e411 (Roche Diagnostics). Using this system, the intra-assay coefficient of variation for human serum samples is <2% for sFlt-1 and PlGF, and the inter-assay coefficients of variation are 2.3 to 4.3% for the sFlt-1 assay and 2.7 to 4.1% for the PlGF assay. Screen positive was defined as sFlt-1:PlGF ratio of >38.4 We also studied more severe elevation of the ratio, namely, >85 at 28wkGA and >110 at 36wkGA.5
Statistical analysis
Full details of the statistical analysis are described in the Analysis plan (Supplemental Material). In brief, standard screening summary statistics were calculated from 2x2 tables. In addition, sFlt-1:PlGF ratio was analyzed as a continuous variable using the area under the receiver operating characteristic curve (AUROCC). Time to event analysis was performed where delivery with the given outcome was the event and delivery without the outcome was treated as a competing risk, and this method was used to generate plots of the cumulative incidence of the outcome from the time of measurement. All analyses were performed using Stata 14.1.
Exclusions and missing data
Of the 4,512 women recruited, 67 (1.5%) women withdrew and 233 (5.2%) delivered elsewhere, leaving 4,212 eligible women.8 Of these, 5 (0.1%) did not have preeclampsia status available and 108 (2.6%) did not have any sFlt-1:PlGF measurements available from the 28wkGA or 36wkGA visits.
Results
Description of the study cohort
The study group consisted of 4,099 women, of whom 3,953 had sFlt-1:PlGF measurement available from the 20wkGA visit, 3,989 from the 28wkGA visit and 3,776 from the 36wkGA visit. The overall incidence of preeclampsia was 6.5% (265/4,099). The incidence of preterm preeclampsia with onset prior to 28wkGA following the 20wkGA measurement was 0.10% (4/3,953). The incidence of preeclampsia leading to preterm delivery following the 28wkGA measurement was 0.65% (26/3,989). The incidence of delivery with severe preeclampsia following the 36wkGA measurement was 2.8% (106/3,776). The characteristics of the cohort are tabulated according to their experience of hypertensive complications of pregnancy (Table 1). In normotensive women, the median sFlt-1:PlGF ratios were 6.5, 2.7 and 11.2 at 20, 28 and 36wkGA, respectively. The median ratio at 28wkGA was 6.5 (interquartile range [IQR] 3.4-22.5) in women who developed preterm preeclampsia, and the median ratio at 36wkGA was 42.3 (IQR 22.6-88.2) in women who had severe preeclampsia.
Table 1.
Characteristics of the study cohort (N=4,099).
| Characteristic | No hypertensive disorder | Preterm preeclampsia | Preeclampsia with severe features* | Preeclampsia without severe features* | Gestational hypertension |
|---|---|---|---|---|---|
| n (%) | 3,751 (91.5%) | 26 (0.6%) | 111 (2.7%) | 128 (3.1%) | 83 (2.0%) |
| Maternal characteristics | |||||
| Age, years | 30 (27 to 33) | 29 (25 to 34) | 30 (26 to 34) | 29 (26 to 33) | 29 (25 to 33) |
| Age stopped FTE†, years | 21 (18 to 23) | 18 (16 to 21) | 19 (17 to 22) | 21 (18 to 23) | 21 (17 to 22) |
| Missing | 105 (2.8) | 1 (3.9) | 4 (3.6) | 7 (5.5) | 5 (6.0) |
| Height, cm | 165 (161 to 170) | 163 (158 to 167) | 165 (160 to 168) | 165 (161 to 168) | 165 (160 to 168) |
| Deprivation quartile | |||||
| 1 (lowest) | 917 (24) | 7 (27) | 26 (23) | 27 (21) | 20 (24) |
| 2 | 899 (24) | 2 (7.7) | 28 (25) | 33 (26) | 14 (17) |
| 3 | 897 (24) | 12 (46) | 26 (23) | 28 (22) | 21 (25) |
| 4 (highest) | 889 (24) | 5 (19) | 28 (25) | 34 (27) | 18 (22) |
| Missing | 149 (3.7) | 0 (0.0) | 3 (2.7) | 6 (4.7) | 10 (12) |
| Ethnicity | |||||
| Non-white | 211 (5.6) | 2 (7.7) | 5 (4.5) | 4 (3.1) | 5 (6.0) |
| White | 3478 (93) | 23 (88) | 105 (95) | 124 (97) | 73 (88) |
| Missing | 62 (1.7) | 1 (3.9) | 1 (0.9) | 0 (0.0) | 5 (6.0) |
| Married | 2558 (68) | 21 (81) | 74 (67) | 80 (63) | 56 (67) |
| Smoker | 182 (4.9) | 1 (3.9) | 3 (2.7) | 7 (5.5) | 8 (9.6) |
| Any alcohol consumption | 172 (4.6) | 0 (0.0) | 4 (3.6) | 4 (3.1) | 6 (7.2) |
| Missing | 1 (<0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Body mass index, kg/m2 | 24 (22 to 27) | 28 (26 to 30) | 25 (23 to 32) | 28 (23 to 32) | 27 (24 to 31) |
| Missing | 1 (<0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Type 1 or type 2 DM† | 7 (0.2) | 3 (12) | 0 (0.0) | 2 (1.6) | 2 (2.4) |
| Chronic hypertension | 95 (2.5) | 9 (35) | 34 (31) | 74 (58) | 0 (0.0) |
| Renal disease | 28 (0.8) | 1 (3.9) | 3 (2.7) | 8 (6.3) | 0 (0.0) |
| UtA mean PI† highest decile | 335 (8.9) | 11 (42) | 23 (21) | 21 (16) | 9 (11) |
| Missing | 91 (2.4) | 2 (7.7) | 1 (0.9) | 7 (5.5) | 3 (3.6) |
| Birth outcomes | |||||
| Birth weight, g | 3425 (3110 to 3735) | 2210 (1650 to 2660) | 3425 (3050 to 3750) | 3383 (3100 to 3785) | 3501 (3190 to 3830) |
| Birth weight, z score | -0.15 (-0.71 to 0.41) | -0.61 (-1.15 to 0.06) | -0.14 (-0.73 to 0.63) | -0.03 (-0.69 to 0.40) | -0.15 (-0.59 to 0.71) |
| Birth weight, centile | 44 (24 to 66) | 28 (13 to 52) | 45 (23 to 73) | 49 (25 to 66) | 44 (28 to 76) |
| Missing | 1 (<0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gestational age, weeks | 40 (39 to 41) | 35 (34 to 36) | 40 (39 to 41) | 40 (39 to 41) | 40 (39 to 41) |
| Induction of labor | 1115 (30) | 6 (23) | 70 (63) | 82 (64) | 37 (45) |
| Mode of delivery | |||||
| Spontaneous vaginal | 1896 (51) | 6 (23) | 25 (23) | 42 (33) | 31 (37) |
| Assisted vaginal | 873 (23) | 0 (0.0) | 29 (26) | 41 (32) | 23 (28) |
| Intrapartum caesarean | 616 (16) | 3 (12) | 43 (39) | 31 (24) | 21 (25) |
| Pre-labor caesarean | 356 (9.5) | 17 (65) | 13 (12) | 14 (11) | 8 (10) |
| Missing | 10 (0.3) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| sFlt-1:PlGF ratio | |||||
| At 20wkGA | 6.5 (4.4 to 9.4) | 9.1 (5.1 to 15.9) | 6.9 (5.0 to 9.1) | 6.9 (4.5 to 10.6) | 6.3 (4.2 to 8.4) |
| Missing | 125 (3.3) | 4 (15) | 6 (5.4) | 7 (5.5) | 4 (4.8) |
| At 28wkGA | 2.7 (1.7 to 4.2) | 6.5 (3.4 to 22.5) | 3.9 (2.4 to 7.8) | 3.9 (2.3 to 6.9) | 2.6 (1.8 to 4.3) |
| Missing | 101 (2.7) | 0 (0.0) | 4 (3.6) | 3 (2.3) | 2 (2.4) |
| At 36wkGA | 11.2 (5.1 to 22.6) | 126.2 (64.5 to 187.7) | 42.3 (22.6 to 88.2) | 35.3 (18.4 to 65.3) | 20.2 (8.2 to 38.3) |
| Missing | 274 (7.3) | 20 (77) | 9 (8.1) | 11 (8.6) | 9 (11) |
Confined to women delivering ≥37wkGA.
Abbreviations: FTE denotes full time education, DM denotes diabetes mellitus, UtA denotes uterine artery and PI denotes pulsatility index.
Data are expressed as median (IQR) or n (column %) as appropriate. For fields where there is no category labelled "missing", data were 100% complete.
Maternal age was defined as age at recruitment. All other maternal characteristics were defined by self-report at the 20wkGA interview, from examination of the clinical case record, or linkage to the hospital’s electronic databases. Deprivation was quantified using the Index of Multiple Deprivation 200710, which is based on census data from the area of the mother’s postcode. Birth weight percentiles and z scores were calculated using a population-based UK reference.11 Gestational hypertension was defined as development of hypertension in the second half of pregnancy in a previously normotensive woman who did not fulfill the diagnostic criteria for preeclampsia. See Supplemental Material for details.
Screening performance at 20wkGA
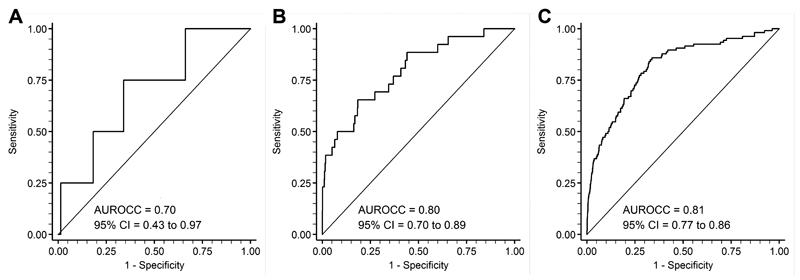
None of the four women who experienced the primary outcome following the 20wkGA measurement had an sFlt-1:PlGF ratio >38. The AUROCC for the sFlt-1:PlGF ratio was 0.70 (95% CI 0.43-0.97) (Figure 1A). Ten women had a ratio >38 and one woman had a ratio >85 at 20wkGA. Further analysis was not performed due to the very small number of events.
Figure 1.
Receiver operating characteristic curve analysis of the relationship between A. sFlt-1:PlGF ratio at 20wkGA and (i) preeclampsia with delivery prior to 28wkGA or (ii) preeclampsia with delivery prior to 37 weeks where the onset of hypertension was prior to 28wkGA (n=4), B. sFlt-1:PlGF at 28wkGA and preeclampsia leading to preterm birth (n=26), and C. sFlt-1:PlGF at 36wkGA and severe preeclampsia (n=106). The continuous sFlt-1:PlGF ratio is used and the area under the receiver operating characteristic curve (AUROCC) with 95% confidence interval (CI) is given for each analysis.
Screening performance at 28wkGA
The AUROCC for the sFlt-1:PlGF ratio was 0.80 (95% CI 0.70-0.89) (Figure 1B). Women with an sFlt-1:PlGF ratio >38 (n=19) had an incidence of preeclampsia leading to preterm delivery of 32% (Table 2). The positive predictive value (PPV) was similar in low and high risk women (33% vs 31%, respectively, P=0.91).
Table 2.
Screening statistics for the primary outcomes by maternal risk status using the threshold of sFlt-1:PlGF ratio of >38 at 28wkGA and 36wkGA.
| 28wkGA* Preeclampsia with preterm delivery |
36wkGA* Preeclampsia with severe features |
|||||
|---|---|---|---|---|---|---|
| Screening statistic | All | High risk | Low risk | All | High risk | Low risk |
| Sensitivity (%) | 23.1 (6.9-39.3) |
22.2 (3.0-41.4) |
25.0 (0.0-55.0) |
54.7 (45.2-64.2) |
53.3 (40.7-66.0) |
56.5 (42.2-70.8) |
| Specificity (%) | 99.7 (99.5-99.8) |
98.8 (98.0-99.6) |
99.9 (99.8-100.0) |
86.2 (85.0-87.3) |
80.6 (77.5-83.6) |
87.4 (86.2-88.5) |
| Positive predictive value (%) | 31.6 (10.7-52.5) |
30.8 (5.7-55.9) |
33.3 (0.0-71.1) |
10.2 (7.7-12.7) |
20.3 (14.0-26.5) |
6.4 (4.0-8.7) |
| Negative predictive value (%) | 99.5 (99.3-99.7) |
98.2 (97.2-99.1) |
99.8 (99.7-100.0) |
98.5 (98.1-98.9) |
94.9 (93.1-96.7) |
99.2 (98.9-99.6) |
| Positive likelihood ratio† | 70.3 (29.0-170.8) |
18.6 (6.3-54.9) |
200.6 (42.6-944.1) |
4.0 (3.3-4.8) |
2.7 (2.1-3.6) |
4.5 (3.4-5.9) |
| Negative likelihood ratio‡ | 0.77 (0.63-0.95) |
0.79 (0.61-1.01) |
0.75 (0.50-1.12) |
0.53 (0.43-0.65) |
0.58 (0.44-0.76) |
0.50 (0.36-0.69) |
wkGA denotes weeks of gestational age (of measurement of the sFlt-1:PlGF ratio).
Positive likelihood ratio was defined as the ratio between the proportions of screen positives among cases and screen positives among non-cases ([S+|D+]/[S+|D-]).
Negative likelihood ratio was defined as the ratio between the proportions of screen negatives among cases and screen negatives among non-cases ([S-|D+]/[S-|D-]).
See Supplemental Tables for raw data from 2x2 tables.
Screening performance at 36wkGA
The AUROCC for the sFlt-1:PlGF ratio was 0.81 (95% CI 0.77-0.86) (Figure 1C). Women with an sFlt-1:PlGF ratio >38 (n=566) had an incidence of severe preeclampsia of 10% (Table 2). The PPV was 20% in high risk women and 6.4% in low risk women. Among women with no prior risk factors, an sFlt-1:PlGF ratio ≤38 had a high negative predictive value for subsequent development of severe preeclampsia (>99%).
Analysis of severe elevation of the sFlt1:PlGF ratio
We studied more severe elevation of the ratio, using pre-defined thresholds, namely, 85 at 28wkGA and 110 at 36wkGA (Table 3). Only 7 women had an sFlt1:PlGF ratio>85 at 28wkGA. However, 4 out of 7 delivered preterm with a diagnosis of preeclampsia (PPV=57%). At 36wkGA, 70 women had an sFlt1:PlGF ratio >110 and 21 developed severe preeclampsia (PPV=30%). The PPV was similar comparing women with and without prior risk factors (36% and 24%, respectively).
Table 3.
Screening statistics for the primary outcomes by maternal risk status using the threshold of sFlt-1:PlGF ratio of >85 at 28wkGA and >110 at 36wkGA.
| 28wkGA* Preeclampsia with preterm delivery |
36wkGA* Preeclampsia with severe features |
|||
|---|---|---|---|---|
| Screening statistic | All | All | High risk | Low risk |
| Sensitivity (%) | 15.4 (1.5-29.3) | 19.8 (12.2-27.4) | 20.0 (9.9-30.1) | 19.6 (8.1-31.0) |
| Specificity (%) | 99.9 (99.8-100.0) | 98.7 (98.3-99.0) | 96.8 (95.4-98.1) | 99.1 (98.7-99.4) |
| Positive predictive value (%) | 57.1 (20.5-93.8) | 30.0 (19.3-40.7) | 36.4 (20.0-52.8) | 24.3 (10.5-38.1) |
| Negative predictive value (%) | 99.4 (99.2-99.7) | 97.7 (97.2-98.2) | 92.9 (90.9-94.8) | 98.8 (98.4-99.2) |
| Positive likelihood ratio | 203.2 (47.8-863.3) | 14.8 (9.2-23.8) | 6.2 (3.2-11.9) | 21.1 (10.6-42.2) |
| Negative likelihood ratio | 0.85 (0.72-1.00) | 0.81 (0.74-0.89) | 0.83 (0.73-0.94) | 0.81 (0.70-0.94) |
wkGA denotes weeks of gestational age (of measurement of the sFlt-1:PlGF ratio).
See Methods for definition of risk status and Supplemental Tables for raw data from 2x2 tables.
Screening performance at 36wkGA using the sFlt1:PlGF ratio combined with maternal risk factors
We then analyzed a composite definition of screen positive at 36wkGA, namely, an sFlt-1:PlGF ratio of >110 irrespective of maternal risk factors or an sFlt-1:PlGF ratio >38 combined with maternal risk factors. A total of 195 (5.2%) women screened positive by this definition and 43% of them subsequently delivered with a diagnosis of preeclampsia: 41 women (PPV=21%) developed pre-eclampsia with severe features and 43 (PPV=22%) developed preeclampsia without severe features. The characteristics and outcomes for the 195 women are summarized (Supplemental Material).
Time to event analysis
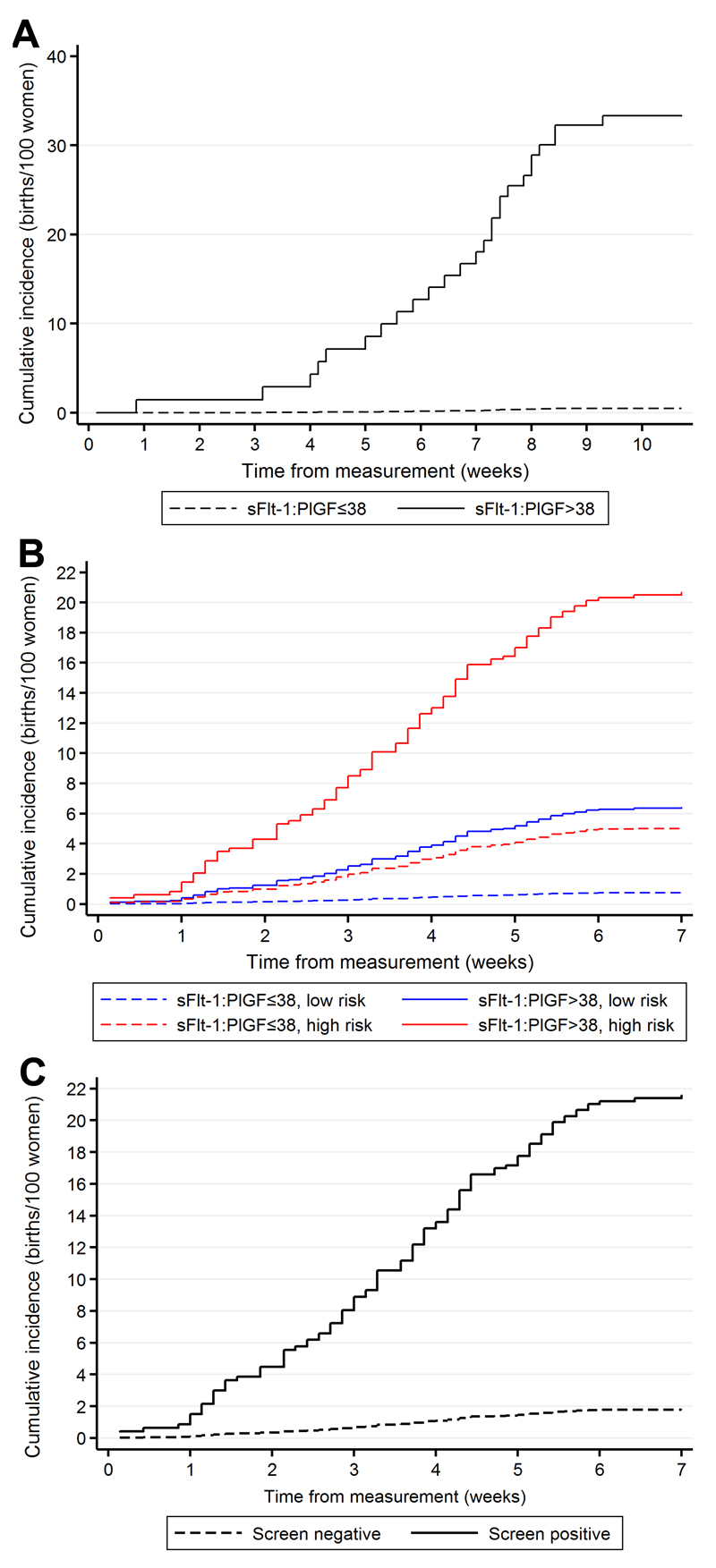
We plotted the cumulative incidence of the primary outcomes following the 28wkGA and 36wkGA measurements of the sFlt-1:PlGF ratio using a competing risks model (Figure 2A & B). The 36wkGA measurements were stratified by maternal risk status (Figure 2B). In both cases, the curves started to deviate at least 1 week after the time of measurement and the proportional increase in risk was maintained over the 7-8 weeks following the test. We also plotted the cumulative incidence of preeclampsia for women at 36wkGA with the composite definition of screen positive (Figure 2C), i.e. a ratio >38 plus risk factors or a ratio of >110 irrespective of risk factors. Delivery without the primary outcome was treated as a competing risk in all three analyses. In all three plots, it is evident that more than 90% of the deliveries in the highest risk group occurred >1 week from the time of measurement of the ratio.
Figure 2.
Cumulative incidence of the primary outcomes (see methods) by the sFlt1:PlGF ratio: A. sFlt-1:PlGF at 28wkGA and preeclampsia leading to preterm birth, B. sFlt-1:PlGF at 36wkGA and severe preeclampsia, stratified by maternal risk. High risk was defined on the basis of maternal risk factors or 20wkGA uterine artery Doppler (see Methods for details), and C. Composite risk status at 36wkGA. Screen positive was defined as (i) sFlt-1:PlGF ratio of >38 AND maternal risk factors OR (ii) sFlt-1:PlGF ratio >110 irrespective of maternal risk factors. Screen negative was defined as all other women. Delivery without the given primary outcome was treated as a competing risk in all three analyses. Hence, the maximum value of the cumulative incidence is the same as the positive predictive value and the curve illustrates the distribution of the timing of the deliveries with the outcome in question.
Discussion
The main finding of the present study is that, in a cohort of unselected, first singleton pregnancies, measurement of the sFlt-1:PlGF ratio identified women with a high absolute risk of experiencing the clinically most important manifestations of preeclampsia. At 28wkGA, an sFlt-1:PlGF ratio >38 identified women with a high risk (>30%) of subsequently delivering preterm with preeclampsia. Women who had a more severe elevation of the ratio (>85) had nearly 60% risk of delivering preterm with preeclampsia, whereas >99% of women who had a ratio of <38 did not develop the outcome. At 36wkGA, about 5% of women were identified as high risk on the basis of either an sFlt-1:PlGF ratio of >110 or an sFlt-1:PlGF ratio >38 plus maternal risk factors. Of this group, 43% were subsequently delivered with a diagnosis of preeclampsia, and about half of these cases were severe. Approximately 70% of women were identified as low risk at 36wkGA, i.e. they had no maternal risk factors and an sFlt-1:PlGF ratio ≤38: their risk of developing severe preeclampsia was <1%.
Screening is generally only conducted when there are evidence-based interventions which mitigate the risk. A key element in the study design was to make a measurement close to term (36wkGA), the rationale being that delivery is the main intervention to prevent preeclampsia. This can more easily and more safely be performed at term.13 Moreover, a randomized controlled trial has shown improved outcome following immediate induction of labor compared with expectant management in women with gestational hypertension or mild preeclampsia near term.14 In the current study we evaluated the diagnostic effectiveness of the sFlt-1:PlGF ratio in identifying women at risk of developing preeclampsia. In light of our results, we hypothesize that one approach to reducing the burden of morbidity associated with preeclampsia could be to screen nulliparous women at 36wkGA using maternal risk factors and sFlt-1:PlGF ratio, monitor screen positive women closely and perform induction of labor prior to development of severe disease. This hypothesis could be readily tested in a randomized controlled trial evaluating whether the introduction of the screening test improves pregnancy outcome (clinical effectiveness). Such an intervention is unlikely to cause harm, and we have recently demonstrated that routine induction of labor at 39wkGA does not increase the risk of caesarean delivery or perinatal morbidity in another high risk population of nulliparous women, namely, those aged 35 or more.15
The sFlt-1:PlGF ratio was also informative for the risk of women experiencing preterm preeclampsia. Women with a ratio >38 at 28wkGA had a 32% risk of preterm delivery with preeclampsia, and the ratio >38 had a very high positive likelihood ratio (˜70). This may reflect the fact that this threshold represents much more significant elevation at 28wkGA (99.5th percentile) than 36wkGA (85th percentile). However, the sensitivity of the sFlt-1:PlGF ratio >38 at 28wkGA was only 23%. Although the POP study included mostly healthy women, this is consistent with findings in women clinically suspected to have preeclampsia, where an elevated sFlt-1:PlGF ratio between 24wkGA and 37wkGA was also associated with an increased risk of developing the disorder within 4 weeks after the measurement.16 Hence, while the test provides clinically useful prediction of risk for a small proportion of women, the majority of women experiencing the disease would not be identified using the test at 28wkGA. However, the AUROCC for the sFlt-1:PlGF ratio at 28wkGA for preterm preeclampsia was 0.80. It is possible that combining the ratio with other measurements (clinical, biomarker and ultrasonic) in a multivariable model might provide better risk prediction. This is an area of further investigation, which will be hopefully paralleled by studies aiming at the implementation of better treatment options. Currently the main limitation of the clinical usefulness of the 28wkGA measurement is the lack of a clearly effective intervention mitigating the risks for those who screen positive, other than close monitoring of the patient. Another important area for further study is to refine the estimation of risk in women whose 36wkGA assessment identified them as being at intermediate risk (5% to 6%) of severe preeclampsia, namely, women with an sFlt-1:PlGF ratio >38 and no risk factors, or risk factors but a ratio of ≤38. Possible approaches include identifying other informative biomarkers, or repeating measurement of the sFlt-1:PlGF ratio after 36wkGA.
The present study had a number of methodological strengths over previous studies. First, the size was sufficiently large that we were able to study the variants of preeclampsia associated with the most severe complications. Second, we used a clinically validated assay where the definition of screen positive was based on prior studies which had identified and validated the chosen threshold. Moreover, the sFlt-1:PlGF ratio can be calculated without modelling in relation to gestational age or maternal characteristics, and in this study clinical care was provided without knowledge of the test result. Finally, the analyses in the present study were planned and specified in advance.
A large scale study of screening women using the sFlt-1:PlGF ratio has recently been reported.17 However, their findings are difficult to compare to the present study as they pooled the results from a wide range of gestational ages (30 to 37wkGA). Moreover, almost half of their population consisted of multiparous women who had not previously experienced preeclampsia, and this is a group with a very low prior risk of disease. The PPV of a test depends both on the a priori risk and the positive likelihood ratio. It is difficult to interpret a summary estimate of PPV when almost half the population has a very low prior risk of the outcome. Another large study based on a multi-ethnic cohort of nulliparous women and high risk parous women concluded that angiogenic biomarkers measured in the first half of pregnancy performed poorly for predicting later development of preeclampsia.18 They also observed, however, that the measurements became more strongly predictive when made closer to disease onset, but the analyses of late pregnancy data were limited by high rates of missing biomarker data (20-30%). The focus of the present study on nulliparous women was purposeful. One of the strongest clinical predictors of the risk of preeclampsia is whether a woman has a prior history of pregnancy affected by the condition. This information dominates risk prediction in multiparous women but is necessarily absent among nulliparous women. Another purposeful feature of the design of the present study was measurement of biomarkers throughout gestation. Results reported in the current and previous studies13 suggest that screening tests for pregnancy complications have a better predictive value when performed close to disease onset.
A number of studies have evaluated trying to predict preeclampsia solely using measurements made in the first trimester. Statistical models are used to determine prior risk. Further modelling is used to process values of first trimester uterine artery Doppler flow velocimetry, and to convert first trimester protein concentrations into gestational age corrected multiples of the median. Two models were externally evaluated in a prospective cohort study in Norway, where measurements were performed between 11wkGA and 14wkGA.19 A study-derived threshold (10% false positive rate) yielded positive predictive values of 5-12%, a sensitivity of 40% and positive likelihood ratios of 1.5 to 3.6 for all preeclampsia cases. Although the nature of that study does not allow direct comparison with this analysis, the current approach may be more likely to be clinically applicable, given that (i) the definition of screen positive was externally defined, (ii) the positive predictive values were higher, (iii) the outcome was confined to the clinically most significant cases, and (iv) the handling of clinical and biochemical predictors is simpler.
Perspectives
We conclude that measurement of the sFlt-1:PlGF ratio at 36wkGA, combined with maternal risk factors, provides clinically useful prediction of the risk of preeclampsia at term for about three quarters of unselected nulliparous women, identifying 5% of them as high risk and 70% as low risk. Screening the pregnant nulliparous population in late pregnancy using this measurement could plausibly improve maternal and perinatal outcome when coupled with close monitoring and/or induction of labor, and this would be an appropriate focus for future randomized controlled trials. Women with extreme elevation of the ratio at 28wkGA have high absolute risks of preterm disease and the test may be useful to identify women for evaluation of candidate disease-modifying therapies as they become available.
Supplementary Material
Novelty and Significance.
What is New?
Among a population of nulliparous women at mixed risk of disease:
An sFlt-1:PlGF ratio >38 at 28 weeks of gestational age identified women with a high risk (>30%) of subsequently delivering preterm with preeclampsia.
An sFlt-1:PlGF ratio >110 at 36 weeks of gestational age identified women with a high risk (>30%) of subsequently experiencing severe preeclampsia.
An sFlt-1:PlGF ratio between >38 and <110 at 36 weeks of gestational age was only associated with a high absolute risk (>20%) of subsequently experiencing severe preeclampsia if the mother had additional risk factors.
What is Relevant?
Measurement of the sFlt-1:PlGF ratio provides clinically useful information on risk of the most clinically important manifestations of preeclampsia among women having first pregnancies.
This identifies potential new approaches to trials of screening and intervention.
Summary
Very high elevation of the sFlt-1:PlGF ratio identifies women with high absolute risks of preterm or severe preeclampsia, and moderate elevation is informative of risk when combined with maternal risk factors.
Acknowledgements
We are extremely grateful to the participants in the Pregnancy Outcome Prediction study. We are very grateful to Leah Bibby, Samudra Ranawaka, Katrina Holmes, Josephine Gill and Ryan Millar for technical assistance in performing the biochemical assays, and to Dr. Rabia Zill-e-Huma and Dr. Amr Gebril for reviewing the clinical case records.
Sources of funding
The work was supported by the National Institute for Health Research (NIHR) Cambridge Comprehensive Biomedical Research Centre (Women's Health theme), and project grants from the Medical Research Council (UK) (G1100221) and the Stillbirth and neonatal death society (Sands). The study was also supported by Roche Diagnostics (provision of equipment and reagents for analysis of sFlt-1 and PlGF), by GE Healthcare (donation of two Voluson i ultrasound systems for this study), and by the NIHR Cambridge Clinical Research Facility, where all research visits took place.
Footnotes
Disclosures
Dr. Hund reports being an employee of Roche Diagnostics, holding stock in Roche, having a pending patent related to the sFtl-1:PlGF or endoglin:PlGF ratio to rule out onset of preeclampsia in pregnant women within a certain time period (PCT/EP2013/063115); holding pending patents related to the dynamic of sFlt-1 or endoglin:PlGF ratio as an indicator for imminent preeclampsia or the HELLP syndrome or both (PCT/EP2012/072157) and the prediction of postpartum HELLP syndrome, postpartum eclampsia, or postpartum preeclampsia (PCT/EP2015/051457). Dr. Smith reports equipment loans and consumable support from Roche Diagnostics. Dr. Sovio, Dr. Gaccioli and Dr. Charnock-Jones report no conflicts of interest.
References
- (1).Lyall F, Belfort M. Pre-eclampsia: etiology and clinical practice. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- (2).Levine RJ, Maynard SE, Qian C, Lim K-H, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- (3).Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA, for the CPEP Study Group Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- (4).Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- (5).Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P, Sabria J, Markfeld-Erol F, Galindo A, Schoofs K, Denk B, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352. doi: 10.1161/HYPERTENSIONAHA.113.01787. [DOI] [PubMed] [Google Scholar]
- (6).Liu Y, Zhao Y, Yu A, Zhao B, Gao Y, Niu H. Diagnostic accuracy of the soluble Fms-like tyrosine kinase-1/placental growth factor ratio for preeclampsia: a meta-analysis based on 20 studies. Arch Gynecol Obstet. 2015;292:507–518. doi: 10.1007/s00404-015-3671-8. [DOI] [PubMed] [Google Scholar]
- (7).Pasupathy D, Dacey A, Cook E, Charnock-Jones DS, White IR, Smith GC. Study protocol. A prospective cohort study of unselected primiparous women: the pregnancy outcome prediction study. BMC Pregnancy Childbirth. 2008;8:51. doi: 10.1186/1471-2393-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sovio U, White IR, Dacey A, Pasupathy D, Smith GCS. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet. 2015;386:2089–2097. doi: 10.1016/S0140-6736(15)00131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).ACOG. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- (10).Noble M, McLennan D, Wilkinson K, Whitworth A, Barnes H, Dibben C. The English Indices of Deprivation 2007. London, UK: Department for Communities and Local Government; 2008. [Google Scholar]
- (11).Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).National Collaborating Centre for Women's and Children's Health. NICE Guideline: Hypertension in pregnancy. London, UK: Royal College of Obstetricians and Gynaecologists; 2011. [Google Scholar]
- (13).Smith GC. Researching new methods of screening for adverse pregnancy outcome: lessons from pre-eclampsia. PLoS Med. 2012;9:e1001274. doi: 10.1371/journal.pmed.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks' gestation (HYPITAT): a multicentre, open-label randomised controlled trial. Lancet. 2009;374:979–988. doi: 10.1016/S0140-6736(09)60736-4. [DOI] [PubMed] [Google Scholar]
- (15).Walker KF, Bugg GJ, Macpherson M, McCormick C, Grace N, Wildsmith C, Bradshaw L, Smith GC, Thornton JG, for the 35/39 Trial Group Randomized Trial of Labor Induction in Women 35 Years of Age or Older. N Engl J Med. 2016;374:813–822. doi: 10.1056/NEJMoa1509117. [DOI] [PubMed] [Google Scholar]
- (16).Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dinkel C, et al. Soluble fms-Like Tyrosine Kinase-1-to-Placental Growth Factor Ratio and Time to Delivery in Women With Suspected Preeclampsia. Obstet Gynecol. 2016;128:261–269. doi: 10.1097/AOG.0000000000001525. [DOI] [PubMed] [Google Scholar]
- (17).Dragan I, Georgiou T, Prodan N, Akolekar R, Nicolaides KH. Screening for preeclampsia by the sFLT to PLGF ratio cut-off of 38 at 30-37 weeks' gestation. Ultrasound Obstet Gynecol. 2016 doi: 10.1002/uog.17301. Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- (18).Widmer M, Cuesta C, Khan KS, Conde-Aqudelo A, Carroli G, Fusey S, Karumanchi SA, Lapaire O, Lumbiganon P, Sequeira E, Zavaleta N, et al. Accuracy of angiogenic biomarkers at 20weeks' gestation in predicting the risk of pre-eclampsia: A WHO multicentre study. Pregnancy Hypertens. 2015;5:330–338. doi: 10.1016/j.preghy.2015.09.004. [DOI] [PubMed] [Google Scholar]
- (19).Skrastad RB, Hov GG, Blaas HG, Romundstad PR, Salvesen KA. Risk assessment for preeclampsia in nulliparous women at 11-13 weeks gestational age: prospective evaluation of two algorithms. BJOG. 2015;122:1781–1788. doi: 10.1111/1471-0528.13194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.