Abstract
Background. Malaria remains an important public health issue in Benin, with Anopheles gambiae s.l. and Anopheles funestus s.s being the predominant vectors. This study was designed to generate information on An. funestus distribution, molecular speciation, Plasmodium infection rate and insecticide susceptibility status across Benin. Methods. Mosquito samples were collected from December 2014 to January 2016 in 46 localities in Benin. These samples were mapped and An. funestus collected were speciated to the molecular level. Plasmodium infection rate was determined using a Taqman assay and susceptibility to insecticides was assessed using the WHO guidelines. The genotyping of the L119F- Gste2 mutation was also carried out. Results. An. funestus was found in 8 out of the 46 localities surveyed with a high presence in Tanongou (wet Sudanese ecological zone), Kpome, Doukonta and Pahou (sub-equatorial ecological zone). Molecular identifications revealed that only An. funestus s.s was present in southern Benin, whereas in Tanongou (northern Benin) An. funestus s.s. and An. leesoni were found in sympatry at proportions of 77.7% and 22.3% respectively. Plasmodium infection rate of An. funestus was higher in southern Benin at a range of 13 to 18% compared to 5.6% recorded in Tanongou. High DDT (8±0.5%) and permethrin (11±0.5%) resistance were observed in Doukonta, Kpome and Pahou, contrasting with relatively low resistance profiles: mortality-DDT=90±3.18% and mortality-permethrin=100% in Tanongou. Genotyping analysis revealed high frequency of the resistant 119F allele in the South (Kpome and Doukonta) compared to the North (Tanongou). Discussion and Conclusion. The high presence of An. funestus in the South compared to the North could be due to favorable environmental and climatic conditions found in both regions. A significant Plasmodium infection rate was recorded across the country. A high resistance profile was recorded in the southern Benin; this raises the need for further investigations on resistance selection factors.
Keywords: Anopheles funestus, distribution, Plasmodium infection, insecticide resistance, South-North, Benin
Background
Malaria remains a major public health challenge in Benin, with the most vulnerable populations being children less than five years and pregnant women 1. It accounts for around 37% of hospital consultations in the country 2. Efforts to eradicate this disease in Africa have focused on treatment of diagnosed cases and preventive strategies, which are mainly based on vector control, such as the use of insecticide treated nets, indoor residual spraying of insecticides and larviciding 1.
In the past decade, vector control interventions have massively contributed to the significant decrease observed in the burden of malaria across Africa, notably in Benin 3. To sustain such progress, national control programs need better knowledge on key malaria vectors nationwide, including their geographical distribution, susceptibility profile to insecticides and contribution to malaria transmission, as well as understanding the vectorial complexity of these species. Such information already exists for Anopheles gambiae across Benin 2, 4, 5, but this is not the case for the other major vector An. funestus, for which only limited information is available, mainly from few coastal populations 6, 7
An. funestus Giles is one of the key malaria-transmitting mosquitoes in Africa. The vectorial capacity of this mosquito vector is close to and could exceed that of An. gambiae, the most documented malaria vector in some countries 8. An. funestus Giles group is made up of nine species distributed across sub-Saharan Africa 9, 10. These nine species of the An. funestus group are as follows: An. funestus Giles (s.s), An. vaneedeni Gillies and Coetzee , An. leesoni Evans, An. parensis Gillies, An. rivulorum Leeson , An. fuscivenosus Leeson, An. brucei Service, An. aruni Sobti and An. confusus Evans and Leeson. These species are not easily distinguishable using morphological keys 9, 10.
The vectorial capacity of members of the An. funestus group varies significantly, with most species being zoophilic, except An. funestus s.s., which is the main Plasmodium vector in this group. Indeed high infection rates have been reported for An. funestus s.s., such as 22% 11 and 27% 12 documented in South Africa, 11% in Tanzania 13, 50% in Burkina Faso 14 and 18% in Benin 7. However, other members of the group, such as An. rivulorum has a high anthropophilic rate of 40% (42/106) in the southern region of Nigeria 15, but presents a low contribution to malaria transmission in Tanzania 16. As for An. vaneedeni, this species could be either exophilic or anthrophilic, but can easily carry the Plasmodium parasite under laboratory conditions 17, whereas An. parensis is endophilic, but does not carry the malaria parasite 15, 18, 19. In most parts of Africa, An. funestus s.s. and other members of the An. funestus group live in sympatry 9, 15, 18, and if appropriate identification is not made this could lead to wrong vectorial characterization of An. funestus s.s. This relevant information on An. funestus in Benin has been documented in some parts of the southern coastal localities of Ouidah, Kpomasse, Tori and Pahou 6, 20, but no extensive study has so far been carried out in a North-South Benin transect to determine the extent of the distribution of this species in the country and its contribution to malaria transmission.
The resistance profile of An. funestus s.s has only been explored for some coastal populations with a multiple resistance to pyrethroids, DDT and carbamates reported in the locations of Pahou 6 and Kpome 7. It remains to be established whether such resistance is distributed nationwide or not. The resistance of An. funestus species to several insecticides used in public health has been well documented in many other African countries, and for some the resistance pattern and underlying resistance mechanisms have been the same nationwide, for example in Uganda 19, whereas variations have also been observed, such as in Malawi 21. Across Africa, the resistance profile of An. funestus s.s. significantly varies with resistance to pyrethroids and carbamates observed in southern Africa (Mozambique, Malawi and South-Africa) 22– 25, whereas East African (Uganda and Kenya) populations of An. funestus are resistant to pyrethroids and DDT, but susceptible to carbamates 19, 26. Central (Cameroon) 27, 28 and West African (Ghana, Benin) populations are resistant to pyrethroid, organochlorines and carbamates 6, 29. In Benin, An. funestus s.s. population in the coastal locality of Pahou is resistant to pyrethroids, carbamates and is highly resistant to DDT 6. Furthermore, it was demonstrated that the GSTe2 gene with the L119F mutation accounts for its capacity to metabolize DDT 30.
This study aims to generate information on the distribution, Plasmodium infection rate and resistance status of An. funestus in the South-North transect of Benin to help control programs to have a better assessment of the contribution of this species nationwide and how best to control it.
Methods
Ethical statement
No ethical permit was required for this study. However, there was a focus group discussion with the community and household heads where verbal consent was obtained for mosquito collections in the community after the study aims and objectives were explained. During this research study, we did not perform insecticide spraying, night collections, or human bait for mosquito collection. All mosquitoes were sampled during daytime using electrical aspirators activated with batteries.
Study sites and mosquito collection
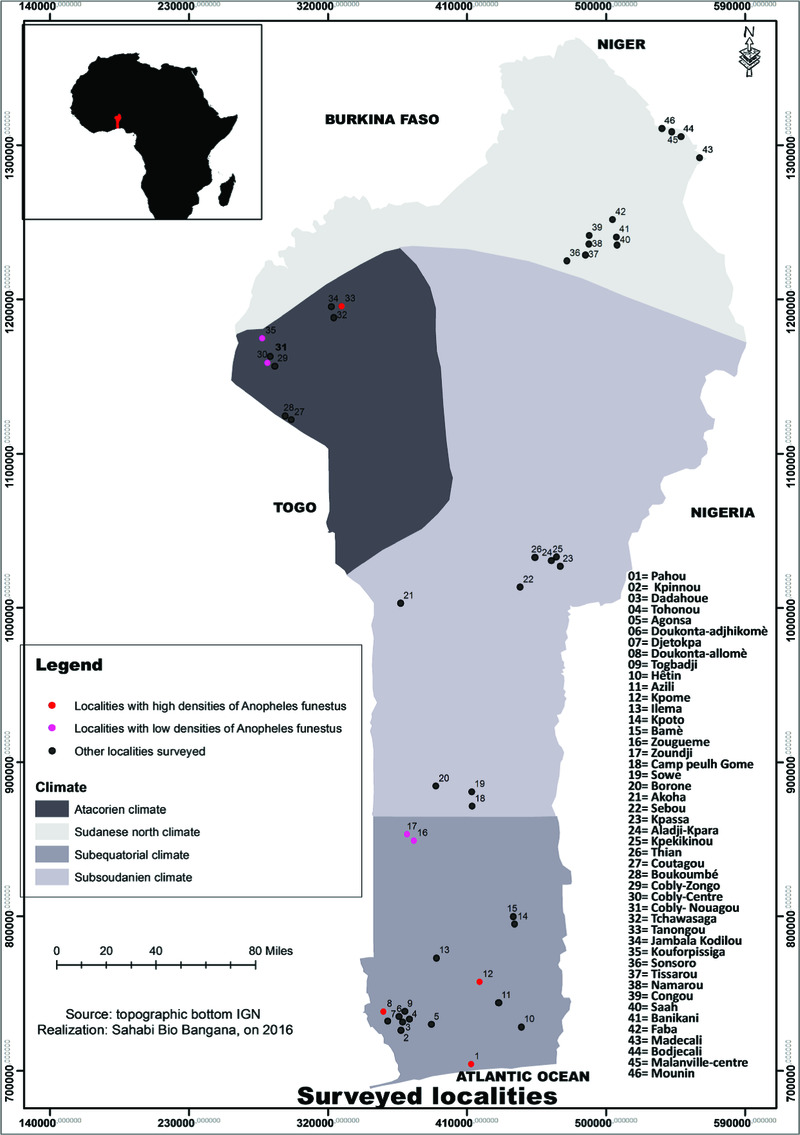
Study site description. Benin lies between the Equator and the Tropic of Cancer at latitudes ranging from 6°30′ N to 12°30′ N and longitude from 1° E to 3°40′ E. This country shares boundaries with Togo in the West, Burkina Faso and Niger in the North, and Nigeria in the East. Four main climatic zones are found in the country. The North Sudanese climatic region, which is characterized by one long dry season and a short rainy season, with low relative humidity and rainfall that is the lowest in the country (800 to 1000 mm per year). Large water bodies are found in this region and temperatures are the highest, and could reach 45°C during dry seasons. The second region is the wet Sudanese climatic zone (Atacorian). This climatic region is dominated by hills of up to 800 m of altitude and several small water bodies, which makes the region colder. Annual rainfall ranges from 1200 to 1300 mm per year, the vegetation is partially of wet savanna type, the temperature in this part of the country is the lowest. The third region is the sub-Sudanese climatic region that covers the center of the country and part of the South. This climatic region has one long rainy season and one short dry season. Rainfall is between 900 and 1200 mm, the region is less hilly and the vegetation is of wet savanna type. The fourth region is the southern sub-equatorial climatic region that spans the southern part of the country and extends up to coastal areas of Benin. This region is made up of two rainy seasons and two dry seasons. The relative humidity is high, temperatures are relatively low and the vegetation is a mosaic of coastal, wetlands, forest, and wet savanna type. Several water bodies join together in this part of the country before being channeled into the sea (Figure 1).
Figure 1. Surveyed localities in South-North of Benin.
Mosquito sampling. From December 2014 to January 2016, indoor collections of adult female mosquito were made between 06 to 10am in several localities along South-North transect of Benin using four electric aspirators. Mosquito collections were carried out in different localities and the GPS was used to determine the latitude and longitude for each sampled locality. Maps of surveyed sites and the distribution of An. funestus in Benin were developed using recorded latitudes and longitudes. For each surveyed locality, a minimum of 30 rooms were randomly selected for mosquito aspirations. These rooms were selected in a way to cover the various ecologies found in each locality. At least three days were spent in each surveyed site but for localities where An. funestus were found, the number of days was extended to five days to obtain a good number of mosquitoes to be used for various analyses. Aspirated mosquitoes were identified morphologically 9, counted and the total number for each species was recorded. All blood-fed and gravid An. funestus (F 0) collected inside houses were taken to the IITA insectary in Cotonou (Benin), where they were kept in small cups until fully gravid. The forced egg laying technique described by Morgan et al. 26 was then used to induce female An. funestus to lay eggs. Egg batches and emerging larvae from the same female mosquito were reared together and later pooled with larvae from other females, if these females were found belonging to the same molecular species. Larvae were fed daily with Tetramin™ baby fish food and the water of each larvae bowl was changed every two days to reduce the mortality. The F 1 adults generated were randomly mixed in cages for subsequent experiments.
Seasonal estimation of mosquito densities per room
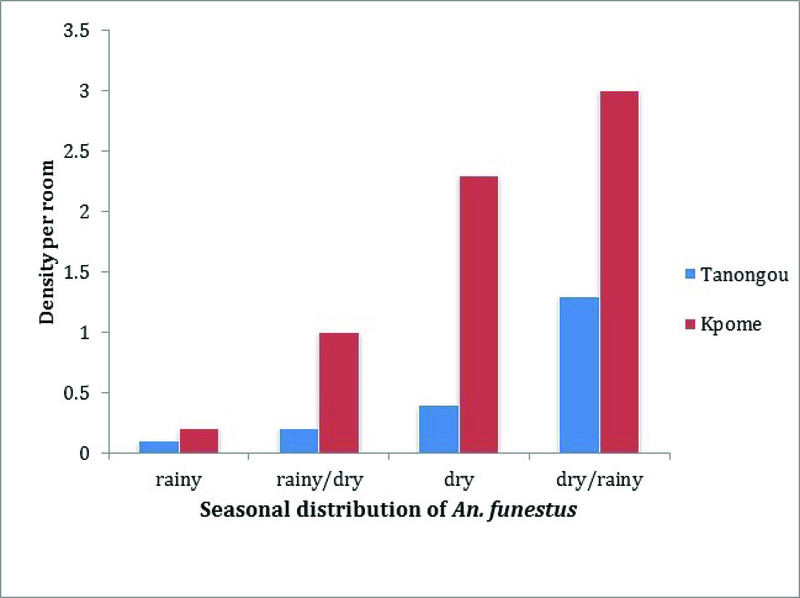
Mosquito densities per room (m/r) were estimated during four annual climatic seasons: rainy season, transition from rainy to dry season, dry season and transition from dry to rainy season. This estimation was based on the total number of An. funestus s.l. collected during each season divided by the number of rooms surveyed for mosquito collections in that season. Seasonal variations of An. funestus densities were determined per room in two localities in Benin: the locality of Tanongou in the North (wet Sudanese/Atacorian climatic region) and the locality of Kpome in the South (subequatorial climatic region). Kpome and Tanongou were selected to represent the southern and northern regions respectively, due to the high density of An. funestus recorded in these localities.
PCR species identification
For each locality, female mosquito specimens that were morphologically identified as belonging to An. funestus group 9 were subjected to DNA extractions using Qiagen DNeasy Kit followed by PCR for species identification, as described by Koekemoer et al. 31.
Plasmodium infection rate of An. funestus populations from surveyed localities
The Plasmodium infection rate was determined using the TaqMan assay 32. The reaction was performed in a 10µl final volume reaction containing 1×SensiMix (Bioline), 800 nM of each primer and 200 nM of probes labeled with fluorophores: FAM for detecting P. falciparum, and HEX for P. ovale, P. vivax and P. malariae (P. ovm). P. falciparum sample and a mixture of P. ovale, P. vivax and P. malariae were used as positive controls. The real-time PCR Agilent MX 3005 system was used for amplification with the following cycling conditions: 95°C for 10 minutes for denaturation, followed by 40 cycles of 15 seconds at 92°C and 1 minute at 60°C.
Insecticide susceptibility tests
Protocols and standard insecticide treated papers supplied by WHO 33 were used to test for insecticide susceptibility of An. funestus from selected localities in the northern and southern where there was a consistent number of ovipositing females. These selected localities were Tanongou, northern Benin in the wet Sudanese climatic region (Atacorian region), and Doukonta, southern Benin in the sub-equatorial climatic region. We assessed the susceptibility pattern of An. funestus s.s. from both localities to two insecticides of public health interest: pyrethroids type I permethrin (0.75%) used for insecticide treated nets (ITNs), and organochlorines DDT (4%) used in insecticide residual spraying (IRS). Exposed mosquitoes were fed with 10% sugar solution after 1hr of insecticide exposure after which mortalities were recorded 24hrs post exposure to insecticide treated papers 33. The wild population of An. funestus was exposed to non-treated insecticide papers as a control 33 due to lack of susceptible strains of An. funestus, (An. funestus FANG). Prior to the experiment, the effectiveness of insecticide treated papers was confirmed by exposing the susceptible strain An. gambiae kisumu to insecticide impregnated papers. WHO criteria were used to determine resistance status with mortality between 98–100% indicating susceptibility, 90–97% potential resistance, and less than 90% resistance 33.
Distribution of L119F-GSTe2 resistance allele using TaqMan assay
To assess the role of L119F mutation in DDT resistance, wild female An. funestus s.s. collected from each selected location were genotyped using the Taqman assay, as previously demonstrated 30. The reaction was performed in a 10μl final volume containing 1×SensiMix (Bioline, London, UK), 800 nM of each primer and 200 nM of each probe using an Agilent MX3005P machine. The following cycling conditions were used: 10 min at 95°C, 40 cycles of 15s at 92°C and 1 min at 60°C. Two probes labelled with fluorochromes FAM and HEX were used. The FAM was used to detect the mutant allele, while the HEX detected the wild type allele.
Data analysis
MedCalc easy-to-use online statistical software 34 was used to test for significant difference of Plasmodium infection rate and L119F-GSTe2 genotyping data in the South compared to the North of Benin.
Results
Distribution of Anopheles funestus species in a South-North transect of Benin
Out of the 46 surveyed localities (Figure 1 and Supplementary Table 1) in this study, An. funestus species were found in eight localities, generally in sympatry with An. gambiae, and spread in two geo-climatic regions of Benin. In addition, most of the sites where An. funestus species were collected were found in the western part of the country (six out of eight localities with An. funestus; Figure 1).
A total of 3179 mosquitoes belonging to different species were caught during this survey. These mosquito populations from indoor collections were dominated by Anopheles spp. 82.89% (2635), followed by Culex spp. 14.90% (474), Mansonia spp. 1.38% (44) and Aedes spp. 0.81% (26) (Supplementary Table 1). Out of the morphologically identified Anopheles spp., An. gambiae s.l. constituted 79% (2083), followed by An. funestus s.l. with 21% (552). No other Anopheles species was collected during the sampling period.
Distribution of An. funestus in various geo-climatic regions of Benin
An. funestus was not found in either the dry Sudanese climatic region (no An. funestus collected in the 11 surveyed localities), nor in the transition region between the Sudanese and the sub-equatorial climatic regions (the sub-Sudanese climatic region), where no An. funestus was found in the nine surveyed localities (Figure 1 and Supplementary Table 1). All An. funestus samples collected were either from the southern sub-equatorial region (An. funestus found in five out of the 17 surveyed localities) or the northern wet Sudanese climatic region of the Atacora (three localities with An. funestus out of nine surveyed). It is worth indicating that most sampled specimens of An. funestus were found in the western part of Benin (six localities out of eight with An. funestus). Out of the 552 morphologically identified An. funestus sampled during this survey, 319 samples were from localities situated in the sub-equatorial climatic region and 233 from the wet Sudanese climatic region (Atacorian region). High densities of An. funestus were recorded in Kpome (243 An. funestus) and Tanongou (229 An. funestus), localities from the sub-equatorial climatic region and the wet Sudanese climatic region, respectively (Figure 1).
Seasonal variations of An. funestus density in the northern (Tanongou) and the southern (Kpome) localities of Benin
Generated data from Kpome during the four monitored seasons revealed a higher An. funestus density per room (m/r) during the transition period from dry to rainy season (3 m/r). The lowest number of An. funestus (0.2 m/r) was recorded during rainy season. Densities of 1 and 2.3 m/r were documented during the transition from rainy to dry season and the dry season, respectively. A similar trend was observed in Tanongou with a higher density of An. funestus recorded during the transition from dry to rainy season (1.3 m/r), followed by the dry season with a density of 0.4 m/r, the transition from rainy to dry season and the rainy season had densities of 0.2 and 0.1 m/r, respectively. Comparative analysis of An. funestus densities at Kpome and Tanongou revealed a relatively higher rate of An. funestus mosquitoes per room at Kpome throughout all the four identified seasons compared to Tanongou (Figure 2).
Figure 2. Seasonal distribution of Anopheles funestus (densities per room) in Kpome and Tanongou.
Distribution of members of An. funestus group across Benin
PCR species detection of the 552 morphologically identified An. funestus individuals revealed a predominance of An. funestus s.s. in the two climatic regions where An. funestus was found in Benin. In the wet Sudanese climatic region, and more specifically in Tanongou, An. funestus s.s. was found in sympatry with its sister species An. leesoni. Out of the 229 An. funestus s.l. aspirated indoors at Tanongou, 178 were An. funestus s.s. and 51 were An. leesoni. In contrast, in the southern locality of Kpome where the highest density of An. funestus was recorded (243 An. funestus s.l.), as well as Doukonta and Pahou, no other member of the group apart from An. funestus s.s. was found (Table 1).
Table 1. Distribution of members of Anopheles funestus group in the North-South Benin.
| Localities |
An. funestus s.l. subjected
to molecular speciation |
An. funestus s.s. | An. leesoni |
|---|---|---|---|
| Doukonta | 15 | 15 | 0 |
| Zoundji | 3 | 3 | 0 |
| Zougueme | 1 | 1 | 0 |
| Kouforpissiga | 3 | 3 | 0 |
| Cobly centre | 1 | 1 | 0 |
| Pahou | 57 | 57 | 0 |
| Tanongou | 229 | 178 | 51 |
| Kpome | 243 | 243 | 0 |
| Total | 552 | 501 | 51 |
Plasmodium infection rate of identified members of An. funestus group
Taqman results (n=552) showed that An. funestus mosquitoes from the sub-equatorial climatic localities of the southern Benin were significantly infected with Plasmodium compared with those from the wet Sudanese localities of the northwestern Benin (Atacorian region) (P=0.0001). An. funestus from Kpome, Pahou and Doukonta in southern Benin had Plasmodium infection rates of 18.51, 15.78 and 13.33%, respectively. However, in northwestern Benin, only An. funestus s.s. from Tanongou was infected with Plasmodium with an infection rate of 5.62% (Table 2). Plasmodium infection was absent in all the 51 An. leesoni specimens analysed during this course of research (Table 2).
Table 2. Plasmodium infection rate of members of Anopheles funestus group in different localities of Benin.
| Locality | Species | Mosquito
analyzed |
Total
infected |
Plasmodium
infection rate (%) |
|---|---|---|---|---|
| Kpome | An. funestus s.s. | 243 | 45 | 18.51 |
| Pahou | An. funestus s.s. | 57 | 9 | 15,78 |
| Doukonta | An. funestus s.s. | 15 | 2 | 13.33 |
| Cobly | An. funestus s.s. | 1 | 0 | 0 |
| Koufforpissiga | An. funestus s.s. | 3 | 0 | 0 |
| Zoundji | An. funestus s.s. | 3 | 0 | 0 |
| Zoungueme | An. funestus s.s. | 1 | 0 | 0 |
| Tanongou | An. funestus s.s. | 178 | 10 | 5.62 |
| An. leesoni | 51 | 0 | 0 | |
| Total | 552 | 66 |
Comparative insecticide susceptibility tests of An. funestus s.s. in the northern (Tanongou) and the southern (Doukonta) localities of Benin
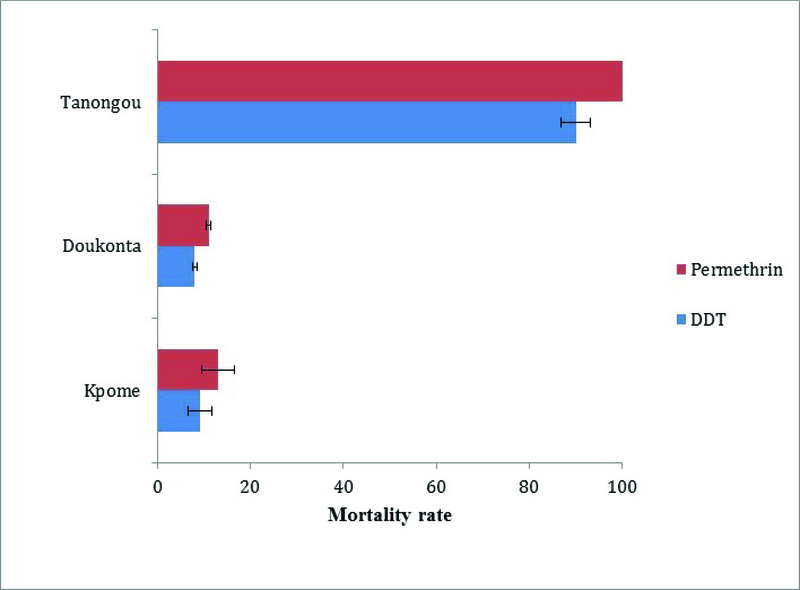
Insecticide susceptibility tests of An. funestus s.s. from Doukonta, Pahou 6 and Kpome 7 in the South, and Tanongou in northern Benin were assessed. In total, 100 females each (F 1 generated from F 0 oviposition) of An. funestus s.s. from Doukonta were exposed to DDT and permethrin. Similarly, 100 An. funestus s.s. from Tanongou were exposed to permethrin and DDT. Results revealed low mortalities to DDT (8±0.5%) and permethrin (11±0.5%) for An. funestus s.s. from Doukonta, whereas the Tanongou population had higher mortality rates to DDT (90±3.18%) and permethrin (100%). This shows that there is a higher resistance in Doukonta compared to Tanongou (Figure 3). Similarly, high resistance levels have been previously documented in southern localities of Pahou and Kpome 6, 7.
Figure 3. Insecticide resistance profiles of Anopheles funestus populations in Kpome (South Benin), Doukonta (South Benin) and Tanongou (North Benin).
Screening of L119F- GSTe2 mutation in a wild population of Anopheles funestus from Benin
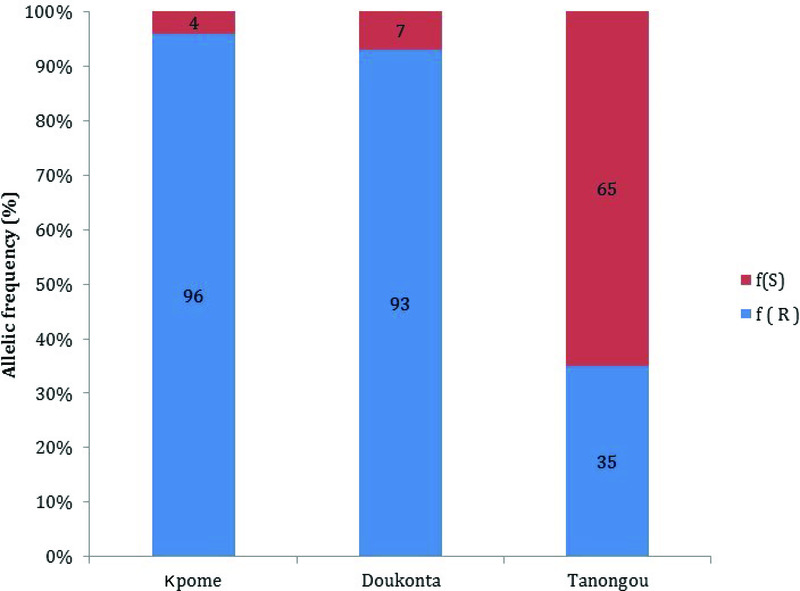
Genotyping of the L119F-Gste2 mutation in wild An. funestus population from each of the selected locations revealed the presence of the resistant 119F allele at a high frequency: 96% in Kpome 7, 83.2% in Doukonta (southern Benin), while in Tanongou (North Benin), 35% mutant allelic frequency was recorded. No susceptible allele (SS) was observed either in Kpome or Doukonta mosquitoes, showing that the 119F gene is close to fixation in the An. funestus populations of these two locations in the southern Benin. A significant difference (P≤0.0001) was observed between the 119F allelic frequency recorded in Kpome and Doukonta, where a high resistance to DDT was observed compared to Tanongou (Figure 4).
Figure 4. Allelic frequency of the L119F-GSTe2 mutation in wild Anopheles funestus populations (F 0) from Kpome (South Benin), Doukonta (South Benin) and Tanongou (North Benin).
Discussion
This research was designed to map the distribution of An. funestus in Benin and compare the insecticide resistance profile of this malaria vector in the North-South transect, as well as their infection rates with Plasmodium species, for improved knowledge on this malaria vector and enhanced performances of current malaria control tools.
Distribution of An. funestus and its implication in malaria transmission in the various geo-climatic settings of Benin
An. funestus was mainly found in the southern and the northwestern localities of Benin in this study. In these two geo-climatic regions, there seem to be a high tendency of this species to colonize the western areas of the country (north and southwestern). The relatively high presence of this vector in the western part of Benin could be explained by the humidity, relatively low temperatures associated with the hilly landscape, and the presence of rivers and streams covered with vegetation 35. This shows that this species prefers more permanent water bodies with vegetation usually found along rivers, streams and lakes 36, whereas An. gambiae tends to oviposit in temporary breeding sites, such as puddles and animal foot prints 37. Very little or no population of An. funestus was found in the dry Sudanese climatic region of northeastern Benin. The low presence of this mosquito species in this dry hot region (low rain falls and temperature reaching 45°C during dry seasons) is either due to the period of sampling or the low presence of permanent fresh water bodies covered with vegetation coupled with dryness of the region 38.
The density of An. funestus species collected indoor in this research further confirms their endophilic behavior 39. Two species of the An. funestus group were identified during this study: An. funestus s.s. and An. leesoni. Contrary to An. funestus s.s., there was no trace of Plasmodium DNA in the 51 samples of An. leesoni analyzed. This result confirms the low/no implication of An. leesoni in the transmission of malaria, as previously documented 14, 39, which is notable in West Africa as this species is known to be highly zoophilic. While placing a low epidemiological interest on An. leesoni, this study further highlights the need for a high focus on An. funestus s.s. for improved control of malaria in Benin 7. Recorded infection rates of An. funestus were more than three times higher in screened localities of southern localities (Kpome, Pahou, Doukonta) compared to the North (Tanongou), suggesting a higher implication of An. funestus in malaria transmission in the southern part of the country where its density is also high. The high Plasmodium infection rates observed in southern Benin are similar to some infection rates documented in several African countries in this species; Plasmodium falciparum infection rates of 22 11 and 27% 12 have been found in An. funestus populations of South Africa. In countries from the western part of Africa, a mean rate of infectivity between 3 and 15% has been observed, including in Burkina Faso 14, 40 and recently in Ghana 41. In Burkina Faso, Dabire et al. 40 documented the presence of Plasmodium in An. funestus (20% infection rate) from Lena during the month of August 2000. In Benin, two studies recently conducted in southern localities revealed Plasmodium infection rates of 13.6 and 18.27% in An. funestus 7, 42. This study has shown a similar trend in the densities of An. funestus in both screened ecological zones throughout the year. High densities of An. funestus mosquitoes were recorded during the transition from dry to rainy season followed by the dry season, then the transition from the rainy to dry season and finally the rainy season, where the least density of An. funestus were recorded. The involvement of An. funestus in the transmission of malaria during dry seasons was also documented in Ghana 43, Nigeria 15, Burkina Faso 14, 40, and more recently in southern Benin 44.
Comparative insecticides susceptibility tests of An. funestus s.s. from southern (Doukonta) and northern (Tanongou) localities of Benin
Comparative analysis of insecticide resistance profiles in An. funestus populations from Doukonta (southern Benin) and Tanongou (northern Benin) reveals that An. funestus s.s. from Doukonta are relatively more resistant to DDT and permethrin (mortality rates of 8±0.5 and 11±0.5%, respectively) than those from Tanongou, where only a moderate resistance was observed to DDT (mortality rate of 90±3.18%) and a full susceptibility to permethrin (100%). High resistance to DDT and permethrin had previously been reported in populations of An. funestus from two other localities of southern Benin, Pahou and Kpome 6, 7. In addition to the use of agricultural insecticides in both the northern and southern surveyed sites, the high insecticide resistance observed in the South could be associated with environmental factors, such as urbanization, which increases the level of xenobiotics (pollution) in Anopheles breeding sites and could favor the selection of cross resistance to permethrin and DDT in southern Benin compared to northwestern Benin with less urbanization and pollution 45. Recorded resistance profiles could also be associated with a relatively high flow of genes among An. funestus populations in southern Benin compared to the North, particularly if there are some barriers to gene flow, which needs to be investigated further. Other factors of resistance selection, such as the relatively high use of ITNs/IRS (use of public health insecticides) in the southern Benin compared to the North, might have also contributed to observed high resistance profile of mosquitoes 6, 46– 50. Similar observations have been documented on An. gambiae s.l. in the North and South of Benin where increased pyrethroid resistance is also prevalent in An. gambiae s.l. species in South Benin 51– 53 than in the North, mirroring the pattern that was observed here for An. funestus. Resistance to DDT and permethrin is also widely distributed in An. gambiae in Benin 4, 54.
Distribution of L119F-GSTe2 mutation in An. funestus populations in Benin
The high frequency of the 119F-GSTe2 resistant allele in Kpome and Doukonta where high phenotypic resistance to DDT was also observed; both results suggest that this mutation plays an important role in DDT resistance in West Africa, as previously documented 30. Indeed, consistent frequencies of this resistance allele were also recorded in other DDT resistant populations in Central and West Africa notably in Cameroon (52%), Ghana (44%) and Burkina Faso (25%) in accordance with the previously reported prevalence of DDT resistance in these countries 27– 29. The resistant 119F allele was detected in An. funestus populations from Tanongou, but with a relatively low frequency (35%), reflecting the moderate level of DDT resistance recorded. This result is in line with the detection of low frequencies of this resistant allele in the eastern African An. funestus of Uganda (20.4%) and Kenya (7.8%), which is associated with a moderate level of DDT phenotypic resistance observed in this region 19, 26. However, this observation is different in southern Africa where this mutation is completely absent despite recent reports of DDT resistance 25, suggesting that DDT resistance in southern Africa is driven by a different mechanism to that observed in West and Central Africa. These heterogeneities in L119F frequencies suggest that there are different mechanisms responsible for the DDT resistance in An. funestus populations across Africa.
Conclusion
This study has generated key relevant information on the bionomics of An. funestus in Benin, including its seasonal distribution in a South-North transect, its Plasmodium infection rate and its resistance profiles to permethrin and DDT in the southern and northern ecological zones. The contrasting profiles observed between southern and northern populations of An. funestus were evident in the present study in terms of density, contribution to malaria transmission and resistance to insecticides. The factors behind these differences need further investigation. Overall, the high density of An. funestus in the south and northwestern Benin coupled with the consistent high Plasmodium infection level of this Anopheles species and its high resistance to insecticides in the South strengthens the need for more research on this species for improved performances of malaria control programs in Benin.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2016 Djouaka R et al.
Raw data are available at the Open Science Framework: DOI, 10.17605/OSF.IO/Y3B8P 55.
Abbreviations
INSAE: Institut National de la Statistique et de l'Analyse Economique; DDT: Dichlorodiphenyltrichloroethane; m/r: mosquito per room; spp: Species; PCR: Polymerase Chain Reaction; WHO: World Health Organization.
Acknowledgements
We appreciate all surveyed communities for their cooperation and assistance during fieldwork. We thank Claude Gande and Murielle Soglo for their technical assistance and relevant advice in the course this study.
Grant Information
This work is supported by the Wellcome Trust [099864], [101893].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
Supplementary material
Supplementary Table 1: The surveyed 46 localities, and the species of mosquitoes collected in each locality.
.
References
- 1. WHO: World malaria report. Geneva, Switzerland, World Health Organization;2016. Reference Source [Google Scholar]
- 2. Ossè R, Gnanguenon V, Sèzonlin M, et al. : Relationship between the presence of kdr and Ace-1 mutations and the infection with Plasmodium falciparum in Anopheles gambiae s.s. in Benin. J Parasitol Vector Biol. 2012;4(3):31–9. Reference Source [Google Scholar]
- 3. WHO: A new malaria framework for Africa. Geneva, Switzerland, World Health Organization;2016. Reference Source [Google Scholar]
- 4. Aïkpon R, Ossè R, Govoetchan R, et al. : Entomological baseline data on malaria transmission and susceptibility of Anopheles gambiae to insecticides in preparation for Indoor Residual Spraying (IRS) in Atacora, (Benin). J Parasitol Vector Biol. 2013;5(7):102–11. Reference Source [Google Scholar]
- 5. Gnanguenon V, Govoetchan R, Agossa FR, et al. : Transmission patterns of Plasmodium falciparum by Anopheles gambiae in Benin. Malar J. 2014;13:444. 10.1186/1475-2875-13-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Djouaka R, Irving H, Tukur Z, et al. : Exploring mechanisms of multiple insecticide resistance in a population of the malaria vector Anopheles funestus in Benin. PLoS One. 2011;6(11):e27760. 10.1371/journal.pone.0027760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Djouaka R, Riveron JM, Yessoufou A, et al. : Multiple insecticide resistance in an infected population of the malaria vector Anopheles funestus in Benin. Parasit Vectors. 2016;9:453. 10.1186/s13071-016-1723-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fontenille D, Lochouarn L, Diagne N, et al. : High annual and seasonal variations in malaria transmission by Anophelines and vector species composition in Dielmo, a holoendemic area in Senegal. Am J Trop Med Hyg. 1997;56(3):247–53. [DOI] [PubMed] [Google Scholar]
- 9. Gillies MT, Coetzee M: A Supplement to the Anophelinae of Africa South of the Sahara. Publ South African Inst Med Res. 1987;55:63 Reference Source [Google Scholar]
- 10. Gillies MT, De Meillon B: The Anophelinae of Africa south of the Sahara. 2nd ed. The South African Institute for Medical Research, Johannesburg, South Africa.1968; 54:343 Reference Source [Google Scholar]
- 11. De Meillon B: On Anopheles funestus and its allies in the Transvaal. Ann Trop Med Parasitol. 1933;27(1):83–97. 10.1080/00034983.1933.11684741 [DOI] [Google Scholar]
- 12. Swellengrebel NH, Annecke S, De Meillon B: Malaria investigations in some parts of the Transvaal and Zululand. Publ S Afr Inst Med Res. 1931;4(27):245–74. Reference Source [Google Scholar]
- 13. Temu EA, Minjas JN, Coetzee M, et al. : The role of four anopheline species (Diptera: Culicidae) in malaria transmission in coastal Tanzania. Trans R Soc Trop Med Hyg. 1998;92(2):152–8. 10.1016/S0035-9203(98)90724-6 [DOI] [PubMed] [Google Scholar]
- 14. Costantini C, Sagnon N, Ilboudo-Sanogo E, et al. : Chromosomal and bionomic heterogeneities suggest incipient speciation in Anopheles funestus from Burkina Faso. Parassitologia. 1999;41(4):595–611. [PubMed] [Google Scholar]
- 15. Awolola TS, Oyewole IO, Koekemoer LL, et al. : Identification of three members of the Anopheles funestus (Diptera: Culicidae) group and their role in malaria transmission in two ecological zones in Nigeria. Trans R Soc Trop Med Hyg. 2005;99(7):525–31. 10.1016/j.trstmh.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 16. Wilkes TJ, Matola YG, Charlwood JD: Anopheles rivulorum, a vector of human malaria in Africa. Med Vet Entomol. 1996;10(1):108–10. 10.1111/j.1365-2915.1996.tb00092.x [DOI] [PubMed] [Google Scholar]
- 17. De Meillon B, Van Eeden GJ, Coetzee L, et al. : Observation on a species of the Anopheles funestus subgroup, a suspected exophilic vector of malaria parasites in northeastern Transvaal, South Africa. Mosq News. 1977;37(4):657–61. Reference Source [Google Scholar]
- 18. Kamau L, Koekemoer LL, Hunt RH, et al. : Anopheles parensis: the main member of the Anopheles funestus species group found resting inside human dwellings in Mwea area of central Kenya toward the end of the rainy season. J Am Mosq Control Assoc. 2003;19(2):130–3. [PubMed] [Google Scholar]
- 19. Mulamba C, Riveron JM, Ibrahim SS, et al. : Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS One. 2014;9(10):e110058. 10.1371/journal.pone.0110058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Djènontin A, Bio-Bangana S, Moiroux N, et al. : Culicidae diversity, malaria transmission and insecticide resistance alleles in malaria vectors in Ouidah-Kpomasse-Tori district from Benin (West Africa): A pre-intervention study. Parasit Vectors. 2010;3:83. 10.1186/1756-3305-3-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnes KG, Irving H, Chiumia M, et al. : Restriction to gene flow is linked to changes in the molecular basis of pyrethroid resistance in the malaria vector Anopheles funestus. Proc Natl Acad Sci U S A.In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hargreaves K, Koekemoer LL, Brooke BD, et al. : Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14(2):181–9. 10.1046/j.1365-2915.2000.00234.x [DOI] [PubMed] [Google Scholar]
- 23. Cuamba N, Morgan JC, Irving H, et al. : High level of pyrethroid resistance in an Anopheles funestus population of the chokwe district in mozambique. PLoS One. 2010;5(6):e11010. 10.1371/journal.pone.0011010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wondji CS, Coleman M, Kleinschmidt I, et al. : Impact of pyrethroid resistance on operational malaria control in Malawi. Proc Natl Acad Sci U S A. 2012;109(47):19063–70. 10.1073/pnas.1217229109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riveron JM, Chiumia M, Menze BD, et al. : Rise of multiple insecticide resistance in Anopheles funestus in Malawi: a major concern for malaria vector control. Malar J. 2015;14:344. 10.1186/s12936-015-0877-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgan JC, Irving H, Okedi LM, et al. : Pyrethroid resistance in an Anopheles funestus population from uganda. PLoS One. 2010;5(7):e11872. 10.1371/journal.pone.0011872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wondji CS, Dabire RK, Tukur Z: Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect Biochem Mol Biol. 2011;41(7):484–91. 10.1016/j.ibmb.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Menze BD, Riveron JM, Ibrahim SS, et al. : Multiple Insecticide Resistance in the Malaria Vector Anopheles funestus from Northern Cameroon Is Mediated by Metabolic Resistance Alongside Potential Target Site Insensitivity Mutations. PLoS One. 2016;11(10):e0163261. 10.1371/journal.pone.0163261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okoye PN, Brooke BD, Koekemoer LL, et al. : Characterisation of DDT, pyrethroid and carbamate resistance in Anopheles funestus from Obuasi, Ghana. Trans R Soc Trop Med Hyg. 2008;102(6):591–8. 10.1016/j.trstmh.2008.02.022 [DOI] [PubMed] [Google Scholar]
- 30. Riveron JM, Yunta C, Ibrahim SS, et al. : A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014;15(2):R27. 10.1186/gb-2014-15-2-r27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koekemoer LL, Kamau L, Hunt RH, et al. : A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66(6):804–11. [DOI] [PubMed] [Google Scholar]
- 32. Bass C, Nikou D, Blagborough AM, et al. : PCR-based detection of Plasmodium in Anopheles mosquitoes: a comparison of a new high-throughput assay with existing methods. Malar J. 2008;7:177. 10.1186/1475-2875-7-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. WHO: World malaria report. Geneva, Switzerland, World Health Organization. Geneva, Switzerland.2013. Reference Source [Google Scholar]
- 34. MedCalc: Easy-to-use online statistical software. Reference Source [Google Scholar]
- 35. INSAE: Projection démographique révisée. Atacora.2004;29. [Google Scholar]
- 36. Coetzee M, Fontenille D: Advances in the study of Anopheles funestus, a major vector of malaria in Africa. Insect Biochem Mol Biol. 2004;34(7): 599–605. 10.1016/j.ibmb.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 37. Minakawa N, Sonye G, Yan G: Relationships between occurrence of Anopheles gambiae s.l. (Diptera: Culicidae) and size and stability of larval habitats. J Med Entomol. 2005;42(3):295–300. 10.1093/jmedent/42.3.295 [DOI] [PubMed] [Google Scholar]
- 38. INSAE: Cahier des villages et quartiers de ville Département du BORGOU. Direction des Etudes Démographiques Cotonou,2004. Reference Source [Google Scholar]
- 39. Dia I, Guelbeogo MW, Ayala D: Advances and Perspectives in the Study of the Malaria Mosquito Anopheles funestus .2013. 10.5772/55389 [DOI] [Google Scholar]
- 40. Dabiré KR, Baldet T, Diabaté A, et al. : Anopheles funestus (Diptera: Culicidae) in a humid savannah area of western Burkina Faso: bionomics, insecticide resistance status, and role in malaria transmission. J Med Entomol. 2007;44(6):990–7. 10.1093/jmedent/44.6.990 [DOI] [PubMed] [Google Scholar]
- 41. Riveron JM, Osae M, Egyir-Yawson A, et al. : Multiple insecticide resistance in the major malaria vector Anopheles funestus in southern Ghana: implications for malaria control. Parasit Vectors. 2016;9(1):504. 10.1186/s13071-016-1787-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandeu MM, Moussiliou A, Moiroux N, et al. : Optimized Pan-species and speciation duplex real-time PCR assays for Plasmodium parasites detection in malaria vectors. PLoS One. 2012;7(12):e52719. 10.1371/journal.pone.0052719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dadzie SK, Brenyah R, Appawu MA: Role of species composition in malaria transmission by the Anopheles funestus group (Diptera: Culicidae) in Ghana. J Vector Ecol. 2013;38(1):105–10. 10.1111/j.1948-7134.2013.12015.x [DOI] [PubMed] [Google Scholar]
- 44. Moiroux N, Gomez MB, Pennetier C, et al. : Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;206(10):1622–9. 10.1093/infdis/jis565 [DOI] [PubMed] [Google Scholar]
- 45. Akogbéto MC, Djouaka RF, Kindé-Gazard DA: Screening of pesticide residues in soil and water samples from agricultural settings. Malar J. 2006;5:22. 10.1186/1475-2875-5-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Djouaka RF, Bakare AA, Bankole HS, et al. : Does the spillage of petroleum products in Anopheles breeding sites have an impact on the pyrethroid resistance? Malar J. 2007;6:159. 10.1186/1475-2875-6-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Djouaka RF, Bakare AA, Coulibaly ON, et al. : Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from Southern Benin and Nigeria. BMC Genomics. 2008;9:538. 10.1186/1471-2164-9-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nkya TE, Akhouayri I, Poupardin R, et al. : Insecticide resistance mechanisms associated with different environments in the malaria vector Anopheles gambiae: a case study in Tanzania. Malar J. 2014;13:28. 10.1186/1475-2875-13-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Antonio-Nkondjio C, Fossog BT, Ndo C, et al. : Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaoundé (Cameroon): influence of urban agriculture and pollution. Malar J. 2011;10:154. 10.1186/1475-2875-10-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Czeher C, Labbo R, Arzika I, et al. : Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189. 10.1186/1475-2875-7-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Corbel V, N’Guessan R, Brengues C, et al. : Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101(3):207–16. 10.1016/j.actatropica.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 52. Djègbè I, Boussari O, Sidick A, et al. : Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar J. 2011;10:261. 10.1186/1475-2875-10-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Djogbénou L, Pasteur N, Bio-Bangana S, et al. : Malaria vectors in the Republic of Benin: distribution of species and molecular forms of the Anopheles gambiae complex. Acta Trop. 2010;114(2):116–22. 10.1016/j.actatropica.2010.02.001 [DOI] [PubMed] [Google Scholar]
- 54. Yadouleton AW, Padonou G, Asidi A, et al. : Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J. 2010;9:83. 10.1186/1475-2875-9-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Djouaka R, Akoton R, Tchigossou GM, et al. : Mapping of the distribution, plasmodium infection rate and insecticide susceptibility of Anopheles funestus in Benin.2016. Data Source [DOI] [PMC free article] [PubMed]