Abstract
Objective
Aspirin together with thienopyridine P2Y12 inhibitors, commonly clopidogrel, is a cornerstone of antiplatelet therapy. However, many patients receiving this therapy display high on-treatment platelet reactivity (HTPR) which is a major therapeutic hurdle to the prevention of recurrent thrombotic events. The emergence of uninhibited platelets following thrombopoiesis has been proposed as a contributing factor to HTPR. Here we investigate the influences of platelet turnover on platelet aggregation in the face of different dual antiplatelet therapy strategies.
Approach and Results
Traditional light transmission aggregometry, cytometry, advanced flow cytometric imaging and confocal microscopy were used to follow the interactions of populations of platelets from healthy volunteers and patients with stable cardiovascular disease. Newly formed, reticulated platelets over-proportionately contributed to, and clustered at, the core of forming aggregates. This phenomenon was particularly observed in samples from patients treated with aspirin plus a thienopyridine, but was absent in samples taken from patients treated with aspirin plus ticagrelor.
Conclusions
Reticulated platelets are more reactive than older platelets, and act as seeds for the formation of platelet aggregates even in the presence of antiplatelet therapy. This is coherent with the emergence of an uninhibited subpopulation of reticulated platelets during treatment with aspirin plus thienopyridine, explained by the short pharmacokinetic half-lives of these drugs. This phenomenon is absent during treatment with ticagrelor, due to its longer half-life and ability to act as a circulating inhibitor. These data highlight the importance influences of pharmacokinetics on anti-platelet drug efficacies especially in diseases associated with increased platelet turnover.
Keywords: aspirin, P2Y12, anti-platelet therapy, platelet
Subject codes: platelets, pharmacology, acute coronary syndromes, thrombosis
Introduction
Platelets are central to the processes underlying atherothrombotic events and consequently are the target of well established prophylactic therapy. The drug dosing regimen referred to as dual antiplatelet therapy (DAPT) typically comprises aspirin combined with a P2Y12 receptor antagonist, commonly the thienopyridine compound clopidogrel. 1–4 The reoccurrence of thrombotic events during therapy represents a major therapeutic hurdle and is associated with high on-treatment platelet reactivity (HTPR).3, 4 However, the causes of HTPR and thrombotic complications are complex and require deeper investigation to improve anti-thrombotic therapies.3–5
One potential contributing factor to HTPR is an increased rate of platelet turnover. There are a notable number of pathological states linked to HTPR where platelet turnover and the circulating levels of newly formed immature platelets are increased. In particular, a recent study has associated elevated immature platelet counts, a measure of platelet turnover, with adverse cardiovascular outcomes.6 This relationship is clearly demonstrated in patients with chronic renal failure requiring haemodialysis whose reticulated platelet proportion increases threefold.7 Additionally there is a strong association between poor clopidogrel responsiveness and increased thrombotic risk.8, 9 Nonetheless a pathophysiological mechanism has yet to be identified.
Aspirin and clopidogrel, the most widely used P2Y12 receptor antagonist, irreversibly bind their respective targets, but are short-lived in the circulation. This suggests that as standard daily doses of either drug are quickly cleared newly formed platelets subsequently entering the circulation will remain uninhibited until the next dose is taken. We have recently demonstrated that uninhibited platelets can act as seeds for aggregate formation during antiplatelet therapy.10 Therefore, in patients with pathologies in which platelet production is increased larger subpopulations of these uninhibited platelets will arise. Compounding this, newly produced immature, or reticulated, platelets appear to be generally more reactive.11
The more recently developed non-thienopyridine P2Y12 antagonist ticagrelor is, unlike thienopyridines, pharmacokinetically long-lived with a circulating half-life of about 8 hours. With standard twice daily dosing it consistently circulates at inhibitory concentrations. Also unlike thienopyridines, ticagrelor acts directly as a reversibly-binding antagonist of P2Y12 receptors, and may consequently provide more thorough anti-thrombotic cover than short-lived thienopyridines.12
Here we compare pharmacokinetically different anti-thrombotic regimens in healthy volunteers, and examine their relationship with uninhibited platelets. In a previous in vitro study we mimicked the in vivo interaction of differently inhibited platelet populations by separately labelling and recombining platelets.10 In this ex vivo study we have for the first time directly examined the functionality of reticulated platelets, those most likely to be uninhibited, in samples from patients with stable cardiovascular disease and receiving DAPT. Finally we describe a mechanism by which newly formed reticulated platelets may promote HTPR and potentially explain the reported increased effectiveness of ticagrelor over thienopyridines.13, 14
Methods
Materials and Methods are available in the online-only Data Supplement.
Results
In vitro modelling identifies differential inhibition between thienopyridines and ticagrelor
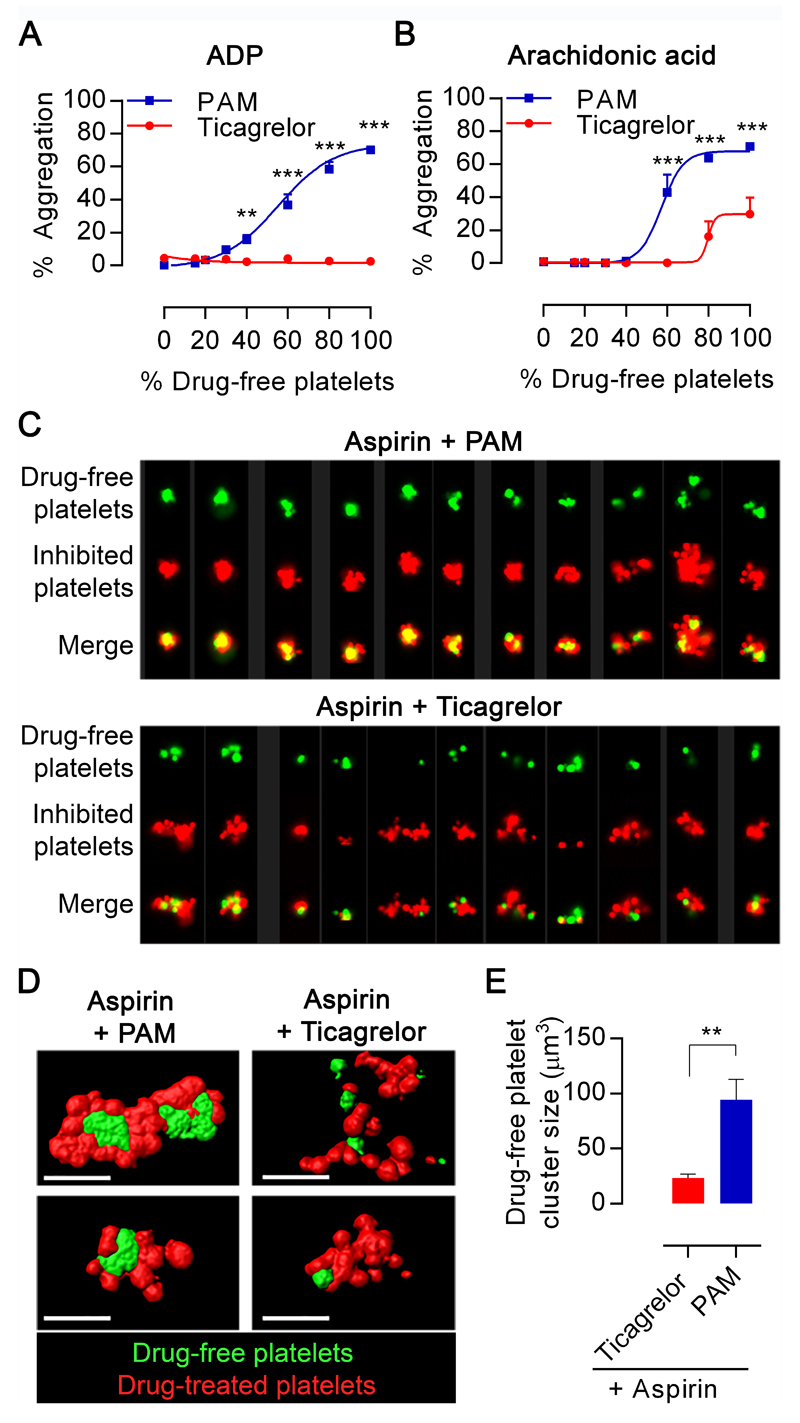
Drug-free platelets were added to platelets pre-incubated with drug to model the in vitro functional consequences of platelet turnover during DAPT treatment comprising aspirin+prasugrel or aspirin+ticagrelor. This modelling demonstrated that for samples treated with prasugrel active metabolite (PAM) responses to ADP returned with increases in the proportion of inhibitor-free platelets. In contrast, aggregatory responses remained inhibited in samples treated with ticagrelor (Figure 1A & 1B). Analysis by flow cytometry and confocal microscopy of aggregates formed from labelled platelet subpopulations indicated that drug-free platelets were over-proportionately recruited to the formed aggregates in samples treated with aspirin+PAM. This was evidenced by clear clustering of uninhibited platelets at the cores of the formed aggregates. In contrast, analysis of samples treated with aspirin+ticagrelor did not show this bias (Figure 1C). Quantitative analyses of aggregate images obtained by confocal microscopy demonstrated a significantly bigger core volume when drug-free platelets were mixed with aspirin+PAM-treated samples (95±18 µm3) than with aspirin+ticagrelor-treated samples (24±4 µm3; Figure 1D & 1E).
Figure 1. Ticagrelor reduces aggregation and prevents formation of drug-free platelet cores during aggregate formation in vitro.
PRP derived from blood pre-incubated with aspirin (30µM) and PAM (3µM) or ticagrelor (1.35µM) was mixed in a range of proportions with PRP from blood pre-incubated with respective vehicles or with ticagrelor (1.35µM) to reflect mid-dose t=6hour levels. Aggregation in response to (A) ADP 20µM or (B) AA 1mM was determined by LTA. Data presented as mean±SEM and compared by two-way ANOVA (n=4, **p<0.01, ***p<0.001). (C) Multiple images captured by ImageStreamX of aggregates (mixtures of 85% aspirin+PAM-pretreated platelets or aspirin+ticagrelor pretreated platelets plus 15% uninhibited platelets). Each panel contains columns with following image sets: drug-free (green), inhibited platelets (red), merged image. (D) Representative confocal images of aggregates (left) formed from mixtures comprising 85% aspirin+PAM-pretreated platelets or aspirin+ticagrelor-pretreated (green) and 15% uninhibited platelets (red). (E) Images were analysed for size of the uninhibited platelet particles. Data is presented as mean±SEM and compared by t-test (n=4, **p<0.01).
Ticagrelor but not prasugrel therapy inhibits drug-free platelets upon in vitro transfusion
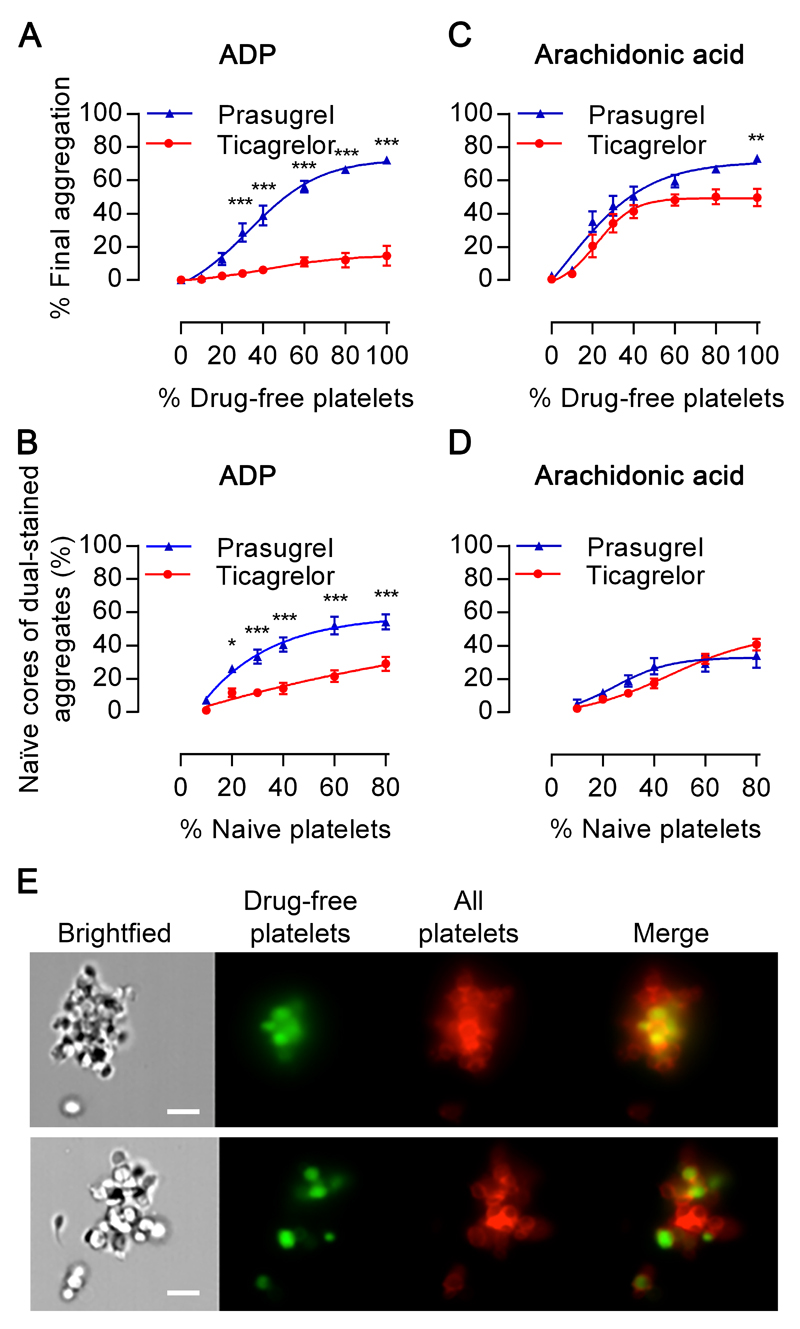
Having identified pharmacological differences between PAM and ticagrelor in vitro, we tested differences between prasugrel and ticagrelor in their ability to inhibit drug-free platelets in a larger inhibited environment using an ex vivo approach in healthy volunteers who had either taken aspirin+prasugrel or aspirin+ticagrelor for 7 days. Blood was collected at estimated peak concentrations of prasugrel active metabolite (30 minutes after last dose) and ticagrelor (120 minutes after last dose), as well as at 6 hours after the last dose and PRP was made. Drug-free platelets from healthy volunteers were then combined with PRP collected from treated subjects to model various rates of platelet turnover, ranging from no turnover (x=0%) to full turnover of new platelets (x=100%), and aggregation responses determined. In samples derived from volunteers treated with aspirin+prasugrel, responses to ADP recovered as the proportion of drug-free platelets was increased, whereas responses remained strongly inhibited in samples from volunteers who had received aspirin+ticagrelor (Figure 2A & Supplemental Figure II). In aspirin+ticagrelor samples the addition of naïve drug-free platelets also produced a smaller increase in the response to AA than in aspirin+prasugrel samples (Figure 2C & Supplemental Figure II).
Figure 2. Drug-free platelets restore ex vivo aggregation responses differentially in presence of prasugrel or ticagrelor.
PRP isolated from individuals 6h after receiving aspirin+prasugrel or aspirin+ticagrelor was mixed with increasing proportions of drug-free platelets and then stimulated. Final aggregation of samples stimulated with (A) ADP (20 µM) or (C) arachidonic acid (1 mM). (B, D) Aggregates (%) where cores comprise naïve platelets were blind scored and calculated from flow cytometric images of platelet aggregates from corresponding samples. 104 aggregates assessed per individual sample, with data presented as mean±SEM and compared by two-way ANOVA (n=10 samples, *p<0.05, **p<0.01, ***p<0.001). (E) Representative flow cytometric imaging (x60 objective) of aggregates formed in response to ADP (20µM) from 80%:20% mixtures of aspirin+prasugrel-inhibited PRP or aspirin+ticagrelor-inhibited PRP obtained 6 hours after the last drug dose was administered (red) and drug-free platelets (green). Scale bars equal to 7μm.
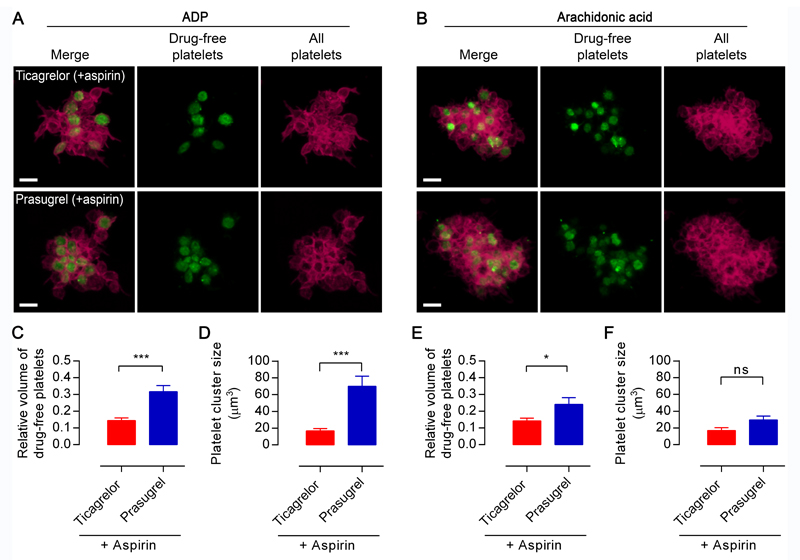
Following LTA, PRP underwent high-throughput flow cytometric imaging to assess aggregate structures. Aggregates (mean=104 per individual sample) were blindly assessed for the proportion of drug-free core aggregates relative to total aggregates (Figures 2E & Supplemental Figure II). Following stimulation by ADP, aggregates formed in PRP derived from volunteers treated with aspirin+prasugrel contained a higher proportion of drug-free cores than those formed in PRP derived from volunteers treated with aspirin+ticagrelor (Figure 2B). In contrast, no such difference in proportion was observed between volunteer groups for aggregates formed following AA stimulation (Figure 2D). Further confocal imaging of aggregates from populations comprising 20% drug-free and 80% inhibited platelets confirmed in response to ADP the formation of platelet aggregates with a core of drug-free platelets in aspirin+prasugrel-treated samples, but not in aspirin+ticagrelor-treated samples (Figure 3a). Moreover quantitative analysis of these images demonstrated fewer drug-free platelets were recruited (relative volumes 0.14±0.02 µm3 vs 0.32±0.04 µm3 respectively p<0.001; Figure 3C) and smaller drug-free cores formed (17±3 µm3vs 70±12 µm3; p<0.001; Figure 3D) in aspirin+ticagrelor-treated samples compared to aspirin+prasugrel-treated samples. Differences in distribution of platelet subpopulations following stimulation by AA were less pronounced (Figure 3B, 3E & 3F) but were similarly observed at ‘plasma peak’ time-points (Supplementary Figure III).
Figure 3. Drug-free platelets form cores within aggregates in presence of prasugrel but not ticagrelor.
Representative confocal images of (A) ADP-stimulated or (B) AA-stimulated aggregates formed from 80%:20% mixtures of aspirin+prasugrel-inhibited PRP or aspirin+ticagrelor-inhibited PRP obtained 6 hours after the last drug dose was administered (red) and drug-free platelets (green), conditions as in Figure 2. Images were analysed for (C, E) volume of the drug free platelet particles relative to the total aggregate volume and (D, F) average size of drug-free platelet clusters. Scale bars represent 5 µm. Data is presented as mean±SEM and compared by t-test (n=7-10; *p<0.05, ***p<0.001).
Reticulated platelets are more reactive than older platelets and locate to the core of aggregates
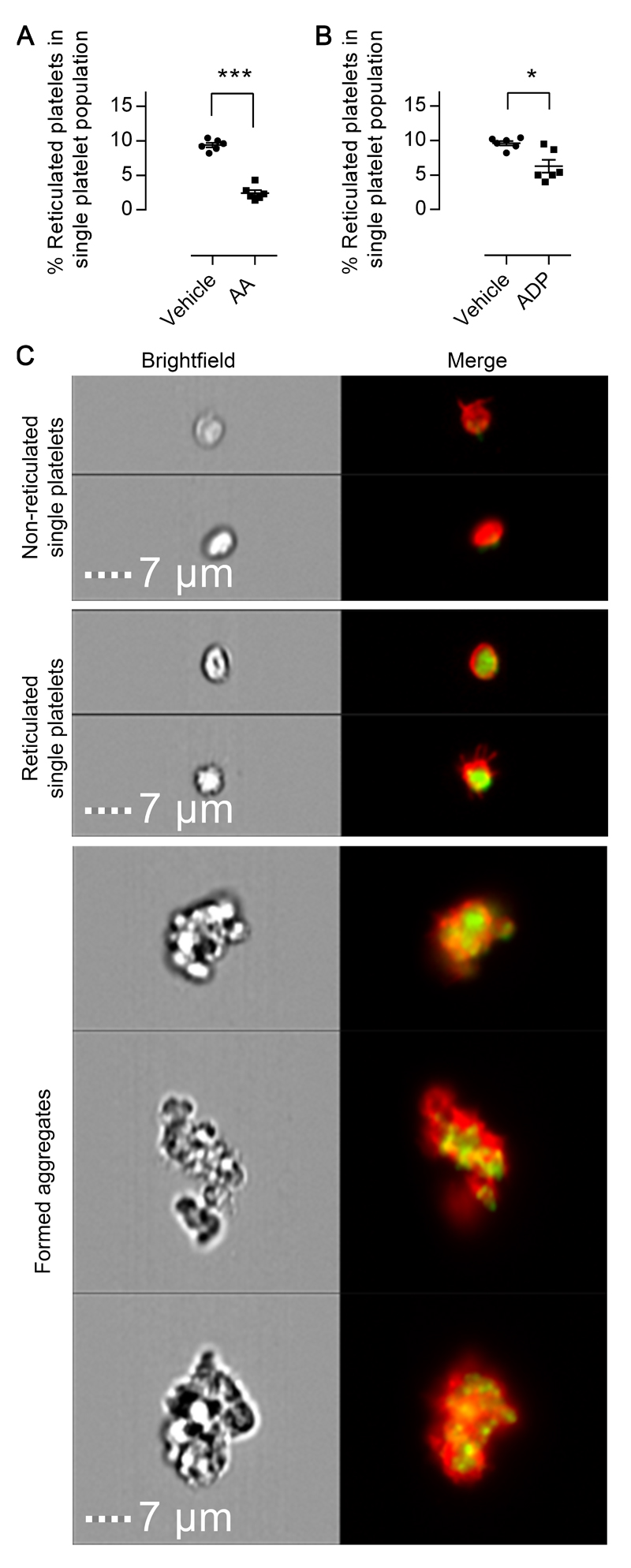
To monitor the reactivity of newly formed platelets, also called reticulated platelets due to the presence of mRNA, platelets were labelled ex vivo with the nucleic dye, thiazole orange (TO).15 We devised a gating strategy (Supplemental Figure IV) to determine the proportional usage of these newly formed platelets relative to older non-reticulated platelets16 during aggregate formation. Using PRP from untreated healthy volunteers stimulated by AA (1 mmol/L) or ADP (20 μmol/L) until 40% of platelets were aggregated the relative composition of the non-aggregated platelet population was assessed by flow cytometry. Following aggregation there was a significant reduction in the relative proportion of reticulated platelets indicating that reticulated platelets contributed over-proportionately to aggregate formation (Figure 4A & 4B). Subsequent examination of formed aggregates by flow cytometric imaging confirmed the presence of reticulated platelets in the majority of aggregates (Figure 4C).
Figure 4. Reticulated platelets display elevated reactivity in response to both arachidonic acid and ADP.
The proportion of reticulated platelets among non-aggregated platelets was assessed by flow cytometry in platelet rich plasma incubated with vehicle, (A) arachidonic acid, or (B) ADP. (C) Representative flow cytometric images of non-reticulated and reticulated (mRNA stain green) single platelets (red), as well as aggregates formed in response to ADP. Scale bars equal to 7μm. Data presented as individual data points with overlaid mean±SEM and compared by paired t-test (n=6; *p<0.05, ***p<0.001).
Reticulated platelets undermine platelet inhibition by DAPT in patients taking thienopyridines but not in patients taking ticagrelor
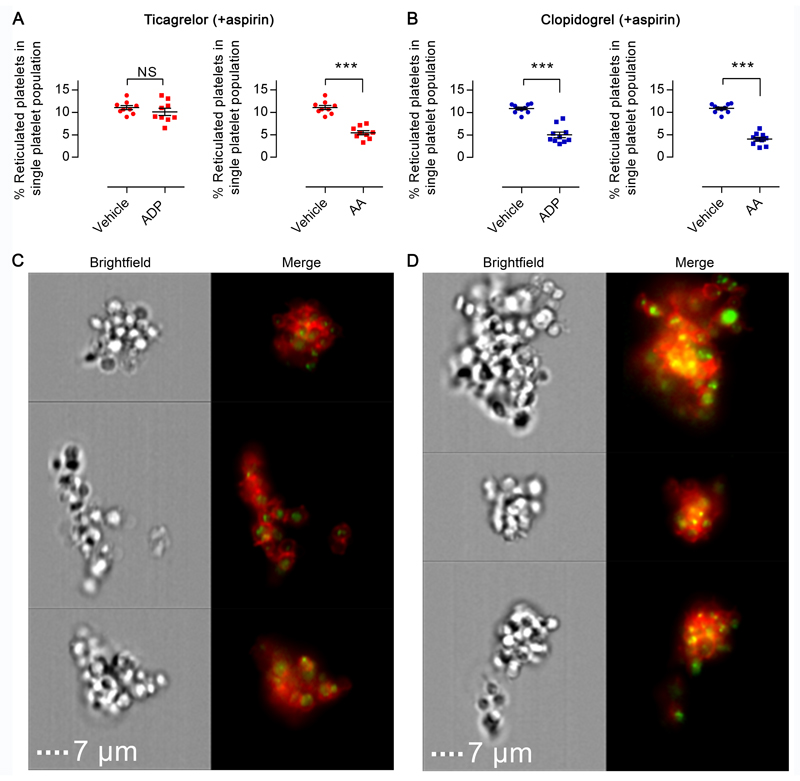
Differential effects of thienopyridine- and ticagrelor-mediated inhibition on reticulated platelet populations were assessed in patients with established stable coronary artery disease who received DAPT comprising either aspirin+clopidogrel or aspirin+ticagrelor. Patients were assessed for pharmacological efficacy by testing of platelet reactivity using LTA. All patients had a final aggregation to ADP (20 μmol/L) of <43%, with those receiving ticagrelor considerably lower (Supplemental Table I). TO stained PRP was incubated with vehicle, AA or ADP for 5 min and reticulated proportion of the non-aggregated platelets assessed by flow cytometric imaging. In both therapy groups, upon stimulation by AA, reticulated platelets disappeared from the non-aggregated platelet population; reducing from 10.9±0.3 % to 4.0±0.4 % (p<0.001) in aspirin+clopidogrel patient samples and from 11.1±0.5 % to 5.4±0.5 % (p<0.001) in aspirin+ticagrelor patient samples (Figures 5A & 5B). This indicated an over-proportional recruitment of reticulated platelets into the formed aggregates. In line with above data from AA-stimulated samples, stimulation by ADP of samples from patients receiving aspirin+clopidogrel caused a significant reduction in the relative population of reticulated platelets in the non-aggregated population, from 10.9±0.3 % to 5.0±0.6 % (Figure 5B, p<0.01). However, in contrast to these observations, stimulation with ADP of PRP from patients receiving aspirin+ticagrelor did not result in a drop of the reticulated platelet population (11.1±0.5 % to 10.1±0.8 %; Figure 5A, p>0.05). The absence of a change in proportion indicates a proportionally equivalent recruitment of reticulated and non-reticulated platelets to aggregates. Qualitative analysis of the imaged formed aggregates from each patient group demonstrated that in samples from patients receiving aspirin+ticagrelor there were few reticulated platelets dispersed throughout the aggregate (Figure 5C) whereas in samples from patients receiving aspirin+prasugrel there were increased numbers of reticulated platelets primarily located in the core of the formed aggregates (Figure 5D).
Figure 5. In patients the response of reticulated platelets to ADP is inhibited to a greater extent by ticagrelor than by clopidogrel.
The reticulated platelet subpopulation among non-aggregated single platelets was assessed by flow cytometry in PRP incubated with vehicle, ADP, or arachidonic acid. Samples were obtained from patients taking (A) ticagrelor or (B) clopidogrel (in addition to aspirin). Representative flow cytometric images of ADP-stimulated platelet aggregates (platelets red; mRNA green) formed in samples from patients taking aspirin plus (C) ticagrelor or (D) clopidogrel. Scale bars equal to 7μm. Data presented as individual data points with overlaid mean±SEM and compared by paired t-test (n=9-10; ***p<0.001).
Discussion
In our previous study, we demonstrated through in vitro modelling that drug-free platelets can act as seeds for aggregate formation during antiplatelet therapy.10 Here we have studied the impact of platelet turnover, including the influences of reticulated platelets, during standard dual antiplatelet therapy in both healthy volunteers and stable cardiovascular patients. Furthermore, we have compared thienopyridines with ticagrelor and from our results provide a potential pathophysiological mechanism that unites previous, but separate, associations between differential effectiveness of P2Y12 receptor inhibition, HTPR, immature platelet counts and thrombotic risk.3, 6, 13, 14 We directly demonstrate that following stimulation reticulated platelets are over-proportionately recruited to aggregates where they can act as seeds for larger aggregate formation and by interplay with drug pharmacokinetics provide a causative mechanism for observed HTPR.
Key to explaining our observations is an understanding that the formation of a drug-free, uninhibited, subpopulation of platelets during DAPT occurs as a result of platelet turnover and drug pharmacokinetics. In terms of standard therapy, aspirin is a short-lived but irreversible inhibitor of platelet COX-1. Similarly, the thienopyridines, prasugrel or clopidogrel acting through their active metabolites, are pharmacokinetically short-lived and irreversible antagonists of platelet P2Y12 receptors. When used as DAPT this combination of aspirin plus thienopyridine produces inhibition of circulating platelets. However, as we model in vitro and demonstrate ex vivo, neither aspirin nor prasugrel (or prasugrel active metabolite) appears present in circulating blood at sufficient levels to inhibit the responses of exogenous platelets added in vitro. One potential explanation for this observation is that these drugs are present in effective inhibitory concentrations only within the portal circulation and so inhibit platelets as they pass through, as has been suggested for aspirin for over 30 years.17 This would explain also why platelets newly released from the bone marrow are either poorly, or not, inhibited by aspirin and thienopyridines.18, 19 One should not overlook, however, the alternative explanation that because of their short half-lives within the circulating blood the active forms of thienopyridines may have insufficient time to interact with exogenously added platelets in our test system. In contrast, as expected, ticagrelor as a longer lasting (plasma half-life of about 8 hours) direct acting reversible antagonist of P2Y12 receptors 12 inhibited the responses to ADP of exogenously added platelets.
As well as modelling these interactions with regard to standard tests of platelet reactivity4, our imaging techniques demonstrated that exogenously added uninhibited platelets were clustered at the cores of aggregates formed in response to ADP in samples from volunteers receiving aspirin+clopidogrel, consistent with their ability to act as seeds for aggregate formation. As previously hypothesised12 the longer half-life and reversible binding of ticagrelor, in contrast to the irreversible binding of prasugrel, means it is present and able to act upon the exogenous drug-free, uninhibited, platelet subpopulation. It can be noted that ticagrelor might in addition act pleiotropically upon adenosine uptake to influence platelet function20, 21, but we did not test this possibility. It was notable that the recovery of the response to AA caused by the addition of exogenous drug-free platelets was blunted in samples prepared from individuals receiving aspirin+ticagrelor compared to those receiving aspirin+prasugrel. This is consistent with P2Y12 blockade reducing the amplifying effects of TxA2 produced in response to AA22–26, and confirmed our in vitro observation that circulating ticagrelor, unlike prasugrel, may provide additional compensation for the loss of COX-1 inhibition noted in individuals with elevated platelet turnover 26, 27.
In our in vitro and ex vivo models we stained or labelled un-inhibited platelets to allow determination of their function as a subpopulation. Examination of the definitive drug-free population in patients is less straightforward. Newly formed immature platelets are also called reticulated platelets due to the presence of residual cytosolic mRNA. Dyes such as TO, which stain nucleic acids, are routinely used for determining the percentage of reticulocytes (including platelets) in blood samples.28 Accepting that the emergence of a drug-free platelet subpopulation occurs as a result of platelet turnover and the associated release of newly formed platelets, analyses of newly formed platelets in samples can be used to inform on the behaviour of drug-free platelets. We have demonstrated under our particular conditions that TO staining of PRP identifies those platelets with the highest mRNA content (manuscript under review). We therefore utilised this approach to track newly formed platelets during aggregate formation. In samples from healthy volunteers not taking anti-platelet drugs reticulated platelets were over-proportionally recruited to the formation of aggregates, indicating that under normal physiological conditions they are important drivers of the aggregation process. This finding concurs with previous reports by ourselves, and others, that newly formed reticulated platelets possess inherently greater reactivity and have a greater propensity for recruitment to thrombi.29
Finally, we sought to determine whether such a mechanism was also present in patient samples. We recruited patients with established, stable coronary artery disease taking clopidogrel or ticagrelor plus aspirin, and confirmed drug efficacy to ensure patients exhibiting HTPR were not included in our analyses. As in our in vitro and ex vivo modelling, the behaviour of reticulated platelets matched that of uninhibited platelets. In samples taken from patients receiving aspirin+clopidogrel there was a significant loss in the proportion of reticulated platelets from the non-aggregated single platelet population following ADP stimulation, whereas strikingly, in patients receiving aspirin & ticagrelor no proportional change was observed. Similarly, examination of the formed aggregates revealed that in response to ADP reticulated platelets were clustered in the core and present in greater proportion in samples from patients receiving clopidogrel+aspirin than in samples from patients receiving ticagrelor+aspirin.
Our results support a mechanism through which newly formed uninhibited reticulated platelets play a key role in the limiting the effectiveness of particular antiplatelet therapies. Furthermore, we provide functional evidence and unique images substantiating the observed impact of subpopulations of reticulated drug-free platelets on the formation of platelet aggregates under recommended clinical testing settings.30, 31 Together these are consistent with emerging evidence establishing a link between reticulated platelets and platelet responsiveness to short-lived P2Y12 antagonist (thienopyridines)11, 32 but not ticagrelor.33 Whilst there have been recent indications of similar short-term efficacy for prasugrel and ticagrelor in patients with acute myocardial infarction34, no comparisons or sub-group analyses has yet been conducted in conditions of high platelet turnover. Moreover our data suggests that in vivo the use of ticagrelor rather than clopidogrel or prasugrel may mitigate incomplete inhibition of TXA2 formation by prophylactic aspirin4, consistent with our previous reports regarding the importance of P2Y12 receptors in amplifying responses to platelet produced TXA2.35–38
In conclusion, we demonstrate a functional mechanism for newly formed reticulated platelets to drive thrombus formation even during standard DAPT. Furthermore our study demonstrates that ticagrelor may be more efficacious than thienopyridine (prasugrel or clopidogrel) therapy for mitigating HTPR associated with the generation of new platelets during standard anti-thrombotic regimens. In turn this illustrates the importance of considering platelet turnover and the pharmacological inhibition of the reticulated platelet subpopulation in attaining optimal anti-thrombotic potential. Finally given the central role for platelet turnover in our model, patients with conditions such as diabetes and chronic kidney disease, where increased platelet turnover has been identified or suspected, may particularly benefit from such considerations.
Supplementary Material
Highlights.
-
1
We demonstrate a functional mechanism by which newly formed reticulated platelets can drive thrombus formation even during standard DAPT.
-
2
Study reveals underlying reasons to consider platelet turnover and drug pharmacokinetics in the selection of appropriate antiplatelet therapies for optimal anti-thrombotic protection.
Acknowledgments
We are grateful to Professor Sussan Nourshargh for use of confocal microscopes.
Sources of Funding
This work was supported by grants from the Medical Research Council, the British Heart Foundation (PG/12/68/29779, PG/15/47/31591 and FS/12/53/29643), Wellcome Trust (101604/Z/13/Z), AstraZeneca and the William Harvey Research Foundation.
Abbreviations
- AA
Arachidonic acid
- ADP
Adenosine Diphosphate
- COX
Cyclooxygenase
- DAPT
Dual anti-platelet therapy
- HTPR
High on treatment platelet reactivity
- LTA
Light transmission aggregometry
- PAM
Prasugrel active metabolite
- PRP
Platelet rich plasma
- TO
Thiazole Orange
- TXA2
Thromboxane A2
Footnotes
Disclosures
Conflict-of-interest disclosure: T.D.W. has received research grant funding and consultancy fees from Astra Zeneca.
References
- 1.Antiplatelet Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Bmj. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, Copland I, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The pci-cure study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 3.Tantry US, Gurbel PA. Antiplatelet drug resistance and variability in response: The role of antiplatelet therapy monitoring. Curr Pharm Des. 2013;19:3795–3815. doi: 10.2174/1381612811319210006. [DOI] [PubMed] [Google Scholar]
- 4.Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adp associated with ischemia and bleeding. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 5.Reny JL, Fontana P, Hochholzer W, et al. Vascular risk levels affect the predictive value of platelet reactivity for the occurrence of mace in patients on clopidogrel. Systematic review and meta-analysis of individual patient data. Thromb Haemost. 2016;115:844–855. doi: 10.1160/TH15-09-0742. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim H, Schutt RC, Hannawi B, DeLao T, Barker CM, Kleiman NS. Association of immature platelets with adverse cardiovascular outcomes. J Am Coll Cardiol. 2014;64:2122–2129. doi: 10.1016/j.jacc.2014.06.1210. [DOI] [PubMed] [Google Scholar]
- 7.Himmelfarb J, Holbrook D, McMonagle E, Ault K. Increased reticulated platelets in dialysis patients. Kidney Int. 1997;51:834–839. doi: 10.1038/ki.1997.117. [DOI] [PubMed] [Google Scholar]
- 8.Htun P, Fateh-Moghadam S, Bischofs C, Banya W, Muller K, Bigalke B, Stellos K, May AE, Flather M, Gawaz M, Geisler T. Low responsiveness to clopidogrel increases risk among ckd patients undergoing coronary intervention. J Am Soc Nephrol. doi: 10.1681/ASN.2010020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel ML, Caillard S, Campia U, Moulin B, Gachet C, Ohlmann P. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired p2y(12) inhibition by clopidogrel. J Am Coll Cardiol. 57:399–408. doi: 10.1016/j.jacc.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Hoefer T, Armstrong PC, Finsterbusch M, Chan MV, Kirkby NS, Warner TD. Drug-free platelets can act as seeds for aggregate formation during antiplatelet therapy. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:2122–2133. doi: 10.1161/ATVBAHA.115.306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernlochner I, Goedel A, Plischke C, Schupke S, Haller B, Schulz C, Mayer K, Morath T, Braun S, Schunkert H, Siess W, et al. Impact of immature platelets on platelet response to ticagrelor and prasugrel in patients with acute coronary syndrome. Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv326. [DOI] [PubMed] [Google Scholar]
- 12.Nylander S, Schulz R. Effects of p2y12 receptor antagonists beyond platelet inhibition - comparison of ticagrelor with thienopyridines. Br J Pharmacol. 2016;173:1163–1178. doi: 10.1111/bph.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James S, Budaj A, Aylward P, et al. Ticagrelor versus clopidogrel in acute coronary syndromes in relation to renal function: Results from the platelet inhibition and patient outcomes (plato) trial. Circulation. 2010;122:1056–1067. doi: 10.1161/CIRCULATIONAHA.109.933796. [DOI] [PubMed] [Google Scholar]
- 14.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 15.Kienast J, Schmitz G. Flow cytometric analysis of thiazole orange uptake by platelets: A diagnostic aid in the evaluation of thrombocytopenic disorders. Blood. 1990;75:116–121. [PubMed] [Google Scholar]
- 16.Angenieux C, Maitre B, Eckly A, Lanza F, Gachet C, de la Salle H. Time-dependent decay of mrna and ribosomal rna during platelet aging and its correlation with translation activity. PLoS One. 2016;11:e0148064. doi: 10.1371/journal.pone.0148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Gaetano G, Cerletti C, Dejana E, Latini R. Pharmacology of platelet inhibition in humans: Implications of the salicylate-aspirin interaction. Circulation. 1985;72:1185–1193. doi: 10.1161/01.cir.72.6.1185. [DOI] [PubMed] [Google Scholar]
- 18.Baaten CC, Veenstra LF, Wetzels R, van Geffen JP, Swieringa F, de Witt SM, Henskens YM, Crijns H, Nylander S, van Giezen JJ, Heemskerk JW, et al. Gradual increase in thrombogenicity of juvenile platelets formed upon offset of prasugrel medication. Haematologica. 2015;100:1131–1138. doi: 10.3324/haematol.2014.122457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuijpers MJ, Megens RT, Nikookhesal E, Feijge MA, De Mey JG, oude Egbrink MG, van Giezen JJ, Heemskerk JW. Role of newly formed platelets in thrombus formation in rat after clopidogrel treatment: Comparison to the reversible binding p2y(1)(2) antagonist ticagrelor. Thromb Haemost. 2011;106:1179–1188. doi: 10.1160/TH11-04-0252. [DOI] [PubMed] [Google Scholar]
- 20.Nylander S, Femia EA, Scavone M, Berntsson P, Asztely AK, Nelander K, Lofgren L, Nilsson RG, Cattaneo M. Ticagrelor inhibits human platelet aggregation via adenosine in addition to p2y12 antagonism. J Thromb Haemost. 2013;11:1867–1876. doi: 10.1111/jth.12360. [DOI] [PubMed] [Google Scholar]
- 21.Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, Gariboldi V, Condo J, Thuny F, Frere C, Camoin-Jau L, et al. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014;63:872–877. doi: 10.1016/j.jacc.2013.09.067. [DOI] [PubMed] [Google Scholar]
- 22.Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: A novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev. 2007;25:357–374. doi: 10.1111/j.1527-3466.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 23.Gachet C. Regulation of platelet functions by p2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 24.Cattaneo M. Adp receptors: Inhibitory strategies for antiplatelet therapy. Drug News Perspect. 2006;19:253–259. doi: 10.1358/dnp.2006.19.5.985936. [DOI] [PubMed] [Google Scholar]
- 25.Gachet C. The platelet p2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther. 2005;108:180–192. doi: 10.1016/j.pharmthera.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Leadbeater PD, Kirkby NS, Thomas S, Dhanji AR, Tucker AT, Milne GL, Mitchell JA, Warner TD. Aspirin has little additional anti-platelet effect in healthy volunteers receiving prasugrel. Journal of thrombosis and haemostasis : JTH. 2011;9:2050–2056. doi: 10.1111/j.1538-7836.2011.04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkby NS, Leadbeater PD, Chan MV, Nylander S, Mitchell JA, Warner TD. Antiplatelet effects of aspirin vary with level of p2y(1)(2) receptor blockade supplied by either ticagrelor or prasugrel. Journal of thrombosis and haemostasis : JTH. 2011;9:2103–2105. doi: 10.1111/j.1538-7836.2011.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujii T, Shimomura T, Fujimoto TT, Kimura A, Fujimura K. A new approach to detect reticulated platelets stained with thiazole orange in thrombocytopenic patients. Thromb Res. 2000;97:431–440. doi: 10.1016/s0049-3848(99)00182-6. [DOI] [PubMed] [Google Scholar]
- 29.McBane RD, 2nd, Gonzalez C, Hodge DO, Wysokinski WE. Propensity for young reticulated platelet recruitment into arterial thrombi. J Thromb Thrombolysis. 2014;37:148–154. doi: 10.1007/s11239-013-0932-x. [DOI] [PubMed] [Google Scholar]
- 30.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the onset and offset of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The onset/offset study. Circulation. 2009;120:2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 31.Guthikonda S, Alviar CL, Vaduganathan M, Arikan M, Tellez A, DeLao T, Granada JF, Dong JF, Kleiman NS, Lev EI. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. Journal of the American College of Cardiology. 2008;52:743–749. doi: 10.1016/j.jacc.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 32.Perl L, Lerman-Shivek H, Rechavia E, Vaduganathan M, Leshem-Lev D, Zemer-Wassercug N, Dadush O, Codner P, Bental T, Battler A, Kornowski R, et al. Response to prasugrel and levels of circulating reticulated platelets in patients with st-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;63:513–517. doi: 10.1016/j.jacc.2013.07.110. [DOI] [PubMed] [Google Scholar]
- 33.Vaduganathan M, Zemer-Wassercug N, Rechavia E, Lerman-Shivek H, Perl L, Leshem-Lev D, Orvin K, Kornowski R, Lev EI. Relation between ticagrelor response and levels of circulating reticulated platelets in patients with non-st elevation acute coronary syndromes. J Thromb Thrombolysis. 2015;40:211–217. doi: 10.1007/s11239-015-1178-6. [DOI] [PubMed] [Google Scholar]
- 34.Motovska Z, Hlinomaz O, Miklik R, et al. Prasugrel versus ticagrelor in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: Multicenter randomized prague-18 study. Circulation. 2016;134:1603–1612. doi: 10.1161/CIRCULATIONAHA.116.024823. [DOI] [PubMed] [Google Scholar]
- 35.Rocca B, Santilli F, Pitocco D, et al. The recovery of platelet cyclooxygenase activity explains interindividual variability in responsiveness to low-dose aspirin in patients with and without diabetes. Journal of thrombosis and haemostasis : JTH. 2012 doi: 10.1111/j.1538-7836.2012.04723.x. [DOI] [PubMed] [Google Scholar]
- 36.Pascale S, Petrucci G, Dragani A, Habib A, Zaccardi F, Pagliaccia F, Pocaterra D, Ragazzoni E, Rolandi G, Rocca B, Patrono C. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood. 2012;119:3595–3603. doi: 10.1182/blood-2011-06-359224. [DOI] [PubMed] [Google Scholar]
- 37.Spectre G, Arnetz L, Ostenson CG, Brismar K, Li N, Hjemdahl P. Twice daily dosing of aspirin improves platelet inhibition in whole blood in patients with type 2 diabetes mellitus and micro- or macrovascular complications. Thromb Haemost. 2011;106:491–499. doi: 10.1160/TH11-04-0216. [DOI] [PubMed] [Google Scholar]
- 38.Capodanno D, Patel A, Dharmashankar K, Ferreiro JL, Ueno M, Kodali M, Tomasello SD, Capranzano P, Seecheran N, Darlington A, Tello-Montoliu A, et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv. 2011;4:180–187. doi: 10.1161/CIRCINTERVENTIONS.110.960187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.