Abstract
Primary aldosteronism is a common cause of hypertension, which becomes refractory if undiagnosed, but potentially curable when due to an aldosterone-producing adenoma (APA). The discovery of somatic mutations and differences in clinical presentations led to recognition of small but common zona glomerulosa (ZG)-like adenomas, distinct from classical large zona fasciculata (ZF)-like adenomas. The inverse correlation between APA size and aldosterone synthase expression prompted us to undertake a systematic study of genotype-phenotype relationships.
Following a microarray comparing tumor subtypes, in which nephronectin (NPNT) was the most highly (>12-fold) upregulated gene in ZG-like APAs, we aimed to determine its role in physiological and pathological aldosterone production.
NPNT was identified by immunohistochemistry as a secreted matrix protein expressed exclusively around aldosterone-producing glomeruli in normal adrenal ZG, and in aldosterone-dense ZG-like APAs; the highest expression was in ZG-like APAs with gain-of-function CTNNB1 mutations, whose removal cured hypertension in our patients. NPNT was absent from normal ZF, ZF-like APAs, and ZG adjacent to an APA. Its production was regulated by canonical Wnt and NPNT transfection or silencing increased or reduced aldosterone production respectively. NPNT was pro-adhesive in primary adrenal and APA cells, but anti-adhesive and anti-apoptotic in immortalized adrenocortical cells.
The discovery of NPNT in the adrenal helped recognition of a common subtype of APAs, and a pathway by which Wnt regulates aldosterone production. We propose that this arises through NPNT’s binding to cell-surface integrins, stimulating cell-cell contact within glomeruli which define ZG. Therefore, NPNT or its cognate integrin could present a novel therapeutic target.
Keywords: hypertension, aldosterone, adenoma, extracellular matrix protein, Wnt pathway
Introduction
5-13% of all hypertension and 20% of resistant hypertension can be attributed to primary aldosteronism, of which unilateral aldosterone-producing adenoma (APA) is the most common curable cause1,2. Early detection of APA is important due to significant increases in cardiovascular morbidity and mortality: congestive cardiac failure and ischemic heart disease are 2–5 times more prevalent3, with an increase in 14-year mortality in these patients over matched patients with essential hypertension4. Whether this additional risk is due directly to aldosterone excess, independent of high blood pressure, or reflects greater average duration of hypertension prior to diagnosis, clinical outcome is considered to benefit from prompt recognition and removal of APAs5.
Over the past decade, new molecular stratifications have enabled the recognition of a group of smaller zona glomerulosa (ZG)-like APAs6–8. Compared to the classical large lipid-laden zona fasciculata (ZF)-like APA with mutations in inward rectifier potassium channel 4 (KCNJ5)6, not only is this ZG-like subtype of APA histologically and biochemically different, it also harbors hallmark somatic mutations in genes encoding a subunit of the voltage-gated calcium channel (CACNA1D)8, Na+/K+-ATPase (ATP1A1)8,9, Ca2+-ATPase (ATP2B3)9, or the Wnt pathway mediator β-catenin (CTNNB1)10. Biochemically, these smaller ZG-like APAs have a higher capacity for aldosterone production; semiquantitative analysis of immunohistochemical staining has revealed that CYP11B2 score is inversely correlated with tumor size and volume11,12. In addition, ZG-like APAs are more responsive to angiotensin II (All), with higher levels of type 1 AII receptor mRNA13,14. However, due to their small size, they are readily overlooked on cross-sectional imaging, as CT is unable to reliably detect adrenal tumors smaller than 5-6mm15. We hypothesized that specific gene products are responsible for the increased hormone production in these aldosterone-dense APAs and can potentially be used as a diagnostic biomarker when imaging proves inconclusive.
In seeking transcriptomic evidence to identify possible pathways of aldosterone production specific to the more compact, aldosterone-synthase dense APAs, we compared CACNA1D/ATP1A1-mutant with KCNJ5-mutant APAs8. Extracellular matrix (ECM) gene nephronectin (NPNT) was found to be the most upregulated, by 12-fold, in the former, and confirmed a categorical difference between APA genotypes by immunohistochemistry. Discovered in the kidney in 200116, NPNT was recently found to be a downstream Wnt target in the epidermis17. This may be relevant in the adrenal, where normal adrenocortical development and steroidogenic activity of the ZG, are dependent on the canonical Wnt pathway18.
Further examination of NPNT’s distribution presented in this paper led us to hypothesize a key role in bringing ZG cells together to form functional units for aldosterone production through intercellular communication. This is supported by the observation, in rat ZG cells, of numerous tight junctions likely to be important in the establishment of electrical coupling19, and the more recent discovery, only in intact adrenal slices, of oscillating membrane potentials regulating aldosterone secretion20.
In this study, we have found that NPNT is directly involved in the physiological secretion of aldosterone in the adrenal. By using both cell-lines and primary human adrenal cells, we have also uncovered a previously unknown role of NPNT in adhesion and cell survival.
Methods
Human subjects
Human adrenal tissues from patients who underwent adrenalectomy after being diagnosed with unilateral APA or phaeochromocytoma were obtained from Cambridge University Hospitals’ Human Research Tissue Bank post-surgery at Addenbrooke’s Hospital, Cambridge, United Kingdom. All tissues were obtained with approval from the Cambridgeshire Research Ethics Committee with written informed consent prior to surgery. Further details are provided in the Data Supplement and clinical features in Table S1.
Cell culture
Human adrenocortical carcinoma H295R cells were obtained from the American Type Culture Collection (ATCC CRL-2128), and grown in DMEM/Nutrient F-12 Ham supplemented with 10% FBS, 100 U penicillin, 0.1 mg/ml streptomycin, 0.4 mM L-glutamine and ITS at 37 °C in 5% CO2. HEK cells were obtained from the American Type Culture Collection (ATCC CRL-1573), and grown in DMEM supplemented with 10% FBS.
Gene overexpression and silencing
Gene overexpression was carried out using lipid-mediated cell transfection Lipofectamine 3000 (Thermo Fisher), while gene silencing was achieved using DharmaFECT 1 lipid transfection reagent (Dharmacon), both according to manufacturer’s instructions. Cells were harvested for analysis of mRNA and protein expression after 48h. Further details are provided in the Data Supplement.
RNA extraction, reverse transcription and quantitative real-time polymerase chain reaction (qRT-PCR)
50-100mg of tissue or 1x105 cells were used for each RNA extraction. Further details are provided in the Data Supplement. qRT-PCR was performed using TaqMan ABI probes (Applied Biosystems) for NPNT (Hs01568126), ITGB1 (Hs00559595) and BCL2 (Hs00608023). CYP11B2 expression was quantified using custom-made TaqMan probes (Invitrogen) previously validated for specificity21.
Immunohistochemistry
Immunohistochemistry was carried out using the peroxidase-antiperoxidase method on fresh frozen human tissue. In cases where fresh frozen tissue was unavailable, immunohistochemistry was performed on formalin-fixed, paraffin-embedded adrenal sections (4 μm) using an automated immunostainer with cover tile technology (Bond-III system, Leica Biosystems). NPNT antibody (HPA003711, Sigma; 1:50 dilution), and CYP11B2 antibody (custom mouse anti-human antibody from Dr Celso E. Gomez-Sanchez)22 were used as the primary antibodies. Further details are provided in the Data Supplement.
Aldosterone measurement
Supernatant from cultured cells was used for aldosterone quantification using the Homogenous Time Resolved Fluorescence (HTRF) assay (Cisbio assays) based on the Fluorescence Resonance Energy Transfer (FRET) technology, according to manufacturer’s instructions. The final fluorescence readout was conducted using a Pherastar FS microplate reader (BMG Labtech). Aldosterone concentrations were then normalized to total cell protein, quantified by the bicinchoninic acid (BCA) protein assay (Pierce Biotechnology).
Firefly/ renilla luciferase assay
To measure the activity of Wnt transcriptional complex TCF/LEF, firefly and renilla luciferase activity was measured 48h after co-transfection with the Dual-Glo Luciferase Assay System (Promega) and normalized to the empty pCMV6 vector as described in the manufacturer’s protocol. Canonical Wnt signaling was quantified using the Cignal TCF/LEF Reporter (luc) Kit (SABiosciences).
Cell confluency and cytotoxicity assay
To measure changes in H295R cell confluency post-NPNT silencing, time-lapsed images were obtained using an Incucyte system (Essen BioScience). To differentiate changes in cell proliferation from cytotoxicity, cell-impermeant cyanine dimer nucleic acid stain YOYO-1 (Y3601, Life Technologies) was utilized. Further details are provided in the Data Supplement.
Annexin V-propidium iodide dual stain
To assess apoptosis over time, cells were double-labelled with Annexin V-APC (550474, BD Pharmingen) and propidium iodide (PI) (Sigma). After silencing, adherent cells were trypsinized, added to any detached cells in the supernatant as previously described23, stained with Annexin V-PI, and analyzed with the FACSCanto II flow cytometer (Becton-Dickinson). Further details are provided in the Data Supplement.
Xcelligence cell impedance measurement and Hoechst stain assay
To evaluate changes in adhesion in response to NPNT, wells of an E-Plate 16 (ACEA Biosciences) were pre-coated with PBS, or 10μg/ml of BSA, NPNT or laminin for 1 h at 37°C as previously described24. Cell adhesion was also measured by Hoechst dye quantification of cells remaining on pre-coated wells post-wash. Further details are provided in the Data Supplement.
Proteins and chemicals
Proteins used in this study were BSA (A9576, Sigma), NPNT (4298-NP-050, R&D systems), Fibronectin (FC010, Millipore) and laminin (AG56P, Millipore). The selective porcupine inhibitor, LGK-974 (1 μM; Selleck Chemicals), as used previously25, was used to analyze the effect of blocking all Wnt secretion.
Statistical analysis
Results are expressed as mean values with standard error of the mean (SEM) and compared using the two-sided Student’s t-test or by one-way ANOVA followed by Tukey's post hoc test. Significance level of P<0.05 was considered to indicate statistical significance. Statistical analysis was performed using Graphpad Prism (Graphpad Software Inc.)
Results
NPNT is selectively expressed in the subtype of smaller ZG-like APAs and able to distinguish between the two APA classes
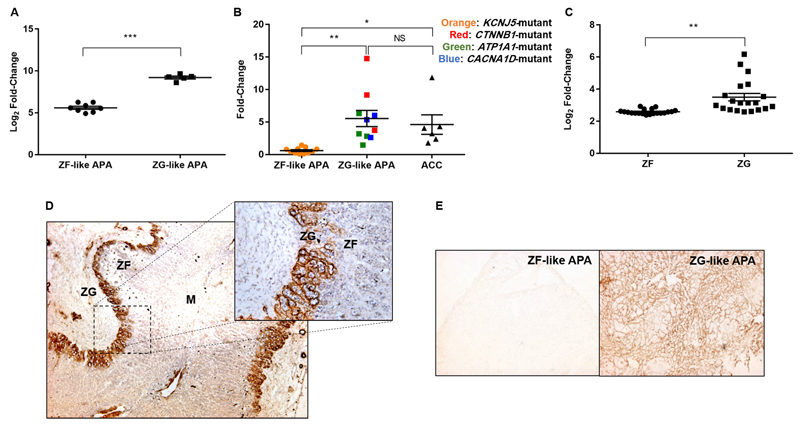
Microarray analysis revealed NPNT to be 12.2-fold upregulated in the smaller ZG-like APAs with higher aldosterone synthetic capacity (Figure 1A). Validation with qRT-PCR revealed a nine-fold difference; both ZG-like APAs and adrenocortical carcinomas (ACCs) had significantly elevated levels of NPNT compared to the ZF-like APAs (Figure 1B). The two APAs with the highest levels of NPNT harbored gain-of-function mutations in the Wnt gene CTNNB1.
Figure 1. NPNT is selectively expressed in normal adrenal ZG, ZG-like APAs and ACCs.
(a). Microarray analysis of NPNT, comparing 8 ZF-like adenomas with 5 ZG-like adenomas
(b). qRT-PCR validation of NPNT, on mRNA extracted from 11 ZF-like APAs and 10 ZG-like APAs differentiated based on their genotypic mutations, as well as 6 ACCs.
(c). Microarray analysis of NPNT, comparing 20 paired ZF and ZG (each pair from the same patient), isolated via laser capture microdissection.
(d). Representative immunohistochemistry of NPNT showing selective extracellular localization in the ZG of adrenal adjacent to a phaeochromocytoma (4x magnification) (inset: 20x magnification).
e). Representative immunohistochemistry of NPNT comparing staining between ZF-like APA and ZG-like APA mounted on the same slide (4x magnification).
In a-c, bars represent mean expression per group±s.e.m. Statistical analyses were conducted by Student's t-test (a,c) or one-way ANOVA followed by Tukey's post hoc test (b). *P<0.05; **P<0.005; ***P<0.0005; NS, not significant. M, medulla; ZG, zona glomerulosa; ZF, zona fasciculata.
In a further microarray comparing the ZG and ZF of 20 human adrenals isolated via laser capture microdissection26, NPNT was on average two-fold more highly-expressed in the outer, aldosterone-producing zone of the ZG (Figure 1C). This selective expression was evident in protein immunohistochemistry, in which NPNT staining is localized exclusively to the ZG (Figure 1D, Figure S1). When mounted on the same slide, NPNT also makes it easy to differentiate between the two APA subtypes as there is negligible staining in ZF–like APAs (Figure 1E, Figure S2). In all cases, NPNT expression appeared extracellular and strikingly peri-glomerular, surrounding clusters of cells.
NPNT expression corresponds to aldosterone synthase CYP11B2 expression
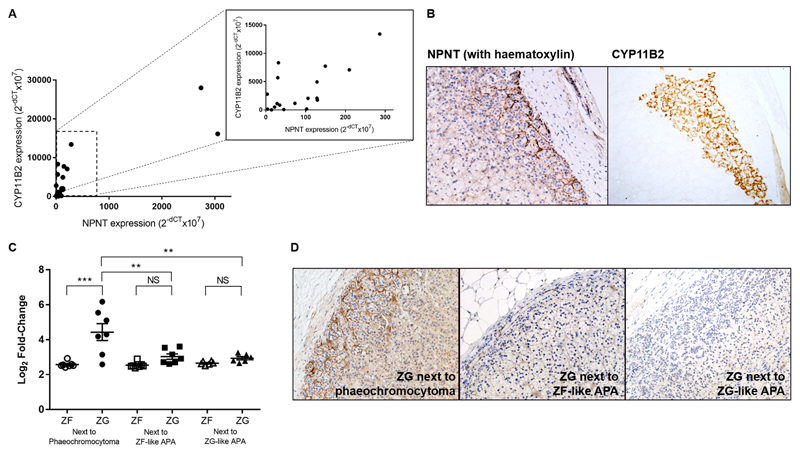
Although the ZG is the zone of physiological aldosterone production, CYP11B2 staining is patchy22. The overall analysis of 20 normal adrenal and APA samples revealed a significant positive correlation between NPNT and CYP11B2 encoding aldosterone synthase (r=0.82, p<0.0001) (Figure 2A). At the protein level, areas of CYP11B2 expression also corresponded consistently with NPNT staining (Figure 2B, Figure S3). We also compared the ZG expression of genes in adrenal adjacent to a phaeochromocytoma versus that next to an APA (i.e. in a state of aldosterone excess). NPNT was 3.6-fold upregulated in ZG, compared to ZF, when adjacent to a phaeochromocytoma, but diminished or absent when adjacent to an APA (Figure 2C). The same observations were made at the protein level (Figure 2D). Similarly, CYP11B2 was two-fold upregulated in ZG next to a phaeochromocytoma vs next to an APA, and 7.8-fold higher on qRT-PCR validation.
Figure 2. NPNT expression corresponds to CYP11B2 expression, with presence of negative feedback.
(a). Strong positive linear correlation between NPNT and CYP11B2 expression in 10 pairs of adenomas and their adjacent adrenal, r(18)=0.8273, p<0.0001. Inset: correlation plot excluding the 2 samples with highest NPNT expression, r(16)=0.6806, p=0.0019. Statistical analysis was conducted by Pearson product-moment correlation.
(b). Representative immunohistochemistry of NPNT and CYP11B2 in corresponding ZG areas in serial sections of the same adrenal tissue (20x magnification).
(c). Microarray expression of NPNT in 7 paired ZG and ZF samples adjacent to a phaeochromocytoma and 13 pairs next to an APA. Bars represent mean expression per group±s.e.m. Statistical analysis was conducted by one-way ANOVA followed by Tukey's post hoc test. *P<0.05; **P<0.005; ***P<0.0005; NS, not significant.
(d). Negative feedback shown by representative immunohistochemistry of NPNT, in ZG adjacent to phaeochromocytoma compared to that adjacent to ZF-like or ZG-like APAs (20x magnification).
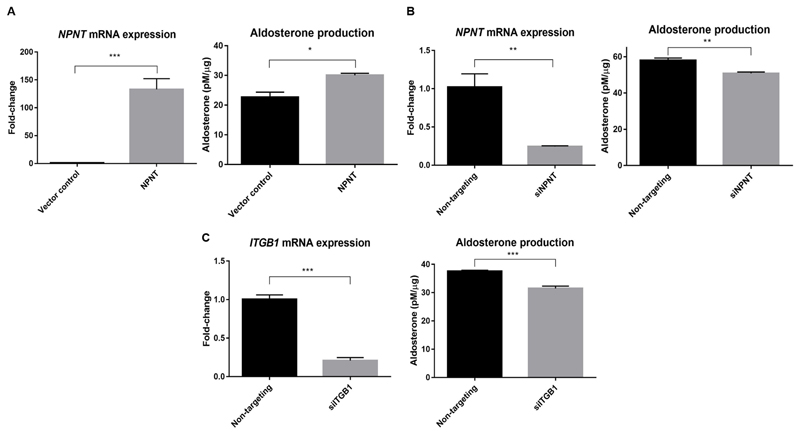
NPNT drives aldosterone production
NPNT overexpression in H295R cells increased aldosterone synthesis compared to control (Figure 3A). Similarly, silencing NPNT by over 75% reduced hormone production (Figure 3B). NPNT has been previously found to bind strongly and specifically to integrin receptor α8β116, with approximately 100-fold higher affinity compared to other RGD motif-containing proteins such as fibronectin or vitronectin27. In our study, silencing of ITGB1, encoding integrin subunit β1, by approximately 80%, caused a similar reduction in aldosterone production comparable to silencing of NPNT (Figure 3C). This receptor silencing was accompanied by a 3.8-fold increase in NPNT mRNA expression (p=0.01).
Figure 3. NPNT drives aldosterone production, likely through receptor ITGB1.
(a). NPNT overexpression increases protein-normalized aldosterone production, compared to vector control (n=4).
(b). NPNT silencing decreases protein-normalized aldosterone production, compared to non-targeting control (n=4).
(c). ITGB1 silencing decreases protein-normalized aldosterone production, compared to non-targeting control (n=4).
Bars represent mean expression per group±s.e.m. Statistical analyses were conducted by Student's t-test. *P<0.05; **P<0.005; ***P<0.0005
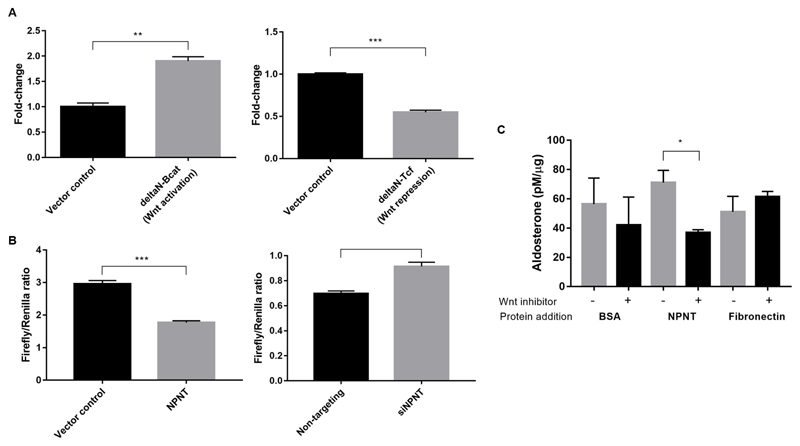
NPNT is a Wnt target gene and produces aldosterone via this pathway
NPNT was found to be a Wnt/β-catenin target gene in skin, being induced by Wnt activation in the bulge and hair germ cells17. We investigated the influence of Wnt on NPNT mRNA expression in H295R cells, using plasmids modulating Wnt signaling. To activate the Wnt canonical pathway, ΔN47 β-catenin, a strong constitutive inducer encoding an N-terminally truncated form of β-catenin resistant to proteolysis28, was expressed. This led to a near-doubling of NPNT expression levels. Conversely, to inhibit β-catenin-dependent gene transcription, we expressed ΔN TCF4, a Wnt constitutive repressor due to an N-terminally truncated, dominant-negative TCF4 protein lacking the β-catenin interaction domain29. Wnt repression caused NPNT mRNA levels to halve (Figure 4A). To investigate the potential for negative feedback of NPNT on its own release, TCF-LEF activity was measured following changes in NPNT expression. Overexpressing NPNT caused a reduction in Wnt transcriptional activity, while silencing NPNT had the opposite effect (Figure 4B).
Figure 4. NPNT is a Wnt target gene and produces aldosterone via Wnt.
(a). Constitutive Wnt activation (ΔN-Bcat) induces NPNT mRNA expression, while constitutive Wnt repression (ΔN-TCF4) decreased NPNT expression compared to vector control (n=3).
(b). Wnt transcriptional complex TCF/LEF activity decreased in NPNT-overexpressed and increased in NPNT-silenced samples, as measured by firefly/renilla luciferase assay (n=6, n=4).
(c). Wnt inhibitor LGK-974 attenuated the increase in protein-normalized aldosterone production with addition of NPNT protein, compared to negative controls bovine serum albumin (BSA) and fibronectin (n=3).
Bars represent mean expression per group±s.e.m. Statistical analyses were conducted by Student's t-test. *P<0.05; **P<0.005; ***P<0.0005
In cases where NPNT protein was added to the cell medium, upon addition of Wnt inhibitor LGK-974, which blocks Wnt pathways upstream by binding the Wnt chaperone, porcupine, aldosterone production was nearly diminished by half compared to controls (Figure 4C).
NPNT is pro-adhesive in normal adrenal and APA cells
NPNT promotes cell adhesion in kidney mesangial cells30 and cardiomyocytes31. Cell impedance was recorded as a measure of cell adhesion in wells coated with phosphate-buffered saline (PBS), negative control bovine serum albumin (BSA), NPNT or positive control laminin. NPNT was pro-adhesive in human embryonic kidney (HEK) cells, normal primary adrenal cells and cells cultured from ZF-like and ZG-like APAs (Figure 5Ai-iv). These findings are consistent with the hypothesized physiological role of NPNT in adrenal cell-clustering for aldosterone production.
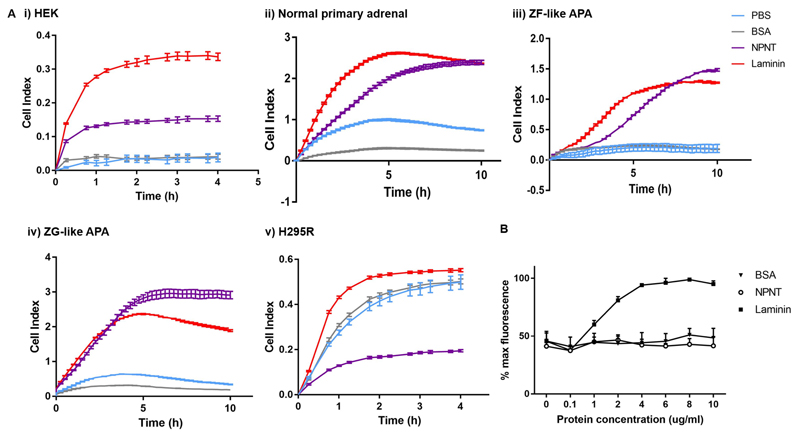
Figure 5. NPNT is pro-adhesive in normal adrenal and APA cells, but anti-adhesive in H295R.
(a). NPNT is pro-adhesive in i. HEK, ii. Normal primary adrenal, iii. ZF-like APA, iv. ZG-like APA, but anti-adhesive in v. H295R, as demonstrated by wells pre-coated with PBS, BSA, NPNT or laminin, and measured by Xcelligence cell impedance as cell index over time (4h for cell-lines, 10h for primary adrenal cells) (n=2 for ZF-like APA and ZG-like APA, n=4 for the rest).
(b). NPNT is anti-adhesive in H295R cells, as confirmed by Hoechst fluorescent stain assay measuring % maximum fluorescence as a representation of number of cells adhered to well with increasing concentrations of BSA, NPNT and laminin precoating (n=3 for each protein at each concentration).
Bars represent mean values per group±s.e.m.
Intriguingly, NPNT had the opposite effect on H295R cells, with cell index reaching less than 50% of that in BSA-coated wells even after 4h (Figure 5Av). This suggested that NPNT was anti-adhesive towards H295R cells, with cells tending to repel from the well surface. To re-affirm this finding, Hoechst stain assays were carried out independently, with increasing concentrations of matrix coating on wells. Post-wash, there was a concentration-dependent increase in number of cells remaining (as measured by fluorescence) in positive control laminin-coated wells. However, in NPNT-coated wells, the number of remaining cells showed no significant difference from that in BSA-coated wells at every protein concentration, indicating only background levels of adhesion (Figure 5B).
NPNT protects H295R cells from apoptosis
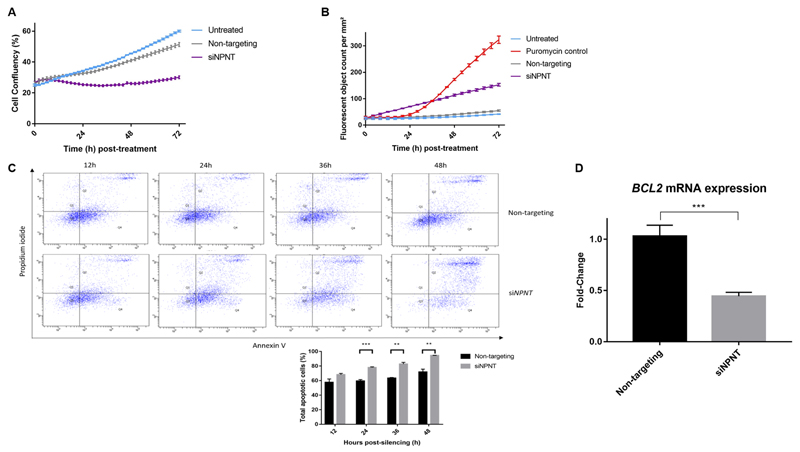
Cell confluency was 41% in the non-targeting controls compared to 26% in the NPNT-silenced samples at 48h, as observed using kinetic live-cell imaging (Figure 6A). Kinetic measurement of cytotoxicity via the YOYO1-iodide reagent revealed over three-fold increase in fluorescent (dead) cells when NPNT was silenced (Figure 6B). Following this, annexin V-propidium iodide dual assay was used to monitor H295R cell staining over time. NPNT-silenced cells exhibited greater apoptosis over time, whereas samples with non-targeting siRNA consistently showed basal levels of apoptosis (Figure 6C).
Figure 6. NPNT protects H295R cells from apoptosis.
(a). NPNT silencing causes cell confluency to remain low over 72h, as measured by kinetic live-cell imaging in groups that are untreated, treated with non-targeting siRNA or specific siRNA against NPNT (n=3, p<0.0001 between siNPNT and non-targeting).
(b). NPNT silencing drives active cell death, as shown by kinetic measurement of cytotoxicity using YOYO-1 iodide reagent, comparing number of fluorescent (dead) cells in groups that are untreated, treated with positive control puromycin, non-targeting siRNA or specific siRNA against NPNT (n=4, p<0.0001 between siNPNT and non-targeting)
(c). NPNT silencing causes cell death via apoptosis, as shown by flow cytometric analysis using annexin V-APC and PI double staining in groups that are treated with non-targeting siRNA or specific siRNA against NPNT. Quadrant analysis of the gated cells in FL-1 versus FL-2 channels was from 10,000 events. Annexin V+/PI− (lower right quadrant) areas stand for early apoptotic cells, and Annexin V+/PI+ (upper right quadrant) areas stand for late apoptotic or necrotic cells. Graph below shows percentage of total apoptotic cells at 12, 24, 36 and 48h post-silencing.
(d). NPNT silencing causes apoptosis through reduction of BCL2, a pro-survival factor in the intrinsic apoptotic pathway, as shown by mRNA expression in groups that are treated with non-targeting siRNA or specific siRNA against NPNT (n=6).
Bars represent mean expression per group±s.e.m. Statistical analyses were conducted by Student's t-test. *P<0.05; **P<0.005; ***P<0.0005.
A pro-survival factor in the intrinsic apoptotic pathway32, transcription of B-cell lymphoma 2 (BCL2) was greatly suppressed (>56%) in NPNT-silenced (siNPNT) cells (Figure 6D).
Discussion
The zona glomerulosa of human adrenal is unusual among endocrine organs in that few cells produce its signature hormone, aldosterone; and yet there is a high incidence of APA occurrence which is a common curable cause of hypertension. The ZG has likely evolved primarily to protect mammals from the scarcity of salt that has been the prevailing natural state, including for most of human history. It clearly also has the ability to adapt to chronic salt excess, to which the sparsity of aldosterone synthase is usually attributed25, but perhaps imperfectly, and hence the frequent somatic mutations permitted by high rates of ZG cell migration and renewal. Our discovery of NPNT, and its putative roles, in the adrenal may help to explain the link between physiology and pathology.
We first discovered NPNT in the adrenal as the most upregulated gene in the smaller ZG-like APAs with higher aldosterone synthetic capacity, harboring mutations of CACNA1D or ATP1A1, when compared to those with a ZF-like phenotype and mutations of KCNJ5. We also noted the exquisite ZG selectivity of its distribution in normal adrenal cortex8. These original findings have been reproduced by Akerstrom et al33. Interest in finding a mechanistic link between NPNT expression and blood pressure control has also been raised by a large-scale genome-wide association study in which a common single-nucleotide polymorphism in NPNT was associated with blood pressure regulation34.
The investigations which we now report show that NPNT is not just a marker of ZG cells, but plays an essential role in normal adrenal physiology as a peri-glomerular ECM protein. Coupled with its steroidogenic and pro-adhesive properties, this is consistent with a physiological role in adrenal cell-clustering to form functional aldosterone-producing units in the ZG. Our work provides evidence that a matrix protein can play a role in driving hormone synthesis, and together with the regulation of ZG cell behavior, helps us understand why the ZG may be structured as it is, and why tumors which resemble ZG are able to have a higher density of aldosterone production.
The ZG-selective staining suggests that the ECM of which NPNT is a major constituent supports zone-specific cellular behavior. In 2012, Hu et al made the crucial observation that isolated mouse ZG cells are too hyperpolarized to permit calcium entry; however, ZG cells in an intact adrenal slice generate spontaneous membrane oscillations sufficient for recurrent Ca2+ signals, whose periodicity could sustain aldosterone production20. Therefore, these findings suggest that the aldosterone-producing ability of adrenal cells requires them to co-exist in whole glomeruli, and this process could be regulated by NPNT.
Although the ZG is the zone of physiological aldosterone production, this is not carried out by all cells in the area, as evidenced by previous reports of patchy CYP11B2 staining22. But the mechanism underlying why some ZG cells express CYP11B2, whilst others do not, is yet to be determined. Together with the consistent correlation between NPNT and CYP11B2 staining, as well as evidence showing NPNT increases aldosterone production, we propose that the role of this matrix protein is to cluster ZG cells together to form a functional unit as indicated by its peri-glomerular staining. This is supported by the recent proposal that Ca2+ and Ca2+-activated K+ channels in ZG cells, when grouped in “rosette” structures, act as a pacemaker generating the oscillations which regulate aldosterone production35. Upon NPNT silencing, although the cell-corrected fall in aldosterone appears relatively small (Figure 3B), the absolute change consequent on reduction in both secretion and cell number is more substantial, and likely to have considerable impact in intact ZG.
In addition, our own finding of NPNT as an exquisitely-selective ZG protein, 3.6-fold upregulated in ZG compared to ZF when adjacent to a phaeochromocytoma, but diminished or absent when adjacent to an APA, suggests the disappearance of NPNT when physiological aldosterone secretion is not required. Analogous negative feedback has been reported in ZG adjacent to APA, where both subtypes of 3β-hydroxysteroid dehydrogenase-isomerase (3β-HSD), responsible for synthesizing progesterone from pregnenolone, were found to be suppressed36.
Our results also showed that NPNT promotes adhesion in normal adrenal cells, and in both subtypes of APAs. This is consistent with previous reports of NPNT expression in hepatitis, inducing the development of granuloma-like cell clusters of hepatocytes37. Cardiomyocytes grown on NPNT not only exhibited increased adhesion, but also expressed high amounts of connexin-43 along their intercellular junctions, indicating well-established intercellular communication, and couple electrically with each other resulting in synchronous beating31.
The link between increased cell adhesion and aldosterone production lies within the Wnt signaling system. Intracellular Wnt signaling diversifies into several major pathways, including: (1) the β-catenin/TCF-LEF pathway (canonical Wnt), which activates nuclear target genes; (2) the planar cell polarity (PCP) pathway; and (3) the Wnt/Ca2+ pathway, with the last two being classified as the non-canonical Wnt pathways38. While NPNT expression is itself under control of the canonical pathway, the non-canonical Wnt/PCP pathway is likely to be involved in mediating NPNT’s control of cell-cell adhesion and the localized assembly of ECM (Figure 4C)39. It has been shown that an aberrant PCP pathway leads to disruption of integrin β1-mediated interactions and in turn, disorganization of the ECM40. In addition, in line with the proposed role of NPNT, integrin β1 expression was reported to be crucial for adhesion of endothelial cells, with its absence causing focal adhesions to become short and disorganized41.
Although our experiments concentrated on the physiological roles of NPNT in normal adrenal and benign APAs, its anti-adhesive and anti-apoptotic effect on H295R cells have drawn our attention to a potential role in malignancy. ACCs, although rare, are much more devastating than APAs. NPNT has been shown to confer apoptosis resistance in H295R cells by modulating the expression of pro-survival protein BCL2, whose role is to block caspase activation32. This detachment of cells from the ECM often results in apoptotic cell death known as anoikis42. Excess secretion of ECM components suppresses the physiological induction of anoikis in maintaining normal tissue architecture43, and could explain the high levels of NPNT expression in adrenocortical carcinoma and immortalized H295R cells (Figure 1B).
However, benign APAs are much commoner than ACCs, and the main translational potential of NPNT may lie in its use as a diagnostic marker in patients with sub-centimeter APAs, whose CT scan and/or adrenal vein sampling results are inconclusive. Recent publications have reported only 50% concordance between AVS and CT, with >10% of patients aged >50 lateralizing to opposite sides44,45. Therefore, in vivo measurement of adrenal NPNT could be a more accurate predictor of APA presence and even genotype, as the secreted protein may be measurable in adrenal vein samples routinely collected for unilateral APA diagnoses.
A limitation of the work to date is that the H295R cell-line is not a perfect model for native ZG cells or ZG-like APAs, even though H295R cells have proven invaluable in studying mechanisms involved in the physiological regulation of aldosterone production46. Therefore, in the adhesion studies, primary adrenal cell types were also utilized. In addition, the H295R cell-line is already known to harbour a CTNNB1 mutation47 with high levels of NPNT, so whenever applicable, silencing NPNT was prioritised. Furthermore, our work on malignant cells in this study has been carried out only on H295R, due to the lack of other human adrenal carcinoma cell-lines amenable to transfection. A further limitation is that centripetal migration of adrenocortical cells cannot be studied in human adrenal. Due to the dispersed-cell nature of the experiments which we can currently undertake, the effects of NPNT’s various roles in the adrenal may be under-estimated in this study. The full impact of NPNT will become apparent during experiments on intact adrenal with preserved cell-cell contacts. Future work may involve a ZG-selective conditional-knockout of NPNT, by crossing a floxed-NPNT mouse48 with an aldosterone synthase-Cre recombinase mouse49.
In conclusion, we have discovered NPNT to be an exquisitely ZG-selective ECM protein in the adrenal. The distribution of NPNT defines the glomeruli anatomically, and its actions explain the critical role of glomerular structure in regulation of aldosterone production and hence blood pressure control. The high levels of NPNT in the smaller aldosterone-dense ZG-like APA subtype, as well as in ACCs, suggest future potential as a diagnostic marker, or target for novel therapies.
Perspectives
Primary aldosteronism is the most common secondary cause of hypertension, of which APAs make up 30-50% of cases, with hypertension potentially curable by adrenalectomy. Recently, the discovery of somatic mutations in CACNA1D/ATP1A1/ATP2B3/CTNNB1 characterize a subtype of small ZG-like APAs that are also histologically and biochemically distinct from the classical large KCNJ5-mutant ZF-like APAs. Investigations into transcriptomic differences between the two subtypes revealed NPNT, encoding the matrix protein nephronectin, to be the most upregulated gene in ZG-like vs ZF-like APAs. Subsequent investigations have shown NPNT to be not just a biomarker of ZG-type APAs, but to play an important role in normal ZG. Found to be under canonical Wnt control, the distribution and effects of NPNT suggest that it defines the anatomy and function of normal adrenal glomeruli, driving steroidogenesis and adhesion physiologically. In contrast, in immortalized adrenocortical carcinoma cells, NPNT exerts anti-adhesive and anti-apoptotic effects. Clinical measurement of NPNT in adrenal vein blood may have application in diagnosis of unilateral APAs. Complete cure of hypertension, upon removal of CTNNB1-mutant APAs, may be predicted through unilateral detection of secreted proteins such as NPNT during adrenal vein sampling. Apart from its potential as a diagnostic marker, the high levels of NPNT in the smaller aldosterone-dense ZG-like APA subtype, and in ACCs, make it an attractive molecular target for novel therapies.
Supplementary Material
Novelty and Significance.
1). What is new?
NPNT, selectively expressed in the zona glomerulosa of human adrenal cortex, and of APAs arising from this, is shown to have roles in steroidogenesis, cell-adhesion, and protection of adrenocortical cells from apoptosis.
2). What is relevant?
APAs are greatly under-diagnosed, one reason being the small size of those appearing to arise in the ZG of adrenal cortex. Recognition of their separate identity, and of the role of NPNT in steroidogenesis, will encourage clinicians to give greater consideration to small adenomas.
3). Summary
NPNT is an exquisitely selective, Wnt-driven ZG protein, which appears to play an important role in steroidogenesis, by promoting adhesion of ZG cells, and preventing apoptosis.
Acknowledgements
We thank the Cambridge NIHR BRC Cell Phenotyping Hub, in particular, Anna Petrunkina Harrison and Simon McCallum and for their advice and support in flow cytometry.
Sources of Funding
This research was funded by grants from the National Institute for Health Research (NIHR) Senior Investigator award (NF-SI-0512-10052) to M.J.B. A.E.D.T. is supported by the Agency for Science, Technology and Research (A*STAR) Singapore, and the Wellcome Trust Translational Medicine and Therapeutics award to M.J.B. (085686/Z/08/A); S.G. is supported by the British Heart Foundation (FS/14/75/31134).; and J.Z. by the Cambridge Overseas Trust. Additional support was provided by the NIHR Cambridge Biomedical Research Centre (Cardiovascular and Metabolic, and Human Tissue Bank).
Footnotes
Conflict of Interest/Disclosure
None.
References
- 1.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 2.Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–336. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 4.Reincke M, Fischer E, Gerum S, et al. Observational study mortality in treated primary aldosteronism: The German conn’s registry. Hypertension. 2012;60:618–624. doi: 10.1161/HYPERTENSIONAHA.112.197111. [DOI] [PubMed] [Google Scholar]
- 5.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 6.Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azizan EAB, Lam BYH, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa-and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- 8.Azizan EAB, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- 9.Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–442. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 10.Teo AED, Garg S, Haris Shaikh L, Zhou J, Karet Frankl FE, Gurnell M, Happerfield L, Marker A, Bienz M, Azizan EaB, Brown MJ. Pregnancy, Primary Aldosteronism, and Adrenal CTNNB1 Mutations. N Engl J Med. 2015;373:1429–1436. doi: 10.1056/NEJMoa1504869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A, Katabami T, et al. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab. 2013;98:1567–1574. doi: 10.1210/jc.2012-3726. [DOI] [PubMed] [Google Scholar]
- 12.Ono Y, Nakamura Y, Maekawa T, Felizola SJ, Morimoto R, Iwakura Y, Kudo M, Seiji K, Takase K, Arai Y, Gomez-Sanchez CE, et al. Different expression of 11β-hydroxylase and aldosterone synthase between aldosterone-producing microadenomas and macroadenomas. Hypertension. 2014;64:438–444. doi: 10.1161/HYPERTENSIONAHA.113.02944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YM, Wu KD, Hu-Tsai MI, Chu JS, Lai MK, Hsieh BS. Differential expression of type 1 angiotensin II receptor mRNA and aldosterone responsiveness to angiotensin in aldosterone-producing adenoma. Mol Cell Endocrinol. 1999;152:47–55. doi: 10.1016/s0303-7207(99)00059-3. [DOI] [PubMed] [Google Scholar]
- 14.Azizan EAB, Murthy M, Stowasser M, Gordon R, Kowalski B, Xu S, Brown MJ, Shaughnessy KM. Somatic mutations affecting the selectivity filter of KCNJ5 are frequent in 2 large unselected collections of adrenal aldosteronomas. Hypertension. 2012;59:587–591. doi: 10.1161/HYPERTENSIONAHA.111.186239. [DOI] [PubMed] [Google Scholar]
- 15.Omura M, Sasano H, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Clinical characteristics of aldosterone-producing microadenoma, macroadenoma, and idiopathic hyperaldosteronism in 93 patients with primary aldosteronism. Hypertens Res. 2006;29:883–889. doi: 10.1291/hypres.29.883. [DOI] [PubMed] [Google Scholar]
- 16.Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Müller U, Reichardt LF. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin α8β1 in the embryonic kidney. J Cell Biol. 2001;154:447–458. doi: 10.1083/jcb.200103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602. doi: 10.1242/dev.021493. [DOI] [PubMed] [Google Scholar]
- 19.Palacios G. Cell junctions in the adrenal cortex of the postnatal rat. J Anat. 1979;129:695–701. [PMC free article] [PubMed] [Google Scholar]
- 20.Hu C, Rusin CG, Tan Z, Guagliardo NA, Barrett PQ. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J Clin Invest. 2012;122:2046–2053. doi: 10.1172/JCI61996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fallo F, Pezzi V, Barzon L, Mulatero P, Veglio F, Sonino N, Mathis JM. Quantitative assessment of CYP11B1 and CYP11B2 expression in aldosterone-producing adenomas. Eur J Endocrinol. 2002;147:795–802. doi: 10.1530/eje.0.1470795. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383:111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Engeland M, Ramaekers FCS, Schutte B, Reutelingsperger CPM. A novel assay to measure loss of plasma membrane asymmetry during apoptosis of adherent cells in culture. Cytometry. 1996;24:131–139. doi: 10.1002/(SICI)1097-0320(19960601)24:2<131::AID-CYTO5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Atienza JM, Zhu J, Wang X, Xu X, Abassi Y. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J Biomol Screen. 2005;10:795–805. doi: 10.1177/1087057105279635. [DOI] [PubMed] [Google Scholar]
- 25.Shaikh LH, Zhou J, Teo AED, Garg S, Neogi SG, Figg N, Yeo GS, Yu H, Maguire JJ, Zhao W, Bennett MR, et al. LGR5 activates noncanonical Wnt signaling and inhibits aldosterone production in the human adrenal. J Clin Endocrinol Metab. 2015;100:E836–E844. doi: 10.1210/jc.2015-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Shaikh LH, Neogi SG, McFarlane I, Zhao W, Figg N, Brighton CA, Maniero C, Teo AED, Azizan EAB, Brown MJ. DACH1, a Zona Glomerulosa Selective Gene in the Human Adrenal, Activates Transforming Growth Factor-Signaling and Suppresses Aldosterone Secretion. Hypertension. 2015;65:1103–1110. doi: 10.1161/HYP.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato Y, Uemura T, Morimitsu K, Sato-Nishiuchi R, Manabe R-I, Takagi J, Yamada M, Sekiguchi K. Molecular basis of the recognition of nephronectin by integrin alpha8beta1. J Biol Chem. 2009;284:14524–14536. doi: 10.1074/jbc.M900200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolligs F, Hu G, Dang C, Fearon E. Neoplastic transformation of RK3E by mutant beta-catenin requires deregulation of Tcf/Lef transcription but not activation of c-myc expression. Mol Cell Biol. 1999;19:5696–5706. doi: 10.1128/mcb.19.8.5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science (80- ) 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 30.Bieritz B, Spessotto P, Colombatti A, Jahn A, Prols F, Hartner A. Role of alpha8 integrin in mesangial cell adhesion, migration, and proliferation. Kidney Int. 2003;64:119–127. doi: 10.1046/j.1523-1755.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 31.Patra C, Ricciardi F, Engel FBF. The functional properties of nephronectin: an adhesion molecule for cardiac tissue engineering. Biomaterials. 2012;33:4327–4335. doi: 10.1016/j.biomaterials.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Kelly P, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–1424. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Åkerström T, Willenberg HS, Cupisti K, et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. 2015;22:735–744. doi: 10.1530/ERC-15-0321. [DOI] [PubMed] [Google Scholar]
- 34.Warren HR, Evangelou E, Cabrera CP, et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet. 2017;49:403–415. doi: 10.1038/ng.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett PQ, Guagliardo NA, Klein PM, Hu C, Breault DT, Beenhakker MP. Role of voltage-gated calcium channels in the regulation of aldosterone production from zona glomerulosa cells of the adrenal cortex. J Physiol. 2016;594:5851–5860. doi: 10.1113/JP271896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okamura H, Doi M, Goto K, Kojima R. Clock genes and salt-sensitive hypertension: a new type of aldosterone-synthesizing enzyme controlled by the circadian clock and angiotensin II. Hypertens Res. 2016;39:681–687. doi: 10.1038/hr.2016.91. [DOI] [PubMed] [Google Scholar]
- 37.Inagaki FF, Tanaka M, Inagaki NF, Yagai T, Sato Y, Sekiguchi K, Oyaizu N, Kokudo N, Miyajima A. Nephronectin is upregulated in acute and chronic hepatitis and aggravates liver injury by recruiting CD4 positive cells. Biochem Biophys Res Commun. 2013;430:751–756. doi: 10.1016/j.bbrc.2012.11.076. [DOI] [PubMed] [Google Scholar]
- 38.Rao TP, Kühl M. An updated overview on wnt signaling pathways: A prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 39.Dzamba BJ, Jakab KR, Marsden M, Schwartz MA, DeSimone DW. Cadherin Adhesion, Tissue Tension, and Noncanonical Wnt Signaling Regulate Fibronectin Matrix Organization. Dev Cell. 2009;16:421–432. doi: 10.1016/j.devcel.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Astudillo P, Larrain J. Wnt Signaling and Cell-Matrix Adhesion. Curr Mol Med. 2014;14:209–220. doi: 10.2174/1566524014666140128105352. [DOI] [PubMed] [Google Scholar]
- 41.Abraham S, Kogata N, Fässler R, Adams RH. Integrin β1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- 42.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 44.Dekkers T, Prejbisz A, Kool LJS, et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: An outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016;4:739–746. doi: 10.1016/S2213-8587(16)30100-0. [DOI] [PubMed] [Google Scholar]
- 45.Zhu L, Zhang Y, Zhang H, Zhou W, Shen Z, Zheng F, Tang X, Tao B, Zhang J. Comparison between adrenal venous sampling and computed tomography in the diagnosis of primary aldosteronism and in the guidance of adrenalectomy. Medicine (Baltimore) 2016;95:e4986. doi: 10.1097/MD.0000000000004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: A model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993;133:1555–1561. doi: 10.1210/endo.133.4.8404594. [DOI] [PubMed] [Google Scholar]
- 47.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagneré AM, René-Corail F, Jullian E, Gicquel C, Bertagna X, Vacher-Lavenu MC, et al. Mutations of β-catenin in adrenocortical tumors: Activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65:7622–7627. doi: 10.1158/0008-5472.CAN-05-0593. [DOI] [PubMed] [Google Scholar]
- 48.Linton JM, Martin GR, Reichardt LF. The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development. 2007;134:2501–2509. doi: 10.1242/dev.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedman B, Kempna P, Carlone D, Shah MS, Guagliardo N, Barrett P, Gomez-Sanchez C, Majzoub J, Breault DT. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26:666–673. doi: 10.1016/j.devcel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.