Abstract
In tuberculosis (TB), the innate inflammatory immune response drives tissue destruction, morbidity and mortality. Monocytes secrete matrix metalloproteinases (MMPs) which have key roles in local tissue destruction and cavitation. We hypothesized that integrin signaling might regulate monocyte MMP secretion in pulmonary TB during cell adhesion to the extracellular matrix (ECM). Adhesion to type I collagen and fibronectin by Mycobacterium tuberculosis (Mtb)-stimulated monocytes, increased MMP-1 gene expression by 2.6-fold and 4.3-fold respectively, and secretion by 60% (from 1208.1±186pg/ml to 1934.4±135pg/ml; p<0.0001) and 63% (1970.3±95pg/ml; p<0.001). MMP-10 secretion increased by 90% with binding to type I collagen and 55% with fibronectin, while MMP-7 increased 57% with collagen. ECM did not affect secretion of tissue inhibitors of metalloproteinases-1/-2. Integrin αVβ3 surface expression was specifically upregulated in stimulated monocytes and was further increased after adhesion to type I collagen. Binding of either β3 or αV integrin subunits increased MMP-1/10 secretion in Mtb-stimulated monocytes. In a cohort of TB patients, significantly increased integrin β3 mRNA accumulation in induced sputum was detected for the first time, compared to control subjects (p<0.05). Integrin αVβ3 co-localized with areas of increased and functionally active MMP-1 on infected monocytes, and αVβ3 blockade markedly decreased type I collagen breakdown, and impaired both monocyte adhesion and leukocyte migration in a trans-well system (p<0.0001). In summary, our data demonstrate that Mtb-stimulation upregulates integrin αVβ3 expression on monocytes which upregulates secretion of MMP-1/-10 on adhesion to the ECM. This leads to increased monocyte recruitment and collagenase activity which will drive inflammatory tissue damage.
Introduction
Tuberculosis (TB) remains an important global health problem with 8.6 million new cases annually, of which at least 480,000 are multi-drug resistant (1). Lung cavitation is the hallmark of TB and results from extracellular matrix (ECM) destruction, creating an immuno-privileged site within which mycobacteria can proliferate and spread to new hosts. In addition, tissue damage impairs organ function and results in patient morbidity and mortality. Pulmonary ECM is composed of a network of molecules including type I, III and IV collagen, fibronectin, laminin, elastin and proteoglycans. Type I collagen is the primary structural fibril of the lung and is highly resistant to enzymatic degradation. In addition to its biomechanical properties, type I collagen has important roles in cell survival, adhesion, proliferation and migration (2). Fibronectin is present in lower amounts and has important functions in cell adhesion, growth and migration (3).
Human monocytes are a key element in formation of TB granuloma which is the main cellular host response to infection. Integrins are a family of receptors involved in regulation of immune responses (4), and peripheral blood monocytes express 8 integrin heterodimers: α1β1, α3β1, α4β1, α5β1, αXβ2, αMβ2, αLβ2 and αVβ3 (4, 5). These are key in interactions with other cells and with the ECM. Monocyte recruitment in acute inflammation is mediated in part by β2-integrin receptors (6, 7) while integrin α4β1 promotes arrest and adhesion to VCAM-1(8). Engagement of β2-integrins is also involved in downregulation of NF-κB-dependent genes encoding for pro-inflammatory cytokines via inhibition of TLR signaling (9). Integrin αvβ3 modulates αLβ2 integrin-dependent monocyte adhesion to ICAM-1(10). Mtb-infection of macrophages was reported to increase cellular adhesion and decrease surface expression of the phagocytic complement receptors (CR)3 (integrin αMβ2), CR4 (integrin αXβ2) (11).
Matrix Metalloproteinases (MMPs) are zinc-containing endopeptidases with diverse functions in inflammation and tissue repair. Most MMPs are able to degrade components of the pulmonary ECM, and some are released during the innate inflammatory immune response to Mtb infection. Our group have shown that MMPs are expressed within TB granulomas (12–14) and associated with disease severity (15) and tissue damage (16–18). MMP-1 is the main collagenase responsible for tissue destruction in pulmonary TB (19). In TB patients, including those with TB/HIV co-infection, elevated plasma MMP-1 concentrations were associated with collagen breakdown (20).
In TB, extensive tissue destruction may occur even with a low bacterial load, indicating a role of immune intercellular networks that drive MMP secretion. MMP expression is initially upregulated by Mtb-infected monocytes/macrophages, which then recruit peripheral blood monocytes by secretion of cytokines and chemokine, amplifying this response. MMP-dependent tissue destruction is, therefore, driven by activated uninfected neighboring cells, such as monocytes or respiratory epithelial cells, due to signaling networks within the granuloma (21, 22). MMP-1 is activated from a precursor zymogen (pro-MMP-1) a process which can be promoted by other MMPs, such as the stromelysins MMP-3 and -10 (23, 24). MMP-1 is inhibited by the tissue inhibitors of metalloproteinases (TIMP)-1 and -2 (25–27).
Although integrins are required for adhesion and cellular response to cues from the ECM environment, it is not known whether integrin-dependent adhesion to the lung ECM plays a role in the regulation of MMP gene expression and secretion by human monocytes recruited to the lung in TB. In the present study, we investigated whether contact with ECM components affects the Mtb-associated MMP gene expression and secretion from monocytes and dissected the mechanisms by which integrin signaling regulates MMP secretion in Mtb infection.
Material and Methods
Antibodies
To study integrin regulation of MMP expression, primary mouse anti-human integrin β1 (clone P4C10), integrin β2 (clone MEM48), integrin β3 (clone B3A), FITC-conjugated anti-human integrin β1, integrin αV (clone 272-17E6) antibodies were used (all from Millipore, Hertfordshire, UK). FITC conjugated goat anti-mouse IgG1 (Sigma-Aldrich, Dorset, UK), and Cy5 conjugated goat anti-rabbit (Abcam, Cambridge, UK) were used as secondary antibodies. Mouse IgG1 and FITC-conjugated mouse IgG1 were the isotype controls (BD Diagnostics, Oxford, UK).
Mycobacterium tuberculosis H37RV culture
Mycobacterium tuberculosis (Mtb) strain H37Rv was cultured in Middlebrook 7H9 medium supplemented with 10% ADC enrichment medium (BD Diagnostics), 0.2% glycerol and 0.02% Tween 80 (Sigma-Aldrich) with agitation at 10rpm. For infection experiments, mycobacteria were used at mid-logarithmic growth at an optical density of 0.60 (Biowave cell density meter, WPA, Cambridge, UK).
Primary monocyte isolation and culture
Ethical approval for obtaining healthy human volunteer blood was provided by the Outer West London Research Ethics Committee and written informed consent was obtained from individuals. Peripheral blood mononuclear cells were isolated by gradient density centrifugation with Ficoll-Paque PLUS (GE Healthcare, Buckinghamshire, UK) and CD16-monocytes were purified by negative magnetic-activated cell sorting (MACS monocyte isolation kit II; Miltenyi Biotec Ltd., Surrey, UK) according to manufacturer’s instructions. Purity was confirmed by CD64 staining and FACS analysis and was ≥95%. Viability assessed by trypan blue exclusion was ≥98%.
Monocytes were seeded at a density of 2.5x105 cells/cm2 in RPMI supplemented with 2mM glutamine and 10µg/ml ampicillin and 10% heat inactivated FBS. Monocytes were allowed to rest for 1h before adding 1:5 diluted CoMtb or CoMCont or infecting with Mtb H37Rv at a MOI=1. Monocytes were incubated for 24h and supernatants were collected for protein concentrations, while for investigation of gene expression, monocytes were incubated for 6h, rinsed with sterile PBS and lysed with Tri-Reagent.
Conditioned medium preparation (CoMtb)
Preparation of CoMtb was performed as previously described (28). Briefly, human monocytes cultured in RPMI (Life Technologies, Paisley, UK) medium supplemented with 2mM glutamine and 10 mg/ml ampicillin, were infected with Mtb strain H37Rv at a multiplicity of infection (MOI) of 1 and incubated for 24h at 37°C and 5% CO2. Supernatants were sterile filtered and aliquots stored at -20°C. Control medium from uninfected monocytes was termed CoMCont. CoMCont and CoMtb used in each experiment were donor-matched.
Coating of tissue culture plates with ECM components or integrin antibodies
Tissue culture plates were pre-coated with 100µg/ml native type I collagen from human fibroblasts (VitroCol), 250µg/ml type IV collagen from human placenta, or 20µg/ml human plasma Fibronectin (all from Advanced BioMatrix, California, USA). In brief, for type I collagen, wells were coated at a desired concentration of VitroCol diluted in sterile distilled water and incubated at room temperature for 1h, rinsed with sterile PBS or Hank’s balanced salt solution (HBSS). Type IV collagen was diluted in a 0.25% acetic acid solution and coated plates incubated for 1h and rinsed with HBSS. Fibronectin was diluted in HBSS, plates incubated for one hour and rinsed with sterile distilled water. After incubation any excess material was aspirated and plates were blocked with sterile 2% heat denatured BSA, rinsed with PBS/ HBSS and allowed to air dry for at least 45min.
To find optimal concentration of ECM ligands initial titration was performed using the human monocyte cell line THP-1 (Fig. S1A-S1C). 96-well plates were coated with increasing concentrations of type I collagen: (0, 1, 10, 100, and 200µg/ml); type IV collagen (0, 10, 100, 250, and 300 µg/ml) and fibronectin (0, 0.2, 2, 20, and 40µg/ml), and blocked with 2% heat denatured BSA to prevent unspecific adhesion. Control wells without ECM were only blocked with BSA. THP-1 monocytes were pre-labelled with 5µM Calcein and integrin αMβ2 (Mac-1) was blocked with 10µg/ml anti-αM antibody (clone LPM19C) to prevent binding to BSA. 5X104 labelled monocytes were added per well in RPMI without phenol red, activated with 20nM PMA and let to adhere for 1.5h. A standard curve was generated from serial dilutions of a known concentration of monocytes, and fluorescence measured in a in a FLUOstar Omega microplate reader (BMG Labtech, Bucks, UK).
To selectively analyze engagement of specific integrins, tissue culture plates were coated with goat anti-mouse IgG H+L polyclonal Ab (Sigma-Aldrich) overnight at 4°C, washed with PBS and coated for 2h at room temperature with 20µg/ml of anti-integrin antibodies (Millipore).
Measurement of MMP and TIMP concentrations
MMP and TIMP concentrations were analyzed by ELISA (Duoset, R&D Systems, Abingdon, UK) or by Luminex bead array (Luminex 200, Bio-Rad, Hertfordshire, UK) using the Fluorokine MAP kit (R&D Systems) according to manufacturer’s instructions. Lower limits of sensitivity for the Duoset kits are: 21.2pg/ml for TIMP-1, 31.2pg/ml for TIMP-2, 156pg/mL for MMP-1 and 31.2pg/ml for MMP-10. In the Fluorokine Luminex kit the lower limits are: 1.1pg/ml for MMP-1, 12.6pg/ml for MMP-2, 7.3pg/ml for MMP-3, 6.6pg/ml for MMP-7, 13.7pg/ml for MMP-9 and 3.2pg/mL for MMP-10. Variation on secreted concentrations of MMPs and TIMP between individual donors is shown in supplementary data.
RNA extraction and real-time reverse transcription PCR
Total RNA was extracted from monocyte lysates using PureLink RNA Mini Kit (Life Technologies) according to manufacturer’s instructions. Quantitative real-time RT-PCR was performed using the OneStep RT-PCR master mix (Qiagen Crawley, UK) according to the manufacturer’s instruction on a Stratagene Mx3000P platform (SABiosciences, Crawley, UK) using 15µg of RNA per sample. To determine the quantitative change in RNA, standard curves were prepared from a known concentration of the genes of interest and subjected to real-time PCR as above. MMP-1 primers and probes were custom made and supplied by Sigma-Aldrich (forward primer: 5’- AAGATGAAAGGTGGACCAACAATT -3’; reverse primer: 5’-CCAAGAGAATGGCCGAGTTC-3’; probe: 5’- FAM-CAGAGAGTACAACTTACATCGTGTTGCGGCTC-TAMRA-3’) and 18S rRNA primer and probe mix was supplied by Life Technologies.
RNA extraction and Real-Time Polymerase Chain Reaction (RT-PCR) from induced sputum
The study was approved by the University of Cape Town (HREC Ref 516/2011), as previously described (29). Informed consent was obtained in all cases. 1ml of RNA later (Qiagen) was added on site to the sputum samples and mucolysis was performed by adding an equal volume of 0.1% Dithiothreitol (Sigma-Aldrich) and agitating gently at room temperature for 20 min. The mucoid layer was filtered through 100μm pore size strainer and centrifuged at 500g for 10 min. The cell pellet was aspirated and 1.5ml of cold TRI reagent added before vortexing. Total RNA was extracted using the Purelink RNA Mini Kit (Life Technologies) and reverse transcription RT-PCR was performed using 15ng RNA and the OneStep RT-PCR Kit (Qiagen), on a Stratagene Mx3000Pro using integrin β3and β-actin primers and probes (Life Technologies). Analysis was performed using the Pfaffl comparative Ct method, applying the equation: Ratio (integrin β3:β-actin mRNA)= E β3ΔCt(calibrator-sample) /E β-actinΔCt (calibrator-sample); E is the real-time PCR efficiency of one cycle in the exponential phase, calculated according to the equation: E= 10[–1/slope]. Samples without a Ct value for integrin β3 after 43 cycles but with a Ct for β-actin were considered negative.
Flow Cytometry
Monocytes were detached with 0.5M EDTA (Life Technologies), washed with PBS, fixed with cold 4% paraformaldehyde and blocked with 1% BSA/5% human serum buffer. Cells were incubated for 1h at 4°C with primary mouse anti-human integrin antibody or mouse isotype IgG1 control and FITC conjugated anti-mouse secondary antibody. For Mtb-infected monocytes, cells were labeled with the antibodies first and fixed for 1h at 4°C with 4% paraformaldehyde. Flow cytometry was performed on a FACS Calibur cytometer which was calibrated using FACS CaliBRITE beads (BD Biosciences). The baseline Forward Scatter, Side Scatter and FL1H settings were adjusted using an unstained, unstimulated monocyte sample. Mean fluorescence intensities (MFI) were compared after normalization to the isotype control. Data was analyzed using FlowJo vX.0.6 (Tree star, Ashland, USA). Analysis of integrin αVβ3 in the presence of ECM components in Mtb or CoMtb stimulated monocytes was performed by plotting MFI data normalized to respective control cells (with control media or CoMCont) from 4 different healthy volunteers (n=4).
Confocal Microscopy
8-well glass slides were pre-coated type I collagen and monocytes were seeded and incubated as described. Cells were fixed, blocked and stained with primary anti-integrin antibodies and FITC-conjugated goat anti-mouse secondary antibody. MMP-1 was stained with a mouse anti-human MMP-1 primary antibody (Abcam) and alexa fluor 633 conjugated with anti-mouse IgG (Life Technologies) as secondary antibody. Integrin β3 was stained with a mouse anti-human β3 primary antibody and alexa fluor 488 conjugated goat anti-mouse IgG secondary antibody. Staining with secondary antibody alone was used as control. DQ collagen coated slides were used to analyze MMP collagenolytic activity by confocal microscopy. Integrin β3 was blocked using 20µg/ml functional blocking antibody, and a mouse IgG1 antibody was used as a control. For all experiments DAPI was used as nuclear counterstain. Phalloidin conjugated with alexa fluor 633 was used to stain F-actin. Images were scanned on a Leica TCS SP5 confocal microscope equipped with 405nm diode laser, 488nm argon laser, 543nm and 633nm HeNe lasers and using the Leica Application Suite 2.6.2 software (Milton Keynes, UK). Images were processed using ImageJ software v1.46r (NIH, Maryland, USA).
Transmigration assay
1x106 primary human monocytes were loaded on the apical side of a trans-well (5μm pore) pre-coated with type I collagen. CoMtb or CoMCont (1:2 dilution) was loaded on the basal side to act as a chemotactic stimulus. Monocytes were let to migrate for 2h at 37°C, 5%CO2. Migrating monocytes were collected from the basal side, centrifuged, and resuspended in 400μL PBS and 50μL of CountBrigh Absolute Counting Beads (Life technologies) were added to each tube. 5.5x106 unstimulated monocytes were used to adjust baseline Forward Scatter, Side Scatter and exclude cell debris. Total number of transmigrated monocytes was assessed by comparing the ratio of bead to cell events.
Adhesion assay
Primary monocytes were stained for 40 min with 10µM Cell Tracker green CMFDA (Life Technologies), washed, and incubated for 1h with 20µg/ml mouse anti-human integrin β3, 20µg/ml mouse IgG1 isotype control antibodies or left untreated. Monocytes were loaded on 96-well plates pre-coated with type I collagen and incubated with CoMCont or CoMtb for 18h. Non-adherent cells were removed by rising with PBS and serum-free RPMI without phenol red was loaded in the wells. Fluorescence was measured in a FLUOstar Omega microplate reader.
Statistical Analysis
Data analysis was performed using GraphPad Prism v5.02 (GraphPad software Inc., California, USA). Data are presented as mean ±s.d. of 3 replicate samples and are representative of at least 3 independent experiments, unless otherwise stated. Statistical analysis was performed using two-tailed Student’s unpaired t-test, or Mann-Whitney, or one-way ANOVA with Tukey’s post hoc analysis were used as appropriate. Differences between variables were considered statistically significant for p-values lower than 0.05. P-values are represented as follows: * p<0.05, ** p<0.01, *** p<0.001, **** p<0.001.
Results
Adhesion to the ECM modulates MMP-1 gene expression and secretion by Mtb-infected monocytes
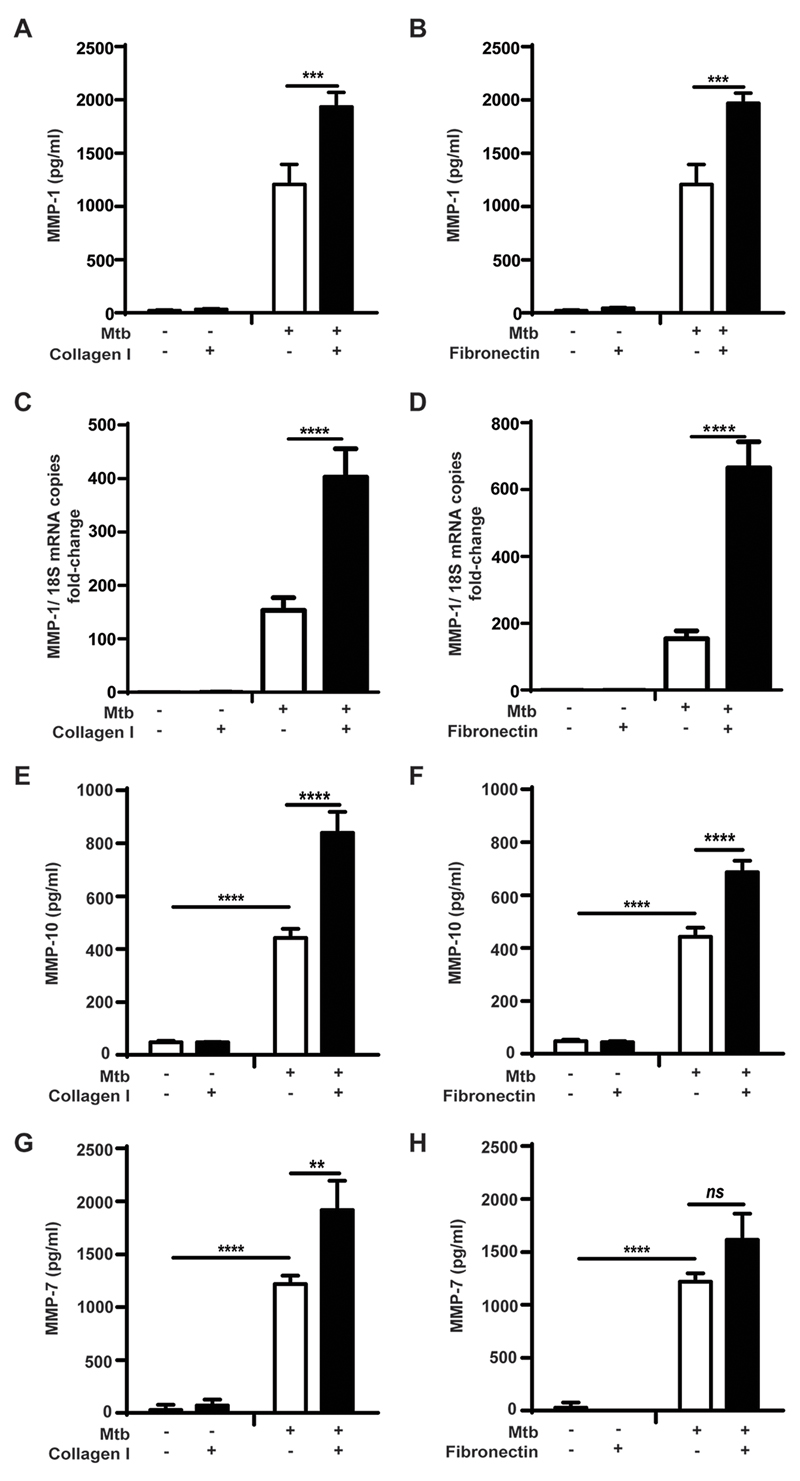
Since MMP-1 is a key mediator of Mtb-associated immunopathology (19), we investigated the role of components of the ECM on the regulation of MMP-1 gene expression and secretion by primary human monocytes in TB. The optimal coating concentration, for each ECM ligand was determined by titration in an adhesion assay using a human monocyte cell line (Fig. S1A-S1C). Mtb-infected monocytes increased MMP-1 secretion after adhesion to collagen or fibronectin by 60% (from 1208.1±186pg/ml to 1934.4±135pg/ml; p<0.0001), and 63% (1970.3±95pg/ml; p<0.001) respectively (Fig. 1A-B). Adhesion to type IV collagen did not alter monocyte MMP-1 secretion compared to cells cultured in the absence of ECM proteins (Fig. S1D). At the transcriptional level, MMP-1 mRNA accumulation in Mtb-stimulated human monocytes increased 2.6-fold with adhesion to type I collagen, and 4.3-fold with fibronectin (all p<0.0001; Fig. 1C-D). Uninfected monocytes were then stimulated with CoMtb to mimic the cytokine networks between infected and uninfected cells that amplify MMP expression within the granuloma. Stimulation with CoMCont was used as control. MMP-1 secretion by CoMtb-stimulated uninfected monocytes was also increased by adhesion to ECM substrates (Fig. S1E, S1F). Again, no significant difference in MMP-1 concentrations was detected with monocyte adhesion to type IV collagen (Fig S1G).
Figure 1. Secretion of MMP-1, -10, and-7 by Mtb-infected primary monocytes is increased by adhesion to type I collagen and fibronectin.
Monocytes in the presence or absence of type I collagen, or fibronectin were infected with Mtb (MOI=1). For secretion analysis supernatants were collected at 24h post-stimulation; while for gene expression cell lysates were collected at 6h. MMP-1 concentrations were upregulated in Mtb-infected monocytes adherent to (A) type I collagen; (B) fibronectin, and MMP-1 mRNA accumulation was also upregulated in the presence of (C) type I collagen and (D) fibronectin. Samples were normalized to 18S ribosomal RNA. Secretion of (E, F) MMP-10, and (G, H) MMP-7 was also upregulated in the presence of type I collagen and fibronectin, after 24h of Mtb-infection. Figures show means ±s.d. and are representative of 3 independent experiments performed in triplicate. **p<0.01;***p<0.001: ****p<0.001; ns- not significant.
Next, we investigated the effect of the ECM on regulation on other MMPs upregulated by stimulation with Mycobacterium tuberculosis. Infected monocytes secreted considerably higher concentrations of the stromelysin MMP-10 which were increased by an additional 90% after adhesion to type I collagen (from 442.5±35pg/ml to 840±78pg/ml; p<0.0001; Fig. 1E) and by 55% with fibronectin (686.2±44pg/ml; p<0.0001; Fig. 1F). MMP-3 secreted concentrations were low, and no differences in secretion were seen with adhesion to ECM proteins (Fig. S2A, S2B). The elastase MMP-7 was also upregulated following Mtb-infection and was further upregulated by 57% with type I collagen (Fig 1G and 1H). Secretion of, MMP-2, a gelatinase, was downregulated in infected monocytes compared with uninfected controls (p<0.0001) but this was not affected by ECM (Fig. S2C, S2D). The secretion of the other major gelatinase MMP-9 was upregulated by Mtb-infection but this too was not affected by monocyte adhesion to ECM proteins (Fig. S2E and S2F).
ECM and control of TIMP secretion in Mtb- and CoMtb-stimulated monocytes
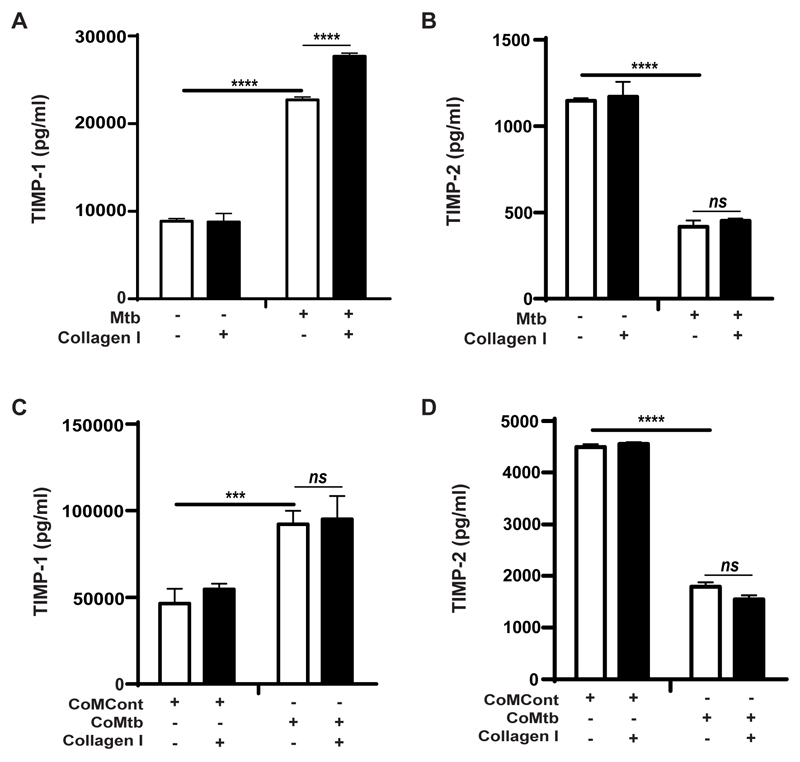
TIMPs are critical regulators of MMP activity and MMP:TIMP ratios regulate net tissue destruction and therefore secreted concentrations of TIMP-1 and -2 were also measured. Mtb-infection increased secretion of TIMP-1 by 2.6-fold (from 8.6ng/ml to 22.7ng/ml; p<0.0001; Fig. 2A), while TIMP-2 concentrations decreased by approximately 64% (Fig. 2B). However, the absolute TIMP-1 concentrations were much greater than the secreted TIMP-2 concentrations. Adhesion to type I collagen increased the TIMP-1 concentrations secreted by Mtb-stimulated monocytes (from 22.71±8.86ng/ml to 27.69±8.78ng/ml; p<0.0001; Fig. 2A). Type I collagen did not affect TIMP-2 (Fig. 2B) and fibronectin had no effect on secretion of either TIMP-1 or -2 by Mtb-stimulated monocytes (data not shown). CoMtb-stimulation similarly increased TIMP-1 and decreased TIMP-2 concentrations and secretion was not modulated by the ECM (Fig. 2C, 2D).
Figure 2. TIMP-1 and -2 secretion by Mtb-infected and CoMtb-stimulated monocytes adherent to type I collagen.
Monocytes adherent to type I collagen, fibronectin or in the absence of matrix were either infected with Mtb (MOI=1) or stimulated with CoMtb (1:5 dilution). Supernatants were collected at 24h post-infection and analyzed for secreted concentrations of: (A) TIMP-1and (B) TIMP-2 with Mtb-infected monocytes, and (C) TIMP-1 and (D) TIMP-2 secretion of CoMtb-stimulated monocytes. CoMCont was used as control of CoMtb. Bars show mean ±s.d. and figures are representative of 3 independent experiments performed in triplicate. ***p<0.001; ****p<0.001; ns- not significant.
Integrin expression by human monocytes in tuberculosis
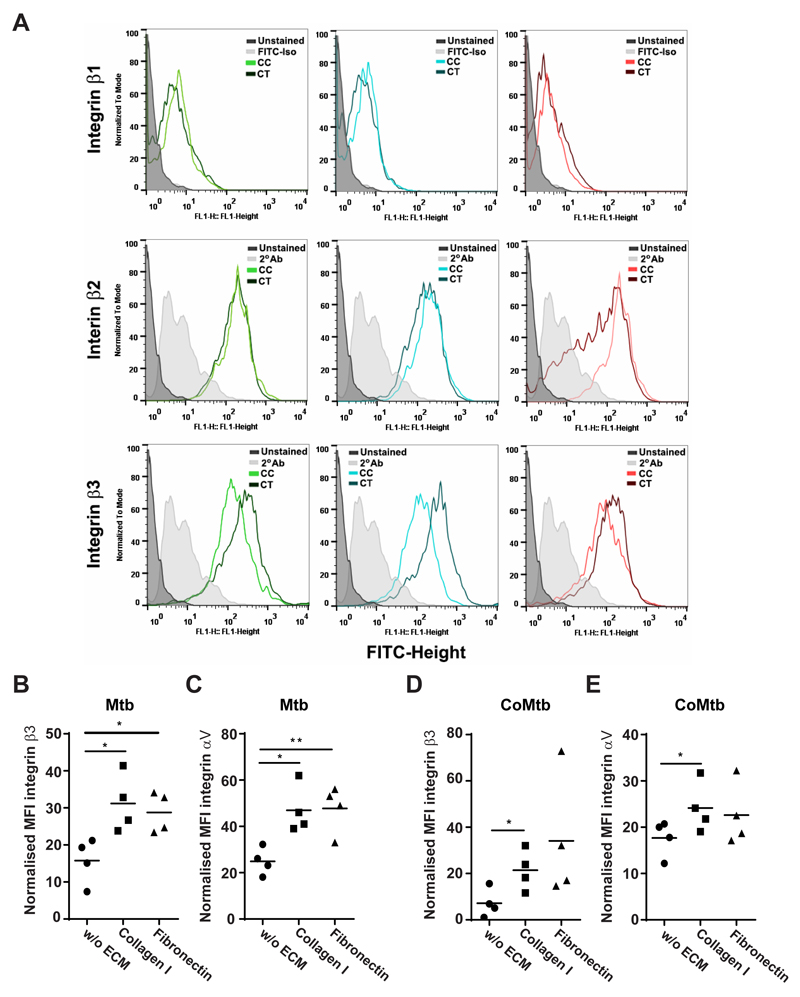
Next, the surface expression of β1, β2 and β3 integrin subunits was analyzed, in order to investigate changes in integrin expression in stimulated monocytes compared with controls and in presence of ECM (Fig. 3). MFIs for β1 integrin were similar in all conditions, while for β2 there was only a small increase with CoMtb stimulation compared to CoMCont. Surface levels of β3 integrin were increased by CoMtb-stimulation (Fig. 3A).
Figure 3. Surface expression of β3 and αV integrin subunits in Mtb-stimulated monocytes adherent to ECM proteins.
Monocytes cultured in the presence or absence of type I collagen or fibronectin were stimulated for 24h with CoMtb (1:5 dilution), and CoMCont as control, or directly infected with Mtb (MOI = 1). Cells were fixed, blocked and stained with FITC-conjugated anti-integrin β1or primary anti-integrin β2, β3 or αV antibodies and secondary FITC-conjugated anti-mouse IgG antibody. Secondary antibody alone or FITC-conjugated IgG1 isotype antibody were used as controls. (A) Histograms of integrin subunits β1, β2 and β3 for control and CoMtb-stimulated monocytes, show an increase in β3 expression with CoMtb stimulation. Mean fluorescence intensities (MFIs) of integrin subunits (B) β3 and (C) αV in monocytes stimulated with Mtb, and MFIs of (D) β3 and (E) αV in monocytes stimulated with CoMtb, in the presence or absence of matrix components (n=4). MFIs were normalized to baseline MFIs of respective controls (control media or CoMCont). Figure shows data points and means; *p<0.05.
Data in which β3 integrin expression in Mtb-stimulated monocytes was normalized by subtracting basal MFIs of respective control, showed that monocyte adhesion to type I collagen and fibronectin increased integrin β3 MFIs by 3-fold (p<0.05) (Fig. 3B). Since the only integrin heterodimer expressed by primary human monocytes containing the β3 subunit is the receptor αVβ3, we next investigated surface expression of the αV subunit. Mtb-stimulated monocytes adherent to type I collagen had significantly greater integrin αV expression compared to cells cultured in the absence of ECM (p<0.05; Fig. 3C). Adhesion to fibronectin also significantly upregulated integrin αV expression compared to Mtb-stimulated cells in the absence of ECM (p<0.01; Fig. 3D). This was also investigated in CoMtb-stimulated monocytes, and we found a similar increase in expression of β3 and αV integrin subunits with adhesion to type I collagen which was further increased by adhesion to ECM (p<0.05; Fig. 3D, 3E).
Integrin regulation of MMP secretion in tuberculosis
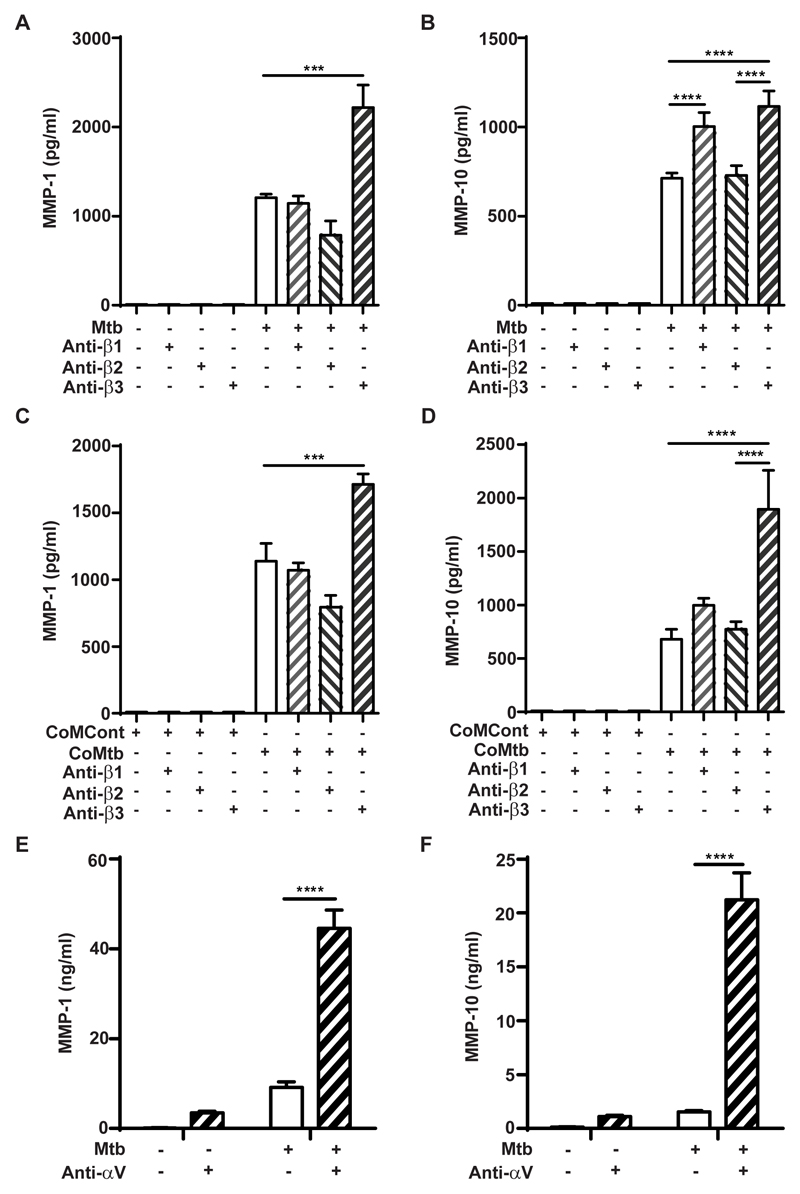
Next, the regulatory effect of integrin αVβ3 activation on MMP-1 and MMP-10 gene expression and secretion by Mtb- and CoMtb-stimulated monocytes was investigated. Integrin activation was performed by pre-coating plates with anti-integrin antibodies to the ligand binding site, to act as ligand mimetic. In Mtb-infected monocytes, activation of the β3 subunit caused an 84% increase in monocyte MMP-1 secretion (from 1207± 41pg/ml to 2218±253pg/ml, p<0.001; Fig. 4A), while no significant differences were detected after activation of β1 subunit and a small decrease in MMP-1 was detected with β2 subunit (p<0.05). MMP-10 secretion was similarly upregulated by integrin β3 activation following Mtb-infection (from 712.8±28.9pg/ml to 1115.9±86pg/ml; Fig. 4B). A significant increase in MMP-10 concentrations was also detected after activation of β1 integrins. Similar data were observed with CoMtb-stimulation of human monocytes following activation of β3 integrin subunit (p<0.001; Fig. 4C-D). A small decrease in MMP-1 was also detected with integrin β2 activation (from 1138±133pg/ml to 794.5±90pg/ml). Monocyte MMP-10 secretion was not altered by activation of β1 or β2 integrins in CoMtb-stimulated monocytes. Binding to αV (which pairs with β3 on monocytes) resulted in a 13-fold increase in MMP-1 secretion (p<0.0001; Fig. 4E) and 14.7-fold increase in MMP-10 concentrations (from 1459.73 ±36.9pg/ml to 21543.41±2485.7pg/ml; p<0.0001; Fig. 4F) following Mtb-infection. Binding to αV also increased MMP-1/10 secretion from uninfected control monocytes.
Figure 4. Regulation of MMP-1 and -10 by integrin beta and αV subunits in Mtb-infected and CoMtb-stimulated primary monocytes.
Plates were pre-coated with goat anti-mouse Fc region, blocked and coated with 20μg/ml either mouse anti-human integrin β1, β2, β3 antibodies or left uncoated and stimulated for 24h with (A, B) Mtb or (C, D) CoMtb. CoMCont was used as control for CoMtb. Supernatants were collected and assayed for MMP-1 and MMP-10. Plates were also coated with 20μg/ml anti-integrin αV antibodies, monocytes infected with Mtb (MOI=1) and supernatants assayed for (E) MMP-1 and (F) MMP-10. Figures show means ±s.d. and are representative of 3 independent experiments performed in triplicate. *p<0.05; ***p<0.001; ****p<0.0001.
Integrin β3 expression by cells extracted from sputum of tuberculosis patients
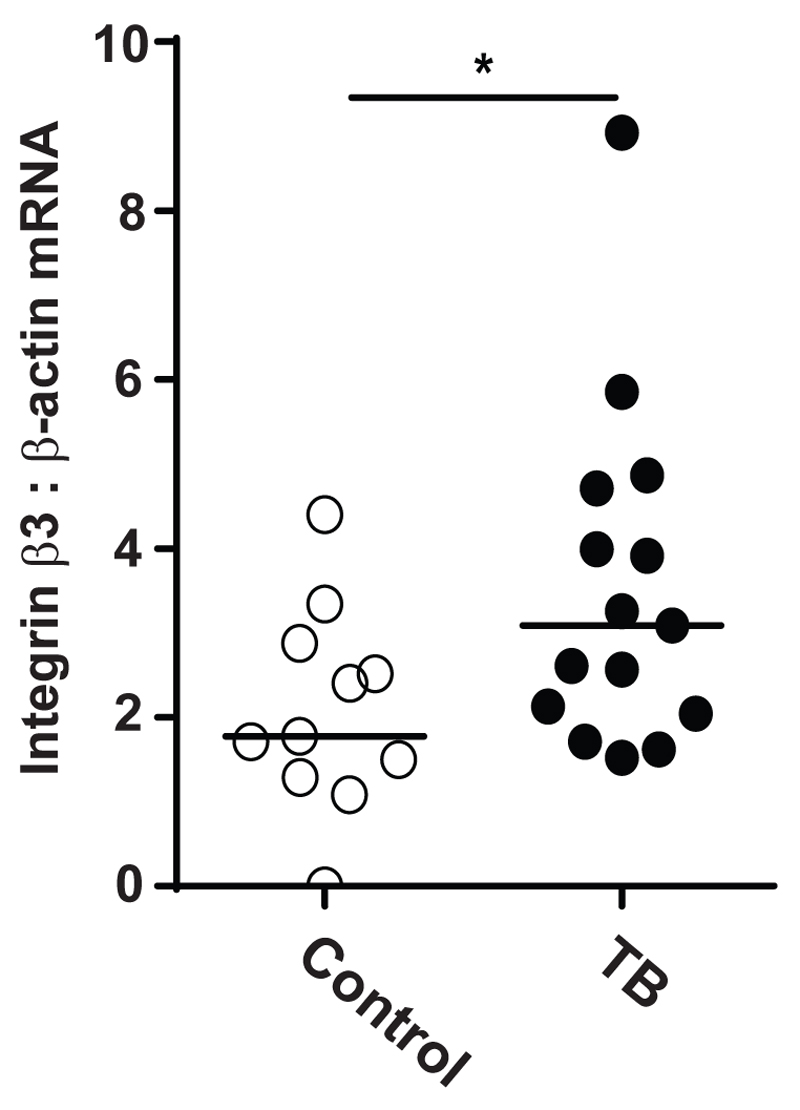
Since integrin αVβ3 was increased in our cellular model of tuberculosis and its activation was associated with increased MMP-1 and -10 secretion, we next investigated whether this might be happening in TB patients. We investigated induced sputum samples from a cohort of TB (n=15) and control subjects (n=11) from Cape Town, South Africa (Fig. 5). This cohort of TB and control subjects has been previously reported (29). Integrin β3 mRNA accumulation in patients with TB was increased by 78.6% compared with subjects, when normalized to β-actin mRNA (p<0.05).
Figure 5. Integrin β3 mRNA is increased in cells from sputum samples of TB patients.
Induced sputum samples were collected prospectively from patients with active pulmonary TB (n=15) and control subjects (n=11) in Cape Town. Integrin β3 mRNA accumulation was analyzed by reverse transcription RT-PCR, and β-actin mRNA was used as the control gene. Horizontal lines represent median values. Statistical analysis was performed using a Mann-Whitney U test (*p<0.05).
Integrin αVβ3, collagen breakdown and monocyte adhesion and migration in TB
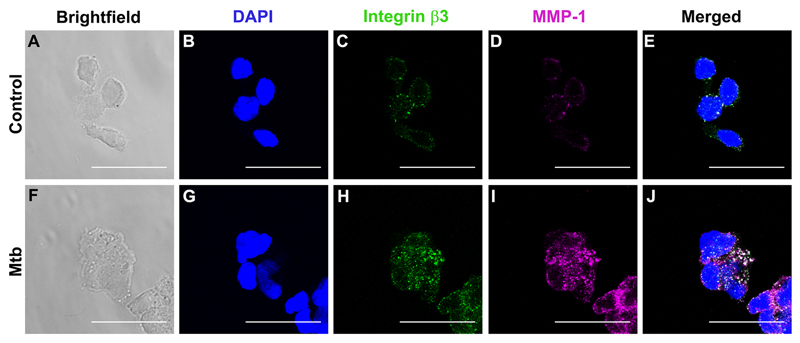
Next, confocal microscopy was used to analyze differences in integrin αVβ3 and MMP-1 localization in both uninfected control monocytes (Fig. 6 A-E) and Mtb-infected cells (Fig. 6 F-J). We observed increased integrin β3 staining with Mtb-infection compared with controls (Fig.6C, 6H). MMP-1 expression was also markedly increased with Mtb-infection (Fig. 6D, and I) and there was co-localization of integrin αVβ3 with MMP-1 in infected monocytes (white staining, Fig. 6J).
Figure 6. Integrin β3 co-localizes with MMP-1 in Mtb infection.
Monocytes seeded in collagen coated chamber slides were infected with Mtb (MOI=1). Cells were fixed, blocked and stained with DAPI for nucleic acids (blue), integrin αVβ3 (green) and MMP-1 (magenta). Panels A and F show bright-field and B-D show fluorescence of control monocytes and G-I fluorescence of Mtb-infected monocytes. Panels E and J are merged fluorescence images. White color corresponds to areas of integrin and MMP co-localization. Scale bar: 25μm.
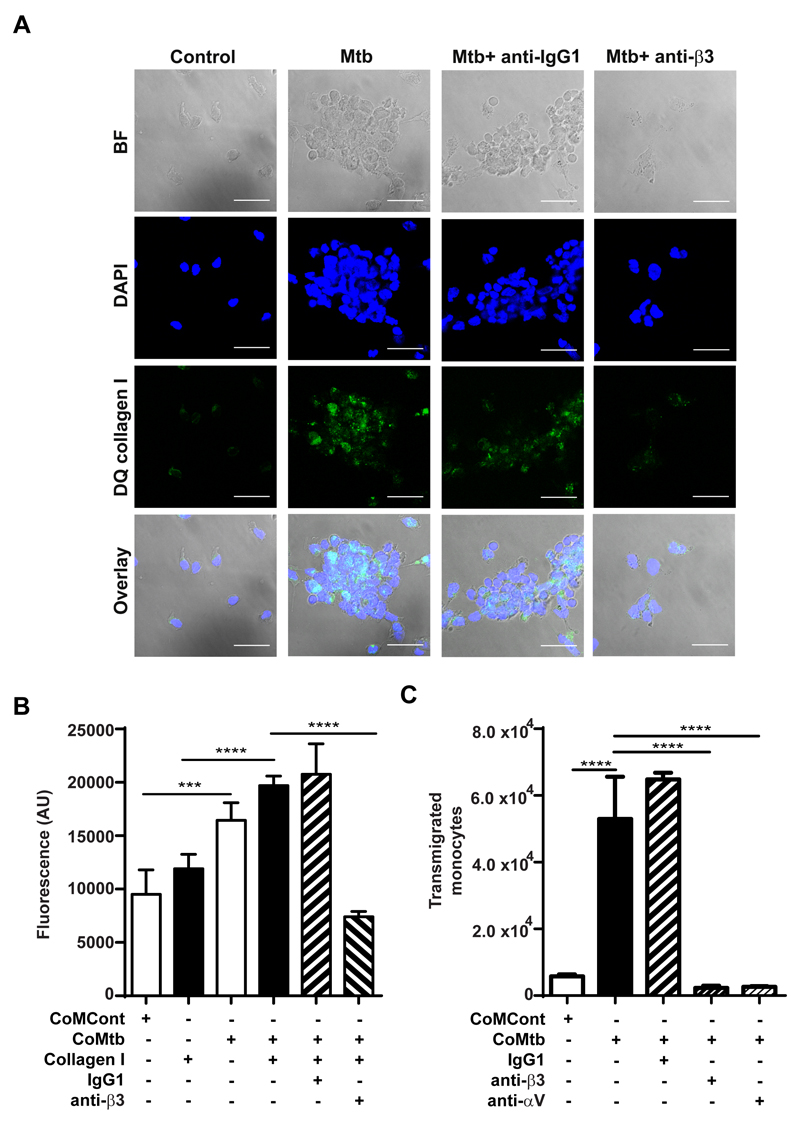
To investigate the functional consequences of integrin αVβ3 binding which resulted in increased MMP-1 secretion, DQ collagen coated slides were used to analyze type I collagen breakdown. Increased DQ collagen fluorescence, which corresponds to areas of collagen breakdown, was markedly increased with Mtb-infection, compared with uninfected controls, but when integrin β3 was inhibited by 20μg/ml anti-integrin β3 antibodies, collagen breakdown decreased, as well as the number of cells adherent to the collagen coated slide, which further implicates integrin αVβ3 on regulation of MMP-1 activity in TB (Fig. 7A). To analyze integrin αVβ3 mediated monocyte adhesion to type I collagen in TB, monocytes labelled with CellTracker green were seeded in either type I collagen coated or uncoated wells prior CoMtb or CoMCont stimulation. Monocyte adhesion was significantly increased with CoMtb stimulation (p<0.001, Fig 7B) and was higher in the presence of type I collagen. Inhibition of integrin β3 activity with blocking antibodies markedly decreased cell adhesion with CoMtb-stimulation. Next, trans-well assays were performed, to analyze integrin αVβ3 involvement in monocyte migration in TB. With CoMtb stimulation, the number of transmigrated monocytes was 2.2-fold higher than in CoMCont controls (p<0.0001; Fig. 7C). However, blockade of either β3 or αV integrin subunits led to a dramatic 96% and 92% decrease in monocyte transmigration respectively (both p<0.0001) demonstrating a key role in monocyte migration. No change in monocyte migration was seen with blockade of β1 integrins (Fig. S3).
Figure 7. Integrin αVβ3 upregulates type I collagen breakdown and increased monocyte adhesion and transmigration in Mtb infection.
Slides were pre-coated with DQ type I collagen in which FITC is quenched and fluorescence is released only in areas of collagen degradation. Monocytes were pre-incubated with or without a function blocking anti-integrin β3 antibody or anti-IgG1 isotype control, followed by Mtb-infection (MOI=1). (A) Panels show brightfield, DAPI nuclear counterstain (blue), collagen degradation (green) and merged images. Scale bar: 25μm. (B) CoMtb stimulation increased monocyte adhesion compared to CoMCont, which was blocked by inhibition of integrin β3. 1x105 monocytes/ well were pre-stained with Cell Tracker green CMFDA dye were stimulated with CoMtb or control media (CoMCont). Integrin β3 mediated adhesion was inhibited with anti-integrin β3 antibody. Control monocytes pre-incubated with IgG1 isotype antibodies. (C) Monocyte migration is increased with CoMtb stimulation, which is blocked with inhibition of integrin αV or β3. Trans-wells were pre-coated with type I collagen and CoMtb or CoMCont was added to the basal side in a 1:2 dilution. Integrins were blocked with anti-integrin αV and β3 antibodies, or an IgG1 isotype control antibody. Bars show mean ±s.d. Figures are representative of 3 independent experiments performed in triplicate. ***p<0.001; ****p<0.0001.
Discussion
In this study, we demonstrate for the first time in Mtb-infection that human monocyte adhesion to type I collagen and fibronectin, which are key in the lung matrix, significantly increase MMP-1, -7 and -10 gene expression and secretion and that this is regulated via activation of integrin αVβ3 on monocytes and in TB patients. In contrast, the gelatinases MMP-2/9 which are also secreted in response to TB were not affected by monocyte binding to ECM.
We show that type I collagen and fibronectin increase MMP-1 gene expression and secretion by monocytes after direct Mtb-infection as well as in response to CoMtb, which mimics in vivo Mtb-dependent signaling networks between infected and uninfected cells. However, type IV collagen, found in basement membranes, did not induce a similar effect, which indicates that this response is ligand-specific. Monocyte-derived MMP-10 was also upregulated by adhesion to type I collagen and fibronectin. Stromelysins although not able to degrade type I collagen, are functionally important in TB because they may activate collagenases such as MMP-1. MMP-3 is secreted at low concentrations by Mtb-infected monocytes, and is therefore less critical than MMP-10. In contrast, gelatinase secretion was not affected by binding to the ECM. Type I collagen had minimal effects on TIMP-1 secretion which is increased by Mtb-stimulation or on TIMP-2 secretion which was decreased by TB.
In dissecting mechanisms by which the lung matrix increased MMP-1/10 secretion, we observed that Mtb-infection was associated with increased surface expression of the integrin β3 subunit. There were no significant differences in expression of the integrin subunits β1 and β2 in infected monocytes, although they do have other immunological functions in TB (30, 31). CoMtb-stimulation of monocytes increased αVβ3 surface expression, which rose further in the presence of type I collagen. Activation of αVβ3 integrin resulted in increased expression of MMP-1 and -10. This is consistent with a murine study showing that overexpression of αvβ3 in breast cancer cells increased formation of osteolytic lesions, indicating a role for this integrin in driving tissue damage (32). Although not directly involved in adhesion to native type I collagen in healthy tissues, in inflammation RGD binding motifs within the collagen fibrils become exposed, allowing adhesion of αVβ3 (33, 34), which provides a positive feedback loop for MMP-1/-10 expression. This is consistent with our observation that inhibition of integrin αVβ3 in Mtb-stimulated monocytes lead to decreased type I collagen breakdown and decreased monocyte adhesion. These findings are potentially clinically important in TB patients since we have demonstrated for the first time that the β3 subunit was upregulated in induced sputum of patients with active TB compared to control subjects.
Importantly, integrin αVβ3 was specifically required for monocyte migration and blockade of either αV or β3 subunits dramatically impaired migration in a trans-well system. In the zebrafish model of TB, macrophage migration was central to the dissemination of infection to new sites in an MMP-9-dependent manner (35), and excessive leukocyte migration may lead to adverse effects in TB including tissue damage (28). Confocal microscopy revealed integrin αVβ3 microclustering and areas of co-localization between integrin αVβ3 and MMP-1, which tended to localize at monocyte protrusions, consistent with trailing uropods of migrating monocyte or areas of monocyte-monocyte adhesion.
Taken together, the present work shows that, in Mtb-infected monocytes, increased integrin αVβ3 expression which is also found in patients, promotes increased monocyte adhesion to ECM and upregulates MMP-1 and -10 secretion, which in turn will drive ECM breakdown. The ECM thereby regulates enzymes required for the influx of inflammatory monocytes, which are also involved in driving tissue damage leading to morbidity and mortality in TB. Leukocyte-ECM interactions are potential targets to develop host-directed therapy aimed at reducing the inflammation and matrix degradation that characterize infection with Mtb.
Supplementary Material
Acknowledgments
We thank the Imperial College NIHR BRC imaging facility for the technical support. The authors are grateful to Dr. Tarangini Sathyamoorthy for providing clinical samples from the Cape Town study. The authors thank Dr. Paras Anand for providing the THP-1 cell line for initial ligand titration assays.
SB was supported by a grant from the Portuguese Foundation for Science and Technology (FCT). AMW was supported by an MRC clinical fellowship. JSF and JCP acknowledge support of the Imperial College NIHR Biomedical Research Centre (BRC) and the University College London Hospitals BRC respectively. The authors are grateful for support from The Rosetrees Trust and Breathing Matters.
Abbreviations.
- CoMCont
conditioned medium from uninfected monocytes
- CoMtb
conditioned medium from Mycobacterium tuberculosis infected monocytes
- ECM
extracellular matrix
- MFI
mean fluorescence intensity
- MMP
matrix metalloproteinase
- MOI
multiplicity of infection
- Mtb
Mycobacterium tuberculosis
- TB
tuberculosis
- TIMP
tissue inhibitor of metalloproteinases
References
- 1.WHO. Global Tuberculosis Report 2016. 2016. [Google Scholar]
- 2.Fassett J, Tobolt D, Hansen LK. Type I collagen structure regulates cell morphology and EGF signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Mol Biol Cell. 2006;17:345–356. doi: 10.1091/mbc.E05-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 4.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 5.Ammon C, Meyer SP, Schwarzfischer L, Krause SW, Andreesen R, Kreutz M. Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells. Immunology. 2000;100:364–369. doi: 10.1046/j.1365-2567.2000.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi C, Zhang X, Chen Z, Sulaiman K, Feinberg MW, Ballantyne CM, Jain MK, Simon DI. Integrin engagement regulates monocyte differentiation through the forkhead transcription factor Foxp1. J Clin Invest. 2004;114:408–418. doi: 10.1172/JCI21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 8.Chu C, Celik E, Rico F, Moy VT. Elongated membrane tethers, individually anchored by high affinity alpha4beta1/VCAM-1 complexes, are the quantal units of monocyte arrests. PLoS One. 2013;8:e64187. doi: 10.1371/journal.pone.0064187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee NK, Hamerman JA. beta(2) integrins inhibit TLR responses by regulating NF-kappaB pathway and p38 MAPK activation. Eur J Immunol. 2013;43:779–792. doi: 10.1002/eji.201242550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weerasinghe D, McHugh KP, Ross FP, Brown EJ, Gisler RH, Imhof BA. A role for the alphavbeta3 integrin in the transmigration of monocytes. J Cell Biol. 1998;142:595–607. doi: 10.1083/jcb.142.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DesJardin LE, Kaufman TM, Potts B, Kutzbach B, Yi H, Schlesinger LS. Mycobacterium tuberculosis-infected human macrophages exhibit enhanced cellular adhesion with increased expression of LFA-1 and ICAM-1 and reduced expression and/or function of complement receptors, FcgammaRII and the mannose receptor. Microbiology. 2002;148:3161–3171. doi: 10.1099/00221287-148-10-3161. [DOI] [PubMed] [Google Scholar]
- 12.Elkington PT, Nuttall RK, Boyle JJ, O'Kane CM, Horncastle DE, Edwards DR, Friedland JS. Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med. 2005;172:1596–1604. doi: 10.1164/rccm.200505-753OC. [DOI] [PubMed] [Google Scholar]
- 13.Elkington PT, Green JA, Emerson JE, Lopez-Pascua LD, Boyle JJ, O'Kane CM, Friedland JS. Synergistic up-regulation of epithelial cell matrix metalloproteinase-9 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2007;37:431–437. doi: 10.1165/rcmb.2007-0011OC. [DOI] [PubMed] [Google Scholar]
- 14.Green JA, Elkington PT, Pennington CJ, Roncaroli F, Dholakia S, Moores RC, Bullen A, Porter JC, Agranoff D, Edwards DR, Friedland JS. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase-1 and -3 expression and secretion via NF-kappaB- and Activator Protein-1-dependent monocyte networks. J Immunol. 2010;184:6492–6503. doi: 10.4049/jimmunol.0903811. [DOI] [PubMed] [Google Scholar]
- 15.Ugarte-Gil CA, Elkington P, Gilman RH, Coronel J, Tezera LB, Bernabe-Ortiz A, Gotuzzo E, Friedland JS, Moore DA. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS One. 2013;8:e61333. doi: 10.1371/journal.pone.0061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright KM, Friedland JS. Regulation of monocyte chemokine and MMP-9 secretion by proinflammatory cytokines in tuberculous osteomyelitis. J Leukoc Biol. 2004;75:1086–1092. doi: 10.1189/jlb.0903433. [DOI] [PubMed] [Google Scholar]
- 17.Elkington PT, Ugarte-Gil CA, Friedland JS. Matrix metalloproteinases in tuberculosis. Eur Respir J. 2011;38:456–464. doi: 10.1183/09031936.00015411. [DOI] [PubMed] [Google Scholar]
- 18.Harris JE, Green JA, Elkington PT, Friedland JS. Monocytes infected with Mycobacterium tuberculosis regulate MAP kinase-dependent astrocyte MMP-9 secretion. J Leukoc Biol. 2007;81:548–556. doi: 10.1189/jlb.0806512. [DOI] [PubMed] [Google Scholar]
- 19.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, Edwards DR, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest. 2011;121:1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seddon J, Kasprowicz V, Walker NF, Yuen HM, Sunpath H, Tezera L, Meintjes G, Wilkinson RJ, Bishai WR, Friedland JS, Elkington PT. Procollagen III N-terminal Propeptide and Desmosine are Released by Matrix Destruction in Pulmonary Tuberculosis. J Infect Dis. 2013;08:1571–9. doi: 10.1093/infdis/jit343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Kane CM, Elkington PT, Friedland JS. Monocyte-dependent oncostatin M and TNF-alpha synergize to stimulate unopposed matrix metalloproteinase-1/3 secretion from human lung fibroblasts in tuberculosis. Eur J Immunol. 2008;38:1321–1330. doi: 10.1002/eji.200737855. [DOI] [PubMed] [Google Scholar]
- 22.Elkington PT, Emerson JE, Lopez-Pascua LD, O'Kane CM, Horncastle DE, Boyle JJ, Friedland JS. Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch. J Immunol. 2005;175:5333–5340. doi: 10.4049/jimmunol.175.8.5333. [DOI] [PubMed] [Google Scholar]
- 23.Barksby HE, Milner JM, Patterson AM, Peake NJ, Hui W, Robson T, Lakey R, Middleton J, Cawston TE, Richards CD, Rowan AD. Matrix metalloproteinase 10 promotion of collagenolysis via procollagenase activation: implications for cartilage degradation in arthritis. Arthritis Rheum. 2006;54:3244–3253. doi: 10.1002/art.22167. [DOI] [PubMed] [Google Scholar]
- 24.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190:9–18. doi: 10.1164/rccm.201311-2106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. pii: a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sathyamoorthy T, Tezera LB, Walker NF, Brilha S, Saraiva L, Mauri FA, Wilkinson RJ, Friedland JS, Elkington PT. Membrane Type 1 Matrix Metalloproteinase Regulates Monocyte Migration and Collagen Destruction in Tuberculosis. J Immunol. 2015;195:882–91. doi: 10.4049/jimmunol.1403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brilha S, Sathyamoorthy T, Stuttaford LH, Walker NF, Wilkinson RJ, Singh S, Moores RC, Elkington PT, Friedland JS. ESAT-6 Drives MMP-10 Gene Expression and Secretion in Tuberculosis. Am J Respir Cell Mol Biol. 2016;56:223–232. doi: 10.1165/rcmb.2016-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts LL, Robinson CM. Mycobacterium tuberculosis infection of human dendritic cells decreases integrin expression, adhesion and migration to chemokines. Immunology. 2014;141:39–51. doi: 10.1111/imm.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walrath JR, Silver RF. The alpha4beta1 integrin in localization of Mycobacterium tuberculosis-specific T helper type 1 cells to the human lung. Am J Respir Cell Mol Biol. 2011;45:24–30. doi: 10.1165/rcmb.2010-0241OC. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, Clement-Lacroix P, Clezardin P. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery AM, Reisfeld RA, Cheresh DA. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci U S A. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 35.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466–469. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.