Abstract
T cell receptor (TCR) stimulation by pMHC complexes on antigen presenting cells (APC), requires precise re-organisation of molecules into the area of cellular contact to form an immunological synapse from where T cell signaling is initiated. Caveolin-1 (Cav1), a widely expressed transmembrane protein, is involved in the regulation of membrane composition, cellular polarity and trafficking, and the organisation of signal transduction pathways. The presence of Cav1 protein in T cells was identified only recently and its function in this context is not well understood. We show that Cav1-knockout (KO) CD8 T cells have a reduction in membrane cholesterol and sphingomyelin, and upon TCR triggering exhibit altered morphology and polarity, with reduced effector function compared to Cav-1 wild-type CD8 T cells. In particular, redistribution of the β2 integrin, LFA-1, to the immunological synapse is compromised in Cav1KO T cells, as is the ability of LFA-1 to form high avidity interactions with ICAM-1. Our results identify a role for Cav1 in membrane organisation and β2 integrin function in primary CD8 T cells.
Introduction
T cells require integrin-mediated cell adhesion in order to interact stably with antigen presenting cells (APC) and initiate optimal T cell receptor (TCR) signaling and activation (1, 2). Integrins are heterodimeric transmembrane proteins, composed of alpha and beta subunits, which are capable of bidirectional signalling across the plasma membrane. In naïve T cells, integrin binding is of low affinity as the molecules are mainly in a low-affinity conformation. Activation through surface receptors, such as TCR by peptide-Major Histocompatibility Complex (pMHC) molecules or chemokine receptor by chemokine, initiates specific intracellular signalling termed ‘inside-out signalling’, which drives conformational changes within the integrin subunits promoting high affinity binding to ligand (3–5). Lateral association of integrins into clusters further promotes ligand binding avidity (6, 7). In turn, ‘outside-in signaling’, whereby high affinity integrin-ligand interactions result in signal transmission into the cell to drive reorganisation of the actin cytoskeleton and mediate cell spreading, increases cell-cell avidity or cell-extracellular matrix (ECM) adhesion.
LFA-1 (αLβ2, CD11a/CD18) and Very late antigen-4 (VLA-4, α4β1, CD49d/CD29) are the major integrins expressed on T cells. LFA-1 is an important structural component of the immunological synapse (IS) formed between T cell and antigen presenting cell (APC), strengthening T cell-APC interactions and facilitating cell polarisation. IS formation reduces the threshold for T cell activation during cell-mediated immune responses (8–12). Integrins play important roles not only in mediating IS formation but also in cell adhesion to the ECM, contractility, motility and growth (13–18). Under conditions of shear flow, high affinity LFA-1 binds Intracellular adhesion molecule (ICAM)-1 and -2 expressed on the endothelial cells surrounding the blood vessels, facilitating firm adhesion for T cell transmigration into lymph nodes. Therefore, active LFA-1 is critical for T cell migration into secondary lymphoid tissues and other sites of inflammation (19, 20).
Caveolin (Cav) proteins have been linked with integrin signalling in multiple cell lineages (21). There are three Cav isoforms, Cav1 and Cav2 which are co-expressed in most cell lineages including adipocytes, endothelial cells, epithelial cells and fibroblasts, whereas Cav3 is muscle cell specific (22, 23). Cav1 has a structural role within the plasma membrane through its direct interaction with cholesterol and lipids, maintaining lipid and cholesterol homeostasis and is the major structural component of caveolae (24). Caveolae are specialised lipid raft microdomains regarded as dynamic signalling centres in which Cav1 facilitates a variety of cellular processes through direct protein-protein interactions with heterotrimeric G proteins, Src-family tyrosine kinases (SFK), H-Ras, endothelial nitric oxide synthase and the insulin receptor (25–27). In addition to its role in caveolae, Cav1 also functions in other subcellular locations including the focal adhesion complex (28, 29). Initial studies failed to detect Cav1 and caveolae in lymphocytes, however Cav1 has now been identified in B cells and T cells (30–32). Moreover Cav1 was shown to influence naïve CD8 T cell activation and cell polarity (32).
To date, there are no reports on the association of Cav1 with integrin function in T cells and we set out to investigate whether Cav1 was involved LFA-1 function. We demonstrate that following TCR engagement, Cav1-deficient CD8 T cells had altered morphology, polarisation and reduced adhesiveness to ICAM-1 under conditions of shear flow. In addition there was impaired homotypic adhesion and impaired LFA-1 recruitment to the IS upon TCR:pMHC association in Cav1-deficient CD8 T cells, together with a reduction in their response to Ag. Loss of Cav1 reduced the cholesterol and sphingomyelin content of CD8 T cells suggesting that Cav1 plays a role in membrane lipid homeostasis, which influenced the redistribution of LFA-1 and its avidity for ICAM-1. Together, these results identify a role for Cav1 in regulating TCR signals required for LFA-1-mediated cellular adhesion and IS formation in naïve CD8 T cells.
Materials and Methods
Mice
Cav1-deficient (Cav1-KO) mice have been described previously (33) and were kindly provided by B. Nichols, Cambridge University. Cav1-KO mice were backcrossed to Rag1-/- OT-1 TCR transgenic mice. CD3ε-deficient (CD3εKO) mice were bred in-house at the University of Edinburgh. All procedures were approved under a project license granted by the UK Home Office and were carried out in accordance with the institutional and ethical guidelines of the University of Edinburgh.
Cell preparation and in vitro analysis of T cell activation
T cell suspensions were prepared from LN of OT-1 or polyclonal C57/Bl6 mice. Where stated, PE-conjugated Abs were used for purification of naive CD8 T cells by negative selection. LN cells were incubated with anti-CD4-PE (eBiosciences) and anti-I-A/I-E-PE (Biolegend) followed by a 15 min incubation with anti-PE MACS beads (Miltenyi Biotech). Cells were added to MACS LS columns (Miltenyi Biotech) and non-binding CD8 T cells were collected. WT and Cav1-KO LN CD8 T cells were cultured in IMDM media (Invitrogen) supplemented with 5% FCS, L-glutamine, antibiotics and 50 μM 2-mercaptoethanol. OT-1 CD8 T cells were activated by the addition of peptides: SIINFEKL (N4), SIITFEKL (T4) or SIIGFEKL (G4) in the presence of CD3ε-KO spleen cells as APC, to culture media as indicated. At the end of culture CD8 T cells were stained with the relevant Abs, run through a MACSQuant flow cytometer (Miltenyi Biotech) and analyzed with FlowJo software (Tree Star Inc). For proliferation assays, OT-1 T cells were labeled with CellTrace Violet (CTV, Molecular Probes) for 10 min before washing and culture. Proliferation was determined by assessing CellTrace Violet dilution using FlowJo software (Tree Star Inc). For LFA-1 blocking assays, M17/4 mAb (eBiosciences) was incubated in 40 μL suspensions of 100,000 cells in 96-well round-bottom plates and incubated at 37°C/5%CO2.
Enzyme linked immunosorbent assay
Cytokines were measured by ELISA with paired capture and detection Abs, anti–TNF-α (tumor necrosis factor-α) Ab and anti-IL-2 Ab from eBiosciences. Plates (NUNC MaxiSorp) were washed with 0.05% Tween 20 in PBS and blocked with 1% bovine serum albumin (BSA) in PBS. After the addition of 100 μL of 3,3′,5,5′ tetramethylbenzidine substrate solution (Sigma-Aldrich) and 100 μL 0.1 M H2SO4, absorbance was read at 450 nm with a Laboratory Systems Multiskan Ascent plate reader.
Flow cytometry and Imagestream analysis
For flow cytometry the following conjugated antibodies were used: anti-CD69-Pe, (eBiosciences), anti-CD11a-Pe (Biolegend), anti-TCRβ-Pe/Cy7 (Biolegend), anti-CD18-PerCP (BDBiosciences), anti-CD29-BV421 (BDBiosciences). Live/Dead Aqua and CTV dyes were from Life Technologies. Samples were acquired with a MACSQuant flow cytometer (Miltenyi) and analyzed with FlowJo software (Treestar).
For Imagestream analysis, in order to accurately determine the frequency and number of 1:1 T-cell:APC hetero-conjugates and subsequently measure the relative level of actin recruitment to the IS, splenocytes from CD3εKO mice were labeled with 1 μM MitoTDR then loaded with 10−6 M N4 peptide for 30 min at 37°C. T cells labelled with 2.5 nM CTV and MitoTDR-labelled peptide-loaded APC were mixed 1:1 in IMDM and at indicated timepoints were fixed in 2% PFA. The cells were rinsed once, permeabilised in 0.1% Triton-X100 for 6 min at RT, washed 3 times and incubated in BODIPY-Fl (Molecular Probes) and CD11a-Pe (Biolegend) for 60 min at RT. Samples were then acquired on a fully ASSIST-calibrated 4-laser ImageStream X MKII Imaging Flow Cytometery (IFC) system (MerckMillipore/Amins). The “high sensitivity” acquisition mode was selected and images collected at 60x magnification. The 488 nm (blue laser) was set to 100 mW, the 561 nm (yellow/green) laser at 100 mW, the 405 nm (violet laser) at 60 mW and the 635 nm (red) laser was set at 120 mW in order to maximise signal resolution but minimise CCD camera saturation. Bright-field illumination was collected in channels 1 and 9. For the purpose of spectral compensation, single stained samples were collected with bright-field illumination and the side scatter (758 nm) laser turned off. Compensation was determined post-acquisition using the standard wizard embedded in the IDEAS analysis software package (MerckMillipore/Amnis) and the associated single stained raw image files (.RIF) (Supplementary Fig. 2F). In all cases the single fluorescent populations used for determining the coefficient of compensation were manually inspected for autofluorescent cells and regated to exclude them as appropriate. For experimental samples a minimum of 10,000 cells were collected excluding cellular debris and focal calibration beads (speed beads) using a gated threshold set on the channel 1 bright-field image. Fully stained experimental samples were then converted to Compensated Image Files (.CIF) using the compensation matrix shown in Supplementary figure 2F. The data analysis pipeline designed to identify true 1:1 T-cell/APC hetero-conjugates is outlined in Supplementary Fig. 2A-C. Briefly potential heteroconjugates were identified and gated based on being dual positive for CTV (CH7) and MitoTDR (CH11). This population was further refined using the area and aspect ratio of the bright-field channel mask (M01). T cell:APC 1:1 hetero-conjugates were defined and gated as having a low aspect ratio and increased area compared to single cells. Next the total integrated intensities values for BODIPY-Fl (F-Actin) and CD11a-PE were used to gate on cells that were positive for both signals prior to measuring the spatial localisation within the synapse. The synapse between the T cell and APC was masked using the “interface” masking adaptation within the IDEAS analysis software. The 7-AAD image (CH05) was used as the cell conjugate input mask (M05) and the CTV image (CH07) as the input mask for the cell of interest. The new mask is shown in Supplementary Fig. 2D image panel “M” and is overlaid on various channel images to show its suitability to mask the synapse between two cells. The mask was generated and validated using an interactive manual process as described elsewhere (34). In order to measure the recruitment of BODIPY-Fl:CD11a to the IS (as defined by the interface mask) the “Internalisation” feature was used (35). A negative internalisation value indicates that the majority of the signal resides outside of the synaptic area whereas increasing positive values correlate with an increasing proportion residing within the synaptic area. Data is expressed as the median internalisation score of the final gated population of BODIPY-Fl:CD11a double positive, 1:1 T-cell:APC heteroconjugates. In all cases the final cell number measured exceeded 100 cells.
Immunoblots
Cells were lysed in 1% Triton X-100, 0.5% n-dodecyl-b-d-maltoside, 50 mM tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1 mM NaF, and 1 mM sodium orthovanadate containing protease inhibitors. Samples were separated by NuPAGE 4-12% Bis-Tris gel electrophoresis (Life technologies). For immunoprecipitation, lysates were immunoprecipitated with anti-Cav1 rabbit Ab (BDBiosciences) coupled to Dynabeads prot A (Life technologies). Immunoprecipitates were washed with lysis buffer, and proteins were transferred onto polyvinylidene difluoride Immobilon-FL membranes (Millipore). Membranes were incubated in blocking reagent (LI-COR Biosciences) before Western blotting analysis with anti-Cav1 rabbit Ab (BD Biosciences) or anti-Cav2 mouse mAb (clone 65, BDBiosciences). Proteins were detected with secondary Abs goat anti-mouse A680 (Life technologies) and goat anti-rabbit IR800 (Thermo scientific) and visualized with an infrared imaging system (Odyssey, LI-COR Biosciences). Fractionation using the Subcellular Fractionation kit was performed according to manufacturer’s instructions (Thermo Scientific).
Confocal microscopy
For adhesion of CD8 T cells to ICAM-1 for morphological studies, 10-well multi-spot glass slides (Hendley-Essex, UK) were coated overnight in 3 μg/mL ICAM-1 (R&D systems). Wells were washed 3 times in PBS and 50 μL naïve CD8 T cells in serum-free media (1x106/mL) treated 10 min prior with 10 μg/mL anti-CD3 (clone 2C11, R&D systems) or 5 μg/mL SDF-1α (R&D systems) were added. Cells were left to attach for 30 min at 37°C. Wells were washed once with PBS before fixing the cells in 4% PFA at RT for 60 min. The cells were rinsed once and then permeabilised in 0.1% Triton-X100 for 6 min at RT. Cells were washed 3 times in PBS/2% BSA and incubated in BODIPY-Fl (Molecular Probes), anti-Lck Ab (Cell Signaling) and anti-Ezrin Ab (EC12, Abcam) for 60 min at RT. Cells were washed in PBS/2% BSA and further incubated with species-specific anti-(Fab’)2-AF488 or 647 conjugates (Molecular Probes) for 15 min at RT. The secondary Ab was removed by washing 3 times in PBS/2% BSA then slides were mounted with Prolong Gold anti-fade (Molecular Probes) containing 1 μg/mL DAPI to stain nuclei. Fluorescent specimens were analysed with a Leica TCS SP5 II confocal imaging system (Leica Microsystems) with lasers exciting at 405, 488 and 647 nm with the 63x objective using LAS AP software (Leica). All confocal analysis were multiple repeats, and at least 100 images were analysed for each condition. Data were rendered and analysed using Volocity 6.3 (Perkin Elmer) and ImageJ (National Institutes of Health). Pearson’s correlation coefficient (R) was calculated using Volocity software to determine the pairwise colocalisation of the signals with a Student’s t-test used to determine statistical significance between two protein data sets. Histograms of MFI signal and protein distribution along the axis of the cell were rendered using the ImageJ plug-in RGB profiler.
RNA isolation, reverse transcription PCR and quantitative real-time PCR
Tissue was isolated from the indicated organs of one mouse, added to Trizol (Invitrogen) and homogenised in a TissueLyser II (QIAGEN). RNA was extracted using 1/5 chloroform. Isoproanol was added approx. 1:1 to the aqueous phase with 30 μg GlycoBlue (Invitrogen). Following a 10 min incubation at RT, the centrifuged pellet was washed in 75% EtOH and allowed to dry. Pellets were resuspended in 20 μL DNase/RNase free water and quantitated on the RNA-40 program of the Nanodrop (Nanodrop Instruments). Reverse transcription of RNA was performed with miScript II (QIAGEN). Quantitative PCR was performed with SYBR Green (QIAGEN) using the following primer pairs: Cav1F: 5’-AACGACGACGTGGTCAAGA-3’, Cav1R: 5’-CACAGTGAAGGTGGTGAAGC-3’, Cav2F: 5’-ATGACGCCTACAGCCACCACAG-3’, Cav2R: 5’-GCAAACAGGATACCCGCAATG-3’, PTRFF: 5’-GAAAGAAAGGCGAGTGA-3’, PTRFR: 5’-TAATGTGTAAGTGCC CCTG-3’ and normalised to the abundance of 18S mRNA.
Aggregation assay
CD8 T cells were resuspended in IMDM supplemented with 10% FCS and 40 mM HEPES at a cell density of 15x106 cells/mL and aliquoted into 96 well plates at 200 μL/well. Cells were activated in 50 ng/mL PdBu (Sigma) or 1 μg/mL anti-CD3 mAb (clone 2C11; R&D Systems) for 60 min to allow for conjugate formation. 20 μL samples were taken in triplicate and the number of free cells counted. Aggregation percentage was calculated as previously described in Fagerholm et. Al.(36); the number of free cells after stimulation subtracted from the number of free cells in unstimulated samples, divided by the number of free cells in the unstimulated samples.
Cell attachment assays
Static adhesion assay. ICAM-1 (6 μg/ml; R&D Systems) was coated onto 96-well Maxisorp plates (Nunc) by overnight incubation at 4°C and the wells were blocked with 1% milk in PBS. Lymphocytes from Cav1-WT or Cav1-KO mice were resuspended in adhesion medium (RPMI1640 supplemented with 0.1% BSA, 40 mM HEPES and 2 mM MgCl2) and added to the plate. Where appropriate, cells were stimulated with 200 nM PdBu (Sigma), 10 μg/mL anti-CD3 (R&D Systems) or 1 μg/mL SDF-1α (R&D Systems) immediately before being added to the plate. Cells were allowed to adhere for 30 min at 37°C. Unbound cells were removed by gentle washing in PBS + 2mM MgCl2 and bound cells were lysed and detected with phosphatase substrate (Sigma).
Shear flow adhesion assay. VI0.4 µslides (Ibidi) were coated with 6 μg/mL murine ICAM-1 (R&D Systems) overnight at 4°C. Where indicated, SDF-1α (R&D Systems) was co-immobilized by addition of 5 μg/ml onto plates for a further 30 min at 37°C. T cells were prepared at a density of 1x106 cells/mL in binding medium (RPMI plus 0.1% BSA, 40mM Hepes and 2mM MgCl2) and, where indicated, were stimulated with 10 µg/mL anti-CD3 (clone 2C11; R&D Systems) for 5 min. Cells were injected into a flow system that used a silicone tubing loop connected to a Multi-phaser NE-1000 syringe pump (New Era Pump Systems), allowing the cells to flow at a continuous shear flow rate of 0.3 dyne/cm2. Cells were monitored by microscopy over a 10 min period, and the number of adhered cells in the field of view was determined at 1 min intervals by manual counting.
LFA-1 affinity modulation assay
CD8 T cells (4x106/mL) were washed and resuspended in HEPES buffer (20 mM HEPES, 140 mM NaCl, 2g/L of glucose) with 0.1% BSA. Where required, 96-well flat-bottom flexiwell plates were coated with 10 μg/mL anti-CD3 (clone 2C11; R&D Systems) at 4°C O/N. T cell aliquots of 50 μL (2x105) were added to flexiwell plates (Dynatech, West Sussex, UK) with or without 5 mM Mg2+ and 1 mM EGTA. Soluble murine ICAM-1-Fc (10 μg/mL) was added to the cells in addition to 50 ng/mL PdBu, 100 μM N4 or 2 μg/mL anti-CD28 (R&D Systems). After 30 min incubation at 37°C, T cells were washed twice in ice-cold assay buffer with 0.1% BSA and incubated with 0.1 μg/mL of Fc-specific FITC-conjugated goat anti-human IgG (Jackson Immunoresearch, West Grove, PA) for 20 min on ice. Cells were washed twice in ice-cold assay buffer with 0.1% BSA to remove excess unbound mAb and fluorescence was detected with a MACSQUANT (Miltenyi Biotech) flow cytometer.
LC-MS analysis
Sphingolipids (SLs) were first extracted from 60 μg of purified CD8 T cells (total protein amount). After injection, samples were recovered and SLs were extracted after alkaline saponification (KOH 1M in MeOH). Prior to the lipids extraction, 0.2 nmol of synthetic standards (Avanti Polar lipids) were used to spike each sample in order to normalize the results and to obtain a relative quantification. As internal non-natural SLs standards, N-dodecanoylsphingosine and N-dodecanoylglucosylsphingosine, were used for ceramide and glucosyl-ceramide respectively and N-dodecanoylsphingosyl phosphorylcholine was the standard chosen for sphingomyelin.
Cholesterol assay
Cholesterol levels were measured in purified CD8 T cells (5 μg protein content) using the Amplex Red cholesterol assay kit (Life technologies) according to the manufacturer instructions.
Statistical analysis
Student’s t-test (paired or unpaired, 2 tails) and ANOVA were performed using Prism 5.0 (GraphPad Software).
Results
Cav1 regulates the lipid composition and antigen response of CD8 T cells
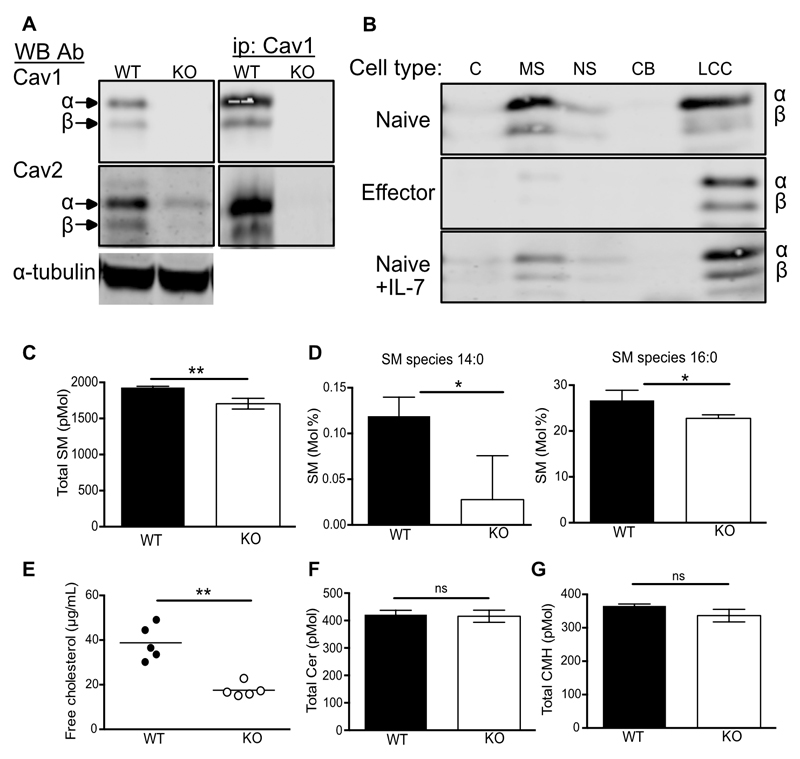
Cav1 was recently shown to be expressed in T cells, having previously been described as absent in T cell lines (37–40) and was shown to influence actin polymerisation, synaptic membrane raft polarity and CD8 T cell function (32). It was unclear whether T cells also expressed Cav2, which is frequently hetero-oligomerised with Cav1. Cav1 is a homodimeric transmembrane protein expressed in two isoforms, Cav1α and Cav1β, derived from alternate translation-initiation sites commencing transcription from a methionine at positions 1 and 32 respectively (41). Comparing lysates of purified CD8 T cells from naïve C57Bl/6 WT and Cav1-KO CD8 T cells, we confirmed that both isoforms of Cav1 were present in WT cells with a preponderance of the α isoform (Fig. 1A). Both isoforms, α and β, of Cav2 were also expressed in naïve WT CD8 T cells. Cav2 could be immunoprecipated with Cav1, as described in other cell types (22). The abundance of Cav2 was greatly reduced in Cav1-KO CD8 T cells (Fig. 1A), consistent with reports showing that when not oligomerised with Cav1, Cav2 was rapidly degraded by the proteosome (42). Cav1, Cav2 and the other critical component of caveolae, cavin/polymerase chain 1 and transcript release factor (PTRF) were expressed in considerably lower abundance in the thymus and secondary lymphoid organs compared to lung and adipose tissue (fig. S1, A to C). As previously suggested (37–40), we could not identify any caveolae-like structures in naïve CD8 T cells while they could be seen by electron microscopy in A431 cells (fig. S1D), confirming that Cav1 in T cells was not associated with caveolae.
Figure 1. Cav1 is localised within soluble and insoluble membrane fractions and regulates cholesterol and lipid composition.
(A) Cav1 and Cav2 protein expression was compared between WT and Cav1-KO CD8 T cell lysates by western blot with anti-Cav1 and Cav2 Ab. Cell lysates were immunoprecipitated with anti-Cav1 and western blots were probed sequentially with anti-Cav1 and Cav2 Ab. (B) Cell lysates were separated into subcellular fractions: C, cytoplasmic; M, membrane soluble; NS, nuclear soluble; CB, chromatin bound; LCC, lipid-cytoskeletal complexes and analysed by Western blot with anti-Cav1 Ab. (C) Total SM content in WT versus Cav1KO CD8 T cells. (D) SM species 14:0 and 16:0 are represented as a percentage of total SM. (E) Free cholesterol from WT and Cav1-KO CD8 T cells is representative of 1 of 3 independent experiments from a total of 12 biological samples of each genotype. (F) Total ceramide and (G) ceramide monohexamide content. Lipidomics data is pooled from 3 biological repeats. Data are shown as mean + SD. NS, not significant; *p ≤0.05, **p ≤0.01, *** p ≤0.001 (Student’s t-test).
Cav1 is found within all membranes of the cell and is also involved in the shuttling of cholesterol from the ER to the plasma membrane, directing Cav1 to cholesterol and sphingolipid enriched membrane domains (43, 44). We asked where Cav1 localised within naïve CD8 T cells and found that approximately 50% of Cav1 protein localised within the membrane soluble (MS) fraction of naïve CD8 T cells with the remaining Cav1 protein found within the insoluble cell pellet, containing lipid-cytoskeletal complexes (LLC) (Fig. 1B). Upon in vitro stimulation of T cells with anti-CD3 Ab for 48 hours, Cav1 wholly redistributed within LLC fractions (Fig. 1B). Redistribution of Cav1 appeared to be driven by in vitro TCR activation, as it was less evident in cells cultured in the cytokine IL-7, which maintains T cell viability without stimulating proliferation (Fig. 1B).
Given that Cav1 directly binds cholesterol and specific sphingolipids, we asked whether the lipid composition was altered in naïve CD8 T cells in the absence of Cav1 by performing lipidomic analysis. Sphingomyelin (SM), one of the most abundant sphingolipids in cells and a major component of lipid rafts was significantly reduced in naive CD8 T cells in the absence of Cav1 (Fig. 1C). Furthermore, analysis of individual SM species revealed a marked reduction in short fatty acids in Cav1-KO CD8 T cells (Fig. 1D). In addition there was a significant decrease in the abundance of free cholesterol in Cav1-KO naïve CD8 T cells compared to WT cells (Fig. 1E). In contrast, Cer and GluCer, other classes of sphingolipids, were not reduced in the absence of Cav1 (Fig. 1, F and G), which suggested a role for Cav1 in the transport of specific lipids to the PM in CD8 T cells.
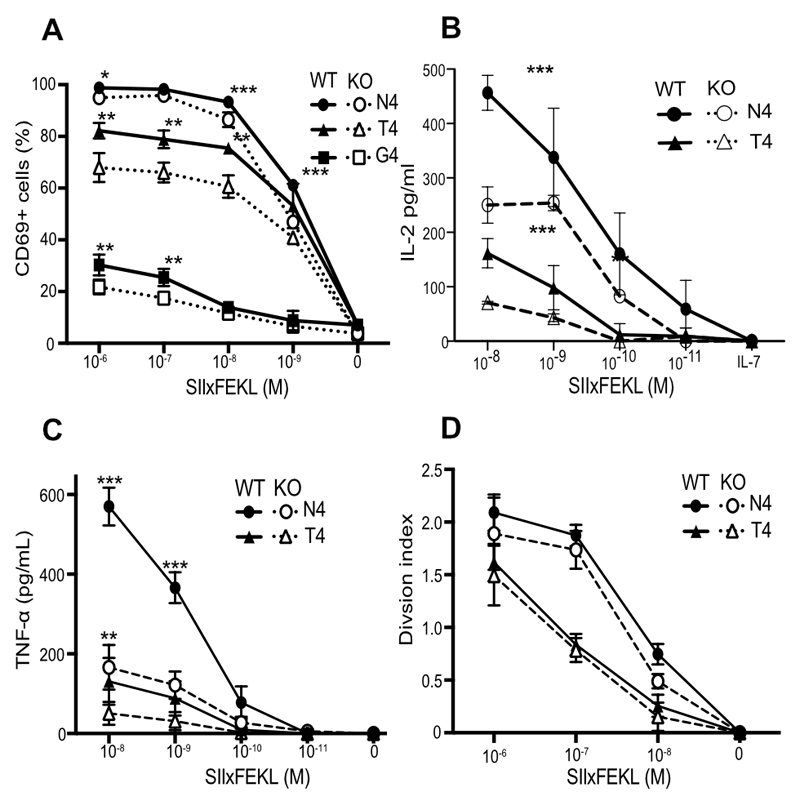
The abundance of cholesterol in the plasma membrane has been linked directly to the ability of CD8 T cells to respond to antigen (45), so we crossed Cav1-KO mice onto the OT-1 class 1 MHC-restricted, TCR transgenic background to enable interrogation of antigen-specific CD8 T cell responses. Responses of Cav1-WT and Cav1-KO OT-1 CD8 T cells were measured to a range of peptides of differing affinities: SIINFEKL (N4) a strong agonist, SIITFEKL (T4) a partial agonist and SIIGFEKL (G4) a very weak agonist. We confirmed that loss of Cav1 impaired the response of CD8 T cells to TCR:pMHC stimulation in vitro (Fig. 2, A to D), as previously reported for polyclonal CD8 T cells stimulated with anti-CD3/28 antibodies (32). The absence of Cav1 had a significant impact on the upregulation of the early activation marker CD69 for all altered peptide ligands (Fig. 2A) and resulted in a significant reduction in the development of effector function, as measured by the production of cytokines IL-2 and TNF-α (Fig. 2, B and C) and, as previously described (32), IFNγ (data not shown). Although activation and effector function were significantly impacted by Cav1 deficiency, there was only a subtle reduction in proliferation in Cav1-KO CD8 T cells (Fig. 2D). At first site these data appear to contradict a previous report (32) showing that Cav-KO CD8 T cells have reduced proliferation to anti-CD3+CD28 stimulation in vitro and expansion to LCMV virus in vivo. However, Tomassian et al. showed that while a reduced proportion of Cav-KO CD8 T cells had entered division by 48h, there was no defect in their ability to undergo multiple divisions. In contrast to anti-CD3+CD28 stimulation, the strong agonist N4 peptide drives all the OT-1 T cells synchronously into division in vitro, therefore, the strength of the stimulus would be likely to minimise any differences in proliferation between WT and KO T cells so that although there was a trend for less proliferation in the Cav1-KOs, this was not significant over multiple experiments. Overall our data confirm that, even though Cav1 was expressed at much lower abundance in T cells than in non-lymphoid tissues and was not associated with caveolae, Cav1 influenced the lipid composition of CD8 T cell membranes, and its absence impaired the activation and the development of effector function of CD8 T cells upon stimulation with antigen.
Figure 2. Cav1 regulates CD8 T cell responses to antigen.
(A) The frequency of CD69+ cells among Cav1-WT and Cav1-KO OT-1 CD8 T cells stimulated for 3 h with a titration of N4, T4 or G4 peptides. (B) and (C) Cav1-WT and Cav1-KO OT-1 CD8 T cells were stimulated and supernatants were assayed at 24h for total IL-2 (B) and TNF-α (C) by ELISA. (D) Proliferation at 72 h of CTV-labeled OT-1 T cells is represented by the division index calculated using FlowJo software. Data representative of 2 independent experiments, each performed on 3 mice per group. Error bars represent SD, *p ≤0.05, **p ≤0.01, *** p ≤0.001 (Student’s t-test, corrected for multiple comparisons using the Holm-Sidak method).
Cav1 influences CD11a recruitment to the immunological synapse
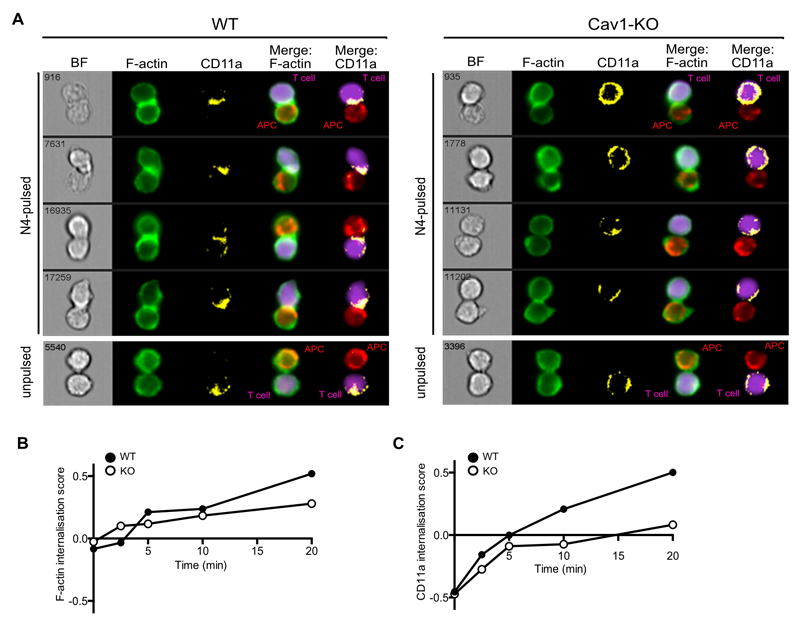
A critical first step in the activation of T cells is the formation of an immunological synapse (IS), a specialised junction between the T cell and the antigen presenting cell (APC). Recent evidence has shown that the IS comprises a highly dynamic periphery in which TCRs in microvesicles engage with intracellular signalling molecules (8) while the structure is stabilised by a peripheral ring (pSMAC) composed of the integrin, LFA-1, interacting with its ligand ICAM1. In order to assess whether the reduced activation of Cav1-KO CD8 T cells was linked to changes in IS formation, we used a high-throughput imaging flow cytometer, Imagestream, to obtain a measure of the spatial distribution of LFA-1 (CD11a) from a large number of conjugates over multiple early time points. Details of spillover coefficients and the analytical and masking workflow used in the analysis are provided in fig. S2. Both Cav1-WT and Cav1-KO naïve OTI CD8 T cells formed conjugates with N4-pulsed APC over the 20 min observation period compared to unpulsed controls (Fig. 3A). LFA-1 surface expression was homogenously dispersed on unconjugated CD8 T cells and on conjugates formed with APC in the absence of Ag (unpulsed) in both control and Cav1-KO CD8 T cells. Upon T cell conjugation with N4-pulsed APC, both F-actin and CD11a redistributed to the IS in Cav1-WT and Cav1-KO CD8 T cells. Calculation of the internalisation score, the amount of protein within the cell:cell contact area, revealed a reduction of both F-actin and CD11a recruitment to the IS in the absence of Cav1 (Fig. 3, B and C), as seen clearly at the 20 min time point shown in Fig. 3A.
Figure 3. Impaired recruitment of F-actin and CD11a to the IS in the absence of Cav1. Imagestream analysis assessing conjugate formation of Cav1-WT or Cav1-KO OT-1 CD8 T cells with N4-pulsed APC for the indicated times (0-20 min). Conjugates were fixed, permeabilised and stained for BODIPY-Fl (F-actin, green) and CD11a (yellow).
(A) The 4 rows show representative cells at 20 min, with distribution of BODIPY-Fl and CD11a within the synapse formed between T cells (purple) and APC (red) shown in Merge columns 4 and 5 respectively. Graphs of the internalization method, previously described in (35), for Cav1-WT and Cav1-KO conjugates stained with (B) BODIPY-Fl and (C) CD11a are representative of 3 independent experiments.
Cav1 alters T cell morphology and polarisation
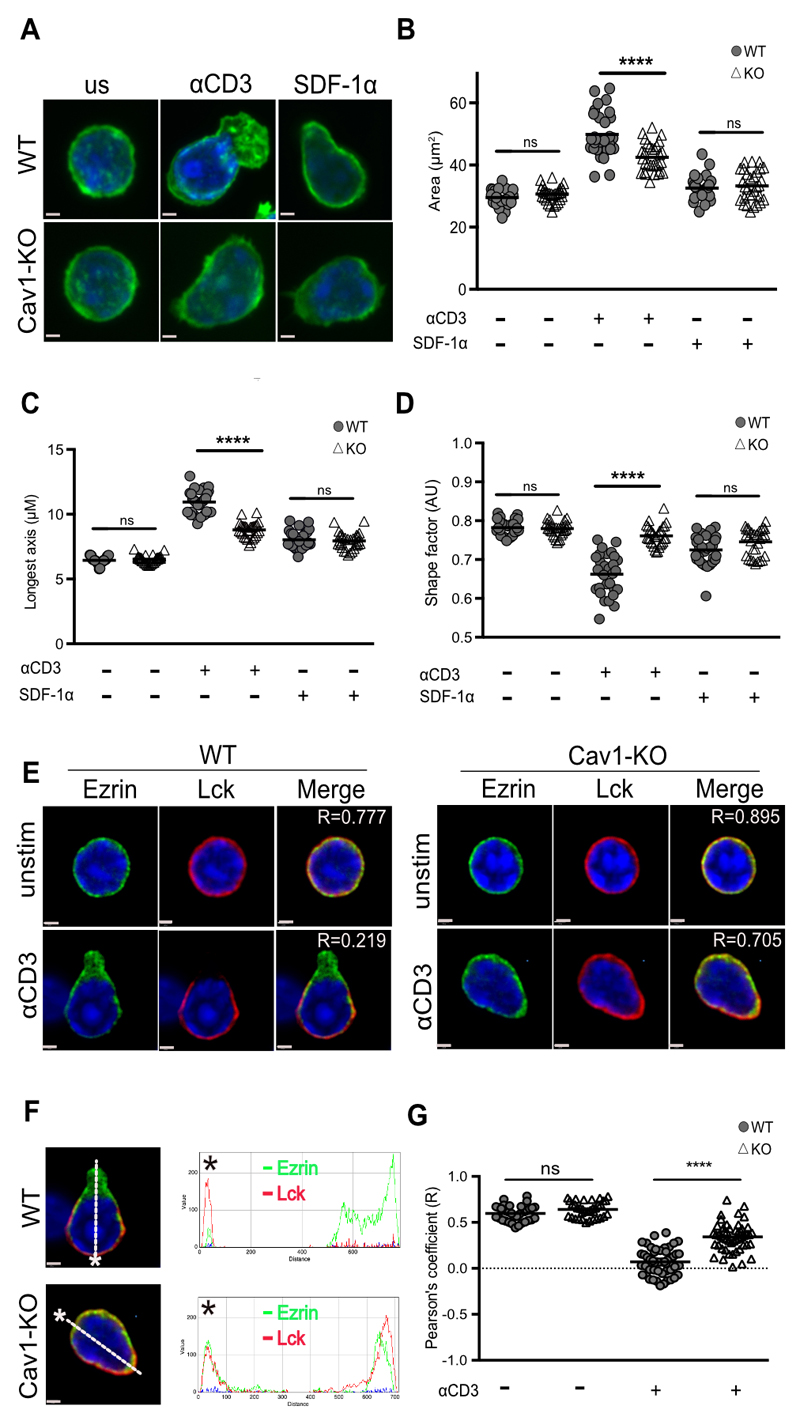
Less LFA-1 was recruited to the IS in the absence of Cav1, which could result from Cav1 directly influencing LFA-1 signaling, as Cav1 is known to influence integrin signaling in other cell types (46–48), or as an indirect effect arising from defective TCR stimulation of inside-out signaling to LFA-1. To better understand how lack of Cav1 influenced LFA-1 interactions with ICAM-1 we looked at the consequences on cell morphology and distribution of particular intracellular signaling molecules following LFA-1 interaction with immobilised ICAM-1. Naïve OT-1 CD8 T cells require either TCR or chemokine CXCR4 stimulation to induce a conformational change in LFA-1 to its active, high affinity conformation. Cav1-WT and Cav1-KO CD8 T cells were activated with anti-CD3 Ab or SDF-1α (CXCL12), the ligand for CXCR4, for 10 min prior to addition to glass slides coated with ICAM-1. Resting T cells have a spherical morphology with F-actin homogenously distributed around the plasma membrane, as shown by both Cav1-WT and Cav1-KO CD8 T cells (Fig. 4A). After 10 min of anti-CD3 stimulation, Cav1-WT and Cav1-KO CD8 T cells were attached to ICAM-1 coated glass coverslips for 30 min, whereupon Cav1-WT cells became polarised and formed the characteristic F-actin extensions at one pole. However Cav1-KO CD8 T cells lacked a defined uropod structure (Fig. 4A). Calculation of the surface area of the anti-CD3 Ab stimulated cells showed that Cav1-KO CD8 T cells had spread significantly less, with a reduced area and mean of the longest axis, than their WT counterparts (Fig. 4, B and C). Accordingly, the shape factor was significantly lower in Cav1-KO CD8 T cells as the cells were more circular than the WT cells which formed distinct uropod protrusions (Fig. 4D).
Figure 4. Absence of Cav1 alters CD8 T cell morphology and polarization upon adhesion to ICAM-1. Cav1-WT and Cav1-KO OT-1 CD8 T cells were allowed to adhere for 30 min to glass slides ±3 μg/mL ICAM-1 coating.
(A) Cells were stained with BODIPY-Fl (green) to define the cell perimeter. Cav1-WT and Cav1-KO CD8 T cells spreading on ICAM-1 were quantified in terms of (B) area, (C) longest axis and (D) shape factor. For shape factor, a value of 1 is circular. (B-D) Small horizontal lines indicate the mean. (E-F) Cells were stained with Ezrin (green) and Lck (red). Merge images were sectioned (white line) from the leading edge, indicated by the asterisk, to the uropod, generating a RGB histogram using ImageJ software. The histogram line indicates the distribution and MFI of proteins throughout the cross-section of the cell. (G) Pearson’s correlation coefficient (R) was calculated by Volocity software and p-values calculated by the Student’s t-test; **** p ≤0.001. Data is representative of 1 of 3 independent experiments with a minimum of 100 cells per condition. Scale bar represents 1 μm.
Cav1 deficiency has been reported to cause Src-dependent removal of focal adhesions from cell edges in fibroblast cells (49), therefore we asked whether loss of morphology was indicative of aberrant polarity of TCR stimulated CD8 T cells upon LFA-1 interaction with ICAM-1. Ezrin, one of the ezrin-radixin-moesin (ERM) proteins which links the plasma membrane to the actomyosin cortex and modulates T cell polarisation through regulating cortical tension (50) was found to be concentrated within the defined uropod of Cav1-WT CD8 T cells following TCR stimulation (Fig. 4E). Strikingly, Ezrin remained homogenously localised around the cell periphery in Cav1-KO CD8 T cells. Loss of Lck clustering with the TCR following early activation of Cav1-KO Tregs was reported recently (51), suggesting that Lck localisation at the leading edge of migrating T cells could be impeded in Cav1-KO CD8 T cells. Indeed, we found that although Cav1-WT CD8 T cells polarised Lck to the leading edge, in Cav1-KO cells Lck maintained a homogenous distribution around the cell membrane. To visualise the proportion of molecules that redistributed toward the uropod and leading edge of TCR stimulated CD8 T cells, we used spectral overlaps and RGB histogram analysis. A merge image sectioned from the leading edge (designated by an asterisk) to the opposing uropod (trajectory represented by a white line) showed that in Cav1-WT CD8 T cells, the majority of Lck was polarised to the leading edge of the migrating cell, with the majority of Ezrin molecules concentrated within the uropod (Fig. 4F). Analysis of Cav1-KO CD8 T cells identified a similar proportion of Lck and Ezrin molecules within both the leading edge and uropod. A comparison of the Pearson’s correlation coefficient (R) between Lck and Ezrin revealed that following TCR stimulation, Cav1-WT CD8 T cells have a significantly lower level of protein co-localisation than Cav1-KO cells (Fig. 4G). Together these results show that Cav1 plays a role downstream of the TCR in maintaining T cell morphology and directing cellular polarisation upon LFA-1-mediated cellular adhesion of CD8 T cells.
Impaired adhesion to ICAM-1 under conditions of shear flow in the absence of Cav1
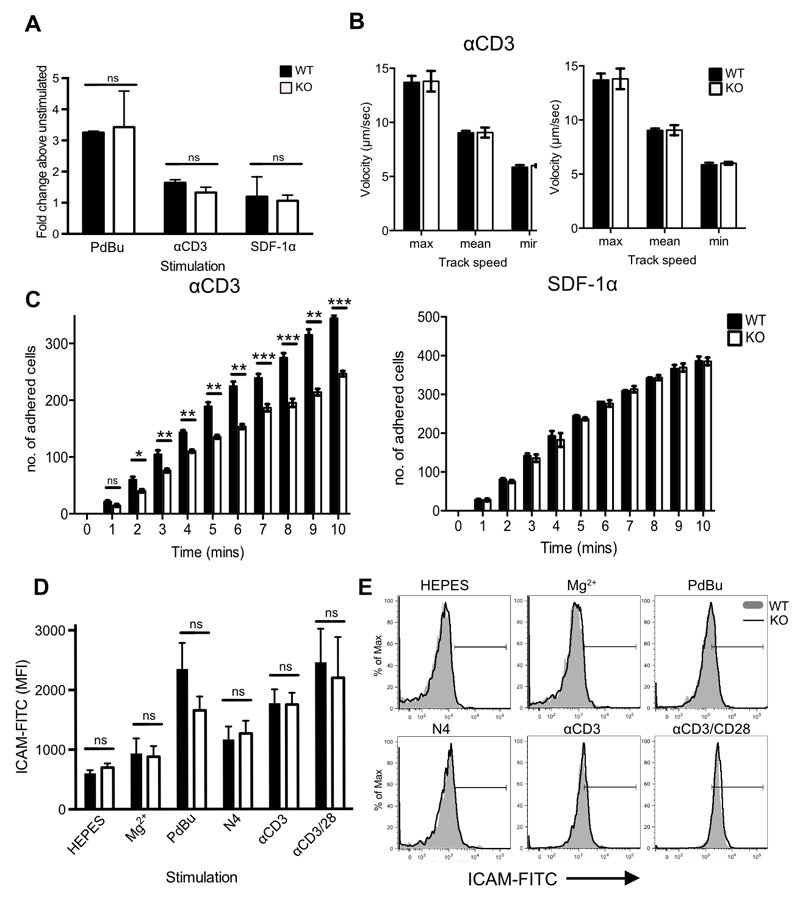
The altered morphology and polarisation of Cav1-KO CD8 T cells interacting with ICAM-1 led us to first analyse cell adhesion using a static adhesion assay in which the capacity of cells to adhere to ligand in the absence of shear flow is quantified. Adhesion of Cav1-KO CD8 T cells to immobilised ICAM-1 was comparable to that observed in WT cells, whether cells were untreated or stimulated with phorbol esters (PdBu), TCR or chemokine ligands (Fig. 5A).
Figure 5. Impaired adhesion to ICAM-1 under conditions of fluid shear stress in the absence of Cav1.
(A) Static adhesion of WT (filled bars) versus Cav1-KO (open bars) CD8 T cells to ICAM-1. CD8 T cells were stimulated with 50nM PdBu, 1μg/mL αCD3 Ab or 5μg/mL SDF-1α before the start of the assay. Fluid shear flow rates were set at 0.3 dynes/cm2. (B) Rolling rates of the cells were analysed in parallel with (C) the number of adherent cells. Data are shown as mean + SEM of data pooled from two independent experiments, each performed in triplicate on two mice per group. NS, not significant; *p ≤0.05, **p ≤0.01, *** p ≤0.001 (Student’s t-test). (D) Cav1-WT or Cav1-KO OT-1 CD8 T cells were incubated for 30 min with chimeric ICAM-1 in HEPES buffer alone or supplemented with 100nM Mg2+, or stimulated with PdBU, N4 peptide, αCD3, or αCD3 + αCD28, as indicated. ICAM-1 bound to T cells was detected by staining with Hu-IgG-FITC. ICAM-1 MFI + SEM from 1 of 3 independent experiments. (E) Representative histograms from each condition, as indicated.
Integrins mediate cell adhesion under shear flow conditions and are essential for the adhesion of T cells to high endothelial venules, a process that is dependent on high-affinity LFA-1 interactions with ICAM-1. Both external (shear-based) and internal (cytoskeleton-based) forces contribute to LFA-1:ICAM-1 interactions and integrin activity (52). We asked whether the absence of Cav1 influenced adhesion of naïve CD8 T cells to immobilised ICAM-1 under conditions of fluid shear stress. Following pre-incubation with anti-CD3 or SDF-1α, T cells were allowed to flow over plates coated with ICAM-1 alone at the shear rate of 0.3 dyne/cm2. The initial transient low affinity interaction between T cells and endothelium in vivo is mediated mainly through P- and L-selectin, and results in T cell rolling. We did not include selectins in this assay so that we could assess rolling as a direct measure of low to intermediate affinity LFA-1:ICAM-1 interactions, which occur at strengths too weak to allow firm attachment. Rolling rates of WT and Cav1-KO CD8 T cells were comparable following either TCR or SDF-1α stimulation (Fig. 5B) indicating these weak LFA-1-ICAM-1 affinities were unaffected by the loss of Cav1. However, when we tested whether the cells could form firm attachments by measuring the number of adherent cells under conditions of flow, we found the Cav-1KO CD8 T cells were 40% less adherent than WT cells to ICAM-1 (Fig. 5C). This difference was only observed following anti-CD3 stimulation of the cells and was not apparent following stimulation with the chemokine SDF-1α. These data indicate that the TCR signals that licence high avidity interactions of LFA-1 with ICAM-1 are compromised in Cav1-KO CD8 T cells.
High avidity interactions between LFA-1 and ICAM-1 are the result of both conformational changes in the LFA molecules themselves, which lead to higher affinity binding termed ‘affinity modulation’ and changes in local integrin density which increases valency and results in ‘avidity modulation’. To ask whether loss of Cav1 impaired LFA-1 affinity modulation, we first examined naïve OT-1 CD8 T cell adhesion to soluble ICAM-1 following activation of LFA-1 directly. Treatment of the cells with the divalent cation Mg2+ in the presence of EGTA, to prevent Ca2+-dependent changes in avidity, can be used to increase LFA-1 affinity (53, 54). When LFA-1 was in a low-affinity state (HEPES buffer only) the amount of soluble ICAM-1 binding was low and equivalent in Cav1-WT and Cav1-KO CD8 T cells (Fig 5, D and E). Addition of Mg2+/EGTA caused only a negligible and comparable MFI shift in ICAM-1 binding to both Cav1-WT and Cav1-KO CD8 T cells. We next addressed the ability of CD8 T cells to bind ICAM-1 following stimulation with PdBu, peptide (N4), anti-CD3 Ab or anti-CD3 plus soluble anti-CD28 Abs (CD3/28). After 30 min stimulation, adhesion of soluble ICAM-1 was enhanced in a comparable manner for Cav1-WT and Cav1-KO CD8 T cells and both bound ICAM-1 maximally following stimulation with PdBu and anti-CD3/28 treatment. These data indicate that there is no defect in upregulation of LFA-1 affinity following TCR stimulation in Cav1-KO CD8 T cells and suggest instead that compared to Cav1-WT, Cav1-KO cells may not be able to re-distribute LFA-1 as effectively to promote high avidity LFA-1:ICAM-1 interactions.
Impaired LFA-1-mediated aggregation in the absence of Cav1
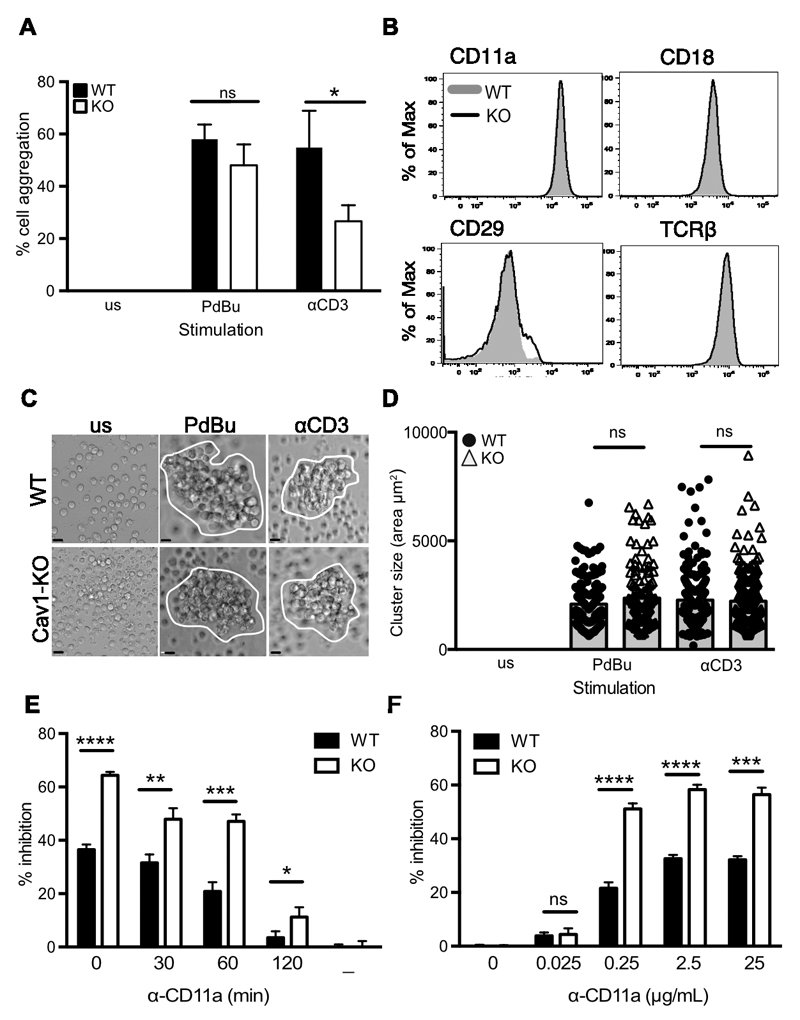
ICAM-1 is expressed on the surface of T cells and in culture LFA-1 mediates cell-cell adhesion by binding in-trans to this ligand in response to stimuli including PdBu (55, 56). Thus activation of LFA-1 can lead to the formation of aggregates of homotypically adherent cells (57), which is considered to be a reflection of ligand-dependent redistribution of LFA-1 molecules (58). We examined the ability of purified CD8 T cells to form aggregates following stimulation with anti-CD3 or with PdBu plus ionomycin which activates integrins while bypassing TCR-proximal signalling (Fig 6A). The percentage of naïve OT-1 CD8 T cells that formed aggregates after 60 min was significantly lower in Cav1-KO T cell cultures compared to Cav1-WT cultures in response to anti-CD3. In contrast, PdBu plus ionomycin stimulation caused equivalent aggregation. Reduced aggregation in the absence of Cav1 was not due to altered expression of adhesion molecules on Cav1KO CD8 T cells as phenotyping for surface markers confirmed that there was equivalent abundance of both the αL (CD11a) and β2 (CD18) chains of LFA-1, as well as other adhesion molecules including the β1 integrin CD29 (VLA4) together with the TCR complex (Fig. 6B). By 24 hours after activation, CD8 T cells formed large clusters that were comparable in size between Cav1-KO and Cav1-WT cells (Fig. 6, C and D). These data indicate that in CD8 T cells, Cav1 plays a role in LFA-1:ICAM-1-dependent cell interactions early after stimulation but does not impact on later aggregate formation, consistent with Cav1 impairing some aspects of T cell activation (Fig 2).
Figure 6. Impaired LFA-1 mediated CD8 T cell homotypic aggregation in the absence of Cav1.
(A) Purified Cav1-WT (filled bars) and Cav1-KO (open bars) OT-1 CD8 T cells were activated with 50ng/mL PdBu or 1μg/mL αCD3 for 60 min and assessed for cell:cell aggregation. Data are shown as a mean + SEM of data representing 1 of 5 independent experiments with each count performed in triplicate on 2-4 mice per group. (B) Expression levels of the indicated surface proteins in WT (grey shaded) and Cav1-KO (black line) naïve OT-1 CD8 T cells. Data are representative of 25 mice per group. Naïve CD8 T cells were cultured for 24 h then (C) imaged and (D) assessed for cluster size using Volocity software. NS, not signficant; *p ≤0.05 (Student’s t-test). Data is pooled from 2 independent experiments with a minimum of 100 cells per condition (mean and SD). Scale bar represents 5 μm. (E) Anti-LFA-1 blocking mAb was added at various timepoints or (F) at various concentrations, following 1μM N4 peptide stimulation of Cav1-WT and Cav1-KO CD8 T cells. Inhibition of CD69 upregulation (% inhibition) was measured at 3h. Data is representative of 1 of 3 independent experiments. NS, not significant; *p ≤0.05, **p ≤0.01, *** p ≤0.001 (Student’s t-test).
LFA-1 mediated homotypic aggregation has been linked to optimal activation of T cells in vitro (59–61) and combined with our observations that there was reduced LFA-1 recruitment to the IS (Fig. 3), suggested that a failure to form early stable LFA-1:ICAM-1 interactions could contribute to the impaired responses to antigen of Cav1-KO OT-1 CD8 T cells. To assess this, we examined the effect upon activation with N4 peptide of disrupted T cell:APC conjugates by addition of blocking LFA-1 mAb. CD69 upregulation, normalised against cells stimulated without blocking antibody, was measured at 3h. We found that Cav1-KO CD8 T cells were more readily inhibited than Cav1-WT cells by addition of anti-LFA-1 Ab at all timepoints up to 120 min after peptide stimulation (Fig. 6E). Moreover titration of the blocking anti-LFA-1 Ab showed that Cav1-KO CD8 T cells were inhibited by lower concentrations of Ab than Cav1-WT cells (Fig 6F). Together these data suggest that Cav1 plays a role in the dynamic regulation of molecules within T cell membranes, and in particular influences LFA-1 redistribution to the IS and thus the avidity of its interaction with ligand with consequent impairment of T cell activation.
Discussion
Cav1 has been shown to influence membrane dynamics and modulate cell adhesion, cytoskeletal organisation and polarity in a variety of cell types (62, 63). We show here that lack of Cav1 in primary CD8 T cells directly impacted on the lipid content of the T cell membrane, with a specific overall reduction in the abundance of short chain fatty acid sphingomyelins and cholesterol. Additionally, Cav1-KO CD8 T cells showed several changes from WT T cells upon stimulation through the TCR, including an altered morphology and an inability to appropriately redistribute key molecules including integrins and Lck. Moreover Cav1-KO CD8 T cells responded less efficiently to antigen stimulation and showed defects in the acquisition of effector function, as has been documented previously (32).
Our data suggest that Cav1 plays a role in influencing the distribution of molecules in the plasma membrane following TCR stimulation. Migrating T cells establish a polarized morphology, with an actin-rich leading edge and a uropod formed at the trailing edge, which is required for efficient crawling. LFA-1 distributes between both poles of the cell, participating in the pulling or pushing of the cell within the migrating gradient. Similarly, during cell migration Cav1 has been reported to accumulate at the anterior, leading edge of cultured fibroblasts (64), smooth muscle cells (65) and endothelial cells (66), and posterior, trailing edge of bovine aortic endothelial cells and fibroblasts (67, 68). Furthermore, Cav1 has been shown to influence cell polarity directly. Cav1-deficient fibroblasts displayed loss of polarised cell morphology with associated removal of focal adhesions at cell edges and loss of directional cell migration upon stimulation, defining an essential role for Cav1 in cell adhesion and motility of fibroblasts (49). We found that Cav1-KO CD8 T cells displayed reduced spreading with altered cellular morphology upon interaction with ICAM-1. Our data indicate that Cav1 is involved also in CD8 T cell polarity, as uropod formation was compromised in TCR-stimulated Cav1-KO cells migrating on ICAM-1, as was the redistribution of Ezrin and Lck. Flotillins, caveolae-associated membrane proteins, have been shown to have a role in uropod formation in neutrophils through direct interactions with the cortical cytoskeleton through myosin-IIa (69). Indeed, Filamin-A, a ligand of Cav1, has been shown to interact with β2 and β3 integrins through cytoskeletal association (70–73). Using co-immunoprecipitation followed by western blot, we were unable to detect a direct association between Cav1 and Filamin A, Csk, Fyn, or CD18, all of which have been implicated in mediating TCR-induced signals in T cells and in regulating membrane dynamics in association with Cav1 in other cell models (74–77). However, due to the very low abundance of Cav1 in primary T cells compared to other cell types, the association of other molecules with Cav1 may be below the level of detection by these biochemical assays.
Cav1 has been shown to impact on membrane dynamics by directly binding cholesterol and sphingolipids at the plasma membrane (44, 62) or through co-ordination of signalling proteins that regulate the actin cytoskeleton (62, 78). Moreover Cav1 has been shown to transport cholesterol from the ER to the PM in fibroblasts, thereby regulating the abundance of cholesterol in the PM (44). The reduction in the abundance of cholesterol and sphingolipids in Cav1KO CD8 T cells suggested that Cav1 might play a similar role in T cells. Upon activation we observed Cav1 redistribution from the PM, in naïve T cells, to an almost exclusive location within a detergent-resistant cellular pellet that is highly enriched in lipids and cytoskeletal components, consistent with a dynamic role for Cav1 in membrane homeostasis.
In CD8 T cells the abundance of cholesterol in the membrane directly influences T cell function, with increased cholesterol potentiating effector function and conversely, depletion of cholesterol impairing function (45). Thus the reduced responsiveness of Cav1KO OT-1 T cells is consistent with the reduction in cholesterol and sphingomyelin content of the cells we observed. LFA-1 has been postulated to associate specifically with a subset of lipid rafts that are high in cholesterol in primary T cells and this association regulated clustering and LFA-1-mediated adhesion (79). Other studies also correlated LFA-1 activity and lipid raft localisation, by showing the clustering of high affinity LFA-1 (by exposure to the divalent cation Mn2+ or following domain I removal) and high avidity LFA-1 (by exposure to PdbU) within lipid rafts, and the exclusion of inactivated LFA-1 by actin cytoskeleton associations (80–82). Our data are most consistent with the absence of Cav1 influencing LFA-1 avidity, which is promoted by redistribution and thus increased valency, rather than by changes in affinity arising from re-positioning or conformation of the integrin I domains to regulate LFA-1 adhesion. We found that in Cav1-KO CD8 T cells the CD11a subunit of LFA-1 as well as F-actin were less efficiently relocated to the IS, which is an area high in lipid raft content, supports the view that Cav1 may be involved in organisation of these signalling rich regions in T cells. A lessening in LFA-1 avidity would also account for the reduction in homotypic adhesion and reduced adhesion under flow observed after TCR stimulation of Cav1-KO T cells. Together this reduction in LFA-1 adhesion was insufficient to abort T cell signalling but could account for the impairment in T cell responses to antigen as shown here and previously (32).
The inability to detect caveolae-like structures on naïve CD8 T cells and the relatively low levels of Cav1, Cav2 and PTRF transcript in lymphoid organs compared to adipose tissue which is enriched in the specialised membrane microdomains, suggests that in T cells, Cav1 plays a distinctly different role than in other cell types where its main role is in caveolae formation. Indeed the combination of proteomics with subdiffraction-limit microscopy has confirmed that Cav1 scaffolds are structurally and functionally distinct from caveolae (30). Expression of Cav1 at low levels can result in the assembly of stable oligomerised Cav1 microdomains, or scaffolds, comprised of approximately 15 Cav1 molecules (78, 83, 84). However the role of non-caveolar Cav1 remains elusive.
In conclusion, we report that Cav1 is involved in the transduction of TCR signals required for optimal regulation of integrin adhesion and function in primary CD8 T cells. These findings support the emerging view that membrane adaptor proteins serve as check points for controlling signalling thresholds and cellular output. TCR signalling pathways are not linear but diverge, facilitated by numerous membrane adaptors, which may include Cav1. Integrin signalling is fundamental to T cell activation and plays a major role in the formation of the IS, cell adhesion and migration. Cav1 is clearly involved in these processes in primary T cells and is therefore a potential target for manipulation of TCR signals to improve T effector function during an immune response.
Supplementary Material
Acknowledgments
We would like to thank the members of the Zamoyska lab for technical assistance and discussions.
2This work was supported by MRC project grant G1100116 and Wellcome Trust Investigator Award WT096669AIA and a strategic award from the Wellcome Trust for the Centre for Immunity, Infection and Evolution 095831. 3VLM, LMU and SCF were supported by the BBSRC, Academy of Finland, and the Sigrid Juselius foundation.
References
- 1.Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The Leukocyte Integrins. J Biol Chem. 2000;275:23409–23412. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- 2.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand Binding to Integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 3.Hogg N, Patzak I, Willenbrock F. The Insider's Guide to Leukocyte Integrin Signalling and Function. Nat Rev Immunol. 2011;11:416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 4.Gahmberg CG, Fagerholm SC, Nurmi SM, Chavakis T, Marchesan S, Gronholm M. Regulation of Integrin Activity and Signalling. Biochim Biophys Acta. 2009;1790:431–444. doi: 10.1016/j.bbagen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larjava H, Plow EF, Wu C. Kindlins: Essential Regulators of Integrin Signalling and Cell-Matrix Adhesion. EMBO Rep. 2008;9:1203–1208. doi: 10.1038/embor.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart M, Hogg N. Regulation of Leukocyte Integrin Function: Affinity Vs. Avidity. J Cell Biochem. 1996;61:554–561. doi: 10.1002/(sici)1097-4644(19960616)61:4<554::aid-jcb8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.van Kooyk Y, Figdor CG. Avidity Regulation of Integrins: The Driving Force in Leukocyte Adhesion. Curr Opin Cell Biol. 2000;12:542–547. doi: 10.1016/s0955-0674(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 8.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The Immunological Synapse: A Molecular Machine Controlling T Cell Activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 9.Carrasco YR, Fleire SJ, Cameron T, Dustin ML, Batista FD. Lfa-1/Icam-1 Interaction Lowers the Threshold of B Cell Activation by Facilitating B Cell Adhesion and Synapse Formation. Immunity. 2004;20:589–599. doi: 10.1016/s1074-7613(04)00105-0. [DOI] [PubMed] [Google Scholar]
- 10.Schmits R, Kundig TM, Baker DM, Shumaker G, Simard JJ, Duncan G, Wakeham A, Shahinian A, van der Heiden A, Bachmann MF, Ohashi PS, et al. Lfa-1-Deficient Mice Show Normal Ctl Responses to Virus but Fail to Reject Immunogenic Tumor. J Exp Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, et al. Spontaneous Skin Ulceration and Defective T Cell Function in Cd18 Null Mice. J Exp Med. 1998;188:119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graf B, Bushnell T, Miller J. Lfa-1-Mediated T Cell Costimulation through Increased Localization of Tcr/Class Ii Complexes to the Central Supramolecular Activation Cluster and Exclusion of Cd45 from the Immunological Synapse. J Immunol. 2007;179:1616–1624. doi: 10.4049/jimmunol.179.3.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aplin AE, Juliano RL. Integrin and Cytoskeletal Regulation of Growth Factor Signaling to the Map Kinase Pathway. J Cell Sci. 1999;112(Pt 5):695–706. doi: 10.1242/jcs.112.5.695. [DOI] [PubMed] [Google Scholar]
- 14.Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K. Beta 1d Integrin Displaces the Beta 1a Isoform in Striated Muscles: Localization at Junctional Structures and Signaling Potential in Nonmuscle Cells. J Cell Biol. 1996;132:211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe AK, Juliano RL. Distinct Mechanisms Mediate the Initial and Sustained Phases of Integrin-Mediated Activation of the Raf/Mek/Mitogen-Activated Protein Kinase Cascade. J Biol Chem. 1998;273:27268–27274. doi: 10.1074/jbc.273.42.27268. [DOI] [PubMed] [Google Scholar]
- 16.Hynes RO. Integrins: Versatility, Modulation, and Signaling in Cell Adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 17.Hynes RO. Integrins: Bidirectional, Allosteric Signaling Machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 18.Hynes RO, Lander AD. Contact and Adhesive Specificities in the Associations, Migrations, and Targeting of Cells and Axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- 19.Andrew DP, Spellberg JP, Takimoto H, Schmits R, Mak TW, Zukowski MM. Transendothelial Migration and Trafficking of Leukocytes in Lfa-1-Deficient Mice. Eur J Immunol. 1998;28:1959–1969. doi: 10.1002/(SICI)1521-4141(199806)28:06<1959::AID-IMMU1959>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte Migration in Lymphocyte Function-Associated Antigen (Lfa)-1-Deficient Mice. J Exp Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salanueva IJ, Cerezo A, Guadamillas MC, del Pozo MA. Integrin Regulation of Caveolin Function. J Cell Mol Med. 2007;11:969–980. doi: 10.1111/j.1582-4934.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-Type and Tissue-Specific Expression of Caveolin-2. Caveolins 1 and 2 Co-Localize and Form a Stable Hetero-Oligomeric Complex in Vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 23.Tang Z, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular Cloning of Caveolin-3, a Novel Member of the Caveolin Gene Family Expressed Predominantly in Muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- 24.van Meer G. Caveolin, Cholesterol, and Lipid Droplets? J Cell Biol. 2001;152:F29–34. doi: 10.1083/jcb.152.5.f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nystrom FH, Chen H, Cong LN, Li Y, Quon MJ. Caveolin-1 Interacts with the Insulin Receptor and Can Differentially Modulate Insulin Signaling in Transfected Cos-7 Cells and Rat Adipose Cells. Mol Endocrinol. 1999;13:2013–2024. doi: 10.1210/mend.13.12.0392. [DOI] [PubMed] [Google Scholar]
- 26.Razani B, Rubin CS, Lisanti MP. Regulation of Camp-Mediated Signal Transduction Via Interaction of Caveolins with the Catalytic Subunit of Protein Kinase A. J Biol Chem. 1999;274:26353–26360. doi: 10.1074/jbc.274.37.26353. [DOI] [PubMed] [Google Scholar]
- 27.Toya Y, Schwencke C, Couet J, Lisanti MP, Ishikawa Y. Inhibition of Adenylyl Cyclase by Caveolin Peptides. Endocrinology. 1998;139:2025–2031. doi: 10.1210/endo.139.4.5957. [DOI] [PubMed] [Google Scholar]
- 28.Fridolfsson HN, Roth DM, Insel PA, Patel HH. Regulation of Intracellular Signaling and Function by Caveolin. FASEB J. 2014;28:3823–3831. doi: 10.1096/fj.14-252320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Head BP, Patel HH, Insel PA. Interaction of Membrane/Lipid Rafts with the Cytoskeleton: Impact on Signaling and Function: Membrane/Lipid Rafts, Mediators of Cytoskeletal Arrangement and Cell Signaling. Biochim Biophys Acta. 2014;1838:532–545. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng YZ, Boscher C, Inder KL, Fairbank M, Loo D, Hill MM, Nabi IR, Foster LJ. Differential Impact of Caveolae and Caveolin-1 Scaffolds on the Membrane Raft Proteome. Mol Cell Proteomics. 2011;10:M110 007146. doi: 10.1074/mcp.M110.007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina FA, Williams TM, Sotgia F, Tanowitz HB, Lisanti MP. A Novel Role for Caveolin-1 in B Lymphocyte Function and the Development of Thymus-Independent Immune Responses. Cell Cycle. 2006;5:1865–1871. doi: 10.4161/cc.5.16.3132. [DOI] [PubMed] [Google Scholar]
- 32.Tomassian T, Humphries LA, Liu SD, Silva O, Brooks DG, Miceli MC. Caveolin-1 Orchestrates Tcr Synaptic Polarity, Signal Specificity, and Function in Cd8 T Cells. J Immunol. 2011;187:2993–3002. doi: 10.4049/jimmunol.1101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, et al. Caveolin-1 Null Mice Are Viable but Show Evidence of Hyperproliferative and Vascular Abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 34.Filby A, Davies D. Reporting Imaging Flow Cytometry Data for Publication: Why Mask the Detail? Cytometry A. 2012;81:637–642. doi: 10.1002/cyto.a.22091. [DOI] [PubMed] [Google Scholar]
- 35.Gandillet A, Park S, Lassailly F, Griessinger E, Vargaftig J, Filby A, Lister TA, Bonnet D. Heterogeneous Sensitivity of Human Acute Myeloid Leukemia to Beta-Catenin Down-Modulation. Leukemia. 2011;25:770–780. doi: 10.1038/leu.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagerholm SC, Prescott A, Cohen P, Gahmberg CG. An Essential Role for Calmodulin in Regulating Human T Cell Aggregation. FEBS Lett. 2001;491:131–136. doi: 10.1016/s0014-5793(01)02182-2. [DOI] [PubMed] [Google Scholar]
- 37.Fra AM, Williamson E, Simons K, Parton RG. Detergent-Insoluble Glycolipid Microdomains in Lymphocytes in the Absence of Caveolae. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- 38.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane Compartmentation Is Required for Efficient T Cell Activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 39.Harder T, Simons K. Caveolae, Digs, and the Dynamics of Sphingolipid-Cholesterol Microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 40.Nabi IR, Le PU. Caveolae/Raft-Dependent Endocytosis. J Cell Biol. 2003;161:673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin Isoforms Differ in Their N-Terminal Protein Sequence and Subcellular Distribution. Identification and Epitope Mapping of an Isoform-Specific Monoclonal Antibody Probe. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- 42.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, Li M, Tang B, Jelicks LA, Scherer PE, Lisanti MP. Caveolin-1-Deficient Mice Are Lean, Resistant to Diet-Induced Obesity, and Show Hypertriglyceridemia with Adipocyte Abnormalities. J Biol Chem. 2002;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 43.Kurzchalia TV, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M, Simons K. Vip21, a 21-Kd Membrane Protein Is an Integral Component of Trans-Golgi-Network-Derived Transport Vesicles. J Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smart EJ, Ying Y, Donzell WC, Anderson RG. A Role for Caveolin in Transport of Cholesterol from Endoplasmic Reticulum to Plasma Membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- 45.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, Yan C, et al. Potentiating the Antitumour Response of Cd8(+) T Cells by Modulating Cholesterol Metabolism. Nature. 2016;531:651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borza CM, Chen X, Mathew S, Mont S, Sanders CR, Zent R, Pozzi A. Integrin {Alpha}1{Beta}1 Promotes Caveolin-1 Dephosphorylation by Activating T Cell Protein-Tyrosine Phosphatase. J Biol Chem. 2010;285:40114–40124. doi: 10.1074/jbc.M110.156729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A Requirement for Caveolin-1 and Associated Kinase Fyn in Integrin Signaling and Anchorage-Dependent Cell Growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 48.Wei Y, Yang X, Liu Q, Wilkins JA, Chapman HA. A Role for Caveolin and the Urokinase Receptor in Integrin-Mediated Adhesion and Signaling. J Cell Biol. 1999;144:1285–1294. doi: 10.1083/jcb.144.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grande-Garcia A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA. Caveolin-1 Regulates Cell Polarization and Directional Migration through Src Kinase and Rho Gtpases. J Cell Biol. 2007;177:683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Belkina NV, Park C, Nambiar R, Loughhead SM, Patino-Lopez G, Ben-Aissa K, Hao JJ, Kruhlak MJ, Qi H, von Andrian UH, et al. Constitutively Active Ezrin Increases Membrane Tension, Slows Migration, and Impedes Endothelial Transmigration of Lymphocytes in Vivo in Mice. Blood. 2012;119:445–453. doi: 10.1182/blood-2011-07-368860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schonle A, Hartl FA, Mentzel J, Noltner T, Rauch KS, Prestipino A, Wohlfeil SA, Apostolova P, Hechinger AK, Melchinger W, Fehrenbach K, et al. Caveolin-1 Regulates Tcr Signal Strength and Regulatory T Cell Differentiation into Alloreactive T Cells. Blood. 2016;127:1930–1939. doi: 10.1182/blood-2015-09-672428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sung KL, Kuhlman P, Maldonado F, Lollo BA, Chien S, Brian AA. Force Contribution of the Lfa-1/Icam-1 Complex to T Cell Adhesion. J Cell Sci. 1992;103(Pt 1):259–266. doi: 10.1242/jcs.103.1.259. [DOI] [PubMed] [Google Scholar]
- 53.Stewart MP, Cabanas C, Hogg N. T Cell Adhesion to Intercellular Adhesion Molecule-1 (Icam-1) Is Controlled by Cell Spreading and the Activation of Integrin Lfa-1. J Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- 54.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1a Positively Regulates T Cells Via Integrin Activation Rather Than Inhibiting Lymphocyte Signaling. Nat Immunol. 2002;3:251–258. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 55.Patarroyo M, Beatty PG, Fabre JW, Gahmberg CG. Identification of a Cell Surface Protein Complex Mediating Phorbol Ester-Induced Adhesion (Binding) among Human Mononuclear Leukocytes. Scand J Immunol. 1985;22:171–182. doi: 10.1111/j.1365-3083.1985.tb01869.x. [DOI] [PubMed] [Google Scholar]
- 56.Rothlein R, Springer TA. The Requirement for Lymphocyte Function-Associated Antigen 1 in Homotypic Leukocyte Adhesion Stimulated by Phorbol Ester. J Exp Med. 1986;163:1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Fougerolles AR, Springer TA. Intercellular Adhesion Molecule 3, a Third Adhesion Counter-Receptor for Lymphocyte Function-Associated Molecule 1 on Resting Lymphocytes. J Exp Med. 1992;175:185–190. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim M, Carman CV, Yang W, Salas A, Springer TA. The Primacy of Affinity over Clustering in Regulation of Adhesiveness of the Integrin {Alpha}L{Beta}2. J Cell Biol. 2004;167:1241–1253. doi: 10.1083/jcb.200404160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudnicka W, English N, Farrant J, North ME, Bryant AE, Edwards AJ, Stackpoole A, Webster AD, Balfour BM. Lfa-1-Dependent Okt3-Driven T Cell Clusters in Common Variable Immunodeficiency. Clin Exp Immunol. 1992;87:46–52. doi: 10.1111/j.1365-2249.1992.tb06411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doh J, Krummel MF. Immunological Synapses within Context: Patterns of Cell-Cell Communication and Their Application in T-T Interactions. Curr Top Microbiol Immunol. 2010;340:25–50. doi: 10.1007/978-3-642-03858-7_2. [DOI] [PubMed] [Google Scholar]
- 61.Sabatos CA, Doh J, Chakravarti S, Friedman RS, Pandurangi PG, Tooley AJ, Krummel MF. A Synaptic Basis for Paracrine Interleukin-2 Signaling During Homotypic T Cell Interaction. Immunity. 2008;29:238–248. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonnino S, Prinetti A. Sphingolipids and Membrane Environments for Caveolin. FEBS Lett. 2009;583:597–606. doi: 10.1016/j.febslet.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Liu P, Rudick M, Anderson RG. Multiple Functions of Caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 64.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a Protein Component of Caveolae Membrane Coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 65.Okada SS, Tomaszewski JE, Barnathan ES. Migrating Vascular Smooth Muscle Cells Polarize Cell Surface Urokinase Receptors after Injury in Vitro. Exp Cell Res. 1995;217:180–187. doi: 10.1006/excr.1995.1077. [DOI] [PubMed] [Google Scholar]
- 66.Parat MO, Anand-Apte B, Fox PL. Differential Caveolin-1 Polarization in Endothelial Cells During Migration in Two and Three Dimensions. Mol Biol Cell. 2003;14:3156–3168. doi: 10.1091/mbc.E02-11-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Isshiki M, Ando J, Yamamoto K, Fujita T, Ying Y, Anderson RG. Sites of Ca(2+) Wave Initiation Move with Caveolae to the Trailing Edge of Migrating Cells. J Cell Sci. 2002;115:475–484. doi: 10.1242/jcs.115.3.475. [DOI] [PubMed] [Google Scholar]
- 68.Sanguinetti AR, Mastick CC. C-Abl Is Required for Oxidative Stress-Induced Phosphorylation of Caveolin-1 on Tyrosine 14. Cell Signal. 2003;15:289–298. doi: 10.1016/s0898-6568(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 69.Ludwig A, Otto GP, Riento K, Hams E, Fallon PG, Nichols BJ. Flotillin Microdomains Interact with the Cortical Cytoskeleton to Control Uropod Formation and Neutrophil Recruitment. J Cell Biol. 2010;191:771–781. doi: 10.1083/jcb.201005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu J, Das M, Yang J, Ithychanda SS, Yakubenko VP, Plow EF, Qin J. Structural Mechanism of Integrin Inactivation by Filamin. Nat Struct Mol Biol. 2015;22:383–389. doi: 10.1038/nsmb.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stahlhut M, van Deurs B. Identification of Filamin as a Novel Ligand for Caveolin-1: Evidence for the Organization of Caveolin-1-Associated Membrane Domains by the Actin Cytoskeleton. Mol Biol Cell. 2000;11:325–337. doi: 10.1091/mbc.11.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ravid D, Chuderland D, Landsman L, Lavie Y, Reich R, Liscovitch M. Filamin a Is a Novel Caveolin-1-Dependent Target in Igf-I-Stimulated Cancer Cell Migration. Exp Cell Res. 2008;314:2762–2773. doi: 10.1016/j.yexcr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Takala H, Nurminen E, Nurmi SM, Aatonen M, Strandin T, Takatalo M, Kiema T, Gahmberg CG, Ylanne J, Fagerholm SC. Beta2 Integrin Phosphorylation on Thr758 Acts as a Molecular Switch to Regulate 14-3-3 and Filamin Binding. Blood. 2008;112:1853–1862. doi: 10.1182/blood-2007-12-127795. [DOI] [PubMed] [Google Scholar]
- 74.Yao Q, Chen J, Cao H, Orth JD, McCaffery JM, Stan RV, McNiven MA. Caveolin-1 Interacts Directly with Dynamin-2. J Mol Biol. 2005;348:491–501. doi: 10.1016/j.jmb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 Maintains Activated Akt in Prostate Cancer Cells through Scaffolding Domain Binding Site Interactions with and Inhibition of Serine/Threonine Protein Phosphatases Pp1 and Pp2a. Mol Cell Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sanguinetti AR, Cao H, Corley Mastick C. Fyn Is Required for Oxidative- and Hyperosmotic-Stress-Induced Tyrosine Phosphorylation of Caveolin-1. Biochem J. 2003;376:159–168. doi: 10.1042/BJ20030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao H, Courchesne WE, Mastick CC. A Phosphotyrosine-Dependent Protein Interaction Screen Reveals a Role for Phosphorylation of Caveolin-1 on Tyrosine 14: Recruitment of C-Terminal Src Kinase. J Biol Chem. 2002;277:8771–8774. doi: 10.1074/jbc.C100661200. [DOI] [PubMed] [Google Scholar]
- 78.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, Rafts, and Scaffolds: Domain Regulation of Receptor Signaling at the Plasma Membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marwali MR, Rey-Ladino J, Dreolini L, Shaw D, Takei F. Membrane Cholesterol Regulates Lfa-1 Function and Lipid Raft Heterogeneity. Blood. 2003;102:215–222. doi: 10.1182/blood-2002-10-3195. [DOI] [PubMed] [Google Scholar]
- 80.Leitinger B, Hogg N. The Involvement of Lipid Rafts in the Regulation of Integrin Function. J Cell Sci. 2002;115:963–972. doi: 10.1242/jcs.115.5.963. [DOI] [PubMed] [Google Scholar]
- 81.Lub M, van Kooyk Y, van Vliet SJ, Figdor CG. Dual Role of the Actin Cytoskeleton in Regulating Cell Adhesion Mediated by the Integrin Lymphocyte Function-Associated Molecule-1. Mol Biol Cell. 1997;8:341–351. doi: 10.1091/mbc.8.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stewart MP, McDowall A, Hogg N. Lfa-1-Mediated Adhesion Is Regulated by Cytoskeletal Restraint and by a Ca2+-Dependent Protease, Calpain. J Cell Biol. 1998;140:699–707. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lajoie P, Partridge EA, Guay G, Goetz JG, Pawling J, Lagana A, Joshi B, Dennis JW, Nabi IR. Plasma Membrane Domain Organization Regulates Egfr Signaling in Tumor Cells. J Cell Biol. 2007;179:341–356. doi: 10.1083/jcb.200611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV. Vip21-Caveolin, a Membrane Protein Constituent of the Caveolar Coat, Oligomerizes in Vivo and in Vitro. Mol Biol Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.