Summary
The fruit fly Drosophila melanogaster offers a host of advantages for studying the biology of ageing: a well understood biology, a wide range of genetic reagents, well defined dietary requirements and a relatively short lifespan, with a median of ~80 days and maximum ~100 days. Several phenotypes can be used to assess the ageing process, but the simplest and most widely used metric is length of life. Here we describe a standard lifespan assay for Drosophila housed on a simple sugar/yeast diet.
Keywords: Drosophila melanogaster, lifespan, ageing, method, diet, genetic interventions, pharmacological interventions, backcross
1. Introduction
As populations around the world age, increasing effort is being devoted to the development of new approaches to improve the health of older people. Remarkably, experimental work on worms, flies and mice over the last 20 years has provided a positive outlook on this prospect (Kenyon, 2010; Partridge et al., 2011). For these model organisms, genetic, environmental and pharmacological interventions have been described that extend healthy lifespan (Niccoli and Partridge, 2012). Even more remarkably given the very different lifespans of these model organisms, these interventions often act on common mechanisms to extend lifespan, implying some degree of evolutionary conservation of mechanisms of ageing. Thus there is great promise that studies of ageing in laboratory model organisms will yield insights into ageing that will ultimately benefit humans.
The challenges of experimental gerontology are enormous. Experiments require long time-scales, genetic manipulations, large populations and well-controlled animal stocks and conditions. These factors make the work perfectly suited to the small, short-lived and well-characterized model organisms such as the fruit fly Drosophila melanogaster.
Lifespan experiments have been conducted on Drosophila for the last 100 years (Loeb and Northrop, 1916) and over time the conditions have been refined (Piper and Partridge, 2007). In general, the protocol can be simple, but small and seemingly insignificant modifications to experimental protocols can have large effects on outcomes. For example, by not controlling for diet quality, genetic background or the interactions between mating frequency and diet, the experiment may report the effects on lifespan of an uncontrolled, trivial experimental procedure, rather than the focal intervention of the study. (Partridge and Gems, 2007).
Here we outline the basic procedure for rearing, isolating and maintaining flies for lifespan experiments, highlighting a number of the known pitfalls that have misled researchers in the past. We provide a basic protocol for wild type flies housed under our standard laboratory conditions and then we provide modified protocols for studying the effects on lifespan of diet, drugs or genetic interventions.
2. Materials
2.1. Media
All media are prepared using reverse osmosis water. Cooking can be done on a gas hob using a standard saucepan and stirring with a heavy-duty whisk (see note 1)
Egg collection medium (volume sufficient for ~10 x 15cm petri dishes): to 250ml cold water add 12.5g agar and stir to mix. Bring to boil while stirring and maintain boiling for ~2min to ensure agar is completely melted. Add 150ml red grape juice (see note 2) and stir until the mixture returns to the boil. Remove from heat. Add 25ml cold water and stir until temperature drops to ~65ºC. Mix in10.5ml 10% nipagin (methyl 4-hydroxybenzoate in 95% ethanol) and pour solution into petri dishes. Allow to cool at room temperature, allowing steam to escape. Ensure to protect the plates from any flies at this stage to avoid contamination. Cover and store at 4ºC.
Fly food for rearing and maintenance (makes 1L of 1SY (Bass et al, Exp Gerontol., 2007; see note 3): add 15g agar to 700ml cold water and stir. Heat until boiling. While continuing to stir, add 50g table sugar (sucrose) and 100g yeast (whole yeast autolysate and not water soluble yeast extract). After returned to boil, remove from heat and add cold water to make up to final volume of 1L. Stir and allow to cool to ~65ºC. Mix in 30ml 10% nipagin and 3ml propionic acid to act as preservatives. This is also the point at which to mix in any small volume additions of drugs/ transgene inducers/ vehicle control. For larger volume additions, reduce the cold water addition after cooking to ensure final total volume remains at 1L. Using a peristaltic pump with clean, sterilized tubing, dispense into clean vials or bottles. Allow to cool at room temperature for several hours (see note 4). To avoid contamination, ensure to protect cooling food from flies (see note 5). Plug individual vials with cotton balls (see note 6 7). Store at 4ºC.
Live yeast paste for stimulating egg laying: Mix dried baker’s yeast granules with cold water at a ratio of approx. 1:1 by weight to make a stiff paste (ice cream consistency). Best when used immediately, but can be stored covered at 4ºC for 2 weeks.
2.2. Plastic / glassware for housing and handling eggs and flies8
15cm diameter plastic petri dishes (see note 9)
fly “cage” for housing parental flies: an ~15cm long plastic cylinder that fits a petri dish snugly at one end, and is covered with mesh at the other
Bottles: ~250ml (polypropylene or glass ; ~60mm OD x 130mm H)
Vials / tubes: ~15ml (polystyrene, polypropylene or glass; ~ 25mm O.D. x 95mm H)
Cotton wool balls or high density synthetic bungs to close tubes
squeeze bottle
pipette (20 – 100μl)
wide bore pipette tips (note 10)
CO2 stream – supplied via a water bubbler and low-static porous diffusion pad
fine paint brush (size 000 - 0000)
handle-mounted metal pick
2.3. Solutions for handling eggs and flies
Phosphate buffered saline. Mix pre-formulated tablets (Sigma, P4417) with water according to instructions on container. This yields 0.01M phosphate buffer, 0.0027M potassium chloride, 0.137M sodium chloride, pH 7.4.
3. Methods
3.1. Parental generation
Preparing stocks for egg collection: most laboratory stocks are kept in small numbers and under crowded conditions, both of which alter adult lifespan (Partridge and Gems, 2007; Zwaan et al., 1991)
-
). It is important to implement procedures to control these factors so that they do not confound interpretations of alterations in fly lifespan.
“wild types”: to escape the trans-generational effects of stock crowding on lifespan, we passage stock-derived flies through two generations of our standard density procedure before use in lifespan experiments.
Genetic crosses: it is extremely important to standardize the genetic background of all mutant lines to be compared in a lifespan experiment. Failure to do so is common and leads to incorrect conclusions about the effects of genetic interventions to extend life. Most experimental transgenic flies are generated by crossing two inbred lines, with one containing the transgene to be activated and the other containing a genetic construct that drives the expression of the first. This cross also produces a hybrid genetic background, and this will generally increase lifespan when compared with that of the inbred controls, as a consequence of heterosis and irrespective of any effect of the transgenes (Pearl, 1921). To avoid this problem, all transgenes and mutants should first be back-crossed into a standardised genetic background for at least 6 generations. To maintain the lines an additional 2-3 backcrosses should be repeated every 6-12 months (see note 11). Furthermore, each of the transgenic lines used to construct the experimental line should be included as a control in the lifespan experiment, because transgenes can cause insertional mutagenesis and which can in turn modify longevity.
-
To collect staged embryos:
-
a.
house parental flies in “cages”. Provide a generous smear of live yeast paste (~1 tsp) at the centre of the egg laying plate
-
b.
after 48h, replace egg laying surface (see note 12) with a fresh plate harbouring a fresh aliquot of live yeast paste (egg laying peaks ~72h after introduction to rich food) (see note 13)
-
c.
leave overnight (see note 14)
-
d.
Collect embryos for development at standard density. To achieve this, we either use a pipette to allocate a fixed volume of a dense embryo suspension into new media for development, or use a mounted metal pick to collect and transfer individual larvae to development media (see note 15):
Pipetting method (ideal for robust genotypes, to yield large numbers of experimental flies):
anaesthetise flies in cage and remove egg laying plate on which fertilized eggs lie (see note 16)
using a squeeze bottle containing PBS, cover the plate with a thin layer of buffer
dislodge eggs by “brushing” the egg laying surface with a fine paint brush
pour egg / PBS suspension into a 15ml falcon tube and allow eggs to settle
pour off most of the PBS and add more fresh PBS to wash the eggs
allow eggs to settle and pour off most of the PBS, leaving only sufficient to just cover the settled egg mass
allow eggs to settle
using 100μl pipette with wide bore tip (see note17), set volume to 18-20ul and insert tip into the solution so the tip is level with the top of settled egg mass; quickly release plunger while dropping tip into the mass of eggs
inspect tip for a dense, even, mass of eggs (see note 18)
dispense egg mass on to surface of ~70ml SY medium in a 250ml bottle
-
Picking method (more labour intensive than pipetting, but more fragile genotypes tend to fare better using this method):.
Incubate egg laying plate with staged embryos for 24h at 25ºC (see note19)
using a dissecting microscope, locate 1st instar larvae (see note 20) on plate and touch with the metal pick. They will stick.
With practice, up to ~20 larvae can be collected on one pick
Gently transfer picked larvae into a fresh vial with food for development by wiping larvae off the needle on to the surface of the food (see note 21 22)
-
e.
plug vial / bottle tightly with cotton wool ball(s) (see note 23)
-
f.
incubate at 25ºC with 65% humidity and 12:12 h light:dark
-
a.
3.2. Experimental generation
3.2.1. measuring lifespan of one batch of mated wild type flies on one food type
after 10 days, transfer freshly emerged flies to fresh bottles or vials containing SY medium (see note 24 25)
return bottles of flies to controlled environment (25ºC, 65% humidity and 12:12 h light:dark) for 48h to allow all flies to mate
anaesthetise flies with CO2 and manipulate carefully using a soft brush (see note 26)
separate males from females and allocate target individuals to experimental containers (refer to notes 27 28 29 30 31). Various aspects of courtship and mating modify lifespan of the different sexes to different extents (Partridge and Andrews, 1985; Partridge and Farquhar, 1981; Partridge et al., 1986, 1987; Sgrò and Partridge, 1999). Also, genotype and food quality interact with courtship and mating frequency (Chapman and Partridge, 1996; Wigby et al., 2011). Housing experimental flies as a single sex population avoids the confounding effects of sex X treatment interactions that modify lifespan.
store vials at 25ºC, 65% humidity, 12:12h light:dark (refer to note 32)
-
transfer flies to fresh food every 2 -3 days (refer to notes 33 34 35 36)
-
if recording female egg laying of experimental flies, a good and simple summary can be generated by counting all eggs in all vials once or twice a week for the first 4 – 5 weeks
-
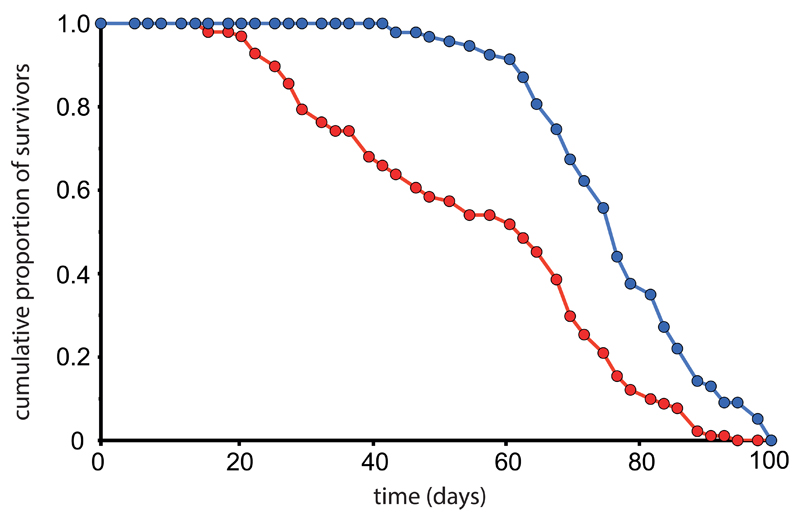
for each transfer, score deaths and censors until all flies are dead (see Figure 1 for survival data examples).
Figure 1.
Examples of good and poor quality survival data. The survival characteristics of a healthy population of flies is demonstrated in blue. There are relatively few deaths up until day 60, from which point there is rapid loss of life. By contrast, the population illustrated by the red line suffers substantial numbers of deaths beginning at day 20. Thus many flies are dying at young and middle ages, rather than predominantly at old age. This is a sign of poor housing conditions or a genetically fragile stock.
3.2.2. protocol modifications for measuring the effects of dietary interventions
-
1.
repeat steps 1-3 from 3.2
-
4.
separate males from females and allocate target individuals to experimental containers (refer to note 40)
-
5.
store vials at 25ºC, 65% humidity, 12:12h light:dark
-
6.
transfer to fresh food every 2-3 days.
conduct egg counts as described in section 3.2
-
7.
at each transfer score deaths and censors until all flies are dead
3.2.3. protocol modifications for measuring effects of genetic interventions to modify lifespan
-
1.
repeat steps 1-3 from 3.2 (see note 41)
-
4.
separate males from females and allocate target individuals to experimental containers (refer to notes 42)
-
5.
store vials at 25ºC, 65% humidity, 12:12h light:dark
-
6.
transfer to fresh food every other day
conduct egg counts as described in section 3.2
-
7.
For each transfer, score deaths and censors until all flies are dead
3.2.4. modifications for measuring the effects of pharmacological interventions to alter lifespan
-
1.
repeat steps 1-3 from 3.2
-
4.
separate males from females and allocate target individuals to experimental containers (refer to notes 43)
-
5.
store vials at 25ºC, 65% humidity, 12:12h light:dark
-
6.
transfer to fresh food every other day
conduct egg counts as described in section 3.2
-
7.
For each transfer, score deaths and censors until all flies are dead
3.3. Data handling
For each lifespan, record the date on which the experiment started, the genotype and conditions used in the experimental setup as well as any notes about the experimental setup that will modify or help interpretation of the outcomes. For consensus guidelines on what constitutes the minimal information to be recorded for lifespan experiments, see (Ziehm et al., 2015).
Throughout the experiment, record deaths and censors for each vial for each day on which they were observed (see note 44)
These data can be used to generate lifespan curves for comparison using standard life table analyses (Lee and Wang, 2003)
An important recent advance has been the publication of an openly available database for storing lifespan data, called SurvCurv (Ziehm and Thornton, 2013; Ziehm et al., 2013, 2015). Users can upload data for secure storage as well as use an array of statistical tools to analyse the experimental outcomes. Additional tools available on the site allow the lifespans to be compared to others in the database and so can be used to aid further biological discoveries.
Acknowledgements
We would like to thank members of the Partridge and Piper laboratories who contributed helpful suggestions and tips. In particular: S. Grönke, T. Niccoli, A. Tillmann and N. Woodling. We acknowledge the following sources of funding: the Royal Society (UF100158 & RG110303; MDWP); the Wellcome Trust UK (098565/Z/12/Z), Max Planck Society and the European Research Council under the European Unions Seventh Framework Programme (FP7/2007-2013), European Research Council grant agreement 268739 (L.P.)
Notes
Automatic cookers with built-in stirrers like the Joni Multimix (Joni Foodline) are useful for standardising large volume cooks.
We use red grape juice that is designed for use in home wine production. Many laboratories use apple juice.
Our simple recipe of sugar and whole yeast lysate provides nutrition for optimal development and lifespan. Many alternatives exist, but not all are optimal (see supplement to (Piper and Partridge, 2007)). Most recently, we have described a standardized holidic diet that contains all necessary nutrients to support long life (Piper et al., 2014). It is important to note that our recipes contain the nutritional complement of whole yeast preparations, which cannot simply be replaced by water soluble yeast extract that does not support long life (Bass et al., 2007).
In a relatively cool climate where room temperatures do not exceed 22ºC, this can be overnight. If medium shrinks in vials and pulls away from the edges, this is a sign of over-drying.
Housing trays of vials / bottles in pillowslips as they cool is a useful way to protect them from stray flies.
Alternatives to cotton wool balls exist: for example polyurethane foam plugs (available from www.drosophilacenter.com) are mite resistant, retain their structure and are reusable after washing.
To avoid the need to plug hundreds of vials before storage, it is possible to seal trays with Glad® Press‘n Seal. If doing so, it is extremely important to ensure the seal is sound, there are no holes in the plastic film and all vials are covered to avoid both contamination and food from excessive drying when cooled.
a useful resource for equipment suitable for use in Drosophila research is the supplier: www.flystuff.com (a division of Genesee Scientific)
in situations where small numbers of parental flies are used for egg lays, it is more space and resource efficient to use small (~5cm diameter) petri dishes and cages.
we use tips from StarLab (Cat Number: E1011-5100), but it is also possible to cut back a standard pipette tip a few mm to make a wide opening.
back-crossing for 6 generations is, in almost all cases, sufficient to eliminate the confounding effects of genetic background. It should be noted that this should be performed to each laboratory’s own genetic stocks since even inbred lines with the same name will differ between laboratories (Berger et al., 2001).
If not experienced with fly handling, replacing the egg laying plate may require flies to be lightly anaesthetized with CO2.
it is important not to use too much live yeast for the egg collection plate as it interferes with egg collection. Nor do you want to use too little such that the yeast supply is exhausted. Aiming to have a small amount left at the egg lay is ideal. A cage of ~300 flies will consume ~1 tsp of live yeast overnight.
While overnight egg lays produce adequate synchronisation for most lifespan experiments, this egg collection window can be reduced.
To time the emergence of adults so that it falls on a weekday, transfer embryos to fresh food for development on a Friday. Emerging flies will be available on Monday, 9.5 days later.
If egg yield is a problem, the same parents can be used for an additional lay on a fresh plate containing live yeast.
Wide bore racked 200μl pipette tips are available from StarLab (Cat Number: E1011-5100). Without these, standard pipette tips can be cut back using scissors
A dense mass of eggs yields ~300 adult flies
before incubating, it is best to remove any leftover yeast paste from the egg laying plate as emerging larvae will burrow into it, making them hard to collect
this is the smallest of three larval stages
before transferring larvae into fresh media, make a dent in the food to make it easier to wipe off the larvae against a slope of food
for practical reasons, collecting larvae by picking is more manageable using 30ml vials containing ~7ml of SY food. Overcrowding can be avoided with 30-50 larvae.
At the larval densities recommended in this protocol, larvae will migrate to the cotton wool to pupate. If the container is not tightly plugged, the larvae will escape from the bottle
in order to collect virgin flies, check bottles at 9 days after egg transfer and clear any flies that have emerged. Check the bottle every ~4h for newly emerged flies – these will all be virgins. Transfer virgin flies to a cold, clean bottle on ice and sort males from females while they remain in a chill coma. CO2 should be avoided as the fly’s cuticle is immature, and exposure to the gas can lead to adverse effects on lifespan. Genders can only be distinguished with the use of a dissecting microscope to examine the genitalia.
It is best to transfer newly emerged flies without using CO2.
In order to anaesthetize a whole bottle of flies rapidly, fill a fresh empty (without food), dry bottle with CO2 and transfer all flies into it. When sorting anaesthetized flies, work on a perforated plate through which a stream of CO2 is passing. To avoid desiccating the flies, it is ideal to bubble the CO2 through water before it reaches the flies
When allocating experimental flies to treatment, ensure the representation of individuals from each rearing container is balanced between experimental treatments.
for standard experiments, 100 – 150 flies per treatment housed as groups of 10 - 15 flies per vial is a manageable size. The lifetime outcome for all flies per vial should be recorded. Although the population across all vials for a treatment is treated as one during analysis, this approach allows the performance of individual vials to be revisited if outliers are suspected.
experimental conditions can be blinded to the experimenter at this stage
fly lifespan varies with the size of their housing. Experiments comparing the lifespans of flies kept in 25ml versus 500ml flasks, but at a standard density per container volume, found that lifespan was significantly shorter in the larger volume flasks. Shorter lifespan was associated with higher levels of flying activity (Magwere et al., 2006).
There is a range of densities of flies per container that is optimum for lifespan. In a series of 30ml vials (Pearl, 1928) found the lifespan optimum to be for 2-15 flies per container and above this density saw a decrease in lifespan for each increase in population density.
As flies age and become frail, they have an increased risk of falling and becoming stuck in their food. They will also spend more time at the base of the vial. Storing the vials on their sides during lifespans, so the food is a vertical surface at one end of the vial rather than the floor, reduces the risk of these accidental causes of death.
Depending on experience with handling Drosophila, during the first two weeks of a lifespan experiment the flies may be too fast to transfer between vials without light anaesthesia. Males are more active and move more quickly than females and so are more likely to escape without anaesthesia. With practice and good technique, it should be possible to transfer flies without CO2.
When transferring flies, record deaths and censors (accidental deaths or escapees). Remember to note any dead flies transferred to new vials so that they can be deducted from the number of deaths recorded during the next transfer.
Some flies are bang sensitive, and appear to become mores so with age. These can appear dead during the disturbance of transfer. To avoid counting these as dead, first scan vials for deaths, then transfer all vials to new food and after that, check vials for dead flies transferred to fresh media.
To reduce labour and use of resources, it is possible to re-use the cotton ball that stoppers a vial by transferring it to the fresh vial to which flies are transferred. However, over time the cotton balls will deteriorate and so it is best to replace them at least once a month.
A sample timetable for transferring flies and counting eggs can be: transfer flies to fresh food on Mon, Wed and Fri afternoons, count eggs on Tue and Thur mornings.
Record the time the flies go on the food and the time at which eggs in the vial were counted.
For young flies, there may be a lot of eggs. If there are too many to count with 10-15 flies in a vial, consider setting up a parallel cohort of flies with fewer females per vial. These flies will not contribute information to the lifespan experiment (as their density of housing is different from those in the experiment) and they can be discarded when egg counting is complete.
To control for rearing conditions in larger experiments, it is good practice to use one rearing bottle per experimental replicate vial. For example: for an experiment with15 vials of flies per food type, generate 15 rearing bottles; when allocating flies to treatment, anaesthetize rearing bottle 1 and allocate 10 flies to experimental vial number 1 for treatment A, then B, C, D and so on until all treatments have one vial populated from the same rearing bottle. Repeat this system with a new rearing bottle for the second replicate vial for each treatment.
Some genetically modified lines will have altered (usually longer) development time. In order to synchronise the start of the lifespan experiment, initiate the parental crosses for the retarded lines so that egg collection is performed before that of the non-delayed lines. To buffer against slight variations in the delay, it is best to rear multiple batches of the experimental generation, derived from consecutive days of egg laying. This way it will be possible to collect flies from all lines that have emerged within 24h of each other.
It may not be possible to control for rearing conditions when using different genotypes in the same way as for single genotypes between multiple experimental foods. However, if the genetic scheme allows, it may be possible to use sibling flies as controls for experimental flies. Alternatively, it may be possible to rear multiple genotypes in a single rearing container. However, it is important to determine first that these larval conditions to not interact with lifespan outcomes.
To control for rearing conditions, use the protocol employed for testing the effects of multiple food types on one genotype (note 41).
Each laboratory has its own method of recording and plotting these data. An excel sheet used in our laboratories can be found at: www.ucl.ac.uk/iha/people/matt-piper/protocols_links_downloads. More sophisticated and automated packages can be found through the Pletcher laboratory (see (Linford et al., 2013) and associated URLs)
References
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc Biol Sci. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Lee ET, Wang J. Statistical methods for survival data analysis. New Jersey: John Wiley & Sons; 2003. [Google Scholar]
- Loeb J, Northrop JH. Is There a Temperature Coefficient for the Duration of Life? Proc Natl Acad Sci USA. 1916;2:456–457. doi: 10.1073/pnas.2.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–52. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Partridge L, Andrews R. The effect of reproductive activity on the longevity of male Drosophila melanogaster is not caused by an acceleration of ageing. Journal of Insect Physiology. 1985;31:393–395. [Google Scholar]
- Partridge L, Farquhar M. Sexual activity reduces lifespan of male fruitflies. Nature. 1981;294:580–582. [Google Scholar]
- Partridge L, Gems D. Benchmarks for ageing studies. Nature. 2007;450:165–167. doi: 10.1038/450165a. [DOI] [PubMed] [Google Scholar]
- Partridge L, Fowler K, Trevitt S, Sharp W. An examination of the effects of males on the survival and egg-production rates of female Drosophila melanogaster. 1986;32:925–929. [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. Journal of Insect Physiology. 1987;33:745–749. [Google Scholar]
- Partridge L, Alic N, Bjedov I, Piper MD. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp Gerontol. 2011;46:376–381. doi: 10.1016/j.exger.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl R. The Biology of Death--VI. Experimental Studies on the Duration of Life. The Scientific Monthly. 1921;13:144–164. [Google Scholar]
- Piper MD, Partridge L. Dietary restriction in Drosophila: delayed aging or experimental artefact? PLoS Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgrò CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- Wigby S, Slack C, Grönke S, Martinez P, Calboli FC, Chapman T, Partridge L. Insulin signalling regulates remating in female Drosophila. Proc Biol Sci. 2011;278:424–431. doi: 10.1098/rspb.2010.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehm M, Thornton JM. Unlocking the potential of survival data for model organisms through a new database and online analysis platform: SurvCurv. Aging Cell. 2013;12:910–916. doi: 10.1111/acel.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehm M, Piper MD, Thornton JM. Analysing variation in Drosophila aging across independent experimental studies: a meta-analysis of survival data. Aging Cell. 2013;12:917–922. doi: 10.1111/acel.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehm M, Ivanov DK, Bhat A, Partridge L, Thornton JM. Bioinformatics (Oxford, England) 2015. SurvCurv database and online survival analysis platform update. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan BJ, Bijlsma R, Hoekstra RF. On the developmental theory of ageing. I. starvation resistance and longevity in Drosophila melanogaster in relation to pre-adult breeding conditions. Heredity. 1991;66(Pt 1):29–39. doi: 10.1038/hdy.1991.4. [DOI] [PubMed] [Google Scholar]