Abstract
Son of Sevenless 1 (SOS1) is a dual guanine nucleotide exchange factor (GEF) that activates the small GTPases RAC and RAS. While the molecular mechanisms of RAS GEF catalysis have been unveiled, how SOS1 acquires RAC GEF activity and what is the physio-pathological relevance of this activity is much less understood. Here, we show that SOS1 is tyrosine phosphorylated on Y1196 by ABL. Phosphorylation of Y1196 controls SOS1 inter-molecular interaction, is required to promote the exchange of nucleotides on RAC in vitro, and for PDGF activation of RAC and RAC-dependent actin remodeling and cell migration. SOS1 is also phosphorylated on Y1196 by BCR-ABL in chronic myelogenous leukemic (CML) cells. Importantly, in these cells, SOS1 is required for BCR-ABL-mediated activation of RAC, cell proliferation and transformation in vitro and in a xenograft mouse model. Finally, genetic removal of Sos1 in bone marrow derived cells (BMDC) from Sos1fl/fl mice and infected with BCR-ABL causes a significant delay in the onset of leukemogenesis once BMDC are injected into recipient, lethally irradiated mice. Thus, SOS1 is required for full transformation and critically contribute to the leukemogenic potential of BCR-ABL.
Introduction
SOS1 plays a central role in signal transduction from receptor tyrosine kinases (RTK) to small G proteins RAS and RAC1–4. Structural and biochemical studies revealed that SOS1 is a multi-domains protein with extensive intramolecular interaction that tightly constrains its activity5. For example, SOS1 possesses two binding sites for RAS, one of which is an allosteric site that is distal to the active one6. Binding of active RAS at this site stimulates the nucleotide exchange activity of SOS1 by causing conformational changes that allow substrate RAS to bind2, 7–9.
An additional important domain is the DH-PH (Dbl and Pleckstrin Homology domain), which is a structural hallmark for exchange factors of the Rho family of GTPases, and was proposed to act as a RAC-specific GEF10–20. However, the DH-PH unit is folded into a closed conformation, implying that SOS1 RAC GEF activity is auto-inhibited and requires specific molecular events to be activated8, 21, 22. Two distinct mechanisms of SOS1 activation have been proposed: i) SOS1 must assemble into a multimolecular complex with EPS8, ABI1 to become catalytically active12, 17–20. The second mechanism, instead, involves tyrosine phosphorylation of SOS1 by ABL kinase15. However, how tyrosine phosphorylation induces SOS1 RAC GEF activity and its pathophysiological relevance remains ill defined.
Physiologically, ABL kinase participates in RTK-induced actin cytoskeleton remodeling, a signaling pathway in which the function of the SOS1-RAC axis is pivotal15. Hyperactive, uncontrolled kinase activity of ABL is also responsible for its oncogenic potential. The p210-BCR-ABL fusion oncoprotein is, indeed, necessary and sufficient to cause Chronic Myelogenous Leukemia (CML)23, 24. Although the role of BCR-ABL in CML has been well studied, the critical signaling pathways mediating its transforming ability are incompletely clarified. Among them, the one leading to the activation of RAC GTPases critically mediates BCR-ABL-transformation25–27. Indeed, RAC3, one of the three mammalian RAC isoforms, is activated in p190-BCR-ABL malignant precursor B-lineage lymphoblast and its removal increases survival in p190-BCR-ABL-transgenic, RAC3-null mice [a model of Acute Lymphoid Leukemia (ALL)]28. Additionally, the ubiquitously expressed RAC1 and hematopoietic-restricted RAC2 were shown to be critical for p210-BCR-ABL-mediated leukemogenesis and myelo-proliferative disease29. How RAC becomes activated by ABL, thus, becomes a key question to be addressed. SOS1, which is expressed in myeloid cells and is regulated by ABL15 may serve this role.
Here, we show that phosphorylation of Y1196 on the C-terminal proline rich region of SOS1 is sufficient to elicit its RAC GEF activity in vitro and in vivo in response to the activation of various receptor and non-receptor tyrosine kinases, including ABL. We further demonstrate that Y1196 of SOS1 is phosphorylated in BCR-ABL leukemic human and murine blasts and is required for full RAC activation, cell proliferation and transformation in vitro and in mouse models. Genetic removal of SOS1 delays the onset of BCR-ABL leukemogenesis.
Material and Methods are described in details in Supplementary Information
Results
SOS1 is tyrosine phosphorylated on Y1196 in vitro
To investigate the molecular, biochemical and functional consequences of ABL-mediated tyrosine phosphorylation of SOS1 we sought to identify the target tyrosine(s). As an initial approach, we subjected full length SOS1 and various recombinant shorter fragments to an in vitro kinase assay with ABL kinase (Figure S1A-top). Only the fragment encompassing the C-terminal proline-rich region was efficiently tyrosine phosphorylated (Figure S1A), hence restricting the region targeted by ABL.
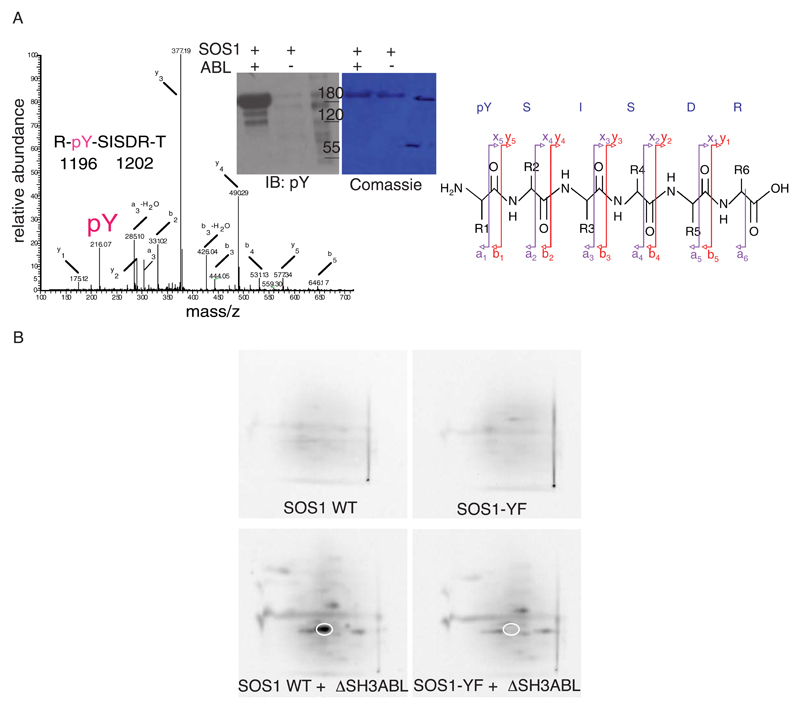
Next, we used in vitro tyrosine phosphorylated SOS1 and titanium Oxide (TiO2)-based affinity chromatography to enrich for phosphorylated peptides coupled with nano LC-MS/MS analysis30, 31(Figure 1A). We generated a peak list, which was searched against the IPI human database using Mascot 2.0. However, no pY-containing peptide could be identified automatically by Mascot search, but spotted, instead, by searching the MS/MS dataset for pTyr immonium ion at m/z 216.04332, 33 and subsequently sequenced manually. This procedure revealed Y1196, located in the proline-rich C-terminus of SOS1, as the major putative ABL kinase target (Figure 1A). We validated this result using 2D phospho-peptide mapping that revealed a single, major phosphorylated spot derived from WT-SOS1, but not from Y1196F-SOS1 mutant (Figure 1B). Thus, tyrosine 1196 is the major ABL phosphorylation site of SOS1 in vitro.
Figure 1. Tyrosine 1196 of SOS1 is the major target residue of ABL kinase.
A. SOS1 immuno-purified from SOS1-expressing 293T cells (1 mg lysates) with an anti-SOS1 antibody was in vitro phosphorylated with ABL kinase, resolved on SDS-Page and detected by Coomassie staining or by immunoblotting (IB) using the indicated antibody. SOS1 band was digested with trypsin. Phosphorylated peptides were enriched with Titanium Oxide beads and analyzed by tandem mass spectrometry. Left: the diagram shows the detected fragments of the phosphorylated pYSISDR peptide. Fragments are presented according to their mass/charge ratio (m/z) and their intensity (Relative abundance). The intensity of the highest peak corresponds to a relative abundance of 100%. Right: The possible fragmentation of the peptide is shown. The peak (pY) in the diagram (left) with an m/z of 216,07 corresponds to the a1 fragment on the right. Mw markers are indicated on the side of the immunoblots.
B. WT-SOS1 and Y1196F-SOS1 immunopurified from SOS1 overexpressing 293T cells (1mg lysate) were incubated in the presence or absence (Control) of ABL kinase and 32P-ATP and subjected to SDS-PAGE. After PonceauS staining, the SOS1 band was excised and digested with trypsin. Oxidized peptides were separated by electrophoresis and thin layer chromatography.
SOS1 is tyrosine phosphorylated on Y1196 in vivo
To assess whether Y1196 is phosphorylated in vivo, we undertook two distinct approaches. Firstly, we ectopically expressed WT-SOS1 or Y1196F-SOS1 mutant in combination with an activated form of ABL (ABL-ΔSH3)34 in 293T cells. Immunoblotting with anti-pY antibodies of SOS1 immunoprecipitates revealed that tyrosine phosphorylation of Y1196F-SOS1 was significantly reduced with respect to WT-SOS1 (Figure S1C). Next, we raised monoclonal, anti-pY1196 antibodies the specificity of which was tested using WT- and Y1196-SOS1 in various assays (Figure S2A-B). We detected Y1196 phosphorylation in lysates of cells expressing activated ABL and WT-SOS1, but not Y1196F-SOS1 mutant, both when we immuno-precipitated total SOS1 with anti-HA antibody, followed by immunoblotting with anti-pY1196 or vice versa (Figure S3A). Additionally, since SOS1 and ABL act on RTK-dependent signaling pathways15, 35, 36, we found that PDGF stimulation efficiently phosphorylated endogenous SOS1 on Y1196 in mouse embryo fibroblasts (MEF) (Figure S3B). Similarly, stimulation of ERBB family members with either Heregulin or EGF in breast cancer cell lines increased Y1196-SOS1 phosphorylation, which was abrogated by the ABL kinase inhibitor Imatinib (STI571) (Figure S3B). We concluded that phosphorylation of Y1196 occurs physiologically in response to a variety of RTK-dependent stimuli, presumably through ABL kinase activity.
Phosphorylation of Y1196 is sufficient to promote the exchange of nucleotides on RAC1, but not on H-RAS in vitro
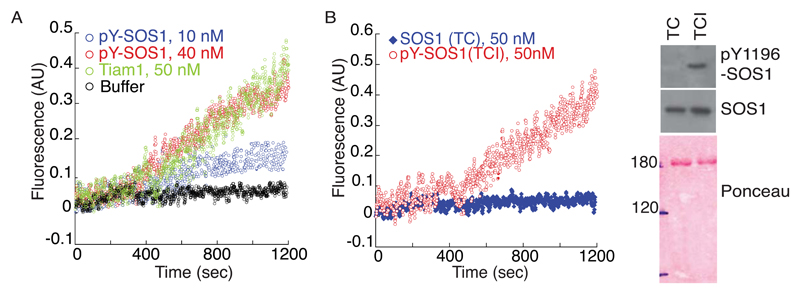
Next, we tested GEF activities on RAC1 and H-RAS of tyrosine phosphorylated SOS1. Tyrosine phosphorylated SOS1, purified from cells co-expressing activated ABL, but not Y1196F-SOS1 mutant displayed detectable RAC1-GEF activity in in vitro assays (Figure S4A-B). Importantly, under the same conditions, WT- and Y1196F-SOS1 showed similar levels of H-RAS-GEF activity, indicating that tyrosine phosphorylation specifically affects RAC1, but not H-RAS-GEF activity (Figure S4A). To confirm that the Y1196F mutation abrogates ABL-mediated SOS1 RAC1 GEF activity, we performed a double affinity purification of SOS1 from lysates of cells expressing activated ABL and SOS1 using a GRB2-GST column in tandem with an anti-pY1196 antibody-protein G-sepharose column (Figure S4C). The final elution step with phosphorylated antigenic SOS1 peptides yielded highly purified pY1196-SOS1 (Figure S4C). Using a fluorescent–nucleotide-based RAC1-GEF assay, we showed that pY1196-SOS1, promoted guanine nucleotide exchange on RAC1 at catalytic concentrations, similarly to the DH-PH domain of TIAM1, used as positive control (Figure 2A). Finally, de-phosphorylation of pY-SOS1 with a tyrosine-specific phosphatase, abrogated its RAC1-GEF activity (Figure 2B). Thus, tyrosine phosphorylated SOS1 acts as a RAC1-GEF in vitro.
Figure 2. Phosphorylation of Y1196 by ABL is sufficient to elicit SOS1 RAC GEF activity.
A-B. MANT-GDP-fluorescence RAC GEF assay of purified pY1196F-SOS1. GST-RAC1 was equilibrated with 2 μM MANT-GDP. After 300s, we added: (B) purified, phospho-enriched SOS1 (30 µl and 120 µl, corresponding to ~ 10 and 40 nM, respectively) or 50 nM of His-DH-PH of Tiam1 (used as positive control). Buffer indicates the non-stimulated nucleotide exchange rate of RAC1: (C) purified, phospho-enriched SOS1 or un-phosphorylated SOS1 [treated with T-cell specific tyrosine phosphatase either in the presence (TCI) or the absence (TC) of T-cell phosphatase inhibitor]. The reaction containing MANT-GDP was excited at 360nm. The emission of MANT-GDP-bound to RAC was measured at 440nm. Right panels: Aliquots of phosphorylated (TCI) and phosphatases dephosphorylated (TC) SOS1 was immunoblotted with the Abs indicated on the right.
SOS1 inter-molecular interaction is inhibited by activated ABL
These findings raise the question as to the underlying molecular mechanism. Crystallization studies revealed that SOS1 is engaged into extensive intra-molecular and possibly inter-molecular interactions8, 37. However, none of the solved structures of SOS1 includes the proline-rich region, which contains Y1196. We hypothesized that also this region undergoes intramolecular or intermolecular interactions with other SOS1 domains. We initially tested the ability of various, immobilized SOS1 fragments to bind the soluble proline-rich region in far western assays. We found that SOS1 proline-rich region binds with a micromolar (apparent) affinity to its DH-PH domain, and further restricted the region of interaction to the PH domain (Figure S5A-C). The SOS1 PH domain has been proposed to exert an inhibitory effect on the DH domain by blocking its accessibility to RAC122. A significant change in the fold must therefore be invoked for this domain to become catalytically active. Loss of binding of the proline-rich region to the DH-PH domain may be implicated in initiating these structural changes. We, thus, performed a binding assay between the DH-PH domain and either the phosphorylated or a non-phosphorylated Y1196-containing peptide. The phosphorylated peptide bound the DH-PH domain with a much lower apparent affinity (Kd > 200 μM), as compared to the non-phosphorylated peptide (Kd ~ 30 μM) (Figure S5D). These findings are also compatible with the possibility of trans, rather than cis interaction between two SOS1 molecules, where the proline rich region of one molecule may bind to the DH-PH domain of the other. If this were the case then, a fraction of SOS1 may form dimers, as previously suggested37, whose stability should be controlled by Y1196 phosphorylation. Consistently, by exploiting differentially HA- and GFP-tagged SOS1 constructs we showed that: i) a sizable fraction of HA-tagged SOS1 co-immunoprecipated with GFP-SOS1 (Figure S5E); ii) the concomitant expression of an activated ABL robustly reduced this inter-molecular interaction (Figure S5E); iii) mutation of Y1196F that prevents ABL-mediated phosphorylation and the acquisition of RAC1-GEF activity also inhibited the inter-molecular interaction (Figure S5F). These results indicate that SOS1 needs to be in its tyrosine phosphorylated, monomeric form to act as RAC1 GEF. A corollary of this mode of action is that a SOS1-Y1196F mutant should not only be deficient in RAC1 activation in vitro (as we showed above), but may also act as a dominant negative. Consistently, the expression of SOS1-Y1196F completely inhibited RAC1-GTP levels in SOS1 proficient cells, acting in a dominant negative fashion (Figure S5G).
While more work is needed to precisely decipher the sequence of molecular events, the sum of this finding indicates that tyrosine phosphorylation of Y1196 promotes the dissociation of SOS1 inter-molecular interaction, resulting in the formation of monomers which, either alone or through subsequent incorporation into larger molecular weight complexes (see discussion), leads to optimal activation of SOS1 RAC1-GEF.
Phosphorylation of SOS1 Y1196 is required for optimal PDGF-dependent activation of RAC1, RAC1-dependent actin remodeling and migration
To validate the physiological relevance of Y1196 phosphorylation of SOS1 in controlling the activation of RAC, we measured the cellular levels of GTP-bound RAC. Initially, we used cells co-expressing either WT- or Y1196F-SOS1 together with activated ABL. Activated ABL increased RAC1-GTP levels, which are not significantly altered by the concomitant expression of SOS1, indicating that the latter protein is not a limiting factor in this pathway (Figure S6A). Conversely, co-expression of activated ABL and Y1196F-SOS1 robustly reduced ABL-mediated RAC activation (Figure S6A), consistent with Y1196F-SOS1 exerting a dominant negative function.
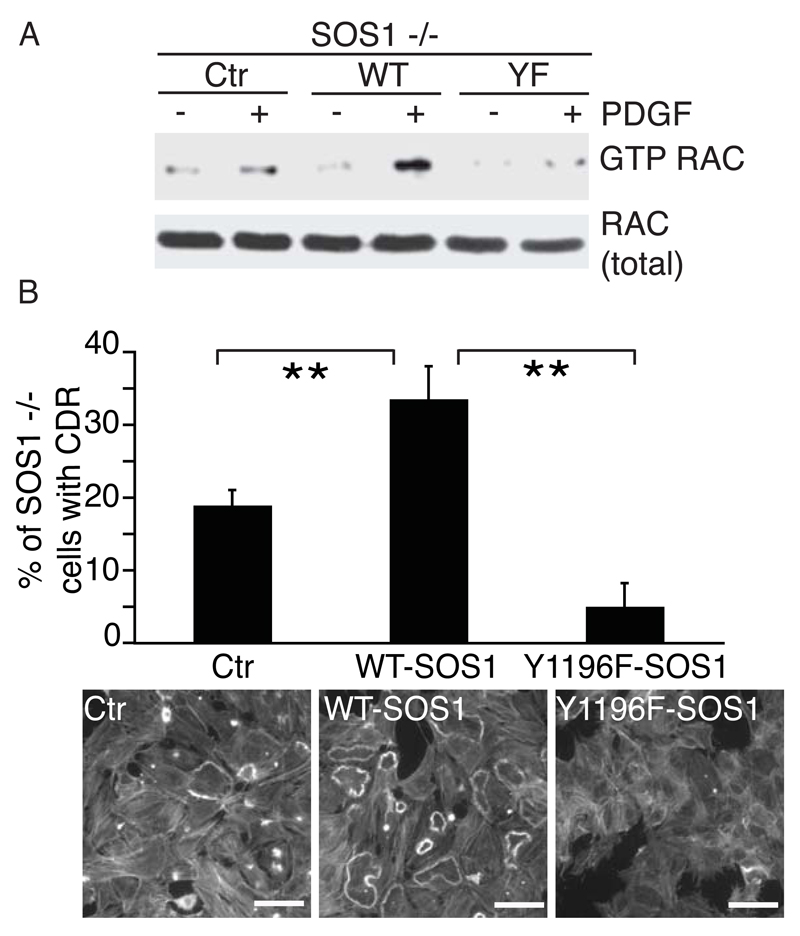
Next, we assessed whether this post-translational modification impacted on RAC activation in response to PDGF stimulation15, 35 using Sos1-/- mouse embryo fibroblasts (MEF) reconstituted with either WT- or Y1196F-SOS1 (Figure S6B). Sos1-/- MEF showed reduced RAC-GTP levels as compared to Sos1-/- MEF reconstituted with WT-SOS1 (Figure 3A). Conversely, the expression of Y1196F-SOS1 did not restore RAC activation (Figure 3A). Notably, PDGF-induced ERK and AKT activities were unaffected by either SOS1 removal or the expression of Y1196F-SOS1 mutant (Figure S6C). In agreement with the observation that pY1196 of SOS1 mediates not only RAC activation, but also PDGF-induced, RAC-dependent actin remodeling, Sos1-/- MEFs were impaired in the formation of PDGF-induced circular dorsal ruffles (CDR) (Figure 3B), where SOS1 localizes (Figure S6D). Circular dorsal ruffles are RAC-dependent migratory and endocytic structures that mark the acquisition of a mesenchymal mode of cell locomotion38. This response was restored by WT-SOS1, but not Y1196F-SOS1 (Figure 3B). Notably, Y1196F-SOS1 expression not only failed in rescuing CDR, but also inhibited their formation (Figure 3B). The specific requirement of SOS1 as a RAC-GEF in PDGF-induced actin remodeling is underscored by the finding that functional interference with VAV, a RAC-GEF family protein regulated by tyrosine phosphorylation, had no effect on CDR formation (Figure S6E). Finally, Sos1-/- MEFs were impaired in cell motility, which was increased by the expression of WT, but not of Y1196-SOS1 (Figure S6F and Movie S1). Thus, SOS1 and phosphorylation of Y1196 are required for optimal RAC1 activation and RAC1-dependent actin remodeling and cell motility in response to PDGF stimulation.
Figure 3. Phosphorylation of SOS1-Y1196 is required to promote optimal RAC activation, RAC-dependent actin remodeling in fibroblasts in response to PDGF.
A. Wild type-, but not Y1196F-SOS1 reconstitutes RAC activation in Sos1-/- MEFs. Serum starved Sos1-/- MEFs, reconstituted with HA-WT-SOS1 or HA-Y1196F-SOS1 (YF) or empty vector, as control (Ctr) (see Figure S6A), were stimulated with 10 ng/ml of PDGF for 7 min. Activated, RAC-GTP was pull down from 1mg of total lysates using 20 μg of recombinant GST-CRIB. Lysates (20μg) and bound proteins were immunoblotted with the indicated Abs.
B. Serum-starved Sos1-/- MEFs, reconstituted with HA-WT-SOS1 or HA-Y1196F-SOS1 or empty vector (Ctr), were stimulated with 10 ng/ml of PDGF for 7 min. Cells were stained for F-actin with Tritc-Phalloidine. The number of cells with CDR is expressed as percent of total. At least 100 cells were scored for each condition. The data represents the mean ± s.e.m (n=4 performed in triplicates). Right panels: Representative pictures of MEFs forming CDR. Bar, 40 μm.
SOS1 is phosphorylated by oncogenic BCR-ABL in leukemic blast and is required for BCR-ABL-mediated full transformation
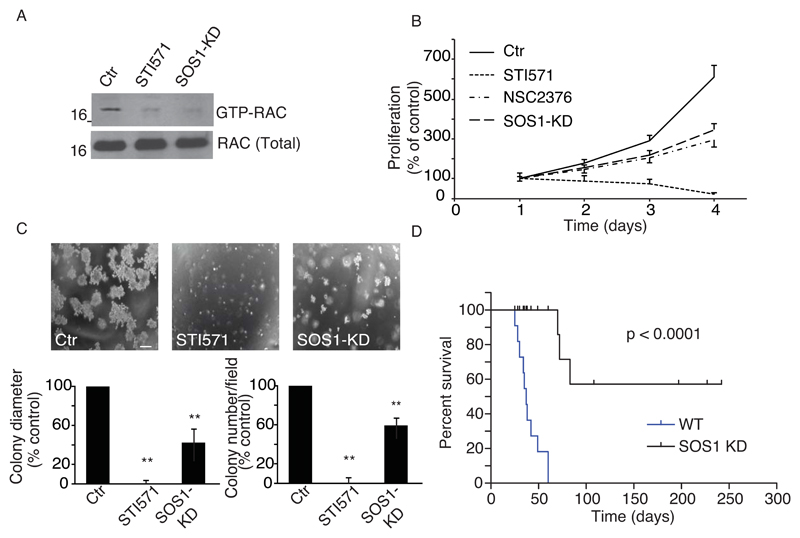
We next assessed whether phosphorylation of SOS1 Y1196 might also be critical in pathological conditions characterized by a hyperactive ABL kinases activity. The fusion oncoprotein BCR-ABL originating from a balance chromosome translocation leading to CML is a case in point39. Among BCR-ABL-stimulated oncogenic pathways the one leading to activation of RAC proteins has been shown to be pivotal for full transformation25, 29, 40. We thus tested whether SOS1 contributes to BCR-ABL-mediated RAC activation and transforming potential. Using pY1196-phospho-specific antibody, we showed that SOS1 is tyrosine phosphorylated in K562, a human cell line derived from a CML patient, and in BCR-ABL-expressing murine hematopoietic progenitor 32D cell line (Figure S7A-B). The administration of the ABL kinase inhibitor STI571 abolished SOS1 phosphorylation in both cell lines (Figure S7B). Next, we generated K562 cells stably knocked down for SOS1 (SOS1-KD) with 3 independent shRNAs (see also Suppl. Methods). SOS1 silencing reduced RAC-GTP levels, similar to STI571 treatment (Figure 4A), but had no impact on ERK1/2 activity (Figure S7C). Additionally, SOS1-KD K562 cells had a significantly reduced rate of proliferation compared to scramble-transfected control cells (Figure 4B), but similar to the one achieved by treatment with the RAC inhibitor, NSC27633 (Figure 4B). Notably, treatment with STI571 was, as expected39, more effective in reducing cell proliferation (Figure 4B), also as a consequence of increase cell death, which instead was not observed after SOS1 removal or RAC inhibition (Figure S7D). The slower proliferation rate of SOS1-KD K562 cells was also accompanied by increased expression of hemoglobin, a prototypical erythroid marker (Figure S7E).
Figure 4. SOS1 phosphorylation is required for RAC1 activation and BCR-ABL-mediated full transformation.
A. K562 cells infected with pSuperRetroPuro Control vector (Ctr) or pSuperRetroPuro SOS1 interfering vector (SOS1-KD) were treated for 10 min with 10 μM STI571 or vehicle as control (Ctr). We used 3 independent shRNA to generate 3 mass SOS1-KD populations (see Suppl. Methods) that gave similar results. Cell Lysates were incubated for 1h with 20 μg of GST-CRIB to pull down GTP-loaded RAC and immunoblotted to detect activated RAC.
B. An equal number (50.000 cells/ml) of K562 control cells (Ctr), STI571-treated, NSC2376-treated or SOS1-KD cells (SOS1 KD) were plated and counted at the indicated times. Data are the mean ± s.e.m. of 4 independent experiments done in triplicates.
C. An equal number (100.000 cells/ml) of control (Ctr), or STI571-treated, or SOS1-KD K562cells (SOS1 KD) were plated in soft agar. Upper panels, representative pictures of the colonies obtained after 14 days. Lower graphs, quantification of colony number (left) and colony diameter (right). At least 50 colonies were counted. Data are the mean ± s.e.m (n=3 independent experiments). ** = P < 0.005 Student’s t-test. Bar, 200 µm.
D. An equal number (5×105) of control and SOS-1 KD K562 were injected into the tail vein of immunodeficient mice. Mice were monitored for survival (n=15 of a representative experiment run in duplicate). P-values (Pearson) were measured by Chi Square test.
Next, we tested weather SOS1 ablation impairs transformation and tumorigenesis. In soft agar growth assays, under conditions in which STI571 treatment completely abrogated transformation, SOS1 silencing significantly reduced both the number and the size of colony as compared to untreated, control K562 cells (Figure 4C). In xeno-transplantation mouse model, NOD/SCID mice injected with control K562 rapidly died due, as expected (Figure 4D), to the expansion of leukemic blasts (not shown). The removal of SOS1 from K562 cells drastically increased mice survival (Figure 4D).
To provide direct evidence that the above phenotypic alterations are caused by the loss of Sos1 and of Y1196 phosphorylation, we restored the expression of either WT or Y1196F-SOS1 mutant into SOS1-KD K562 cells using shRNA-resistant variants fused to GFP. GFP-WT and GFP-Y1196F-SOS1 expression levels were comparable to those of endogenous SOS1 of control K562, while endogenous SOS1 levels in the ectopically reconstituted cells remain significantly down-regulated (Figure S8A). Re-expression of WT-SOS1, but not of Y1196F-SOS1 mutant reduced hemoglobin, suggesting that differentiation blockade was restored (Figure S8A), restored RAC-GTP levels (Figure S8B), cell proliferation (Figure S8C) and transformation potential (Figure S8D).
The transient nature of SOS1 knocked down and Y1196F-re-expression, which were counter selected and lasted only for a limited number of passages (similar data were obtained using CRISPR-SOS1-KO K562 cells –not shown), prevented us to assess whether also in vivo we could restore leukemogenesis. To overcome this limitation, we employed a murine model in which the loss of Sos1 can be induced by tamoxifen (TAM)-mediated CRE recombination (Herein on referred to as Sos1fl/fl 41). BMDC from control and Sos1fl/fl mice were retro-virally infected with EGFP-p210-BCR-ABL-fusion gene-expressing retroviral vectors (MigBCR-ABLp210), treated with TAM to induce Sos1 deletion, and tested in vitro and in vivo to assess BCR-ABL transforming and leukemogenic ability (Figure 5). Consistently with the results obtained with human K562 cells, genetic removal of Sos1 significantly reduced colony formation (Figure S9A), without altering the immunophenotypes of BCR-ABL-transformed Sos1 null BMDC as compared to control cells (Figure S9A). More relevantly, tamoxifen-mediated Sos1 loss of BCR-ABL-infected Sos1fl/fl BMDC significantly delayed the development of leukemia upon BMDC transplantation into recipient mice (Figure 5A-C), without affecting engraftment (Figure S9B-C) and stemness potential of the leukemic cells (Figure S9E-G).
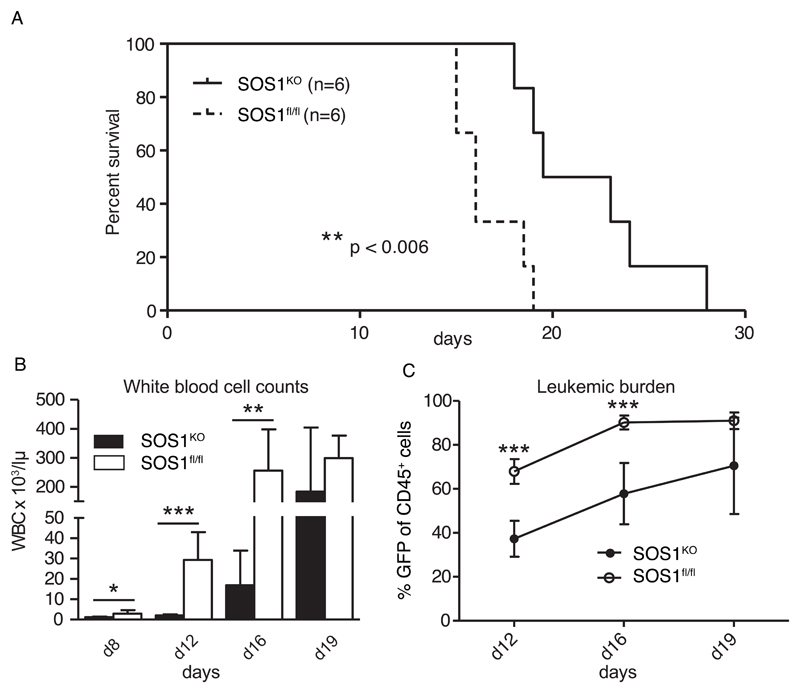
Figure 5. Sos1 deletion abolishes BCR-ABL induced transformation of HSCs in vitro and delays leukemogenesis in vivo.
A. 5-FU enriched BMDCs were retrovirally-infected with pMigBCR-ABLp210 before being treated with (SOS1KO) or without (SOS1fl/fl) tamoxifen and transplanted into lethally irradiated recipient mice. Recipient mice were injected i.p. with tamoxifen or solvent for two days after TX to induce Sos1 deletion and monitored for leukemia induction. Tamoxifen had no effect on cell engraftment or leukemic development (not shown). In addition, it did not affect the efficiency of infection, which was about 20% in the presence or the absence of TX (not shown). Kaplan-Meier plot details the overall survival of transplanted mice (n=6) of 2 independent experiments. P value was determined by Log-rank Test.
B-C. Peripheral blood measurement of mice that received a Sos1 deleted BMDC transplant showed a significantly reduction in leukemic white blood cells (C) gain as well as a reduced leukemic burden (D) of Sos1 deleted BMDCs transplanted mice during the course of disease. Results are from a representative transplantation experiment (n=6) out of two independent ones at the indicated time points. Leukemic burden was measured by the percentage of EGFP positive CD45 cells in the PB. P-values were determined by Students t-test: * p < 0.05; ** p < 0.01; *** p < 0.005.
Collectively, our result supports the notion that SOS1 is phosphorylated by oncogenic BCR-ABL in leukemic cells, is required for BCR-ABL-mediated full transformation and contributes to leukemogenesis.
Discussion
In this work, we provide evidence that SOS1 is phosphorylated by ABL on Y1196. This post-translational modification endows SOS1 with the capacity to activate RAC in response to physiological stimulation of RTKs and in BCR-ABL pathological context. In the former, case an RTK-ABL-pY1196-SOS1 pathway operates to control RAC-mediated actin-based protrusions leading to enhanced directed migration, consistent with a role of ABL and RAC in these processes42, 43. A BCR-ABL-pY1196SOS1-RAC axis is, instead, critical to promote full transforming and leukemogenic ability of the fusion protein.
Our findings are of relevance both for the understanding of the molecular mechanisms through which SOS1 RAC-GEF activity is controlled and further unveil an unexpected role of SOS1-RAC in BCR-ABL transformation. How SOS1 acquires RAC-GEF activity has remained elusive and difficult to reconcile with the finding that the isolated DH-PH domain of SOS1 folds into a catalytically inactive conformation22, 37. A substantial conformational change of the DH-PH domain must occur for SOS1 to become catalytically proficient. Our findings suggest that phosphorylation of Y1196 may be a way to promote such structural rearrangements. Consistently, we found that the isolated proline–rich region binds directly to the DH domain through residues encompassing Y1196. Additionally, phosphorylation of Y1196 reduces this interaction, induces SOS1 RAC-GEF in vitro and is required to elevate RAC-GTP levels in vivo. Thus, while we cannot exclude that Y1196 might be the binding site of yet-to-be found regulators of SOS1, a plausible possibility is that phosphorylation of this tyrosine is necessary to liberate an inhibitory molecular proline–rich/DH-PH interaction. The latter interaction may not necessarily occur intra-molecularly and might drive the formation of inter-molecular interactions. Within this context, ABL-mediated phosphorylation of Y1196 disrupt this linkage, leading to liberation of monomeric SOS1, that may, in turn, become incorporated into large macromolecular complex, necessary for full activation of its RAC1 GEF. Indeed, we have previously shown that one additional way to modulate SOS1 RAC-GEF is via assembly into a macromolecular complex that contains EPS8 and ABI112, 18, 19. Intriguingly, the latter protein is an interactor and activator of ABL44, 45, suggesting that SOS1 tyrosine phosphorylation may ensue following the assembly of this complex. In keeping with this notion, preliminary evidence indicates that ABL co-immunoprecipates with SOS1 and EPS8 in an ABI1-dependent manner. Under this condition, ABL activity is elevated and SOS1 becomes phosphorylated on tyrosine: an event that might be critical for promoting the RAC-GEF activity of the macromolecular complex.
Biochemical and genetic studies in mice models have unequivocally shown that the RAC GTPases are critical downstream signaling targets used by BCR-ABL to promote its full leukemogenic and transforming potential25, 28, 29, 40, 46. Mechanistically, the GEFs VAV1, 2 and 3 have been shown to biochemically link BCR-ABL to RAC proteins47. Indeed, VAV proteins are tyrosine phosphorylated and activated by ABL42, 47, 48. Their genetic loss, however, results in complex BCR-ABL-dependent pathological phenotypes and delays, but does not abrogate BCR-ABL leukemogenesis49, suggesting that other GEFs must play important roles. Our findings are consistent with the latter notion by showing that SOS1 is one additional critical exchange factors mediating BCR-ABL-dependent activation of RAC proteins and transforming ability. In none of our assays, we could distinguish whether tyrosine phosphorylated SOS1 displays any preference for the different RAC proteins. However, it would not be unfeasible that different GEFs target specific GTPases (different RACs in this case), in turn required to control a define set of biological outputs. In support of this contention, we noticed, that both RAC2 and VAV3 deficiency reduced cell proliferation and increased apoptosis, possibly interfering with phosphorylation of the pro-apoptotic BAD protein40, 49. Loss of Sos1, instead, reduces cell proliferation, but has no effect on apoptosis, while pushing K562 cells to acquire expression of hemoglobin. K562 cells are bi-potent progenitor cells, which are blocked in the differentiation program due to the expression of the BCR-ABL50. Thus, removal of SOS1 may induce these cells to acquire erythrocyte lineage properties, suggesting that SOS1 is required for cell proliferation and, possibly for the maintenance of a more undifferentiated state, rather than controlling apoptosis.
In summary, our data reveal a novel molecular mechanism through which the poorly studied SOS1 RAC-GEF activity becomes activated by BCR-ABL, contributing to its pathogenic, transforming functions. They further suggest that targeting this interaction and signaling axis might be beneficial in combination with BCR-ABL pharmacological treatment to prevent or delay the onset of BCR-ABL resistance, at least in part, by reducing the proliferation potential of leukemic progenitors.
Supplementary Material
Key points.
ABL-mediated phosphorylation of Y1196 SOS1 elicits its RAC GEF activity in vitro and in vivo
A SOS1pY1196-RAC axis delays the onset of BCR-ABL leukemogenesis
Acknowledgments
This work has been supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC #10168 and # 18621), the Italian Ministries of Education- University-Research (MIUR-PRIN- 2009X23L78), the International Association For Cancer Research (AICR-09-0582 & 14-0335), the CARIPLO Foundation (#2010-0737); the European Research Council (Advanced-ERC#268836). AL Illert was supported by a Grant from der German Jose Carreras Stiftung (DJCLS R14/22) and a Grant from the Government Baden-Württemberg (MWK). C.M. was supported form a AECC fellowship, Spain.
Footnotes
Author Contribution
S. Gerboth designed and performed and analyzed data; E. Frittoli performed and analyzed data; FC Baltanas and C. Gomez contributed in generating vital new Sos1 mouse models; A. Palamidessi performed and analyzed data (CRIB assays); S. Mogjiborahman performed and analyzed mass spectrometry data; J Rappsilber designed and analyzed mass spectrometry data; F. Troglio, C. Giuliani, Y. Rolland performed and analyzed data on K562; G. Pruneri designed and analyzed ISH data; S. Kreutmair performed and analyzed murine blast data; I. Pallavicini and S. Minucci performed and analyzed murine data; M. Zobel and M. Cinquanta knocked down experiments E. Santos provided key murine models; AL. Illert designed and analyzed primary BMC data; G. Scita designed analyzed data and wrote the manuscript.
Disclosure
The authors declare no potential conflicts of interest.
References
- 1.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007 Jun;9(6):706–712. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 2.Gureasko J, Galush WJ, Boykevisch S, Sondermann H, Bar-Sagi D, Groves JT, et al. Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat Struct Mol Biol. 2008 May;15(5):452–461. doi: 10.1038/nsmb.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- 4.Lu TY, Doherty J, Freeman MR. DRK/DOS/SOS converge with Crk/Mbc/dCed-12 to activate Rac1 during glial engulfment of axonal debris. Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):12544–12549. doi: 10.1073/pnas.1403450111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickerson S, Joy ST, Arora PS, Bar-Sagi D. An orthosteric inhibitor of the RAS-SOS interaction. Enzymes. 2013;34(Pt. B):25–39. doi: 10.1016/B978-0-12-420146-0.00002-0. [DOI] [PubMed] [Google Scholar]
- 6.Margarit SM, Sondermann H, Hall BE, Nagar B, Hoelz A, Pirruccello M, et al. Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003 Mar 7;112(5):685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 7.Freedman TS, Sondermann H, Kuchment O, Friedland GD, Kortemme T, Kuriyan J. Differences in flexibility underlie functional differences in the Ras activators son of sevenless and Ras guanine nucleotide releasing factor 1. Structure. 2009 Jan 14;17(1):41–53. doi: 10.1016/j.str.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sondermann H, Soisson SM, Boykevisch S, Yang SS, Bar-Sagi D, Kuriyan J. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell. 2004 Oct 29;119(3):393–405. doi: 10.1016/j.cell.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Yadav KK, Bar-Sagi D. Allosteric gating of Son of sevenless activity by the histone domain. Proceedings of the National Academy of Sciences of the United States of America. 2010 Feb 23;107(8):3436–3440. doi: 10.1073/pnas.0914315107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimnual AS, Yatsula BA, Bar-Sagi D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279(5350):560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 11.Salojin KV, Zhang J, Meagher C, Delovitch TL. ZAP-70 is essential for the T cell antigen receptor-induced plasma membrane targeting of SOS and Vav in T cells. J Biol Chem. 2000;275(8):5966–5975. doi: 10.1074/jbc.275.8.5966. [DOI] [PubMed] [Google Scholar]
- 12.Innocenti M, Tenca P, Frittoli E, Faretta M, Tocchetti A, Di Fiore PP, et al. Mechanisms through which Sos-1 coordinates the activation of Ras and Rac. J Cell Biol. 2002;156(1):125–136. doi: 10.1083/jcb.200108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Bashaw GJ. Son of sevenless directly links the Robo receptor to rac activation to control axon repulsion at the midline. Neuron. 2006 Nov 22;52(4):595–607. doi: 10.1016/j.neuron.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 14.Das B, Shu X, Day GJ, Han J, Krishna UM, Falck JR, et al. Control of intramolecular interactions between the pleckstrin homology and Dbl homology domains of Vav and Sos1 regulates Rac binding. J Biol Chem. 2000;275(20):15074–15081. doi: 10.1074/jbc.M907269199. [DOI] [PubMed] [Google Scholar]
- 15.Sini P, Cannas A, Koleske AJ, Di Fiore PP, Scita G. Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat Cell Biol. 2004 Mar;6(3):268–274. doi: 10.1038/ncb1096. [DOI] [PubMed] [Google Scholar]
- 16.Hwang HS, Hwang SG, Cho JH, Chae JS, Yoon KW, Cho SG, et al. CIIA functions as a molecular switch for the Rac1-specific GEF activity of SOS1. The Journal of cell biology. 2011 Oct 31;195(3):377–386. doi: 10.1083/jcb.201106138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, et al. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401(6750):290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 18.Scita G, Tenca P, Areces LB, Tocchetti A, Frittoli E, Giardina G, et al. An effector region in Eps8 is responsible for the activation of the Rac- specific GEF activity of Sos-1 and for the proper localization of the Rac-based actin-polymerizing machine. J Cell Biol. 2001;154(5):1031–1044. doi: 10.1083/jcb.200103146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Innocenti M, Frittoli E, Ponzanelli I, Falck JR, Brachmann SM, Di Fiore PP, et al. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J Cell Biol. 2003 Jan 6;160(1):17–23. doi: 10.1083/jcb.200206079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanday FA, Santhanam L, Kasuno K, Yamamori T, Naqvi A, Dericco J, et al. Sos-mediated activation of rac1 by p66shc. J Cell Biol. 2006 Mar 6; doi: 10.1083/jcb.200506001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder JT, Worthylake DK, Rossman KL, Betts L, Pruitt WM, Siderovski DP, et al. Structural basis for the selective activation of Rho GTPases by Dbl exchange factors. Nat Struct Biol. 2002;9(6):468–475. doi: 10.1038/nsb796. [DOI] [PubMed] [Google Scholar]
- 22.Soisson SM, Nimnual AS, Uy M, Bar-Sagi D, Kuriyan J. Crystal structure of the Dbl and pleckstrin homology domains from the human Son of sevenless protein. Cell. 1998;95(2):259–268. doi: 10.1016/s0092-8674(00)81756-0. [DOI] [PubMed] [Google Scholar]
- 23.Daley GQ, McLaughlin J, Witte ON, Baltimore D. The CML-specific P210 bcr/abl protein, unlike v-abl, does not transform NIH/3T3 fibroblasts. Science. 1987 Jul 31;237(4814):532–535. doi: 10.1126/science.2440107. [DOI] [PubMed] [Google Scholar]
- 24.Kogan SC, Ward JM, Anver MR, Berman JJ, Brayton C, Cardiff RD, et al. Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood. 2002 Jul 1;100(1):238–245. doi: 10.1182/blood.v100.1.238. [DOI] [PubMed] [Google Scholar]
- 25.Thomas EK, Cancelas JA, Zheng Y, Williams DA. Rac GTPases as key regulators of p210-BCR-ABL-dependent leukemogenesis. Leukemia. 2008 May;22(5):898–904. doi: 10.1038/leu.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pai SY, Kim C, Williams DA. Rac GTPases in human diseases. Disease markers. 2010;29(3–4):177–187. doi: 10.3233/DMA-2010-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skorski T, Wlodarski P, Daheron L, Salomoni P, Nieborowska-Skorska M, Majewski M, et al. BCR/ABL-mediated leukemogenesis requires the activity of the small GTP-binding protein Rac. Proc Natl Acad Sci U S A. 1998 Sep 29;95(20):11858–11862. doi: 10.1073/pnas.95.20.11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho YJ, Zhang B, Kaartinen V, Haataja L, de Curtis I, Groffen J, et al. Generation of rac3 null mutant mice: role of Rac3 in Bcr/Abl-caused lymphoblastic leukemia. Mol Cell Biol. 2005 Jul;25(13):5777–5785. doi: 10.1128/MCB.25.13.5777-5785.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, et al. Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell. 2007 Nov;12(5):467–478. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJ. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Molecular & cellular proteomics : MCP. 2005 Jul;4(7):873–886. doi: 10.1074/mcp.T500007-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Pinkse MW, Uitto PM, Hilhorst MJ, Ooms B, Heck AJ. Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal Chem. 2004 Jul 15;76(14):3935–3943. doi: 10.1021/ac0498617. [DOI] [PubMed] [Google Scholar]
- 32.Steen H, Kuster B, Mann M. Quadrupole time-of-flight versus triple-quadrupole mass spectrometry for the determination of phosphopeptides by precursor ion scanning. Journal of mass spectrometry : JMS. 2001 Jul;36(7):782–790. doi: 10.1002/jms.174. [DOI] [PubMed] [Google Scholar]
- 33.Salek M, Alonso A, Pipkorn R, Lehmann WD. Analysis of protein tyrosine phosphorylation by nanoelectrospray ionization high-resolution tandem mass spectrometry and tyrosine-targeted product ion scanning. Analytical chemistry. 2003 Jun 1;75(11):2724–2729. doi: 10.1021/ac020657y. [DOI] [PubMed] [Google Scholar]
- 34.Pendergast AM, Muller AJ, Havlik MH, Clark R, McCormick F, Witte ON. Evidence for regulation of the human ABL tyrosine kinase by a cellular inhibitor. Proc Natl Acad Sci U S A. 1991;88(13):5927–5931. doi: 10.1073/pnas.88.13.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13(18):2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plattner R, Koleske AJ, Kazlauskas A, Pendergast AM. Bidirectional signaling links the Abelson kinases to the platelet-derived growth factor receptor. Mol Cell Biol. 2004 Mar;24(6):2573–2583. doi: 10.1128/MCB.24.6.2573-2583.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sondermann H, Soisson SM, Bar-Sagi D, Kuriyan J. Tandem histone folds in the structure of the N-terminal segment of the ras activator Son of Sevenless. Structure. 2003 Dec;11(12):1583–1593. doi: 10.1016/j.str.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Hoon JL, Wong WK, Koh CG. Functions and regulation of circular dorsal ruffles. Mol Cell Biol. 2012 Nov;32(21):4246–4257. doi: 10.1128/MCB.00551-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mauro MJ, Druker BJ. STI571: targeting BCR-ABL as therapy for CML. Oncologist. 2001;6(3):233–238. doi: 10.1634/theoncologist.6-3-233. [DOI] [PubMed] [Google Scholar]
- 40.Sengupta A, Arnett J, Dunn S, Williams DA, Cancelas JA. Rac2 GTPase deficiency depletes BCR-ABL+ leukemic stem cells and progenitors in vivo. Blood. 2010 Jul 8;116(1):81–84. doi: 10.1182/blood-2009-10-247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baltanas FC, Perez-Andres M, Ginel-Picardo A, Diaz D, Jimeno D, Liceras-Boillos P, et al. Functional redundancy of Sos1 and Sos2 for lymphopoiesis and organismal homeostasis and survival. Mol Cell Biol. 2013 Nov;33(22):4562–4578. doi: 10.1128/MCB.01026-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daubon T, Chasseriau J, El Ali A, Rivet J, Kitzis A, Constantin B, et al. Differential motility of p190bcr-abl- and p210bcr-abl-expressing cells: respective roles of Vav and Bcr-Abl GEFs. Oncogene. 2008 Apr 24;27(19):2673–2685. doi: 10.1038/sj.onc.1210933. [DOI] [PubMed] [Google Scholar]
- 43.Ridley AJ. Life at the leading edge. Cell. 2011 Jun 24;145(7):1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, Jenkins EC, et al. Identification of a candidate human spectrin src homology 3 domain- binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton [In Process Citation] J Biol Chem. 1998;273(22):13681–13692. doi: 10.1074/jbc.273.22.13681. [DOI] [PubMed] [Google Scholar]
- 45.Taki T, Shibuya N, Taniwaki M, Hanada R, Morishita K, Bessho F, et al. ABI-1, a human homolog to mouse Abl-interactor 1, fuses the MLL gene in acute myeloid leukemia with t(10;11)(p11.2;q23) Blood. 1998;92(4):1125–1130. [PubMed] [Google Scholar]
- 46.Williams DA, Zheng Y, Cancelas JA. Rho GTPases and regulation of hematopoietic stem cell localization. Methods Enzymol. 2008;439:365–393. doi: 10.1016/S0076-6879(07)00427-2. [DOI] [PubMed] [Google Scholar]
- 47.Bustelo XR. Vav family exchange factors: an integrated regulatory and functional view. Small GTPases. 2014;5(2):9. doi: 10.4161/21541248.2014.973757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bassermann F, Jahn T, Miething C, Seipel P, Bai RY, Coutinho S, et al. Association of Bcr-Abl with the proto-oncogene Vav is implicated in activation of the Rac-1 pathway. J Biol Chem. 2002 Apr 5;277(14):12437–12445. doi: 10.1074/jbc.M112397200. [DOI] [PubMed] [Google Scholar]
- 49.Chang KH, Sanchez-Aguilera A, Shen S, Sengupta A, Madhu MN, Ficker AM, et al. Vav3 collaborates with p190-BCR-ABL in lymphoid progenitor leukemogenesis, proliferation, and survival. Blood. 2012 Jul 26;120(4):800–811. doi: 10.1182/blood-2011-06-361709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang CD, Do IR, Kim KW, Ahn BK, Kim SH, Chung BS, et al. Role of Ras/ERK-dependent pathway in the erythroid differentiation of K562 cells. Experimental & molecular medicine. 1999 Jun 30;31(2):76–82. doi: 10.1038/emm.1999.13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.