Abstract
Mucosal-associated invariant T (MAIT) cells are innate T cells recognising intermediates of the vitamin B2 biosynthetic pathway presented by the monomorphic MR1 molecule. To date it remains unclear whether in addition to their cytolytic activity important in antimicrobial defence, MAIT cells have also immune-modulatory functions, which could enhance DC maturation. Here, we investigated the molecular mechanisms dictating the interactions between human MAIT cells and dendritic cells (DC) and demonstrate that human MAIT cells mature monocyte-derived and primary DC in an MR1- and CD40L-dependent manner. Furthermore, we show that MAIT cell derived signals synergise with microbial stimuli to induce secretion of bioactive IL-12 by DC. Activation of human MAIT cells in whole blood leads to MR1- and cytokine-dependent NK cell transactivation. Our results underscore an important property of MAIT cells, which can be of translational relevance to rapidly orchestrate adaptive immunity through DC maturation.
Keywords: MAIT, MR1, dendritic cells, adaptive immunity, CD40L
Introduction
The innate and adaptive arms of the immune system require tight regulation for the induction of protective immunity against pathogens and prevention of autoimmunity. In addition to conventional peptide-specific MHC-restricted T cells, an expanding population of “in-betweeners” or unconventional cells exists (1). These cells bear adaptive rearranged TCRs yet with limited diversity, display innate-like behaviour, a memory phenotype, can be rapidly activated, and orchestrate adaptive immunity through dendritic cell (DC) maturation (1). Unconventional T cell populations include CD1-restricted T cells, γδ T cells with various restriction and MHC class-Ib restricted T cells (2). Of the latter, MR1-restricted MAIT cells are a recently described addition to the unconventional T cell family, recognising unstable adducts derived from a precursor of the riboflavin (vitamin B2) pathway, present in a number of bacterial and fungal species (3). While the details of MR1-restricted antigen presentation are being unravelled (4), a number of questions on the biology of these cells still remain unanswered. MR1-deficient mice, which lack MAIT cells, are more susceptible to some bacterial infections, such as Klebsiella, Mycobacterium bovis BCG and F. tularensis, suggesting an important role for MAIT cells in antibacterial immunity (reviewed in (5)). Recently, in a mouse model of Francisella tularensis infection, it has been shown that MAIT cells promote GM-CSF dependent but MR1-independent differentiation of inflammatory monocytes into monocyte-derived DC, influencing early activation and recruitment of T cells (6). These results suggest that cross talk between MAIT cells and myeloid cells may be important to shape antigen specific adaptive immunity, as previously observed for CD1d-restricted iNKT cells. We therefore investigated the molecular mechanisms dictating the outcome of interactions between human MAIT cells and DC and demonstrate the ability of human MAIT cells to mature monocyte-derived and primary DCs.
Materials and methods
Medium and reagents
The complete medium (CM) used throughout was RPMI 1640 (Gibco) for DC and IMDM (Gibco) for human MAIT cells. CM was supplemented with 2 mM L-glutamine, 1% non-essential amino acids, 1% sodium pyruvate, 1% pen/strep, 5 x10-5 2ME (all from Gibco) and serum: 10% FCS (Sigma) or 5% Human AB Serum (Sigma) for MAIT cells. MAIT cell medium was supplemented with 1000U/ml recombinant human IL-2, produced in our laboratory as described (7).
Ultrapure LPS and R848 were purchased from InvivoGen. Methyl Glyoxal (40% in water) was from Sigma. DH5α E Coli bacteria (Invitrogen) were grown overnight to stationary phase in Luria broth (LB), and supernatant was centrifuged and sterile filtered before use as an enriched source of MAIT ligands (30μl, 10μl or 3μl in a 200μl assay).
5-A-RU was synthesized as follows. 6-Ribitylaminouracil was synthesised from D-ribose as previously reported (8) and purified by ion exchange chromatography (9). Nitrosation at the 5-position was carried out by slightly modifying the literature procedure (8), using sodium nitrite and barium acetate in place of barium nitrite. The product, 5-nitroso-6-ribitylaminouracil, was purified by ion exchange chromatography (9). Reduction of the nitroso group using sodium dithionite gave 5-A-RU (3), which was used without further purification. The product was analysed by LCMS on an Agilent 1260 HPLC equipped with an Agilent 6130 single quadrupole mass spectroscopic detector. Chromatographic conditions: Phenomenex Synergi Fusion-RP column (2.5 µm. 100Å, 50x3 mm), eluting with isocratic 10 mM aq ammonium acetate (0.5 mL/min, 30 °C). 5-A-RU was detected by UV (214, 254 nm) and MS (ESI +ve, m/z = 277 [M+H]; ESI -ve, m/z = 275 [M-H]) at 1.05 min.
Generation of MAIT cells and Dendritic Cells
Blood was purchased from the UK National Blood Service. Human MAIT cells were isolated by cell sorting CD2 MACS enriched leukocytes with CD161 and Vα7.2 antibodies (Biolegend). MAIT cells were grown for a few weeks in CM supplemented with IL-2. Control CD8+ CD161+ and CD8+ CD161+ were sorted from CD2 enriched leukocytes at the same time as MAIT cells and simultaneously cultured.
Dendritic Cells were differentiated culturing CD14 MACS purified monocytes in CM supplemented with human GM-CSF (50ng/ml) and human IL-4 (1000U/ml, both from Peprotech).
Whole blood assays
Freshly drawn blood was distributed in 5ml polypropylene conical tubes (BD Falcon). 1ml blood was activated with 5-A-RU and MG (1μg/ml and 100μM respectively), in the presence of 30μg/ml of isotype controls, anti-MR1 (clone 26.5) or anti-CD40L (clone 24.31) antibodies. Anti IL-18R1 (clone H44) was used for the NK experiments. LPS was used at 1μg/ml. After two hours of stimulation, protein transport inhibitor containing Brefeldin A (BD) was added and stimulation was continued for additional 16 hours. Cells were surface stained in Brilliant violet buffer (BD), fixed and permeabilized (eBioscience) and stained for intracellular cytokines. The following antibodies were used throughout: BV785 CD123 (7G3, Biolegend), BUV737 IFN-γ (4S.B3, BD), BUV395 TNF-α (MAb11, BD), BUV661 CD3 (UCHT1, BD), BUV563 CD56 (NCAM16.2, BD), CD33 PE or APC (P67.6, Biolegend), PE/Dazzle CD137 (VI C-7, Biolegend), PE IL-12p40 (C11.5, Biolegend), APC or BV510 CD161 (HP-3G10, Biolegend), BV605 Vα7.2 (3C10, Biolegend), BV605 CD14 (M5E2, Biolegend), BV510 CD19 (HIB19, Biolegend), FITC CD40L (TRAP1, BD), Alexa700 CD11b (M1/70, Biolegend), BV650 CD16 (3G8, Biolegend), PECy7 CD1c (L161, Bioegend), APCCy7 HLA DR (L243, Biolegend), APC BDCA2 (201A, Biolegend), BV711 CD40 (5C3, Biolegend), BV421 CD80 (2D10, Biolegend), BB515 CD86 (FUN-1, BD), PerCPCy5.5 CD141 (M80, Biolegend), BV785 CD38 (HIT2, Biolegend), FITC CD28 (28.2, Biolegend), PE/Dazzle CD27 (1G3A10, Biolegend), PECy7 NKG2D (1D11, Biolegend), PE NKG2A (REA110, Miltenyi), PerCPCy5.5 KIR2DL1 (HP MA4, Biolegend). Samples were acquired on a X50 BD symphony machine and analysed with Flowjo 10. Viability was assessed with live/dead Aqua staining, according to the manufacturer’s instructions (Invitrogen).
Stimulation assays
DC were plated at 50000 cells/well in 96 well flat bottom plates in CM and incubated with MAIT cells (20000 cells/well, in triplicate) in the presence or absence of different concentrations of 5-A-RU/MG, bacterial supernatant, LPS or R848. Depending on availability, autologous and/or allogeneic MAIT cells were used in different experiments as MR1 lacks polymorphism. For blocking experiments, DC were pre-incubated for 1 hour with the ligands, subsequently for 2 hours with 30μg/ml isotype controls, anti-MR1 (clone 26.5), anti-CD40L (clone 24.31) or anti IL-12 (clone C8.6) antibodies before addition of MAIT cells. MAIT and DC activation was assessed by flow cytometry after 36 hours with the following antibodies: FITC CD83 (HB15e, BD), APC CD86 (FUN1, BD), PE CD80 (L307.4 BD), PE-Cy7 PDL1 (29E.2A3, Biolegend), PE-CF594 CD25 (M-A251, BD). IFNγ, IL12p40 and IL12p70 were also measured by ELISA (antibodies from BD) on supernatants harvested after 36 hours. We did not detect any IL-17 or IL-23 in our supernatants (ELISA antibody pairs from eBioscience, sensitivity 0.04ng/ml and 0.39ng/ml, respectively).
Transwell assay
Costar 24 well plates with an insert with pore size 0.4μm were used (cat 3470). 100000 DC were plated in the bottom well in 800μl medium; in the top well 60000 DC were plated with 50000 allogeneic MAIT cells in 200μl medium. 5-A-RU was used at 100ng/ml, with 100μM MG, blocking antibodies at 30μg/ml, as described above. As a positive control for maturation, 50μl supernatant of a 6ml overnight culture of spun DH5α bacteria were added to the DC. DC were collected and stained after 36 hours of culture.
Simoa immunoassay
In order to detect very low levels of IL12p70, supernatants were subject to automatized ELISA analysis with the Simoa HD1-Analyzer and Single Molecule Array (Simoa) technology (Quanterix), using the Simoa Human IL12 p70 kit, following the recommendations of the manufacturer (10).
Statistical analyses
Statistical analyses were made with GraphPad Prism software, version 7. Comparisons were performed using Wilcoxon matched pairs signed rank test and differences with p<0.05 were deemed significant. In experiments with n=2 a statistical test was not performed because of the low sample size issue.
Results
Activated human MAIT cells up-regulate CD40L
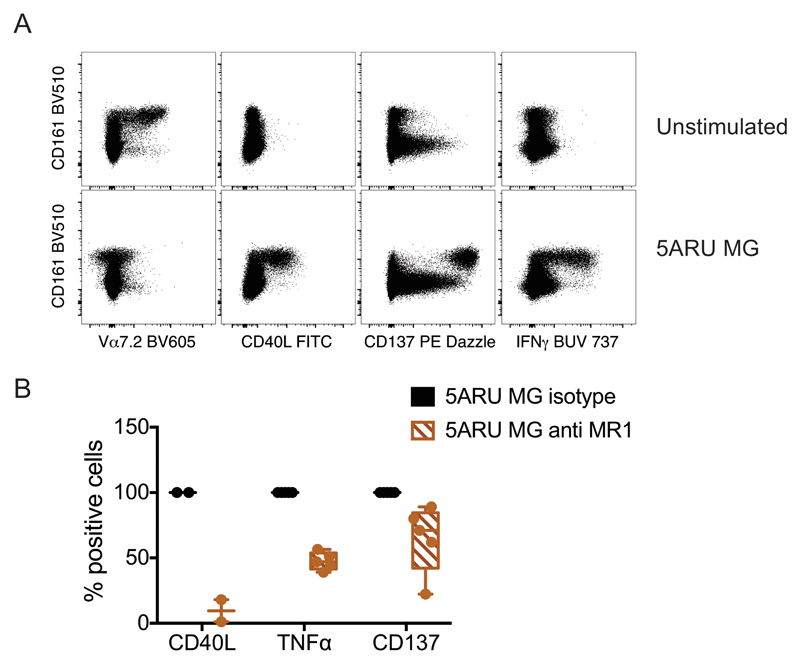
We synthesized 5-amino-6-d-ribitylaminouracil (5-A-RU), an early intermediate in bacterial riboflavin synthesis that can form simple adducts with cellular metabolites to provide MR1-binding MAIT cell agonists (11, 12). This compound induced potent dose-dependent and MR1-restricted activation of MAIT cell lines derived from healthy donors (Supplemental Fig.1A). As previously described, MAIT cell activation was much stronger in the presence of exogenous methyl glyoxal (MG), likely due to the formation of the potent adduct 5-OP-RU, which is known to be a potent agonist (Supplemental Fig. 1B) (11). We next tested 5-A-RU/MG on unfractionated MAIT cells in whole blood, and identified MAIT cells by Vα7.2 and CD161 co-staining (13). Upon MAIT cell activation, we observed strong Vα7.2 TCR down-regulation, accompanied by IFN-γ secretion and up-regulation of the activation marker CD137, both of which were confined to the CD161 bright cells (Fig. 1A). Interestingly, MAIT cell activation was accompanied by CD40L up-regulation (Fig. 1 and Supplemental Fig.1C). In these co-cultures, CD40L was specifically expressed on IFN-γ secreting, CD137+ CD161bright cells (Fig. 1 and Supplemental Fig.1C). The specificity of MAIT cell activation was demonstrated with anti-MR1 antibodies, which blocked CD40L expression and TNF-α secretion (Fig. 1B). However, anti-MR1 antibodies only marginally reduced CD137 expression (Fig. 1B and Supplemental Fig. 4).
Figure 1.
Human MAIT cell activation leads to MR1-dependent CD40L up-regulation. Whole blood was incubated 16 hours with 5-A-RU and MG in the presence of protein transport inhibitors. Expression of Vα7.2 TCR, CD40L, CD137 and IFN-γ was detected by intracellular staining and is depicted in the FACS dot plots, in parallel with CD161 expression. B) Effect of MR1 blockade on CD40L (n=2), TNF-α (n=5) and CD137 (n=5) expression in the intracellular assay performed as depicted in panel A. Values represent mean +/- SD; for TNF-α and CD137 p= 0.06 (Wilcoxon signed rank test; statistical test was not performed for CD40L because of the low sample size). For CD137 expressing cells, the ratio of mean fluorescence intensity with and without anti-MR1 is plotted, as the overall percentage of positive cells remains unchanged (see also Supplemental Fig. 4B).
Human MAIT cell lines induce CD40L-dependent DC maturation
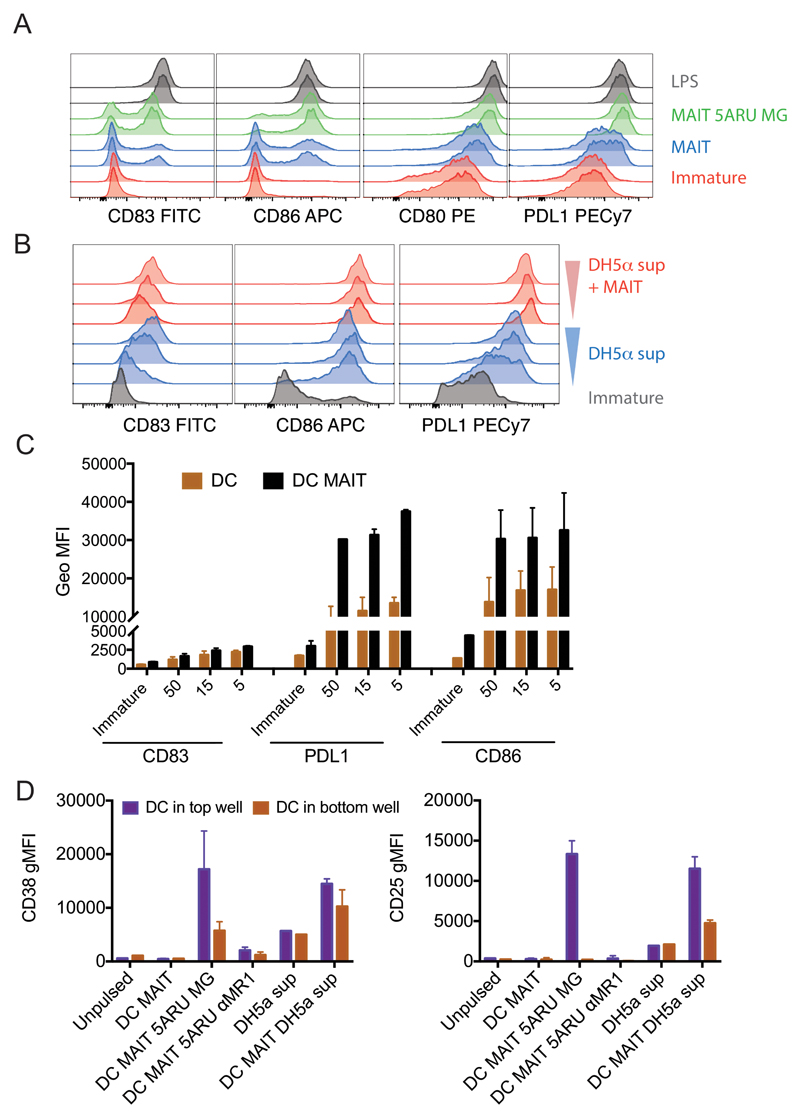
Ligation of CD40 on human and murine dendritic cells triggers production of high levels of IL-12 and provides a source of help required for efficient priming of cytotoxic T lymphocytes (CTL) (14–16). In addition to CD4+ T cells, iNKT cells have been shown to be potent inducer of CD40-dependent DC maturation (17, 18). We therefore investigated whether MAIT cells could also trigger DC maturation and IL-12 secretion. When co-incubated with monocyte-derived immature DC in the absence of exogenous ligands, we observed that MAIT cells induced partial DC maturation, as defined by increase in the expression of CD80, CD83, CD86 and PDL-1 (Fig. 2A, blue lines). DC maturation was clearly enhanced in the presence of the synthetic cognate ligand 5-A-RU/MG (Fig. 2A, green lines), almost reaching levels observed with low dose LPS (Fig. 2A, grey lines). Furthermore, MAIT cells enhanced maturation induced by supernatant of DH5α E. coli (Fig. 2B and C), which has been shown to contain vitamin B2-derived activating ligands (3, 19). This effect could be partially blocked with anti-MR1 antibodies (Supplemental Fig. 1D). To further dissect the contribution of MR1 and soluble factors in dictating DC maturation, we performed transwell experiments (Fig. 2D). DC were plated in the top and bottom wells of a tranwell chamber, while MAIT cells and stimuli were added in the top well. We observed that 5-A-RU/MG dependent DC maturation was mostly driven by cognate interactions, as we detected it mainly in the DC harvested from the top wells. In addition, in both wells, DC maturation could be blocked by anti-MR1 (Fig. 2D). Finally, we observed that MAIT cells enhanced DC maturation induced by DH5α E. coli supernatants in both wells, suggesting a possible contribution of soluble factors in this specific setting (Fig. 2D). We excluded the possibility that DC maturation was due to endotoxin or other innate signalling contaminants, as in the absence of MAIT cells, 5-A-RU/MG did not induce DC maturation, even at the high concentration of 150μg/ml (Supplemental Fig. 1E).
Figure 2.
Human MAIT cells induce DC maturation. A) Immature DC (red histograms) were incubated with allogeneic MAIT cells in the presence (green histograms) or absence (blue histograms) of 5-A-RU/MG. LPS (grey histograms) was used as positive controls. Staining profile of duplicate wells is shown. B) Immature DC (grey histograms) were incubated with three concentrations of DH5-α supernatant in the presence (red histograms) or absence (blue histograms) of autologous MAIT cells. Depicted are expression levels of CD83, CD86, CD80 and PDL1 as detected by flow cytometry after 36 hours. One representative experiment of four; data from two donors, one autologous and one allogeneic to the DC are tabulated in panel C, where 50, 15 and 5 on the x-axis refer to different amounts of DH5-α supernatant. D) Expression of CD38 and CD25 (geometric mean+/- SD of two donors) on DC harvested from the top and bottom wells of a transwell assay. When indicated, allogeneic MAIT cells were added in the top wells, in the presence or absence of 5-A-RU/MG or supernatant of DH5-α and anti MR1 blocking antibodies.
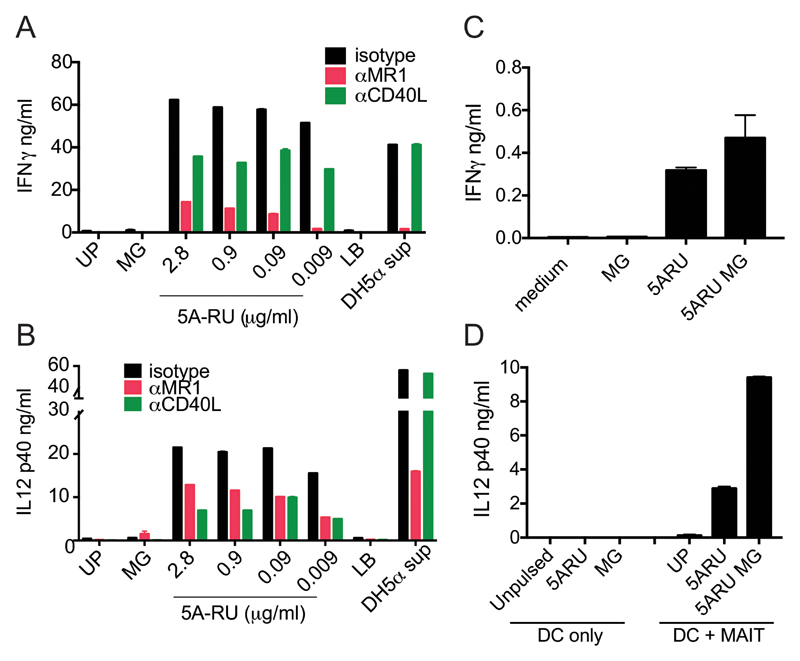
We next investigated by ELISA whether cytokines were released into the supernatant when human MAIT cells were co-cultured with 5-A-RU/MG pulsed DC. We observed dose-dependent release of IFN-γ and IL-12p40, which could largely be blocked with anti-MR1, likely reflecting release by MAIT cells and DCs, respectively (Fig. 3A and B). In keeping with the concept that MAIT cells can licence DCs via CD40, IL-12 p40 production was blocked with anti-CD40L (Fig. 3B). Interestingly, anti-CD40L also reduced IFN- γ secretion by MAIT cells (Fig. 3A), which may be a consequence of the reduction of cytokines such as IL-12, that could feedback on MAIT cells via highly expressed receptors (20). Since MR1 is ubiquitously expressed (21), we ruled out that MAIT cell activation was due to presentation of 5-A-RU/MG by neighbouring MAIT cells (Fig. 3C), and also demonstrated that DC did not release any IL-12 in the absence of MAIT cells (Fig. 3D). We did not detect any IL-23 or IL-17 in our co-culture experiments (data not shown).
Figure 3.
Human MAIT cells induce CD40L-dependent DC maturation. A, B) IFN-γ (A) and IL-12p40 (B) levels in the supernatant of MAIT-allogeneic DC co-cultures pulsed with the indicated concentration of 5-A-RU and constant MG (50μM) in the presence or absence of anti-MR1 or anti-CD40L blocking antibodies. C) IFN-γ levels in the supernatant of MAIT cells exposed to 5-A-RU and/or MG in the absence of allogeneic DC. Note the difference in the y-axis scale. D) IL-12p40 in the supernatant of DC alone or DC and allogeneic MAIT co-cultures pulsed with 5-A-RU and/or MG. One experiment representative of three, values represent mean +/- SD.
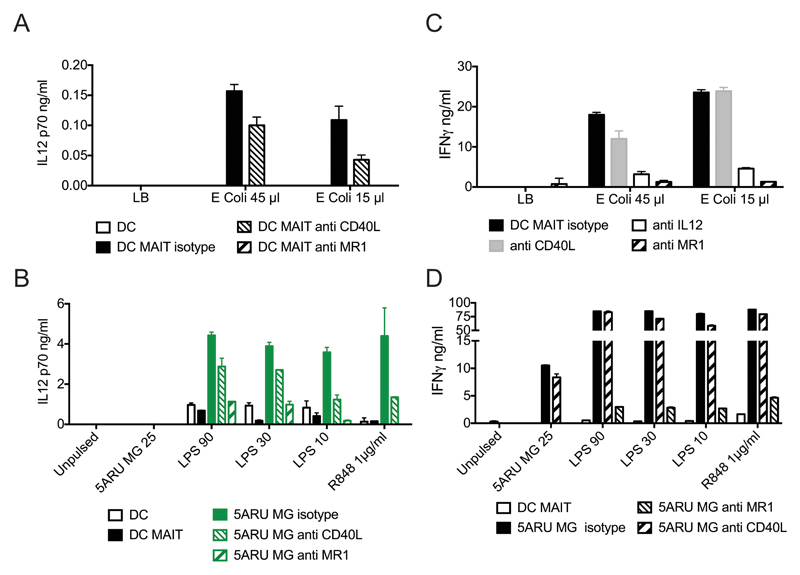
In the above co-culture experiments, IL-12p40 was easily detected while IL-12p70 was below the detection limit of our ELISA. We therefore investigated whether signals from activated MAIT cells could cooperate with stimuli via pathogen-derived pattern recognition receptors to induce release of the bioactive form of IL-12 by DC. First, we observed that when DC were incubated with supernatants of E. coli DH5α bacteria, they secreted IL-12p70 only in the presence of MAIT cells, and this could be completely abrogated by anti-MR1 antibodies and partially by anti-CD40L antibodies (Fig. 4A, Supplemental Fig. 2A). We next extended these results and demonstrated that, in the presence of low doses of 5-A-RU/MG, MAIT cells significantly enhanced IL-12p70 secretion induced by the TLR agonists LPS and R848 (Fig. 4B, Supplemental Fig. 2C, D). This effect was entirely MR1 dependent, with some contribution of CD40L at low concentrations of TLR ligands. In turn, we observed an IL-12 and MR1-dependent MAIT cell activation by E. coli pulsed DC (Fig. 4C, Supplemental Fig. 2B). In addition, at the concentrations tested, TLR ligands potently synergized with 5-A-RU/MG in inducing MR1-dependent MAIT cell activation (Fig. 4D, Supplemental Fig. 2E).
Figure 4.
Synergy between human MAIT cell agonists and TLR agonists. A) Bioactive IL-12p70 in the supernatant of DC pulsed with E. coli supernatant in the presence or absence of allogeneic MAIT cells and blocking anti-CD40L or anti-MR1 antibodies. B) Bioactive IL-12p70 in the supernatant of DC pulsed with the indicated concentrations (ng/ml) of 5-A-RU/MG, LPS or R848 (μg/ml) in the presence or absence of allogeneic MAIT cells and blocking anti-CD40L or anti-MR1 antibodies. C) IFN-γ levels in the supernatant of MAIT-allogeneic DC co-cultures pulsed with E. coli supernatant in the presence or absence of blocking anti-CD40L, anti IL-12 or anti-MR1 antibodies. D) IFN-γ levels in the supernatant of MAIT-allogeneic DC co-cultures pulsed with the indicated concentrations (ng/ml) of 5-A-RU MG, LPS or R848 (μg/ml) in the presence or absence of blocking anti-CD40L or anti-MR1 antibodies. Data are from one experiment representative of four, values represent mean +/- SD. Two more donors are shown in Supplemental Fig. 2.
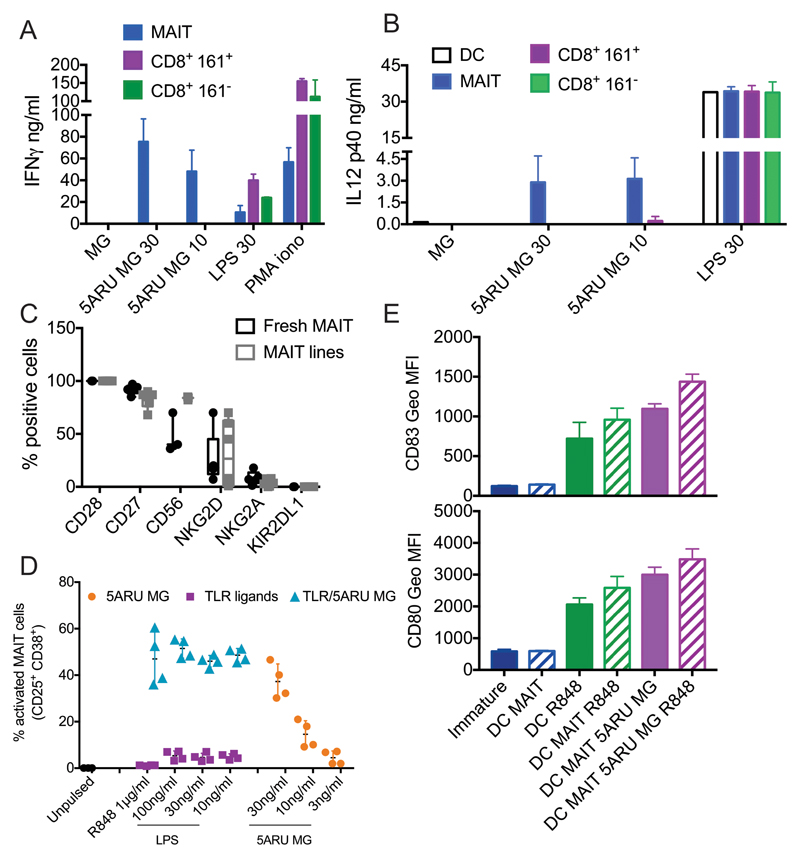
Only MAIT cells induced 5-A-RU/MG-dependent DC maturation, as neither CD8+161+ T cells nor CD8+161- T cells sorted from the same donors were activated by the vitamin B2 intermediate (Fig. 5A) or induced DC activation (Fig. 5B). As expected, however, CD8 cells were responsive to TLR-matured DCs (Fig. 5A).
Figure 5.
A and B) MAIT but not CD8 T cells induce 5-A-RU/MG dependent DC maturation. IFN-γ (A) and IL-12 (B) secretion in cocultures of DC and MAIT, CD8+161+ or CD8+161- cells, activated by the indicated concentration of 5-A-RU/MG or LPS. PMA and ionomycin was used as a control for T cell activation. Data are from two experiments, with one MAIT cell donor autologous and one allogeneic to the DC; values represent mean +/- SD. C, D, E) Freshly sorted MAIT cells induce DC maturation. C) Phenotype of freshly sorted and MAIT lines. Plotted is the percentage of cells positive for each indicated marker, depicted as box and whiskers, with all points indicated. N=5 for fresh MAIT, 6 for MAIT lines (only 3 of each were tested for CD56). For two donors both fresh and cultured MAIT cells were tested. D) Percentage of freshly sorted MAIT cells activated in response to DC pulsed with the indicated concentration of 5-A-RU/MG, TLR ligands or a combination of the two. E) Expression of CD83 and CD80 (geometric mean +/- SD) in DC activated by freshly sorted MAIT cells, R848 or a combination of the two. Data in panels D and E are from two donors, both allogeneic to the DC. Further data related to this experiment are shown in supplemental Fig 3.
Freshly sorted human MAIT cells induce DC maturation
It has been shown that MAIT cell cytotoxicity can be enhanced upon in vitro culture, following up-regulation of Granzyme B and perforin (22). To exclude a possible effect of the in vitro culture on MAIT cells function, we investigated whether freshly sorted MAIT cells could also induce DC maturation. In line with recently published results (23), both fresh MAIT and MAIT lines expressed homogeneously CD27 and CD28, while they up-regulated CD56 in culture (Fig. 5C). NKG2D expression varied the most across the donors tested, particularly upon culture (Fig. 5C). A small percentage of cells expressed NKG2A, while we did not observe any KIR2DL1 expression (Fig. 5C). Freshly sorted MAIT cells were activated by 5-A-RU/MG in a dose dependent manner and their activation was enhanced by TLR ligands (Fig. 5D and Supplementary Fig 3A). Freshly sorted MAIT cells also induced DC maturation and, in the presence of low doses of 5-A-RU/MG, enhanced TLR-dependent DC activation, as shown by upregulation of surface markers (Fig. 5E), secretion of IL-12p70 and p40 (Supplementary Fig. 3B and C).
Maturation of primary DC via MAIT cells leads to transactivation of NK cells
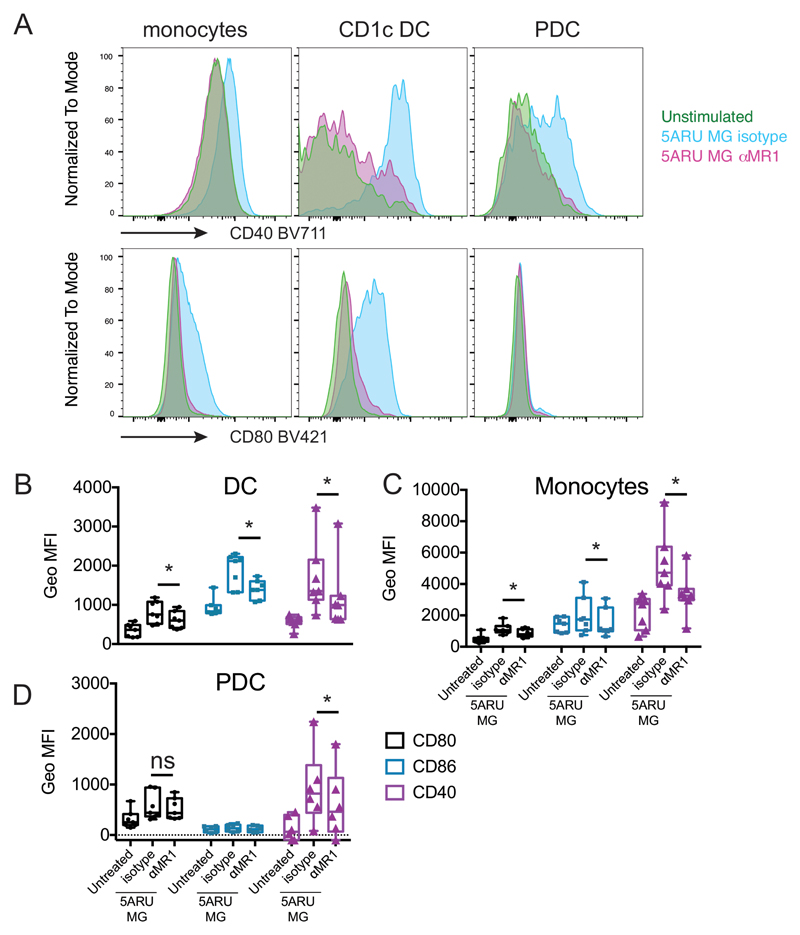
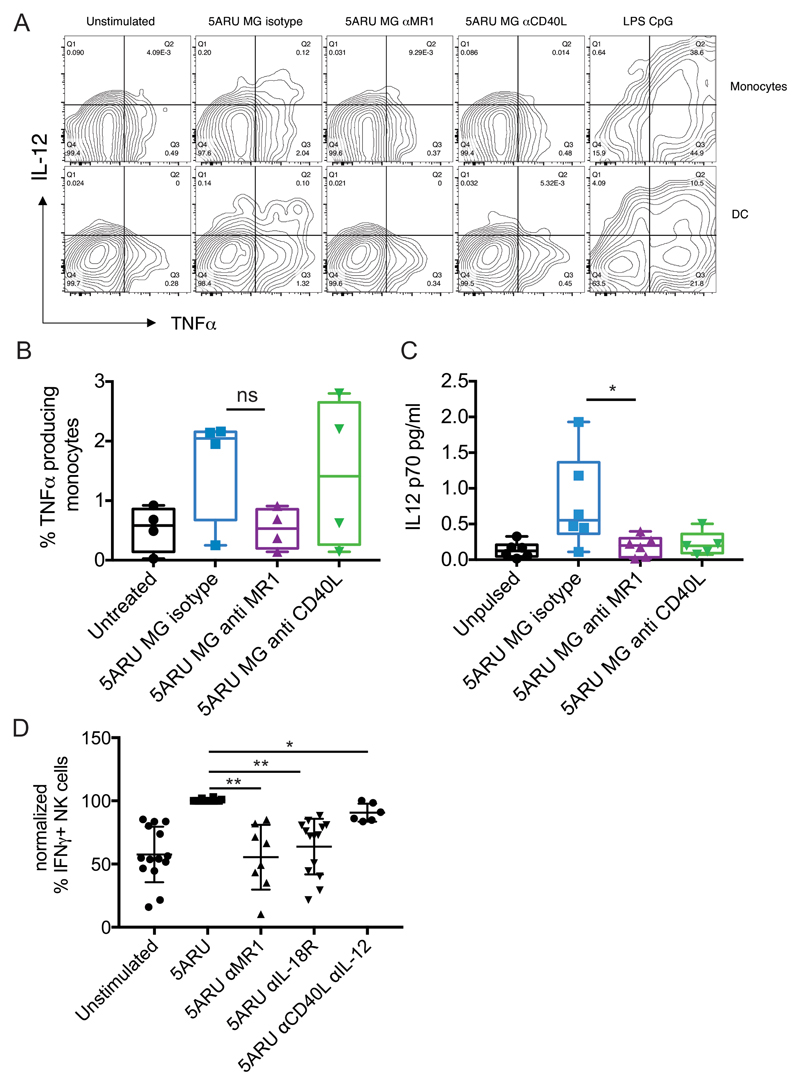
To assess whether our results obtained with monocyte-derived DC could be extended to primary cells, we investigated the immune-modulatory activity of MAIT cells ex vivo, in whole blood. Using an X50 BD Symphony 5 laser flow cytometer, which allows simultaneous measurement of up to 30 parameters we developed a gating strategy to identify myeloid subpopulations, and T, B or NK cells (Supplemental Fig. 4A). Freshly collected blood from healthy volunteers was incubated with 5-A-RU/MG in the presence or absence of anti-MR1 or anti-CD40L antibodies and cells were stained 16 hours later. Activation of MAIT cells in response to 5-A-RU/MG stimulation was revealed by induction of TNF-α secretion, CD137 up-regulation and Vα7.2 TCR down-regulation (Supplemental Fig. 4B). As expected, MAIT cell activation could be blocked by anti-MR1 treatment (Supplemental Fig. 4B). Upon 5-A-RU/MG exposure, monocytes, CD1c DC and plasmacytoid DC (PDC) up-regulated CD40, CD86 and CD80, markers indicative of maturation. Significantly, blocking MAIT cell activation with anti-MR1 also abrogated this maturation process (Fig. 6A, B, C and D). Furthermore, in activated monocytes and DC we detected intracellular IL-12p40 and TNF-α (Fig. 7A and B). TNF-α production could be specifically blocked by anti-MR1 but not by anti-CD40L treatment (Fig. 7 B). By a highly sensitive Simoa Immunoassay, in the above co-cultures we detected secretion of IL-12p70, which was also reduced by anti-MR1 and anti-CD40L (Fig. 7C). Finally, MAIT cell activation also led to NK cell transactivation, in an MR1- and IL-18 dependent manner (Fig. 7D).
Figure 6.
Human MAIT cells induce maturation of primary blood DC. A) Monocytes, CD1c DC and PDC were gated ad shown in supplemental Fig. 4. Depicted is the cell surface expression of CD40 (top) and CD80 (bottom) in cells un-stimulated (green) or stimulated 16 hours with 5-A-RU/MG, in the presence (pink) or absence (light blue) of anti-MR1 blocking antibody. B, C and D) Cumulative data for 7 donors (6 for PDC), depicting geometric MFI for CD80 (black), CD86 (blue) and CD40 (purple) for cells un-stimulated or stimulated with 5-A-RU/MG, in the presence or absence of anti-MR1 blocking antibody. Data are depicted as box and whiskers, with all points indicated, +/- SD; * denotes p<0.05.
Figure 7.
A) FACS dot plots showing intracellular expression of IL-12 and TNF-α in monocytes and DC (gated as in Fig. 6 and supplemental Fig. 4), 16 hours after incubation with 5-A-RU/MG in the presence or absence of anti-MR1 or anti-CD40L blocking antibodies. Profiles of LPS and CpG stimulated cells are shown as positive control. B) Cumulative data for the intracellular TNF-α expression described in A. Data are depicted as box and whiskers, with all points indicated +/ SD (n=4). C) IL-12p70 detected by Simoa immunoassay in whole blood stimulated for 16 hours with 5-A-RU/MG in the presence or absence of anti-MR1 or anti-CD40L blocking antibodies. Cumulative data (n=6, 5 for anti CD40L) depicted as box and whiskers, with all points indicated +/ SD; * denotes p<0.05. D) PBMC were stimulated with 5-A-RU/MG in the presence or absence of blocking antibodies to MR1 (n=8), IL-18R (n=14), IL-12 and CD40L (n=6) and the percentage of IFN-γ secreting NK cells was determined by FACS after 16 hours. The response with antigen was normalised to 100%; we observed background IFN-γ in the majority of the donors tested. Each symbol represents a donor; * denotes p<0.05, ** denotes p<0.01.
Discussion
In this paper, we have demonstrated that human MAIT cells rapidly up-regulate CD40L upon activation by the cognate antigen 5-A-RU/MG presented by the non-classical class I molecule MR1. Furthermore, we have shown that MAIT cells are able to instruct DC maturation in a CD40L and MR1 dependent manner, resulting in secretion of bioactive IL-12. Notably, we have demonstrated MAIT-dependent DC maturation with primary CD1c DC and PDC as well as monocyte-derived DC.
It is now well accepted that DC maturation is key to the successful induction of adaptive T cell responses. Immature DC patrolling peripheral tissues are exposed to a variety of maturation signals (pathogens associated molecular patterns, PAMPs, or danger associated molecular patterns, DAMPs) that, through specific sensors (pattern recognition receptors), initiate the activation/maturation process, leading to DC migration to the draining lymph node and antigen presentation to naïve T cells (24). Pattern recognition receptors tailor the quality of the adaptive immune response to the nature of the pathogen (25). Full DC maturation, and generation of cytotoxic T cell responses, also requires engagement of the TNF superfamily receptor CD40 (15). In addition to rare antigen specific activated CD4+ T cells (26), innate lymphocytes such as NK cells, iNKT cells and γδ T cells, have also been shown to constitutively express CD40L and trigger CD40L-dependent DC maturation (17, 18, 27). We have now demonstrated that, upon antigen specific activation, MAIT cells also rapidly up-regulate CD40L at the cell surface, and in synergy with TLR ligands induce secretion of bioactive IL-12 by DC. Our results complement a previous report demonstrating the presence of soluble CD40L in the supernatant of human MAIT cells cultures activated by bacteria, although the functional relevance of this observation was not addressed at the time (28). Altogether, these results point to an important immune-regulatory function of MAIT cells, in agreement with their observed ability to induce GM-CSF-dependent differentiation of inflammatory monocytes into monocyte-derived DC (6).
We have observed that MAIT cells induced partial DC activation, even in the absence of exogenous ligands. We refer to this as basal MAIT cell auto-reactivity and future work will be required to determine whether it is due to endogenous ligand or medium derived vitamins, or a combination of the two. Activation of murine MAIT hybridomas has been previously observed in response to MR1 over-expressing APC (29–31), and while this was considered evidence of self-ligand presentation, the ligand was not identified. Recently, within the conventional Vα7.2 bearing MAIT population a subset of auto-reactive and folate reactive cells was identified, whose activity was strictly dependent on the TCR-β chain (32). We have also observed variability in the extent of basal auto-reactivity within our Vα7.2+ CD161+ MAIT sorted cells, which could be accounted for by heterogeneous TCR-β repertoire within our blood donor cohort (unpublished results). Although we have observed basal autoreactivity in both autologous as well as allogeneic settings, further experiments are warranted to investigate whether, despite the monomorphic nature of MR1, basal autoreactivity of MAIT cells may be modulated by differential expression of KIR or by HLA mismatch between MAIT cells and DC.
Finally, in addition to DC maturation, MAIT cell activation in whole blood led to NK cell transactivation, in an MR1 and IL-18 dependent manner. NK cell transactivation has also been observed upon iNKT cell activation with a number of iNKT cell agonists, such α-GalCer (33) and its non glycosidic derivatives (34), and it is essential for the anti metastatic activity of iNKT cells and the sustained IFN-γ secretion observed upon iNKT cell activation (33). The investigation of the in vivo consequences of MAIT cell activation upon 5-A-RU/MG triggering will be the subject of future studies.
Similarities and differences have emerged between the two populations of innate-like T cells for which synthetic antigens and tetramers are available, MAIT and iNKT cells. Parallel thymic selection processes on double positive thymocytes lead to appearance of cells which need further shaping in the periphery, in a process dependent on microbial flora (35–37). While a shared developmental niche has been suggested to regulate iNKT cell numbers (35), it is unclear to which extent MAIT and iNKT cell function is co-regulated in the periphery. Nevertheless, the ability of both MAIT and iNKT cells to regulate DC function (this paper) and differentiation (6) warrants further investigation. iNKT cell agonists are currently being tried in the clinic to enhance antigen specific immune responses (38–40), and we anticipate that harnessing MAIT cells in humans may offer important additional strategies to promote the development of mucosal and systemic immunity. Indeed, the higher precursor frequency and memory phenotype of MAIT cells (41) renders them particularly attractive “natural adjuvants”, to enhance immune responses against pathogens and for cancer immunotherapy (42).
Supplementary Material
Acknowledgements
We thank Dr Ted Hansen for the gift of anti MR1 26.5 antibody. We acknowledge the contribution of Craig Waugh and the WIMM Flow cytometry facility for cell sorting of MAIT cells. We thank Dr Hashem Koohy for advice on statistical analysis.
This work was supported by the UK Medical Research Council (MRC Human Immunology Unit) and Cancer Research UK (Programme Grant #C399/A2291). G.S.B. acknowledges support in the form of a Personal Research Chair from James Bardrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow. G.S.B. also acknowledges funding from the MRC (grant MR/K012118/1) and the Wellcome Trust (grant 081569/Z/06/Z).
Nonstandard abbreviations
- MG
methyl glyoxal
Footnotes
Author contributions
MS designed the research, performed the experiments and wrote the manuscript. OG, CGL, AM, UG, JV, GN performed experiments. NV, RA, GP, GSB contributed essential reagents. IH, VC contributed to research design and writing of the manuscript.
References
- 1.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 2.Salio M, Cerundolo V. Regulation of Lipid Specific and Vitamin Specific Non-MHC Restricted T Cells by Antigen Presenting Cells and Their Therapeutic Potentials. Front Immunol. 2015;6:388. doi: 10.3389/fimmu.2015.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, Williamson NA, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 4.McWilliam HE, Eckle SB, Theodossis A, Liu L, Chen Z, Wubben JM, Fairlie DP, Strugnell RA, Mintern JD, McCluskey J, Rossjohn J, et al. The intracellular pathway for the presentation of vitamin B-related antigens by the antigen-presenting molecule MR1. Nat Immunol. 2016;17:531–537. doi: 10.1038/ni.3416. [DOI] [PubMed] [Google Scholar]
- 5.Wong EB, Ndung'u T, Kasprowicz VO. The role of mucosal-associated invariant T cells in infectious diseases. Immunology. 2017;150:45–54. doi: 10.1111/imm.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meierovics AI, Cowley SC. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J Exp Med. 2016;213:2793–2809. doi: 10.1084/jem.20160637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traunecker A, Oliveri F, Karjalainen K. Myeloma based expression system for production of large mammalian proteins. Trends Biotechnol. 1991;9:109–113. doi: 10.1016/0167-7799(91)90038-j. [DOI] [PubMed] [Google Scholar]
- 8.Davoll J, Evans DD. The synthesis of 9-glycitylpurines, 3-glycityl-[1,2,3]-triazolo[d]-pyrimidines, 8-glycitylpteridines, and 10-glycitylbenzo[g]pteridines, including riboflavin and riboflavin 2-imine. J Chem Soc. 1960:5041–5049. [Google Scholar]
- 9.Maley GF, Plaut GW. The isolation, synthesis, and metabolic properties of 6, 7-dimethyl-8-ribityllumazine. J Biol Chem. 1959;234:641–647. [PubMed] [Google Scholar]
- 10.Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, Piech T, Patel PP, Chang L, Rivnak AJ, Ferrell EP, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotech. 2010;28:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, Chen Z, Reantragoon R, Meehan B, Cao H, Williamson NA, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 12.Eckle SB, Corbett AJ, Keller AN, Chen Z, Godfrey DI, Liu L, Mak JY, Fairlie DP, Rossjohn J, McCluskey J. Recognition of Vitamin B Precursors and Byproducts by Mucosal Associated Invariant T Cells. J Biol Chem. 2015;290:30204–30211. doi: 10.1074/jbc.R115.685990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SB, Uldrich AP, Birkinshaw RW, Patel O, Kostenko L, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210:2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanzavecchia A. Immunology. Licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 16.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 17.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT Cells Enhance CD4(+) and CD8(+) T Cell Responses to Soluble Antigen In Vivo through Direct Interaction with Dendritic Cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 19.Soudais C, Samassa F, Sarkis M, Le Bourhis L, Bessoles S, Blanot D, Herve M, Schmidt F, Mengin-Lecreulx D, Lantz O. In Vitro and In Vivo Analysis of the Gram-Negative Bacteria-Derived Riboflavin Precursor Derivatives Activating Mouse MAIT Cells. J Immunol. 2015;194:4641–4649. doi: 10.4049/jimmunol.1403224. [DOI] [PubMed] [Google Scholar]
- 20.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, Mettke E, Kurioka A, Hansen TH, Klenerman P, Willberg CB. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McWilliam HE, Birkinshaw RW, Villadangos JA, McCluskey J, Rossjohn J. MR1 presentation of vitamin B-based metabolite ligands. Curr Opin Immunol. 2015;34C:28–34. doi: 10.1016/j.coi.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, Kang YH, Walker LJ, Hansen TH, Willberg CB, Klenerman P. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8:429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dias J, Leeansyah E, Sandberg JK. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1705759114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 25.Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nat Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- 26.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202:203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, Lee B, Poidinger M, Zolezzi F, Quagliata L, Sander P, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRbeta repertoire. Nat Commun. 2014;5:3866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- 29.Huang SS, Gilfillan C, Cella M, Miley MJ, Lantz O, Lybarger L, Fremont DH, Hansen TH. Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J Biol Chem. 2005;280:21183–21193. doi: 10.1074/jbc.M501087200. [DOI] [PubMed] [Google Scholar]
- 30.Huang S, Gilfillan S, Kim S, Thompson B, Wang X, Sant AJ, Fremont DH, Lantz O, Hansen TH. MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J Exp Med. 2008;205:1201–1211. doi: 10.1084/jem.20072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, Lantz O, Hansen TH. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci U S A. 2009;106:8290–8295. doi: 10.1073/pnas.0903196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gherardin NA, Keller AN, Woolley RE, Le Nours J, Ritchie DS, Neeson PJ, Birkinshaw RW, Eckle SB, Waddington JN, Liu L, Fairlie DP, et al. Diversity of T Cells Restricted by the MHC Class I-Related Molecule MR1 Facilitates Differential Antigen Recognition. Immunity. 2016;44:32–45. doi: 10.1016/j.immuni.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–1266. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 34.Jukes JP, Gileadi U, Ghadbane H, Yu TF, Shepherd D, Cox LR, Besra GS, Cerundolo V. Non-glycosidic compounds can stimulate both human and mouse iNKT cells. Eur J Immunol. 2016;46:1224–1234. doi: 10.1002/eji.201546114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koay HF, Gherardin NA, Enders A, Loh L, Mackay LK, Almeida CF, Russ BE, Nold-Petry CA, Nold MF, Bedoui S, Chen Z, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nature Immunology. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 36.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, Cherif S, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 38.Exley MA, Friedlander P, Alatrakchi N, Vriend L, Yue SC, Sasada T, Zang W, Mizukami Y, Clark J, Nemer D, LeClair K, et al. Adoptive Transfer of Invariant NKT Cells as Immunotherapy for Advanced Melanoma: a Phase 1 Clinical Trial. Clinical Cancer Research. 2017 doi: 10.1158/1078-0432.ccr-16-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunn MK, Hermans IF. Exploiting invariant NKT cells to promote T-cell responses to cancer vaccines. Oncoimmunology. 2013;2:e23789. doi: 10.4161/onci.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McEwen-Smith RM, Salio M, Cerundolo V. The regulatory role of invariant NKT cells in tumor immunity. Cancer Immunol. Res. 2015;3:425–435. doi: 10.1158/2326-6066.CIR-15-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dusseaux M, Martin E, Serriari N, Peguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, Lantz O. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 42.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.