Abstract
Background
Cardiac myosin-binding protein C (cMyC) is a cardiac-restricted protein that is more abundant than cardiac troponins (cTn) and is released more rapidly following acute myocardial infarction (AMI). We evaluated cMyC as an adjunct or alternative to cTn in the early diagnosis of AMI.
Methods
In 1954 unselected patients presenting to the emergency department with symptoms suggestive of AMI, concentrations of cMyC and high (hs) and standard (s) sensitivity cTn were measured at presentation. The final diagnosis of AMI was independently adjudicated using all available clinical and biochemical information without knowledge of cMyC. The prognostic endpoint was long-term mortality.
Results
Final diagnosis was AMI in 340 patients (17%). Concentrations of cMyC at presentation were significantly higher in those with vs. without AMI (median 237 ng/L vs. 13 ng/L, p<0.001). Discriminatory power for AMI, as quantified by the area under the receiver-operating characteristic curve was comparable for cMyC (AUC; 0.924), hs-cTnT (0.927) and hs-cTnI (0.922) and superior to cTnI measured by a contemporary sensitivity assay (0.909). Combination of cMyC with hs-cTnT or s-cTnI (but not hs-cTnI) led to an increase in AUC to 0.931 (p<0.0001) and 0.926 (p=0.003), respectively. Use of cMyC more accurately classified patients with a single blood test into rule-out or rule in categories: Net Reclassification Improvement (NRI) +0.149 vs hs-cTnT, +0.235 vs hs-cTnI (p<0.001). In early presenters (chest pain <3h), the improvement in rule-in/rule-out classification with cMyC was larger compared with hs-cTnT (NRI +0.256) and hs-cTnI (NRI +0.308; both p<0.001). Comparing the C statistics, cMyC was superior to hs-cTnI and s-cTnI (p<0.05 both) and similar to hs-cTnT at predicting death at 3 years.
Conclusions
cMyC at presentation provides discriminatory power comparable to hs-cTnT and hs-cTnI in the diagnosis of acute myocardial infarction, and may perform favorably in patients presenting early after symptom onset.
Trial-Registration: www.clinicaltrials.gov. Identifier, NCT00470587
Keywords: Cardiac myosin-binding protein C, cMyC, Troponin I, Troponin T, myocardial infarction, APACE
Introduction
Of the 130 million attendances to Emergency Departments (ED) in the United States each year, approximately 7 million (6%) are due to acute chest pain.1 The assessment and triage of such patients has become increasingly complex as now only a small proportion of those with acute myocardial infarction (AMI) have the diagnostic ECG change of ST-segment elevation.2 Consequently, the identification of patients with AMI has become almost totally dependent on the measurement in the systemic circulation of cardiac troponin I (cTnI) or cardiac troponin T (cTnT). These biomarkers are released slowly3 - to overcome this hurdle, the analytic performance of the cTn assays has been enhanced markedly to measure the lower concentrations achieved before the late peak.4 Hence, the best assays can reliably measure cTn concentrations below the 99th centile of the healthy population. These high-sensitivity (hs) assays are increasingly available and are the subject of national and international guidelines describing their use to achieve more rapid triage.5,6 In particular, the European guidelines recommend the use of assays for hs-cTnI and hs-cTnT to rapidly rule-in and rule-out AMI. Algorithms using widely based decision limits based on concentrations well below the population defined 99th centile (for rule out) and above the 99th centile (for rule in) markedly improves the sensitivity of rule-out and specificity of rule-in. However, many patients presenting with chest pain have cTn concentrations that place them between these decision limits; in an indeterminate observation zone. These patients require repeat testing and subsequent second or third rounds of triage based on rates of change of cTn concentration over time.6–8 European guidelines also do not support the use of rapid rule-out/rule-in pathways using hs-cTn in patients presenting ‘too early’ after chest pain onset – only after 3 hours is the rule-out threshold at the limit of detection guideline-compliant.6 This introduces systemic delays in allocation of evidence-based treatments and prolongs stay in the pressured and precious environment of the ED.
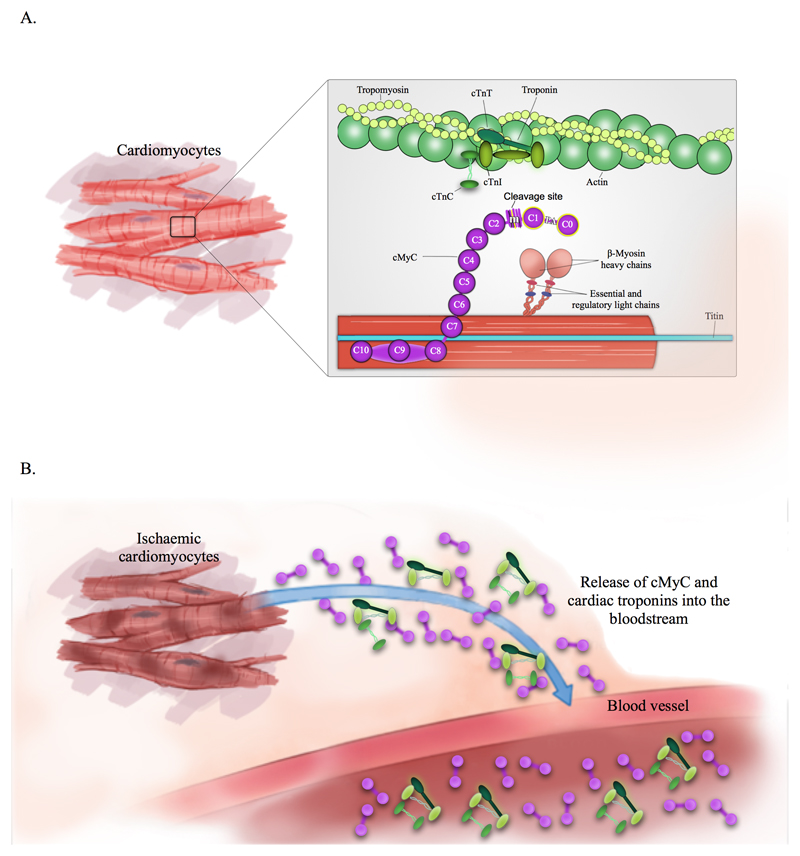
Originally discovered by Offer et al in 19739, the myosin-binding protein C family consists of three isoforms, specific for slow skeletal, fast skeletal and cardiac muscle – the latter being exclusively expressed in the heart from neonatal throughout human development.10,11 Amongst others12–15, we have identified cardiac myosin-binding protein C (cMyC, see figure 1) as a new candidate biomarker of cardiac injury.16 In common with cTnT and cTnI, cMyC expression is restricted to the heart but it is more abundant.17 Moreover, cMyC rises more rapidly in the systemic circulation than hs-TnT after timed, iatrogenic AMI16, perhaps as a result of its higher myocardial concentration.18 Using a recently developed high-sensitivity assay for cMyC19, a pilot study in 26 patients presenting early with AMI suggested that cMyC may rise more rapidly than hs-cTnI.20
Figure 1.
Structure of cardiac Myosin-binding protein C and cardiac troponins in (A) healthy cardiomyocytes and (B) Ischaemia-induced cardiomyocyte damage. The highlighted N-terminal domain C0C1 is the binding site for the previously developed monoclonal antibodies used for detection of the cardiac-specific isoform of cMyC – see Baker et al, 201516
The purpose of the current study is to compare the novel biomarker cMyC (measured on a research platform) against the most accurate currently available biochemical signals, hs-cTnI and hs-cTnT, for the early detection of AMI.
Methods
Study design and population
Advantageous Predictors of Acute Coronary Syndrome Evaluation (APACE) is an ongoing international multicenter diagnostic study (nine study centers in Switzerland, Spain, Poland, the Czech republic, and Italy) designed to advance the early diagnosis of AMI.4,21–23 All patients older than 18 years presenting to the ED with acute chest discomfort possibly indicating AMI were eligible for recruitment if the onset of, or peak chest pain symptoms, were within the preceding 12 hours. Enrolment was independent of renal function, while patients with terminal kidney failure on chronic dialysis were excluded. For this analysis, the following patients were excluded (figure S1): patients presenting with ST-segment elevation myocardial infarction; patients with missing levels of cMyC at presentation; patients in whom the final diagnosis remained unclear after adjudication and at least one hs-cTnT level was elevated. The latter group comprises of patients triaged and discharged following a negative gold-standard test at the time of enrolment (on a conventional cTn assay), who were later found to have an elevated hs-cTn result (comparison see table S1). A proportion of patients had no levels of cMyC measured at presentation due to insufficient sample volume. Demographics of the patients excluded due to missing cMyC values, compared to those of the test cohort, appear in the supplement (table S2). The protocol for routine clinical assessment is also described in the supplement. To obtain follow-up data, patients were contacted 3, 12, 24 and 36 months after discharge via telephone, email or letter. Additionally, information regarding death during follow-up was obtained from the patient’s hospital notes, the family physician’s records and the national registry on mortality.
The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees. Written informed consent was obtained from all patients. TK, RT and CM had full access to all the data in the study and take responsibility for its integrity and the data analysis. The authors designed the study, gathered, and analyzed the data according to the STARD guidelines for studies of diagnostic accuracy (table S3), vouch for the data and analysis, wrote the paper, and decided to publish.
Adjudicated final diagnosis
Adjudication of the final diagnosis was performed centrally according to the universal definition of MI, incorporating levels of hs-cTnT as the adjudicating biomarker.24 It was based on extensive patient documentation derived from two sets of data: First, all clinical data derived from routine clinical investigations including all available medical records - patient history, physical examination, results of laboratory testing including serial local (h)s-cTn, radiologic testing, ECG, echocardiography, cardiac exercise stress test, lesion severity and morphology at coronary angiography - pertaining to the patient from the time of ED presentation to 90-day follow up. Second, study-specific assessment was collected, including 34 chest pain characteristics and serial hs-cTnT measurements in order to take advantage of the higher sensitivity and higher overall diagnostic accuracy offered by the more sensitive assays, as previously published.4,21 In situations of disagreement about the diagnosis, cases were reviewed and adjudicated in conjunction with a third cardiologist. In brief, AMI was diagnosed when there was evidence of myocardial necrosis in association with a clinical setting consistent with myocardial ischemia. Myocardial necrosis was diagnosed by at least one (h)s-cTn value above the 99th percentile together with a significant rise and/or fall.25–27 All other patients were classified into the categories of unstable angina (UA), cardiac but non-coronary disease (e.g. tachyarrhythmias, perimyocarditis), non-cardiac chest pain and symptoms of unknown origin.
Measurement of cMyC, hs-cTnI, hs-cTnT, and s-cTnI
Blood samples for determination of cMyC, hs-cTnI, hs-cTnT, and s-cTnI were collected into heparin plasma and serum tubes at presentation to the ED and serially thereafter (at time points 1 h, 2 h, 3 h and 6 h). Serial sampling was discontinued when a diagnosis of AMI was certain and treatment required patient transfer to the coronary care unit or catheter laboratory. After centrifugation, samples were frozen at -80°C until they were assayed in a blinded fashion in a dedicated core laboratory. cMyC was measured using the previously established high-sensitivity assay on the Erenna platform that was performed by Millipore Sigma (Hayward, California).19 The assay has a Limit of Detection (LoD) of 0.4 ng/L and a lower limit of quantification (LoQ) of 1.2 ng/L. The 99th percentile cut-off point determined previously (in patients without obstructive coronary artery disease on invasive angiography) is 87 ng/L.19 Details of the assays used for hs-cTnI, hs-cTnT, and s-cTnI are described in the supplement.
Early guideline-based triage and Net Reclassification Improvement
The European Society of Cardiology (ESC) has published a rapid rule-in/rule-out pathway in the 2015 NSTEMI guidelines using hs-cTn at 0h and 1h to risk-stratify patients into ‘rule-out’, ‘observe’ and ‘rule-in’ categories.6 Such categorization did not drive clinical decisions in this cohort, but it was used to compare the potential clinical utilities of cMyC and hs-cTn as triage tools. For this purpose, we have compared the categorical discrimination of hs-cTnT, hs-cTnI and cMyC at presentation only (without subsequent delta measurements). In brief, the ESC pathway classifies patients – based on the presentation sample at 0h – into ‘rule-out’ with a hs-cTnT level <5 ng/L; hs-cTnI <2 ng/L; into ‘rule-in’ (for both assays) at ≥52 ng/L.6 The ESC advocates the use of the pathway only in patients with ≥3 hours since chest pain onset; for completeness we have presented results for all patients, <3 and ≥3 hours since chest pain onset alone.
For cMyC we separated the cohort into derivation and validation cohorts (a randomized 3:7 split, for comparison see table S4); the ‘rule-out’ threshold was derived from a pre-defined sensitivity of ≥99.5%, ‘rule-in’ from a pre-defined specificity >95% for the gold-standard diagnosis of AMI. This resulted in a ‘rule-out’ threshold of ≤10 ng/L, and ‘rule-in’ threshold of >120 ng/L for cMyC (figure S2). These thresholds were then used in the validation cohorts to compare cMyC against both hs-cTnT and hs-cTnI. NRI operates as follows: each patient is first assigned a classification (‘rule-out’, ‘observe’ or ‘rule-in’) based on cut-off values of hs-cTnI/T in the presentation blood sample (the initial model). The same cohort is then reclassified to the same three groups based on the cMyC cut-off values (the new model). This reclassification may correctly or incorrectly reallocate a patient, e.g. a patient who went on to be diagnosed with an AMI may be correctly reclassified from ‘observe’ to ‘rule-in’, or incorrectly reclassified from ‘observe’ to ‘rule-out’. The ‘NRI’ analysis defines separate categorical NRI values for those patients who were ultimately diagnosed with AMI (quoted as NRIAMI) and those who were not (NRInoAMI) – range -1 to +1; ‘Dimensionless NRI’ reflects the unweighted, net-movement of all patients regardless of final diagnosis (range -2 to +2). NRIAMI is positive if there is a net movement of patients with adjudicated AMI into higher-risk classifications using cMyC (the new model). NRInoAMI is positive there is a net movement of patients without an adjudicated diagnosis of AMI into lower-risk classifications using cMyC (the new model).28 NRI calculations were performed for the validation cohort, early presenters (<3 hours since onset of chest pain; ESC guideline not applicable) and late presenters (≥3 hours since onset; ESC guideline applicable); tables are presented in full where appropriate.
Statistical analysis
All data are expressed as medians [1st quartile, 3rd quartile] or means (standard deviation) for continuous variables (compared with the Mann-Whitney-U test or student's t-test), and for categorical variables as numbers and percentages (compared with Pearson chi-square). Hypothesis testing was two-tailed, and p values <0.05 were considered statistically significant. No adjustment for multiple comparisons was performed.
Discrimination power was quantified by the area under the receiver-operating characteristics curve (AUC) for each biomarker with all cases available, using 1,000 stratified bootstrap replicates to calculate Confidence intervals (CI). Logistic regression was used to combine cMyC levels with hs-cTnT, hs-cTnI or s-cTnI values for the assessment of an incremental value using two biomarkers at presentation. Sub-group analysis was performed for patients presenting early, defined as chest pain onset within 3 hours of presentation to the Emergency Department. This is a particular limitation of the published ESC guidance on the use of hs-cTn for risk-stratification, as the rapid rule-out/rule-in algorithms are only applicable to patients with chest pain onset >3 hours.
Predictive value of the biomarkers during follow-up was assessed two-fold: We calculated 1) Harrell’s C statistic for each biomarker at presentation for endpoints AMI, death or the composite of AMI and all-cause mortality during follow-up (excluding the index event) – a higher C index indicates a higher probability of an event occurring during follow-up with higher biomarker values29; and 2) Kaplan-Meier survival curves. Cox regression analysis was performed as follows: All available biomarker levels were divided into 1) quintiles and 2) groups according to ‘rule-out’, ‘observe’ and ‘rule-in’ classification. Unadjusted Cox proportional hazard regression models were fitted for 30-day and 3-year follow-up for each group with the lowest quintile (or risk group, respectively) normalized to a hazard ratio of 1 and assessed using the likelihood-ratio test. Cox coefficients and thus hazard ratios were not calculated if the lowest risk group did not suffer any events, which would invalidate the regression model. NRI statistics were calculated as categorical values.28,30 The Integrated Discrimination Improvement (IDI) values quoted reflect a category-free (positive or negative) change in model-performance. Confidence intervals for cut-off thresholds, NRI and IDI statistics were derived using 1,000 bootstrap replicates. All statistical analyses were performed using R, version 3.3.0 GUI 1.68 (The R Foundation for Statistical Computing), including packages ggplot2, R Markdown, RStudio, PredictAbel, survival, Hmisc, compareC and ROCR.
Results
Baseline characteristics
A total of 1954 unselected patients eligible for this analysis were enrolled (Figure S1). Median age was 62 years, 31% were women, and 36% had a prior history of coronary artery disease (table 1). Overall, 1469 patients (75%) had no significant electrocardiographic abnormalities at presentation to the ED. Median time since onset of chest pain was 5 hours [IQR 3, 12], with a median of 3 hours [IQR 2, 7] since peak chest pain severity.
Table 1. Demographics.
| Demographics | All patients (n = 1954) |
AMI (n = 340) |
Other diagnoses (n = 1614) |
p value* |
|---|---|---|---|---|
| Age, years | 62 ± 16 | 69 ± 13 | 60 ± 16 | <0.001 |
| Male | 1341 (69) | 256 (75) | 1085 (67) | 0.004 |
| Risk factors | ||||
| Hypertension | 1247 (64) | 269 (79) | 978 (61) | <0.001 |
| Hyperlipidaemia | 992 (51) | 227 (67) | 765 (47) | <0.001 |
| Diabetes mellitus | 369 (19) | 92 (27) | 256 (16) | <0.001 |
| Current smoking | 500 (25) | 90 (27) | 386 (24) | 0.345 |
| History of smoking | 718 (38) | 141 (42) | 577 (36) | 0.051 |
| History | ||||
| Coronary artery disease | 710 (36) | 174 (51) | 536 (33) | <0.001 |
| Previous myocardial infarction | 474 (24) | 118 (35) | 356 (22) | <0.001 |
| Previous revascularisation (CABG or PCI) | 553 (28) | 127 (37) | 426 (26) | <0.001 |
| Peripheral artery disease | 119 (6) | 43 (13) | 76 (5) | <0.001 |
| Previous stroke | 100 (5) | 23 (7) | 77 (5) | 0.167 |
| Vital status | ||||
| Heart rate, beats/min | 79 ± 20 | 81 ± 20 | 79 ± 20 | 0.092 |
| Systolic blood pressure, mm Hg | 144 ± 24 | 145 ± 27 | 143 ± 24 | 0.421 |
| Diastolic blood pressure, mm Hg | 82 ± 15 | 81 ± 17 | 82 ± 15 | 0.299 |
| Electrocardiographic findings | ||||
| ST-segment depression | 193 (10) | 93 (28) | 100 (6) | <0.001 |
| T-wave inversion | 260 (13) | 82 (24) | 178 (11) | <0.001 |
| No significant electrocardiographic abnormalities | 1469 (75) | 161 (49) | 1308 (83) | <0.001 |
| Laboratory assessment | ||||
| Estimated glomerular filtration rate, ml/min/1.73m2† | 84 ± 26 | 74 ± 26 | 86 ± 25 | <0.001 |
| Presentation time | ||||
| Time since chest pain onset, hours | 5 [3, 12] | 5 [3, 12] | 5 [3, 12] | 0.898 |
| Time since chest pain peak, hours | 3 [2, 7] | 3 [2, 7] | 4 [2, 7] | 0.408 |
p values for comparison AMI group versus all other diagnoses; data are expressed as medians [1st quartile, 3rd quartile] or means ± standard deviation, for categorical variables as numbers (percentages);
AMI = Acute Myocardial Infarction; IQR = Interquartile Range; CABG = Coronary Artery Bypass Graft; PCI = Percutaneous Coronary Intervention;
glomerular filtration rate was estimated using the Modification of Diet in Renal Disease (MDRD) formula
The adjudicated final diagnosis was AMI in 340 (17%) patients, unstable angina in 10%, symptoms of cardiac origin other than coronary artery disease in 14%, non-cardiac symptoms in 54% and symptoms of unknown origin in 5%.
Median follow-up for the entire cohort was 772 days [IQR 731, 907]; of those not sustaining any events in the monitoring period (AMI or death), the median follow-up was 792 days [IQR 738, 923]. A total of n=165 (8%) patients died during 3-year follow-up. 1903 patients (97%) exceeded 90 days of follow-up; of those who did not (n=51, 3%), 27 (1%) sustained a cardiovascular death.
Distribution of biomarker concentrations
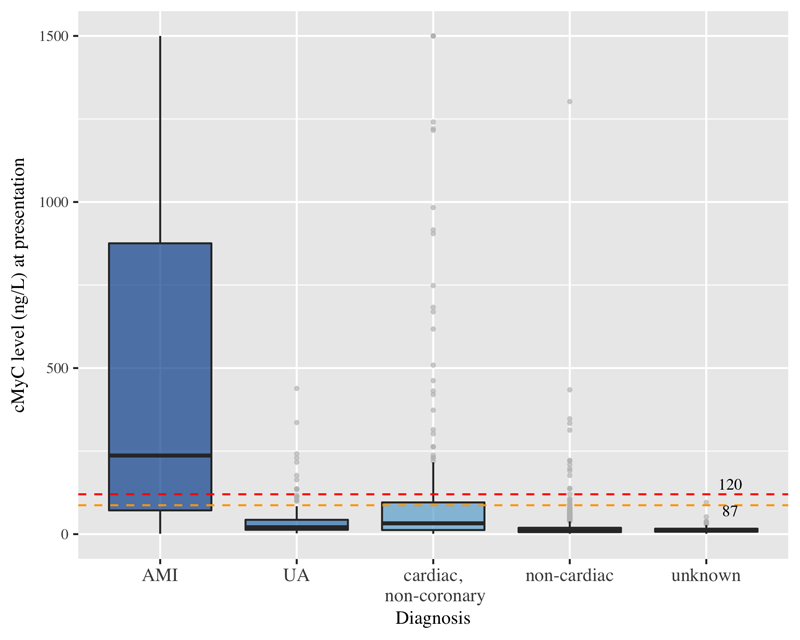
As shown in figure 2, cMyC levels were significantly higher in patients with AMI (n=340) compared to patients with other diagnoses (AMI, median 237 ng/L [IQR 71, 876 ng/L; unstable angina, median 21 ng/L [IQR 13, 43 ng/L]; cardiac symptoms of origin other than coronary artery disease, median 33 ng/L [IQR 12, 96 ng/L]; non-cardiac symptoms, median 10 ng/L [IQR 6, 19 ng/L]; symptoms of unknown origin, median 11 ng/L [IQR 7, 16 ng/L]; p <0.001 for all comparisons with AMI patients). Similarly, blood concentrations of hs-cTnT, hs-cTnI, and s-cTnI were significantly higher in AMI as compared to other final diagnoses (median biomarker concentrations displayed in tables S5+S6). Overall, blood concentrations of cMyC in relation to LoD were higher than those of hs-cTn in all diagnostic categories (Table S5). Non-cardiac sources of cMyC variation were previously investigated in an ambulatory cohort19; results of comparison within the groups with AMI and non-cardiac symptoms have been displayed in tables S7+S8.
Figure 2.
Baseline distribution of cMyC levels at presentation to the emergency department in all patients based on adjudicated final diagnosis. Boxes represent interquartile ranges, whiskers extend to 1.5 * IQR from the hinges (y-axis capped at 1,500 ng/L, outliers represented by light grey bullets). 87 ng/L represents the 99th centile based on a previous study, 120 ng/L the cut-off threshold for diagnostic rule-in of AMI at presentation. AMI, median 237 ng/L [IQR 71, 876 ng/L]; unstable angina, median 21 ng/L [IQR 13, 43 ng/L]; cardiac symptoms of origin other than coronary artery disease, median 33 ng/L [IQR 12, 96 ng/L]; non-cardiac symptoms, median 10 ng/L [IQR 6, 19 ng/L]; symptoms of unknown origin, median 11 ng/L [IQR 7, 16 ng/L]; p<0.001 for all comparisons with AMI patients
Discrimination power
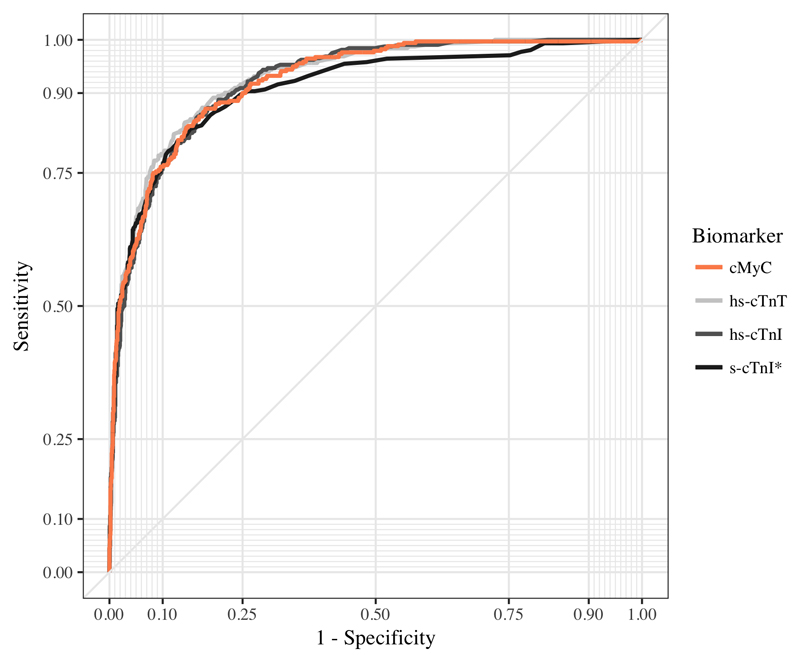
In blood drawn at presentation, the discrimination of cMyC for AMI, as quantified by the AUC, was 0.924 (95% confidence interval [CI], 0.910-0.939), compared to the AUCs for hs-cTnT 0.927 (95% CI, 0.913-0.941; p=0.573 for direct comparison); hs-cTnI 0.922 (95% CI, 0.908-0.936; p=0.993 for direct comparison) and s-cTnI 0.909 (95% CI, 0.889-0.928; p=0.024 for direct comparison, table 2, figure 3).
Table 2. Area under the Receiver-Operating Characteristics Curve – Comparisons between biomarkers.
| All patients - comparison | Area Under the Curve (95% Confidence Interval) | p value* | n |
|---|---|---|---|
| cMyC vs hs-cTnT | 0.924 (0.910-0.939) vs 0.927 (0.913-0.941) | 0.573 | 1554 controls, 322 AMI |
| cMyC vs hs-cTnI | 0.923 (0.908-0.937) vs 0.922 (0.908-0.936) | 0.993 | 1537 controls, 320 AMI |
| cMyC vs s-cTnI | 0.924 (0.906-0.938) vs 0.909 (0.889-0.928) | 0.024 | 1463 controls, 311 AMI |
| Early presenters (≤ 3 hours since chest pain onset) - comparison | |||
| cMyC vs hs-cTnT | 0.915 (0.887-0.941) vs 0.892 (0.857-0.922) | 0.022 | 562 controls, 104 AMI |
| cMyC vs hs-cTnI | 0.915 (0.889-0.939) vs 0.909 (0.879-0.935) | 0.539 | 554 controls, 102 AMI |
| cMyC vs s-cTnI | 0.914 (0.888-0.939) vs 0.892 (0.859-0.925) | 0.060 | 529 controls, 103 AMI |
| All patients - Combination cMyC with… | p value† | n | |
| hs-cTnT | 0.935 (0.921-0.948) | 0.002 | 1548 controls, 322 AMI |
| hs-cTnI | 0.929 (0.913-0.943) | 0.093 | 1537 controls, 320 AMI |
| s-cTnI | 0.928 (0.909-0.943) | <0.001 | 1463 controls, 311 AMI |
p value for direct comparison between biomarkers;
p value for direct comparison between AUC for combination (cMyC with cTn) and respective cTn on its own; AUC = Area under the Curve; AMI = Acute Myocardial Infarction
Figure 3.
ROC curves for individual biomarkers: Diagnostic performance of cMyC, hs-cTnT, hs-cTnI and s-cTnI in the early diagnosis of acute myocardial infarction (AMI), based on presentation blood sample and adjudicated AMI diagnosis. Receiver operating characteristic (ROC) curves describing the performance of cMyC (orange line; Area under the Curve (AUC) 0.924), hs-cTnT (light grey line; AUC 0.927), hs-cTnI (dark grey line; AUC 0.922) and s-cTnI (black line; AUC 0.909*); *p<0.05
Early presenters
In patients presenting within 3 hours of symptom onset (n=694, with AMI adjudicated in 16%) the AUC for cMyC was 0.915 (95% CI, 0.887-0.941), compared to the AUCs for hs-cTnT 0.892 (95% CI, 0.857-0.922; p=0.022); hs-cTnI 0.909 (95% CI, 0.879-0.935; p=0.539) and s-cTnI 0.892 (95% CI, 0.859-0.925; p=0.060) (table 2).
Combination of cMyC with cTn
AUC for the combination of cMyC with hs-cTnT was 0.935 (95% CI, 0.921-0.948; p=0.002 for comparison with hs-cTnT alone), cMyC with hs-cTnI 0.929 (95% CI, 0.913-0.943; p=0.093 for comparison with hs-cTnI alone) and cMyC with s-cTnI 0.928 (95% CI, 0.909-0.943; p<0.001 for comparison with s-cTnI alone) (table 2, figure S3).
Classification function of cut-off values for risk groups
Sensitivity, specificity, negative and positive predictive values were calculated for derivation (tables S9, S10) and validation cohorts based on cut-offs published in the 2015 ESC guideline6: in the validation cohort (n=1,368, 233 events), hs-cTnT has a sensitivity of 99.6% (95% CI, 98.5-100%) and NPV of 99.7% (95% CI, 99-100%) at the rule-out threshold of 5 ng/L, specificity of 97.1% (95% CI, 96.1-98%) and PPV 80.1% (95% CI, 73.2-86.2%) at the rule-in threshold (52 ng/L); hs-cTnI has a sensitivity of 100% (95% CI, 100-100%) and NPV of 100% (95% CI, 100-100%) at 2 ng/L, specificity of 94.5% (95% CI, 93-95.8%) and PPV 70.4% (95% CI, 63.6-76.5%) for rule-in – table 3. After obtaining clinically meaningful cut-off thresholds in the internal derivation cohort (tables S9, S10; figure S2; based on sensitivity ≥99.5%, specificity >95%), these were tested in the validation cohort: at a threshold of 10 ng/L for rule-out, cMyC achieves a sensitivity of 99.6% (95% CI, 98.6-100%) and NPV of 99.8% (95% CI, 99.3-100%). At 120 ng/L for the rule-in threshold, cMyC achieves a specificity of 94.7% (95% CI, 93.3-95.9%) and PPV of 71% (95% CI, 64.9-77.2%); all data in table 3.
Table 3. Net Reclassification Improvement (Validation cohort).
| Initial model | New model - cMyC (10/120) - Validation cohort | |||||
|---|---|---|---|---|---|---|
| hs-cTnT | No AMI (n=1089) | AMI (n=219) | ||||
| Rule-out | Observe | Rule-in | Rule-out | Observe | Rule-in | |
| Rule-out | 249 | 77 | 0 | 0 | 1 | 0 |
| Observe | 190 | 509 | 32 | 1 | 66 | 24 |
| Rule-in | 0 | 7 | 25 | 0 | 9 | 118 |
| NRI | 0.081 (95% CI, 0.029-0.113) | 0.068 (95% CI, 0.016-0.121) | ||||
| NRI (dimensionless) | 0.149 (95% CI, 0.089-0.210); p value <0.001 | IDI | 0.050 (95% CI, 0.029-0.070) | |||
| Thresholds | Sensitivity (95% CI) | NPV (95% CI) | Specificity (95% CI) | PPV (95% CI) | ||
| hs-cTnT 5 ng/L | 99.6% (98.5-100%) | 99.7% (99-100%) | 29.9% (27.3-32.5%) | 22.2% (19.6-24.8%) | ||
| hs-cTnT 52 ng/L | 58.1% (51.6-64%) | 92% (90.5-93.5%) | 97.1% (96.1-98%) | 80.1% (73.2-86.2%) | ||
| cMyC 10 ng/L | 99.5% (98.6-100%) | 99.8% (99.3-100%) | 38.8% (35.8-41.7%) | 24.6% (21.8-27.4%) | ||
| cMyC 120 ng/L | 64.9% (58.5-71.2%) | 93.1% (91.4-94.5%) | 94.8% (93.5-96%) | 71.5% (64.7-78%) | ||
| Initial model | New model - cMyC (10/120) – Validation cohort | |||||
|---|---|---|---|---|---|---|
| hs-cTnI | No AMI (n=1080) | AMI (n=224) | ||||
| Rule-out | Observe | Rule-in | Rule-out | Observe | Rule-in | |
| Rule-out | 167 | 32 | 0 | 0 | 0 | 0 |
| Observe | 273 | 526 | 22 | 1 | 63 | 19 |
| Rule-in | 0 | 25 | 35 | 0 | 16 | 125 |
| NRI | 0.226 (95% CI, 0.174-0.258) | 0.009 (95% CI, -0.044-0.062) | ||||
| NRI (dimensionless) | 0.235 (95% CI, 0.174-0.296); p value <0.001 | IDI | 0.078 (95% CI, 0.057-0.098) | |||
| Thresholds | Sensitivity (95% CI) | NPV (95% CI) | Specificity (95% CI) | PPV (95% CI) | ||
| hs-cTnI 2 ng/L | 100% (100-100%) | 100% (100-100%) | 18.4% (16-20.8%) | 20.3% (18-22.7%) | ||
| hs-cTnI 52 ng/L | 62.9% (56.4-68.9%) | 92.5% (90.9-93.9%) | 94.5% (93-95.8%) | 70.4% (63.6-76.5%) | ||
| cMyC 10 ng/L | 99.6% (98.6-100%) | 99.8% (99.3-100%) | 39.4% (36.3-42.4%) | 25.5% (22.9-28.5%) | ||
| cMyC 120 ng/L | 64.3% (58.1-70.7%) | 92.8% (91.2-94.3%) | 94.7% (93.2-96%) | 71.8% (65.3-77.9%) | ||
Legend: NRI = Net Reclassification Improvement; IDI = Integrated Discrimination Improvement; CI = Confidence Interval; NPV = Negative Predictive Value; PPV = Positive Predictive Value; AMI = Acute Myocardial Infarction, based on the adjudicated gold-standard diagnosis
All data for the groups of early (<3 hours of chest pain) and late presenters (≥3 hours of chest pain) is presented in tables S11 and S12. In short, in early presenters cMyC demonstrates higher sensitivity than hs-cTnT (100% vs 98.8%) and greater specificity (46.4% vs 33.3%) at the rule-out threshold (10 ng/L). Sensitivity is similar for cMyC and hs-cTnI, however again with greater specificity for cMyC (47.1% vs 23.2%). In the group of late presenters, cMyC yields higher specificity (37.3% vs hs-cTnT 28.4%, 38.1% vs hs-cTnI 15.9%) at the rule-out threshold with otherwise comparable sensitivity. Specificity for adjudicated diagnosis of AMI was individually assessed at the 99th centile in table S13.
Risk group reclassification
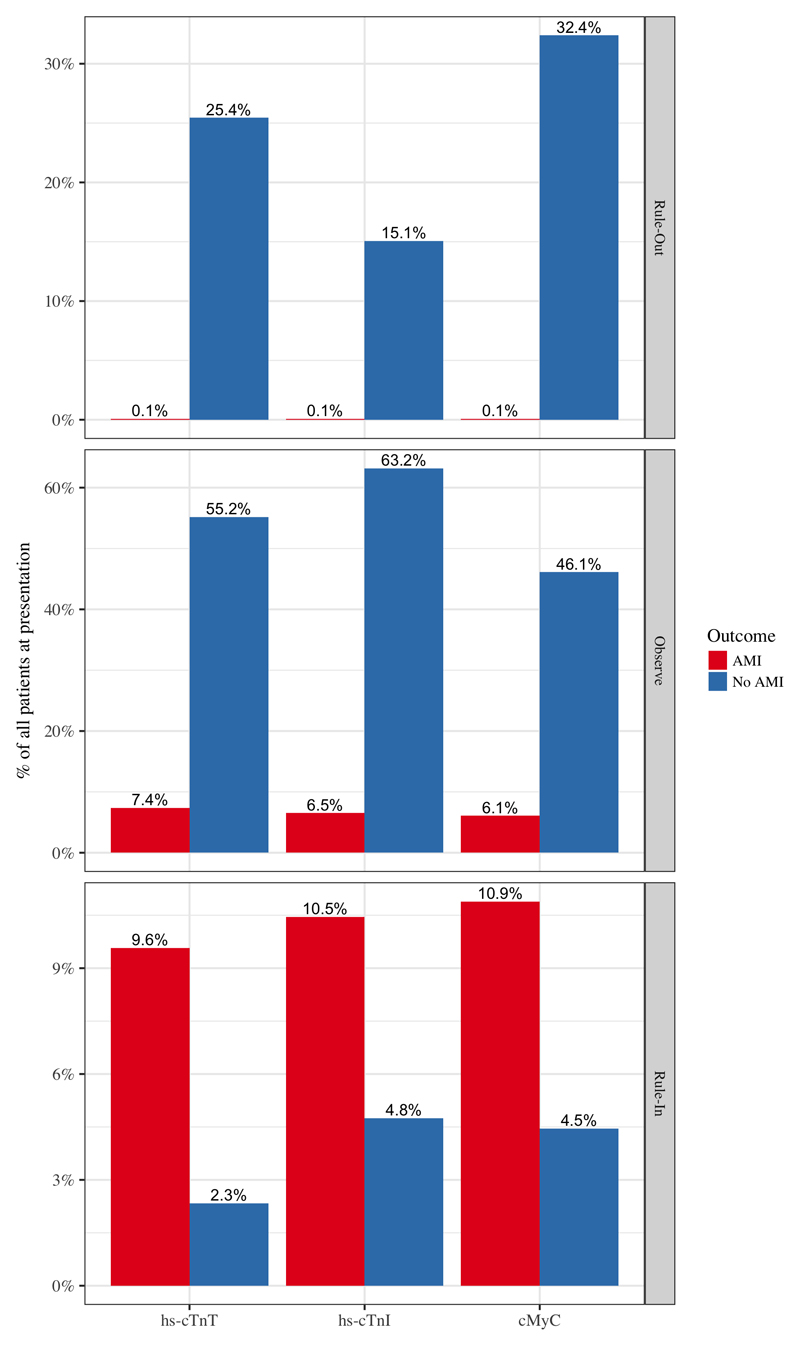
The distribution of patients in risk groups ‘rule-out’, ‘observe’ and ‘rule-in’ based on the initial blood test (either hs-cTnT, hs-cTnI or cMyC) is displayed in figure 4 (validation cohort, n=1368, AMI in 17%). cMyC classified 443 patients (32.4%) safely as rule-out, compared to 348 (25.4%) with hs-cTnT and 206 (15.1%) with hs-cTnI – predominantly by reducing the size of the observation group.
Figure 4.
Distribution of patients in different risk categories after presentation blood tests, based on ESC guideline 20156 for hs-cTnT and hs-cTnI, and newly developed cut-off thresholds for cMyC at ≤10 ng/L for rule-out and >120 ng/L for rule-in of myocardial infarction.
In the validation cohort (table 3), cMyC at presentation was superior to hs-cTnT with NRI +0.149 (NRInoAMI +0.081, NRIAMI +0.068; p <0.001), and to hs-cTnI with NRI +23.5 (NRInoAMI +0.226, NRIAMI +0.009; p <0.001). In the cohort of early presenters (<3 hours of chest pain), cMyC was superior to hs-cTnT with NRI +0.256 (NRInoAMI +0.256, NRIAMI +0.128; p <0.001), and to hs-cTnI with NRI +0.308 (NRInoAMI +0.257, NRIAMI +0.051; p <0.001); table S11. In the cohort of late presenters (≥3 hours of chest pain), cMyC was superior to hs-cTnT with NRI +0.133 (NRInoAMI +0.084, NRIAMI +0.049; p <0.001), and to hs-cTnI with NRI +0.227 (NRInoAMI +0.240, NRIAMI -0.012; p <0.001); table S12.
Prognostic performance of cMyC
As quantified by Harrell’s C statistic calculated from the presentation sample (table S14), cMyC matched the performance of hs-cTnT in predicting AMI (excluding index event), death and the composite endpoint within a 3-year follow-up. Compared to hs-cTnI, there was a statistically different but numerically small improvement in predicting death and the composite endpoint at 3 years: cMyC C index 0.767 vs hs-cTnI 0.732 (p=0.001) and 0.746 vs 0.722 (p=0.008), respectively; AMI was comparable. cMyC was significantly better at predicting AMI, death or the composite endpoint when compared to cTnI.
For the calculation of cumulative hazard ratios (HR) for all-cause mortality using a Cox regression model, each biomarker was separated into quintiles. HR for hs-cTnT at three year follow-up was 2.3 (95% CI, 0.6-9.0) in the second quintile, 7.7 (95% CI, 2.3-25.8) in the third, 17.7 (95% CI, 5.5-57.1) in the fourth and 33.6 (95% CI, 10.6-106.3) in the fifth quintile - p <0.05 for all except 2nd quintile. The HR for hs-cTnI was 6.6 (95% CI, 1.5-29.2), 11.3 (95% CI, 2.7-48.3), 25.1 (95% CI, 6.1-103.3) and 39.7 (95% CI, 9.7-161.8), respectively - p <0.05 for all quintiles. The HR for cMyC was 2.6 (95% CI, 0.7-10.0), 7.8 (95% CI, 2.3-25.9), 17.2 (95% CI, 5.4-55.0) and 29.4 (95% CI, 9.3-93.2) – p <0.05 for all except 2nd quintile. Survival curves for cMyC and hs-cTn assays are displayed in figures S4A-C for three-year and 30-day follow-up.
Discussion
To our knowledge, cMyC is the first cardiac-restricted protein to be analyzed as a diagnostic test for AMI since cTn. In this diagnostic multicenter study we compared its diagnostic performance to cTnI and cTnT, measured using the leading high-sensitivity assays recommended in current practice guidelines6, in a well-characterized and large cohort of patients presenting with symptoms suggestive of AMI. Discrimination for MI with cMyC was similar to that of hs-cTnT and hs-cTnI and superior to s-cTnI. In patients presenting within 3 hours of chest pain onset, cMyC was superior to hs-cTnT, despite the latter’s use as the adjudicating biomarker. Using cut-offs for cMyC calibrated against those recommended in guidelines6, cMyC correctly and safely rules-out and rules-in AMI in a greater proportion of patients than either hs-cTnT or hs-cTnI. These findings indicate that cMyC may be better able to triage patients presenting to the ED with suspected AMI.
cTnT and cTnI have transformed the management of patients with suspected AMI and their importance is enshrined in the Universal Definition of Myocardial Infarction.31 Consequently, AMI events are identified/adjudicated based on a significant rise (and/or fall) in cTnT/I blood concentration. This definition has harmonized clinical care and clinical research, but also introduced an inherent bias in favor of cTnT/I versus novel diagnostic biomarkers in studies such as ours. cMyC is not part of the troponin complex and has a distinct location within the cardiac sarcomere (figure 1). For these reasons, our findings regarding the performance of cMyC against the hs-cTnT and hs-cTnI “gold-standard”, are notable. Since cMyC was not measured through the patients’ journey, it is a matter of speculation if the outcome would have been different with cMyC as the adjudicating biomarker.
After iatrogenic or spontaneous AMI cMyC appears more rapidly in the systemic circulation than either hs-cTnT or hs-cTnI.16,20 This is probably due to a combination of cMyC’s greater myocardial abundance, distinct sarcomeric location and loose association with myosin and actin.16 This biological distinctiveness of cMyC likely underpins the diagnostic advantage we observed over hs-cTnT/I in patients presenting within 3 hours of symptom onset. Moreover, the more rapid appearance of cMyC in the systemic circulation after cardiac injury is also likely to explain the net reclassification improvement over both hs-cTnT and hs-cTnI.
There are no large prospective clinical trials comparing the effect of different biomarkers of cardiac necrosis on clinical outcome. Nonetheless it is interesting to speculate what effect the improved classification of events by cMyC could have in clinical practice. The current guidelines identify three risk groups, where only hs-cTn concentrations at the limit of detection or significantly above the 99th centile clearly triages patients towards rule-out or rule-in of AMI, respectively.6 This leaves a significant proportion of patients within the ‘observe’ zone of clinical uncertainty requiring repeat cTn measurement and further investigation.32 In the current study, of the patients who ultimately did not have AMI, the proportion in the observe-zone after the first measurement at presentation was 55.2% using hs-cTnT, 63.2% using hs-cTnI and 46.1% using cMyC. It is expected that the greater diagnostic certainty afforded on a single presentation blood draw by cMyC may reduce median time to discharge and costs of investigations.
As yet, near-patient, point-of-care devices have not been able to rule-out AMI since they have struggled to achieve the required analytic sensitivity to measure low concentrations of cTnT or cTnI. In addition, the development of reliable large-platform based hs-cTn assays has proved more challenging than expected. Until now, only two manufacturers have overcome the difficulties of developing and introducing hs-cTn assays into clinical practice6, of which one had major quality issues initially.33–35 These uncertainties and concerns have led to delays in the approval of these assays for clinical care in the United States.36 The Food and Drug Administration has only recently ratified the use of the 5th generation hs-cTnT assay.37 Since cMyC is more abundant and rises more rapidly, migration to a point-of-care format may be less challenging. Risk prediction appears grossly similar when comparing hs-cTn and cMyC and could therefore be performed on either. Notably, a cMyC level below 10 ng/L (the threshold resembling 25-times the Limit of Detection) offers both very high NPV and 30-day mortality rates approaching zero.
There are a number of limitations to our study. First, the diagnostic cut-offs for cMyC require external validation: Despite its size, a single cohort cannot entirely safeguard against calibration-issues and is inherently subject to potential, institutional bias. We have attempted to mitigate these risks by employing both randomization and bootstrapping, but in an ideal scenario the findings were validated in an independent cohort. Second, the analyses within this manuscript are confined to the concentration of necrosis biomarker on first blood draw. We have not analyzed the effect on the grey zone of repeat blood draws after set intervals. This is an area of active research for which there is no consensus regarding resampling interval, magnitude of concentration change, use of absolute or relative change in concentration or effect of assay vendor.4,5,21,38–40 Third, as a prospective diagnostic study, we cannot exactly quantify the clinical benefit associated with the use of cMyC as an alternative or addition to hs-cTn – further cluster-randomized studies will be required to address this issue. Fourth, we cannot comment on the accuracy of cMyC among patients with terminal kidney failure on renal replacement therapy or ST elevation myocardial infarction, since such patients were excluded from this study. Currently, biomarkers have no role in the assessment of patients with STEMI and hence this group was not analyzed. Fifth, of 3029 patients recruited, 875 had no baseline cMyC measured; however, a comparison between the analyzed cohort and the excluded patient sample has not demonstrated substantial differences in baseline characteristics (suppl. table 3S). Sixth, in patients with low levels of cMyC (e.g. the non-cardiac chest pain group), we have observed a significant difference in biomarker values dependent on certain underlying conditions (such as prior coronary artery disease; suppl. tables S7-8); however, this effect is muted in patients with AMI, and indeed did not negatively influence specificity. Finally, cMyC was measured using a research platform, whilst hs-cTnI and hs-cTnT were measured using widely available clinical laboratory analyzers. The sandwich immunoassay is comparable to the setup used to test for hs-cTn, but cMyC is not yet available on a random-access laboratory analyzer for routine clinical use.
In summary, cMyC is a promising new biomarker of myocardial necrosis with overall discriminatory power comparable with the leading troponin assays in AMI diagnosis. A potential advantage of cMyC is its ability to more effectively rule-out AMI at presentation, particularly among those presenting early after symptom onset.
Supplementary Material
Clinical Perspective.
What is new?
Cardiac myosin-binding protein C (cMyC) is a recently described novel biomarker of cardiac injury and in small “proof-of concept” studies its serum concentration rises and falls more rapidly than that of troponin T and I.
This is the first study to assess the diagnostic and prognostic value of cMyC in patients presenting with possible acute myocardial infarction (AMI).
A rule-in/rule-out pathway using the novel biomarker was designed to compare discriminative power in a clinical setting.
What are the clinical implications?
Diagnostic accuracy of cMyC for AMI was similar to that of hs-cTnT and hs-cTnI in the entire cohort but superior for those with chest pain of less than 3 hours duration (early presenters) when compared to hs-cTnT.
cMyC has correctly triaged more patients to “rule-out” or “rule-in” groups than either hs-cTnI or hs-cTnT leaving a much smaller proportion in the observation groups. This advantage may facilitate early discharge of low-risk patients.
Sources of Funding
Advantageous Predictors of Acute Coronary Syndrome Evaluation (APACE) was supported by research grants from the Swiss National Science Foundation, the European Union, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Brahms, Biomerieux, Beckman Coulter, Nanosphere, Roche, Singulex, 8sense, and Siemens. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
This work was further supported by grants from the Medical Research Council (United Kingdom) (G1000737), Guy's and St Thomas' Charity (R060701, R100404), British Heart Foundation (TG/15/1/31518, FS/15/13/31320), and the United Kingdom Department of Health through the National Institute for Health Research Biomedical Research Centre award to Guy's & St Thomas' National Health Service Foundation Trust.
Footnotes
Disclosures:
Dr. Twerenbold has received a research grant from the Swiss National Science Foundation (P300PB-167803) and speaker/consulting honoraria from Roche, Abbott and B.R.A.H.M.S. Dr. Rubini has received speaker honoraria from Abbott. Dr. Reichlin has received research grants from Goldschmidt-Jacobson-Foundation, the Swiss National Science Foundation (PASMP3-136995), the Swiss Heart Foundation, the University of Basel, the Professor Max Cloetta Foundation, the Uniscientia Foundation Vaduz, and the Department of Internal Medicine, University Hospital Basel as well as speaker honoraria from B.R.A.H.M.S. and Roche. Dr. Mueller has received research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the European Union, the Swiss Commission for Technology and Innovation (CTI), the Cardiovascular Research Foundation Basel, the University Hospital Basel, Abbott, Alere, Astra Zeneca, Beckman Coulter, BG medicine, Biomerieux, BRAHMS, Critical Diagnostics, Nanosphere, Roche, Siemens, Singulex, Sphingotec, Department of Internal Medicine (University Hospital Basel), 8sense as well as speaker/consulting honoraria from Abbott, Alere, Astra Zeneca, Biomerieux, BMS, Boehringer Ingelheim, B.R.A.H.M.S., Cardiorentis, Duke University, Eli Lilly, Novartis, Radiometer, Roche, Sanofi, Siemens, and Singulex.
Millipore Sigma was contracted to undertake the analyses of cMyC on a fee-for-service basis and holds no commercial interest. Prof Marber is named as an inventor on a patent held by King’s College London for the detection of cardiac myosin–binding protein C as a biomarker of myocardial injury.
References
- 1.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey: 2012 Emergency Department Summary Tables. [cited 2017 May 3];:1–37. CDC.gov. Available from: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2012_ed_web_tables.pdf.
- 2.McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent Trends in the Incidence, Treatment, and Outcomes of Patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. doi: 10.1016/j.amjmed.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katus HA, Remppis A, Neumann FJ, Scheffold T, Diederich KW, Vinar G, Noe A, Matern G, Kuebler W. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83:902–912. doi: 10.1161/01.cir.83.3.902. [DOI] [PubMed] [Google Scholar]
- 4.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 5.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 7.Reichlin T, Twerenbold R, Maushart C, Reiter M, Moehring B, Schaub N, Balmelli C, Rubini Giménez M, Hoeller R, Sakarikos K, Drexler B, et al. Risk stratification in patients with unstable angina using absolute serial changes of 3 high-sensitive troponin assays. Am Heart J. 2013;165:371–8.e3. doi: 10.1016/j.ahj.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Boeddinghaus J, Reichlin T, Cullen L, Greenslade JH, Parsonage WA, Hammett C, Pickering JW, Hawkins T, Aldous S, Twerenbold R, Wildi K, et al. Two-Hour Algorithm for Triage toward Rule-Out and Rule-In of Acute Myocardial Infarction by Use of High-Sensitivity Cardiac Troponin I. Clin Chem. 2016;62:494–504. doi: 10.1373/clinchem.2015.249508. [DOI] [PubMed] [Google Scholar]
- 9.Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973;74:653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- 10.Fougerousse F, Delezoide AL, Fiszman MY, Schwartz K, Beckmann JS, Carrier L. Cardiac myosin binding protein C gene is specifically expressed in heart during murine and human development. Circ Res. 1998;82:130–133. doi: 10.1161/01.res.82.1.130. [DOI] [PubMed] [Google Scholar]
- 11.The Human Protein Atlas. MYBPC3. [cited 2017 May 3]; proteinatlas.org. Available from: http://www.proteinatlas.org/ENSG00000134571-MYBPC3/tissue.
- 12.Kuster DWD, Barefield D, Govindan S, Sadayappan S. A sensitive and specific quantitation method for determination of serum cardiac myosin binding protein-C by electrochemiluminescence immunoassay. J Vis Exp. 2013 doi: 10.3791/50786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuster DWD, Cardenas-Ospina A, Miller L, Liebetrau C, Troidl C, Nef HM, Möllmann H, Hamm CW, Pieper KS, Mahaffey KW, Kleiman NS, et al. Release kinetics of circulating cardiac myosin binding protein-C following cardiac injury. Am J Physiol Heart Circ Physiol. 2014;306:H547–56. doi: 10.1152/ajpheart.00846.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch TL, Sadayappan S. Surviving the infarct: A profile of cardiac myosin binding protein-C pathogenicity, diagnostic utility, and proteomics in the ischemic myocardium. Prot Clin Appl. 2014;8:569–577. doi: 10.1002/prca.201400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govindan S, Kuster DW, Lin B, Kahn DJ, Jeske WP, Walenga JM, Leya F, Hoppensteadt D, Fareed J, Sadayappan S. Increase in cardiac myosin binding protein-C plasma levels is a sensitive and cardiac-specific biomarker of myocardial infarction. Am J Cardiovasc Dis. 2013;3:60–70. [PMC free article] [PubMed] [Google Scholar]
- 16.Baker JO, Tyther R, Liebetrau C, Clark J, Howarth R, Patterson T, Möllmann H, Nef H, Sicard P, Kailey B, Devaraj R, et al. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res Cardiol. 2015;110:23. doi: 10.1007/s00395-015-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacquet S, Yin X, Sicard P, Clark J, Kanaganayagam GS, Mayr M, Marber MS. Identification of cardiac myosin-binding protein C as a candidate biomarker of myocardial infarction by proteomics analysis. Mol Cell Proteomics. 2009;8:2687–2699. doi: 10.1074/mcp.M900176-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marjot J, Kaier TE, Martin ED, Reji SS, Copeland O, Iqbal M, Goodson B, Hamren S, Harding SE, Marber MS. Quantifying the Release of Biomarkers of Myocardial Necrosis from Cardiac Myocytes and Intact Myocardium. Clin Chem. 2017;63:990–996. doi: 10.1373/clinchem.2016.264648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marjot J, Liebetrau C, Goodson RJ, Kaier T, Weber E, Heseltine P, Marber MS. The development and application of a high-sensitivity immunoassay for cardiac myosin-binding protein C. Transl Res. 2016;170:17–25. doi: 10.1016/j.trsl.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaier TE, Anand A, Shah ASV, Mills NL, Marber M. Temporal Relationship between Cardiac Myosin-Binding Protein C and Cardiac Troponin I in Type 1 Myocardial Infarction. Clin Chem. 2016;62:1153–1155. doi: 10.1373/clinchem.2016.257188. [DOI] [PubMed] [Google Scholar]
- 21.Rubini Gimenez M, Twerenbold R, Reichlin T, Wildi K, Haaf P, Schaefer M, Zellweger C, Moehring B, Stallone F, Sou SM, Mueller M, et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur Heart J. 2014;35:2303–2311. doi: 10.1093/eurheartj/ehu188. [DOI] [PubMed] [Google Scholar]
- 22.Reichlin T, Hochholzer W, Stelzig C, Laule K, Freidank H, Morgenthaler NG, Bergmann A, Potocki M, Noveanu M, Breidthardt T, Christ A, et al. Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol. 2009;54:60–68. doi: 10.1016/j.jacc.2009.01.076. [DOI] [PubMed] [Google Scholar]
- 23.Haaf P, Reichlin T, Twerenbold R, Hoeller R, Rubini Giménez M, Zellweger C, Moehring B, Fischer C, Meller B, Wildi K, Freese M, et al. Risk stratification in patients with acute chest pain using three high-sensitivity cardiac troponin assays. Eur Heart J. 2014;35:365–375. doi: 10.1093/eurheartj/eht218. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 25.Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, et al. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J. 2012;33:2252–2257. doi: 10.1093/eurheartj/ehs154. [DOI] [PubMed] [Google Scholar]
- 26.Apple FS, Wu AHB, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: how to use existing assays clinically and for clinical trials. Am Heart J. 2002;144:981–986. doi: 10.1067/mhj.2002.124048. [DOI] [PubMed] [Google Scholar]
- 27.Apple FS, Jesse RL, Newby LK, Wu AHB, Christenson RH, Cannon CP, Francis G, Christenson RH, Morrow DA, Ravkilde J, Apple FS, et al. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Clin Chem. 2007;53:547–551. doi: 10.1373/clinchem.2006.084715. [DOI] [PubMed] [Google Scholar]
- 28.Pickering JW, Endre ZH. New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol. 2012;7:1355–1364. doi: 10.2215/CJN.09590911. [DOI] [PubMed] [Google Scholar]
- 29.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 30.Pencina MJ, D' Agostino RB, D' Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 31.Alpert JS, Chaitman BR, Authors/Task Force Members Chairpersons, Biomarker Subcommittee. Katus HA, Thygesen K, Apple FS, Lindahl B, White HD, Morrow DA, Jaffe AS, ECG Subcommittee et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Nestelberger T, Wildi K, Boeddinghaus J, Twerenbold R, Reichlin T, Gimenez MR, Puelacher C, Jaeger C, Grimm K, Sabti Z, Hillinger P, et al. Characterization of the observe zone of the ESC 2015 high-sensitivity cardiac troponin 0h/1h-algorithm for the early diagnosis of acute myocardial infarction. Int J Cardiol. 2016;207:238–245. doi: 10.1016/j.ijcard.2016.01.112. [DOI] [PubMed] [Google Scholar]
- 33.Hallermayer K, Jarausch J, Menassanch-Volker S, Zaugg C, Ziegler A. Implications of adjustment of high-sensitivity cardiac troponin T assay. Clin Chem. 2013;59:572–574. doi: 10.1373/clinchem.2012.197103. [DOI] [PubMed] [Google Scholar]
- 34.Kavsak PA, Hill SA, McQueen MJ, Devereaux PJ. Implications of adjustment of high-sensitivity cardiac troponin T assay. Clin Chem. 2013;59:574–576. doi: 10.1373/clinchem.2012.197434. [DOI] [PubMed] [Google Scholar]
- 35.Wildi K, Twerenbold R, Jaeger C, Rubini Giménez M, Reichlin T, Stoll M, Hillinger P, Puelacher C, Boeddinghaus J, Nestelberger T, Grimm K, et al. Clinical impact of the 2010-2012 low-end shift of high-sensitivity cardiac troponin T. Eur Heart J Acute Cardiovasc Care. 2016;5:399–408. doi: 10.1177/2048872616642952. [DOI] [PubMed] [Google Scholar]
- 36.Apple FS, Hollander J, Wu AHB, Jaffe AS. Improving the 510(k) FDA Process for Cardiac Troponin Assays: In Search of Common Ground. Clin Chem. 2014;60:1273–1275. doi: 10.1373/clinchem.2014.229286. [DOI] [PubMed] [Google Scholar]
- 37.U.S. Food and Drug Administration. 510(k) Premarket Notification. [cited 2017 Apr 3];2017 FDA.gov. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm?ID=K162895.
- 38.Haaf P, Drexler B, Reichlin T, Twerenbold R, Reiter M, Meissner J, Schaub N, Stelzig C, Freese M, Heinzelmann A, Meune C, et al. High-sensitivity cardiac troponin in the distinction of acute myocardial infarction from acute cardiac noncoronary artery disease. Circulation. 2012;126:31–40. doi: 10.1161/CIRCULATIONAHA.112.100867. [DOI] [PubMed] [Google Scholar]
- 39.Hoeller R, Rubini Giménez M, Reichlin T, Twerenbold R, Zellweger C, Moehring B, Wildi K, Freese M, Stelzig C, Hartmann B, Stoll M, et al. Normal presenting levels of high-sensitivity troponin and myocardial infarction. Heart. 2013;99:1567–1572. doi: 10.1136/heartjnl-2013-303643. [DOI] [PubMed] [Google Scholar]
- 40.Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, Wild P, Genth-Zotz S, Warnholtz A, Giannitsis E, Möckel M, et al. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.