Abstract
The apoptosis-associated speck-like protein with a caspase-recruitment domain (ASC) adaptor protein bridges inflammasome sensors and caspase-1. Upon inflammasome activation, ASC nucleates in a prion-like manner into a large and single platform responsible for the recruitment and the activation of caspase-1. Active caspase-1 will in turn promote the proteolytic maturation of the pro-inflammatory cytokine IL-1β. ASC oligomerization is a direct evidence for inflammasome activation and its detection allows a read-out independent of caspase-1 and IL-1β. This protocol describes how to detect the oligomerization of ASC by Western blot.
Keywords: Inflammasome, Caspase-1, Pyroptosis, Biochemistry, Auto-inflammation
Background
Inflammasomes are large multiprotein platforms that sense a variety of microbial, endogenous and environmental stressors leading to the maturation of the pro-inflammatory IL-1 family of cytokines (Martinon et al., 2002; Sharma and Kanneganti, 2016). Upon activation, inflammasome sensors recruit the adaptor protein ASC through pyrin domain (PYD)-PYD homotypic interactions. ASC will in turn bind to caspase-1 via caspase activation and recruitment domain (CARD)-CARD interactions and favor auto-proteolytic cleavage of caspase-1, leading to maturation of IL-1β and IL-18 (Hoss et al., 2016). Inflammasome activation triggers supramolecular oligomerization of ASC dimers into large interweaving fibrils also termed ‘ASC-specks’ or ‘pyroptosome’ (Fernandes-Alnemri et al., 2007). ASC-speck/pyroptosomes is a hallmark of inflammasome activation that correlates with caspase-1 cleavage and release of mature IL-1β (Dick et al., 2016). Recently we showed that Nelfinavir, an HIV-protease inhibitor, promotes the release of self-DNA into the cytosol, activates the DNA sensing inflammasome AIM2 and subsequent ASC oligomerization (Di Micco et al., 2016). This protocol aims at detecting endogenous ASC oligomerization in immortalized bone marrow-derived macrophages (iBMDMs) by Western blot analysis. It is adapted from the publication of Fernandes-Alnemri et al. (2007) that used this technique to detect ASC oligomerization in THP-1 cells.
Materials and Reagents
6 well culture dishes (TPP, catalog number: 92406)
Syringe 1 ml (BD, catalog number: 300013)
21 gauge needle (B. Braun Melsungen, Sterican®, catalog number: 4657527)
1.5 ml Eppendorf tubes (Corning, Axygen®, catalog number: MCT-150-C-S)
200 μl pipette tips (STARLAB INTERNATIONAL, TipOne, catalog number: S1111-1000)
1,000 μl pipette tips (STARLAB INTERNATIONAL, TipOne, catalog number: S1111-6001)
Cell scrapers (Corning, Falcon®, catalog number: 352340)
Nitrocellulose blotting membrane (Amersham) (GE Healthcare, catalog number: 10600003)
Immortalized Murine Bone-Marrow-Derived Macrophages (iBMDMs), obtained from Professor Petr Broz, Biozentrum, University of Basel, Switzerland
Opti-MEM (Thermo Fisher Scientific, Gibco™, catalog number: 31985070)
Nigericin sodium salt resuspended at 5 mg/ml in 100% ethanol (Sigma-Aldrich, catalog number: N7143)
poly(dA:dT) resuspended at 1 mg/ml (InvivoGen, catalog number: tlrl-patn-1)
Lipofectamine 2000 (Thermo Fischer Scientific, Invitrogen™, catalog number: 11668019)
EDTA (Acros Organics, catalog number: 118432500)
Disuccinimidyl suberate (DSS) (Thermo Fisher Scientific, Thermo Scientific™, catalog number: 21655)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 41640)
Rabbit anti-ASC antibody (Santa Cruz Biotechnology, catalog number: sc-22514-R or Martin Oeggerli, Adipogen, catalog number: AG-25B-0006-C100)
Peroxidase-conjugated goat anti-Rabbit IgG (H+L) (Jackson ImmunoResearch, catalog number: 115-035-146)
Nelfinavir Mesylate 10 mM in DMSO (Axon Medchem, catalog number: AG-1342)
ECL Western blotting detection reagent (GE Healthcare, catalog number: RPN2106)
Sodium chloride (NaCl)
Potassium chloride (KCl) (AppliChem, catalog number: A1362)
Sodium phosphate dibasic (Na2HPO4) (AppliChem, catalog number: 131965.1210)
Potassium dihydrogen phosphate (KH2PO4) (AppliChem, catalog number: A1042)
RPMI 1640 (Thermo Fisher Scientific, Gibco™, catalog number: 681870010)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, Gibco™, catalog number: 10270106)
Penicillin and streptomycin (Thermo Fisher Scientific, Gibco™, catalog number: 15640055)
HEPES-KOH (BioConcept, Amimed, catalog number: 5-31F00-H)
Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266)
EGTA (Sigma-Aldrich, catalog number: 03777)
Sucrose (Sigma-Aldrich, catalog number: 84097)
PMSF (Sigma-Aldrich, catalog number: P7626)
CHAPS (AppliChem, catalog number: A1099)
SDS 20% (AppliChem, catalog number: A3942)
Glycerol (AppliChem, catalog number: A0970)
Bromophenol blue (Sigma-Aldrich, catalog number: B0126)
Acrylamide (Applichem, catalog number: A1672)
TEMED (AppliChem, catalog number: A1148)
Ammonium persulfate (APS) (GE Healthcare, catalog number: 17-1311-01)
Tris base (Biosolve, catalog number: 20092391)
Ethanol (Fisher Scientific, catalog number: 10437341)
-
Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 84422)
Note: This product has been discontinued.
Tween-20 (Applichem, catalog number: A1389)
Non-fat dry milk 5% (Migros, Rapilait)
Lipopolysaccharide (LPS) resuspended at 5 mg/ml in endotoxin free water (InvivoGen, catalog number: tlrl-eklps)
1x PBS (see Recipes)
Growth media (see Recipes)
Buffer A (see Recipes)
CHAPS buffer (see Recipes)
4x protein loading buffer (see Recipes)
15% SDS-PAGE (see Recipes)
Stacking gel (see Recipes)
Migration buffer SDS-PAGE (see Recipes)
Transfer buffer (see Recipes)
PBS-Tween (PBS-T) (see Recipes)
Blocking buffer (see Recipes)
Equipment
Pipettes (Gilson, Pipetman Classic)
Pipette aid (INTEGRA Biosciences, model: PIPETBOY acu 2)
37 °C, 5% CO2 cell culture incubator (Thermo Fischer Scientific, Thermo Scientific™, model: Series 8000 Direct-Heat CO2 Incubators)
Bench top refrigerated centrifuge for 1.5 ml tubes (LabNet International, model: Prism™ R)
Tissue culture class II laminar flow hood (Gelaire, model: TC48)
SDS-PAGE Migration System (VWR, Peqlab, model: PerfectBlue™ Dual Gel System Twin ExWS)
Electrotransfer Blotter System (VWR, Peqlab, model: PerfectBlue™ Tank Electro Blotter)
Procedure
-
Sample preparation and cross-linking
At 18 h before stimulation, seed macrophages in 6-well plates containing 2 ml growth media in each well, 1 well per condition (in simplicate), at a density of 1.5 x 106 cells per well.
Wash cells twice with PBS and prime cells with 1 μg/ml LPS for 2 h in 1 ml Opti-MEM.
Wash cells twice with PBS, and activate inflammasome by stimulating cells with 5 μM nigericin for 30 min or 25 μM nelfinavir for 6 h or transfect for 4 h with 2 μg/ml poly(dA:dT) using Lipofectamine 2000 (use DNA/Lipofectamine 2000 in the ratio of 1 μg/2 μl) in 1 ml Opti-MEM.
-
Optional: harvest supernatants to assess the release of mature IL-1β and caspase-1 cleavage by Western blot.
Note: Proteins from the supernatants can be precipitated using methanol/chloroform. If required supernatants can be stored frozen and proteins precipitated later. Precipitated proteins resuspended in 2x protein loading buffer can also be stored frozen for further Western blotting.
Detach cells by scraping in 1 ml ice cold PBS containing 2 mM EDTA and centrifuge for 5 min at 1,500 x g at 4 °C.
Resuspend cell pellets in 0.5 ml of ice-cold buffer A and lyse by shearing 30 times through a 21-gauge needle in microcentrifuge tubes. Centrifuge lysates in 1.5 ml Eppendorf tubes for 8 min at 1,800 x g, 4 °C to remove bulk nuclei. Keep 30 μl of lysates for Western blots of ASC as input controls.
Dilute the remaining supernatants in a 1:1 ratio with buffer A and centrifuge at 2,000 x g for 5 min at 4 °C. The dilution is required to optimize harvest.
After centrifugation, collect and dilute supernatants with 1 volume of CHAPS buffer and centrifuge at 5,000 x g for 8 min to pellet ASC oligomers.
Discard supernatants and resuspend pellets in 50 μl of CHAPS buffer containing 4 mM of disuccinimidyl suberate (dissolved in DMSO). Incubate for 30 min at room temperature to cross-link proteins.
-
Centrifuge at 5,000 x g for 8 min at 4 °C, discard supernatants and resuspend pellets in 30 μl of 2x protein loading buffer. Heat the samples for 2 min at 90 °C.
Note: It is possible to stop at this point and freeze protein lysates at -20 °C before continuing the protocol with Western blotting steps.
-
Western blot analysis
-
Assemble a 15% SDS-PAGE.
Note: A 15% gel with a distance of migration of 7 to 8 cm is sufficient to give a good resolution of the ASC monomers and oligomers.
Load 30 μl of resuspended ASC containing pellets on the 15% SDS-PAGE. (This sample should contain the cross-linked oligomerized ASC)
Add 30 μl of protein loading buffer to 30 μl of input isolated during step A6, heat the samples for 2 min at 90 °C and load 30 μl on 15% SDS-PAGE. (This sample should contain the input ASC amounts)
Separate at constant 160 V until protein loading buffer reaches the end of the gel.
Transfer at 80 V for 2 h using a wet transfer system.
Rinse membrane with distilled water.
Block membrane in blocking buffer for 1 h at room temperature with agitation.
Incubate membrane overnight at 4 °C with gentle agitation with anti-ASC antibody diluted 1:1,000 in blocking buffer.
Wash membrane 3 times for 10 min with PBS-T (each wash).
Incubate for 1 h with a 1:5,000 dilution of secondary antibody in blocking buffer at room temperature with agitation.
Wash membrane 3 times for 10 min with PBS-T (each wash).
Remove excess of PBS-T.
Incubate with ECL and proceed to detection of chemiluminescence.
-
Data analysis
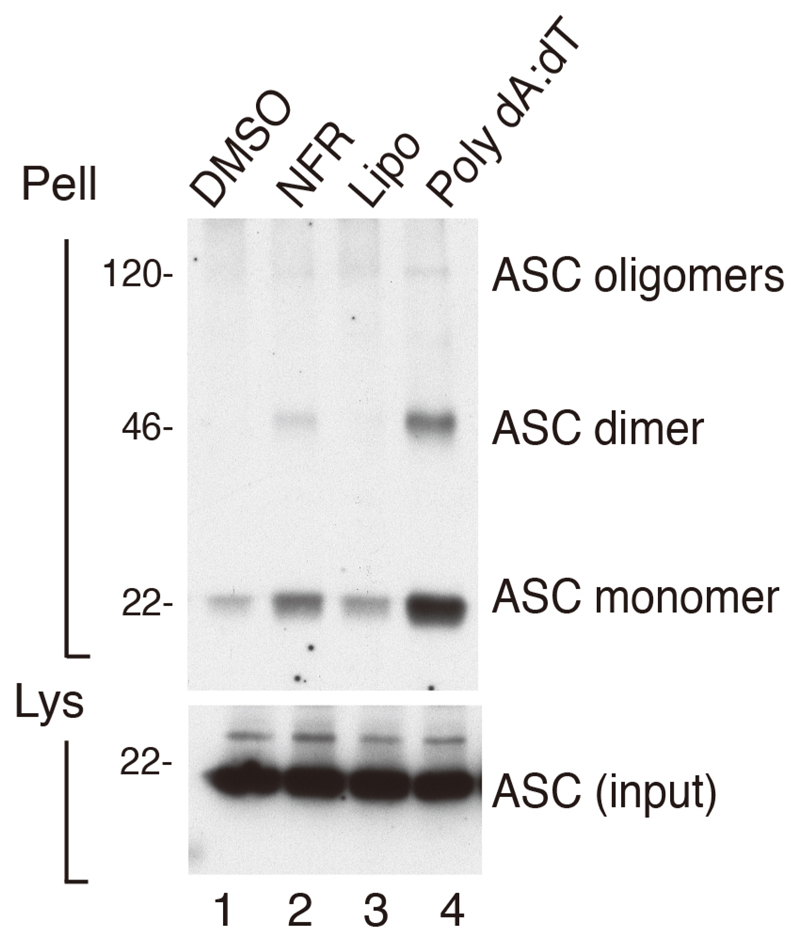
Immunoreactive bands for ASC oligomers appear at molecular weights corresponding to ASC monomers, dimers, and higher orders of oligomers. A positive control for ASC expression in the total cell lysates of each condition needs to be done. Other positive controls for equal protein loading such as tubulin can be included. Three independent experiments should be done. An example of data analysis can be found in Figure 1 and in Figure 3A of (Di Micco et al., 2016). In this study immortalized bone marrow-derived macrophages (iBMDMs) left unchallenged (lane 1) or challenged with NLRP3 inflammasome activator Nigericin (lane 2) or AIM2 inflammasome activators poly(dA:dT) (lane 3) and Nelfinavir (lane 4). In the first lane, resting iBMDMs did not exhibit ASC oligomerization in the pelleted fraction. In lanes 2, 3 and 4, iBMDMs challenged with inflammasome activators exhibited strong ASC oligomerization in their cross-linked pellets.
Figure 1. BMDMs were primed with LPS and treated for 6 h with vehicle, DMSO (Lane1) Nelfinavir, (Lane 2), lipofectamine (Lipo) (lane 3) Lipofectamine plus poly(dA:dT) (Lane 4), as indicated.
Cross-linked pellets (Pell) or soluble lysates (Lys) were immunoblotted for ASC or caspase-1. More details on the experiment can be found in Di Micco et al., 2016.
Notes
Proteins from cell culture supernatants should be precipitated and loaded on 15% SDS-PAGE in order to simultaneously determine the release of mature IL-1β and caspase-1 cleavage to ASC oligomerization.
All stimulations are performed in Opti-MEM without serum. Presence of serum in culture media will drastically interfere with Western blotting of proteins precipitated from cell culture supernatants.
LPS and Nigericin are toxic compounds that should be handled with care.
LPS is resuspended at 5 μg/μl in water and stored aliquoted at -20 °C for up to 12 months. Nigericin sodium salt is resuspended in EtOH at a concentration of 5 mM and conserved aliquoted at 4 °C for up to 12 months. Nelfinavir mesylate is resuspended in DMSO at 50 mM and stored in aliquots at -20 °C for up to 12 months. Poly(dA:dT) is resuspended in H2O at 1 μg/μl and stored in aliquots at -20 °C for up to 12 months. All stimuli are made fresh from these stock solutions immediately before stimulations.
Recipes
-
1x PBS
137 mM NaCl
2.7 mM KCl
10 mM Na2HPO4
1.8 mM KH2PO4
-
Growth media
RPMI 1640
10% FBS
1% penicillin-streptomycin
-
Buffer A
20 mM HEPES-KOH, pH 7.5
10 mM KCl
1.5 mM MgCl2
1 mM EDTA
1 mM EGTA
320 mM sucrose
-
CHAPS buffer
20 mM HEPES-KOH, pH 7.5
5 mM MgCl2
0.5 mM EGTA
0.1 mM PMSF
0.1% CHAPS
-
4x protein loading buffer
50 mM Tris-HCl, pH 6.8
2% SDS
10% glycerol
12.5 mM EDTA
0.02% bromophenol blue
Note: 2x loading buffer is made by diluting the 4x protein loading buffer 2x in distilled water and does NOT contain reducing agent like DTT or 2-mercaptoethanol.
-
15% SDS-PAGE
375 mM Tris pH 8.8
15% acrylamide
1:1,000 (v:v) TEMED
0.1% APS
-
Stacking gel
125 mM Tris pH 6.8
4.2% acrylamide
1:1,000 (v:v) TEMED
0.1% APS
-
Migration buffer SDS-PAGE
25 mM Tris base, pH 8.3
250 mM glycine
0.1% SDS
-
Transfer buffer
25 mM Tris base
190 mM glycine
20% ethanol
Adjust pH to 8.3 with HCl
-
PBS-Tween (PBS-T)
1x PBS
0.1% Tween-20
-
Blocking buffer
PBS-T
Non-fat dry milk 5% (w/v)
Acknowledgments
This work was supported by European Research Council Starting Grant 281996 and is adapted from (Di Micco et al., 2016).
References
- 1.Dick MS, Sborgi L, Ruhl S, Hiller S, Broz P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun. 2016;7:11929. doi: 10.1038/ncomms11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Micco A, Frera G, Lugrin J, Jamilloux Y, Hsu ET, Tardivel A, De Gassart A, Zaffalon L, Bujisic B, Siegert S, Quadroni M, et al. AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc Natl Acad Sci U S A. 2016;113(32):E4671–4680. doi: 10.1073/pnas.1602419113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14(9):1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoss F, Rodriguez-Alcazar JF, Latz E. Assembly and regulation of ASC specks. Cell Mol Life Sci. 2016 doi: 10.1007/s00018-016-2396-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 6.Sharma D, Kanneganti TD. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213(6):617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]