To the Editor
The interferon gene signature (IGS) is of increasing interest in patients with autoimmune diseases. In patients with established rheumatoid arthritis (RA), an IGS is present in approximately 20% of patients and associates with poor response to rituximab.1,2 Nevertheless, in patients with established RA, the IGS does not associate with disease activity, and little is known regarding its role in RA pathogenesis.3 However, glucocorticoids, which are commonly administered in patients with RA, can modulate the IGS,2 potentially confounding reported findings.
For the first time, we examined the IGS in glucocorticoid and disease-modifying antirheumatic drug (DMARD)–naive patients at their initial diagnosis of RA, as classified by a consultant rheumatologist (early RA, n = 50; male/female ratio, 2:3; median age, 57 years [range, 30-91 years]). Clinical parameters, including autoantibody status, scores on the disease activity score for rheumatic arthritis (DAS28) score and its components (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP] level, tender joint count, swollen joint count, and a patient-reported measure [the visual analog scale]) were recorded. The DAS28 was repeated at 6 months (n = 32), and drug history was recorded, including glucocorticoid administration. Healthy control subjects with no history of autoimmunity were also recruited (n = 23; male/female sex, 1:1; median age, 36.5 years [25-62 years]), as were patients with established (disease duration >12 months) RA (n = 25; male/female ratio, 2:3; median age, 68 years [range, 28-81 years]) and systemic lupus erythematous (SLE; n = 23; male/female sex, 1:8; median age, 55 years [range, 33-69 years]). Notably, disease activity (DAS28 score), serostatus, ESR/CRP levels, age, and sex were comparable between the early and established RA cohorts. Full demographic data are shown in Table E1 in this article’s Online Repository at www.jacionline.org.
Whole blood RNA (Tempus Spin RNA Isolation Kit; Thermo Fisher Scientific, Waltham, Mass) was reverse transcribed to cDNA with Superscript II (Thermo Fisher Scientific). RT-PCR (TaqMan; Thermo Fisher Scientific) determined the expression of MxA, IFI6, OAS1, ISG15, and IFI44L, the mean of which was termed the IGS (full details are shown in Table E2 in this article’s Online Repository at www.jacionline.org). Patients had a positive IGS if their score was 2 or more SDs greater than the mean healthy control IGS. Eleven patients with early RA had repeated longitudinal IGS measurements at 1, 3, and 6 months after diagnosis. Treatment for this cohort included a single baseline intramuscular glucocorticoid depot (n = 10) and methotrexate monotherapy (n = 8), hydroxychloroquine monotherapy (n = 1), or both methotrexate with hydroxychloroquine (n = 2). Analysis used GraphPad Prism software (version 5.0; GraphPad Software, La Jolla, Calif) and JMP Statistical Visualization Software (version 11; SAS Institute, Cary, NC). Significance was determined at a P value of less than .05. Age and sex had no effect on IGS; nonetheless, all analyses were corrected for this.
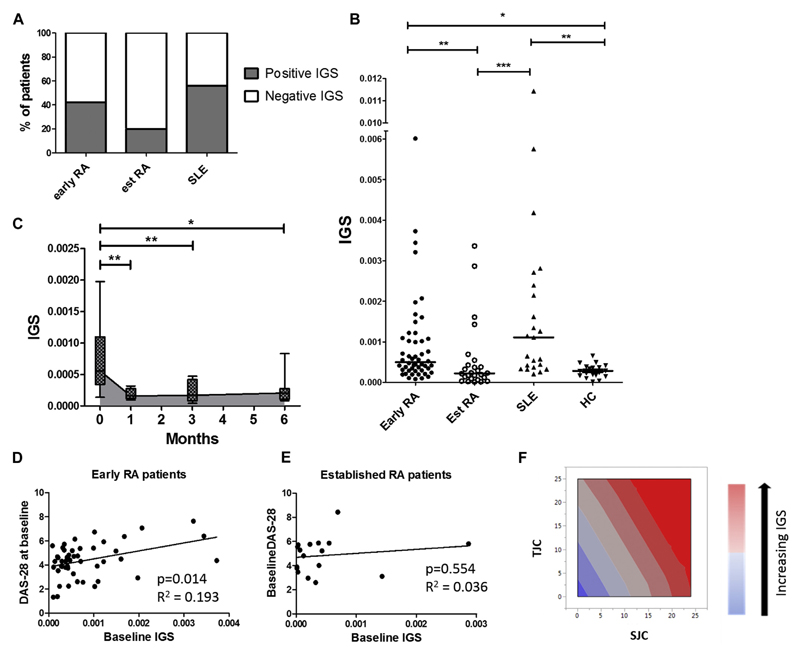
IGS prevalence in our established RA and SLE cohorts was similar to that in previous reportsE1,E2; however, there were twice as many patients with a positive IGS among those with early RA than among those with established RA (Fig 1, A). Indeed, the mean IGS was significantly higher in patients with early RA than in those with established RA (Fig 1, B). Corroborating this observation, there was a significant and sustained decrease in IGS in patients with early RA after 6 months of treatment (Fig 1, C). At diagnosis (baseline), most patients received a depot intramuscular glucocorticoid injection, but to reduce confounding,2 we excluded from longitudinal IGS analysis patients receiving additional glucocorticoids. Thus glucocorticoid exposure might have influenced the IGS at 1 month, but by 6 months, other factors will have contributed to the reduced IGS. These could include reduced disease activity, as well as direct DMARD effects, although the potential for drugs, such as methotrexate, to modulate the IGS has not been appreciated previously. However, we did not have adequate power in this cohort to dissect the effects of different DMARDs on this response. Nonetheless, the observed increased prevalence of type 1 interferons in patients with early RA could mirror other human autoimmune conditions in which early exposure can reinforce aberrant signaling pathways and promote breach of tolerance.4,5 Indeed, genetic variants associated with the type 1 interferon pathway, such as interferon regulatory factor 5, increase RA susceptibility6; furthermore, IFN-α therapy can induce a seropositive polyarthritis, and IGS in seropositive patients with arthralgia has been reported to predict progression to RA.7
Fig 1.
IGS in patients with early RA: Prevalence and disease activity associations. Patients with early RA (n = 50), established RA (n = 25), and established SLE (n = 23) and healthy control subjects (HC;n = 23) had their whole blood IGS measured. A, Proportion of patients with a positive IGS in patients with early RA (42%), established RA (21%), and SLE (56%). B, IGS expression across different disease cohorts. C, Patients with early RA (n = 11): longitudinal IGS expression at baseline (0 months) and 1, 3, and 6 months after initiation of DMARD therapy. Gray shading depicts median IGS. Box and whisker plots show medians and interquartile ranges. For both Fig 1, B and C, analysis included rank transformation of IGS scores, which were tested for differences between groups after adjustment for age and sex with the Tukey multiple comparison test applied. D and E, Plot depicts age- and sex-matched multiple regression between baseline IGS and baseline DAS28 scores in patients with early (Fig 1, D) and established (Fig 1, E) RA. F, Swollen joint count (SJC; P = .017) and tender joint count (TJC; P = .043) were the key drivers of association between baseline DAS28 scores and baseline IGS. Multiple regression analysis: *P < .05, **P < .01, and ***P < .001.
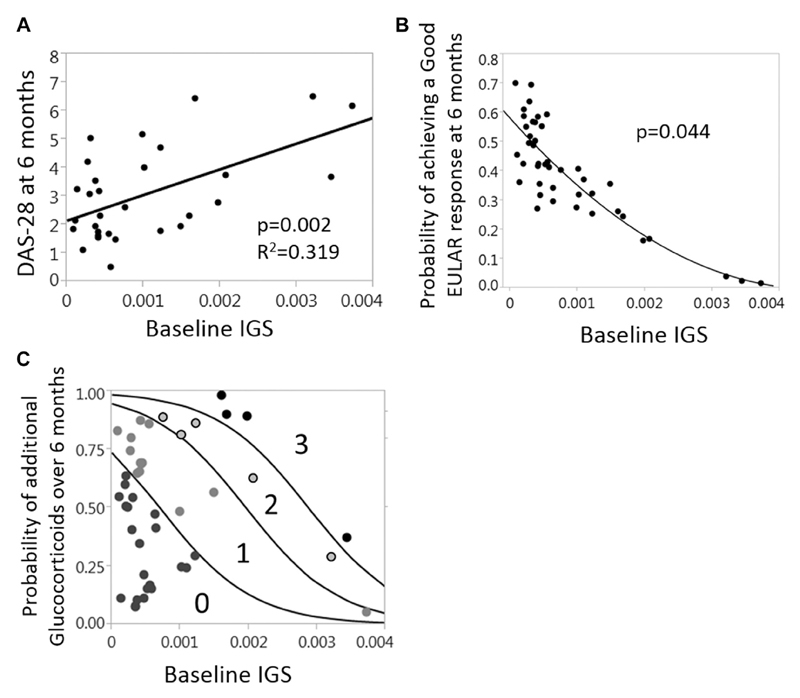
Intriguingly, there was a significant association between baseline IGS and contemporary DAS28 scores (Fig 1, D). This observation was driven predominantly by the swollen joint count and tender joint count DAS28 components (Fig 1, F). However, in keeping with previous literature,3 there was no association between DAS28 scores and IGS in patients with established RA (Fig 1, E), thereby highlighting the potential importance of examining the IGS in an early DMARD-naive cohort. Others demonstrate a positive IGS, predicting an inferior response to biological therapies in patients with established RA1,2; however, we also demonstrated uniquely that in patients with early RA, the IGS behaves as a prognostic marker, predicting disease activity at 6 months (Fig 2, A). Indeed, logistic regression confirmed a significant inverse association between baseline IGS and the probability of a good European League Against Rheumatism (EULAR) response at 6 months despite treatment according to best current practice (Fig 2, B). Finally, the baseline IGS also predicted additional glucocorticoid administration in the initial 6 months after diagnosis (Fig 2, C). Crucially, baseline DAS28 scores and CRP/ESR levels did not significantly predict these disease activity outcomes (see Fig E1 in this article’s Online Repository at www.jacionline.org). In other words patients with a higher IGS were significantly less likely to respond well to initial therapies, and this was independent of conventional disease activity markers.
Fig 2.
IGS in patients with early RA predicts worse disease activity, a poorer response to initial therapy, and increased glucocorticoid requirements. Patients with early RA (n = 32) had baseline IGS calculated and disease activity parameters assessed at 6 months after diagnosis and initiation of treatment. A, Plot depicts age- and sex-corrected multiple regression between baseline IGS and DAS28 scores at 6 months. B, Relationship between the probability of achieving a good EULAR response at 6 months and baseline IGS (nominal logistic regression, age and sex corrected). C, The plot depicts the relationship between the probability of receiving additional glucocorticoids in the first 6 months after diagnosis and baseline IGS. Numbers denote the number of additional glucocorticoid administrations (0-3) in the first 6 months. Baseline IGS significantly predicted additional glucocorticoid requirements (ordinal logistic regression with bootstrap probabilities included, P = .0003). The higher the baseline IGS, the greater the probability that patients fall into the regions indicating 1 or more additional glucocorticoid doses.
Consequently, the baseline IGS predicts disease activity at 6 months despite itself falling during the intervening period. Therefore type 1 interferon exposure might leave an early “imprint” (eg, an epigenetic modification) on pathways that underpin disease activity, rendering them less responsive to treatment. Indeed, in animal models type 1 interferon–producing dendritic cells promoted an inflammatory arthritis phenotype that persisted beyond the type 1 interferon production.8 Additionally, in patients with SLE, simultaneous Toll-like receptor 7/9 signaling in plasmacytoid dendritic cells, as well as stimulating IFN-α release, causes relative glucocorticoid resistance.9 In that situation glucocorticoids can mask the type 1 interferon/IGS response despite cellular resistance to their anti-inflammatory and disease-modulating effects. These examples illustrate that type 1 interferon exposure can establish or be associated with pathogenic pathways that are maintained independently of sustained type 1 interferon signaling. Whether such mechanisms are involved in IGS-positive patients with early RA remains to be elucidated, but our data are suggestive.
RA is highly heterogeneous, and we hypothesize that, in a subgroup of patients with a susceptible genetic background, type 1 interferons contribute to initiation or reinforcement of early pathogenic pathways. This predicts lack of response to initial treatment, as we demonstrate here, as well as a differential response to later therapies.1,2 RA therapeutic strategies are increasingly focused on early treatment and prompt disease control. Therefore although validation of this work is required, we suggest that in a subset of patients with RA at disease onset, there could be future therapeutic merit in targeting type 1 interferon pathways.
Supplementary Material
Acknowledgments
Supported by the Medical Research Council and JWG Patterson Foundation. The research was supported by the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Disclosure of potential conflict of interest: F. A. H. Cooles received a grant from the Medical Research Council for a Fellowship. J. D. Isaacs received a grant from the Medical Research Council, the fellowship paid to Dr Cooles. The rest of the authors declare that they have no relevant conflicts of interest.
We thank Ben Hargreaves for his contribution to the Newcastle Early Arthritis Clinic Database.
References
- 1.Raterman HG, Vosslamber S, de Ridder S, Nurmohamed MT, Lems WF, Boers M, et al. The interferon type I signature towards prediction of non-response to rituximab in rheumatoid arthritis patients. Arthritis Res Ther. 2012;14:R95. doi: 10.1186/ar3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong TD, Vosslamber S, Blits M, Wolbink G, Nurmohamed MT, van der Laken CJ, et al. Effect of prednisone on type I interferon signature in rheumatoid arthritis: consequences for response prediction to rituximab. Arthritis Res Ther. 2015;17:78. doi: 10.1186/s13075-015-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Jong TD, Blits M, de Ridder S, Vosslamber S, Wolbink G, Nurmohamed MT, et al. Type I interferon response gene expression in established rheumatoid arthritis is not associated with clinical parameters. Arthritis Res Ther. 2016;18:290. doi: 10.1186/s13075-016-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira RC, Guo H, Coulson RM, Smyth DJ, Pekalski ML, Burren OS, et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes. 2014;63:2538–50. doi: 10.2337/db13-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brkic Z, van Bon L, Cossu M, van Helden-Meeuwsen CG, Vonk MC, Knaapen H, et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann Rheum Dis. 2016;75:1567–73. doi: 10.1136/annrheumdis-2015-207392. [DOI] [PubMed] [Google Scholar]
- 6.Shimane K, Kochi Y, Yamada R, Okada Y, Suzuki A, Miyatake A, et al. A single nucleotide polymorphism in the IRF5 promoter region is associated with susceptibility to rheumatoid arthritis in the Japanese population. Ann Rheum Dis. 2009;68:377–83. doi: 10.1136/ard.2007.085704. [DOI] [PubMed] [Google Scholar]
- 7.Lubbers J, Brink M, van de Stadt LA, Vosslamber S, Wesseling JG, van Schaardenburg D, et al. The type I IFN signature as a biomarker of preclinical rheumatoid arthritis. Ann Rheum Dis. 2013;72:776–80. doi: 10.1136/annrheumdis-2012-202753. [DOI] [PubMed] [Google Scholar]
- 8.Narendra SC, Chalise JP, Hook N, Magnusson M. Dendritic cells activated by double-stranded RNA induce arthritis via autocrine type I IFN signaling. J Leukoc Biol. 2014;95:661–6. doi: 10.1189/jlb.0613320. [DOI] [PubMed] [Google Scholar]
- 9.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–41. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.