Abstract
Following activation, T cells rapidly divide and acquire effector functions. This energetically demanding process depends upon the ability of T cells to undergo metabolic remodelling from oxidative phosphorylation to aerobic glycolysis, during which glucose is converted into lactate and released extracellularly.
Here we demonstrate that extracellular lactate can be used to dynamically assess human T cell responses in vitro. Extracellular lactate levels strongly correlated with T cell proliferation, and measuring lactate compared favorably with traditional methods of determining T cell responses, namely 3H-thymidine incorporation and the use of cell proliferation tracking dyes. Furthermore, we demonstrate the utility of measuring lactate as a read-out in conventional suppression assays and high throughput peptide screening assays. Extracellular lactate was stably produced over 7 days and results were reproducibly performed over several freeze-thaw cycles.
We conclude that the use of extracellular lactate measurements can be a sensitive, safe, stable and easy to implement research tool for measuring T cell responses and cellular metabolic changes in vitro.
Introduction
T cells have complex, environment dependent, metabolic profiles, with different T cell subsets utilizing different metabolic pathways to fuel their energy requirements (1,2). For example in the presence of oxygen resting naïve and memory effector T cells (Teffs) primarily metabolize glucose to pyruvate, which then enters the mitochondrial TCA cycle to produce NADH, which acts as an electron donor for the electron transport chain, fueling ATP production via oxidative phosphorylation (OXPHOS) (2,3). However, upon stimulation through the TCR and in the presence of CD28 co-stimulation, Teffs rapidly switch from OXPHOS to glycolysis in order to meet the increased energy and biosynthesis demands of cellular activation and proliferation (4,5). This shift causes an increase in the production and subsequent excretion of lactate by the cell, which is formed in the cytosol by the action of lactate dehydrogenase. The upregulation of glycolytic metabolism in Teffs upon stimulation (even in the presence of oxygen) is akin to the ‘Warburg effect’ observed in oncological cell lines, where cancerous cells increase their lactate output with respect to healthy tissue, to fuel their ever-increasing metabolic demands for rapid cellular proliferation and expansion (4,6,7). In contrast to Teffs, in normoxic conditions, resting Tregs utilize fatty acids, rather than glucose, as their primary energy source. And they do not switch their metabolism from OXPHOS to aerobic glycolysis following in vitro TCR/CD28 stimulation (8,9,10).
Given the reliance of activated Teffs cells on Warburg metabolism we set out to explore the utility of quantifying extracellular lactate as a measure of Teff cell proliferation, under standard laboratory normoxic conditions. Here we show that extracellular lactate compares favorably to more traditional measures of T cell proliferation – namely thymidine DNA incorporation and cell division tracking dye dilution assessed by flow cytometry. As naturally occurring Tregs do not increase their production of lactate in response to CD3/28 stimulation in vitro, we demonstrate the utility of measuring lactate as a read-out of Treg mediated suppression of Teff cell proliferation. And finally, given the stability of lactate and the speed and ease with which it can be measured, we demonstrate the potential of using lactate in T cell screening assays, for example as a read-out of CMV exposure status (11).
Materials and Methods
Cell Preparation
Human PBMC were isolated from whole blood of healthy donors by ficoll centrifugation (Amersham Pharmacia Biotech, UK). All individuals gave written consent and the study was approved by a local ethical review committee (REC: 11/EE/0007). PBMCs were immediately suspended in culture medium (RPMI, life technologies, California), containing 1% penicillin, 1% streptomycin, and 10% fetal calf serum (S5394; Sigma-Aldrich, UK), and adjusted to a concentration of 106 viable cells/mL for subsequent assays. Pan T cells were separated magnetically (T cell negative separation kit II, Miltenyi Biotec), according to the manufacturer’s instructions. CD4+CD25- and CD8+CD25- Teffs and CD4+CD127lowCD25Hi Tregs were isolated from Pan T cells by Flow Assisted Cell Sorting (FACS) (BD Influx) following staining of cell surface CD4, CD8, CD25 and CD127 with relevant antibodies (ebioscience, BD and BioLegend).
For the CMV study, healthy seropositive and negative donors were recruited from the National Institute of Health Research (NIHR) Cambridge BioResource (HBREC.2014.07). PBMC were isolated using Lymphoprep (Axis-shield, Oslo, Norway) density gradient centrifugation and the samples frozen in 10% DMSO (Sigma Aldrich) and 90% Fetal Bovine Serum (FBS) (Gibco, ThermoFisher Scientific, Paisley, UK). Cryopreserved PBMC were resuscitated before use in pre-warmed D-MEM (Sigma Aldrich) in the presence of 10U/ml Benzonase nuclease (Millipore, Watford, UK), followed by a 1 hour incubation in warmed X-VIVO-15 media (Lonza, Slough, UK) supplemented with Benzonase nuclease at 37°C. The cells were then further rested overnight at 37°C in X-VIVO 15 media or RPMI (Sigma Aldrich) supplemented with penicillin, streptomycin and 10% FBS. CMV sero-status of all donors was confirmed by serological assessment of CMV IgG levels using Captia CMV IgG EIA test (Trinity Biotech, Ireland) following the manufacturer’s instructions.
Proliferation assays
CD4+ and CD8+ T cells were cultured in normoxic conditions at 37°C with 5% CO2 with and without anti CD3/28 stimulation, cell:bead ratio 4:1. At varying time-points (as indicated in the text), supernatant was collected and frozen at -20 °C for lactate measurement and the number of viable cells at the end of culture counted using a hemocytometer. To correlate lactate with proliferation and/or activation 106 cell tracker labelled CD4+ cells were cultured with and without anti-CD3 stimulation for 6 days. At days 1, 4, and 6 the supernatant was taken for lactate measurements, the cells counterstained with live/dead exclusion dye (zombie NIR, Biolegend) and anti-CD25 and CD69 antibodies (BD) and analysed by flow cytometry. Lactate measurements were compared with output for cell proliferation (cell tracker dye analysis by flow cytometry, cell tracker high cells marked as non-proliferating versus cell tracker dye low cells marked as proliferating). To assess the sensitivity of lactate measurements, compared to thymidine incorporation, human PBMC were cultured at 105-1.5x103 cells per well in triplicates, with and without stimulation using 1µg/mL plate bound anti-CD3/soluble 28. Thymidine (25miC/mL) was added during the last 18 hours of culture and plates pulsed on a Becton Coulter cell harvester at days 3 and 5.
Lactate Measurements
Media and supernatant lactate was measured spectrophotometrically using a Dimension EXL autoanalyser (Siemens, Germany) with Flex© reagent cartridges (product code DF16). In brief, LDH, NAD, dihydrazine sulphate, and tris buffer (40U, 10mmolL-1, 180mmolL-1, and 100mmolL-1 respectively) were added to cell free supernatants. The subsequent exchange of lactate to pyruvate, captured by the hydrazine compound, is directly proportional to the change in NAD+ to reduced NAD+ (NADH) concentration measured at 340-383 nm, from which the initial lactate pool size was inferred. Lactate was not measurable in RPMI and 10% FBS alone. To assess the freeze-thaw stability of lactate 3x106 Pan T cells were cultured in three separate 2 mL wells. All supernatant was removed from the wells at day 2, and three 50 μL fresh supernatant samples from each well were analysed for extracellular lactate. Supernatants were subsequently also taken for analysis after 1, 2, and 3 freeze-thaw cycles, with samples left for at least 24 hours in the freezer before subsequent thawing.
T regulatory cell suppression Assay
Teffs (CD4+CD25- T cells) and Tregs were sorted using an Influx cell sorter (BD) according to their expression of CD4, CD25, and CD127. Cells were stained with antibodies (BD) and sorted as follows: CD3+CD4+CD25hiCD127low cells were taken as Tregs and CD3+CD4+CD25- cells as CD4+Teffs. To discriminate between Teffs and Tregs on final analysis, the Teffs were labelled with 5 µM cell proliferation dye V450 (ebioscience) and Tregs were labelled with 5 µM V670 cell proliferation dye (ebioscience). Teffs were plated at 104 cells/well in triplicate with and without Tregs in RPMI+ 5% Human AB serum. For final analysis, dead cells were excluded using dead cell exclusion staining (zombie NIR, ebioscience) and cells counterstained with antibodies to CD4. Tregs were titrated in doubling dilutions so that the ratio of Treg: Teffs was 1:1 to 1:16. Triplicate control wells of CD4+ T cells without stimulus, and Tregs, at varying dilutions, with and without stimulus were also cultured. Miltenyi Treg suppression inspector beads were used to stimulate the assay (used according to manufacturer’s instructions).
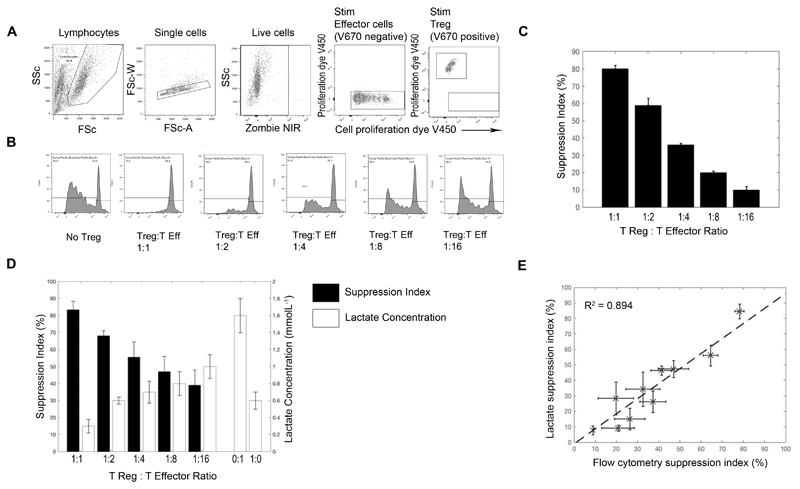
Supernatant was removed for lactate analysis, and the cells analysed by flow cytometry at day 6. A lactate suppression index, derived from lactate and flow results, was calculated using equations 1 and 2.
Equation 1 – Lactate Suppression Index
Equation 1 shows the suppression index calculated from the extracellular lactate measurements, where Ts is the lactate concentration in a stimulated well of 104 Teffs alone (i.e. Treg:Teff ratio 0:1), Tsup is the lactate concentration with Treg:Teff ratio 1:n, where n > 0, and TReg,Stim is the lactate concentration from the 1:0 well (i.e. 104 stimulated Tregs alone). This was selected because (i) our preliminary data demonstrated that the lactate suppression index was not significantly altered by correcting for the exact number of Tregs in any given well (supplementary Figure 2), (ii) it would be impractical for most researchers to set up multiple Treg control wells and (iii) because most researchers report the 1:1 suppression index (showing the other ratios to demonstrate that suppression can be diluted out).
For analysis of suppression by flow cytometry, live Teffs were gated (see figure 5 for analysis strategy). Flow cytometry suppression index was calculated by taking the ratio between the proliferating and non-proliferating populations, as defined by equation 2.
Figure 5. Use of lactate in analyzing a Treg suppression assay.
Treg suppression assays were performed by co-culturing V450 cell tracker labelled CD4+ effector T cells with V670 cell tracker labelled CD4+CD25hiCD127low Tregs at ratio of 1:1 and doubling dilutions thereafter, in triplicate. (A) demonstrates the flow gating strategy of this assay. Live, V670 negative (non-Treg) cells were taken as effector cells. (B) shows the V450 proliferation dye dilution of the effector T cells (gated in A) without Treg and with Treg at various dilutions. Percentage suppression of the effector T cell response in the presence of Treg was calculated according to the given equation (see methods), suppression by Tregs was titratable (C). D shows the amount of lactate produced by: 104 effectors (0:1), 1x104 Tregs (1:0) and 104 effectors cultured with varying numbers of Tregs, corrected for the amount of lactate in the 1:0 well. The black bars represent the calculated lactate suppression index. E shows combined data from 3 suppression assays, correlating the suppression index calculated by flow with that calculated by lactate.
Equation 2 – Flow Cytometry Suppression Index
Where is the proportion of proliferating, V670 low (Teff) in the simulated culture, and is the proportion of proliferating, V670 low (Teff) in stimulated culture with Tregs. The suppression index for each well was calculated, and averaged for each cell set.
CMV peptide response assay
106 PBMC from ten healthy donors were labelled with cell tracker dye efluorV450 (ebioscience) and cultured at 105 cells per well in triplicate, either alone, with soluble anti-CD3 at 1 µg/mL or with two overlapping peptide pools of CMV immunodominant epitopes, IE1 and gB (1 µg/mL). The peptide pools were consecutive 15mer peptides overlapping by 10 amino acids (libraries synthesized by ProImmune PEPScreen, Oxford, UK, from sequences previously published) (11, 12). Five donors were naive to CMV, and five had known previous exposure. Donor status was assessed using CMV serology as previously described. Cells were cultured in a 96 well plate for six days. At days two, five, and six triplicate supernatants were removed from the plate for lactate analysis. At the final time point (day 6) the cells were taken for flow cytometric analysis. Total lactate responses were corrected by subtracting the unstimulated pool concentrations from their counterpart anti-CD3 and CMV peptide stimulated wells. Lactate data were analysed in a blinded fashion. Positive wells were those with lactate concentrations =>2 standard deviations above the mean lactate concentration of the unstimulated wells. The lactate assay was set up in parallel with an IFNγ Fluorspot assay, using the same PBMC and peptide pools. The Flurospot was processed at day 2, as previously described (13). In brief, 2 x 105 PBMC were incubated in pre-coated Fluorospot plates (Mabtech AB, Nacka Strand, Sweden) in triplicate with either gB or IE1 protein mix peptides (at a final concentration of 2µg/ml/peptide) or unstimulated, or in the presence of anti-CD3 stimulation. The cells and medium were decanted from the plate and the assay developed following the manufacturer’s instructions. Developed plates were read using an AID iSpot reader (Oxford Biosystems, Oxford, UK) and counted using AID Fluorospot v7 software (Autoimmun Diagnostika GmbH, Strasberg, Germany). All data were corrected for background cytokine production. The positive response cut-off was taken as a mean of 100 spot forming units (sfu)/million as previously determined in a larger study (13).
Statistical Analysis
All flow cytometry data was analysed in house with FlowJo (V.10.1), and all other data analysis and statistical fitting was performed in house with Matlab (The Mathworks, MA) or graphpad (Prism). Where appropriate, ANOVA and two tailed t-tests have been performed to assess for differences between lactate concentrations in cell well cultures. Co-efficient of variation analysis was performed on freeze-thaw samples. To assess for correlation between techniques in the suppression assay experiments, a least squares linear fit was performed. Sensitivity and specificity calculations were performed in the peptide response experiments, using serology as the gold standard measurement technique.
Results
Activated CD4+ and CD8+ effector cells produce lactate in normoxic conditions
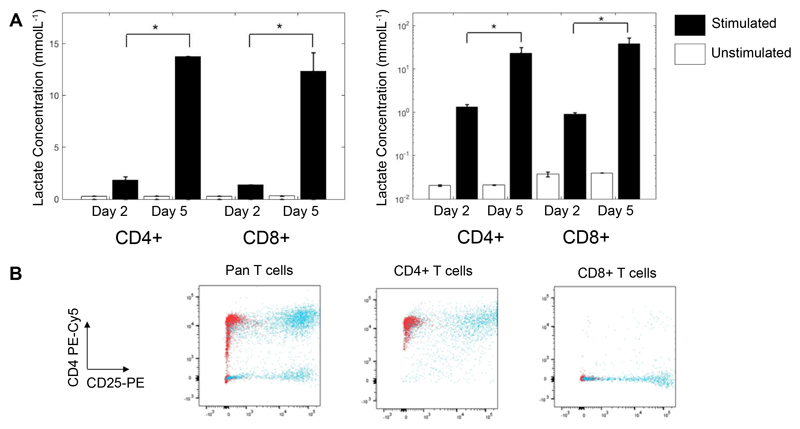
By day 2, the concentration of lactate measured in supernatant derived from CD3/28 stimulated CD4+ and CD8+ cells cultured in standard laboratory conditions (normoxia at 37°C with 5% CO2) was significantly higher than that derived from unstimulated cells (0.3±0.1 vs 1.9 ± 0.3 and 0.4 ± 0.1 vs 1.46 ± 0.03 mmolL-1, respectively, p<0.01). Between days 2 and 5 lactate continued to accumulate in the supernatant of stimulated CD4+ and CD8+ cells (0.4 ± 0.2 vs 14.16 ± 1.82, 0.4 ± 0.1 vs 13.7 ± 0.4 mmolL-1, p<0.01). Correcting this for the number of live CD4+ and CD8+ cells at the end of culture revealed an increase in the amount of lactate produced per cell (Figure 1, p<0.01 in all cases). Lactate did not accumulate in the supernatant of unstimulated cells. Flow cytometric analysis confirmed purity and activation status of the cells (Figure 1B).
Figure 1. Extracellular lactate production by Human T Cells.
CD4+ and CD8+ T cells from 2 donors were cultured for 2 and 5 days, with and without anti-CD3/28 dynabeads and supernatants taken in triplicate for analysis of extracellular lactate. Lactate was normalized to viable cell number at each time point (A). On day 5 the remaining cells were analyzed by flow cytometry for purity and activation status, as shown by CD4-PEcy5 vs CD25-PE staining in (B). Red dots show unstimulated cells and blue dots show stimulated cells. Unstimulated cell lactate output showed no change between days 2 and 5. * = Significant between days 2 and 5 (p < 0.01).
Extracellular lactate correlates well with T cell proliferation
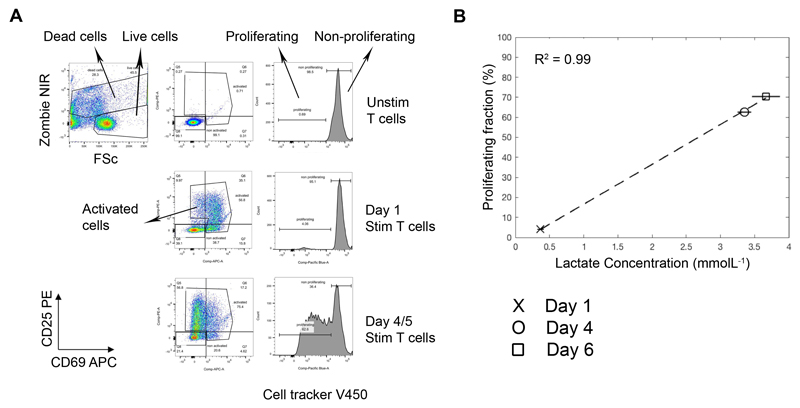
Comparison of the temporal changes in total lactate obtained from the supernatants of CD3/28 stimulated T cells after day 1, 4 and 6, with flow cytometry data from the same culture well, revealed a very strong correlation between T cell proliferation (defined by cell division dye dilution) and total lactate (R2 = 0.99, Figure 2). Cultures of activated T cells, expressing the early activation marker CD69 and/or CD25 but not yet proliferating, did not contain high levels of lactate indicating that cells undergoing cellular proliferation utilize glycolysis to a greater extent than non-dividing, activated T cells. Extracellular lactate concentration was unaffected by cell death (Supplementary Figure 1).
Figure 2. Increased total extracellular lactate correlates with increasing proliferation in stimulated T cell cultures.
Cell proliferation tracker V450 labelled T cells were cultured in the presence and absence of anti-CD3/28 dynabeads for 6 days. At days 1, 4 and 6, cell culture supernatants were sampled for lactate analysis and cells were analysed by flow cytometry for proliferation status (cell proliferation dye dilution) as well as counterstained with dead cell marker (zombie NIR) and antibodies to CD69-APC and CD25-PE. The flow gating strategy is shown in (A): dead cells, activated cells, proliferating and non-proliferating cells were gated. The correlation of total extracellular lactate concentration with T cell proliferation is shown in (B).
The sensitivity of lactate as a measure of T cell proliferation is comparable to thymidine at later time points
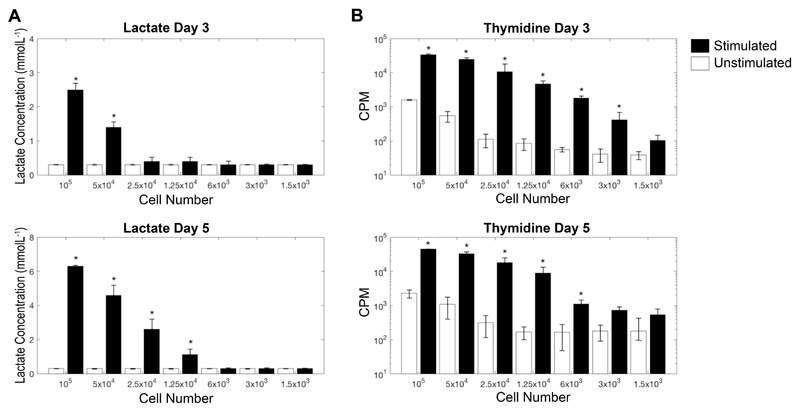
Next we went on to compare the sensitivity of extracellular lactate as a measure of T cell proliferation with the commonly used technique of 3H-thymidine incorporation, by culturing between 105 to 1.5 x 103 whole PBMCs with plate-bound anti-CD3 and soluble anti-CD28 for 3 and 5 days. Thymidine was able to discriminate between unstimulated and stimulated wells at a lower limit of 6 x 103 PBMCs, at both day 3 and 5 (p < 0.01); although at both time points the lowest PBMC count that was significantly above the commonly accepted median counts per minute (CPM) cut-off of 1000 was 1.25 x 104 PBMCs (14). Lactate performed less well at day 3 (lower limit of sensitivity 5 x 104 PBMCs; p < 0.01), however at day 5 its sensitivity was comparable to that of thymidine (lower limit of sensitivity 1.25 x 104; p < 0.01). As this work was undertaken by stimulating T cells within whole PBMC cultures, the number of proliferating T cells that can be detected by both methods will be significantly fewer than 1.25 x 104. Results from the sensitivity assay are shown in Figure 3.
Figure 3. Comparison of extracellular lactate proliferation assay with thymidine incorporation proliferation assay.
Whole PBMC were stimulated, in triplicate, with plate-bound anti-CD3/sol anti-CD28 and supernatants taken for lactate analysis prior to pulsing cells with thymidine. To test for Sensitivity of the assay cell numbers with titrated from 105 per well to 500 cells/well. Total lactate at day 3 and day 5 are shown in (A). Counts per minute (CPM) of incorporated thymidine at day 3 and day 5 are shown in (B). * Denotes significant from unstimulated control, p < 0.05. Lactate data is shown on a linear scale, and thymidine on a log scale.
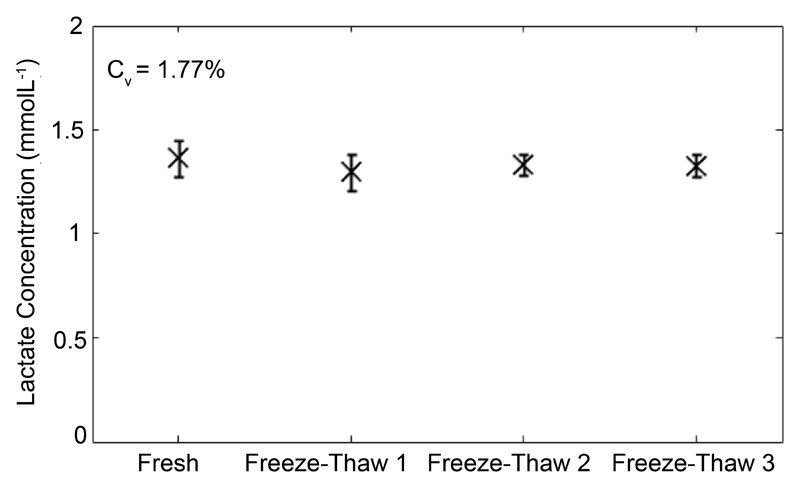
Lactate is stable over multiple freeze-thaw cycles
To determine the stability of lactate to freeze-thawing, 106 T cells were stimulated with anti-CD3/28 dynabeads for 5 days and the concentration of lactate in aliquots put through 1 to 3 rounds of -20°C freeze-thawing was compared to the concentration of lactate measured in fresh supernatant. Analysis of the variance in results revealed highly reproducible results over a course of three freeze-thaw cycles with a coefficient of variation of 1.77% over four test re-test cycles (Figure 4).
Figure 4. Stability of lactate measurments.
Extracellular lactate is stable over several freeze-thaw cycles. 3x106 Pan T cells were stimulated in 24 well plate with anti-CD3/28 dynabeads for 5 days and 1 mL supernatant taken for analysis. The figure shows total lactate concentration in the same cell culture supernatant that was repeatedly freeze-thawed.
Extracellular lactate is as accurate as cell division tracking dye in determining T regulatory cell suppression
In keeping with the literature (10) under normoxic conditions in vitro, Treg lactate production was relatively low (compared to Teffs), and did not increase following activation (Supplementary Figure 2). Therefore,we went on to explore the utility of measuring lactate in a classical Treg suppression assay, where the co-culture would not be complicated by the increased background production of metabolites from the regulatory cell pool. Cell division tracking dye dilution was used as the comparator. Example flow cytometric analysis is shown in Figure 5A-C and lactate analysis in Figure 5D. As expected, a titratable effect of modifying Treg:Teff ratio was observed using both methods (Figure 5C and D). Furthermore, calculation and comparison of the suppression index obtained over multiple suppression assays revealed a strong correlation between estimates of suppression obtained from lactate and those obtained by flow cytometry (see equations 1 and 2, R2 = 0.894).
Extracellular lactate provides high specificity and sensitivity in predicting CMV status
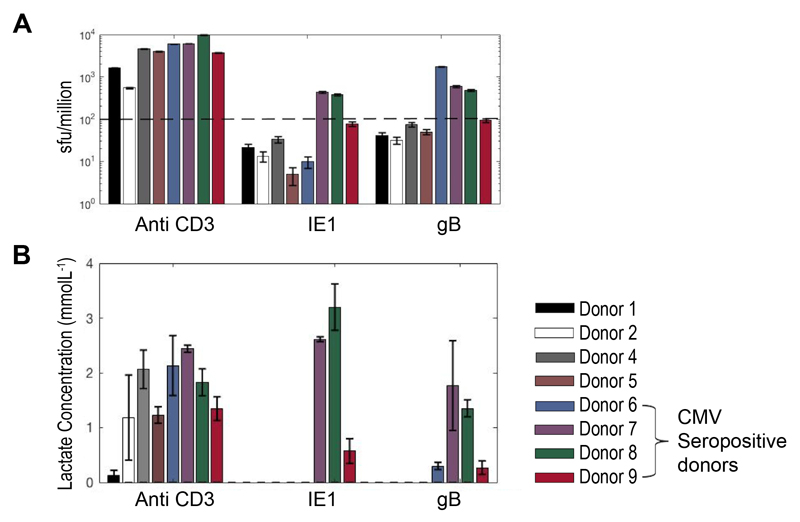
Given the ease with which lactate can be measured, its sensitivity, stability and low cost, we went on to test its utility in interrogating T cell responses to libraries of overlapping peptides; a relatively high-throughput technique commonly used to assess vaccine induced or other immunological responses. In brief, cryopreserved PBMCs from 10 healthy individuals – 5 known to be CMV seropositive and 5 seronegative, were cultured with IE1 and gB protein CMV peptide pools, both proteins have been shown to trigger T cell responses in many donors (15). Supernatant lactate production was compared to IFNγ production as measured by Fluorospot. CMV serology was taken as the “gold standard”; CD3 stimulation was included as a positive control. Two donors were excluded from the analysis owing to a poor anti-CD3 response in both the lactate and Fluorospot assays, possibly as a result of using cryopreserved PBMCs which did not recover well. The remaining 8 donors made a positive anti-CD3 response and were therefore included in the analysis. Of note, donor 1 made very little lactate in response to CD3 stimulation (just above background) whereas their cells made significant amounts of IFNγ; highlighting the recognized difference between proliferation (which we have shown correlates strongly with lactate production) and cytokine secretion (16).
Two independent, blinded, analysts concluded donors 6, 7, 8, and 9 were CMV positive from the lactate results, this was in 100% agreement with the serology and Fluorospot results after un-blinding (Figure 6). Further analysis of using lactate production as a predictor for CMV status revealed 100% specificity and sensitivity of the technique, in comparison to serology and Fluorospot.
Figure 6. Screening of blood donors for responses to CMV peptides.
Data from PBMC of 8 healthy donors, cultured with soluble anti-CD3 or one of 2 different CMV peptide pools (IE1 and gB) at 1ug/mL. PBMC were plated at 105 cells per well in triplicates in 96 well plates. (A) IFNγ Elispot was performed at 48 hours and a mean sfu/million >100 was taken as a positive responder. Extracellular lactate was measured in cell culture supernatants at day 2 and day 5; (B) shows day 5 positive total lactate concentrations. All 4 seropositive donors were identified as responders to one, or both peptides by both methods.
Discussion
By making use of what is known about the reliance of activated Teffs on Warburg metabolism, we have demonstrated that extracellular lactate can be used to accurately determine T cell proliferation in vitro, with utility across a range of assays.
Here we used spectrophotometry to quantify lactate via the lactate to pyruvate exchange reaction catalyzed by lactate dehydrogenase. This represents a simple, inexpensive and widely available approach to assess T cell activation. Culture cell supernatants can be simply harvested and stored at -20°C until the researcher is ready to process them. There are no additional time consuming steps to be performed, and unlike thymidine, there is no need for radioactive and potentially carcinogenic material to be handled. The stability of lactate over multiple freeze-thaw cycles also opens up the possibility that this method could be used to measure T cell proliferation post hoc, in stored supernatants collected for other purposes, such as the measurement of cytokines. Commercial lactate measurement kits are also available as a simple to use colorimetric assay kit. These assays were originally designed for the analysis of tumor cells in culture, however here we suggest that they could be repurposed to effectively measure T cell metabolism and proliferation.
In contrast to thymidine, lactate is not a “snap-shot” measure of cell proliferation at a single time point; rather it is an endpoint/cumulative measure that provides information regarding the replicative history of the cultured cells. In that regard, it is more akin to cell division tracking dyes. As a result, lactate is less sensitive than thymidine at early time points, however at later time points its sensitivity is comparable.
Several other non-radioactive single time point methods of analyzing T cell proliferation exist: for example the MTT assay which is a metabolism-based colorimetric assay. However, this suffers a relative lack of sensitivity, and has issues with toxicity. MTT requires cells to be pulsed with substrate and therefore, multiplexing this assay with other methods is not possible. In contrast, lactate accumulates naturally throughout the period of cell culture, with no need for additional cytotoxic reagents to be added, so it can be readily used alongside other assays – for example cytokine analysis, or flow cytometry. Through co-staining with antibodies against additional cellular markers, flow cytometric methods enable significant additional details about the nature of the cellular response to be interrogated. However, flow cytometry is labor-intensive and relatively costly. Furthermore, the level of detailed information that flow cytometry can provide is not always required.
We have demonstrated that under standard laboratory normoxic conditions, extracellular lactate strongly correlates with T cell proliferation (R2 = 0.99). We assume that this would not be the case if cells were to be cultured in hypoxic conditions, where both proliferating and non-proliferating cells would utilize glycolysis in order to satisfy their energy needs (17).
Although the literature on Treg metabolism is complex, and recent studies have demonstrated that the in vivo environment may influence their metabolic requirements, it is generally accepted that in vitro FoxP3 positive Tregs rely more on fatty acid oxidation and do not undergo glycolysis. In addition, it has recently been suggested that FoxP3, the key transcription factor in Tregs, actively turns off glycolysis (10,17,18,19). In keeping with this, we have shown that in normoxic conditions, in vitro cultured Tregs cells produce relatively little lactate, and that production does not increase with activation. Given this, we reasoned that lactate may be a particularly attractive method for assessing Treg suppression of Teffs, since the co-cultures would not be complicated by significant background production of metabolites from the regulatory cell pool. Indeed, this was demonstrated to be the case, with suppression of lactate production showing strong correlation with suppression of T cell proliferation as determined by cell division tracking dye and flow cytometry (R2 = 0.894). As described in the methods, the amount of lactate produced by Teffs in the presence of Tregs was corrected by the amount of lactate produced by 104 stimulated Tregs (regardless of the actual number of Tregs in any given well), raising the possibility that our method may overestimate suppression at the lower Treg:Teff ratios. However this effect was found to be small, and was not statistically significant. Given, its simplicity, sensitivity, stability and cost effectiveness, we have shown that lactate could be useful in interrogating T cell responses in high-throughput assays – such as measuring responses to libraries of overlapping peptides in order to assess vaccine induced, or other immunological responses. To assess this, we examined its utility in identifying CMV seropositive healthy individuals; and found it to be both 100% sensitive and specific in the sample tested. A summary of the techniques used in this paper, including their cost and time for analysis can be seen in Table I.
Table I.
Summary of cell culture analysis techniques.
| Technique Name | Type of measurement | Cost per well (£) | Radiation Exposure | Experimental Time |
|---|---|---|---|---|
| Lactate | Cumulative | 1 | No | Approx. 30 Minutes
|
| Flow Cytometry | Cumulative | 5 | No | Several Hours
|
| Thymidine | Snapshot | 1 | Yes | >12 hours
|
In conclusion, we believe that the measurement of extracellular lactate is a safe, reliable, sensitive, fully quantifiable and cost-effective method for analyzing T cell proliferative responses in vitro, that can complement, and in selected circumstances replace, more traditional methods. In particular, we suggest that lactate may prove to be particularly useful in high-throughput screening assays.
Supplementary Material
Acknowledgments
This research was performed at the Cambridge NIHR BRC Cell Phenotyping Hub and the authors would like to thank Dr Andy Sage (Department of Cardiovascular medicine, University of Cambridge) for help with thymidine assays.
Funding:
This study was funded by the Medical Research Council, Wellcome Trust, and CRUK.
Footnotes
Authorship Contributions
Writing of manuscript: JTG, LBJ, JLJ
Study Design: JTG, LBJ, JLJ, FAG,
Data Acquisition: LBJ, JTG, HK, ZG, SJ,
Data Analysis: JTG, LBJ, SJ
Data Interpretation: JTG, LBJ, FAG, JLJ, SJ, MW
Reviewing Final Manuscript: All
Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Michalek Ryan D, Rathmell Jeffrey C. The metabolic life and times of a T cell. Immunol Rev. 2011;236:190–202. doi: 10.1111/j.1600-065X.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearce EL, Pearce EJ. Metabolic Pathways In Immune Cell Activation And Quiescence. 2014;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Matteson EL, Goronzy JJ, Weyand CM. T-cell metabolism in autoimmune disease. Arthritis Res Ther. 2016:1–10. doi: 10.1186/s13075-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill LAJ, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–65. doi: 10.1038/nri.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang C-H, Pearce EL. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol. 2016;17:364–368. doi: 10.1038/ni.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buck MD, Sullivan DO, Pearce EL. T cell metabolism drives immunity. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–52. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 8.Coe DJ, Kishore M, Marelli-Berg F. Metabolic regulation of regulatory T cell development and function. Front Immunol. 2014;5:1–6. doi: 10.3389/fimmu.2014.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newton R, Priyadharshini B, Turka LA. Immunometabolism of regulatory T cells. Nat Immunol. 2016;17:618–625. doi: 10.1038/ni.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michalek Ryan D, Gerriets Valerie A, Jacobs Sarah R, Macintyre Andrew N, MacIver Nancie J, Mason Emily F, Sullivan Sarah A, Nichols Amanda G, Rathmel JC. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J Immunol. 2012;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sylwester A, Mitchell B, Edgar J, Taormina C, Pelte C, Ruchti F, Sleath P, Grabstein K, Hosken N, Kern F, Nelson J, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson SE, Mason GM, Okecha G, Sissons JGP, Wills MR. Diverse specificities, phenotypes, and antiviral activities of cytomegalovirus-specific CD8+ T cells. J Virol. 2014;88:10894–908. doi: 10.1128/JVI.01477-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson SE, Sedikides GX, Mason GM, Okecha G, Wills MR. Human Cytomegalovirus (HCMV)-Specific CD4+ T Cells Are Polyfunctional and Can Respond to HCMV-Infected Dendritic Cells In Vitro. J Virol. 2017;91:1–20. doi: 10.1128/JVI.02128-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collison LW, Vignali DAA. In Vitro Treg Suppression Assays. Methods. 2011;707:21–37. doi: 10.1007/978-1-61737-979-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. Table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinclair E, Black D, Epling CL, Carvidi A, Josefowicz SZ, Bredt BM, Jacobson MA. CMV antigen-specific CD4+ and CD8+ T cell IFNgamma expression and proliferation responses in healthy CMV-seropositive individuals. Viral Immunol. 2004;17:445–454. doi: 10.1089/0882824041857049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Ertl HCJ. Starved and asphyxiated: How can CD8+ T cells within a tumor microenvironment prevent tumor progression. Front. Immunol. 2016;7:1–7. doi: 10.3389/fimmu.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelin A, Gil-de-go L, Dahiya S, Wallace DC, Hancock WW, Beier UH. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metabolism Ref. 2017;25:1–12. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matarese G, Galgani M, De Rosa V, La Cava A. Regulatory T Cells Role of Metabolism in the Immunobiology of Role of Metabolism in the Immunobiology of Regulatory T Cells. J Immunol Ref. 2017;197:2567–2575. doi: 10.4049/jimmunol.1600242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.