Abstract
Context
Offspring exposed to maternal obesity in-utero are at an increased risk of later obesity; however the underlying mechanisms remain unknown.
Objective
To assess the effect of an antenatal lifestyle intervention in obese women on the offspring’s cord blood metabolic profile, and determine association between the maternal clinical characteristics, cord blood metabolic profile and offspring body composition at birth and 6 months of age.
Design
Randomised controlled trial and cohort study.
Setting
Data from the UK Pregnancies Better Eating and Activity randomised controlled trial (UPBEAT).
Participants
344 mother-offspring pairs.
Intervention
Antenatal behavioural lifestyle (diet and physical activity) intervention.
Main outcome measures
Untargeted cord blood metabolic profile, including candidate hormone and metabolomic analyses.
Results
The lifestyle intervention was not associated with change in any measures of the cord blood metabolic profile. Higher maternal glycaemia, specifically fasting glucose at 28 weeks’ gestation had a linear association with higher cord blood concentrations of lysophosphatidylcholines 16.1 (β=0.65; 95%CI 0.03 to 0.10) and 18.1 (0.52; 0.02 to 0.80). A principal component of cord blood phosphatidylcholines and lysophosphatidylcholines was associated with infant z-scores of birthweight (0.04; 0.02 to 0.07) and weight at age 6 months (0.05; 0.00 to 0.10). Cord blood IGF-1 and adiponectin concentrations were also positively associated with infant weight z-scores at birth and at 6 months.
Conclusions
We provide novel evidence that lysophosphatidylcholines and IGF-1 measured in cord blood are related to infant weight. These findings lend support to the hypothesis that susceptibility to childhood obesity may be programmed in-utero, but further investigation is required to establish whether these associations are causally related.
Keywords: Maternal obesity, cord blood, gestational diabetes, offspring body composition, catch up growth
Introduction
The increasing incidence of childhood obesity is a major public health concern. Recent global estimates from the WHO, suggest that 41 million children under the age of 5 years are overweight or obese (1). Observational cohort studies and experimental animal studies have strongly suggested that both the pre- and postnatal environments modulate developmental pathways that increase susceptibility to later obesity (2). Offspring exposed to maternal obesity, excessive gestational weight gain (GWG) and/or gestational diabetes (GDM) in-utero are at an increased risk of obesity and altered glucose metabolism throughout the life-course (2, 3). Exposure to maternal obesity in-utero is proposed to set the offspring on a trajectory of increased adiposity throughout life due to persistent changes in metabolic function (4).
Metabolomics enables the investigation of low-molecular weight molecules such as intermediate metabolites and signalling molecules and can be used as a tool to provide insight in the systemic perturbations of an individual as a result of pathophysiological in-utero exposure (5). Investigations of cord blood metabolic profiles have previously been conducted within small cases-control studies assessing associations with birth weight or post-natal trajectories and with limited adjustment for in-utero confounding variables. In a large birth cohort from Germany, certain cord blood metabolites were associated with birth weight (6). However, neonatal adiposity explains only 40% of the observed variation in birthweight.
To date, no investigations have addressed the relations between maternal clinical and biochemical characteristics in obese women and fetal metabolism, in association with neonatal and early infancy weight and anthropometric measures of adiposity. We examined these relations in a group of obese pregnant women and their offspring who had taken part in the UK Pregnancies Better Eating and Activity Trial (UPBEAT); a randomised controlled trial assessing a behavioural lifestyle intervention in 1555 obese pregnant women (7). Although the trial intervention did not reduce the incidence of GDM and delivery of a large for gestational age infant (primary outcomes), we have recently reported a reduction in infant subscapular skinfold thickness at 6 months of age mediated through significant improvements in maternal antenatal diet, measures of adiposity and GWG initiated by the UPBEAT intervention (8).
Our primary aim was to determine if the intervention resulted in changes in an untargeted cord blood metabolic profile and candidate hormones previously implicated with obesity and fetal growth. The secondary aim was to explore the relations between maternal antenatal characteristics including total GWG, pre-pregnancy BMI and GDM and cord blood metabolic profile. As weight and adiposity have been shown to track through childhood, further assessment was made for potential relations between metabolites in the cord blood and measures of weight and anthropometry in offspring at birth and at 6 months of age.
Subjects and Methods
Study Design
This study was a secondary analysis from the UPBEAT trial(7). To assess the primary aim of this study; the influence of the UPBEAT lifestyle intervention on the cord blood metabolic profile, the UPBEAT study was treated as an RCT (7). As the secondary aim of the study was to assess the relationship of the cord blood metabolic profile with maternal clinical characteristics and neonatal and infant anthropometry, an analysis based on a cohort study approach was chosen using both active treatment and control groups, and taking into account the original randomisation allocation.
Study population
Primary aim
Women over the age of 16 years were recruited to the UPBEAT trial between 15+0-18+6 weeks’ gestation. The participants were from inner-city populations with high socioeconomic deprivation. The detailed study design including inclusion and exclusion has been previously published (7).
In summary, the UPBEAT study recruited 1555 obese women from 8 tertiary maternity units located within inner city populations at 15+0-18+6 weeks’ gestation. A behavioural intervention was devised based on psychological models of health behaviour including control and social cognitive theory, delivered via weekly sessions to increase physical activity and reduce maternal glycaemic load and saturated fat intake. The primary maternal outcome was a reduction in the incidence of GDM at 27-28+6 weeks gestation and the neonatal outcome was a reduction in the delivery of a large for gestational age infant. Women were randomised using an online database with minimisation for ethnicity, parity and BMI, to ensure that the groups were comparable at baseline. 47.3% of offspring were followed up at 6 months postpartum. In comparison to those who did not take part, the mothers were older, more likely to be White, nulliparous and less likely to be current smokers (See Supplement, Table 2). There was no significant difference in sessions covered between those who did and did not take part in the present study (p=0.09).
Secondary aim
Mother-neonate pairs were included in the analyses if detailed neonatal anthropometric and cord blood metabolic data were available. Infants were included within the further analysis of data at 6months of age if they attended that follow-up appointment and did not suffer from major ill health.
Cord blood analyses
Cord blood biomarkers
Candidate cord blood biomarkers assessed in this study include cord blood insulin, C-peptide, glucose, LDL-c, HDL-c, triglycerides, adiponectin, leptin, IGF I, II, IL-6 and TNF-α (Supplement Text 1).
Metabolomic analyses
An untargeted cord plasma metabolome was analysed using mass spectroscopy, enabling the quantification of phospholipids, acylcarnitines, non-esterified fatty acids (NEFA), carboxylic acids and amino acids as described previously (Supplement Material Text 1) (9, 10). Analysis was undertaken in eight batches.
Maternal variables
Maternal clinical characteristics investigated, included maternal early pregnancy BMI (kg/m2); total GWG (kg) defined from pre-pregnancy to 34-36 weeks’ gestation; GDM defined using the IADPSG’s diagnostic criteria at 24-28+6 weeks’ gestation, and the fasting glucose, 1 and 2 hour glucose concentrations at the time of the OGTT (11).
Offspring anthropometry
Neonate
Anthropometric measurements were made within 72 hours of birth by a trained midwife. Birthweight was recorded from maternal medical records and birthweight z-scores calculated using a UK reference population adjusted for sex and gestation at delivery (12). Neonatal subscapular and triceps skin fold thicknesses (SFT) were measured using Harpenden skinfold callipers in triplicate and the sum of skin fold thickesses (SSFT) were calculated. Neonatal length was assessed using a neonatometer. Abdominal and mid upper arm circumferences were assessed.
Infant
Anthropometric measurements were collected at 6 months of age by a trained midwife. Weight was assessed using SECA® scales, and length assessed in the supine position using an infantometer. Triceps and subscapular SFT were measured in triplicate using Holtain callipers. Where reference WHO population data were available, z-scores were calculated, adjusting for infant sex and age at measurement (13). Early catch-up growth was defined using the WHO definition of catch up growth, defined as an increase ≥0.67SDs in weight-z-scores from birth to 6 months of age (13). Similarly catch down growth was defined as a decrease in weight-z-scores from birth to 6 months of age of ≥0.67SDs (13).
Statistical analyses
Cord blood metabolic profile
To correct for potential batch effects with the cord blood metabolomics analysis, linear regression models were applied and the residuals were used for further statistical analysis. Metabolites were standardised to average metabolite concentrations and standard deviation over all eight batches for statistical analysis. Metabolites were included in the analyses if they had > 70% complete data. Cord blood biomarkers and metabolomic variables were assessed for normality and transformed appropriately. Variables were summarised using mean (SD) and median (IQR) where appropriate.
Principal component analysis was undertaken for the metabolomic data only, to reduce the number of metabolites based on a series of uncorrelated linear combinations of variables containing the most variance. Following orthogonal rotation, metabolites with a loading ≥0.1 were considered to have a strong association with the cluster.
Effect of a lifestyle intervention on the cord blood metabolic profile
Assessment was made for any differences in maternal characteristics and birth outcomes between those included vs. those excluded from the analysis. Adjustment was made for any apparent differences in maternal characteristics at trial entry (15+0-18+6 weeks’ gestation) between the two arms. The effect of the UPBEAT intervention was assessed using linear regression adjusting for minimisation variables used at trial randomisation (ethnicity, parity and maternal early pregnancy BMI).
Maternal associations with cord blood metabolic profile
Maternal antenatal variables (including early pregnancy BMI, GWG and GDM) were assessed in relation to the cord blood metabolic profile. To assess for potential relationships, multivariable linear regression was undertaken, where components of the cord blood metabolic profile were treated as the outcome and maternal antenatal variables as the exposure adjusting for offspring sex, gestational age at delivery and randomisation to the UPBEAT intervention.
Cord blood metabolic profile and offspring anthropometry
To assess the association between the cord blood metabolic profile (exposure) and subsequent offspring anthropometry (outcome) at birth and at 6 months, multivariable linear or logistic regression was undertaken where appropriate.
Adjustment was made for confounders, selected a-priori based on clinical knowledge with the aid of directed acyclic graphs (DAGs) (Supplement Text 2). Unless a systematic approach is taken, adjusting for potential confounders may increase bias. The proper use of DAGs in selecting covariates is likely to reduce the degree of bias (14). Selected confounders included age at anthropometric measurement, offspring sex where appropriate and randomisation to the UPBEAT intervention (Model 1). Further adjustment was made for maternal parity, ethnicity (reference white ethnicity), current smoker in early pregnancy, GDM, GWG (Model 2). For potential associations of metabolic profile at birth and infant anthropometry at 6 months of age, further adjustment was made for mode of feeding (reference exclusive breastfeeding ≥4 months of age).
All linear regression models were further assessed for data points exhibiting high leverage by using Cook’s Distance (defined as Di>4/n), heteroscedasticity and linearity. Correction for multiple testing was undertaken using a false discovery rate utilising the Benjamin & Hochberg procedure. Presented significance levels were corrected for multiple testing (statistical significance p<0.05) (15).
Sensitivity analyses
Sensitivity analysis were undertaken by assessing demographic characteristics for those included within the analyses versus the mother-offspring pairs excluded. Sensitivity analyses were performed excluding offspring born <34 weeks’ gestation. A further sensitivity analyses was undertaken excluding mothers diagnosed with GDM. As mode of delivery has been shown to influence the cord blood metabolic profile, a fourth sensitivity analysis was undertaken with statistical models further adjusted for mode of delivery (reference category; unassisted vaginal delivery).
All statistical analyses were performed using Stata Version 14.1.
Results
Demography
Of the 608 cord samples available from neonates born to women randomised to the UPBEAT trial, 343 mother-offspring pairs were included (Supplement Figure 2). Median maternal BMI was 35.6 kg/m2 (IQR 33.0, 38.9), 71.7% were of a white ethnic group and 87.8% were in the highest quintiles of socioeconomic deprivation. Median neonatal birthweight was 3.5 kg (IQR 3.21, 3.82 kg) and 26.0% of offspring demonstrated significant catch-up growth as defined as >0.67 SD increase in infant weight z-scores between offspring birthweight and assessment at 6 months of age. Further maternal, neonatal and infant demographics and anthropometric characteristics are provided in Table 1. To assess for potential selection bias, comparisons were made between mother-offspring pairs included and excluded from the analysis. The incidence of black ethnicity, neonatal birthweight and subscapular SFT were different between the two groups (Supplement Table 2). There was no difference in the incidence of GDM, total GWG or infant anthropometric measures between the two groups (Supplement Table 2).
Table 1.
Maternal, neonatal and infant demographic, anthropometry and clinical characteristics of mother-offspring pairs by randomisation allocation (n=343).
| Intervention | Control | All | |||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD)/Median (IQR)/N(%) | N | Mean (SD)/Median (IQR)/N(%) | N | Mean (SD)/Median (IQR)/N(%) | ||
| Maternal | |||||||
| Age (years) | 169 | 31.0 (28.0, 35.0) | 174 | 31.0 (27.0, 35.0) | 343 | 31.0 (27.0, 35.0) | |
| BMI (kg/m2) | 169 | 35.5 (33.0, 39.1) | 174 | 35.7 (33.0, 38.5) | 343 | 35.6 (33.0, 38.9) | |
| Ethnicity | Asian | 169 | 12 (7.1) | 174 | 7 (4.0) | 343 | 19 (5.5) |
| Black | 32 (18.9) | 31 (17.8) | 63 (18.4) | ||||
| Other | 7 (4.1) | 8 (4.6) | 15 (4.4) | ||||
| White | 118 (69.8) | 128 (73.6) | 246 (71.7) | ||||
| Multiparous | 169 | 86 (50.9) | 174 | 79 (45.4) | 343 | 165 (48.1) | |
| Smoker in early pregnancy | 169 | 7 (4.1) | 174 | 12 (6.9) | 343 | 19 (5.5) | |
| Socioeconomic deprivation | 169 | 145 (85.7) | 174 | 151 (86.8) | 343 | 295 (87.8) | |
| Gestational weight gain (kg) | 169 | 6.94 (4.27) | 174 | 8.00 (3.79) | 343 | 7.78 (4.07) | |
| Gestational diabetes | 169 | 59 (34.9) | 174 | 52 (29.9) | 343 | 111 (32.4) | |
| Neonate | |||||||
| Mode of delivery | Vaginal | 169 | 73 (43.2) | 174 | 65 (37.4) | 343 | 138 (40.2) |
| Operative vaginal | 25 (14.8) | 19 (10.9) | 44 (12.8) | ||||
| Emergency C-section | 28 (16.6) | 50 (28.7) | 78 (22.7) | ||||
| Elective C-section | 43 (25.4) | 40 (23.0) | 83 (24.2) | ||||
| Gestation at delivery (weeks) | 169 | 39.7 (38.7, 40.7) | 174 | 40.0 (38.7, 41.0) | 343 | 39.9 (38.7, 40.9) | |
| Birthweight (kg) | 169 | 3.51 (3.22, 3.79) | 174 | 3.59 (3.19, 3.86) | 343 | 3.6 (3.2, 3.8) | |
| Birthweight z scores | 169 | 0.12 (0.99) | 174 | 0.13 (0.99) | 343 | 0.21 (0.76) | |
| Skinfold thickness-subscapular (mm) | 169 | 5.81 (1.46) | 174 | 5.75 (1.37) | 343 | 5.78 (1.42) | |
| Skinfold thickness triceps (mm) | 169 | 5.33 (1.42) | 174 | 5.34 (1.49) | 343 | 5.34 (1.45) | |
| Sum of skinfold thicknesses (mm) | 169 | 11.14 (2.61) | 174 | 11.09 (2.58) | 343 | 11.12 (2.59) | |
| Midarm circumference (cm) | 169 | 11.57 (0.98) | 174 | 11.59 (0.97) | 343 | 11.58 (0.97) | |
| Abdominal circumference (cm) | 169 | 32.71 (2.11) | 174 | 32.57 (1.99) | 343 | 32.63 (2.05) | |
| Infant | |||||||
| Weight for age z-score | 125 | 0.17 (1.04) | 123 | 0.42 (0.97) | 247 | 0.29 (1.01) | |
| Length for age z-score | 120 | 0.36 (1.71) | 119 | 0.54 (1.99) | 238 | 0.44 (1.85) | |
| BMI for age z-score | 120 | -0.00 (1.62) | 119 | 0.21 (2.02) | 238 | 0.10 (1.84) | |
| Arm circumference z-score | 125 | 1.11 (0.92) | 122 | 1.40 (1.86) | 246 | 1.25 (1.47) | |
| Triceps skinfold thickness z-score | 124 | 0.17 (1.59) | 119 | 0.44 (1.42) | 242 | 0.29 (1.51) | |
| Subscapular skinfold thickness z-score | 107 | 0.23 (1.42) | 103 | 0.37 (1.49) | 209 | 0.30 (1.45) | |
| Catch up growth* | 125 | 31 (24.8) | 122 | 34 (27.9) | 246 | 64 (26.0) | |
| Catch down growth** | 125 | 36 (28.8) | 122 | 24 (19.7) | 246 | 60 (24.4) | |
Abbreviations BMI-Body Mass Index.
Catch up growth defined as a ≥ 0.67 SDs increase in weight-z-scores from birth to 6 months of age.
Catch down growth defined as a ≥0.67 SDS decrease in weight-z-scores from birth to 6 months of age.
191 cord blood metabolites and 12 candidate biochemical markers were included in the analyses. Summary statistics of cord biochemical analyses including candidate biomarkers and metabolomic analyses are shown in Supplement Table 3. Following PCA, 4 distinct principal components of metabolites were identified which were; “Phosphatidylcholines”, “Non-esterified fatty acids”, “Long-chain Acylcarnitines and TCA metabolites” and “Amino acids” (Supplement Figure 3a-d).
Effect of the UPBEAT intervention
Mother’s included in this analysis were older, more likely to be nulliparous and less likely to be of black ethnic origin compared to those without a cord blood sample (Supplement Table 4). Following correction for multiple testing, there were no significant differences in the cord blood metabolic profile including clusters derived from PCA, between intervention and control arms (Supplement Figure 4).
Relationships between maternal exposures and cord blood metabolic profile
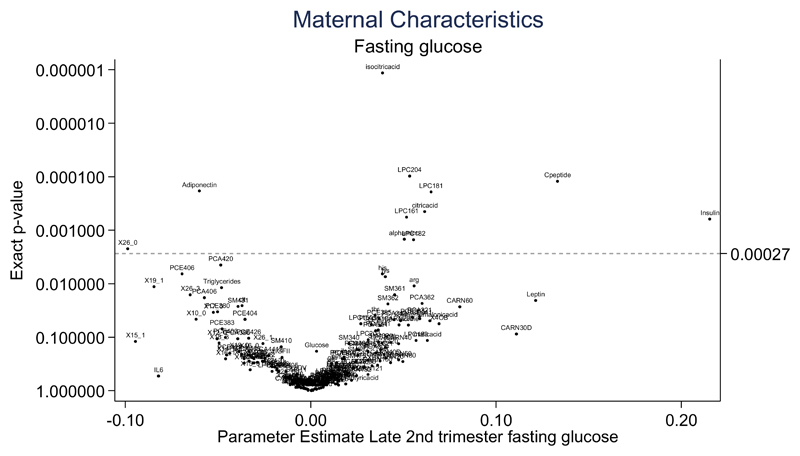
Diagnosis of maternal GDM was associated with reduced cord blood adiponectin and increased isocitric acid and lysophosphatidylcholine (LPC) 18.1 concentrations following correction for multiple testing by using a FDR (Supplement Figure 5). Both maternal early pregnancy BMI and total GWG were not associated with the cord blood metabolic profile (Supplement Figure 6 and 7). Maternal fasting glucose collected at the time of the OGTT (28 weeks’ gestation) was positively associated with higher cord insulin, C-peptide, LPC 18.1, 18.2 and 20.4, alpha aminoadipic acid and citric acid following correction for multiple testing. Maternal fasting glucose was also associated with lower cord adiponectin and NEFA 26.0 (Figure 1). Associations between maternal glucose at 1 hour and 2 hour post OGTT are illustrated in Supplement Figure 8 and 9.
Figure 1. Volcano plot demonstrating the association of maternal fasting glucose at the time of the oral glucose tolerance test with the cord blood metabolic profile from infants born to obese pregnant women (n=607).
Parameter estimates are graphically represented for each biochemical variable in relation to maternal clinical characteristics following adjustment using a false discovery rate (Benjamin & Hochberg procedure) (17). Statistical significance p<0.0027. Abbreviations; CARN-Carnitines; HDL-High density lipoprotein; IGFI- Insulin growth factor I; IGF II- Insulin growth factor II; IL6- Interleukin 6; LPC- lysophosphatidylcholines; LPCE- lysophosphatidylethanolamine; PC-phosphatidylcholines; PCA- diacylphosphatidylcholines; PCE- acylalkylphosphatidylcholines; SM- sphingomyelins. TNFalpha- Tumor necrosis factor; X30B- x3methyl2oxobutanoicacid; X30V-- x4methyl2oxovalvericacid. NEFAs are described using the nomenclature CX:Y, where X is the length of the carbon chain, Y is the number of double bonds, OH in the formula means the molecule contains a hydroxyl-group.
Cord blood metabolic profile and neonatal anthropometry
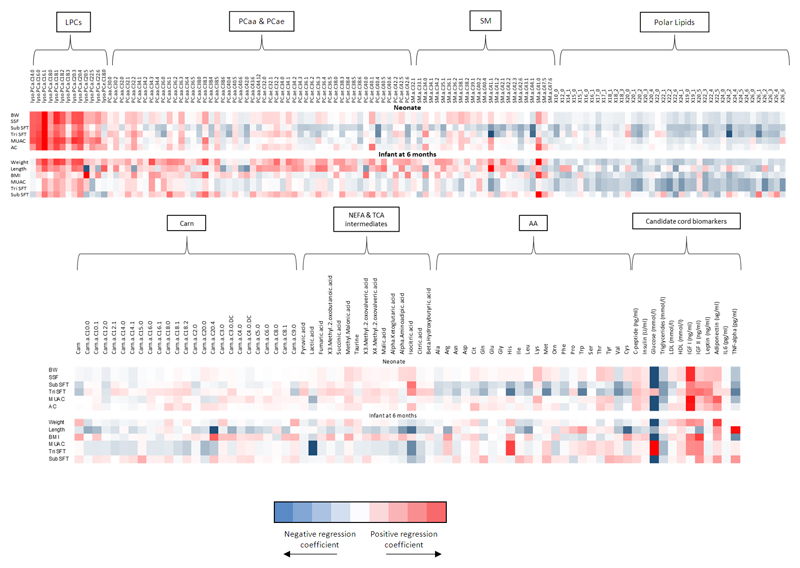
There was a positive linear relationship between cord C-peptide, insulin, IGF-1, leptin and neonatal birthweight z-scores, SSFT, subscapular SFT, triceps SFT (except for insulin, and C-peptide), mid upper arm and abdominal circumference (Table 2, Supplement Figure 10). Principal components of NEFAs as assessed in the metabolome and cord blood triglycerides were inversely associated with neonatal birthweight z-scores, SSFT, subscapular SFT, triceps SFT and mid upper arm circumference at birth (Table 2, Supplement Figure 10). HDL, adiponectin and principal components of phosphatidylcholines were linearly associated with birthweight z-score only. LPC 16.1 and 18.0 were positively associated with neonatal birth weight, SSFT, subscapular and triceps SFTs following correction for multiple testing (Figure 2). Cord blood cholesterol was not associated with any measure of neonatal anthropometry (Supplement Figure 10). Interleukin-6 and TNF-α were negatively associated with neonatal birthweight z-scores. There were no associations between cord principal components of acylcarnitines, amino acids and IGF-II with any measure of neonatal anthropometry (Table 2, Figure 2 and Supplement Figure 10).
Table 2.
Associations of the cord blood metabolic profile with neonatal anthropomety in infants born to obese pregnant women (n=344)
| Birthweight z-scores (SDS) | SSFT (mm) | Subscapular SFT (mm) | Triceps SFT (mm) | MUAC (cm) | Abdominal circumference (cm) | |
|---|---|---|---|---|---|---|
| Coef (95% CI) | Coef (95% CI) | Coef (95% CI) | Coef (95% CI) | Coef (95% CI) | Coef (95% CI) | |
| PCA-Phosphatidylcholines | 0.04 (0.02 to 0.07)** | -0.11 (-0.25 to 0.03) | -0.06 (-0.12 to 0.01) | -0.05 (-0.15 to 0.04) | 0.03 (-0.02 to 0.07) | 0.01 (-0.10 to 0.11) |
| PCA- NEFA | -0.04 (-0.08 to 0.00)* | -0.28 (-0.49 to -0.08)* | -0.11 (-0.21 to 0.00)* | -0.18 (-0.32 to -0.03)* | 0.02 (-0.05 to 0.10) | -0.01 (-0.17 to 0.15) |
| PCA- Long chain acylcatnitines and TCA metabolites | 0.00 (-0.04 to 0.04) | -0.05 (-0.27 to 0.17) | -0.02 (-0.13 to 0.09) | -0.03 (-0.18 to 0.12) | -0.01 (-0.08 to 0.07) | 0.01 (-0.15 to 0.17) |
| PCA-Amino acids | 0.05 (0.00 to 0.11) | -0.05 (-0.36 to 0.25) | 0.06 (-0.09 to 0.20) | -0.11 (-0.32 to 0.10) | 0.06 (-0.04 to 0.16) | 0.19 (-0.03 to 0.40) |
| Cpeptide (log2) ng/ml | 0.27 (0.14 to 0.39)** | 0.86 (0.22 to 1.50)* | 0.52 (0.17 to 0.87)* | 0.34 (-0.02 to 0.69) | 0.25 (0.00 to 0.49)* | 0.73 (0.22 to 1.25)* |
| Insulin (log2) U/ml | 0.16 (0.09 to 0.23)** | 0.47 (0.12 to 0.82)* | 0.31 (0.11 to 0.50)* | 0.17 (-0.03 to 0.37) | 0.16 (0.02 to 0.29)* | 0.38 (0.10 to 0.67)* |
| Glucose (log2) mmol/l | -1.80 (-3.59 to 0.00)* | -2.94 (-12.96 to 7.08) | -3.68 (-9.32 to 1.96) | 0.48 (-4.94 to 5.90) | -2.13 (-6.01 to 1.75) | -6.19 (-14.48 to 2.09) |
| Triglycerides mmol/l | -0.47 (-0.74 to -0.20)** | -1.65 (-3.18 to -0.11)* | -0.95 (-1.82 to -0.08)* | -0.73 (-1.56 to 0.10) | -0.90 (-1.48 to -0.33)* | -1.05 (-2.32 to 0.22) |
| Cholesterol mmol/l | 0.13 (-0.06 to 0.32) | -0.75 (-1.68 to 0.17) | -0.45 (-0.97 to 0.07) | -0.31 (-0.81 to 0.19) | -0.11 (-0.48 to 0.25) | 0.40 (-0.38 to 1.17) |
| HDL mmol/l | 0.22 (0.06 to 0.38)* | -0.11 (-0.89 to 0.67) | -0.25 (-0.68 to 0.17) | 0.16 (-0.28 to 0.60) | 0.02 (-0.27 to 0.32) | 0.38 (-0.25 to 1.01) |
| IGFI (log2) ng/ml | 1.13 (0.97 to 1.29)** | 1.80 (0.89 to 2.72)** | 1.08 (0.58 to 1.59)** | 0.72 (0.20 to 1.24)* | 1.19 (0.87 to 1.51)** | 2.41 (1.70 to 3.12)** |
| IGFII (log2) ng/ml | 0.31 (-0.02 to 0.65) | 1.57 (-0.15 to 3.29) | 0.68 (-0.27 to 1.63) | 0.89 (-0.08 to 1.85) | 0.29 (-0.37 to 0.96) | 0.54 (-0.86 to 1.93) |
| Leptin (log2) ng/ml | 0.38 (0.30 to 0.46)** | 1.59 (1.21 to 1.97)** | 0.83 (0.62 to 1.04)** | 0.76 (0.54 to 0.98)** | 0.50 (0.35 to 0.66)** | 0.80 (0.47 to 1.13)** |
| Adiponectin (log2) ug/ml | 0.40 (0.12 to 0.68) | 0.64 (-0.79 to 2.08) | 0.48 (-0.31 to 1.27) | 0.07 (-0.73 to 0.87) | 0.84 (0.31 to 1.37)* | 1.16 (0.00 to 2.31)* |
| IL6 (log2) pg/ml | -0.04 (-0.07 to 0.00)* | 0.06 (-0.11 to 0.23) | -0.02 (-0.11 to 0.08) | 0.08 (-0.02 to 0.17) | -0.04 (-0.11 to 0.03) | -0.14 (-0.28 to 0.00)* |
| TNF alpha (log2) pg/ml | -0.58 (-1.12 to -0.03)* | -1.44 (-4.21 to 1.32) | -0.72 (-2.25 to 0.80) | -0.86 (-2.41 to 0.69) | -0.84 (-1.87 to 0.19) | -0.61 (-2.82 to 1.60) |
Regression coefficients with corresponding 95% confidence intervals presented are adjusted for maternal parity, ethnicity, smoker in early pregnancy, gestational diabetes, gestational weight gain, offspring sex, gestation at delivery, randomisation to UPBEAT Intervention. Abbreviations; HDL- High density lipoprotein; IGFI- Insulin growth factor I; IGFII- Insulin growth factor II; IL6-Interleukin 6; MUAC-Mid upper arm circumference; NEFA- Non-esterified fatty acids; SDS- Standard deviation scores; SFT- skinfold thickness; TCA- Tricarboxylic acid; TNFalpha- Tumour Necrosis Factor alpha. *p<0.05; ** p<0.001.
Figure 2. Heat map demonstrating associations between cord blood metabolites with anthropometric measurements in neonates (n=344) and six month old infants (n=209). Data from infants born to obese pregnant women in the UPBEAT study.
Regression coefficients plots with adjustment made for maternal parity, ethnicity, smoker in early pregnancy, gestational diabetes, gestational weight gain, offspring sex, gestation at delivery, randomisation to UPBEAT Intervention. Additional adjustment is made for early mode of infant feeding for infant anthropometry data at 6 months of age. Abbreviations; BW- Neonatal birthweight z-scores (SDs); CARN-Carnitines; LPC- lysophosphatidylcholines; LPCE- lysophosphatidylethanolamine; MUAC- Mid upper arm circumference (cm); PC-phosphatidylcholines; PCA- diacylphosphatidylcholines; PCE- acylalkylphosphatidylcholines SM- sphingomyelins; SSF- Sum of skinfold thicknesses (mm); Tri SFT- Triceps skinfold thickness (mm); Sub SFT- Subscapular skinfold thickness (mm); X30B- 3methyl2oxobutanoicacid; X30V- 3methyl2oxovalvericacid; X40B- x4methyl2oxovalvericacid. NEFAs are described using the nomenclature CX:Y, where X is the length of the carbon chain, Y is the number of double bonds, OH in the formula means the molecule contains a hydroxyl-group.
Cord blood metabolic profile and infant anthropometry at 6 months of age
Of those biochemical variables, which were significantly associated with neonatal body composition, clusters of phosphatidylcholines and adiponectin were linearly associated with infant weight and length z-scores at 6 months of age (Figure 2, Supplement Table 5). In particular, LPC 16.1 and 18.1 were linearly associated with infant weight z-scores at 6 months of age (Figure 2, Supplement Table 5). Cord IGF-I was linearly associated with infant weight z-scores, BMI z-score and mid upper arm circumference z-score (Figure 2, Supplement Table 5). Cord leptin and triglycerides were negatively associated with infant mid-upper arm circumference z-scores following adjustment for maternal and infant confounding (Figure 2, Supplement Table 5). There were no associations between cord insulin, glucose, C-peptide and IL-6 with infant anthropometry at 6 months of age (Figure 2, Supplement Table 5).
For every unit increase in principal components of phosphatidylcholines, the odds of catch up growth at 6 months of age increased by 1.35 (1.04 to 1.75), whereas leptin decreased by 0.33 (0.17 to 0.52) (Supplement Table 6). IGF-1 and leptin were positively associated with increased odds of catch down growth at 6 months of age (Supplement Table 6).
Sensitivity analyses
The associations between cord blood metabolic profile and neonatal or infant body composition remained unchanged following removal of offspring born <34 weeks’ gestation (N=36) (Supplement Figure 11 & 12), those participants exposed to GDM (n=111) (Supplement Figure 13 & 14) and following further adjustment for mode of delivery (Supplement Figure 15 & 16).
Discussion
This study reports a comprehensive cord blood metabolic profile, including candidate biochemical markers and metabolome, in offspring born to obese mothers. By demonstrating associations with fasting glucose, it has been shown that in-utero exposure to maternal dysglycaemia in obese pregnancies has the potential to modify the cord blood metabolic profile at birth. Although there was no effect of the UPBEAT antenatal lifestyle intervention on the cord blood metabolic profile, when treating the data as a cohort, associations were observed between the cord blood metabolic profile and offspring growth in early life. The novel associations between cord lysophosphatidylcholines and neonatal adiposity, together with the relation with maternal hyperglycaemia may provide mechanistic insight into the early-life origins of obesity.
The lack of effect of the UPBEAT intervention on the cord metabolic profile may suggest that the differences observed in the maternal secondary outcomes including reduction in adiposity and gestational weight gain were inadequate to have a major impact on fetal metabolism, although more subtle molecular effects could have occurred to influence adiposity in the six month infants as recently reported (8). Principal components of phosphatidylcholines and lysophosphatidylcholines were found to be positively associated with early growth velocities and weight z-scores, providing possible mechanistic insight of the mechanisms contributing to early postnatal growth in offspring born to obese women. The finding that principal components of cord lysophosphatidylcholines, primarily lysophosphatidylcholines 16.1 and lysophosphatidylcholines 18.1 were not only associated with neonatal weight-z-scores, but also infant growth and catch-up growth within the first 6 months of age provides novel evidence, suggesting a role in the early life growth velocities. Of relevance, associations with cord lysophosphatidylcholines and birthweight were recently reported in a birth cohort from Germany(16). In the present study lysophosphatidylcholines were associated with neonatal adiposity as well as birthweight; supporting a role in body fat accretion. It may be of relevance to these observations that the infant growth trajectory in the first 6 months of life has been shown to be predictive of adolescence and early adulthood obesity (17) and that The European Childhood Obesity Programme has shown that lysophosphatidylcholines 14.0 correlate with rapid growth in infancy and subsequent obesity at 6 years of age (16). Together these findings would suggest a possible role for lysophosphatidylcholines in the early life ‘programming’ of obesity risk (18).
Further interrogation of the dataset demonstrated a significant linear relationship between cord blood insulin, C-peptide and IGF-1 with cord lysophosphatidylcholines (Supplement Figure 17 &18) supporting the suggestion of an interaction between fetal glucose homeostasis and these molecules(19). This observation is in part supported by one small case-control study (n=46) that identified an inverse relationship between maternal gestational diabetes and placental uptake of lysophosphatidylcholines 22:6, in women of heterogeneous BMI (20). A role in fetal metabolism for lysophosphatidylcholines has also been suggested in the non-pregnant state with the development of visceral fat obesity, unrelated to genetic origin but associated with nutritional status (21). A study from the USA Project Viva cohort, demonstrated that associations between cord blood metabolites from a metabolome, particularly those related to one-carbon metabolism may contribute to rapid postnatal weight gain in offspring born to women of heterogeneous BMI (22). Taken together, studies of the cord blood metabolic profile suggest that obesity risk may be determined at birth (4). Antenatal interventions directed towards optimising adverse fetal exposures may therefore contribute to curbing the incidence of childhood obesity.
The positive associations between cord blood IGF-1 with neonatal measures of growth and body composition together with infant weight and mid upper arm circumference z-scores at 6 months also suggests a persistent influence of in-utero exposures on early growth. Whilst the relationship between the cord blood metabolic profile and differential growth in early infancy suggests a potential persistent effect on growth at 6 months of age, mechanisms must remain conjectural and causal inference should be made with caution. However, several studies have suggested that the IGF-1 gene may be prone to epigenetic modification in-utero (23–25), with animal studies shedding some light on this in providing evidence of an interaction with maternal glycaemia status. For example, Zinkhan et al, demonstrated that in-utero exposure to maternal glycaemia in rats led to decreased hepatic H3Me3K36 and mRNA variants of the IGF-1 gene in the offspring (26). Others have implicated a role of these variants to a predisposition to later obesity and insulin resistance (25). Whether epigenetic modification may also influence lipid metabolism, including that of lysophosphatidylcholines, remains conjectural.
The linear associations with cord adiponectin and measures of weight, length and subscapular z-scores at 6 months are in keeping with recent evidence from a prospective cohort study from Germany, in children born to women of heterogeneous BMI (n=141); suggesting a potential long term influence in children at 5 years of age (27). We also found that cord blood leptin was associated with measures of neonatal growth and body composition and increased odds of catch up growth from birth to 6 months of age, suggesting a potential mediatory role of early infancy growth. Cord blood leptin has been implicated as a proxy for neonatal fat mass as it is synthesised by the adipocyte Ob gene and is proportional to adipose tissue mass (28). This study has demonstrated an inverse relationship with cord leptin and catch up growth independent of birthweight which may be explainable by a state of leptin resistance in early infancy, as observed in previous studies (29).
The linear associations between cord blood anabolic hormones including cord blood C-peptide, insulin and IGF-1 with measures of growth and body composition at birth, agree with previous studies in offspring born to women of heterogeneous BMI (30, 31) and concur with the knowledge that insulin and IGF-1 are the most important regulators of fetal growth in the 2nd and 3rd trimester (32). IGF-1 has been shown consistently to be raised in cord blood of offspring born to obese women, predominately as a consequence of maternal dysglycaemia (33).
Triglycerides and non-esterified fatty acids in the maternal circulation have been widely implicated as determinants of fetal growth in obese and diabetic women (32). However, in this study, an inverse relationship between clusters of non-esterified fatty acids and triglycerides with neonatal growth and adiposity was observed, which has also been reported by others(34, 35). This association with low rather than high birth weight could reflect mobilisation of lipids as an alternative fuel source (34). Importantly, this study adds to others that have questioned the role of triglycerides in the determination of neonatal adiposity.
Despite the suggestion that inflammatory mediators (interleukin-6 and tumour necrosis factor-α) may contribute to the development of neonatal adiposity in-utero, through regulation of central pathways of satiety and appetite, (36, 37). We found an inverse association with neonatal body composition. There is no obvious explanation for this observation.
Strengths of this study include an extensive assessment of the cord blood metabolic profile at birth, detailed neonatal and infant anthropometric data collection and prospective collection of maternal early pregnancy BMI, total gestational weight gain and measures of maternal insulin resistance. Using data reduction techniques for the cord blood metabolome, metabolite clusters of biological importance associated with measures of neonatal and infant anthropometry were identified.
Limitations include the collection of mixed cord blood (umbilical artery and vein), which weakens conclusions regarding fetal or maternal origin of the metabolites in this study, as well as previously published reports (22). Whilst treatment of GDM has the potential to influence cord insulin, C-peptide and IGF-1 concentrations, this was not adjusted for within this analysis, however a sensitivity analyses removing women with GDM did not modify the observed relationships. It must be recognised that the metabolome and the candidate markers measured provide only an incomplete profile of the late pregnancy in-utero fetal exposures as unmeasured micronutrients, essential fatty acids and steroid hormones may also contribute to neonatal and early life growth and body composition.
In summary, this study of more than 300 infants describes for the first time a comprehensive cord blood metabolic profile in offspring born to obese women. Known associations of metabolic variables with infant adiposity were confirmed and questions raised regarding previous associations derived from smaller cohorts. Importantly we have highlighted novel associations with lipid sub-species and early postnatal growth, and provide supporting evidence that IGF-1 at birth may be a determinant of later growth trajectories. Current investigation of the maternal metabolome and neonatal epigenome may shed light on the causative mechanisms and further insight into growth trajectories. Ongoing studies of the cord epigenome may provide further mechanistic insight into potential pathways. Replication in other cohorts including the use of Mendelian randomisation methods are required to determine causality. Ongoing follow-up of the UPBEAT offspring will address the long-term implications of these observed associations.
Supplementary Material
Precis.
Investigation of the cord blood metabolic profile was undertaken in offspring born to obese women identifying a novel role of lipid sub-species as a potential determinant of early infancy weight. (Patel N et al)
Funding
This work was supported by the European Union's 7th Framework Programme (FP7/2007–2013), project EarlyNutrition under grant agreement no. 289346, Action Medical Research Council (GN2456), the National Institute for Health Research (NIHR) (UK) Programme Grants for Applied Research Programme (RP-0407-10452), and the European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG – no. 322605). The views expressed in this paper are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health or any other listed funders. Support was also provided from the Biomedical Research Centre at Guy’s and St.Thomas’ NHS Foundation Trust and King’s College London, the Chief Scientist Office Scotland, Guy’s and St Thomas’ Charity and Tommy’s Charity (Registered charity no. 1060508). KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre. The funders had no role in study design, data collection, data analysis, data interpretation or writing of the final report. The corresponding author had access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Disclosure Summary: The authors report no conflicts of interest in this work.
I, the designated corresponding author, on behalf of myself and my co-authors, hereby transfer and assign all right, title, and interest, including copyright and any moral rights, in and to the manuscript named in this submission (called the Work hereafter) to the Endocrine Society (ES). If ES ultimately declines to publish the Work in an ES journal, all rights in and to the Work will revert to the author(s).
2. I, and all co-authors, warrant that the Work intended for publication is original and has not been published other than as an abstract or preprint in any language or format and has not been submitted elsewhere for print or electronic publication consideration. We further warrant that the Work does not contain any material that is defamatory or the publication of which would violate any copyright or other personal, intellectual, property, contract, or proprietary right of any person or entity.
3. I warrant that each person listed as an author participated in the Work in a substantive way and is prepared to take public responsibility for it. All authors consent to the investigation of any improprieties that may be alleged regarding the Work. Each author further releases and holds harmless the Endocrine Society from any claim or liability that may arise therefrom.
4. I warrant that I am authorized to accept the terms of this agreement on behalf of myself and all co-authors.
Clinical trial registration number: This trial is registered with Current Controlled Trials, ISCRTN89971375
References
- 1.WHO. Ending childhood obesity report World Health Organization. 2016 accessed online http://apps.who.int/iris/bitstream/10665/204176/1/9789241510066_eng.pdf on 24.06.2015.
- 2.Patel N, Pasupathy D, Poston L. Determining the consequences of maternal obesity for offspring health. Experimental Physiology. 2015;100:1421–1428. doi: 10.1113/EP085132. [DOI] [PubMed] [Google Scholar]
- 3.Fraser A, Lawlor DA. Long-Term Health Outcomes in Offspring Born to Women with Diabetes in Pregnancy. Current Diabetes Reports. 2014;14:1–8. doi: 10.1007/s11892-014-0489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giles LC, Whitrow MJ, Davies MJ, Davies CE, Rumbold AR, Moore VM. Growth trajectories in early childhood, their relationship with antenatal and postnatal factors, and development of obesity by age 9 years: results from an Australian birth cohort study. International Journal of Obesity. 2015;39:1049–1056. doi: 10.1038/ijo.2015.42. [DOI] [PubMed] [Google Scholar]
- 5.Hivert MF, Perng W, Watkins SM, Newgard CS, Kenny LC, Kristal BS, Patti ME, Isganaitis E, DeMeo DL, Oken E, Gillman MW. Metabolomics in the developmental origins of obesity and its cardiometabolic consequences. Journal of Developmental Origins of Health and Disease. 2015;6:65–78. doi: 10.1017/S204017441500001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmuth C, Uhl O, Standl M, Demmelmair H, Heinrich J, Koletzko B, Thiering E. Cord blood metabolome is highly associated with birth weight, but less predictive for later weight development. Obesity facts. 2017;10:85–100. doi: 10.1159/000453001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, Hayes L, Khazaezadeh N, Nelson SM, Oteng-Ntim E, Pasupathy D, et al. Effect of a behavioural intervention in obese pregnant women (the UK Pregnancies Better Eating and Activity Trial study): a multicentre, randomised controlled trial. Lancet Diabetes & Endocrinology. 2015;3:767–777. doi: 10.1016/S2213-8587(15)00227-2. [DOI] [PubMed] [Google Scholar]
- 8.Patel N, Godfrey KM, Pasupathy D, Levin J, Flynn AC, Hayes L, Briley AL, Bell R, Lawlor DA, Oteng-Ntim E, Nelson SM, et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. International Journal of Obesity. 2017;41:1018–1026. doi: 10.1038/ijo.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harder U, Koletzko B, Peissner W. Quantification of 22 plasma amino acids combining derivatization and ion-pair LC-MS/MS. Journal of Chromatography. 2011;879:495–504. doi: 10.1016/j.jchromb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Hellmuth C, Weber M, Koletzko B, Peissner W. Nonesterified fatty acid determination for functional lipidomics: comprehensive ultrahigh performance liquid chromatography-tandem mass spectrometry quantitation, qualification, and parameter prediction. Analytical chemistry. 2012;84:1483–1490. doi: 10.1021/ac202602u. [DOI] [PubMed] [Google Scholar]
- 11.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, Lowe LP, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright CM, Booth IW, Buckler JMH, Cameron N, Cole TJ, Healy MJR, Hulse JA, Preece MA, Reilly JJ, Williams AF. Growth reference charts for use in the United Kingdom. Archives of Disease in Childhood. 2002;86:11–14. doi: 10.1136/adc.86.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. World Health Organisation Child Growth Standards based on length/height, weight and age. Acta Pædiatrica. 2006;95:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 14.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Medical Research Methodology. 2008;8:1–15. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;1:289–300. [Google Scholar]
- 16.Rzehak P, Hellmuth C, Uhl O, Kirchberg FF, Peissner W, Harder U, Grote V, Weber M, Xhonneux A, Langhendries J-P. Rapid growth and childhood obesity are strongly associated with lysoPC (14: 0) Annals of Nutrition and Metabolism. 2014;64:294–303. doi: 10.1159/000365037. [DOI] [PubMed] [Google Scholar]
- 17.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatrica. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 18.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. British Journal of Obstetrics and Gynaecology. 2006;113:1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 19.Metzger BE, Persson B, Lowe LP, Dyer AR, Cruickshank JK, Deerochanawong C, Halliday HL, Hennis AJ, Liley H, Ng PC, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics. 2010;126:1545–1552. doi: 10.1542/peds.2009-2257. [DOI] [PubMed] [Google Scholar]
- 20.Prieto-Sanchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, Peissner W, Ruiz-Alcaraz AJ, Parrilla JJ, Koletzko B, Larque E. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clinical Nutrition. 2016;1:1–9. doi: 10.1016/j.clnu.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Lee SY, Kim M, Jung S, Lee S-H, Lee JH. Altered plasma lysophosphatidylcholines and amides in non-obese and non-diabetic subjects with borderline-to-moderate hypertriglyceridemia: a case-control study. PLoS ONE. 2015;10:1–14. doi: 10.1371/journal.pone.0123306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isganaitis E, Rifas-Shiman SL, Oken E, Dreyfuss JM, Gall W, Gillman MW, Patti ME. Associations of cord blood metabolites with early childhood obesity risk. International Journal of Obesity. 2015;39:1041–1048. doi: 10.1038/ijo.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao PC, Matheny AP, Jr, Lang CA. Insulin-like growth factor-I comparisons in healthy twin children. Journal of Clinical Endocrinolology and Metabolism. 1994;78:310–312. doi: 10.1210/jcem.78.2.8106617. [DOI] [PubMed] [Google Scholar]
- 24.Baker J, Liu J-P, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- 25.Fu Q, Yu X, Callaway CW, Lane RH, McKnight RA. Epigenetics: intrauterine growth retardation modifies the histone code along the rat hepatic IGF-1 gene. Federation of American Societies for Experimental Biology. 2009;23:2438–2449. doi: 10.1096/fj.08-124768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zinkhan EK, Fu Q, Wang Y, Yu X, Callaway CW, Segar JL, Scholz TD, McKnight RA, Joss-Moore L, Lane RH. Maternal hyperglycemia disrupts histone 3 lysine 36 trimethylation of the IGF-1 gene. Journal of Nutrition and Metabolism. 2012;2012:1–7. doi: 10.1155/2012/930364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer DM, Brei C, Stecher L, Much D, Brunner S, Hauner H. Cord blood and child plasma adiponectin levels in relation to childhood obesity risk and fat distribution up to 5 years. Pediatric Research. 2017;1:1–7. doi: 10.1038/pr.2016.275. [DOI] [PubMed] [Google Scholar]
- 28.Zimmet P, Hodge A, Nicolson M, Staten M, de Courten M, Moore J, Morawiecki A, Lubina J, Collier G, Alberti G, Dowse G. Serum leptin concentration, obesity, and insulin resistance in Western Samoans: cross sectional study. British Medical Journal. 1996;313:965–969. doi: 10.1136/bmj.313.7063.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, Dunger DB. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. Journal of Clinical Endocrinology and Metabolism. 1999;84:1145–1148. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]
- 30.Wang HS, Lim J, English J, Irvine L, Chard T. The concentration of insulin-like growth factor-I and insulin-like growth factor-binding protein-1 in human umbilical cord serum at delivery: relation to fetal weight. Journal of Endocrinology. 1991;129:459–464. doi: 10.1677/joe.0.1290459. [DOI] [PubMed] [Google Scholar]
- 31.Carlsen EM, Renault KM, Jensen RB, Nørgaard K, Jensen J-EB, Nilas L, Cortes D, Michaelsen KF, Pryds O. The association between newborn regional body composition and cord blood concentrations of C-Peptide and insulin-like growth factor I. PLoS ONE. 2015;10:1–14. doi: 10.1371/journal.pone.0121350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of Obese Mothers Develop Insulin Resistance in Utero. Diabetes Care. 2009;32:1076–1080. doi: 10.2337/dc08-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawlor DA, West J, Fairley L, Nelson SM, Bhopal RS, Tuffnell D, Freeman DJ, Wright J, Whitelaw DC, Sattar N. Pregnancy glycaemia and cord-blood levels of insulin and leptin in Pakistani and white British mother-offspring pairs: findings from a prospective pregnancy cohort. Diabetologia. 2014;57:2492–2500. doi: 10.1007/s00125-014-3386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelishadi R, Badiee Z, Adeli K. Cord blood lipid profile and associated factors: baseline data of a birth cohort study. Paediatric Perinatal Epidemiology. 2007;21:518–524. doi: 10.1111/j.1365-3016.2007.00870.x. [DOI] [PubMed] [Google Scholar]
- 35.Geraghty AA, Alberdi G, O’Sullivan EJ, O’Brien EC, Crosbie B, Twomey PJ, McAuliffe FM. Maternal blood lipid profile during pregnancy and associations with child adiposity: findings from the ROLO study. PLOS ONE. 2016;11:1–13. doi: 10.1371/journal.pone.0161206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory”. Experimental Diabetes Research. 2011;1:1–13. doi: 10.1155/2011/218598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cesar HC, Pisani LP. Fatty-acid-mediated hypothalamic inflammation and epigenetic programming. Journal of Nutritional Biochemistry. 2016;42:1–5. doi: 10.1016/j.jnutbio.2016.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.