Abstract
Background
The majority of prosthetic heart valves currently implanted are tissue valves that can be expected to calcify with time and eventually fail. Surgical or percutaneous redux valve replacement is associated with higher rate of complications. We propose a novel non-invasive therapeutic approach based on the use of pulsed cavitational ultrasound (PCU) to improve the valvular function of degenerative calcified bioprosthesis.
Objectives
Our study aims to demonstrate in vitro and in vivo on an ovine model that PCU can significantly improve the bioprosthesis opening by softening remotely the calcified stiff cusps.
Methods
All the experiments were performed on calcified bioprosthetic valves explanted from human patients. PCU was performed in vitro on calcified bioprosthesis mounted on a hydraulic bench with pulsatile flow (n=8) and in vivo on an ovine model with implanted calcified bioprosthesis (n=7). We used 3D echocardiography, pressure and flow sensors, quantitative stiffness evaluation using shear wave elastography, micro-CT imaging and histology to evaluate in vitro and in vivo the effect of PCU.
Results
The transvalvular gradient was found to decrease by a mean of 50% after PCU in both in vitro (from 21.1±3.9 to 9.6±1.7 mmHg, p<0.001) and in vivo setup (from 16.2±3.2 to 8.2±1.3 mmHg, p<0.001), with a decrease of valve stiffness (in vitro: from 105.8±9 to 46.6±4 kPa, p<0.001; in vivo: from 82.6±10 to 41.7±7 kPa, p<0.001) and an increase of valve area (from 1.10±0.1 to 1.58±0.1 cm2, p<0.001). Histology and micro-CT imaging showed modifications of calcification structure without loss of calcification volume or alteration of the leaflet superficial structures.
Conclusions
We have demonstrated in vitro and in vivo that PCU can decrease a calcified bioprosthesis stenosis by softening the leaflets remotely. This new non-invasive approach has the potential to improve the outcome of patients with severe bioprosthesis stenosis.
Keywords: ultrasonic, bioprosthesis, therapy
Introduction
Bioprosthetic valves are becoming increasingly common in patients with valvular heart diseases and are often favored over mechanical ones. This is because of a lower risk of thrombotic or bleeding events and as well as the desire to avoid lifetime anticoagulation medications1. However, bioprosthetic valves have limited durability, with a progressive deterioration of the bioprosthesis after 12-15 years, mainly due to intravalvular calcifications2. The need for a redo surgery due to the incidence of structural valve deterioration is expected to increase3. Nevertheless, redo valve surgery is associated with significant morbidity and mortality4. Transcatheter valve-in-valve implantation has emerged as a promising less invasive alternative to redo surgery. It does however, still come with its own various set of complications5.
In parallel, for about 30 years, other therapeutic strategies6,7 have been investigated to treat calcified valves (native or bioprosthetic). One promising approach was ultrasound-based8 but it remained limited at the time by the need of an open surgery with cardiopulmonary bypass (CPB). Nevertheless, this limitation must be challenged again with the recent improvement of technologies and concepts of pulsed cavitational focused ultrasound (PCU) or histotripsy. Histotripsy is a non-invasive, cavitation-based therapy (mechanical effect) based on very short, high-pressure ultrasound pulses focused in tissues to generate a dense, energetic, lesion-producing bubble cloud. Although histotripsy can be used to produce sharp lesions in soft tissues9,10 , recent studies have suggested that cavitation activity can also soften biological tissues11.
The objective of our study was to evaluate the efficacy of PCU to improve significantly valve opening of severe degenerative calcified bioprosthetic valves. First, we demonstrate in vitro the efficacy of PCU on human explanted calcified bioprosthesis mounted on an artificial heart pump and quantify the improvement of the valvular function. Then, we demonstrate its feasibility and efficacy in vivo in the beating heart of an ovine model with implanted calcified bioprosthesis.
Methodology
Model of heart calcified valve
We used Carpentier-Edwards Perimount Magna (stented bovine pericardial bioprosthesis), explanted on a human, as a model of heart calcified valve stenosis. For all patients, the indication of explant was a severe stenosis with calcification. Each valve was fixed in glutaraldehyde 0.6% immediately after explant. Before each experimentation, the valve was immersed in saline serum (0.9% NaCl) for the duration of 5 minutes, three consecutive times.
This protocol was in agreement with institutional guidelines (national reference number of the study: 02255.02).
Ultrasound Generation and PCU Acoustic Parameters
A 1.25 MHz focused single-element transducer (Imasonic®, Besançon, France), called a therapy transducer, was used to generate PCU. This transductor had a 100 mm focal length (f-number = 1) and was driven by a high-voltage amplifier (RITEC GA-2500A, Warwick, USA). We produced PCU using 10-cycle pulses, each 8 µs long, delivered at a pulse repetition frequency (PRF) of 100 Hz. We estimated the pressure peak amplitudes at the focal spot to be 70 MPa and -19 MPa respectively for the positive and negative peak pressure.
Ultrasound Cavitational Treatment guidance and monitoring
3D Echocardiography was used to guide and monitor the treatment. An IE33 (Philips) scanner and X5-1 probe (xMATRIX array, 3 MHz, 3040 elements with microbeam-forming) were used. The imaging probe was fixed through a hole in the center of the therapy transducer (figure 1). The focal spot of the therapy transducer was positioned on the central axis of the imaging probe at a depth of 100mm. A bi-plane imaging mode with two imaging planes set at 90° was used during the whole procedure. The cavitation bubble cloud generated at the PCU focal spot was visible within the two imaging planes. The combination of therapy transducer and imaging probe was called the "therapy device". The same material was used for in vitro and in vivo procedures.
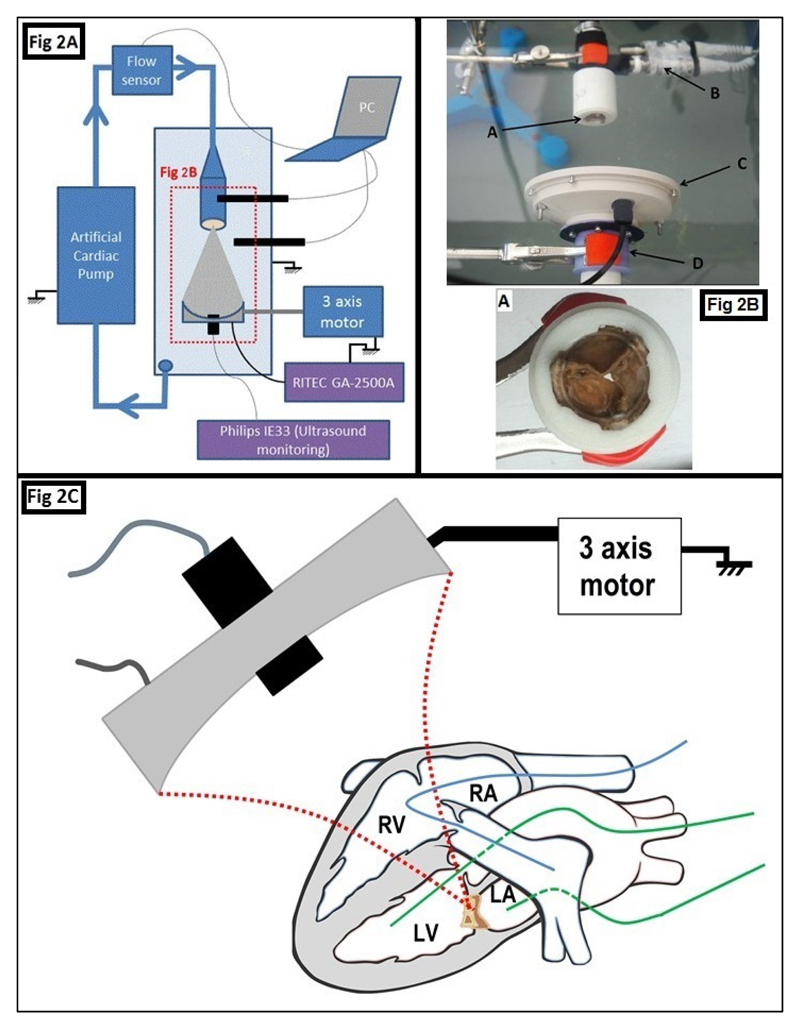
Figure 1. Image guided therapy setup.
The imaging probe was fixed through a hole in the center of the pulsed cavitational ultrasound therapy transducer.
For all the procedures, 10 minutes sequences of PCU were applied, and repeated until reaching a stabilization of the transvalvular gradient for 3 consecutive sequences (considered as the ineffective threshold for PCU procedures). The therapy device was controlled by a 3-axis motor to scan the PCU continuously and uniformly over the entire valve.
Shear wave elastography evaluation
The assessment of valve leaflet biomechanical properties remains challenging and requires destructive strain-stress mechanical tests. In order to assess non-invasively the modification of the biomechanical properties induced by PCU, we used shear wave elastography, an ultrasound-based tool for non-invasive evaluation of soft tissue’s stiffness. We used the Aixplorer ultrasound imaging system (Aixplorer, Supersonic Imagine, Aix-en-Provence, France) with a linear probe (SL10-2) to evaluate the stiffness of each valvular leaflet. Three acquisitions were made for each leaflet, using the shear wave elastography imaging mode (SWE™) of the Aixplorer scanner in the ‘penetration’ setting. A “QBox™” region of interest (mean diameter 1 mm) was positioned inside the elasticity image after each acquisition to obtain a mean stiffness value.
In vitro procedure (figure 2A-2B)
Figure 2. Experimental procedures.
Figure 2A. In vitro procedure setup.
Figure 2B. Photography of a part of the in vitro setup. The bioprosthesis (A) was placed in front of the therapy device composed of a therapy transducer (C) and animaging transducer (D). The two pressure sensors (B) were positioned before and after the valve.
Figure 2C. In vivo procedure, in beating heart
The bioprosthesis was implanted in mitral position, between the LA and the LV. The Swan-Ganz catheter (in blue, to estimate the cardiac flow in real time), was positioned into the right heart (RV and RA) and the pulmonary artery. The Millar catheters (in green), were positioned into the LA et le LV to estimate the transvalvular gradient in real time. PCU was then applied all overthe bioprosthesis by moving the therapy device thanks to the 3 axis motorized stage.
LA: left atrium; LV: left ventricle; RA: right atrium; RV: right ventricle
The objective of this procedure was to analyze the impact of the PCU on the anterograde transvalvular flow, with a pulsatile flow equivalent to the cardiac flow. The flow was generated by an artificial heart pump (Harvard Apparatus Pulsatile Blood Pump®), with flow controlled variation. The flow rates were applied at 3L, 4L and 5L per minute, monitored by a flow sensor (Small flow Meter Kit, Atlas scientific®; accuracy +/- 1 ml/min). The valve and the therapy device were immersed in degassed water. Eight bioprosthesis were explanted and used for this procedure. The transvalvular pressure gradient was estimated by:
-
-
A pulsed Doppler ultrasound assessment by applying the Bernoulli equation [ΔP = 4(Vmax)2]
-
-
Hemodynamic assessment by pressure sensor before and after the valve (sensor IXIAN™ 0-7.5 PSI Industrial Control Pressure Sensor, Atlas scientific® ; accuracy +/- 1 mmHg)
The pump operated during 2 hours at a 4 L/min flow rate (70 cycles per minute, ejection volume equal to 57 mL) to control the variation of the gradient before PCU, after which sequences of PCU were applied. All post PCU transvalvular gradients were re-assessed one month after the procedure (each valve was fixed in glutaraldehyde between these evaluations).
Elastography was performed before and after the procedure on each valve.
Finally, the bioprostheses were sent to the department of pathology for histopathological analysis.
In vivo procedure - Sheep model (figure 2C)
The animal procedure was approved by the Institutional Animal Care and Use Committee of the Hôpital Européen Georges Pompidou (PARCC) according to the European Commission guiding principles (2010/63/EU).
The sheep were anesthetized with thiopentothal (0.5 mL/kg), intubated, ventilated at 15 mL/kg with 2% isoflurane, and given glycopyrrolate (0.4 mg intravenous) and vancomycin (0.5 grams intravenous). A sterile sternotomy was performed. The calcified bioprosthesis was implanted in mitral position, after CPB. Vital signs (including heart rate (HR), oxygen saturation, arterial blood pressure (BP)), left atrial and ventricle pressure (by two Mikro-Tip® Millar Catheter Transducers, to have the transvalvular pressure gradient in real time) and cardiac flow (by a Swan-Ganz CCOmbo Pulmonary Artery Catheter, Edwards Lifesciences®) were monitored. The CPB was stopped and removed to restore independent cardiac activity. Sternotomy was maintained and the thorax was filled with degassed saline water. A complete echocardiography was realized, especially to evaluate the calcified bioprosthesis12,13,14. Fourteen explanted bioprosthesis were used for this procedure.
We then applied the sequences of PCU. An echocardiographic evaluation was realized between each 10 min sequence, concurrent with the catheters evaluation (pressure and cardiac flow).
At the end of the procedure, the animal was sacrificed (Dolethal intravenous injection, 1 ml/kg) and an anatomical macroscopic evaluation of the cardiac structure was performed. Elastography examination of the bioprosthesis was done before and after each procedure. The bioprosthesis was then explanted and sent after elastography to the department of pathology for histopathological analysis.
Microscopic analysis
Histological exploration
Immediately after the procedure (in vitro and in vivo), the bioprosthesis was dissected, fixed in formalin and embedded in individual paraffin blocks. Regions of interest, like macroscopic calcification on leaflet, were labelled with tattoo ink. Serial sections were stained with H&E (hematoxylin and eosin) for histopathological analysis.
In addition, we also analyzed five calcified bioprosthesis directly after their explantation from human, without any PCU procedure. The objective was to perform a histopathological comparison between bioprosthesis with or without PCU.
Micro-CT Imaging
The micro-CT device used in this study is a “Quantum FX Caliper, Life Sciences, Perkin Elmer, Waltham, MA”. Sample up to a Field of View of 10 mm diameter and three-dimensional acquisitions were performed using an isotropic voxel size of 20x20x20 μm3. Full 3D high-resolution raw datas were obtained by rotating both the X-ray source and the flat panel detector 360° around the sample, with a rotation step of 0.1°. The corresponding 3,600 image projections were then automatically reconstructed (RigakuSW software, Caliper) into a DICOM stack of 512 files, using standard back-projection techniques.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD). Univariate analyses of continuous variables were performed with the Wilcoxon matched-pairs signed-ranks test (non-parametric distributions). The level of significance was set at an alpha level of 0.05 or less. The analysis was conducted using Medcalc (MedCalc Software, Mariakerke, Belgium).
Results
All of our results consistently showed a softening of the valve leaflets allowing a decrease of the anterograde gradient after PCU. This decrease was persistent one month after the procedure. The decrease of transvalvular gradient measured by Doppler echocardiography was confirmed by invasive pressure sensors in both in vitro and in vivo setup.
In vitro procedure (figure 3)
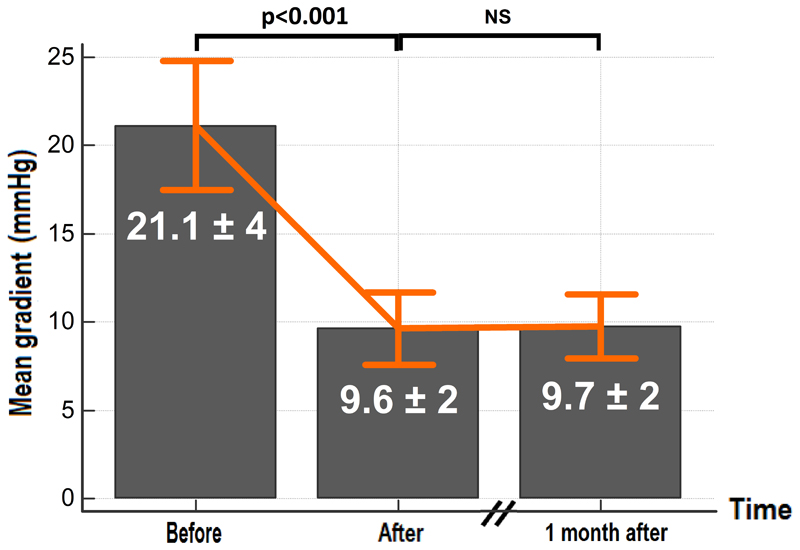
Figure 3. In vitro results.
Mean transvalvular gradient measured before and after PCU.
Eight bioprosthesis were explanted and used for this procedure. At a flow rate of 4L/min, the mean transvalvular gradient over the set of valves was 21.1 ± 3.9 mmHg (max=38, min=10, figure 3) and the maximum gradient was 39 ± 6.9 mmHg (max=73, min=22). After two hours of controlled pulsatile flow without PCU, we observed no statistically significant change of the transvalvular gradients. The mean duration of PCU was 70 ± 12 minutes with a maximum duration of 90 minutes and a minimum of 50 minutes. After the procedure, the mean transvalvular gradient was 9.6±1.7 mmHg (max=19; min=4), corresponding to a decrease of 55±10 % (p<0.001). The maximum gradient was 19.6±3.5 mmHg (max=37; min=10), corresponding to a decrease of 51±9 % (p<0.001). The results of each individual bioprosthesis are shown on supplementary figure 1.
Hemodynamic parameters were also measured at 3L/min and at 5L/min, before and after procedure. The gradients also showed a significant decrease at the different flow rates (p<0.001) (supplementary figure 2). At 3L/min, the mean gradient varied from 14.2±2.5 to 7.1±1.2 mmHg (p<0.001) and the maximum gradient from 29.1±5.1 to 14.9±2.6 mmHg (p<0.001). At 5L/min, the mean gradient varies from 23.8±4.2 to 13±2.3 mmHg (p<0.001) and the maximum gradient from 42.3±7.5 to 24.1±4.3 mmHg (p<0.001).
The gradient dependence with flow rate allowed us to assess the pliability of the valve cusps before and after the procedure. Pliability is indeed inversely linked to the slope of the gradient-flow rate relationship. The slope of the maximum gradient-flow rate relationship was found to decrease from 9±4.3 to 4.9±2.9 mmHg/L/min (p<0.001) which confirmed an improved pliability of the valves cusps after the procedure.
All post PCU transvalvular gradients were re-assessed one month after the procedure and no statistically significant difference was observed (figure 3).
In vivo procedure (table 1, figure 4-5 and see video in supplementary data)
Table 1. In vivo results.
| Before Therapy | After Therapy | Variation* Before/After (%) |
p | |
|---|---|---|---|---|
| Shear Wave Imaging | ||||
| Elastography (kPa) | 76.1 ± 23.7 | 35.6 ± 7.2 | - 52 ± 7 | <0.001 |
| Echocardiography | ||||
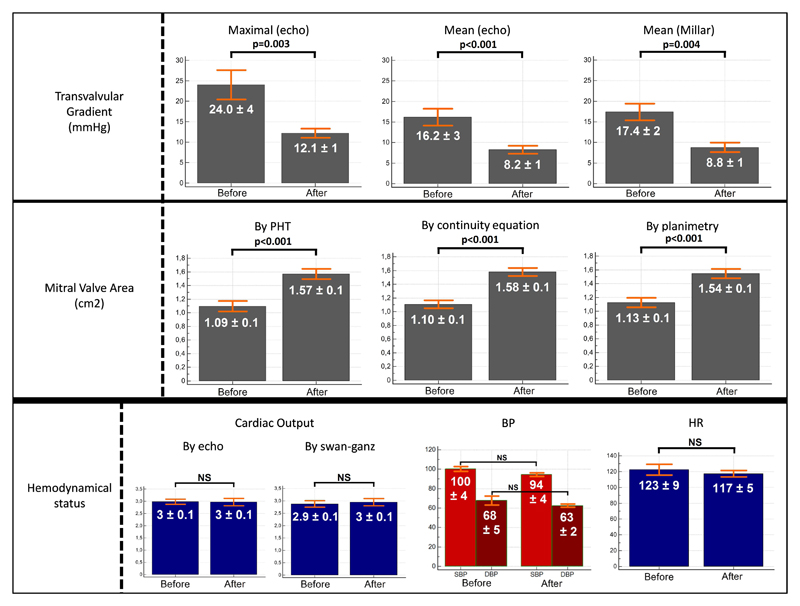
| Doppler Mitral Valve (figure 5A) | ||||
| - Maximal Velocity (m/s) | 2.41 ± 0.50 | 1.73 ± 0.21 | - 28 ± 6 | 0.007 |
| - Maximal Pressure Gradient (mmHg) | 24.0 ± 4.4 | 12.1 ± 1.4 | - 49 ± 11 | 0.003 |
| - Mean Velocity (m/s) | 1.95 ± 0.36 | 1.38 ± 0.24 | - 29 ± 6 | 0.001 |
| - Mean Pressure Gradient (mmHg) | 16.2 ± 3.2 | 8.2 ± 1.3 | - 48 ± 7 | <0.001 |
| Mitral Valve Area (cm2) | ||||
| - By pressure half time (PHT, fig 5B) | 1.09 ± 0.09 | 1.57 ± 0.08 | + 143 ± 18 | <0.001 |
| - By continuity equation | 1.10 ± 0.15 | 1.58 ± 0.15 | + 142 ± 15 | <0.001 |
| - By planimetry (fig 5C) | 1.13 ± 0.13 | 1.54 ± 0.14 | + 137 ± 14 | <0.001 |
| Pulmonary artery pressure (mmHg) | ||||
| - Maximal (Tricuspid Valve) | 64.7 ± 12.8 | 34.1 ± 10.2 | - 47 ± 12 | <0.001 |
| Cardiac Output# (L/min) | 2.98 ± 0.1 | 2.96 ± 0.14 | - 1 ± 0.2 | 0.83 |
| Pressure Sensors (Millar) (mmHg) | ||||
| Mean Diastolic Left Atrium pressure (LAP) | 36.8 ± 6.2 | 20.2 ± 5.1 | - 44 ± 11 | 0.004 |
| Mean Diastolic Left Ventricle pressure (LVP) | 17.4 ± 2.7 | 11.4 ± 1.9 | - 35 ± 10 | 0.014 |
| Mean Diastolic Gradient LVP-LAP | 17.4 ± 2.4 | 8.8 ± 1.2 | - 50 ± 13 | 0.002 |
| Cardiac Flow Captor (L/min) | ||||
| Swan-Ganz catheter | 2.87 ± 0.11 | 2.96 ± 0.14 | + 3 ± 0.4 | 0.74 |
± SD
Variation Before/After = [(Result After – Result Before) / Result Before] x 100
Cardiac Output = HR x LVOT area x LVOT VTI
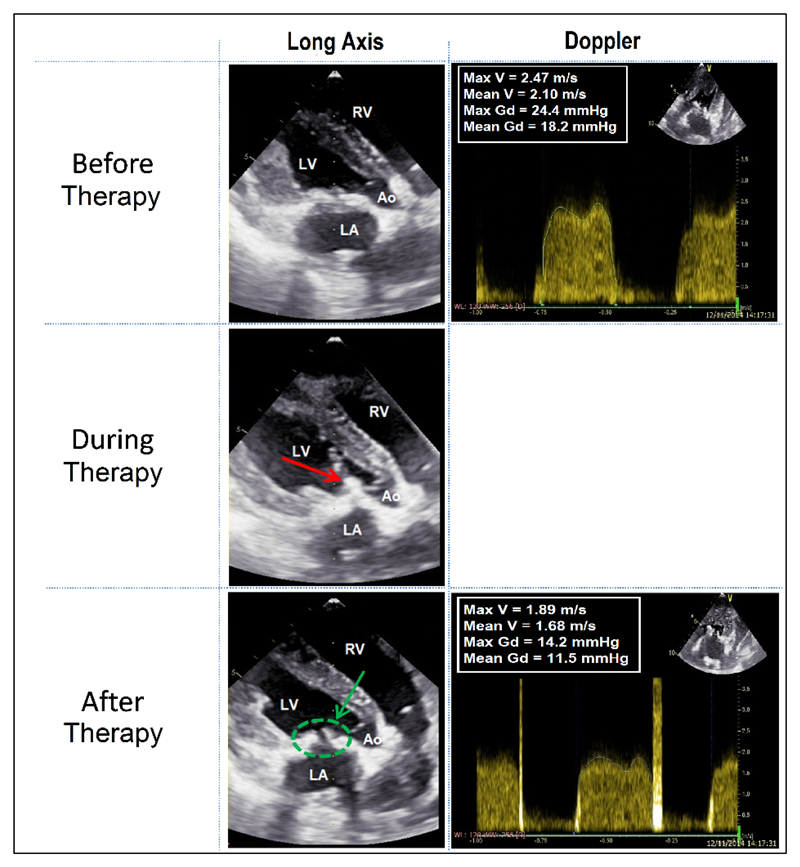
Figure 4. Images of echocardiography during procedure.
During PCU a “cavitation bubble cloud” is visible as a hyperechogenic zone (red arrow). After PCU, the improvement of the bioprosthesis opening is confirmed thanks to the echocardiography (green arrow).
The images are realized during diastasis (closed aortic valve).
LA: left atrium; LV: left ventricle; RV: right ventricle; Ao: Aorta
Figure 5. Results in vivo, before and after pulsed cavitational focused ultrasound.
BP: blood pressure; HR: heart rate; PHT: pressure half-time method
Fourteen explanted bioprosthesis were used for this procedure. Seven of the animals suffered a massive acute pulmonary edema with severe heart failure, just after the implantation of the valve and the cessation of the CPB. These animals died before the PCU procedure. The other animals tolerated the implantation. Seven valves were thus treated and analyzed.
The mean weight of the animals was 37.8±4.6 kg (min=29; max=43).
Just after the valve implantation, we monitored all the parameters during one hour, before any PCU procedure. Between the beginning (just after the valve implantation) and one hour after implantation (before any PCU procedure), there was no statistical significant difference of the transvalvular gradients (p=0.45) and mitral valve areas (planimetry, p=0.38; continuity equation, p=0.74; PHT, p=0.51).
The mean procedure duration was 60 ± 13 minutes with a maximum duration of 100 minutes and a minimum of 40 minutes (see online video of an in vivo procedure). We observed an important decrease of the transvalvular gradient after PCU (see table 1) which was reduced by 50% on average. The results of elastography, of echocardiography and of pressure/flow cardiac catheters after the PCU procedures are synthesized in the table 1. The mean cardiac frequency was 123±9 (min=94; max=154) and all the hemodynamic parameters were stable during the procedures: HR (p=0.24), BP (p=0.27), O2 saturation (p=0.42).
No mitral valve regurgitation was observed at the end of the procedures.
We observed isolated ventricular extrasystoles (VES) in two animals, without any repercussion on hemodynamic parameters. As long as the focal spot of the therapy device remained on the bioprosthesis, no arrhythmia was visible.
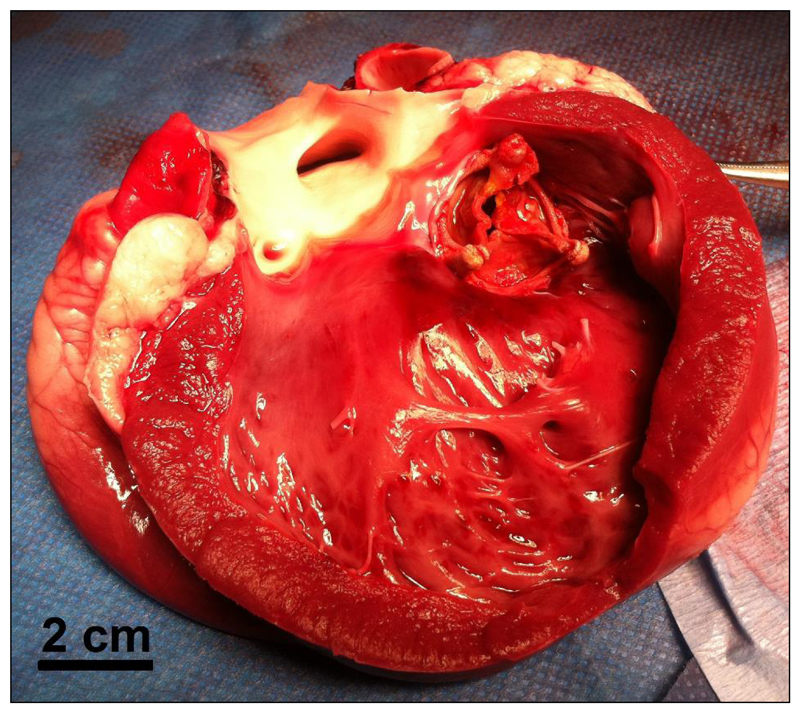
Once the threshold was reached and the reevaluation of all parameters was achieved, the animal was sacrificed and the heart was explanted. Macroscopic analysis showed that all cardiac structures were intact (figure 6), except in one animal in which a superficial hematoma (epicardium) of 7 mm diameter was visible at the lateral left ventricle wall (on the path of the ultrasound beam). This animal was also one of two animals which presented isolated VES.
Figure 6. Macroscopic postmortem analysis.
Macroscopic analysis showed that all cardiac structures were intact. The whole hearts appeared intact from the outside, with no visible damage on the epicardial surfaces (except in one animal in which a superficial hematoma -epicardium- of 7 mm diameter was visible). After dissection, no damage was observed inside the heart.
At the end of the procedure, the bioprosthesis was sent to the department of pathology for histopathological analysis.
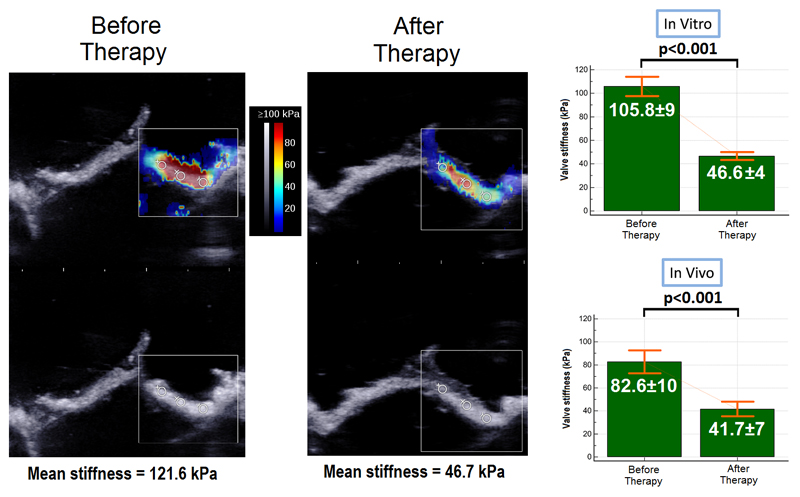
Elastography (figure 7A-7B)
Figure 7. Elastography.
Figure 7A. Example of bioprosthesis elastography in vitro by Shear Wave Elastography
Figure 7B. Elastrography results (in vitro and in vivo)
In vitro, before PCU, the mean stiffness of the valves leaflets measured by elastography was 105.8 ± 9 kPa. After the procedure, the mean stiffness of the valves leaflets measured by elastography was 46.6 ± 4 kPa, corresponding to a decrease of 55 ± 8% (p<0.001).
We observed a similar stiffness decrease for the bioprosthesis used in vivo (82.6 ± 10kPa before the procedure and 41.7 ± 7 kPa after PCU, 49 ± 7 % decrease, p< 0.001).
The results of each individual bioprosthesis are shown on supplementary Figure 3.
Microscopic analysis
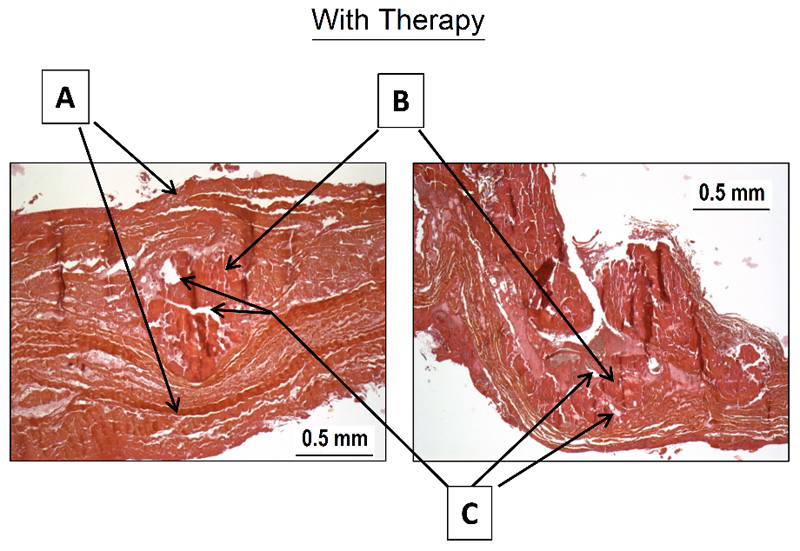
Histological exploration (figure 8)
Figure 8. Histological analysis.
After pulsed cavitational focused ultrasound, the superficial structures remain intact (A). An aspect of calcifications fragmentation is visible (B). Some vacuoles are also found inside of the calcifications (C)
All the superficial structures of the leaflets were intact (fibrosa and ventricularis).
In comparison with the five bioprosthesis explanted without PCU procedure, we observed:
-
-
A fragmentation of the calcification.
-
-
Presence of vacuoles inside the calcification
There was no histological evidence for acute inflammation or acute thrombosis on the bioprosthesis.
Micro-CT Imaging (supplementary figure 4)
Micro-CT imaging did not show modifications of the calcifications shapes. However, we did observe qualitatively multiple fragmentations inside the calcification after PCU. These micro-fragmentations are visible as subvoxel modifications of the CT image (size<20µm) so that quantitative analysis remains limited.
The estimation of calcification volume shows no statistical difference before PCU (mean volume = 294.9 mm3 [min = 45.7; max = 662.3]) and after PCU (mean volume = 288.9 mm3 [min = 42.9; max = 659.9]), with a mean diminution of 2.02 % (p=0.44).
Discussion
In this study we demonstrated in vitro and in vivo that PCU can decrease remotely the transvalvular gradients of calcified bioprosthetic valves. The mean and maximal transvalvular gradients decreased by two-fold on average in both the in vitro and in vivo setups. Moreover, these hemodynamical modifications persisted after one month (evaluated only in vitro). The evolution of other echocardiographic parameters measured in vivo (valve area, PAP) confirmed a consistent decrease of the valvular stenosis. Finally, we showed that PCU induced a decrease of the valves leaflet stiffness.
We believe that PCU can have a real clinical impact on the treatment of calcified bioprosthesis stenosis. Its two main advantages are that it could be potentially applied in a transthoracic configuration completely non-invasively and would allow the preservation of the bioprosthesis valve ad integrum.
Impact of PCU on calcified bioprosthesis valve
Using quantitative shear wave elastography, we demonstrated that the biomechanical properties of calcified leaflets were modified by PCU. The stiffness was decreased on average by two-fold. The softening mechanism induced by PCU may be complex and needs to be further investigated, however, we can hypothesize that calcifications are mostly impacted by the treatment. The histological analyses showed a fragmentation of calcifications with the preservation of the leaflet superficial structures. Micro-CT imaging, which can be considered as a gold standard for the valvular calcification evaluation15,16, confirmed that micro-fragmentations appeared inside the calcification after PCU. This micro-fragmentation of large calcifications could explain the overall change of biomechanical properties which leads to the improvement of the leaflet motion. Further investigations are required to better understand other potential mechanisms of PCU and in particular its impact on soft valvular tissues.
Safety
Accuracy of therapy
For two animals, we observed a few non-persistent ventricular extrasystoles, and post mortem anatomic exploration showed bruising of the cardiac wall, due to off-target cavitation. These two undesirable effects are mostly induced by the actual complexity of the target positioning and motion, and could be greatly reduced with some technical improvements. As our therapeutic transducer is readily focused at a single location, the target position can only be changed by mechanically and slowly moving the therapy device with millimetric motors. To solve this problem, a multi-element transducer could be used to steer the focal spot electronically in real time. 3D motion correction would then be feasible in real-time, based on an accurate ultrasonic speckle-tracking method as it has been demonstrated for HIFU applications in 2004 by our team17 and more recently for histotripsy18. With such a motion correction technique, we would be able to track the valve motion all along the cardiac cycle, and avoid off-target cavitation. An easier strategy would be to trigger PCU exposures by electrocardiogram19. We could for instance select specific moments in the cardiac cycle when the valve is closed. Thus its whole surface would be equally exposed and far away from the cardiac wall.
Emboli
Our study aimed to demonstrate the efficacy proof of concept. We have thus not precisely evaluated the clinical risk of emboli during and after PCU, by endovascular filter or brain MRI/CT, and these explorations should be performed during specific risk evaluation studies. Nevertheless, our histological exploration after in vitro and in vivo procedures have shown the preservation of the leaflet superficial structures (fibrosa and ventricularis). These are fundamental structures around the calcification20, meaning that our action seems to be confined within the leaflet. This result was confirmed by the micro-CT explorations which found no statistical difference for the calcification volumes before and after PCU. This local intravalvular impact is a reassuring argument against the major risk of emboli. Moreover, the specific histological lesions due to PCU noted inside the calcification (vacuole) seem to preserve the overall architecture of the valve leaflet.
Concerning cardiac application of PCU, Xu et al have shown in 2009 that the size of myocardium debris obtained after histotripsy was inferior to the size of a red blood cell for > 99% of the debris21. As of now, no studies have been conducted specifically on valvular calcifications and PCU.
Limitations & Perspectives
In vivo follow up
We observed a stability of the transvalvular gradient in vitro one month after the procedure. However, this was not confirmed for in vivo procedure since we sacrificed the animals directly after the procedure. It is therefore difficult to evaluate the persistence of the therapeutic effect.
Shear Wave Elastography evaluation
Shear wave elastography has never been used for the stiffness evaluation of valve leaflets, therefore, this application needs to be validated in further studies. In particular, previous studies have shown the limitation of shear wave elastography in thin layers such as in the arterial wall. In such a medium, the shear waves are guided inside the two dimensional leaflet, and correspond to leaky lamb wave propagation23. This impacts the elastography measures which prohibits the absolute values of leaflet stiffness to be derived directly. Nevertheless, semi-quantitative evaluation of leaflet stiffness remains possible by performing elastography before and after PCU on the same leaflet24,23.
Transthoracic approach
Transthoracic treatment should be feasible but requires further development of the therapy device. The main challenge of the transthoracic approach is to overcome the effect of the deep heterogeneous tissue layers on the path of the ultrasound beam. The ribs are a particularly strong barrier for ultrasound, allowing the ultrasonic beam to propagate only through the intercostal space. This limited aperture reduces the energy at the focal point as well as the quality of the focus. However, previous work in the field of histotripsy25,26 and HIFU27 have shown the feasibility of transcostal focusing.
Clinical applications
The treatment of calcified bioprosthesis stenosis in mitral or aortic position is still currently a challenge. Redo surgery or transapical transcatheter valve-in-valve implantation expose the patient to significant risks, especially for patients with comorbidities5,28. The results presented in this study may provide a new treatment option for these patients. From a general point of view, the PCU could be applied to calcified bioprosthesis whatever its localization (aortic, mitral, tricuspid or pulmonary), and this should be confirmed in further studies on animal and human patients. If these results are confirmed and stable in long term, the indication of bioprosthesis could be expanded to wider and younger population with the acceptable perspective of non-invasive redux therapy in long term. This application however, still needs to be confirmed by further prospective studies and significant follow up.
Conclusion
We have demonstrated in vitro and in vivo that PCU can significantly decrease the transvalvular gradient of a calcified bioprosthesis stenosis by softening the leaflets remotely and improving valve opening. We believe that this novel ultrasound therapy could become a non-invasive therapeutic strategy in cardiology. This new non-invasive approach has the potential to improve the outcome of patients with severe calcified bioprosthesis stenosis by avoiding risky surgical or transcatheter reintervention.
Supplementary Material
In vivo procedure of pulsed cavitational ultrasound therapy
Acknowledgements
This work was supported by the LABEX WIFI (Laboratory of Excellence ANR-10-LABX-24) and the French Program “Investments for the Future” under reference ANR-10-IDEX-0001-02 PSL.
The authors thank Hicham Serroune (Institut Langevin), Julie Piquet and the staff of PARCC (Paris Research Cardiovascular Center).
Funding: This work was supported the ANR-10-IDEX-0001-02 PSL* Research University.
Abbreviations
- BP
blood pressure
- CUSA
cavitron ultrasonic surgical aspirator
- CBP
cardiopulmonary bypass
- CI
confidence interval
- CT
computerized tomography
- CUSA
cavitron ultrasonic surgical aspirator
- DICOM
digital imaging and communications in medicine
- HIFU
high intensity focused ultrasound
- HR
heart rate
- MRI
magnetic resonance imaging
- LV
left ventricle
- LVOT
left ventricle outflow tract
- PCU
pulsed cavitational ultrasound
- PHT
pressure half time
- PMV
percutaneous balloon mitral valvuloplasty
- PRF
pulse repetition frequency
- SD
standard deviation
- SWE
shear wave elastography
- VES
ventricular extrasystoles
- VTI
velocity time integral
Footnotes
Competing Interests: Emmanuel Messas, Mathieu Pernot and Mickael Tanter are co-founders and shareholders of Cardiawave SAS. Mathieu Rémond is an employee of Cardiawave SAS.
References
- 1.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) 2013;14(3):167–214. doi: 10.1714/1234.13659. [DOI] [PubMed] [Google Scholar]
- 2.Jamieson WRE, Rosado LJ, Munro AI, et al. Carpentier-Edwards Standard Porcine Bioprosthesis: Primary Tissue Failure (Structural Valve Deterioration) by Age Groups. Ann Thorac Surg. 1988;46(2):155–162. doi: 10.1016/s0003-4975(10)65888-2. [DOI] [PubMed] [Google Scholar]
- 3.Thourani VH, Weintraub WS, Guyton RA, et al. Outcomes and Long-Term Survival for Patients Undergoing Mitral Valve Repair Versus Replacement. Circulation. 2003;108(3) doi: 10.1161/01.CIR.0000079169.15862.13. [DOI] [PubMed] [Google Scholar]
- 4.Potter DD, Sundt TM, Zehr KJ, et al. Risk of repeat mitral valve replacement for failed mitral valve prostheses. Ann Thorac Surg. 2004;78(1):67–72. doi: 10.1016/j.athoracsur.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Ye J, Cheung A, Yamashita M, et al. Transcatheter Aortic and Mitral Valve-in-Valve Implantation for Failed Surgical Bioprosthetic Valves. JACC Cardiovasc Interv. 2015;8(13):1735–1744. doi: 10.1016/j.jcin.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Williamson WA, Aretz HT, Weng G, et al. In vitro decalcification of aortic valve leaflets with the Er:YSGG laser, Ho:YAG laser, and the cavitron ultrasound surgical aspirator. Lasers Surg Med. 1993;13(4):421–428. doi: 10.1002/lsm.1900130405. [DOI] [PubMed] [Google Scholar]
- 7.Hutcheson JD, Aikawa E, Merryman WD. Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol. 2014;11(4):218–31. doi: 10.1038/nrcardio.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman WK, Schaff HV, Orszulak TA, Tajik AJ. Ultrasonic aortic valve decalcification: Serial Doppler echocardiographic follow-up. J Am Coll Cardiol. 1990;16(3):623–630. doi: 10.1016/0735-1097(90)90352-p. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Owens G, Gordon D, Cain CA, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121(6):742–9. doi: 10.1161/CIRCULATIONAHA.109.889071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villemain O, Kwiecinski W, Bel A, et al. Pulsed cavitational ultrasound for non-invasive chordal cutting guided by real-time 3D echocardiography. Eur Heart J Cardiovasc Imaging. 2016;17(10):1101–7. doi: 10.1093/ehjci/jew145. [DOI] [PubMed] [Google Scholar]
- 11.Ebbini ES, ter Haar G. Ultrasound-guided therapeutic focused ultrasound: current status and future directions. Int J Hyperthermia. 2015;31(2):77–89. doi: 10.3109/02656736.2014.995238. [DOI] [PubMed] [Google Scholar]
- 12.Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound. J Am Soc Echocardiogr. 2009;22(9):975–1014. doi: 10.1016/j.echo.2009.07.013. -4. [DOI] [PubMed] [Google Scholar]
- 13.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33(19):2451–96. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10(1):1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 15.Baker M. Whole-animal imaging: The whole picture. Nature. 2010;463(7283):977–80. doi: 10.1038/463977a. [DOI] [PubMed] [Google Scholar]
- 16.Gillis K, Bala G, Roosens B, et al. Quantification of Calcium Amount in a New Experimental Model: A Comparison between Ultrasound and Computed Tomography. PLoS One. 2016;11(2):e0148904. doi: 10.1371/journal.pone.0148904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pernot M, Tanter M, Fink M. 3-D real-time motion correction in high-intensity focused ultrasound therapy. Ultrasound Med Biol. 2004;30(9):1239–49. doi: 10.1016/j.ultrasmedbio.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Miller RM, Kim Y, Lin K-W, Cain CA, Owens GE, Xu Z. Histotripsy cardiac therapy system integrated with real-time motion correction. Ultrasound Med Biol. 2013;39(12):2362–73. doi: 10.1016/j.ultrasmedbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe Y, Otsuka R, Muratore R, et al. In vitro mitral chordal cutting by high intensity focused ultrasound. Ultrasound Med Biol. 2008;34(3):400–5. doi: 10.1016/j.ultrasmedbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: a disease of the valve and the myocardium. J Am Coll Cardiol. 2012;60(19):1854–63. doi: 10.1016/j.jacc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Fan Z, Hall TL, Winterroth F. Size measurement of tissue debris particles generated from pulsed ultrasound cavitational therapy–histotripsy. Ultrasound Med. 2009;35(2):245–255. doi: 10.1016/j.ultrasmedbio.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman WK, Schaff HV, Orszulak TA, Tajik AJ. Ultrasonic aortic valve decalcification: Serial Doppler echocardiographic follow-up. J Am Coll Cardiol. 1990;16(3):623–630. doi: 10.1016/0735-1097(90)90352-p. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen T-M, Couade M, Bercoff J, Tanter M. Assessment of viscous and elastic properties of sub-wavelength layered soft tissues using shear wave spectroscopy: theoretical framework and in vitro experimental validation. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58(11):2305–15. doi: 10.1109/TUFFC.2011.2088. [DOI] [PubMed] [Google Scholar]
- 24.Couade M, Pernot M, Prada C, et al. Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med Biol. 2010;36(10):1662–76. doi: 10.1016/j.ultrasmedbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y, Wang T-Y, Xu Z, Cain CA. Lesion generation through ribs using histotripsy therapy without aberration correction. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58(11):2334–43. doi: 10.1109/TUFFC.2011.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, Vlaisavljevich E, Owens GE, Allen SP, Cain CA, Xu Z. In vivo transcostal histotripsy therapy without aberration correction. Phys Med Biol. 2014;59(11):2553–68. doi: 10.1088/0031-9155/59/11/2553. [DOI] [PubMed] [Google Scholar]
- 27.Marquet F, Aubry JF, Pernot M, Fink M, Tanter M. Optimal transcostal high-intensity focused ultrasound with combined real-time 3D movement tracking and correction. Phys Med Biol. 2011;56(22):7061–80. doi: 10.1088/0031-9155/56/22/005. [DOI] [PubMed] [Google Scholar]
- 28.Maciejewski M, Piestrzeniewicz K, Bielecka-Dabrowa A, Piechowiak M, Jaszewski R. Redo surgery risk in patients with cardiac prosthetic valve dysfunction. Arch Med Sci. 2011;7(2):271–7. doi: 10.5114/aoms.2011.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo procedure of pulsed cavitational ultrasound therapy