Abstract
Purpose
BRCA1/BRCA2 predictive test negatives are proven non-carriers of a BRCA1/BRCA2 mutation that is carried by their relatives. The risk of developing breast cancer (BC) or epithelial ovarian cancer (EOC) in these women is uncertain. The study aimed to estimate risks of invasive BC and EOC in a large cohort of BRCA1/BRCA2 predictive test negatives.
Methods
We used cohort analysis to estimate incidences, cumulative risks and standardised incidence ratios (SIR).
Results
A total of 1895 unaffected women were eligible for inclusion in the BC risk analysis and 1736 in the EOC risk analysis. There were 23 incident invasive BCs and 2 EOCs. The cumulative risk of invasive BC was 9.4% (95% CI 5.9%-15%) by age 85 years and the corresponding risk of EOC was 0.6% (95% CI 0.2%-2.6%). The SIR for invasive BC was 0.93 (95% CI 0.62-1.40) in the overall cohort, 0.85 (95% CI 0.48-1.50) in non-carriers from BRCA1 families and 1.03 (95% CI 0.57-1.87) in non-carriers from BRCA2 families. The SIR for EOC was 0.79 (95% CI 0.20-3.17) in the overall cohort.
Conclusions
Our results did not provide evidence for elevated risks of invasive BC or EOC in BRCA1/BRCA2 predictive test negatives.
Keywords: BRCA1/BRCA2, predictive test negatives, breast cancer, ovarian cancer, risks
Introduction
Several breast and ovarian cancer susceptibility genes have been identified to date. The most important genes in the context of genetic counselling are BRCA1 and BRCA2 which are associated with high risks of developing breast, ovarian and other cancers 1–5. Genetic testing for BRCA1 and BRCA2 has become an integral part of genetic counselling; the results are used to inform women’s treatment or clinical management options, which involve a combination of screening, prophylactic surgery and other risk reduction strategies 6–11.
BRCA1/BRCA2 predictive test negatives are individuals who have been offered a predictive test for a specific BRCA mutation that has been found in a biological relative and found to be a non-carrier. Several studies have estimated the risk of developing breast cancer (BC) and epithelial ovarian cancer (EOC) in these women, but estimates vary widely across studies. Although the risks have generally been found be lower than those in BRCA1 and BRCA2 mutation carriers, retrospective studies have estimated the risks of BC to be two- to five-fold higher than the risk in the general population 12,13. Estimates based on retrospective studies may be overestimated if ascertainment is not correctly allowed for in the analysis. The findings from the eight prospective studies published to date have been conflicting. In five of them, risk to non-carriers was not raised (0.52-0.95) 14–18 while in three studies the estimated risks were significantly increased (2.0-4.57) 19–21. An important limitation in all these studies, however, was small sample size, such that the point estimates have wide confidence intervals. Data on EOC risks for BRCA1/BRCA2 predictive test negatives are currently scarce and the only estimates available are from retrospective studies 13,22.
Here, we used data from a large prospective cohort of BRCA1/BRCA2 predictive test negatives from the UK to estimate the risks of developing BC or EOC.
Patients and Methods
Subjects
The “Epidemiological Study of Familial Breast Cancer” (EMBRACE) study is a prospective cohort study in the United Kingdom (UK) and Republic of Ireland, aiming to characterise cancer risks in BRCA1/BRCA2 mutation carriers and their relatives. Individuals are eligible for inclusion in EMBRACE if they: (1) carry a pathogenic mutation in BRCA1 or BRCA2, (2) are non-carriers in families with a pathogenic BRCA1 or BRCA2 mutation, or (3) if they are potential carriers but opt not to undergo a predictive genetic test. Recruitment is organised through regional cancer genetics centres, generally close to the time of genetic testing. Recruitment commenced in 1997 and includes both women and men. The cohort includes individuals with or without personal history of cancer; all participants complete a baseline questionnaire which includes information on BC and EOC risk factors and medical history. The EMBRACE study was approved by the former Anglia and Oxford Medical Research and Ethics Committee (North West Anglia Health Authority, Peterborough, United Kingdom), now East of England – Cambridge South Research Ethics Committee (Nottingham, United Kingdom).
Only non-carrier women were eligible for inclusion in the present study. Information on date and cause of death, and cancer diagnoses, was obtained through linkage with the Health and Social Care Information Centre for England and Wales, and with the National Health Service Central Register for Scotland. For the purpose of the study, the last record linkage was performed on the 15th December 2015. The end of follow-up was set as the 15th July 2015 to ensure that any cancer diagnoses by this date were likely to have been reported at the time of the record linkage.
Cancer risks for BRCA1/BRCA2 predictive test negatives were estimated separately for incident invasive BC and incident EOC. Women were eligible for inclusion in the analysis if they had not been diagnosed with cancer, other than non-melanoma skin cancer (NMSC), before the date at the baseline questionnaire (study recruitment). For the estimation of BC risk, participants were ineligible if they had already undergone risk-reducing bilateral mastectomy (RRBM) at recruitment. In estimating EOC risk, participants were ineligible if they had undergone risk-reducing salpingo-oophorectomy (RRSO) or bilateral salpingectomy (since non-carriers in EMBRACE do not complete follow-up questionnaires after study recruitment, information on prospective prophylactic surgeries occurring after the baseline questionnaire date was not available). Women were followed from baseline until the first of (1) death, (2) a diagnosis of a cancer other than NMSC (3) attained age 85 years or (4) 15th July 2015. For the BC risk estimation, only women diagnosed with an incident invasive BC were assumed to be affected (i.e. non-invasive BCs were ignored but censored as unaffected at diagnosis) and for the EOC analysis only women diagnosed with an EOC were assumed to be affected.
Statistical Analysis
Annual incidences of invasive BC and EOC per 1000 person-years were estimated for the age intervals 18-24, 25-34, 35-44, 45-54, 55-64, 65-74 and 75-84 using standard cohort analysis. Kaplan-Meier analysis was used to estimate the cumulative risks. The probability of experiencing the event of interest up to a given time-point was calculated as 1-Kaplan-Meier estimate of the survival probability. Differences in cancer risks between members of BRCA1 families and members of BRCA2 families were assessed using Cox regression. The hazard ratio (HR) was defined as the ratio between the hazard function at age t for non-carriers from BRCA2 families and the hazard function at age t for non-carriers from BRCA1 families. To account for possible variation in risk due to year of birth (i.e. cohort effect), the analysis was adjusted for year of birth using a categorical variable based on the observed quartiles (1922 to 1953, 1954 to 1963, 1964 to 1972, 1973 to 1995).
We assessed the difference between the estimated incidence rates of invasive BC and EOC in the sample under study and the corresponding incidence rates in a reference population by estimating the standardised incidence ratio (SIR). A SIR is the ratio between the number of observed incident cases and the number of expected incident cases in the study population over the period of observation. For this purpose, we used the population incidences for England 23 and assumed they also apply to women recruited from Scotland and Wales. Expected cases were calculated by applying the calendar period-specific, age-specific and cancer-specific incidences for England to the eligible sample of non-carriers. These rates were available from 1998 to 2014. Rates for 1998 and 2014 were assumed to apply for the years 1997 and 2015, respectively.To allow for the fact that multiple individuals from the same family may be included in the analyses robust standard errors were calculated by clustering on family 24.
Results
The process of generating the eligible sets for inclusion in the BC and EOC analyses are described in the appendix (supplemental figures 1 and 2). Table 1 summarises the eligible cohort characteristics.
Table 1.
Descriptive summary of the cohorts in which risks were calculated, separately for invasive BC and EOC.
| eligible set for invasive BC analysis | eligible set for EOC analysis | |||||
|---|---|---|---|---|---|---|
| overall | BRCA11 | BRCA21 | overall | BRCA11 | BRCA21 | |
| sample, N (%) | 1895 | 1042 (55) | 849 (45) | 1736 | 939 (54) | 793 (46) |
| mean age at questionnaire (SD2) | 45.1 (13.1) | 44.6 (13.1) | 45.6 (13) | 44.2 (13.1) | 43.5 (13) | 45.1 (13.1) |
| mean follow-up in years (SD2) | 7.1 (4.5) | 7.4 (6.6) | 6.6 (4.3) | 7.0 (4.5) | 7.3 (4.7) | 6.6 (4.3) |
| failures | 23 | 12 | 11 | 2 | 0 | 2 |
| mean age at failure (SD2) | 57.2 (8.8) | 60.5 (9.7) | 53.6 (6.2) | 50.5 (8.0) | - | 50.5 (8.0) |
| ethnicity (%) | ||||||
| unknown | 1 (0) | 0 | 1 (0) | 1 (0) | 0 | 1 (0) |
| White | 1810 (96) | 982 (94) | 824 (97) | 1658 (95) | 885 (94) | 769 (97) |
| Indian | 5 (0.3) | 4 (0.4) | 1 (0.1) | 5 (0.4) | 4 (0.5) | 1 (0.1) |
| Black-Caribbean | 3 (0.2) | 3 (0.3) | 0 | 3 (0.2) | 3 (0.3) | 0 |
| Ashkenazi Jewish | 47 (2) | 34 (3.4) | 13 (1.8) | 45 (3) | 32 (3.4) | 13 (1.8) |
| Bangladeshi | 1 (0) | 1 (0.1) | 0 | 1 (0) | 1 (0.1) | 0 |
| Pakistani | 3 (0.2) | 2 (0.2) | 1 (0.1) | 3 (0.2) | 2 (0.2) | 1 (0.1) |
| Black other | 5 (0.3) | 5 (0.5) | 0 | 4 (0.3) | 4 (0.5) | 0 |
| Chinese | 1 (0) | 0 | 1 (0.1) | 0 | 0 | 0 |
| other | 19 (1) | 11 (1.1) | 8 (0.9) | 16 (0.9) | 8 (1) | 8 (1) |
| number of families (%) | 1602 | 887 (55) | 711 (45) | 1488 | 812 (55) | 672 (45) |
| families by number of non-carriers (%) | ||||||
| 1 | 1381 (86) | 767 (86) | 610 (86) | 1300 (87) | 714 (88) | 582 (88) |
| 2 | 179 (11) | 98 (11) | 81 (11) | 152 (11) | 80 (10) | 72 (11) |
| 3 | 25 (2) | 14 (2) | 11 (1.6) | 24 (1.5) | 11 (1.3) | 13 (0.3) |
| 4 | 14 (1) | 6 (0.8) | 8 (1.3) | 9 (0.5) | 5 (0.5) | 4 (0.6) |
| 5 | 1 (0) | 1 (0.1) | 0 | 1 (0) | 1 (0.1) | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 1 (0) | 1 (0.1) | 0 |
| 8 | 1 (0) | 1 (0.1) | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 1 (0) | 0 | 1 (0.1) | 1 (0) | 0 | 1 (0.1) |
In four individuals family identification number and family mutation were missing. In reference to familial clustering, these individuals were assumed to be independent. No assumptions were made on family mutation; therefore, in the stratified analysis by family mutation, these individuals were not considered.
SD, standard deviation.
Risk of Invasive BC
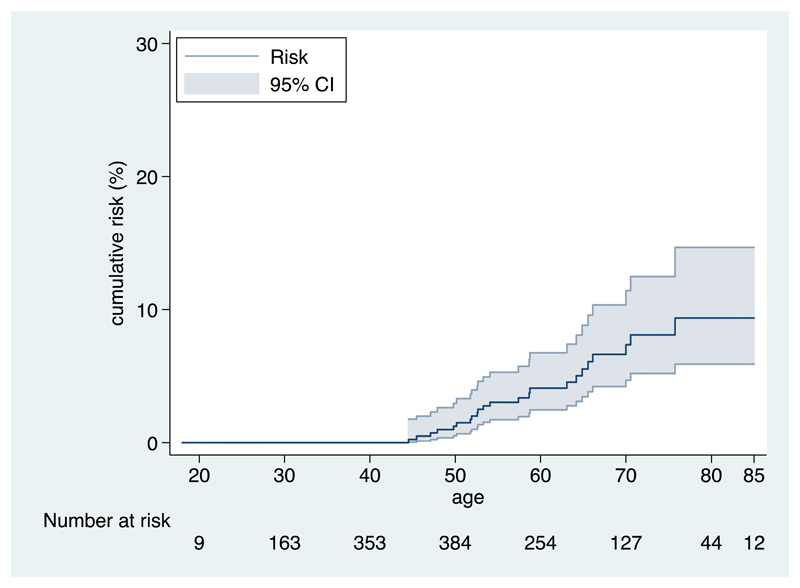
Among 1,895 BRCA1/BRCA2 predictive test negatives eligible for inclusion in the BC analysis, 23 incident invasive BCs occurred, 12 in women from BRCA1 families and 11 in women from BRCA2 families. The estimated incidences, cumulative risks and SIRs are shown in table 2. The crude incidence rate of invasive BC in the overall sample was estimated to be 1.72 per 1000 person years (95% CI 1.16-2.66). The incidence rate increased rapidly at young ages up to age 45 but remained relatively constant after that age, ranging from 2.33 to 3.04 per 1000 person years. The estimate was similar when analysis was restricted to individuals of European ancestry (1.74 per 1000 person years, 95% CI 1.17-2.70). The incidence rate in members of BRCA2 families (1.95/1000 person years ,95% CI 1.11-3.79) was slightly, but not significantly (p-value 0.58), higher than the rate in members of BRCA1 families (1.55 per 1000 person years, 95% CI 0.90-2.92). After adjustment for birth cohort, the hazard ratio estimate for members of BRCA2 families versus members of BRCA1 families was 1.20 (95% CI 0.53-2.70, Wald test p-value=0.6). The SIR estimate for invasive BC was 0.93 (95% CI 0.62-1.40). In the age-specific analysis the highest SIR was estimated for age group 45-55 (SIR=1.30, 95% CI 0.72-2.35), but SIRs were estimated to be <1 for all other age groups (range: 0.35-0.89). There was no apparent trend in the SIRs with age. The SIR was estimated to be 0.85 (95% CI 0.48-1.50) for members of a BRCA1 family with the highest SIR estimated for age group 65-75 (SIR=1.56, 95% CI 0.59-4.16) and 1.03 (95% CI 0.57-1.87) for relatives of BRCA2 mutation carriers with the highest SIR occurring for ages 45-55 (SIR=1.85, 95% CI 0.88-3.87) (table 2). The risk of developing invasive BC in the pooled sample was estimated to be 3% (95% CI 1.7%-5.3%) by age 55 years and 9.4% (95% CI 5.9%-15%) by age 85 years (figure 1). When analysis was restricted to members of BRCA1 families, the cumulative risk of invasive BC was estimated to be 1.8% (95% CI 0.7%-4.8%) by age 55 and 11% (95% CI 5.6%-19%) by age 85. For members of BRCA2 families, the risk of developing invasive BC was 4.5% (95% CI 2.3%-8.8%) by age 55 and 7.7% (95% CI 4.2%-14%) by age 85 (supplemental figure 3). Sensitivity analyses, after excluding women with bilateral oophorectomy, excluding the first year of follow or by including in-situ BC’s yielded similar results (sensitivity analyses described in the appendix (supplemental table 1)).
Table 2.
BC analysis: summary of the estimated parameters. Incidence rates, SIRs and cumulative risks are reported for the overall sample and by family mutation.
| overall sample | relatives of BRCA1 mutation carriers | relatives of BRCA2 mutation carriers | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| py1 | incident invasive BCs | IR2 per 1000 py1 (95%CI) | SIR3 (95%CI) | cumulative risk (95%CI) | py1 | incident invasive BCs | IR2 per 1000 py1 (95%CI) | SIR3 (95% CI) | cumulative risk (95%CI) | py1 | incident invasive BCs | IR2 per 1000 py1 (95%CI) | SIR3 (95% CI) | cumulative risk (95%CI) | |
| overall | 13398.1 | 23 | 1.72 (1.16-2.66) | 0.93 (0.62-1.40) | NA4 | 7738.7 | 12 | 1.55 (0.90-2.92) | 0.85 (0.48-1.50) | NA4 | 5632.4 | 11 | 1.95 (1.11-3.79) | 1.03 (0.57-1.87) | NA4 |
| agegroup | |||||||||||||||
| ≥18-<25 | 187.5 | 0 | 0 | 0 | 0 | 116.1 | 0 | 0 | 0 | 0 | 68.8 | 0 | 0 | 0 | 0 |
| ≥25-<35 | 1556.4 | 0 | 0 | 0 | 0 | 904.8 | 0 | 0 | 0 | 0 | 646.1 | 0 | 0 | 0 | 0 |
| ≥35-<45 | 3426.1 | 1 | 0.29 (-) | 0.35 (0.05-2.45) | 0.3% (0-1.8) | 2114.1 | 0 | 0 | 0 | 0 | 1312 | 1 | 0.76 (-) | 0.90 (0.13-6.42) | 0.7% (0.1-4.7) |
| ≥45-<55 | 3908.9 | 11 | 2.81 (1.59-5.47) | 1.30 (0.72-2.35) | 3% (1.7-5.3) | 2157.6 | 4 | 1.85 (0.70-6.64) | 0.86 (0.32-2.30) | 1.8% (0.7-4.8) | 1751.2 | 7 | 4,00 (1.95-9.52) | 1.85 (0.88-3.87) | 4.5% (2.3-8.8) |
| ≥55-<65 | 2574.4 | 6 | 2.33 (1.06-6.13) | 0.84 (0.38-1.87) | 5.5% (3.5-8.8) | 1437.3 | 3 | 2.09 (0.65-10.2) | 0.75 (0.24-2.31) | 3.9% (1.8-8.2) | 1137.1 | 3 | 2.64 (0.82-12.9) | 0.96 (0.31-2.97) | 7.7% (4.2-14) |
| agegroup | |||||||||||||||
| ≥65-<75 | 1316 | 4 | 3.04 (1.14-10.9) | 0.89 (0.33-2.37) | 8.1% (5.2-12) | 754.7 | 4 | 5.3 (1.99-18.9) | 1.56 (0.59-4.16) | 8.4% (4.5-15) | 555.1 | 0 | 0 | 0 | 7.7% (4.2-14) |
| ≥75-<85 | 428.8 | 1 | 2.33 (-) | 0.65 (0.09-4.59) | 9.4% (5.9-15) | 253.9 | 1 | 3.94 (-) | 1.09 (0.15-7.73) | 11% (5.6-19) | 162 | 0 | 0 | 0 | 7.7% (4.2-14) |
| European Ancestry | 13184.4 | 23 | 1.74 (1.17-2.70) | 0.94 (0.63-1.42) | NA | 7587.7 | 12 | 1.58 (0.91-2.98) | 0.87 (0.49-1.53) | NA | 5569.6 | 11 | 1.97 (1.12-3.83) | 1.04 (0.58-1.88) | NA |
1: py, person years; 2: IR, incidence rate; 3: SIR, standardised incidence ratio; 4: NA, not applicable.
In four individuals family identification number and family mutation were missing.
Figure 1.
Kaplan Meier plot for the risk of invasive BC in the combined sample of non-carriers from families with BRCA1 and BRCA2 mutations. CI: confidence interval.
Risk of EOC
In the cohort of 1,736 non-carriers eligible for the EOC analysis, two incident EOCs were observed, both in individuals from BRCA2 families, with ages at diagnosis 44.8 and 56.1 years. The crude EOC incidence rate in the overall sample was 0.16 per 1000 person years (95% CI 0.03-1.66). The estimated SIR for EOC was 0.79 (95% CI 0.20-3.17) for all age groups. With only two incident EOCs, SIR estimates by age group and by family mutation were either not possible or associated with wide confidence intervals. They are not therefore reported. The estimated absolute risk of EOC in non-carriers was 0.6% (95% CI 0.2%-2.6%) by age of 85 years. In a sensitivity analysis that included women with a prior BC diagnosis in the eligible cohort, results were unchanged (appendix).
Discussion
This is the largest prospective cohort study of BRCA1/BRCA2 predictive test negatives to date. We found no evidence of an increased BC or EOC risk in relatives of BRCA1 or BRCA2 mutation carriers. The BC risk estimates were not influenced by the inclusion of individuals with prior bilateral salpingo-oophorectomy. When the four non-invasive prospective BCs were also considered as events, to rule out the possibility that some cancers were being wrongly excluded, the SIR estimates were similar. Similarly, after censoring individuals at an earlier date (15th June 2014) to allow for possible risk underestimation due to delayed cancer notification, results were unchanged.
Early clinical recommendations on hereditary BC and EOC recommended standard surveillance for BRCA1/BRCA2 predictive test negatives 25. In 2013, the United States Preventive Services Task Force issued a recommendation reiterating that non-carriers were at population-risk 26,27. According to the National Institute for Health and Care Excellence (NICE) guidelines 6, women at population risk should be given standard surveillance and discussion of any further risk-reducing intervention would not be appropriate. If, however, BRCA1/BRCA2 predictive test negatives are at increased risk of developing BC, as recently suggested 19–21, the recommendations for women at moderate to high risk of developing BC may apply.
In five out of the eight prospective studies published to date, the estimated SIR for BC was in line with our results (range of SIRs= 0.52-0.95) 14–18. In three studies a significant two- to five-fold increased risk of BC was reported compared to population risks, but there were inconsistencies between these studies 19–21. Evans et al. found a statistically significant increased risk only for relatives of BRCA2 mutations carriers 19, while Vos et al. found a statistically significant increased risk only for relatives of BRCA1 mutation carriers 21. Both studies only included first degree relatives of known mutation carriers. The authors argued that common genetic variants, which modify risks in BRCA1/BRCA2 carriers, also may modify risk in non-carriers. These predisposing variants would be more likely to segregate in families with multiple affected individuals, such as those ascertained in genetics clinics. Korde et al. 17 and Nielsen et al. 18, however, did not find an increase in the risk of BC for BRCA predictive test-negatives who were first degree relatives of mutation carriers. A limitation of all these studies was the small sample size (none included more than 21 BC cases). In the present study we were not able to restrict analysis to only first degree relatives of known mutation carriers as the exact familial relationships were not available. Similarly, we were not able to investigate the variation in risk by family history of cancer. Korde et al. 17 reported a non significant increase in the risk of BC for non-carriers with an affected first degree relative. In Harvey et al. 16 the estimated SIR of BC in predictive test negatives without affected relatives in the parental lineage not associated with the BRCA mutation was 0.48, albeit statistically not significant.
Only a few published studies provided a SIR for EOC (range of SIRs reported: 0-4.6) 13,22 but all were based on retrospective studies. Three prospective studies 15,20,21 aimed to estimate a SIR for EOC. In Domchek et al. and Rowan et al.15,20 no events occurred during the follow-up, while Vos et al. 21 reported two EOCs but the corresponding SIR was not provided. This is the first study to estimate a SIR for EOC based on a prospective cohort, but the confidence intervals were wide due to the small number of events.
The sample size of this study is larger than previous studies and the confidence limits are correspondingly narrower. Nonetheless, when considering BC risk estimates by age group and family mutation, caution is needed due to the relatively small number of events in each stratum. Since this is a prospective cohort, estimates are less likely to be prone to ascertainment or reporting biases, usually associated with retrospective or historical cohort studies.
Limitations of this study include: (1) The SIRs were calculated using incidences from England, but EMBRACE non-carriers were also recruited in Scotland and Wales. Age-specific estimates for these countries were not readily available; however, data from previous periods indicate that the incidence rates are similar amongst countries. Moreover, the large majority of study participants (83.3%) were from clinics based in England. (2) Surgeries unrelated to cancer diagnoses prior to recruitment were not verified through surgical records, but bias due to misreporting or underreporting was probably small given the life-changing sexual and physical impact of risk-reducing surgery 28–30. (3) No information on prophylactic surgeries after recruitment was available. Such surgeries are unlikely in women testing negative for mutations; however, if risk-reducing surgeries are more frequent in the non-carriers within families with BRCA1 and BRCA2 mutations compared to the general population this may lead to an underestimation of the SIR. In an approximate calculation, assuming the same proportion (i.e. 0.88%) of bilateral mastectomies in the prospective cohort as in the retrospective data, 17 such procedures would be expected. In the unlikely event that all these individuals would have developed invasive BC if they had not undergone surgery, the observed/expected ratio would have been 1.61 (95% CI 1.18-2.2). In the retrospective data, 5.4% of non-carriers from BRCA1 families had undergone bilateral oophorectomy with or without bilateral salpingectomy, compared to 3.3% of non-carriers from BRCA2 families, possibly reflecting differential counselling towards risk-reducing surgery when a BRCA1 mutation segregates in the family. In the current sample, 98% of non-carriers from BRCA1 families who underwent bilateral oophorectomy (or bilateral salpingectomy) at baseline had the procedure before genetic testing, whereas only 3% underwent RRSO after notification of the negative result. If the uptake of RRSO (after notification of mutation carrier status) was similar in the prospective cohort, this may have led to some underestimation of the EOC SIR for relatives of BRCA1 mutation carriers and in the overall sample. However, the size of these biases is most likely small because RRBM and RRSO after the disclosure of a negative result are less likely. (4) The non-carrier’s degree of relationship with the mutation carrier and cancer family history were not available. Although women were ascertained through clinical genetics centres and most would have come from high-risk families, ascertainment criteria with respect to family history can vary. Therefore, it is not possible to rule out that risk in non-carriers may be increased if multiple family members or first degree relatives are affected. (5) No information on the date of the most recent screening mammogram or breast magnetic resonance imaging was available. If BRCA1/BRCA2 predictive test negatives with a normal breast imaging close to the genetic test discontinue surveillance following the notification of non-carrier status, they may be subject to a reverse lead time effect if they are diagnosed with a BC and this may lead to some underestimation of the risk. We investigated this by calculating the BC incidence in 3-year intervals following the genetic test. Although there was a suggestion of a higher BC incidence rate between three and six years following the genetic test compared to the initial years, the differences were not significant. Larger sample sizes will be required to clarify this potential bias. (6) Although this is the largest prospective study, the mean follow-up time in the cohort was still relatively short (7.1 years) and longer follow-up will be required to investigate the risks at older ages with more precision.
Based on the confidence interval of the invasive BC SIRs, a 1.5-fold increase in risk in non-carriers from BRCA1 families and a 1.9-fold increase in non-carriers from BRCA2 families cannot be ruled out. Translating these SIRs to lifetime risk yields an upper bound of risk of ~17% to age 80 years for relatives of BRCA1 mutation carriers and ~20% to age 80 years for relatives of BRCA2 mutation carriers, based on UK general population incidences 31. Hence, even at the upper bound of the 95% CI, BRCA1/BRCA2 predictive test negatives would not be classified as being at high risk of developing invasive BC according to the NICE guidelines 6.
The familial aggregation of BC is known to be determined by other factors, including multiple common genetic variants, in addition to BRCA1 and BRCA2 mutations. Thus, one would predict that BRCA1/BRCA2 predictive test negatives in cohorts such as the present study, who have a family history of BC and are hence more likely to carry other predisposing variants, would still be at increased risk relative to general population. We therefore investigate whether the present estimates are consistent with predictions given by the BOADICEA model, which models the familial aggregation of BC in terms of BRCA1/2 mutation status and a residual polygenic component. We computed the predicted lifetime risk of BC for a 20 year-old true-negative, with two different family histories: (1) a mutation-positive mother diagnosed with BC at age 50 years, and (2) a mother and maternal grandmother both diagnosed with BC at age 50 and carrying a mutation. The predicted BC risks to age 80 years in these two scenarios were 12% and 13% for a BRCA1 positive family, and 14% and 15% for a BRCA2 positive family. These women would be classified as at near population risk according to BOADICEA. Nevertheless, the predicted risks are somewhat higher than the point estimates in the present study, but within or close to the upper confidence interval bounds of the estimated cumulative risks. These observations suggest that the BOADICEA model may overestimate slightly the risks to individuals with a negative predictive test. However, larger sample sizes will be required to resolve this issue: if the true risks were in line with those predicted by BOADICEA, a study with at least 100 events would be required to provide sufficient statistical power.
Our results suggest that BRCA1/BRCA2 predictive test negatives can be classified as being at near-population risk as defined by the NICE guidelines 6. Therefore, it would not be appropriate to offer RRBM and RRSO to these individuals. In practice a proportion of women undergo preventative surgery based solely on the family history. Based on these data, predictive genetic testing of female relatives of a known BRCA1/BRCA2 mutation carrier is strongly beneficial in terms of avoiding unnecessary surgical procedures for those found to be BRCA1/BRCA2 negative. Standard surveillance may be offered, unless personal risk factors warrant further consideration such as strong family history of cancer in the parental lineage not associated with the known mutation or history of lobular carcinoma in situ or atypical ductal hyperplasia. 32,33
Supplementary Material
Acknowledgements
Sources of Funding
This work was supported by Cancer Research – UK grants C12292/A20861, C1287/A10118, C1287/A11990, C1287/A23382, C1287/A17523 and C1287/A16563.
Footnotes
Authors’ Contributions
Fabio Girardi: data analysis and interpretation, manuscript writing, final approval of the manuscript, agreement to be accountable for all aspects of the work.
Daniel R. Barnes: data analysis and interpretation, critical review of the manuscript, final approval of the manuscript, agreement to be accountable for all aspects of the work.
Daniel Barrowdale, Debra Frost, Angela F. Brady, Claire Miller, Alex Henderson, Alan Donaldson, Alex Murray, Carole Brewer, Caroline Pottinger, D. Gareth Evans, Diana Eccles, EMBRACE, Fiona Lalloo, Helen Gregory, Jackie Cook, Jacqueline Eason, Julian Adlard, Julian Barwell, Kai-ren Ong, Lisa Walker, Louise Izatt, Lucy E. Side, M. John Kennedy, Marc Tischkowitz, Mark T. Rogers, Mary E. Porteous, Patrick J. Morrison, Ros Eeles, Rosemarie Davidson, Katie Snape: collection and assembly of data, critical review of the manuscript, final approval of the manuscript, agreement to be accountable for all aspects of the work.
Douglas F. Easton: conception and design, data analysis and interpretation, manuscript writing, final approval of the manuscript, agreement to be accountable for all aspects of the work.
Antonis C. Antoniou: conception and design, data analysis and interpretation, manuscript writing, final approval of the manuscript, agreement to be accountable for all aspects of the work.
Potential Conflict of Interests
Diana Eccles has received honoraria from AstraZeneca and has reported consulting or advisory role in AstraZeneca.
Fabio Girardi, Daniel R. Barnes, Daniel Barrowdale, Debra Frost, Angela F. Brady, Claire Miller, Alex Henderson, Alan Donaldson, Alex Murray, Carole Brewer, Caroline Pottinger, D. Gareth Evans, EMBRACE, Fiona Lalloo, Helen Gregory, Jackie Cook, Jacqueline Eason, Julian Adlard, Julian Barwell, Kai-ren Ong, Lisa Walker, Louise Izatt, Lucy E. Side, M. John Kennedy, Marc Tischkowitz, Mark T. Rogers, Mary E. Porteous, Patrick J. Morrison, Ros Eeles, Rosemarie Davidson, Katie Snape, Douglas F. Easton and Antonis C. Antoniou have reported no potential conflict of interests.
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457–1466. doi: 10.1038/sj.bjc.6604305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The National Institute for Health and Care Excellence. Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. [Accessed March 1, 2017];2013 Available at https://www.nice.org.uk/Guidance/CG164. [PubMed]
- 7.National Comprehensive Cancer Network. NCCN Guidelines for detection, prevention, & risk reduction. [Accessed March 1, 2017];2016 Available at https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf.
- 8.Karlan BY, Berchuck A, Mutch D. The role of genetic testing for cancer susceptibility in gynecologic practice. Obstet Gynecol. 2007;110:155–167. doi: 10.1097/01.AOG.0000269050.79143.84. [DOI] [PubMed] [Google Scholar]
- 9.Greene MH, Mai PL, Schwartz PE. Does bilateral salpingectomy with ovarian retention warrant consideration as a temporary bridge to risk-reducing bilateral oophorectomy in BRCA1/2 mutation carriers? Am J Obstet Gynecol. 2011;204:19.e1–6. doi: 10.1016/j.ajog.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker JL, Powell CB, Chen LM, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121:2108–2120. doi: 10.1002/cncr.29321. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann LC, Lindor NM. The role of risk-reducing surgery in hereditary breast and ovarian cancer. N Engl J Med. 2016;374:454–468. doi: 10.1056/NEJMra1503523. [DOI] [PubMed] [Google Scholar]
- 12.Gronwald J, Cybulski C, Lubinski J, Narod SA. Phenocopies in breast cancer 1 (BRCA1) families: implications for genetic counselling. J Med Genet. 2007;44:e76. doi: 10.1136/jmg.2006.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith A, Moran A, Boyd MC, et al. Phenocopies in BRCA1 and BRCA2 families: evidence for modifier genes and implications for screening. J Med Genet. 2007;44:10–15. doi: 10.1136/jmg.2006.043091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernholtz S, Laitman Y, Kaufman B, Shimon-Paluch S, Friedman E. Phenocopy breast cancer rates in Israeli BRCA1 BRCA2 mutation carrier families: is the risk increased in non-carriers? Breast Cancer Res Treat. 2012;132:669–673. doi: 10.1007/s10549-011-1886-3. [DOI] [PubMed] [Google Scholar]
- 15.Domchek SM, Gaudet MM, Stopfer JE, et al. Breast cancer risks in individuals testing negative for a known family mutation in BRCA1 or BRCA2. Breast Cancer Res Treat. 2010;119:409–414. doi: 10.1007/s10549-009-0611-y. [DOI] [PubMed] [Google Scholar]
- 16.Harvey SL, Milne RL, McLachlan SA, et al. Prospective study of breast cancer risk for mutation negative women from BRCA1 or BRCA2 mutation positive families. Breast Cancer Res Treat. 2011;130:1057–1061. doi: 10.1007/s10549-011-1733-6. [DOI] [PubMed] [Google Scholar]
- 17.Korde LA, Mueller CM, Loud JT, et al. No evidence of excess breast cancer risk among mutation-negative women from BRCA mutation-positive families. Breast Cancer Res Treat. 2011;125:169–173. doi: 10.1007/s10549-010-0923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen HR, Petersen J, Krogh L, Nilbert M, Skytte AB. No evidence of increased breast cancer risk for proven noncarriers from BRCA1 and BRCA2 families. Fam Cancer. 2016;15:523–528. doi: 10.1007/s10689-016-9898-0. [DOI] [PubMed] [Google Scholar]
- 19.Evans DG, Ingham SL, Buchan I, et al. Increased rate of phenocopies in all age groups in BRCA1/BRCA2 mutation kindred, but increased prospective breast cancer risk is confined to BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2013;22:2269–2276. doi: 10.1158/1055-9965.EPI-13-0316-T. [DOI] [PubMed] [Google Scholar]
- 20.Rowan E, Poll A, Narod SA. A prospective study of breast cancer risk in relatives of BRCA1/BRCA2 mutation carriers. J Med Genet. 2007;44:e89. [PMC free article] [PubMed] [Google Scholar]
- 21.Vos JR, de Bock GH, Teixeira N, et al. Proven non-carriers in BRCA families have an earlier age of onset of breast cancer. Eur J Cancer. 2013;49:2101–2106. doi: 10.1016/j.ejca.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 22.van der Kolk DM, de Bock GH, Leegte BK, et al. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat. 2010;124:643–651. doi: 10.1007/s10549-010-0805-3. [DOI] [PubMed] [Google Scholar]
- 23.Office for National Statistics. Cancer registration statistics, England. [Accessed March 1, 2017];2015 Available at https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancerregistrationstatisticscancerregistrationstatisticsengland.
- 24.MacInnis RJ, Bickerstaffe A, Apicella C, et al. Prospective validation of the breast cancer risk prediction model BOADICEA and a batch-mode version BOADICEA Centre. Br J Cancer. 2013;109:1296–1301. doi: 10.1038/bjc.2013.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berliner JL, Fay AM. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: recommendations of the National Society of Genetic Counselors. J Genet Couns. 2007;16:241–260. doi: 10.1007/s10897-007-9090-7. [DOI] [PubMed] [Google Scholar]
- 26.Nelson HD, F R, Goddard K, Mitchell J Priest, Okinaka-Hu L, Pappas M, Zakher B. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: systematic review to update the U.S. Preventive Services Task Force recommendation. Evidence synthesis No. 101. Agency for Healthcare Research and Quality; 2013. publication no 12-05164-EF-1. [PubMed] [Google Scholar]
- 27.United States Preventive Services Task Force recommendation. BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. [Accessed March 1, 2017];2013 Available at http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing.
- 28.Rocca W, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., III Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 29.Stan DL, Shuster LT, Wick MJ, Swanson CL, Pruthi S, Bakkum-Gamez JN. Challenging and complex decisions in the management of the BRCA mutation carrier. J Womens Health (Larchmt) 2013;22:825–834. doi: 10.1089/jwh.2013.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gahm J, Wickman M, Brandberg Y. Bilateral prophylactic mastectomy in women with inherited risk of breast cancer: prevalence of pain and discomfort, impact on sexuality, quality of life and feelings of regret two years after surgery. Breast. 2010;19:462–469. doi: 10.1016/j.breast.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Lee AJ, Cunningham AP, Kuchenbaecker KB, Mavaddat N, Easton DF, Antoniou AC. BOADICEA breast cancer risk prediction model: updates to cancer incidences, tumour pathology and web interface. Br J Cancer. 2014;110:535–545. doi: 10.1038/bjc.2013.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavassoli FA, Millis RR, Boecker W, Lakhani SR. Lobular neoplasia. In: Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs. International Agency for Research on Cancer. Lyon: IARCpress; 2003. pp. 60–62. [Google Scholar]
- 33.Tavassoli FA, Hoefler H, Rosai J, et al. Intraductal proliferative lesions. In: Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs. International Agency for Research on Cancer. Lyon: IARCpress; 2003. pp. 63–73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.