Abstract
Background
C-reactive protein (CRP) is a candidate biomarker for major depressive disorder (MDD), but it is unclear how peripheral CRP levels relate to the heterogeneous clinical phenotypes of the disorder.
Aim
To explore CRP in MDD and its phenotypic associations.
Method
We recruited 102 treatment-resistant, depressed MDD patients, 48 treatment-responsive, non-depressed MDD patients, 48 depressed but un-medicated patients, and 54 healthy volunteers. High sensitivity CRP in peripheral venous blood, body mass index (BMI), and questionnaire assessments of depression, anxiety, and childhood trauma, were measured. Group differences in CRP were estimated, and Partial least squares (PLS) analysis explored the relationships between CRP and specific clinical phenotypes.
Results
Compared to healthy volunteers, BMI-corrected CRP was significantly elevated in treatment-resistant patients (P = 0.007; Cohen’s d = 0.47); but not significantly so in the treatment-responsive (d = 0.29) and untreated (d = 0.18) groups. PLS yielded an optimal two factor solution that accounted for 34.7% of variation in clinical measures, and for 36.0% of variation in CRP. Clinical phenotypes most strongly associated with CRP and heavily weighted on the first PLS component were: vegetative depressive symptoms, BMI, state anxiety, and feeling unloved as a child or wishing for a different childhood.
Conclusion
CRP was elevated in MDD, more so in treatment-resistant cases. Other phenotypes associated with elevated CRP included childhood adversity, and specific depressive and anxious symptoms. We suggest that MDD patients stratified for pro-inflammatory biomarkers, like CRP, have a distinctive clinical profile that might be responsive to second-line treatment with anti-inflammatory drugs.
Introduction
Immunological mechanisms are increasingly implicated in the pathogenesis of depressive symptoms (1–3). Activation of the peripheral immune system has been consistently associated with major depressive disorder (4–8). However, it has also been anticipated that not all patients with MDD will be peripherally inflamed to the same extent. A deeper understanding of how peripheral immune biomarkers relate to some of the dimensions of clinical heterogeneity encompassed by a diagnosis of MDD could be an important step towards mechanistically stratified treatment of depression in the future (3, 9, 10).
C-reactive protein (CRP) is an acute phase protein that is widely used in clinical practice and has also been measured in many prior studies of MDD. A high sensitivity assay for CRP is well validated and accessible. CRP synthesis is induced in the liver by pro-inflammatory cytokines – especially interleukin 6 (IL-6) – in response to infection, inflammation and tissue damage. In a meta-analysis of 20 case-control studies (8), CRP was moderately increased “on average” (Cohen’s d = 0.47) in patients with MDD. However, there was significant heterogeneity of effect size between studies that may be attributable to clinical heterogeneity, with higher CRP in severe depression (Cohen’s d = 0.50) than in mild/moderate depression (Cohen’s d = 0.37), as well as methodological differences between studies (11).
We were motivated to test the hypothesis that the clinically defined subgroup of patients with treatment-resistant depression would have the most abnormally increased CRP. An association between treatment resistance to monoaminergic anti-depressant drugs and increased CRP is hypothetically predictable on clinical and mechanistic grounds. Clinical studies indicate that pro-inflammatory cytokines that induce CRP synthesis are increased in treatment-resistant MDD (12, 13). Pro-inflammatory cytokines can reduce the extracellular availability of serotonin by biasing expression of genes related to serotonin transport and tryptophan metabolism (14, 15). Single studies have also reported that elevated CRP may be associated with other dimensions of clinical heterogeneity, viz atypical depression (16), childhood adversity (17), higher numbers of previous depressive episodes (18), or anxiety in male patients (19).
We measured CRP in four groups of participants: currently depressed but not medicated (untreated) MDD patients; currently depressed and medicated (treatment-resistant) patients; currently medicated but not depressed (treatment-responsive) patients; and healthy volunteers with no history of MDD or monoaminergic drug treatment. The primary hypothesis, that CRP would be most clearly increased above normal levels in treatment-resistant patients with MDD, was tested by planned analyses of between-group differences in mean CRP. In a secondary analysis, we took a more exploratory approach to the question of what other dimensions of clinical heterogeneity in the sample might be related to variation in CRP. We used the multivariate technique of partial least squares (PLS) to explore the relationships between CRP and multiple (139) clinical phenotypes – ranging from BMI to questionnaire items for depressive symptoms, anxiety states or history of childhood adversity (20–23). In this way, we could identify a subset of clinical phenotypes weighted strongly on latent dimensions of clinical heterogeneity that were predictive of higher CRP levels. We also tested the confirmatory hypothesis that scores on these clinical dimensions of peripheral inflammation would be higher in the subgroup of patients with treatment resistance defined a priori.
Patients and Methods
This was a non-interventional study, conducted as part of the Wellcome Trust Consortium for Neuroimmunology of Mood Disorders and Alzheimer’s disease (NIMA). There were five clinical study centres in the UK: Brighton, Cambridge, Glasgow, King’s College London, and Oxford. All procedures were approved by an independent Research Ethics Committee (National Research Ethics Service East of England, Cambridge Central, UK) and the study was conducted according to the Declaration of Helsinki. All participants provided informed consent in writing, and received £100 compensation for taking part.
Sample and eligibility criteria
We recruited four groups of participants: treatment-resistant depression, treatment-responsive depression, untreated depression, and healthy volunteers.
For all participants, the following inclusion criteria applied: age 25-50 years, able to give informed consent; able to fast for 8 hours, and abstain from strenuous exercise for 72h, prior to venous blood sampling; and fluent English. The following exclusion criteria applied: pregnancy or breast feeding; alcohol or substance use disorder in the preceding 12 months; participation in an investigational drug study within the preceding 12 months; lifetime history of any medical disorder or current use of any medication (e.g. statins, corticosteroids, anti-histamines, anti-inflammatory medications) likely to compromise interpretation of CRP (see Supplemental Information).
Adult patients meeting Diagnostic and Statistical Manual Version 5 (DSM-5) criteria for MDD (American Psychiatric Association, 24) were recruited from NHS mental health and primary care services and from the general population by purposive advertising. Lifetime histories of bipolar disorder or non-affective psychosis were additional exclusion criteria. Diagnosis of MDD and other psychiatric disorders was ascertained by Structured Clinical Interview for DSM-5 (25). Current depressive symptom severity was defined using total scores from the 17-item Hamilton Rating Scale for Depression (HAM-D) (26), and lifetime anti-depressant medication use was quantified using the Antidepressant Treatment Response Questionnaire (ATRQ) (27). The ATRQ was completed by a member of the study team via an interview with each participant. This structured instrument records all medications received for at least 6 weeks for treatment of depression, for current and past depressive episodes. For each medication ever received, the percentage improvement experienced by the participant during the corresponding episode was documented (less than 25%, 25-49% improved, 50-75% improved, or >75% improved). Treatment response was conservatively defined as >75% improvement in depressive symptoms, as recalled by the participant. The ATRQ provides definitions for the minimum dose for each medication to be considered an adequate treatment course (27).
Patients were assigned to one of three subgroups or strata, per protocol: (i) treatment-resistant (DEP+MED+) patients who had total HAM-D > 13 and had been medicated with a monoaminergic drug at a therapeutic dose for at least six weeks; (ii) treatment-responsive (DEP-MED+) patients who had total HAM-D < 7 and had been medicated with a monoaminergic drug at a therapeutic dose for at least six weeks; and (iii) untreated (DEP+MED-) patients who had HAM-D > 17 and had not been medicated with a monoaminergic drug for at least six weeks Cut-offs were defined a priori based on the literature. Total HAM-D score > 17 is a standard threshold for entry into placebo-controlled treatment trials of MDD; whereas a lower threshold of total HAM-D > 13 is typically used to define treatment-resistant depression, because there is usually some modest symptomatic response to treatment even if patients remain depressed (28, 29).
A group of healthy volunteers was recruited by advertising with no current or past history of any major psychiatric disorder as defined by DSM-5, and no history of monoaminergic drug treatment for any indication. Healthy volunteers completed the same screening and baseline assessments as patient groups (see below).
Age, gender, medical history, smoking status, and family history were documented by semi-structured clinical interviews. Height and weight were measured for calculation of BMI (kg/m2).
Questionnaire assessments
Psychological symptoms and childhood adversity were assessed by administration of the following questionnaires (see Supplemental Information): the Beck depression inventory (BDI v2.0, 30); the Spielberger State-Trait Anxiety Rating scale (STAI, 31); the Chalder Fatigue Score (CFS, 32); the Snaith-Hamilton Pleasure Scale (SHAPS, 33); and the Childhood Trauma Questionnaire (CTQ, 34).
High sensitivity CRP measurement
C-reactive protein was measured as one of many immunological markers in a venous blood sample drawn from each participant. Here, we focus on CRP since this has established utility as an immune biomarker of depression, having been widely used in case-control and epidemiological studies, and thus informing our hypothesis that CRP would be increased specifically in treatment-resistant depression. Participants fasted for 8h, and abstained from strenuous exercise for 72h, prior to venous blood sampling between 08:00-10:00am. Patients taking psychotropic medication(s) continued their usual medication during the assessment day. High sensitivity CRP was assayed via a central laboratory (see Supplemental Information).
Statistical analysis
For analysis of between-group differences in hs-CRP and other variables we first compared all MDD participants to healthy volunteers using planned t-tests. We then evaluated pairwise group differences using post-hoc t-tests, provided the main effect of group was significant by one-way analysis of variance (ANOVA). When assumptions of normality were violated, data were appropriately transformed and/or non-parametric tests were used for inference. Cohen’s d was reported for the effect size of hs-CRP corrected for BMI in each clinical group compared to healthy volunteers. Additionally, we compared the proportion of participants in each group who had clinically elevated CRP, defined as > 3mg/L (35, 36). The threshold for statistical significance was defined as two-tailed P < 0.05 throughout.
To identify demographic and clinical phenotypes associated with variation in CRP across all study participants, we utilized the method of partial least squares (PLS), as implemented in JMP Pro software Version 13.0 (37). PLS is a multivariate technique for modelling relationships between a set of predictor (X) and response (Y) variables in terms of a set of mutually orthogonal latent factors, or PLS components (21, 22, 38, 39). It requires no distributional assumptions and thus is robust against skewness. This same software was also used for other statistical tests and generation of violin plots for CRP across groups.
Here we modeled hs-CRP as the response variable, Y. The predictor variables, X, comprised gender, age, BMI, and education level; plus each of the 21 HAM-D, 11 CFS, 21 BDI, 28 CTQ, 40 STAI, and 14 SHAPS questionnaire items. Data from all subjects were included and missing data were imputed by sample means. Thus the Y vector was (252 × 1) and the X matrix was (252 × 139). Due to the number of variables, and the expectation that many variables would correlate with each other, other statistical approaches (such as linear regression) would not have been valid. In contrast, PLS is an ideal statistical technique under these circumstances (21, 22, 38, 39). An initial PLS model was fitted including all predictor variables. We then used a two-step approach to identify the subset of predictor variables that significantly contributed to the model: first, we discarded individual X variables with low importance by conventional criteria (variable importance parameter, VIP, <0.8 and standardized absolute model coefficient < absolute magnitude 0.05 (40)); second, we utilized a more conservative approach of excluding variables whose standardized model coefficient had a 95% confidence interval, constructed by bootstrapping the data 1000 times, that included zero. PLS models were fitted using leave-one-out cross-validation (non-linear iterative partial least squares, NIPALS algorithm), and the optimal number of latent factors was selected by minimizing the predictive residual sum of the squares (PRESS). The statistical significance of the final model was confirmed by comparing the percentage of variation in X and Y accounted for in the experimental data compared to the null distributions of the percentage of X or Y variance sampled by bootstrapping (1000 iterations).
Results
Demographic and clinical data
The size of each group and their demographic and clinical characteristics are summarized in Table 1. The groups did not differ significantly in terms of demographic characteristics. As expected, post hoc tests indicated that each group differed significantly from each other group on HAM-D total score (least significant t = 4.19, df = 248, P < 0.001). The mean number of failed pharmacological treatments for MDD episodes (<75% symptomatic response, defined by ATRQ) is listed for each clinical group in Table 1. The treatment-resistant group had more failed treatments than the untreated group (Wilcoxon Z = 2.843, P = 0.005); both the treatment-resistant group and the untreated group had significantly more failed treatments than the treatment-responsive group (Wilcoxon Z = 5.794, P < 0.001 and Wilcoxon Z = 3.079, P = 0.002, respectively). The majority of treatment-resistant patients were taking selective serotonin reuptake inhibitors (see Table 1 footnote). Summary statistics for questionnaire-based measures and comorbidities are provided in Tables S1 and S2.
Table 1. Demographic, clinical and hs-CRP data.
| Mean (95% confidence interval) / N [%] in category | Group test | |||||
|---|---|---|---|---|---|---|
| Healthy volunteers N=54 |
Treatment-responsive MDD N=48 |
Treatment-resistant MDD N=102 # |
Untreated MDD N=48 |
Statistic | P | |
| Age, years | 34.2 (32.3-36.2) |
35.9 (33.6-38.3) |
36.5 (35.1-38.0) |
35.1 (32.6-37.6) |
F=1.14 | 0.34 |
| Gender, female, N [%] | 37 [68.5%] | 32 [66.7%] | 72 [70.6%] | 31 [64.6%] | L=0.61 | 0.89 |
| Education level | 3.7 (3.4-4.0) |
3.4 (3.1-3.7) |
3.3 (3.1-3.5) |
3.3 (3.0-3.6) |
K=6.42 | 0.09 |
| Smoking status Never smoked, N [%] Current smoker, N [%] Ex-smoker, N [%] |
35 [64.8%] 9 [16.7%] 10 [18.5%] |
34 [70.8%] 6 [12.5%] 8 [16.7%] |
63 [61.8%] 18 [17.7%] 21 [20.6%] |
33 [68.8%] 5 [10.4%] 10 [20.8%] |
L=2.36 | 0.88 |
| HAM-D total score | 0.8 (0.5-1.1) |
4.0 (3.2-4.8) |
18.3 (17.5-19.0) |
20.5 (19.6-21.4) |
F=615.0 | <0.001 |
| Number of failed anti-depressant drug treatments (lifetime) | N/A | 1.1 (0.7-1.6) |
2.7 (2.4-3.0) |
1.8 (1.2-2.3) |
K=38.0 | <0.001 |
| BMI, kg/m2 | 25.5 (23.9-27.1) |
27.8 (26.2-29.5) |
27.6 (26.5-28.7) |
26.4 (24.7-28.0) |
K=4.85 | 0.18 |
| CRP, mg/L | 1.3 (0.9-1.6) |
2.1 (1.5-2.8) |
3.1 (2.1-4.2) |
2.50 (1.3-3.7) | K=10.72 | 0.01 |
| CRP > 3mg/L, N [%] | 4 [7.4%] | 11 [22.9%] | 26 [25.5%] | 14 [29.2%] | L=10.50 | 0.015 |
| log10 CRP | -0.1 (-0.2-0.0) |
0.1 (0.0-0.3) |
0.2 (0.1-0.3) |
0.0 (-0.1-0.2) |
F=3.57 | 0.015 |
| BMI-corrected CRP | -0.1 (-0.3 - 0.0) |
0.0 (-0.1 -0.1) |
0.1 (0.0 – 0.2) |
0.0 (-0.2 - 0.0) |
F=2.67 | 0.048 |
| Cohen’s d for case-control difference in BMI-corrected CRP | 0.29 | 0.47 | 0.18 | |||
| Estimated sample size (N; case and control) for 80% power to detect difference in BMI-corrected CRP | 188 | 73 | 486 | |||
F = ANOVA, L = Likelihood ratio, K = Kruskal-Wallis.
The majority of treatment-resistant patients were taking selective serotonin reuptake inhibitors (SSRIs; 70%) with smaller numbers exposed to noradrenergic and specific serotonergic reuptake inhibitors (NSRIs; 15%), mixed reuptake inhibitors (25%), tricyclic antidepressants (4%), mood stabilizers (4%) and dopamine receptor antagonists (3%). Treatment-responsive patients were likewise predominantly treated with SSRIs (85%), followed by mixed reuptake inhibitors (25%), NSRIs (11%), or tricyclic antidepressants (4%). All treatment-resistant patients were taking at least one conventional anti-depressant monoaminergic drug.
C-reactive protein
Mean hs-CRP concentrations (and 95% confidence intervals) are shown in Table 1. Mean CRP was significantly increased in all MDD cases compared to healthy controls (Wilcoxon Z = 2.7, P = 0.007). Both treatment-resistant and treatment-responsive groups had significantly higher mean hs-CRP than controls (Wilcoxon Z = 2.9, P = 0.004 and Wilcoxon Z = 2.6, P = 0.010, respectively). Across all MDD patients, drug treatment class did not significantly affect CRP levels (F = 0.799, P = 0.572), nor did the number of failed treatments in the past (F = 0.245, P = 0.621).
The proportion of participants with hs-CRP levels exceeding the conventional threshold value of 3mg/L was also significantly different between the pooled MDD groups and controls (likelihood ratio chi-square (LR) = 10.01, P = 0.002). Treatment-resistant, untreated, and treatment-responsive MDD groups all had significantly increased proportions of participants with hs-CRP > 3 mg/L compared to healthy volunteers (LR = 8.4, P = 0.004; LR = 8.6, P = 0.003; and LR = 5.0, P = 0.025, respectively). No other post hoc test was statistically significant; i.e. depressed groups did not differ significantly from each other (all P > 0.09).
Log-transformed and BMI-corrected C-reactive protein
The distributions of hs-CRP were positively skewed (moment skewness = 5.08) (41) and therefore were normalized by base 10 log transform; see Figure 1. Log10 CRP was significantly increased in all MDD cases compared to controls (t = 2.81, df = 250, P = 0.004). Only the treatment-resistant and treatment-responsive cases had significantly higher log10 CRP than controls (t = 3.07, df = 248, P = 0.002 and t = 2.32, df = 248, P = 0.021, respectively).
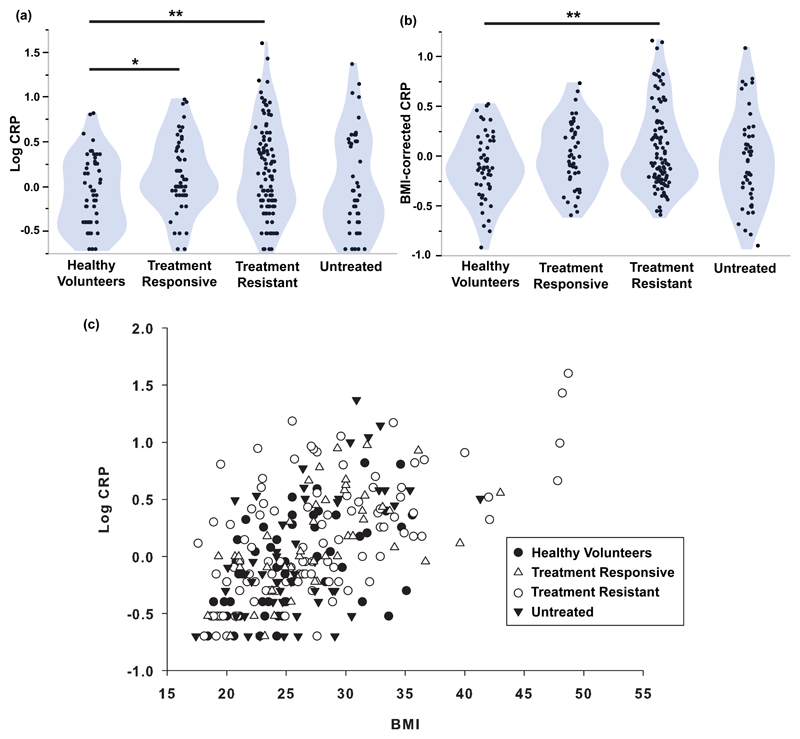
Figure 1. High sensitivity CRP and its relationship with BMI.
(a): Violin plots of log10 CRP for each of three subgroups of MDD patients and healthy volunteers. (b): Violin plots of BMI-corrected CRP (log10 CRP regressed on log10 BMI) for each of three subgroups of MDD patients and healthy volunteers. (c): Scatterplot of BMI versus log10 CRP (Spearman’s rho = + 0.57, P < 0.001), with points coded by sample group. The main effects of group were significant for (a) and (b) (ANOVA, F = 3.57, P = 0.015; F = 2.67, P = 0.048). * P < 0.05, ** P < 0.01 significant pair-wise difference between groups by post hoc t-tests.
As anticipated by prior studies (42–44), there was a significant positive correlation between BMI and log10 CRP across all study participants (Spearman’s rho = 0.56, df = 250, P < 0.001; Figure 1). Since BMI data were also positively skewed (moment skewness = 1.03) (41), we regressed log10 CRP on log10 BMI and used the residuals as estimates of BMI-corrected CRP (Figure 1). BMI-corrected CRP was significantly elevated in all MDD cases compared to controls (t = 2.24, df = 238, P = 0.026). Post hoc t-tests indicated that only the treatment-resistant cases had significantly higher mean BMI-corrected CRP than the controls (t = 2.71, df = 236, P = 0.007; Cohen’s d = 0.47).
To assess the possible confounding effect of symptom severity, we identified the subgroup of treatment-resistant patients (N=48) that had total HAM-D > 17, thereby corresponding to the cut-off used to define the untreated group. We confirmed that BMI-corrected CRP was abnormally increased in treatment-resistant patients with HAM-D > 17 (t = 3.0, P = 0.004) with a case-control difference of similar size (Cohen’s d = 0.43) to that of treatment-resistant patients with HAM-D > 13.
Partial least squares analysis of the relationship between CRP and clinical variables
Thirteen (out of 139) clinical phenotypes passed criterion for an important effect on CRP levels. Iterative cross-validation of the PLS model including only these important variables yielded an optimal two-factor solution (Figure 2 and S2), which accounted in total for 34.7% of variation in clinical measures (X), and for 36.1% of variation in CRP (Y). This differed significantly from the proportions of variance expected under the null hypothesis (% Var (X) =12.3%, 95% CI 12.1-12.5%; % Var (Y) = 2.7%, 95% CI 1.9-3.0%).
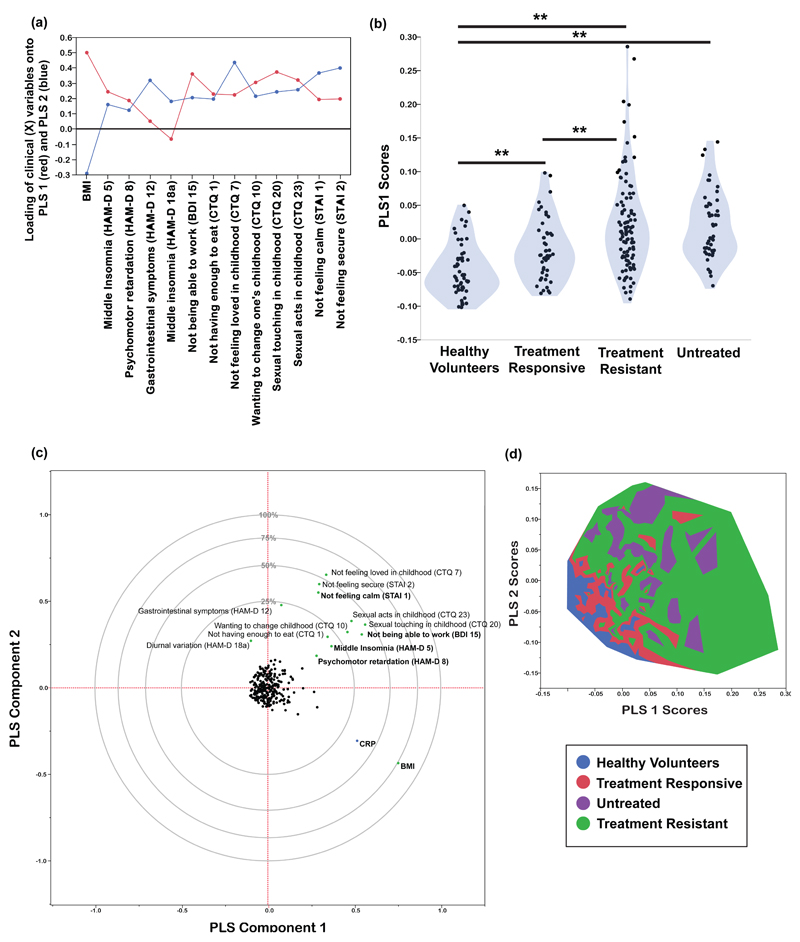
Figure 2. Partial least squares analysis of the relationships between hs-CRP and clinical phenotypes.
(a): Loadings of clinical (X) variables onto the first two PLS components. These 13 variables passed the first criterion for an important effect on CRP variance. (b): Violin plot of PLS1 X scores for each of three subgroups of MDD patients and healthy volunteers. ** P < 0.01 significant pair-wise difference between groups by post hoc t-tests. (c): Plot of the clinical (X) and CRP (Y) variables in the space of the first two PLS components. The clinical variables named in bold font passed both criteria for an important effect on CRP; the variables named in normal font passed the first criterion but not the more conservative second criterion. Panel (d): Contour plot for distribution of study participants in the space of the first two PLS components, color-coded by group, confirming that patients with treatment-resistant depression had high scores on the clinical syndrome of variables represented by PLS1.
The first PLS component (PLS1) accounted for 26.7% of the variation in hs-CRP. Positive scores on PLS1 indicated higher CRP. The clinical phenotypes that were significantly weighted on PLS1 were higher BMI, not feeling loved in childhood (CTQ item 7), not feeling calm (STAI item 1), wanting to change one’s family in childhood (CTQ item 10), psychomotor retardation (HAM-D item 8), middle insomnia (HAM-D item 5) and not being able to work (BDI item 15). The PLS1 scores for individual participants differed significantly between groups (F = 19.88, df = 3,248, P < 0.001; Figure 2). PLS1 scores were highest in the treatment-resistant group, followed by the untreated group, the treatment-responsive group and the healthy volunteers.
The second PLS component (PLS2) orthogonally explained 9.4% of variation in CRP. PLS scores in each group, and variables loading significantly onto PLS2, are summarised in Figure S3. Positive scores on PLS 2 indicated lower CRP. PLS2 scores differed significantly between groups (F = 24.34, df = 3,248, P < 0.001). Untreated patients had the highest PLS2 scores, followed by treatment-resistant patients, treatment-responsive patients and healthy volunteers.
Discussion
This is the first study to measure peripheral C-reactive protein using the same high sensitivity assay across a large sample of MDD patients (N=198) prospectively stratified in terms of their current and past history of treatment with monoaminergic antidepressant drugs. We replicated the well-established finding that CRP is significantly increased “on average” in MDD patients, screened for physical comorbidity, and compared to healthy volunteers who did not differ in terms of age, sex, BMI and cigarette smoking status. However, we also found some evidence for our primary hypothesis that CRP was most abnormally increased in the subgroup of patients with treatment-resistant depression (N=102). The standardized size of the case-control difference in CRP between healthy volunteers and treatment-resistant cases (Cohen’s d = 0.47) was higher than the case-control difference for treatment-responsive cases (0.29), or untreated cases (0.18). Controlling for non-normality of the CRP distribution, and for the strong positive correlation between CRP and obesity, we found that the case-control difference in CRP remained significant only for the subgroup of treatment-resistant cases. These results of planned analysis are consistent with the hypothesis that peripheral inflammation is a marker or risk factor for treatment-resistant depression. It should be noted that CRP was somewhat elevated even in treatment responsive cases, suggesting that a degree of elevated CRP could be trait related rather than related to current symptom or treatment status.
Taking a convergent but more exploratory approach to the data, we used multivariate analysis to identify two dimensions of clinical heterogeneity that were predictive of CRP. We found that a subset of clinically measured phenotypes explained ~36% of the variance in CRP. High BMI, high scores on vegetative symptoms of depression, low scores on calmness, and a history of childhood adversity, were all predictive of increased CRP. As expected from the results of our primary analysis, we confirmed that the group of patients defined a priori in terms of treatment resistance had the highest scores on this clinical profile associated with high CRP.
Treatment-resistant depression and peripheral inflammation
Monoamine reuptake inhibitors and related drugs are evidence-based pharmacological treatments for MDD; but response failure afflicts approximately 30% of patients (45, 46). Because of the global burden of disability attributable to MDD (47), the search for improved understanding of biomarkers of therapeutic resistance to current first-line treatment options is pressing (1, 48). Our results provide fresh evidence that patients with treatment-resistant depression have the most abnormally increased CRP levels compared both to treatment-responsive and currently untreated patients. To our knowledge, a specific relationship between CRP and monoaminergic antidepressant drug treatment resistance has not been demonstrated previously, although there is evidence both for increased pro-inflammatory cytokine concentrations (3, 14, 49) and for increased peripheral expression of cytokine related genes (12) in treatment-resistant depression. There is also some evidence that baseline inflammatory markers may be useful predictors of treatment response in MDD (50, 51). In a rat model of treatment-resistant depression, elevated CRP at baseline differentiated responders from non-responders to ketamine, an NMDA receptor antagonist with anti-inflammatory and anti-depressant effects (52). At a cellular level, neurons, microglia and macrophages respond to inflammatory challenges by activating metabolic pathways that reduce the synaptic availability of serotonin and catalyse the conversion of tryptophan to kynurenine and its putatively neurotoxic, glutamatergic agonist metabolites (15, 53–55). These effects of inflammation on serotonin transport and tryptophan metabolism may constitute a mechanism by which peripheral inflammation is associated with lack of therapeutic response to SSRIs (56).
Clinical phenotypes predictive of increased CRP in depression
Obesity and its cardiovascular sequelae have been repeatedly associated with increased CRP (e.g. 43, 57, 58). In the current study, which excluded patients with a lifetime history of medical disorders including atherosclerosis and diabetes, we confirmed that higher BMI was strongly associated with higher CRP levels. One mechanistic explanation is that macrophages constitute up to 60% of cells in adipose tissue and can release large amounts of IL-6, which is a key driver of CRP synthesis (59). So it is not surprising that inflammation (CRP) and obesity (BMI) were related herein; however, we do not consider that this association trivially accounts for increased CRP in treatment-resistant depression. The groups did not differ significantly in baseline BMI and the case-control difference remained significant for the treatment-resistant patients even after statistical regression to control for individual differences in BMI.
Of all the depressive symptoms measured, so-called vegetative symptoms (psychomotor retardation, insomnia, difficulty getting started / difficulty working) were more important in explaining higher CRP. These findings are consistent with prior reports that somatic but not cognitive symptoms of depression were associated with increased CRP (60). Vegetative symptoms of depression are akin to the illness or sickness behavior that has been repeatedly demonstrated in animal models and experimental medicine studies of humans exposed to acute pro-inflammatory challenge (61) (2). We also found evidence that state anxiety was related with CRP, which is compatible with prior data linking acute endotoxin exposure to anxious and depressive states in healthy volunteers (62).
It is established that childhood trauma increases risk of later mental health disorders, including depression (63). In a meta-analysis, individuals exposed to childhood trauma had significantly elevated levels of CRP in adulthood, albeit with a small effect size (Fisher’s Z = 0.10) (64). In a longitudinal study of female adolescents at risk of depression, childhood adversity was found to promote subsequent clustering of depression and inflammation (65). These results are compatible with our findings that feeling unloved in childhood and wanting to change one’s family in childhood were significantly correlated with higher CRP in adults.
Methodological issues
Due to the case-control design, between-group differences in CRP could theoretically be confounded by other factors influencing peripheral inflammation. However, we excluded patients with inflammatory disorders or anti-inflammatory drug treatment; and the groups did not differ on demographic characteristics. The lack of statistically significant case-control differences in BMI-corrected CRP for the comparisons between healthy volunteers and the treatment-responsive and untreated MDD groups could theoretically reflect the smaller sizes of these groups compared to the treatment-resistant MDD group. However, power calculations indicated that the case-control differences in BMI-corrected CRP would probably not have been significant even if the treatment-responsive group had the same size as the resistant group (Table 1). While the untreated MDD group had not received antidepressant treatment for at least six weeks, the majority (N=28, 58.3%) had received at least one such treatment in the past (the average number of historically failed treatments in this group was 1.8). As such, this group had some degree of heterogeneity, including both treatment-naïve individuals and those who would have been expected to be treatment-resistant if currently medicated. Treatment resistance was defined by inadequate response to the current drug treatment whereas some other criteria for treatment resistance stipulate failed response to at least two drugs of different mechanisms of action. There are multiple definitions of ‘treatment-resistance’ used in the field; our choice is widely used. The study was not planned or powered to test differences in CRP between subgroups defined by dose or type of current anti-depressant medication. We did not find that CRP differed significantly as a function of anti-depressant medication class, nor as a function of total number of previous failed treatments (as measured by the ATRQ). A limitation in assessing prior medications was the use of retrospective self-report, albeit based on a comprehensive structured instrument completed by an interviewer. The sample was recruited from the UK population, which is known to differ from the US and other populations in terms of BMI and other factors that can influence the numerical distribution of CRP and this may mitigate generalizability of our results, as would our exclusion of people with inflammatory disorders. Finally, CRP is only one of many markers of peripheral inflammation that have been, or could be, linked to depression. Although these data demonstrate that CRP is robustly associated with treatment-resistant depression, we do not claim that CRP is necessarily the best of all possible peripheral blood biomarkers of treatment-resistant depression.
Conclusions
Major depressive disorder is associated with increased CRP compared to healthy volunteers and the case-control difference appears higher in treatment-resistant depression. Increased CRP and treatment resistance were also associated with other aspects of clinical heterogeneity in depression including obesity, vegetative symptoms of fatigue and sleep disturbance, state anxiety, and a history of childhood adversity. We suggest there may be a clinically and immunologically diagnosable sub-syndrome of “inflamed depression” comprising the MDD patients most likely to benefit therapeutically from second-line treatment with anti-inflammatory drugs (36).
Supplementary Material
Acknowledgements
The authors would like to thank all study participants, research teams, and laboratory staff, without whom this research would not have been possible.
Members of the NIMA Consortium are thanked and acknowledged:
Cambridge
Edward T. Bullmore (PI, EC)1,2,11, Junaid Bhatti1, Samuel R. Chamberlain1,2, Marta M. Correia1,12, Amber Dickinson*, Andy Foster2, Manfred Kitzbichler1, Clare Knight2, Mary-Ellen Lynall1, Christina Maurice1, Howard Mount13, Ciara O'Donnell1, Linda J. Pointon1, Peter St George Hyslop1,13,14, Lorinda Turner1, Barry Widmer1, Guy B. Williams1,14
Cardiff
B. Paul Morgan (PI)15, Claire Leckey15, Angharad Morgan15, Caroline O'Hagan*, Samuel Touchard15
Glasgow
Jonathan Cavanagh (PI, EC)3, Catherine Deith*, John McClean16, Alison McColl3, Andrew McPherson*, Paul Scouller*, Murray Sutherland16
Independent advisor
H.W.G.M. (Erik) Boddeke (EC)17
GSK
Jill Richardson (EC)18, Shahid Khan11, Phil Murphy19, Christine Parker19, Jai Patel11
Janssen
Declan Jones (EC)6, Peter de Boer4, John Kemp4, Paul Acton6, Wayne C. Drevets6, Jeffrey S. Nye (deceased), Gayle Wittenberg6, John Isaac6, Anindya Bhattacharya6, Nick Carruthers6, Hartmuth Kolb6
Kings College London
Carmine Pariante (PI)10, Gareth Barker20, Heidi Byrom10, Diana Cash20, Antony Gee20, Caitlin Hastings10, Nicole Mariani10, Anna McLaughlin10, Valeria Mondelli10, Maria Nettis10, Naghmeh Nikkheslat10, Karen Randall20, Hannah Sheridan*, Camilla Simmons20, Nisha Singh20, Federico Turkheimer20, Victoria Van Loo*, Marta Vicente Rodriguez20, Tobias Wood20, Courtney Worrell*, Zuzanna Zajkowska*
Lundbeck
Niels Plath (EC)21, Jan Egebjerg21, Hans Eriksson21, Francois Gastambide21, Karen Husted Adams21, Ross Jeggo21, Christian Thomsen21, Jason O'Connor22, Jan Torleif Pederson21, Brian Campbell*, Thomas Möller*, Bob Nelson*, Stevin Zorn*
Oxford
Mary Jane Attenburrow (PI)7,23, Alison Baird, Jithen Benjamin23, Stuart Clare25, Philip Cowen7, I-Shu (Dante) Huang24, Samuel Hurley*, Helen Jones23, Simon Lovestone7, Francesca Mada23, Alejo Nevado-Holgado7, Akintayo Oladejo*, Elena Ribe7, Anviti Vyas*
Pfizer
Zoe Hughes (EC)26, Rita Balice-Gordon*, Brendon Binneman26, James Duerr26, Terence Fullerton26, Justin Piro26, Tarek Samad26, Jonathan Sporn26
Southampton
Hugh Perry (PI)27, Madeleine Cleal*, Gemma Fryatt27, Diego Gomez-Nicola27, Renzo Mancuso27
Sussex
Neil Harrison (PI, EC)28, Mara Cercignani28, Charlotte Clarke28, Elizabeth Hoskins29, Charmaine Kohn29, Rosemary Murray*, Dominika Wlazly30
PI = Principal Investigator
EC = Executive committee member
1 Department of Psychiatry, School of Clinical Medicine, University of Cambridge, CB2 0SZ, UK
2 Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, CB21 5EF, UK
3 Sackler Centre, Institute of Health & Wellbeing, University of Glasgow, Sir Graeme Davies Building, Glasgow, G12 8TA, UK
4 Neuroscience, Janssen Research & Development, Janssen Pharmaceutica NV, Turnhoutseweg 30, B-2340, Beerse, Belgium
5 The Maurice Wohl Clinical Neuroscience Institute, Cutcombe Road, London, SE5 9RT, UK
6 Neuroscience, Janssen Research & Development, LLC, Titusville, NJ, 08560, USA
7 University Department of Psychiatry, Warneford Hospital, Oxford, OX3 7JX, UK
8 Brighton & Sussex Medical School, University of Sussex, Brighton, BN1 9RR, UK
9 Sussex Partnership NHS Foundation Trust, Swandean, BN13 3EP, UK
10 Stress, Psychiatry and Immunology Lab & Perinatal Psychiatry, Maurice Wohl Clinical Neuroscience Institute, Kings College, London, SE5 9RT, UK
11 Immuno-Psychiatry, Immuno-Inflammation Therapeutic Area Unit, GlaxoSmithKline R&D, Stevenage SG1 2NY, UK
12 MRC Cognition and Brain Sciences Unit, 15 Chaucer Road, Cambridge CB2 7EF, UK
13 Tanz Centre for Research in Neurodegenerative Diseases, 60 Leonard Avenue, Toronto, ON M5T 2S8 Canada
14 Department of Clinical Neurosciences, University of Cambridge, CB2 0SZ, UK
15 University of Cardiff, Cardiff CF10 3AT, UK
16 NHS Greater Glasgow and Clyde, 1055 Great Western Rd, Glasgow G12 0XH, UK
17 University of Groningen, 9712 CP Groningen, Netherlands
18 Neurosciences Virtual PoC DPU, GlaxoSmithKline R&D, Stevenage SG1 2NY, UK
19 Experimental Medicine Imaging, GlaxoSmithKline R&D, Stevenage SG1 2NY, UK
20 Centre for Neuroimaging Sciences, Denmark Hill, London SE5 9AF, UK
21 H. Lundbeck A/S Ottiliavej 9, 2500, Valby, Denmark
22 University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr, San Antonio, TX 78229, USA
23 NIHR Oxford cognitive health Clinical Research Facility, Warneford Hospital, Oxford, OX3 7JX, UK
24 The Kennedy Institute of Rheumatology, Roosevelt Dr, Oxford OX3 7FY, UK
25 Oxford Centre for Functional MRI of the Brain, John Radcliffe Hospital, Oxford OX3 9DU, UK
26 Pfizer, Inc, 1 Portland Street, Cambridge MA, USA
27 Centre for Biological Sciences, University of Southampton, Southampton, UK
28 Clinical Imaging Sciences Centre (CISC), University of Sussex, Brighton, BN1 9RR, UK
29 Sussex Partnership NHS Foundation Trust, Nevill Avenue, Hove BN3 7HZ, UK
30 Brighton & Sussex University Hospitals NHS Trust, Brighton BN2 5BE, UK
*Former consortium members
Funding and Disclosure: This work was funded by a Wellcome Trust strategy award to the Neuroimmunology of Mood Disorders and Alzheimer’s Disease (NIMA) Consortium which is also funded by Janssen, GlaxoSmithKline, Lundbeck and Pfizer. Recruitment of patients was supported by the National Institute of Health Research (NIHR) Clinical Research Network: Kent, Surrey and Sussex & Eastern. SRC consults for Cambridge Cognition and Shire; and his input in this project was funded by a Wellcome Trust Clinical Fellowship (110049/Z/15/Z). ETB is employed half-time by the University of Cambridge and half-time by GlaxoSmithKline; he holds stock in GSK. In the last three years PJC has served on an advisory board for Lundbeck. NAH consults for GSK. PdB, DJ and WCD are employees of Janssen Research & Development, LLC., of Johnson & Johnson, and hold stock in Johnson & Johnson. The other authors report no financial disclosures or potential conflicts of interest.
References
- 1.Bhattacharya A, Derecki NC, Lovenberg TW, Drevets WC. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology (Berl) 2016;233(9):1623–36. doi: 10.1007/s00213-016-4214-0. [DOI] [PubMed] [Google Scholar]
- 2.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol. 2015;25(10):1532–43. doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. 1995;34(4):301–9. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- 5.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frasure-Smith N, Lesperance F, Irwin MR, Sauve C, Lesperance J, Theroux P. Depression, C-reactive protein and two-year major adverse cardiac events in men after acute coronary syndromes. Biol Psychiatry. 2007;62(4):302–8. doi: 10.1016/j.biopsych.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 7.Kinney DK, Tanaka M. An evolutionary hypothesis of depression and its symptoms, adaptive value, and risk factors. J Nerv Ment Dis. 2009;197(8):561–7. doi: 10.1097/NMD.0b013e3181b05fa8. [DOI] [PubMed] [Google Scholar]
- 8.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–15. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young JJ, Silber T, Bruno D, Galatzer-Levy IR, Pomara N, Marmar CR. Is there Progress? An Overview of Selecting Biomarker Candidates for Major Depressive Disorder. Front Psychiatry. 2016;7:72. doi: 10.3389/fpsyt.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domenici E, Wille DR, Tozzi F, Prokopenko I, Miller S, McKeown A, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One. 2010;5(2):e9166. doi: 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009–14. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 12.Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology. 2013;38(3):377–85. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattaneo A, Ferrari C, Uher R, Bocchio-Chiavetto L, Riva MA, Consortium MRCI et al. Absolute Measurements of Macrophage Migration Inhibitory Factor and Interleukin-1-beta mRNA Levels Accurately Predict Treatment Response in Depressed Patients. Int J Neuropsychopharmacol. 2016;19(10) doi: 10.1093/ijnp/pyw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen DG, Caniato RN, Verster JC, Baune BT. A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum Psychopharmacol. 2010;25(3):201–15. doi: 10.1002/hup.1103. [DOI] [PubMed] [Google Scholar]
- 15.Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S, et al. Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickman RJ, Khambaty T, Stewart JC. C-reactive protein is elevated in atypical but not nonatypical depression: data from the National Health and Nutrition Examination survey (NHANES) 1999-2004. J Behav Med. 2014;37(4):621–9. doi: 10.1007/s10865-013-9510-0. [DOI] [PubMed] [Google Scholar]
- 17.Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72(1):34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry. 2012;71(1):15–21. doi: 10.1016/j.biopsych.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liukkonen T, Rasanen P, Jokelainen J, Leinonen M, Jarvelin MR, Meyer-Rochow VB, et al. The association between anxiety and C-reactive protein (CRP) levels: results from the Northern Finland 1966 birth cohort study. Eur Psychiatry. 2011;26(6):363–9. doi: 10.1016/j.eurpsy.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Abdi H, Williams LJ. Partial least squares methods: partial least squares correlation and partial least square regression. Methods Mol Biol. 2013;930:549–79. doi: 10.1007/978-1-62703-059-5_23. [DOI] [PubMed] [Google Scholar]
- 21.Wold H. Estmation of principal components and related models by iterative least squares. New York: Academic Press; 1966. [Google Scholar]
- 22.Wold S. Personal memories of the early PLS development. Chemometrcs Intell Lab Syst. 2001;58:83–4. [Google Scholar]
- 23.Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del Campo N, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130(Pt 12):3223–36. doi: 10.1093/brain/awm205. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association (APA) 2012 www.dsm5.org.
- 25.Spitzer MB, Miriam G, Williams JB. Structured Clinical Interview for DSM-IV Axis I disorders, clinician version (SCID) Washington, D.C.: American Psychiatric Press, Inc.; 1996. [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desseilles M, Witte J, Chang TE, Iovieno N, Dording CM, Ashih H, et al. Assessing the adequacy of past antidepressant trials: a clinician's guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72(8):1152–4. doi: 10.4088/JCP.11ac07225. [DOI] [PubMed] [Google Scholar]
- 28.Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(2):156–65. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- 29.Katona CL, Robertson MM, Abou-Saleh MT, Nairac BL, Edwards DR, Lock T, et al. Placebo-controlled trial of lithium augmentation of fluoxetine and lofepramine. Int Clin Psychopharmacol. 1993;8(4):323. doi: 10.1097/00004850-199300840-00020. [DOI] [PubMed] [Google Scholar]
- 30.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory - II. San Antonio, Texas: Psychological Corporation; 1996. [Google Scholar]
- 31.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 32.Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–53. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 33.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 35.Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol. 2004;29(8):439–93. [PubMed] [Google Scholar]
- 36.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.JMP Pro ® Version 13.0. Cary, North Carolina, USA: SAS Institute Inc.; 2017. [Google Scholar]
- 38.Garthwaite PH. An Interpretation of Partial Least Squares. Journal of the American Statistical Association. 1994;89(425):122–7. [Google Scholar]
- 39.Höskuldsson A. PLS regression methods. Journal of Chemometrics. 1988;2(3):211–28. [Google Scholar]
- 40.Cox I, Gaudard M. Discovering Partial Least Squares with JMP. Cary, North Carolina, USA: SAS Institute Inc.; 2013. [Google Scholar]
- 41.Terpening WD. Statistical analysis for business using JMP: A Student's Guide. Cary, North Carolina, USA: SAS Institute Inc.; 2011. [Google Scholar]
- 42.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Lv G. Correlation of C-reactive protein level and obesity in Chinese adults and children: a meta-analysis. J Endocrinol Invest. 2013;36(8):642–7. doi: 10.3275/9004. [DOI] [PubMed] [Google Scholar]
- 44.Janka Z. [Serotonin dysfunctions in the background of the seven deadly sins] Ideggyogyaszati szemle. 2003;56(11–12):376–85. [PubMed] [Google Scholar]
- 45.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schosser A, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, et al. European Group for the Study of Resistant Depression (GSRD) - Where have we gone so far: Review of clinical and genetic findings. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat. 2011;7(Suppl 1):3–7. doi: 10.2147/NDT.S19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharya A, Drevets WC. Role of Neuro-Immunological Factors in the Pathophysiology of Mood Disorders: Implications for Novel Therapeutics for Treatment Resistant Depression. Curr Top Behav Neurosci. 2017;31:339–56. doi: 10.1007/7854_2016_43. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho LA, Torre JP, Papadopoulos AS, Poon L, Juruena MF, Markopoulou K, et al. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord. 2013;148(1):136–40. doi: 10.1016/j.jad.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 50.Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology. 2017;78:105–13. doi: 10.1016/j.psyneuen.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. 2014;171(12):1278–86. doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]
- 52.Walker AJ, Foley BM, Sutor SL, McGillivray JA, Frye MA, Tye SJ. Peripheral proinflammatory markers associated with ketamine response in a preclinical model of antidepressant-resistance. Behav Brain Res. 2015;293:198–202. doi: 10.1016/j.bbr.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 53.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10(6):538–44. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 54.Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6(11):e946. doi: 10.1038/tp.2016.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Postal M, Appenzeller S. The importance of cytokines and autoantibodies in depression. Autoimmun Rev. 2015;14(1):30–5. doi: 10.1016/j.autrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137–62. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 58.Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, et al. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry. 2008;64(10):896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 60.Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA) Psychoneuroendocrinology. 2013;38(9):1573–85. doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26(5):643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 62.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58(5):445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 63.Sachs-Ericsson N, Kendall-Tackett K, Hernandez A. Childhood abuse, chronic pain, and depression in the National Comorbidity Survey. Child Abuse Negl. 2007;31(5):531–47. doi: 10.1016/j.chiabu.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry. 2016;21(5):642–9. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, et al. Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Compr Psychiatry. 2013;54(7):953–61. doi: 10.1016/j.comppsych.2013.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.