Abstract
Rationale
One measure of protein glycosylation (GlycA) has been reported to predict higher cardiovascular risk by reflecting inflammatory pathways
Objective
To assess the role of a comprehensive panel of immunoglobulin (IgG) glycosylation traits on traditional risk factors for cardiovascular disease and on presence of subclinical atherosclerosis in addition to GlycA.
Methods and Results
We measured 76 IgG glycosylation traits in 2970 women (age range 40-79 years) from the TwinsUK cohort and correlated it to their estimated 10-year atherosclerotic cardiovascular disease (ASCVD) risk score and their carotid and femoral plaque measured by ultrasound imaging. Eight IgG glycan traits are associated with the 10-year ASCVD risk score after adjusting for multiple tests and for individual risk factors – 5 with increased risk and 3 with decreased risk. These glycans replicated in 967 women from ORCADES cohort, six of them were also associated in 845 men. A linear combination of IgG glycans and GlycA is also associated with presence of carotid (OR[95%CI]=1.55 [1.25;1.93], P=7.5X10-5) and femoral (OR[95%CI]==1.32[1.06;1.64], P=0.01) plaque in a subset of women with atherosclerosis data after adjustment for traditional risk factors. One specific glycosylation trait, GP18 was negatively correlated with VLDL and triglyceride levels in serum and with presence of carotid plaque (OR[95%CI] = 0.60[0.50;0.71], P = 5x10-4).
Conclusions
We find molecular pathways linking IgG to arterial lesion formation. Glycosylation traits are independently associated with subclinical atherosclerosis. One specific trait related to the sialylated N-glycan is negatively correlated with CVD risk, VLDL and triglyceride serum levels and presence of carotid plaque.
Keywords: IgG glycans, cardiovascular risk, atherosclerotic plaque, cardiovascular research, atherosclerosis
Subject Terms: Atherosclerosis, Biomarkers, Mechanisms, Proteomics, Risk Factors
Introduction
Cardiovascular diseases (CVDs) are the first cause of morbidity and mortality in Western countries1. In addition, the improvement of treatment and the reduction of case fatality are consistently increasing the prevalence of people who are at risk for recurring events and/or cardiac decompensation2. Many, often co-occurring, risk factors, have been identified and account for most of the CVD burden3 and different validated algorithms have been developed to estimate the individual risk of developing specific CVD events4–6.
The 10-year atherosclerotic cardiovascular disease (ASCVD)7 risk score is a gender and race specific single multivariable risk assessment tool used to estimate the 10-year CVD risk of an individual that has replaced clinically the Framingham-10 years cardiovascular risk score (FRS)8. It is based on the age, sex, ethnicity, total and HDL cholesterol, systolic blood pressure, smoking status, use of blood pressure lowering medications, and the presence of type 2 diabetes (T2D). Data on subclinical atherosclerosis, presence of atherosclerotic plaques in carotid and femoral arteries, used in combination with traditional risk factors, provides additional information about the presence of coronary lesion9 and the risk of myocardial infarction, stroke, and CVD mortality10–13. Glycosylation is the most abundant and diverse form of post-transcriptional modification which participates in every physiological process14. Protein glycosylation is driven by specific enzymes and the complex carbohydrates (glycans) attached to, for example, immunoglobulins, have a specific regulatory role and result in differences in immune function15, 16. An altered protein glycosylation pattern has been described as a significant event that occurs during the transition from healthy to diseased tissue14, 17. This type of protein glycosylation is related to disease development in many syndromes such as congenital disorders of glycosylation, cancer, inflammatory bowel diseases, renal disease, rheumatoid arthritis, chronic obstructive pulmonary disease and AIDS18. Some of the most important interactions between the immune system and pathogens are mediated by protein-glycan interactions, and it has been shown that alterations of the glycosylation of immunoglobulin G (IgG), the most abundant immunoglobulin in circulation, have direct impact on its inflammatory properties16. Different IgG glycosylation profiles may provide an at risk phenotype to the developing of CVD since inflammation is known to play a crucial rule in CVD development19. A study of 27941 participants of the Women's Health Study, has previously shown that GlycA, a biomarker of plasma protein glycan N-acetyl methyl groups (located on specific glycan branches of particular plasma proteins mainly α1acid glycoprotein, haptoglobin, α1antitrypsin, α1antichymotrypsin, and transferrin) is related to incident CVD17 which remained significant, when adjusting for traditional risk factors and for C-reactive protein levels17. GlycA, as a measure of protein glycosylation, has also been found to correlate with longitudinal risk of CVD, and mortality in various cohort studies (reviewed in 20). However, besides GlycA, a large number of protein glycosylation traits can be measured21, 22. We hypothesized that these traits may reveal important information on the relationship between protein glycosylation, traditional risk factors and subclinical atherosclerosis.
The aim of this study is to investigate the role of 76 IgG glycosylation traits in the risk of CVD measured with the 10-year ASCVD risk score7 by analyzing the IgG glycome composition in a large population based female cohort from the UK (TwinsUK). We then replicated the significant results in an independent sample from the ORCADES cohort. Finally, we investigate the association between the replicated glycan traits associated with CVD risk and presence of carotid and femoral atherosclerotic plaques in a subset of female individuals from the TwinsUK cohort.
Methods
The TwinsUK data that support the findings of this study are publicly available upon request on the department website (http://www.twinsuk.ac.uk/data-access/accessmanagement/). ITo access the ORCADES data, please email jim.wilson@ed.ac.uk.
Discovery cohort
Study subjects were individuals enrolled in the TwinsUK registry, a national register of adult twins23. In this study we analyzed data from 2970 females, 40 to 79 years old and without CVD. They had glycomics data available and the 10-year ASCVD risk score. The study was approved by St. Thomas’ Hospital Research Ethics Committee, and all twins provided informed written consent.
Replication cohort
The replication sample was drawn from the Orkney Complex Disease Study (ORCADES). ORCADES is a family-based, cross-sectional study that seeks to identify genetic factors influencing cardiovascular and other disease risk in the isolated archipelago of the Orkney Isles in northern Scotland24. 2078 participants aged 16-100 years were recruited between 2005 and 2011, all of them having at least two Orcadian grandparents. Fasting blood samples were collected and many health-related phenotypes and environmental exposures were measured in each individual. Here we included 967 females with glycomics data available and the 10-year ASCVD risk score. All participants gave written informed consent and the study was approved by Research Ethics Committees in Orkney and Aberdeen.
In addition to the replication carried out in women, we further validated our results in 189 men from TwinsUK and 656 men from ORCADES.
Phenotype definitions
Data relevant to the present study include, BMI (body weight in kilograms divided by the square of height in square meters), T2D (defined as fasting glucose ≥7 mmol/L or physician’s letter confirming diagnosis), smoking (defined as current smoker, non smoker), treated and untreated systolic blood pressure (SBP), total and HDL cholesterol and insulin. Fasting insulin levels were measured using the same methods as previously described25. The homeostasis model assessment-estimated insulin resistance (HOMA-IR) was calculated multiplying overnight fasting plasma insulin (FPI) by overnight fasting plasma glucose (FPG), then dividing by the constant 22.5, i.e. HOMA-IR = (FPI×FPG)/22.515. The ASCVD risk score is an algorithm used to estimate the 10-year cardiovascular risk of an individual using the individual’s gender, ethnicity, age, smoking status, cholesterol levels, blood pressure and diabetes status7. The individual risk of CVD was estimated using the 10-year ASCVD risk score7.
Femoral and carotid plaque
Left and right carotid and femoral arteries were visualized with B-mode ultrasound (Siemens CV70, Siemens, Erlangen, Germany, with 13-MHz vascular probe) as previously described26. Briefly, arterial walls were examined for plaque in the common carotids, carotid bifurcations, origins of the internal and external carotid arteries, common femoral arteries, femoral bifurcations, and the origins of the superficial and deep femoral arteries. Plaque was defined in the longitudinal view as focal widening and protrusion into the lumen of ≥1.5-mm thickness relative to neighboring areas and confirmed in transverse view and it was graded according to echogenicity.
Analysis of IgG glycans
IgG glycans were measured by Genos Ltd as previously described27, 28. Briefly, the IgG was isolated using protein G monolithic plates (BIA Separations, Ajdovščina, Slovenia). Dried IgG was denatured with 1.33 % SDS (w/v) and N-glycans were released by digestion with PNGase F (ProZyme, Hayward, CA). After deglycosylation, N-glycans were labelled with 2- aminobenzamide fluorescent dye.
Free label and reducing agent were removed from the samples using hydrophilic interaction chromatography–solid-phase extraction.
Fluorescently labelled N-glycans were separated by hydrophilic interaction chromatography on a Waters Acquity UPLC instrument (Waters, Milford, MA). Data processing was performed using an automatic processing method with a traditional integration algorithm after which each chromatogram was manually corrected to maintain the same intervals of integration for all the samples. The chromatograms were all separated in the same manner into 24 peaks and the amount of glycans in each peak was expressed as percentage of total integrated area. In addition to 24 directly measured glycan structures, 52 derived traits were calculated which is a maximal number of traits we were able to calculate. These derived traits average particular glycosylation features (galactosylation, fucosylation, bisecting N-acetylglucosamine (GlcNAc) and sialylation) (see Online Table I). The derived glycan traits are calculated from directly measured glycans and therefore their measurement error is smaller (at least the random error) 27.
Lipoprotein profiling and glycoprotein by Nuclear Magnetic Resonance
Gycoprotein (GlycA), lipoproteins and triglycerides were measured by Nightingale Health (previously known as Brainshake Ltd, Finland, https://www.brainshake.fi/) from fasting serum samples using 500Mhz proton nuclear magnetic resonance spectroscopy as previously described 29.
Statistical analysis
Statistical analysis was carried out using Stata version 12 and R version 3.3.3.
Glycans were global normalised and log transformed due to right-skewness of their distributions. In order to remove experimental biases, all measurements were adjusted for batch and run-day effects using ComBat (R-package sva). Derived glycan traits were calculated using normalized and batch-corrected glycan measurements (exponential of batch corrected measurements). All variables were centred and scaled to have standard deviation 1. Outliers (more than 6SD from the mean) were excluded from the analysis.
In the discovery cohort, association analyses between the 10-year ASCVD risk score and glycan traits were performed using linear mixed models adjusting for age BMI, and family relatedness as random effect. We used a conservative Bonferroni correction to account for multiple testing assuming 76 independent tests thus giving a significant threshold of (P<6.5x10-4=0.05/76). The Bonferroni significant 10-year ASCVD risk score glycan association were replicated in 967 females from the ORCADES study.
To adjust for kinship in the ORCADES cohort, the 10-year ASCVD risk score traits were set to their grammar+ residuals in GenABEL using the genomic relationship matrix and no other covariates. These residuals are suitable for analysis as an unrelated population30. These kinship adjusted 10-year ASCVD risk score traits were then taken forward using the same (fixed only) effect model as TwinsUK. We then combined the results using inverse variance fixed effect meta-analysis.
Linear mixed model adjusting for covariates and family relatedness were then undertaken in the TwinsUK sample to determine the association between the identified glycan traits with the contributing factors of the 10-year ASCVD risk score (ie. T2D, smoking, total and HDL cholesterol and SBP) as well as with HOMA.
We also looked at the association between the identified glycan traits with carotid and femoral plaque in a subset of 1382 female individuals from TwinsUK with plaque measured.
Finally, we created a glycan risk score in females from TwinsUK to assess the combined effects of all glycan traits identified. We fitted a logistic regression model for the significantly replicated glycans to a binary trait of high 10-year ASCVD risk score. For this we selected the top quintile (corresponding to 10-year ASCVD risk score >5.2%) taking the z-scores of all the significant IgG glycans using both linear and quadratic terms and using a stepwise regression approach to account for the collinearities between glycan traits. The proportion of the variance in the 10-year ASCVD risk score was then assessed in women from TwinsUK and in men and women from ORCADES. The GlycA measure was added to the glycans from the score and this IgG+GlycA was tested for association with carotid and femoral plaque adjusting for log 10-year ASCVD risk score score.
Results
Levels of 76 IgG glycans (24 directly measured and 52 derived traits) (Online Table I) were obtained in 2970 females from the TwinsUK sample and in 967 females from the ORCADES cohort with the ACC/AHA ASCVD risk score available (age range: 40-79 years). The demographic characteristics of the study populations are presented in Table 1. A flowchart of the study design is presented in Figure 1.
Table 1. Demographic characteristics of the study populations.
| Phenotype | TwinsUK Mean(SD) | ORCADES Mean(SD) |
|---|---|---|
| n | 2970 | 967 |
| Female % | 100% | 100% |
| Carotid plaque, yes:no | 336:1046 | NA |
| Femoral plaque, yes:no | 337:1036 | NA |
| Age | 57.41(8.71) | 53.7(15.11) |
| 10-year ASCVD Risk Score | 4.85(5.43) | 6.9(11.24) |
| BMI | 26.61(4.85) | 27.5(3.98) |
| DBP, mmHG | 77.63(9.79) | 72.67(9.23) |
| HDL Cholesterol, mmol/l | 1.56(0.43) | 1.62(0.43) |
| HOMA-IR | 1(0.74) | NA |
| SBP, mmHG | 126.02(15.83) | 125.12(19.52) |
| Current smokers, n(%) | 251(8.45%) | 66(6.812%) |
| Total Cholesterol, mmol/l | 5.63(1.20) | 5.47(1.16) |
| T2D, n(%) | 79 (2.66%) | 28(2.89) |
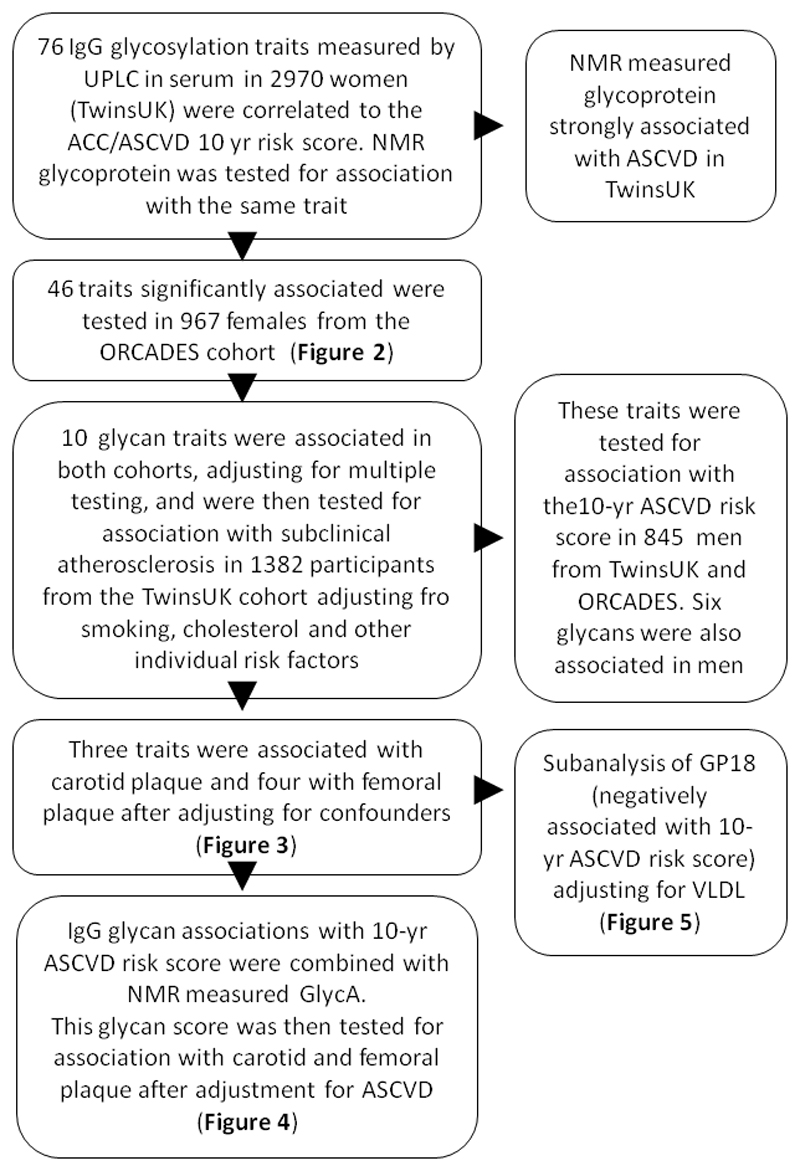
Figure 1.
Discovery: The role of glycan traits on cardiovascular risk estimates was tested on 3281 samples available. Having identified traits significantly associated with CVD risk, we replicated them first, in an independent cohort, validated them in men and then investigated whether any of these associations could be exclusively explained by any of the individual factors that constitute the ACC/AHA10-year ASCVD risk estimate. The traits that remained associated were then tested for association with presence of subclinical atherosclerosis adjusting for the potential confounders. A sub-analysis was performed for the IgG glycan GP18 which is strongly negatively correlated with VLDL.
Discovery and replication in women
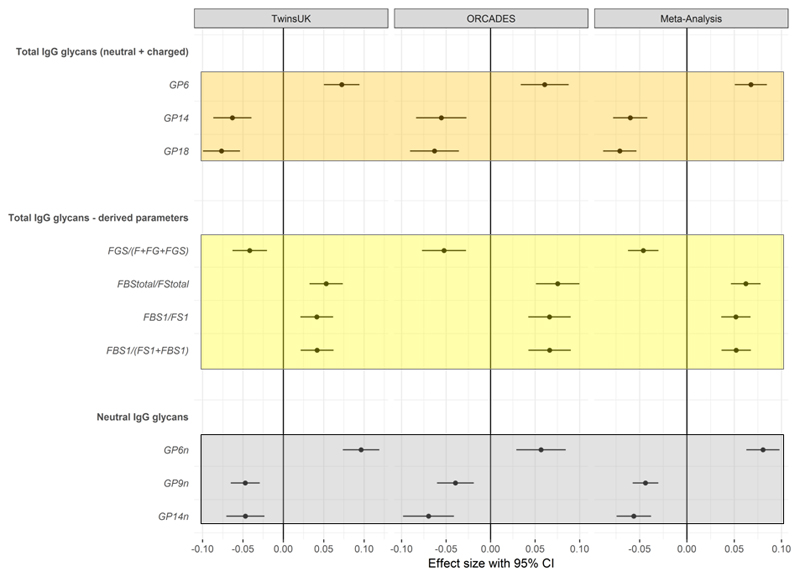
We first ran linear mixed models in the discovery sample adjusting for age, BMI and family relatedness. We controlled for multiple testing using Bonferroni correction (P<6.58x10-4 =0.05/76 glycan traits). This identified 46 glycan traits significantly associated with the 10-year ASCVD risk score; 25 glycan traits were positively associated with the 10-year ASCVD risk score, while 21 were negatively associated (Online Table I). We then assessed whether these associations with the 10-year ASCVD risk score were robust by testing for association these 46 glycans in 967 females from the ORCADES study. Out of those, 24 glycan traits were nominally associated with the 10-year ASCVD risk score (p<0.05) in the replication cohort and 10 glycans were significantly associated the 10-year ASCVD risk score after adjusting for covariates and multiple testing using Bonferroni correction (P<0.05/46). We then combined the results using inverse variance fixed effect meta-analysis. (Figure 2).
Figure 2.
Glycan traits significantly associated with ACC/AHA 10-year ASCVD risk score in the discovery, replication and meta-analysis. Analyses adjusted by age, sex, BMI, family relatedness and multiple testing.
Validation in men
We tested whether these results discovered in women and replicated in women were also associated in men. We find that 6 of the 10 glycans are also significantly associated in men when we meta-analyse IgG glycan data from ORCADES and TwinsUK (n=845. Online Table II, Online Figure I)
Adjustment for risk factors in women
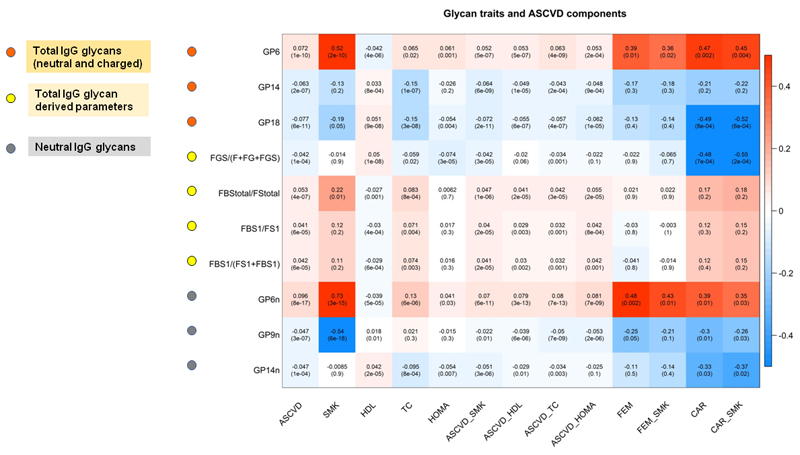
We investigated in TwinsUK women, the association of the 10 replicated glycan traits with HDL and total cholesterol, smoking, SBP, T2D and insulin resistance. While no associations were observed with T2D and SBP (Online Table III), at P<0.05, all the glycan traits were associated with HDL cholesterol, 9 were associated with total cholesterol and 5 were associated with smoking, and 5 were associated with insulin resistance (Figure 3, Online Table III). After adjusting for the contributing risk factors (Figure 3, Online Table IV) we find that 8 of the associations always remain statistically significant.
Figure 3.
Glycan traits and ACVD components. Each cell of the matrix contains the regression coefficient between one glycan trait and a component of the 10-year ASCVD risk score and the corresponding p value. The table is color coded by correlation according to the table legend (red for positive and blue for negative correlations). ASCVD =10 year ASCCVD risk score, SMK= smoking, HDL= HDL cholesterol, TC= total cholesterol, HOMA= insulin resistance, ASCVD _SMK = 10-year ASCVD risk score adjusted for covariates and smoking, ASCVD _HDL=10-year ASCVD risk score adjusted for covariates and HDL cholesterol, ASCVD_ TC = 10-year ASCVD risk score adjusted for covariates and total cholesterol, ASCVD_HOMA= 10-year ASCVD risk score adjusted for covariates and insulin resistance, FEM = femoral plaque, FEM _SMK =femoral plaque adjusted for covariates and smoking, CAR =carotid plaque, CAR _SMK =carotid plaque adjusted for covariates and smoking.
Association with subclinical atherosclerosis
We assessed in TwinsUK women the association between the glycan traits identified as associated with CVD risk after adjustment for individual risk factors and carotid and femoral plaque, which are well known markers of subclinical coronary atherosclerosis 9. We find that 3 of these 8 glycan traits are associated with femoral plaque (P<0.05) and 4 of them are associated with carotid plaque (P<0.05), indicating that indeed these glycan traits are related to atherosclerosis. All but one of these associations remained significant (P<0.05) after adjusting for smoking (Figure 3, Online Table V).
GlycA NMR association with the ACC/AHA 10-year ASCVD risk score
Because a number of authors31–33 have shown the effect of NMR measured glycoprotein on cardiovascular mortality we then investigated the association between this marker (GlycA) and ASCVD risk score. We find that indeed circulating levels of GlycA are positively and significantly correlated with the 10-year ASCVD risk score (0.14(0.02), P= 8.49x10-15) in the TwinsUK cohort. Higher circulating levels of GlycA are also associated with a higher risk of developing both carotid (OR(SE)= 1.41(0.21), P= 0.020 and femoral (1.57(0.26), P=0.005) plaque.
Correlation between GlycA and IgG glycans
The NMR measured GlycA shows a significant correlation with all the 8 glycan traits that are reproducibly associated with the 10-year ASCVD risk score (summarized in Online Table VI). However, the correlation is not very large explaining 6% of variation in any of the IgG CVD-associated glycan trait. IgG glycan associations with the 10-year ASCVD risk score are consistent if we further adjust for GlycA.
Glycan score
To assess the combined effects of all glycan traits we fitted a logistic regression model of the 8 glycans (with and without GlycA) to the top quintile corresponding of the 10-year ASCVD risk score (>5.2%) to compute a linear glycan score in females form the TwinsUK cohort (were who have both UPLC and NMR measures). After stepwise regression the model fitted on standardized (mean zero, variance 1, i.e. z-scores) of the IgG glycan measures was IgG score:
This linear combination was associated with log (10-year ASCVD risk score) in a linear regression with Beta(SE)=0.477 (0.0211), P=2.3 x 10-96 explaining 26.9% of the variance in log(10-year risk ASCVD score) in our data. This score was then tested for association with log(10-year ASCVD risk score) in ORCADES where it explained 54.6% of the variance in log(10-year ASCVD risk score) (Beta(SE)=0.412 (0.012), P=5.1 x 10-168) in women and 39.5% of the variance in log (the 10-year risk ASCVD score)(Beta(SE)=0.443 (0.021), P=1.7 x 10-73) in men.
We then adjusted for GlycA levels in TwinsUK (where the NMR measure was available) the role of this glycan score. Adjusting for GlycA resulted in an association for the IgG score with Beta(SE)=0.441(0.0211) P=4.4 x 10-84 and of Beta(SE)=0.193(0.023) P=5.5 x 10-16 for GlycA indicating a significant contribution for both the combined IgG glycans and NMR measure.
We therefore computed a glycan score based on both IgG glycans plus GlycA. The model identified was IgG+GlycA score:
This measure is strongly associated with log (10-year ASCVD risk score) (Beta(SE)=0.571(0.023), P=2.1x10-110) and explains 30.1% of the variation in log(10-year ASCVD risk score) in the TwinsUK data.
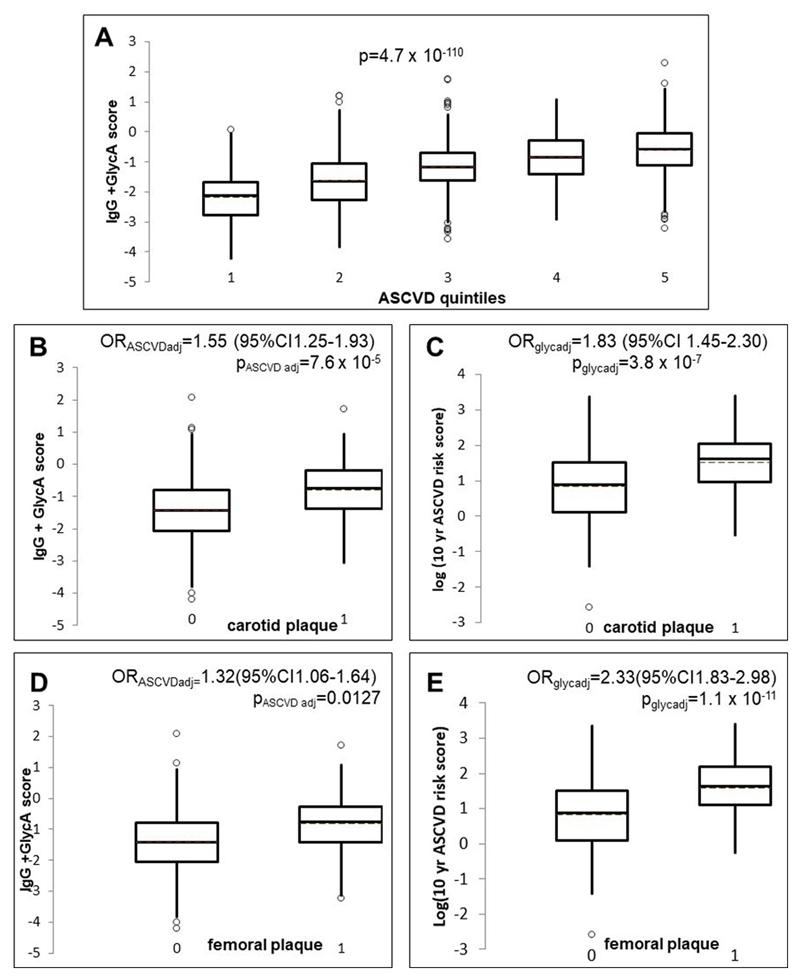
The distribution of this IgG+GlycA score for each of the five quintiles of the 10-year ASCVD risk score distribution is shown as box plots in Figure 4A. We proceeded to compare the association between this the glycan score and 10-year ASCVD risk score on subclinical atherosclerosis. The associations of the glycan score in individuals with carotid and femoral plaque are presented in Figures 4B and 4D, while the distribution of log(10-year ASCVD risk score) in the same individuals is depicted in Figures 4C and 4E.
Figure 4.
(A) Box plot showing the distribution of the glycan score in quintiles of the 10-year ASCVD risks core. (B) Box plot showing the distribution of the glycan (IgG +GlycA) score in individuals with and without carotid plaque. P-values and odds ratios (OR) from logistic regression adjusted for log (10-year ASCVD risk score) (C) Box plot showing the distribution of the log (10-yearASCVD risk score) in individuals with and without carotid plaque, OR and p-value adjusted for glycan score (D) Box plot showing the distribution of the glycan score in individuals with and without femoral plaque. P-value and OR adjusted for log (10-year ASCVD risk score) (E) Box plot showing the distribution of the log (10-year ASCVD risk score) in individuals with and without femoral plaque, OR and p-value adjusted for glycan score.
In quantitative terms, the association between the glycan score and carotid plaque – adjusting for the 10-year ASCVD risk score - is OR[95%CI] = 1.55[1.25;1.93]; P=7.5x10-5) whereas the 10-year ASCVD risk score (adjusted for the glycan score) is associated with OR[95%CI] =1.83[1.45;2.30]; P=3.8x10-7. For femoral plaque, the association of the glycan score (adjusted for the 10-year ASCVD risk score) was OR[95%CI]=1.32[1.06,1.64]; P=0.01) and that of the 10-year ASCVD risk score (adjusted for glycan score) OR[95%CI]=2.33[1.83;2.98] P=1.1x10-11). Thus the glycan score contributes significantly to both measures of subclinical atherosclerosis in addition to the known CVD risk factors.
GP18 and VLDL
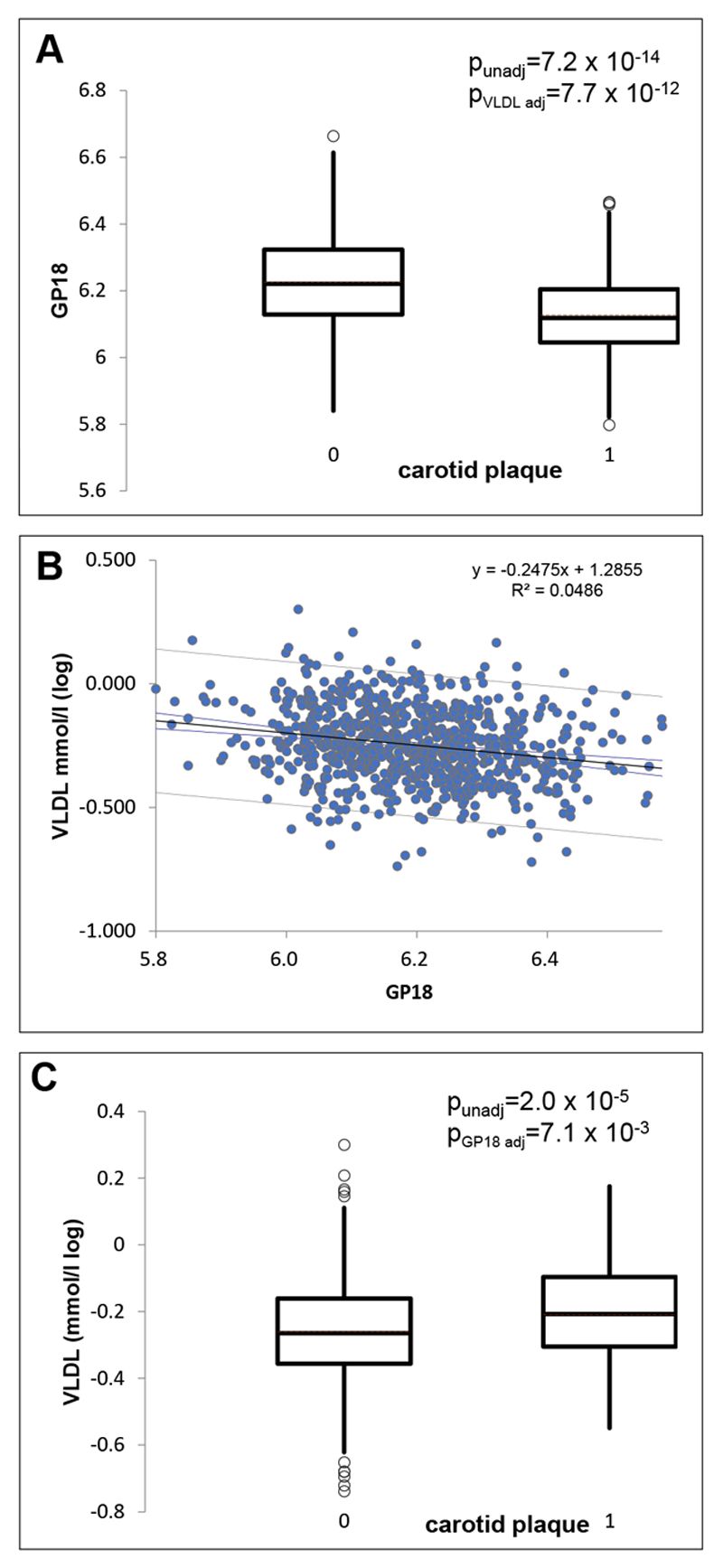
One of the glycan traits, GP18 (FA2G2S1), is negatively associated with ASCVD risk score, total cholesterol and carotid plaque, however the association remains significant after adjusting for total cholesterol. We therefore investigated its relationship to other measures of lipoproteins and triglycerides using the Nightingale platform. This monosialylated glycan with core fucose is strongly negatively correlated with various measures of lipids and triglycerides, in particular with the concentration of VLDL and triglycerides in VLDL (Online Table VII). To illustrate the magnitude of the associations between carotid plaque and glycosylation traits the distribution of GP18 in individuals with and without carotid plaque is shown in Figure 5 side by side to the distribution of 10-year ASCVD risk score.
Figure 5.
(A) Boxplot showing the distribution of the IgG glycan trait GP18 in individuals with and without carotid plaque. The p-values shown are unadjusted and adjusted for circulating levels of VLDL (B) Correlation between circulating VLDL and GP18. (C) Distribution of VLDL in individuals with and without carotid plaque. The p-values shown are unadjusted and adjusted for levels of GP18.
Discussion
In this study we report that there are significant and reproducible IgG glycan traits associations with cardiovascular risk in addition to the previously reported ones with one single measure of protein glycosylation (GlycA). After adjustment for individual risk factors, we identify 8 quantitative IgG glycan traits associated with the 10-year ASCVD risk score in women from two independent cohorts, 6 of which are also associated in men. Four of the glycan traits identified are also associated with presence of subclinical atherosclerosis after adjusting for all traditional risk factors (3 with both femoral and carotid plaque, and 1 with carotid plaque only) indicating that indeed these glycan traits are related to atherosclerosis.
Several recent studies have used targeted metabolomics platforms to examine a glycan signal (referred to as GlycA) thought to identify the concentration of circulating protein-bound N-acetyl methyl groups of GlcNAc and N-acetylgalatosamine glycan moieties based on NMR measures33. One such study33 demonstrated that this signal was associated with longitudinal risk of mortality related to both ASCVD and cancer34.
In this study we have used the NMR GlycA measure as a positive control and find that, this measure of protein glycosylation previously reported to be associated with CVD mortality by Lawler et al33 is also strongly associated in our data. In addition, 8 of the glycan associations we report to be associated with 10-year ASCVD risk score, remain significant after adjusting for individual risk factors indicating that they are independently contributing to CVD risk. This is line with literature data indicating that IgG glycans are only weakly related to the GlycA NMR signal 35. Importantly, we find that a linear combination of IgG glycans predicts a large proportion of the variance in 10-years ASCVD risk score both in men (39%) and women (26-54%) and that this is reproducible.
When we combined the GlycA NMR measure into an IgG+GlycA score and we found that it was strongly associated with both femoral and carotid plaque in the TwinsUK cohort. The association with subclinical atherosclerosis of this measure remained statistically significant after adjustment for 10-year ASCVD risk score, in line with previous reports20 that measures of protein glycosylation contribute to cardiovascular risk in addition to traditional risk factors. It is of interest that the association between the IgG glycan traits and CVD-related study endpoints remain significant after adjusting for GlycA, suggesting incremental information is gained with the different measures of glycans.
It has been hypothesized20 that protein glycosylation may be capturing a combined measure of upstream inflammation related to risk of ASCVD. Recently there has been an emerging focus on reclassification of diseases based on common mechanisms of pathophysiology and away from traditional clinical manifestation-defined approaches36. Our data, with more comprehensive measures of protein glycosylation, highlight the potential value of glycomics in identifying such pathways of disease, the reproducibility of results across different cohorts and the extent to which CVD risk can be captured by these measures.
We also report that some IgG glycans are associated with higher CVD risk and others associated with lower CVD risk. More precisely, glycans that contain exposed three GlcNAcs (GP6) or glycans that contain both bisecting GlcNAc and one sialic acid are positively associated with CVD risk, (consistent with the previous GlycA reports) while sialylated glycans without a bisecting GlcNAc are negatively associated. Increased levels of glycans with a bisecting GlcNAc are reported to associate with higher age while decreased levels were associated with longevity 37. Even though only non-galactosylated glycoforms with a bisecting GlcNAc were associated with familial longevity, our results show that association of bisecting GlcNAc and CVD risk is not dependent on the presence of other sugar residues27, 37; we found agalactosylated, monogalactosylated and sialylated N-glycans with a bisecting GlcNAc positively associated with CVD risk. Besides ageing, increased levels of glycans with a core-fucose and bisecting GlcNAc are known to be present in serum of patients with type 2 diabetes 38, and most of these glycans are coming from IgG 39 thus reflecting the same traits connected with CVD risk in this study.
Age, T2D and smoking are all factors included in the 10-year ASCVD cardiovascular risk score assessment and have a positive association with a bisecting GlcNAc. IgG glycosylation is able to modulate Fc receptor binding, and bisecting GlcNAc was shown to increase antibody-dependent cellular cytotoxicity (ADCC) mediated by binding of the antibody to the Fcγ-receptor 40. Glycan traits known to increase ADCC are involved in pro-inflammatory pathway and inflammation is known to be underlying mechanism of CVD’s development19.
While bisecting GlcNAc is related with pro-inflammatory activity of IgG and the aforementioned conditions, sialylation and core-fucosylation are consistently associated with anti-inflammatory activity 16, 42. Indeed, a core-fucosylated digalactosylated monosialylated glycan, GP18 (also called FA2G2S1), over all glycan traits remains strongly associated with the10-year ASCVD risk score after adjustment for the individual risk factors that constitute the ASCVD risk score. After further investigation, we found that this glycan structure is strongly negatively correlated with VLDL levels. VLDL itself is a risk factor for cardiovascular disease being associated with hypertriglyceridemia and dyslipidemia in general 43. Importantly defects in the cholesterol metabolism pathway (particularly in the generation of non-sterol isoprene compounds) lead to disturbances in the glycosylation of proteins. This suggests a functional link between cholesterol metabolism and protein glycosylation44. Moreover, in rabbits, IgG and VLDL were shown to contribute arterial lesions and that sialic acid plays a crucial role in the prevention of an arterial lesion formation 45; even though our work supports that connection, the complete picture is still missing. Therefore, further studies should be carried out focusing on the role of these glycosylated structures in predicting cardiovascular events, and in particular their interaction with VLDL.
We note some study limitations. The results were discovered and replicated primarily in women, even though most of the results are also replicated in men. Second, the cross-sectional nature of our data does not allow us to draw conclusions as to whether the identified glycan traits are causative of CVD decline or merely correlated with it, although the results from these hypothesis generating findings are consistent with other less comprehensive measures of glycosylations where causative links between glycosylation and CVD outcomes have been shown 33 34. Third, the associations were discovered with the 10-year ASCVD risk score and not with actual CVD events. We report that these associations were also validated with measures of subclinical atherosclerosis after adjusting for all the risk factors in the 10-year ACC/AHA risk score, aiming to show that glycan traits provide molecular information that was not present in the pooled risk equation and thus suggesting an important biological role for these post-translational IgG modifications
In conclusion, our data point to separate pathways whereby immunoglobulin glycosylation may be related to cardiovascular risk, on the one hand, a large number of N-glycan traits related to core-fucose and bisecting GlcNAc are strongly associated with atherosclerotic plaque.
On the other hand, one specific trait related to the sialylated N-glycan appears to be strongly negatively related to circulating VLDL and is supportive of a role of IgG glycosylation in VLDL metabolism and arterial lesion formation also in humans.
Supplementary Material
Novelty and Significance.
What Is Already Known?
Sugar molecules can attach to proteins in a process called glycosylation, which plays an important role in regulating inflammation.
One of these proteins, GlycA, is involved with an increased risk of heart disease.
There are many different types of glycosylated proteins and overall, they constitute the "glycome".
What New Information Does This Article Contribute?
We tested 76 glycosylated immunoglobulin measures for association with the risk of atherosclerosis.
Four of these measures, plus GlycA, were associated with cardiovascular risk and ultrasound measures of atherosclerosis, even after taking into account all other known cardiovascular risk factors.
Glycosylation is the process by which sugar molecules are attached to proteins and it plays an important role in regulating inflammation. One measure of glycosylated protein is GlycA, which is associated with an increased risk of cardiovascular disease. However, there are many different types of glycosylated proteins and collectively they constitute the "glycome". Here, we provide a comprehensive overview of the “glycome” by measuring 76 glycosylated immunoglobulin or “glycans” plus GlycA in 845 men and 3937 women from two independent cohorts. Six of the glycans tested were associated with the summary risk score of atherosclerotic disease. In addition to GlycA, 4 other glycans were also associated with measures of atherosclerosis, even after taking into account other known cardiovascular risk factors. A combination of all significant glycan factors showed stronger association with atherosclerosis, possibly though summation of the total amount of inflammation, which contributes to the risk of cardiovascular disease.
Acknowledgements
We wish to express our appreciation to all study participants of the TwinsUK cohort. We want to acknowledge Dr Benyu Jiang for performing the majority of the carotid measurements in TwinsUK. Toma Keser, Jerko Štambuk, Mirna Šimurina, Tamara Pavić and Jasminka Krištić are acknowledged for their help during the laboratory work on glycan analysis. We would like to acknowledge the invaluable contributions of the research nurses in Orkney, the administrative team in Edinburgh and the people of Orkney.
Sources of Funding
This work was funded by the British Heart Foundation (BHF) Special Project grant SP/12/4/29573 and MRC/BHF AIM HY (MR/M016560/1) grant. Twins UK receives funding from the Wellcome Trust European Community’s Seventh Framework Programme (FP7/2007-2013 to TwinsUK); the National Institute for Health Research (NIHR) Clinical Research Facility at Guy’s & St Thomas’ NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. Glycan analysis was supported by European Commission FP7 grants MIMOmics (contract #305280) and H2020 grants GlySign (contract #722095) and IMforFuture (contract #721815) as well as by the European Structural and Investment Funds IRI (grant #KK.01.2.1.01.0003) and Croatian National Centre of Research Excellence in Personalized Healthcare (grant #KK.01.1.1.01.0010);. ORCADES was supported by the Chief Scientist Office of the Scottish Government (CZB/4/276, CZB/4/710), the Royal Society, the MRC Human Genetics Unit quinquennial programme “QTL in Health and Disease”, Arthritis Research UK and the European Union framework program 6 EUROSPAN project (contract no. LSHG-CT-2006-018947). AMV is supported by the NIHR Nottingham BRC.
Nonstandard Abbreviations and Acronyms
- ASCVD
atherosclerotic cardiovascular disease risk
- BMI
body mass index
- CVD
cardiovascular disease
- DZ
dizygotic twin
- FRS
Framingham Risk Score
- GlcNAc
N-acetylglucosamine
- IgG
immunoglobulin G
- MZ
monozygotic twin
- SBP
systolic blood pressure
- T2D
type 2 diabetes
- TC
total cholesterol
Footnotes
Disclosures
GL is a founder and owner, IG and ITA are employees of Genos Ltd, which offers commercial service of glycomic analysis and has two patents in this field (WO/2014/203010 and WO/2017/215973). All other authors declare no competing financial interests.
References
- 1.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in england between 2002 and 2010: Linked national database study. BMJ. 2012;344:d8059. doi: 10.1136/bmj.d8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. Lancet. 2005;365:434–441. doi: 10.1016/S0140-6736(05)17833-7. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The reynolds risk score. JAMA : the journal of the American Medical Association. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 5.Assmann G, Cullen P, Schulte H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular munster (procam) study. Circulation. 2002;105:310–315. doi: 10.1161/hc0302.102575. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario M, Chiodini P, Chambless LE, Cesana G, Vanuzzo D, Panico S, Sega R, Pilotto L, Palmieri L, Giampaoli S. Prediction of coronary events in a low incidence population. Assessing accuracy of the cuore cohort study prediction equation. International journal of epidemiology. 2005;34:413–421. doi: 10.1093/ije/dyh405. [DOI] [PubMed] [Google Scholar]
- 7.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, et al. 2013 acc/aha guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 8.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 9.Laclaustra M, Casasnovas JA, Fernandez-Ortiz A, Fuster V, Leon-Latre M, Jimenez-Borreguero LJ, Pocovi M, Hurtado-Roca Y, Ordovas JM, Jarauta E, Guallar E, et al. Femoral and carotid subclinical atherosclerosis association with risk factors and coronary calcium: The awhs study. Journal of the American College of Cardiology. 2016;67:1263–1274. doi: 10.1016/j.jacc.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 10.Polonsky TS, Ning H, Daviglus ML, Liu K, Burke GL, Cushman M, Eng J, Folsom AR, Lutsey PL, Nettleton JA, Post WS, et al. Association of cardiovascular health with subclinical disease and incident events: The multi-ethnic study of atherosclerosis. Journal of the American Heart Association. 2017;6 doi: 10.1161/JAHA.116.004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selwaness M, Bos D, van den Bouwhuijsen Q, Portegies ML, Ikram MA, Hofman A, Franco OH, van der Lugt A, Wentzel JJ, Vernooij MW. Carotid atherosclerotic plaque characteristics on magnetic resonance imaging relate with history of stroke and coronary heart disease. Stroke. 2016;47:1542–1547. doi: 10.1161/STROKEAHA.116.012923. [DOI] [PubMed] [Google Scholar]
- 12.Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. The New England journal of medicine. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, Raychaudhuri S, Ruhaak LR, Lebrilla CB. Glycans in the immune system and the altered glycan theory of autoimmunity: A critical review. Journal of autoimmunity. 2015;57:1–13. doi: 10.1016/j.jaut.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nature reviews. Immunology. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 17.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. Journal of the American Heart Association. 2014;3:e001221. doi: 10.1161/JAHA.114.001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauc G, Pezer M, Rudan I, Campbell H. Mechanisms of disease: The human n-glycome. Biochimica et biophysica acta. 2016;1860:1574–1582. doi: 10.1016/j.bbagen.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 20.Lawler PR, Mora S. Glycosylation signatures of inflammation identify cardiovascular risk: Some glyc it hot. Circulation research. 2016;119:1154–1156. doi: 10.1161/CIRCRESAHA.116.310005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Klaric L, Yu X, Thaqi K, Dong J, Novokmet M, Wilson J, Polasek O, Liu Y, Kristic J, Ge S, et al. The association between glycosylation of immunoglobulin g and hypertension: A multiple ethnic cross-sectional study. Medicine. 2016;95:e3379. doi: 10.1097/MD.0000000000003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knezevic A, Gornik O, Polasek O, Pucic M, Redzic I, Novokmet M, Rudd PM, Wright AF, Campbell H, Rudan I, Lauc G. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma n-glycans. Glycobiology. 2010;20:959–969. doi: 10.1093/glycob/cwq051. [DOI] [PubMed] [Google Scholar]
- 23.Moayyeri A, Hammond CJ, Valdes AM, Spector TD. Cohort profile: Twinsuk and healthy ageing twin study. International journal of epidemiology. 2013;42:76–85. doi: 10.1093/ije/dyr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McQuillan R, Leutenegger AL, Abdel-Rahman R, Franklin CS, Pericic M, Barac-Lauc L, Smolej-Narancic N, Janicijevic B, Polasek O, Tenesa A, Macleod AK, et al. Runs of homozygosity in european populations. American journal of human genetics. 2008;83:359–372. doi: 10.1016/j.ajhg.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menni C, Migaud M, Glastonbury CA, Beaumont M, Nikolau A, Small K, Brosnan MJ, Mohney R, Spector TD, Valdes AM. Metabolomic profiling to dissect the role of visceral fat in cardiometabolic health. Obesity (Silver Spring) 2016 doi: 10.1002/oby.21488. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cecelja M, Jiang B, Bevan L, Frost ML, Spector TD, Chowienczyk PJ. Arterial stiffening relates to arterial calcification but not to noncalcified atheroma in women. A twin study. Journal of the American College of Cardiology. 2011;57:1480–1486. doi: 10.1016/j.jacc.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O, Gornik O, Supraha-Goreta S, Wormald MR, Redzic I, Campbell H, et al. High throughput isolation and glycosylation analysis of igg-variability and heritability of the igg glycome in three isolated human populations. Molecular & cellular proteomics : MCP. 2011;10:M111 010090. doi: 10.1074/mcp.M111.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menni C, Keser T, Mangino M, Bell JT, Erte I, Akmacic I, Vuckovic F, Pucic Bakovic M, Gornik O, McCarthy MI, Zoldos V, et al. Glycosylation of immunoglobulin g: Role of genetic and epigenetic influences. PloS one. 2013;8:e82558. doi: 10.1371/journal.pone.0082558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soininen P, Kangas AJ, Wurtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circulation. Cardiovascular genetics. 2015;8:192–206. doi: 10.1161/CIRCGENETICS.114.000216. [DOI] [PubMed] [Google Scholar]
- 30.Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. Genabel: An r library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- 31.Wurtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, Kangas AJ, et al. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation. 2015;131:774–785. doi: 10.1161/CIRCULATIONAHA.114.013116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, Krishnamoorthy P, Ng Q, Aridi TZ, Salahuddin T, Natarajan B, et al. Glyca is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circulation research. 2016;119:1242–1253. doi: 10.1161/CIRCRESAHA.116.309637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawler PR, Akinkuolie AO, Chandler PD, Moorthy MV, Vandenburgh MJ, Schaumberg DA, Lee IM, Glynn RJ, Ridker PM, Buring JE, Mora S. Circulating n-linked glycoprotein acetyls and longitudinal mortality risk. Circulation research. 2016;118:1106–1115. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niyonzima N, Halvorsen B, Sporsheim B, Garred P, Aukrust P, Mollnes TE, Espevik T. Complement activation by cholesterol crystals triggers a subsequent cytokine response. Molecular immunology. 2017;84:43–50. doi: 10.1016/j.molimm.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. Glyca: A composite nuclear magnetic resonance biomarker of systemic inflammation. Clinical chemistry. 2015;61:714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 36.Antman EM, Loscalzo J. Precision medicine in cardiology. Nature reviews. Cardiology. 2016;13:591–602. doi: 10.1038/nrcardio.2016.101. [DOI] [PubMed] [Google Scholar]
- 37.Ruhaak LR, Uh HW, Beekman M, Koeleman CA, Hokke CH, Westendorp RG, Wuhrer M, Houwing-Duistermaat JJ, Slagboom PE, Deelder AM. Decreased levels of bisecting glcnac glycoforms of igg are associated with human longevity. PloS one. 2010;5:e12566. doi: 10.1371/journal.pone.0012566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itoh N, Sakaue S, Nakagawa H, Kurogochi M, Ohira H, Deguchi K, Nishimura S, Nishimura M. Analysis of n-glycan in serum glycoproteins from db/db mice and humans with type 2 diabetes. American journal of physiology. Endocrinology and metabolism. 2007;293:E1069–1077. doi: 10.1152/ajpendo.00182.2007. [DOI] [PubMed] [Google Scholar]
- 39.Clerc F, Reiding KR, Jansen BC, Kammeijer GS, Bondt A, Wuhrer M. Human plasma protein n-glycosylation. Glycoconjugate journal. 2016;33:309–343. doi: 10.1007/s10719-015-9626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umana P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma igg1 with optimized antibody-dependent cellular cytotoxic activity. Nature biotechnology. 1999;17:176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 41.Wahl A, Kasela S, Carnero-Montoro E, van Iterson M, Stambuk J, Sharma S, van den Akker E, Klaric L, Benedetti E, Razdorov G, Trbojevic-Akmacic I, et al. Igg glycosylation and DNA methylation are interconnected with smoking. Biochimica et biophysica acta. 2017;1862:637–648. doi: 10.1016/j.bbagen.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 42.Bohm S, Schwab I, Lux A, Nimmerjahn F. The role of sialic acid as a modulator of the anti-inflammatory activity of igg. Seminars in immunopathology. 2012;34:443–453. doi: 10.1007/s00281-012-0308-x. [DOI] [PubMed] [Google Scholar]
- 43.Tenenbaum A, Klempfner R, Fisman EZ. Hypertriglyceridemia: A too long unfairly neglected major cardiovascular risk factor. Cardiovascular diabetology. 2014;13:159. doi: 10.1186/s12933-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfe LA, Morava E, He M, Vockley J, Gibson KM. Heritable disorders in the metabolism of the dolichols: A bridge from sterol biosynthesis to molecular glycosylation. American journal of medical genetics. Part C, Seminars in medical genetics. 2012;160C:322–328. doi: 10.1002/ajmg.c.31345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarphie TG. Interactions of igg and beta-vldl with aortic valve endothelium from hypercholesterolemic rabbits. Atherosclerosis. 1987;68:199–212. doi: 10.1016/0021-9150(87)90199-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.