Abstract
Strategies to treat Zika virus (ZIKV) infection in dengue virus (DENV) endemic areas are urgently needed. Here we show that a DENV-specific antibody against the E-dimer epitope (EDE) potently cross-neutralizes ZIKV and provides robust therapeutic efficacy as well as prophylactic efficacy against ZIKV in rhesus monkeys. Viral escape was not detected, suggesting a relatively high bar to escape. These data demonstrate the potential for antibody-based therapy and prevention of ZIKV.
Zika virus (ZIKV) has been associated with fetal microcephaly and other congenital abnormalities as well as Guillain-Barre syndrome1,2. Our laboratory and others have shown that ZIKV-specific neutralizing antibodies correlate with vaccine protection in both mice and monkeys3–6 as well as with rapid control of viremia following infection in monkeys7. Several groups have also demonstrated therapeutic efficacy of ZIKV-specific mAbs in immunosuppressed mice8–11, and a cocktail of three ZIKV-specific mAbs that targeted domain III was shown to prevent ZIKV infection in nonhuman primates12. In the present study, we assessed the therapeutic and prophylactic efficacy of a potent ZIKV-specific antibody in rhesus monkeys.
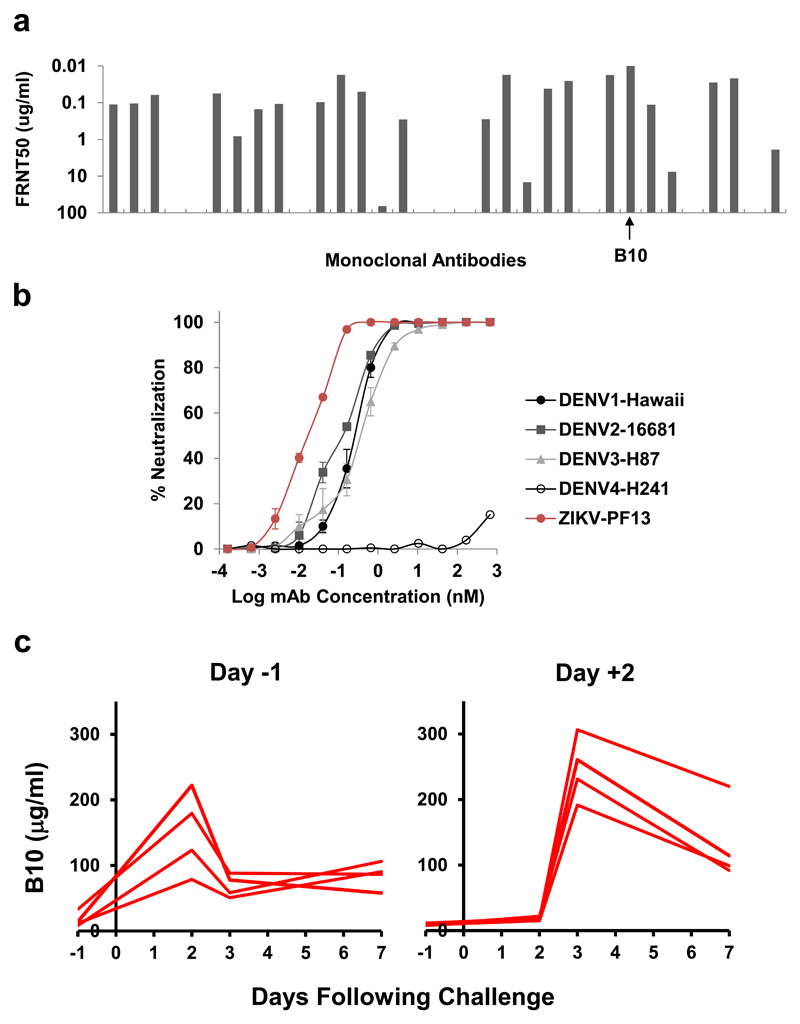
Substantial humoral cross-reactivity exists between DENV and ZIKV, and DENV-specific antibodies have been associated with antibody-dependent enhancement of ZIKV infection in vitro and in certain murine models13–15. We previously reported that DENV E-dimer epitope (EDE)-specific mAbs bind a quaternary epitope formed at the interface of head-to-tail E-dimers and efficiently cross-neutralize ZIKV15–17. EDE-specific mAbs bind poorly to monomeric E-proteins but bind efficiently to stable E-dimers18 and can be subdivided into two groups, EDE1 and EDE2, by their insensitivity or sensitivity, respectively, to removal of N-linked glycan at position 153, with EDE1 mAbs typically exhibiting greater potency15,17. Moreover, the EDE1-specific mAb B10 has been shown to prevent and treat ZIKV infection in mice8. We evaluated 33 EDE1-specific antibodies isolated from DENV infected patients17 and found that B10 was the most potent at neutralizing a French Polynesian ZIKV strain (Fig. 1a). B10 neutralized ZIKV-PF13 (NT50 of 0.016 ± 0.001 nM; NT90 of 0.100 ± 0.009 nM) even more potently than DENV-1/2/3 but showed poor neutralization against DENV4 (Fig. 1b).
Figure 1. Characterization and pharmacokinetics of B10.
(a) Neutralization of ZIKV-PF13/251013-18 (PF13), an Asian strain of Zika virus isolated from French Polynesia in 2013, using a panel of 33 EDE1-specific mAbs originally isolated from DENV-infected patients. B10 was the most potent mAb in this panel. Data are representative of n=3 biologically independent experiments. (b) Neutralization curves of B10 against DENV-1, DENV-2, DENV-3, DENV-4, and ZIKV-PF13. Data are representative of n=3 biologically independent experiments, and mean ± SEM are shown. (c) Levels of B10 (μg/ml) were determined in monkey sera at multiple timepoints in singlet following B10 infusion by ELISA.
To confirm the antiviral activity of B10 against ZIKV in vivo, we performed a titration study in immunocompetent Balb/c mice. Groups of Balb/c mice (N=5/group) received a single infusion of 6.25, 3.12, 1.56, 0.78, 0.39, 0.19, 0.097, 0.048, and 0 μg B10 and were subsequently challenged with 105 viral particles (VP) [102 plaque-forming units (PFU)] of ZIKV-BR by the intravenous route4 (Supplementary Fig. S1). In naïve mice, ZIKV-BR infection led to peak viral loads of 5.24-6.18 log RNA copies/ml, similar to previous findings with this challenge stock4. B10 doses as low as 3.12 μg, corresponding to serum levels of 0.5-0.9 μg/ml (3-6 nM), resulted in complete protection against ZIKV-BR challenge in mice (Supplementary Fig. S1). Sub-protective B10 doses of 0.19-1.56 μg resulted in partial protection of a subset of mice and attenuation of viral loads in infected animals. These data confirm B10 potency against ZIKV challenge in mice.
We next evaluated the therapeutic and prophylactic efficacy of B10 in rhesus monkeys. 16 monkeys received the following antibodies by intravenous infusion either before or after ZIKV-BR challenge (N=4/group): (1) 10 mg/kg B10 on day -1, (2) 10 mg/kg isotype matched control antibody (PGT121)19,20 on day -1, (3) 10 mg/kg B10 on day +2, or (4) 10 mg/kg isotype matched control antibody (PGT121) on day +2. We selected this antibody dose based on our previous experience with therapeutic HIV-1-specific antibody studies in SHIV-infected rhesus monkeys19,20. Antibody pharmacokinetics was monitored by ELISA, and peak B10 levels were 78-306 μg/ml (0.5-2 μM) on the day after infusion (Fig. 1c).
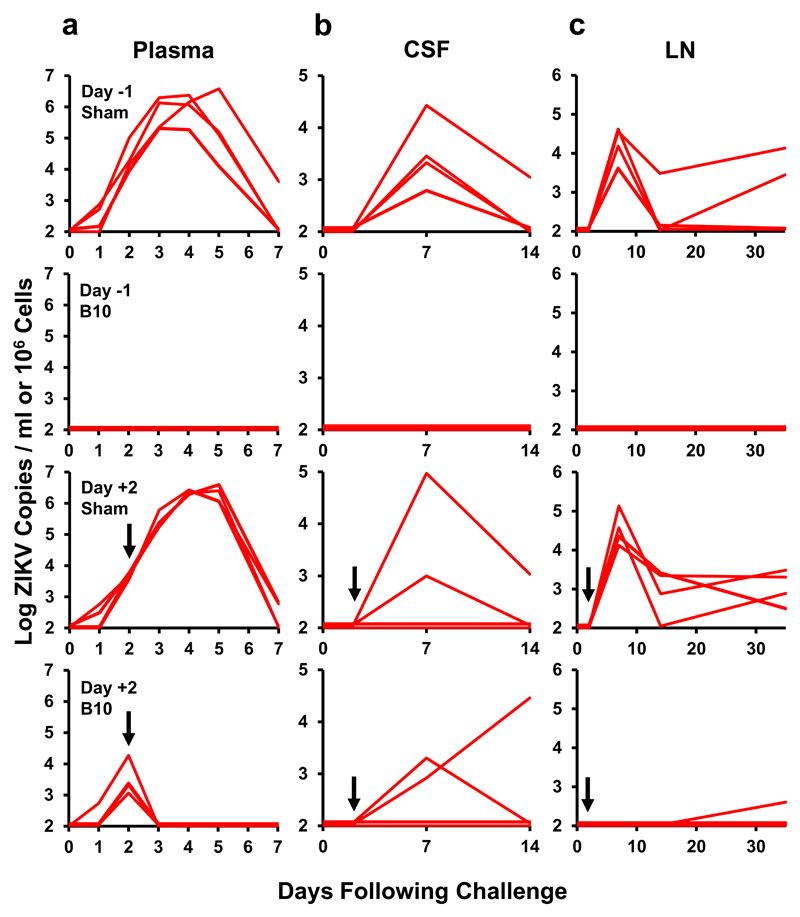
On day 0, all monkeys were challenged by the subcutaneous route with 106 VP (103 PFU) of ZIKV-BR, and viral loads were quantitated by RT-PCR3,7. Animals that received the isotype matched sham control antibody either before or after ZIKV-BR challenge exhibited approximately 7 days of viremia with median peak viral loads of 6.40 (range 5.31-6.60) log RNA copies/ml on day 3-5 following challenge (Fig. 2a), consistent with our previous studies with this ZIKV-BR challenge stock in rhesus monkeys3,7. Administration of B10 on day -1 prior to challenge resulted in complete protection, as evidenced by no detectable plasma viremia at any timepoint (P=0.02 comparing infection of B10 group vs controls, Fisher’s exact test). Administration of B10 on day +2 after challenge, which was during the exponential rise of plasma viremia, resulted in an abrupt termination of viral replication and rapid clearance of virus from peripheral blood by day 3 (Fig. 2a; P=0.02 comparing viremia on days 3-7 of B10 group vs controls).
Figure 2. Therapeutic and prophylactic efficacy of B10 in rhesus monkeys.
Rhesus monkeys (N=4/group) received 10 mg/kg B10 or the isotype matched sham control antibody PGT121 by the i.v. route on day -1 or day +2. All animals were challenged on day 0 by the s.c route with 106 VP (103 PFU) ZIKV-BR. Viral loads are shown in (a) plasma, (b) cerebrospinal fluid (CSF), and (c) lymph nodes (LN). Viral loads were determined on days 0, 1, 2, 3, 4, 5, and 7 for the plasma samples and on days 0, 3, 7, 14, and 35 for the other samples. Assay sensitivity 100 copies/ml or 1x106 cells. Arrows designate the day +2 infusions.
We observed prolonged ZIKV-BR shedding in the sham controls in cerebrospinal fluid (CSF), lymph nodes (LN), and colorectal (CR) biopsies (Fig. 2b-c; Supplementary Fig. S2), consistent with our previous observations7. Monkeys that received B10 on day -1 prior to challenge had no detectable virus in these tissues, consistent with complete protection against infection. Moreover, these animals had no detectable cellular immune responses following ZIKV-BR challenge, as measured by IFN-γ ELISPOT assays to ZIKV Env, NS1, Cap, and prM peptide pools (Supplementary Fig. S3). Monkeys that received B10 on day +2 after challenge also showed substantial reduction of virus in tissues. However, ZIKV-BR was still detected in 2 of 4 animals in CSF on day 7 and in 1 of 4 animals in CSF on day 14. In this animal (12-083), the peak B10 level in CSF was 1 μg/ml (0.5% of plasma levels). The prM-Env sequence from the CSF virus on day 14 was identical to the ZIKV-BR challenge stock (Supplementary Fig. S4), suggesting that the virus did not specifically escape from B10. These data demonstrate that therapeutic B10 administration in acutely ZIKV-infected monkeys rapidly controlled virus replication in the periphery within 24 hours but incompletely cleared virus from immunoprivileged sites, likely due to reduced antibody penetration into these anatomic compartments.
To evaluate further the capacity of ZIKV to escape EDE1-specific mAbs, we incubated ZIKV with escalating concentrations of the antibodies B10 or C815,16 in vitro at 0.002, 0.015, and 0.070 μg/ml (corresponding to FRNT50, FRNT90, and FRNT99) for 2, 3, and 5 passages, respectively. After 10 passages, parental and passaged viruses were analyzed for resistance to neutralization by FRNT assays. We did not observe viral escape under these conditions (Supplementary Fig. S5), suggesting a relatively high bar to resistance. These findings are consistent with the observed therapeutic and prophylactic efficacy with B10 in rhesus monkeys even when delivered as monotherapy (Fig. 2). In contrast, a cocktail of three domain III-specific mAbs was required to prevent ZIKV infection in nonhuman primates12.
Our data demonstrate that a DENV EDE1-specific mAb has potent cross-reactive neutralizing activity against ZIKV and provides robust therapeutic as well as prophylactic efficacy against ZIKV infection in rhesus monkeys. Based on the rapid clearance of plasma virus by 24 hours after B10 infusion, we speculate that this antibody functions therapeutically by opsonization of virus followed by clearance. Previous studies have evaluated ZIKV-specific mAbs in therapeutic studies in immunosuppressed murine models8–11. Our data extend these prior studies by demonstrating the therapeutic and prophylactic efficacy of a ZIKV-specific antibody in nonhuman primates. These findings encourage clinical development of ZIKV-specific mAbs for both therapy and prevention.
The potency of B10 and apparent relatively high bar to escape raise the possibility of antibody monotherapy, which would be logistically far simpler than the development of antibody cocktails12 or bi-specific antibodies9. The structure of B10 remains to be determined, but the related cross-reactive DENV/ZIKV EDE1-specific mAb C8 binds a conserved quaternary site at the interface between the two Env subunits in the dimer at the interaction site of prM16, which may explain its high bar to escape.
A potential challenge for any antibody-based ZIKV therapeutic strategy will likely involve persistent virus in immunoprivileged sites, since the virus may be seeded in these sites quickly within the first few days of infection. Such sites include the central nervous system, lymph nodes, and placental and fetal tissues. We previously reported that ZIKV persists in CSF, lymph nodes, and colorectal mucosa in monkeys for substantial periods of time after viremia resolves, and viral persistence at these sites correlates with activation of mTOR and proinflammatory signaling pathways7. We show here that B10 penetrates poorly into the CSF and thus may not fully clear CSF virus that was seeded prior to antibody administration.
A unique aspect of B10 is that it was derived from a DENV-infected individual prior to the ZIKV epidemic. Certain DENV-specific antibodies have been shown to enhance ZIKV replication in vitro and in mice13–15, although the relevance of these observations for humans remains to be determined. In our experiments, sub-neutralizing doses of B10 did not result in enhanced ZIKV replication in mice (Supplementary Fig. S1), but nevertheless the possibility of antibody-dependent enhancement with a cross-reactive DENV/ZIKV-specific antibody requires further investigation, and if necessary Fc inactivating mutations could be incorporated8. Our data also raise the possibility of developing antibody therapeutics targeting both flaviviruses in endemic areas.
Methods
Animals, vaccines, and challenges
Female 6-8 week old Balb/c mice were housed at Beth Israel Deaconess Medical Center. 16 outbred, Indian-origin male and female rhesus monkeys (Macaca mulatta) were housed at AlphaGenesis, Yemassee, SC. Animals received B10 or isotype matched control antibody (PGT121) infusions by the i.v. route either before or after challenge. Antibodies were negative for endotoxin by Pierce LAL Chromogenic Endotoxin Quantitation kit (Thermo Scientific). Balb/c mice were challenged with 105 viral particles (VP) [102 plaque-forming units (PFU)] ZIKV-BR (Brazil ZKV2015)4. Rhesus monkeys were challenged by the s.c route with 106 VP (103 PFU) ZIKV-BR3. Animals were randomly allocated to groups. Immunologic and virologic assays were performed blinded. Animal studies were approved by the Institutional Animal Care and Use Committees (IACUCs) at AlphaGenesis and Beth Israel Deaconess Medical Center, as well as the Central Animal Welfare Ethical Review Board at Imperial College London.
Focus reduction neutralization assay
Virus was incubated with serial dilutions of antibodies at a 1:1 ratio for 1 h at 37 °C. The mAb/virus mixtures were then inoculated onto Vero cells. After 1 h incubation, the cell monolayers were overlaid with 1.5% (w/v) carboxymethyl cellulose and incubated for 2 d (for ZIKV) or 3 d (for DENV). The viral foci were visualized by staining with mAb 4G2 supernatant (mouse anti-DENV fusion loop that cross-reacts to ZIKV) followed by peroxidase-conjugated goat anti-mouse immunoglobulin at a 1:1,000 dilution (Sigma). The foci (infected cells) were visualized by adding the peroxidase substrate DAB (Sigma).
RT-PCR
RT-PCR assays were utilized to monitor viral loads, essentially as previously described3,4. RNA was extracted from plasma or other samples with a QIAcube HT (Qiagen). The wildtype ZIKV BeH815744 Cap gene was utilized as a standard. RNA was purified (Zymo Research), and RNA quality and concentration was assessed by the BIDMC Molecular Core Facility. Log dilutions of the RNA standard were reverse transcribed and included with each RT-PCR assay. Viral loads were calculated as virus particles (VP) per ml or per 1x106 cells and were confirmed by PFU assays. Assay sensitivity was 100 copies/ml or 1x106 cells.
ELISA
Mice and monkey ZIKV Env ELISA kits (Alpha Diagnostic International) were used to assess B10 levels. 96-well plates coated with ZIKV Env protein were first equilibrated at room temperature with 300 µl of kit working wash buffer for 5 min. 6 µl of serum was added to the top row, and 3-fold serial dilutions were tested in the remaining rows. Samples were incubated at room temperature for 1 h, and plates washed 4 times. 100 µl of anti-mouse or anti-human IgG HRP-conjugate working solution was then added to each well and incubated for 30 min at room temperature. Plates were washed 5 times, developed for 15 min at room temperature with 100 µl of TMB substrate, and stopped by the addition of 100 µl of stop solution. Plates were analyzed at 450nm/550nm on a VersaMax microplate reader using Softmax Pro 6.0 software (Molecular Devices). B10 levels were assessed against a standard curve.
In vitro selection with B10 and C8
To try to select ZIKV mutants resistant to neutralization by B10 of C8, ZIKV was incubated with mAb for 1 h at 37 °C. Viruses were then inoculated onto Vero cells and incubated for 2 days. In parallel, mock-neutralized virus was used as wildtype virus control. Viral titers were determined, and virus containing cell suspension was harvested for the next passage. This process was repeated through 10 passages, with 0.002, 0.015, and 0.070 μg/ml of antibody (FRNT50, FRNT90, and FRNT99) for 2, 3, and 5 passages, respectively. After 10 passages, parental and passaged viruses were analyzed for resistance to B10 or C8 neutralization by FRNT assays.
Viral sequencing
Viral RNA was extracted by QIAamp Viral RNA Mini Kit or Qiacube HT (Qiagen), and RT-PCR was performed to generate cDNA by using SuperScript® III First-Strand Synthesis System (Invitrogen). The prM-Env or Env region was amplified with Q5 high fidelity DNA polymerase (New England Biolabs) or Accuprime Taq DNA polymerase High Fidelity (Invitrogen) and sequenced.
Statistical analyses
Analysis of virologic and immunologic data was performed using GraphPad Prism v6.03 (GraphPad Software). Comparisons of groups were performed using Fischer’s exact tests and Wilcoxon rank-sum tests.
Supplementary Material
Acknowledgements
We thank V. Cao-Lormeau, E. Moseley, K. McMahan, M. Boyd, M. Kirilova, O. Nanayakkara, Z. Li, N. Mercado, A. Badamchi-Zadeh, M. Iampietro, C. Bricault, P. Gandhi, S. Khatiwada, S. Mojta, B. Alimonti, A. Chandrashekar, A. Brinkman, M. Ferguson, and W. Rinaldi for generous advice, assistance, and reagents. We acknowledge support from the National Institute for Health Research Biomedical Research Centre funding scheme UK, MRC-Newton UK (G.R.S.) and the Ragon Institute of MGH, MIT, and Harvard (D.H.B.). G.R.S. is a Wellcome Trust Senior Investigator.
Footnotes
Author Contributions
D.H.B. and G.R.S. designed the studies. W.D., P.S. and J.M. produced and characterized the B10 antibody. R.A.L. conducted the mouse studies. P.A. and R.P. conducted the virologic assays. J.P.N. and E.N.B. conducted the monkey study and immunologic assays. D.H.B. wrote the paper with all co-authors.
Competing Financial Interests Statement
The B10 antibody is the subject of patents held by Imperial College and Institute Pasteur on which G.R.S., W.D., and J.M. are inventors.
References
- 1.Mlakar J, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- 2.Brasil P, et al. Guillain-Barre syndrome associated with Zika virus infection. Lancet. 2016;387:1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- 3.Abbink P, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larocca RA, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowd KA, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016;354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardi N, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017 doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aid M, et al. Zika Virus Persistence in the Central Nervous System and Lymph Nodes of Rhesus Monkeys. Cell. 2017;169:610–620 e614. doi: 10.1016/j.cell.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez E, et al. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat Immunol. 2017;18:1261–1269. doi: 10.1038/ni.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, et al. A Human Bi-specific Antibody against Zika Virus with High Therapeutic Potential. Cell. 2017;171:229–241 e215. doi: 10.1016/j.cell.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kam YW, et al. Cross-reactive dengue human monoclonal antibody prevents severe pathologies and death from Zika virus infections. JCI insight. 2017;2 doi: 10.1172/jci.insight.92428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapparapu G, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016 doi: 10.1038/nature20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnani DM, et al. Neutralizing human monoclonal antibodies prevent Zika virus infection in macaques. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stettler K, et al. Specificity, cross-reactivity and function of antibodies elicited by Zika virus infection. Science. 2016 doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 14.Bardina SV, et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356:175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dejnirattisai W, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17:1102–1108. doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barba-Spaeth G, et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 17.Dejnirattisai W, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol. 2015;16:170–177. doi: 10.1038/ni.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouvinski A, et al. Covalently linked dengue virus envelope glycoprotein dimers reduce exposure of the immunodominant fusion loop epitope. Nature communications. 2017;8:15411. doi: 10.1038/ncomms15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, et al. Antibody-mediated protection against SHIV challenge includes systemic clearance of distal virus. Science. 2016 doi: 10.1126/science.aag0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.