Abstract
Influenza is a major health threat and a broadly protective influenza vaccine (BPIV) would be a significant advance. S-FLU is a candidate BPIV, limited to a single cycle of replication which induces a strong cross-reactive T cell response, but a minimal antibody response to hemagglutinin after intranasal or aerosol administration. We tested whether an H3N2 S-FLU can protect pigs and ferrets from heterosubtypic H1N1 influenza challenge. Aerosol administration of S-FLU to pigs induced lung tissue resident memory T cells and reduced lung pathology, but not the viral load. In contrast, in ferrets S-FLU reduced viral replication and aerosol transmission. Our data show that S-FLU has different protective efficacy in pigs and ferrets, and that in the absence of antibody, lung T cell immunity can reduce disease severity without reducing challenge viral replication.
Introduction
Influenza virus infection is a global health threat to livestock and humans causing substantial mortality. The major obstacle in combating influenza is the rapid evolution of the virus, rendering the host antibody response ineffective. Seasonal influenza virus vaccines are therefore strain specific, do not protect well against drifted viruses from the same haemagglutinin (HA) subtype, and offer no protection against infection with heterologous influenza viruses from different HA subtypes. Furthermore pandemic influenza can arise at any time, originating from either group 1 or 2 avian influenza A viruses, and can cause devastating mortality. Therefore, a broadly protective influenza A vaccine (BPIV), which could protect against both group 1 and 2 viruses, would be a great advance in preventing seasonal infection and reducing mortality from pandemic influenza (1).
S-FLU is a replication incompetent influenza virus, candidate BPIV, limited to a single cycle of replication (2) through inactivation of the HA signal sequence (3). Functional HA protein, required to form infectious virus particles, is provided in trans from a transfected cell line by pseudotyping, and S-FLU vaccine virus can therefore infect the host, but cannot replicate. All of the conserved viral core proteins are expressed in the cytosol of S-FLU infected cells for optimal antigen presentation to T lymphocytes (4). S-FLU induces a strong cross-reactive T cell response in the lung to the conserved core proteins, a specific antibody response to the expressed neuraminidase (NA), but a minimal antibody response to the HA coating the particle when administered to the respiratory tract. Immunization of mice with H1N1 or H5N1 S-FLU results in a high degree of protection against the homologous and heterologous H1N1, H6N1 (group 1), H3N2 and H7N9 (group 2) viruses, with moderate protection against distinct (heterologous) H5N1 (3, 5). Similarly in ferrets immunization with H1N1 or H5N1 S-FLU significantly reduced replication of H1N1, H6N1, H5N1 (group 1) and H7N9 (group 2) viruses in the lung. In pigs immunization with H1N1 or H5N1 S-FLU reduced the viral load in nasal swabs and lungs following challenge with a swine H1N1pdm09 isolate (6).
Because S-FLU can neither replicate nor donate its HA sequence to other influenza strains if administered to infected individuals, it should be safe. For this reason and because immunization via the lower respiratory tract has been shown to be a highly effective means of immunizing against influenza, in all experiments with S-FLU, the vaccine was administered either intranasally to mice and ferrets or intranasally, intra-tracheally or by aerosol in pigs. Our results show also that targeting the lower respiratory tract by aerosol in pigs is more effective than intra-tracheal or intranasal immunization in preventing severe disease (6). The reason for this may be that local immunization induces lung tissue-resident memory T cells (TRM), which have been shown to be important in cross-protective immunity against influenza infection (7–10). Most work on TRM has been performed in mice and the TRM defined as inaccessible to intravenously administered anti-T cell antibody (11). TRM identified in this way are an activated, dividing population capable of responding rapidly to antigen by further cell division in situ in mice. However there are very few data on TRM in large animals.
To further assess whether S-FLU vaccines could protect from a completely heterosubtypic challenge, where the HA and NA of the vaccine and challenge viruses belonged to different genetic groups, we have tested the protective efficacy of an S-FLU coated in contemporary human H3N2 (group 2) glycoproteins against challenge with an H1N1pdm09 (group 1) virus, in both pigs and ferrets.
Materials and Methods
Vaccines and influenza challenge virus
The design and production of pdmH1N1 S-FLU [eGFP*/N1(Eng195)].H1(Eng/195/2009) has been described previously(3, 5). We made a new H3N2 S-FLU [eGFP*/N2(X217)].H3/SW/9725293/2013 (encoding N2 from A/Victoria/361/2011 from the vaccine strain X217, and coated in H3 from A/Switzerland/9725293/2013) at 1.52 x 108/ml TCID 50 (95%CI 1.13 to 2.05x10 8/ml). The internal protein gene segments were from influenza A/Puerto Rico/8/34 (H1N1).
In the ferret studies, a live attenuated influenza A/Switzerland/9715293/2013 cold adapted (ca) vaccine on the influenza A/Ann Arbor/6/60 ca backbone was included as a comparator and a group of mock-immunized control animals received Leibovitz 15 (L-15) media. Influenza A/California/07/2009 (H1N1pdm09) and A/Switzerland/9715293/2013 (H3N2) viruses were used for challenge infection.
The pig isolate of A/swine/England/1353/09pdmH1N1 (1353/09pdmH1N1) was used for challenge infection in pigs. The homologous vaccine consisted of the identical betapropiolactone inactivated 1353/09pdmH1N1 with TS6 adjuvant. TS6 adjuvant was kindly provided by Dr Catherine Charreyre (Merial/Boehringer Ingelheim). It contains an oily phase (comprising sorbitan monooleate, sorbitan trioleate, paraffin oil and sodium mercurothiolate) and an aqueous phase of monopotassium and disodium phosphate.
Aerosol characterization
We first established that passage through the Aerogen Solo vibrating mesh nebuliser (Aerogen, Ireland) did not significantly reduce the titre of S-FLU. The cell supernatant containing S-FLU in Viral Growth Medium (DMEM/0.1% BSA/10mm HEPES pH7, VGM) was passed through a 0.22 micron filter, then aerosolized using the nebulizer, captured and condensed. The effect of nebulisation on the infectious titer of S-FLU was measured on three different batches of S-FLU coated in three different hemagglutinins by comparison of quadruplicate measurements of the means of the number of doubling dilutions (ie Log2 of the dilution factor) giving 50% infection of MDCK-SIAT cells (calculated by linear interpolation) pre and post nebulization by an unpaired t test (Prism v7.0). H5 (A/Vietnam/1203/2004) -.2938 (95% CI -0.5059 to -0.08161, P .0147) = 18.4% reduction; H7 (A/Netherlands/219/2003) -.2067 (95% CI -0.5808 to 0.1674, P .225) = 13.3% reduction; H3 (A/Victoria/361/2011) -.04246 (95% CI -0.2699 to 0.185, P .664) = 2.9% reduction. Although the 18.4% reduction in titre of the H5 S-FLU after nebulisation was statistically significant, the minimal effects on the H7 and H3 batches did not reach statistical significance. We regard these small effects of the vibrating mesh nebuliser on infectious titer of S-FLU as not biologically significant.
We then assessed the aerosol droplet size distribution using a cascade impactor (Next Generation Impactor, Copley Scientific, UK) at 15 litres per minute vacuum flow rate. A known quantity of virus (2.13-3.39x109 CID50 in 4 ml VGM) was passed into the impactor, and subsequently harvested from each of the impactor stages, which fractionate the aerosol droplets by size. In three replicates the mean aerodynamic size of the aerosol droplets was 1.953 micrometers with a geometric standard deviation (GSD) of 1.795. The fine particle fraction, which is the fraction of the aerosol produced with a droplet size less than 5 micrometers, was 92.34 %, indicating that the aerosol produced was highly respirable.
Animals and immunizations
Pigs
All experiments were approved by the ethical review processes at the Pirbright Institute and Animal and Plant Health Agency (APHA), and conducted according to the UK Government Animal (Scientific Procedures) Act 1986. Both Institutes conform to ARRIVE guidelines. Eighteen, 5-6 week old landrace cross, female pigs were obtained from a commercial high health status herd (average weight of 10 kg at the beginning of the experiment). Pigs were screened for absence of influenza A infection by matrix gene real time RT-PCR and for antibody-free status by HI using four swine influenza virus antigens. Pigs were randomly divided into three groups of six and immunized as follows: 1) Control unimmunized; 2) Homologous (1353/09pdmH1N1) vaccine containing 2048 HAU administered I.M. in 1 ml and 3) H3N2 S-FLU administered by aerosol delivering approximately 1.5×108 TCID50 in 1ml. The animals received an identical booster immunization 21 days later. S-FLU was administered using an Aerogen Solo nebuliser attached to a custom made mask held over the animal’s nose and mouth (Supplemental figure 1) following sedation.
For logistical reasons, two influenza A virus challenges were performed, with half of the animals challenged at 28 days post-boost (dpb) and the remainder at 30 dpb. Animals were challenged intranasally with 1.5×106 PFU (plaque forming unit)/pig of 1353/09pdmH1N1. Two milliliters were administered to each nostril using a mucosal atomization device, MAD300 (Wolfe Tory Medical). As the analysis of samples from pigs challenged at days 28 and 30 did not show any significant differences, for simplicity in presentation the results of the assays carried out on pigs challenged on both days have been amalgamated in all figures.
Ferrets
4- to 6-month-old female ferrets that were seronegative by hemagglutination inhibition (HI) assay to circulating influenza A H1N1pdm09, H3N2 viruses were purchased from Triple F Farms, Sayre, PA. The ferret experiments were conducted in animal BSL2 laboratories at the National Institutes of Health (NIH) in compliance with the guidelines of the Institutional Animal Care and Use Committee. Ferrets were lightly anesthetized with isoflurane and immunized intranasally with two doses of 107 TCID50 in 0.5 ml of A/Switzerland/9715293/2013 S-FLU, 107 TCID50 in 0.5 ml of A/Switzerland/9715293/2013 ca or 0.5 ml of L-15 21 days apart. The ferrets were challenged with 106 TCID50 in 1 ml of influenza A/California/07/2009 (H1N1pdm09) or A/Switzerland/9715293/2013 (H3N2) virus.
Pathological and histopathological examination of pig lungs
Animals were humanely killed 5 days post challenge (dpc). At post mortem the lungs were removed and digital photographs taken of the dorsal and ventral aspects. Macroscopic pathology was scored blind as previously reported (12). Five lung tissue samples per animal from the right lung (2 pieces from the apical, 1 from the medial, 1 from the diaphragmatic and 1 from the accessory lobe) were collected into 10% neutral buffered formalin for routine histological processing at the University of Surrey. Formalin fixed tissues were paraffin wax-embedded and 4-μm sections were cut and routinely stained with haematoxylin and eosin (H&E). Immunohistochemical staining of influenza virus nucleoprotein was performed in 4-μm tissue sections as previuolsy described (13). Histopathological changes in the stained lung tissue sections were scored by a veterinary pathologist blinded to the treatment group. Lung histopathology was scored using five parameters (necrosis of the bronchiolar epithelium, airway inflammation, perivascular/bronchiolar cuffing, alveolar exudates and septal inflammation) scored on a 5-point scale of 0 to 4 and then summed to give a total slide score ranging from 0 to 20 and a total animal score from 0 to 100 (6). The Iowa system includes both histological lesions and immunohistochemical staining for nucleoprotein, NP (14).
Tissue sample processing
Pigs
Four nasal swabs (two per nostril) were taken daily after the challenge. Serum and heparin anti-coagulated blood samples were collected at the start of the study (prior to immunization) and at days 7, 14, 21, 28, 35, 42 and 49 after the first immunization. Broncho-alveolar lavage (BAL) and tracheobronchial lymph nodes (TBLN) were processed as previously described (6). Medial and diaghramatic lung cells were dissociated into a single cell suspension with the GentleMACS Octo dissociator (Miltenyi, UK), using C tubes (Miltenyi) with 5ml of serum free RPMI containing collagenase and DNAse (Sigma). Following dissociation the tubes were incubated at 37°C for 20 min and the resulting suspension mashed through a tea strainer using complete RPMI, the single cell suspension was filtered twice using a 100µm cell strainer, washed and red blood cells lysed. Cells were washed and cryopreserved.
Ferrets
Four ferrets from each group were sacrificed at 2 and 4 dpc and their lungs and nasal turbinates (NT) were harvested. Harvested tissues were homogenized in L-15 medium at 10% (w/v) for lung or 5% (w/v) for NT samples clarified by centrifugation at 2500 rpm for 10 min.
Transmission studies in ferrets
We performed airborne transmission studies using a caging system previously described (15). Briefly, four adult ferrets obtained from Triple F farms that were seronegative by HAI to citculating H1N1pdm09 and, H3N2 viruses were anesthetized by i.m. injection of a ketamine-xylazine mixture prior to i.n.immunization with two doses of H3N2 S-FLU or with L-15 medium alone (mock immunized). Twenty-one days after the second dose, ferrets were challenged with 106 TCID50 of A/California/07/2009 virus. Challenged ferrets were placed into the section of the cage closest to the air inlet the day of challenge. One day later, a naive ferret was placed into the cage on the other side of the divider. Environmental conditions inside the laboratory were monitored daily and were consistently 19±0.3°C and 60±2.2% relative humidity. The transmission experiments were conducted in the same room, to minimize any effects of caging and airflow differences on aerobiology. The naive ferret was always handled before the infected ferret. Animals were carefully handled during nasal wash collections and husbandry in order to ensure no direct contact occurred between the ferrets. Nasal washes were collected every other day for 14 days and analyzed for the presence and titer of infectious viruses and expressed as 50% tissue culture infectious doses (TCID50) per mL. On day 14 post-infection, blood was collected from each animal and serology was performed by HAI and MN assays.
Virus titration
Viral titers in nasal swabs and BAL from pigs were determined by plaque assay on MDCK cells as previously described (6). Clarified homogenates of ferret tissues were titrated on MDCK cell monolayers and virus titers were calculated by the Reed and Muench method and expressed as log10 TCID50/g.
Microneutralization assay
Neutralizing antibody titers in serum were determined as previously described (3, 16)
IFN-γ ELISPOT
Frequencies of IFNγ-secreting pig PBMC and BAL cells were determined by ELISPOT using fresh or cryopreserved cells (6). Cells were stimulated with either 1 x 106 PFU live MDCK-grown1353/09pdmH1N1, 1×105 TCID50 H3N2 S-FLU, medium control or 10μg/ml Con A (Sigma-Aldrich). Results were expressed as number of IFNγ-producing cells per 106 cells after subtraction of the average number of IFNγ+ cells in medium control wells.
Flow cytometry
Cryopreserved mononuclear cells from blood, TBLN, BAL, spleen and lung were thawed and stimulated for 12 hours at 37°C with either 1 x106 PFU live MDCK-grown strain 1353/09pdmH1N1 or 1 x 106 TCID50 H3N2 S-FLU or MDCK mock supernatant as control. GolgiPlug (BD Biosciences) was added for a further 4 hours before intracellular cytokine staining. Cells were stained for surface markers with CD3ε-AF647 BB23-8E6-8C8, CD4 clone 74-12-4 PerCpCy5.5, CD8α-FITC 76-2-11 (BD Biosciences) and Near-Infrared Fixable Live/Dead stain (Invitrogen). Cells were permeabilized using Cytofix Cytoperm (BD Biosciences) before intracellular staining with IFN-γ PE P2G10 (BD Biosciences) and cross-reactive anti-human TNFα-AF650 Mab11 (Biolegend). Samples were fixed in 1% paraformaldehyde before analysis using an LSRFortessa (BD Biosciences). Data was analysed using FlowJo v10 (Treestar).
Lung tissue resident memory cells
Before sacrifice three animals from each group were infused intravenously with 10 ml of 3.24 mg/ml purified CD3 antibody (clone PPT3) and sacrificed 10 minutes later. Lymphocytes were isolated and stained ex vivo with anti-mouse Ig-FITC (Southern Biotec) which labels the circulating intravascular cells. The cells are washed and normal mouse serum is then added to block any remaining binding capacity of the anti Ig-FITC. The cell are then washed again and incubated with CD3 antibody labelled with PeCy5 (AbCam). This will bind unsaturated sites of the cirucalting cells which are therefore double labelled as well as all the sites on the TRM which are not accessible to the CD3 antibody given intravenously. TRM as therefore single labelled with PeCy5.
To allow intracytoplasmic staining of TRM the i.v. CD3 was detected with goat anti-mouse IgG BV421 (Biolegend) and blocked using normal mouse serum as above. Surface markers used were CD3ε-biotin PPT3 (AbCam), CD4 clone 74-12-4 PerCpCy5.5, CD8α-FITC 76-2-11 (all BD Biosciences) and Near-Infrared Fixable Live/Dead stain (Invitrogen). Biotinylated CD3 was visualised with a streptavidin AF647 (Biolegend). Cells were permeabilized using Cytofix Cytoperm before intracellular staining with IFN-γ PE P2G10 (BD Biosciences) and cross-reactive anti-human TNFα-AF650 Mab11 (Biolegend). Samples were fixed in 1% paraformaldehyde before analysis using an LSRFortessa.
Statistical analysis
One-way or two-way ANOVA with Dunnets post-test for multiple comparisons were used to compare immunized groups to the control group, analysis was performed using GraphPad Prism 6.
Results
Viral load and lung pathology in pigs
Groups of 6 pigs were immunized twice 3 weeks apart with an inactivated virus with adjuvant intramuscularly (Homologous inactivated) or with H3N2 S-FLU by aerosol (S-FLU). The control group was unimmunized (control). Aerosol immunization was carried out using a purpose built mask and Aerogen Solo nebuliser which allowed efficient vaccine delivery in less than 5 min to the sedated animals after we had established that the nebuliser did not affect the titer of the S-FLU vaccines and provided a droplet size appropriate for delivery to the lower respiratory tract (Supplemental Figure 1). Four weeks after the second immunization the animals were challenged intranasally with swine isolate of pandemic H1N1 A/Sw/Eng/1353/09 (1353/09pdmH1N1) virus and sacrificed 5 days post challenege (dpc).
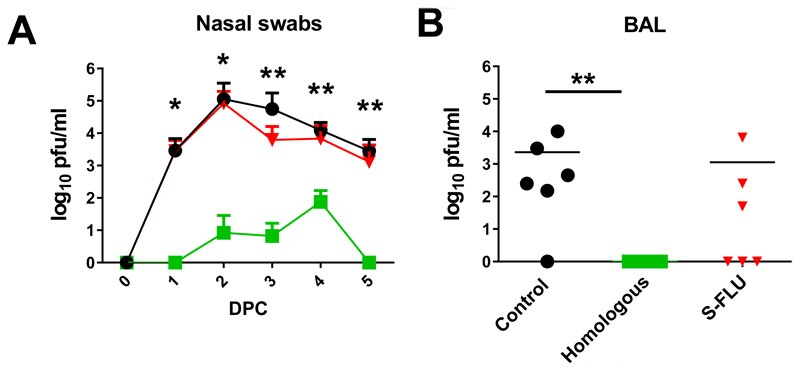
The pigs immunized with the Homologous inactivated vaccine showed the greatest and statisctically significant reduction of challenge virus in the nasal swabs at 1, 2, 3, 4 and 5 dpc (Fig 1A). S-FLU did not reduce viral shedding in nasal swabs significantly at any day post challenge (Fig 1A), although 2 out of the 6 S-FLU immunized animals did not shed virus on day 5. No virus was detected in the BAL of the Homologous inactivated vaccine group, and although S-FLU reduced viral load in the BAL, with no virus in 3 animals, this reduction was not statistically significant (Fig 1B).
Figure 1. Viral load in nasal swabs.
Pigs were immunized twice 21 days apart with either homologous inactivated vaccine by the I.M. route (Homologous) or H3N2 S-FLU by aerosol (S-FLU). Controls were unimmunized pigs. Animals were challenged with 1353/09pdmH1N1 28 or 30 days after the boost. Nasal swabs were taken at 0, 1, 2 3, 4, 5 and pigs sacrificed at 5 dpc. As the analysis of samples from pigs challenged at days 28 and 30 did not show any significant differences, for simplicity in presentation the results of the assays carried out on pigs challenged on both days have been amalgamated in this and other figures.Viral titres in the nasal swabs (A) and BAL (B) were determined by plaque assay. The mean value for shedding for each group is shown over the 5 days (A). Each data point represents an individual within the indicated group and bars represent the mean (B). * denotes significant difference between the indicated groups and controls * p < 0.05, ** p < 0.01 determined using one-way ANOVA with Dunn’s test for multiple comparison
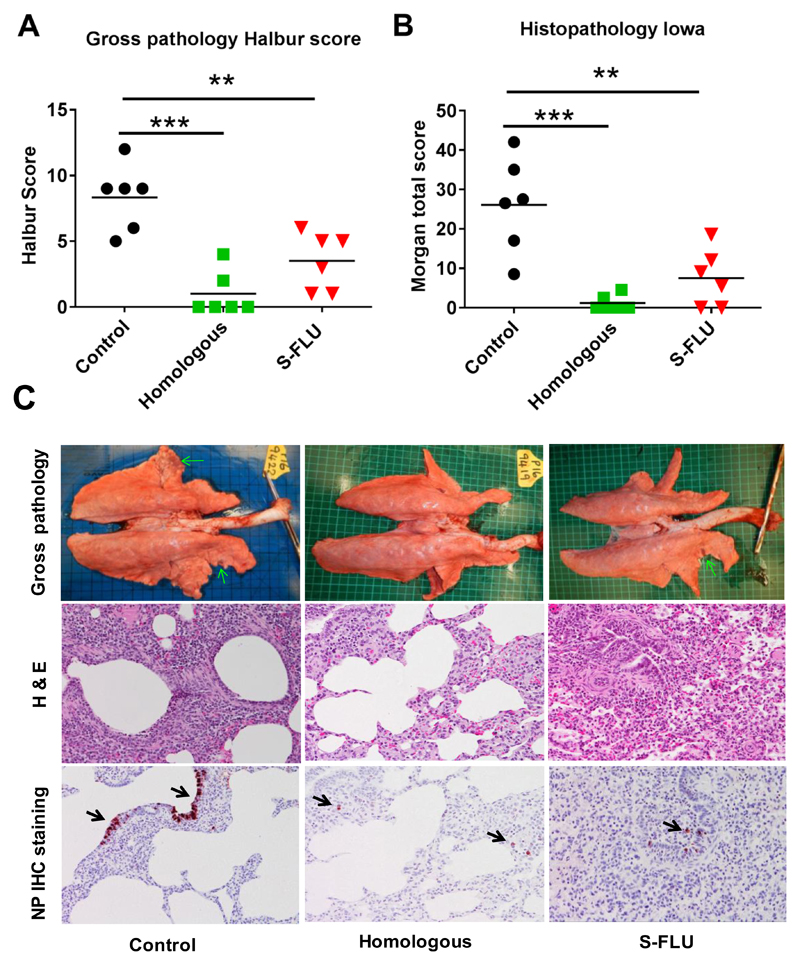
Following challenge the unimmunized animals developed typical gross lesions of influenza virus infection (17). Histopathology showed lesions consisting of severe multifocal interstitial pneumonia, attenuation of the bronchial and bronchiolar epithelium, presence of inflammatory infiltrates within the interalveolar septa and the alveolar lumen, and oedema. Immunohistochemical detection of influenza virus nucleoprotein (NP) showed many positive cells within the endothelium of bronchi and bronchioles (Fig 2).
Figure 2. Gross and histopathology.
Pigs were immunized twice 21 days apart with either homologous inactivated vaccine I.M. (Homologous) or H3N2 S-FLU by aerosol (S-FLU). Controls were unimmunized animals. Animals were challenged with 1353/09pdmH1N1 on 28 or 30 days after the boost. Animals were sacrificed at 5 dpc and lungs scored for appearance of gross pathology (A) and histopathological lesions (B). Each data point represents an individual within the indicated group and bars represent the mean * denotes significant difference from the control group ** p < 0.01, *** p < 0.005. ****p<0.0001 determined using one-way ANOVA with Dunn’s test for multiple comparisons. (C) Representative lungs, H & E and immunohistochemical NP staining of representative sections for each group are shown.
Animals immunized with the Homologous inactivated vaccine showed very few gross pathological lesions. Histologically, only a few lung sections showed mild interstitial pneumonia and necrosis of the bronchial and bronchiolar epithelium. Virus NP immunostaining was restricted to very few inflammatory cells within the interalveolar septa. The S-FLU immunized animals showed small areas of gross pathology. Histopathology showed mild to moderate interstitial pneumonia, oedema and epithelial necrosis within the bronchi and bronchioles. Few bronchiolar epithelial and inflammatory interstitial cells exhibited nucleoprotein immunostaining (Fig 2).
These results indicated that immunization of pigs with a group 2 H3N2 S-FLU significantly reduced gross and histopathology, but did not significantly reduce the viral load in nasal swabs and BAL after heterologous challenge with group 1 H1N1pdm09 virus.
Antibody and IFNγ ELISPOT responses in pigs
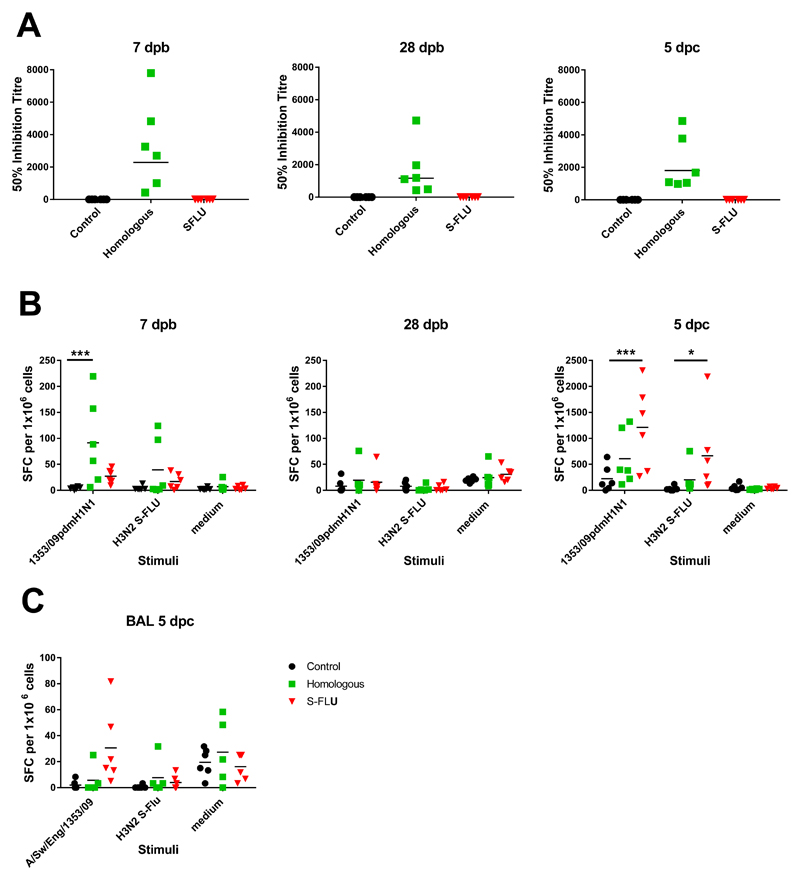
We determined the antibody response in pigs using microneutralization assay. Sera from the Homologous inactivated vaccine group had neutralizing antibody, with mean inhibitory titers of 1:2291 at 7 days post boost (dpb), 1:1166 at 28 dpb and at 1:1801 at 5 dpc. This indicates that the Homologous inactivated vaccine was successfully delivered and induced anti-1353/09pdmH1N1 neutralizing antibodies as expected. Also as expected, no neutralizing antibody to the H1N1 virus was detected in the S-FLU or in the unimmunized controls (Fig 3A).
Figure 3. Antibody and IFNγ ELISPOT responses in pigs.
Pigs were immunized twice 21 days apart with either homologous inactivated vaccine I.M. (Homologous) or S-FLU by aerosol (S-FLU). Animals were challenged with 1353/09pdmH1N1 on 28 or 30 days after the boost. (A) Individual 50% inhibition titers in the serum at 7 days post boost (7 dpb), 28 dpb just before the challenge and 5 days post challenge (dpc). Numbers of IFNγ spot forming cells (SFC) in PBMC (B) and BAL (C) were determined by ELISPOT following stimulation with the challenge virus 1353/09pdmH1N1 or H3N2 S-FLU in vitro. Results for 1353/09pdmH1N1 and H3N2 S-FLU stimulation were expressed as number of IFNγ-producing SFC per 106 cells after subtraction of the average number of IFNγ+ cells in medium control wells. Cells cultured in medium alone are also shown to indicate the background obtained. Each data point represents an individual within the indicated group, * denotes significant difference from the control group * p < 0.05, *** p < 0.005 determined using two-way ANOVA with Dunnet’s test for multiple comparisons.
We determined influenza-specific T cell responses in PBMC in pigs by IFNγ ELISPOT at 7 dpb and 28 dpb, just before the challenge, and at the time of sacrifice 5 dpc. PBMC were stimulated with either the challenge virus 1353/09pdmH1N1 or with H3N2 S-FLU. Both homologous inactivated vaccine and S-FLU immunized animals showed a virus specific response to the challenge virus at 7 days post boost (dpb), which was higher in the homologous inactivated group (mean 91 for the homologous inactivated vaccine and 27 for S-FLU SFC per 106 cells). The response to stimulation with H3N2 S-FLU was minimal (mean 39 SFC in homologous inactivated and 17 SFC in S-FLU group). At 28 dpb, just before the challenge, the response was undetactable in any of the groups. At 5 dpc the S-FLU immunized animals showed the strongest response to both 1353/09pdmH1N1 and H3N2 S-FLU stimulation (mean 665 and 1211 SFC per 106 cells for homologous inactivated vaccine and S-FLU groups respectively) (Fig 3B). The reduced response in the homologous inactivated vaccine group was most likely due to the lack of antigen stimulation because of the greatly reduced influenza A viral load in these animals.
The response in the BAL showed a similar trend. However the detectable response was apparently much weaker (~ 30 SFC per 106 cells for S-FLU) because of the low percentage of T cells in BAL. There was also a high medium control background most likely because of the presence of many activated cells in the BAL (Fig 3C). td. These data show that as expected the Homologous inactivated vaccine induced a strong antibody response, while in contrast S-FLU did not induce detectable neutralizing antibody, but these animals had the highest number of IFNγ producing cells following stimulation with either 1353/09pdmH1N1 or H3N2 S-FLU post challenge.
Analysis of cytokine producing cells
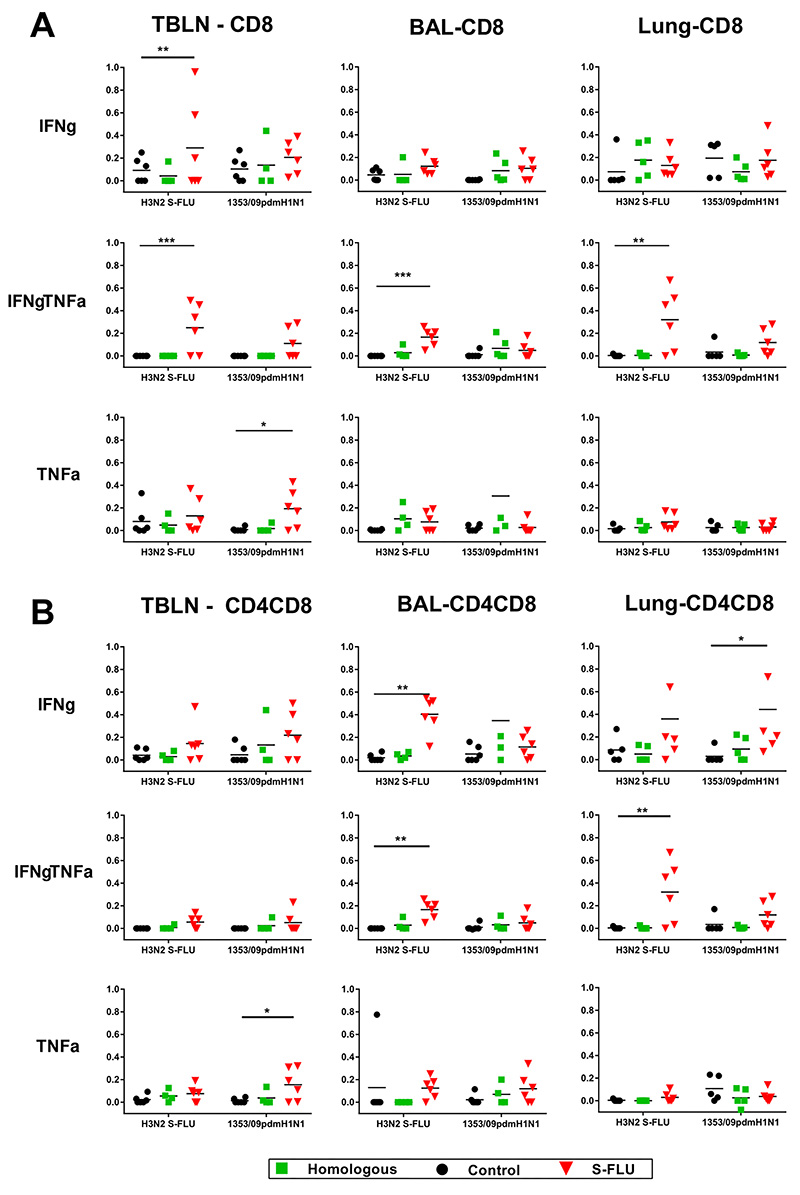
In order to dissect which cells produce cytokines, we performed intracellular staining for IFNγ and TNFα combined with surface staining for CD4, CD8 and CD4CD8 cell subsets. The latter are the activated memory CD4 cells in pigs (18).To analyze local immune responses tracheobronchial lymph nodes (TBLN), lung and BAL cells were stimulated with either the challenge virus 1353/09pdmH1N1 or with H3N2 S-FLU. S-FLU immunization induced the highest proportion of double IFNγTNFα cytokine producing CD8 cells in LN, BAL and lung, followed by single IFNγ or single TNFα producers in the TBLN (Fig 4A). Similarly the S-FLU immunized animals had the highest proportion of CD4CD8 cells producing single IFNγ and double IFNγTNFα cytokines in the BAL and lung (Fig 4B). A statistically significant proportion of single TNFα producers was observed in TBLN CD8 and CD4CD8 cells. The CD4 response was negligible in all tissues and is not shown. In contrast to the local tissues, systemic responses analysed in PBMC and spleen showed lower proportions of CD8 or CD4CD8 antigen specific cells (data not shown).
Figure 4. Cytokine producing cells in pig tracheobronchial lymph nodes (TBLN), BAL and lung tissues.
Flow cytometry was used to quantitate the frequency of IFNγ, IFNγTNFα and TNFα positive cells within CD8hi (A) and CD4+CD8+ (B) cells at 5 dpc. Cells were stimulated with either challenge virus 1353/09pdmH1N1 or H3N2 S-FLU. Each data point represents an individual within the indicated group, * denotes significant difference from the control group * p < 0.05, ** p<0.01, *** p < 0.005 determined using two-way ANOVA with Dunnet’s test for multiple comparisons.
In summary, the S-FLU immunization induced a strong local lung response to influenza A virus and S-FLU. The high frequency of these single and double IFNγ and TNFα producers may account for the protective efficacy of local immunization.
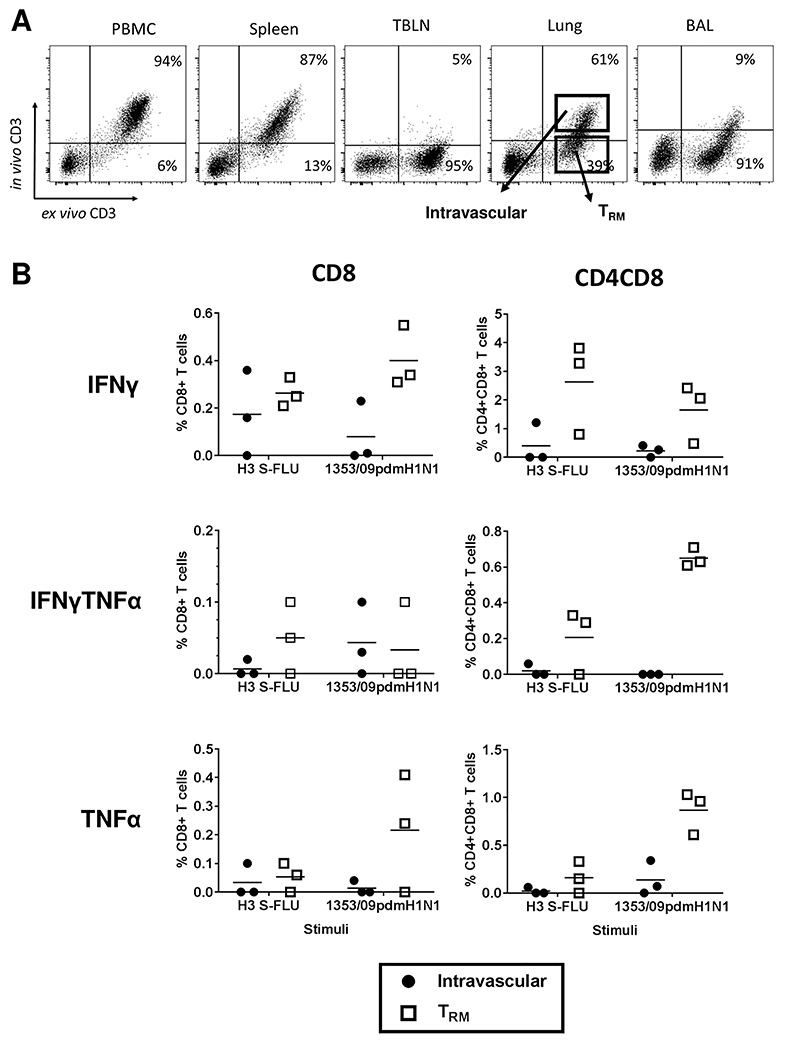
Tissue resident memory cells (TRM)
Since we have shown that aerosol immunization with H3N2 S-FLU induced a strong local immune response, we wished to establish whether the responding cells were part of the TRM population. To distinguish TRM in the lungs of pigs from circulating cells present in the vasculature of the tissue, we administered CD3 monoclonal antibody intravenously 10 minutes before sacrificing the animal. After sacrifice, the lymphocytes were isolated and stained ex vivo with anti-mouse IgG-FITC and with the same clone of CD3 directly labelled with PeCy5. As the infused CD3 does not saturate all CD3 sites, blood, spleen and some lung tissue T cells are double positive (intravascular cells) (Fig 5A). In contrast most BAL and some lung tissue cells are only CD3PeCy5 positive indicating that they are inaccessible to the antibody in the blood (TRM). Lymph nodes were inaccessible to the infused CD3 antibody, as has been shown to be the case by others (19). There was no difference in the proportions of TRM and intravascular cells in the immunised and control pigs (data not shown). A similar pattern has been observed in more than 20 animals from other studies.
Figure 5. Porcine lung TRM.
(A) Before sacrifice three pigs from each group were infused intravenously with CD3 antibody and sacrificed 10 minutes later. Lymphocytes were isolated and stained ex vivo with anti-mouse Ig-FITC and the same CD3 antibody labelled with PeCy5. As the infused CD3 does not saturate all CD3 sites, blood, spleen and some lung tissue T cells are double positive (intravascular cells). BAL and some of lung cells are unstained by intravascular antibody (TRM). (B) Lower panels show IFNγ and TNFα production by intravascular and TRM CD8 and CD4CD8 cells in H3N2 S-FLU immunized animals after in vitro stimulation with either challenge virus 1353/09pdmH1N1 or H3N2 S-FLU.
As BAL is 90% stained only by the ex vivo CD3PeCy5, while the blood and spleen are double labelled, this indicates that most BAL cells are part of the blood inaccessible pool of TRM cells. Because BAL gives a strong antigen specific response we can conclude that S-FLU is inducing lung TRM. Our staining in lung indicates that a proportion of lung T cells (39%) are inaccessible to intravenous CD3 antibody and therefore also part of the TRM population. The TRM in the three S-FLU immunized animals treated with intravenous antibody, had a higher proportion of antigen specific cells producing IFNγ and TNFα than the lung intravascular population (Fig 5B) following stimulation with either the challenge virus 1353/09pdmH1N1 or with H3N2 S-FLU. The comparison between intravascular and TRM cells in the Homologous inactivated and control groups is unreliable, because there were few responding cells and therefore very few events in the gated populations (data not shown). These results demonstrate that aerosol immunization with H3N2 S-FLU induces a large lung tissue resident memory population.
Evaluation of the H3N2 S-FLU vaccine in ferrets
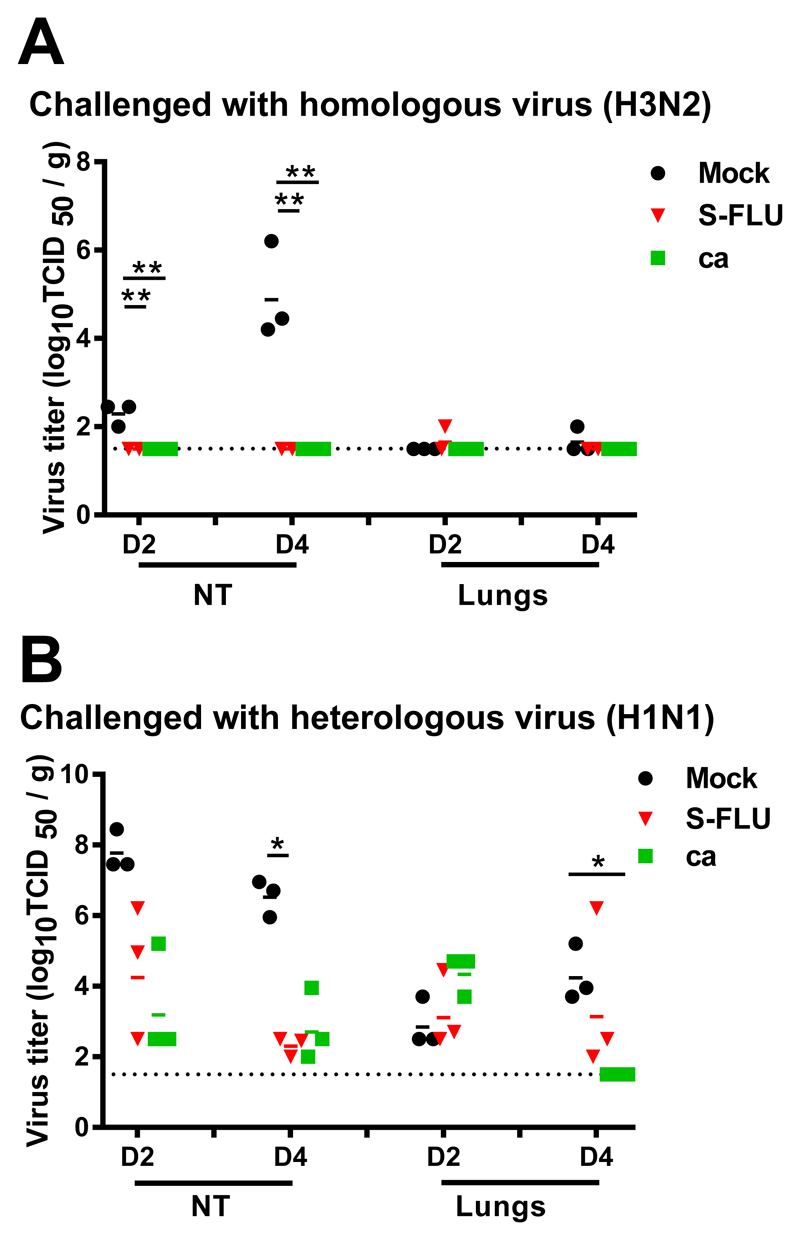
We next determined the protective efficacy of the same batch of H3 SFLU in ferrets, using as a positive control live attenuated virus H3N2 cold-adapted (ca) as we have previously used similar ca viruses for this purpose in ferrets (5). We also immunised intranasally rather than by aerosol as in anesthetized small animals, intranasal administration has been shown to reach the lungs (20, 21). Groups of 12 ferrets were immunized intranasally twice with H3N2 S-FLU or H3N2 ca and 12 ferrets were mock-vaccinated. Three weeks later, half the animals in each group (n=6 per group) were challenged with intranasally delivered homologous wild-type (wt) influenza A/Switzerland/9715293/2013 (H3N2) and the other half with a heterologous influenza A/California/07/2009 (H1N1pdm09) virus. On days 2 and 4 post-challenge, three ferrets in each group were sacrificed and virus titers in lungs and nasal turbinates were assessed. The homologous wt H3N2 virus did not replicate in the lower respiratory tract of mock-immunized (or vaccinated) ferrets (Fig 6A) so the efficacy of the vaccines in protecting against pulmonary virus replication could not be assessed. However wt H3N2 virus replicated to moderately high titer (mean 104.95TCID50/g) in the nasal turbinates (NT) at 4 dpc and both vaccines prevented replication of the challenge virus in the NT (p<0.05).
Figure 6. Virus replication in respiratory tissues of ferrets immunized and challenged with homologous or heterologous virus.
Ferrets were lightly anesthetized with isoflurane and immunized intranasally with two doses of 107 TCID50 in 0.5 ml of A/Switzerland/9715293/2013 S-FLU, 107 TCID50 in 0.5 ml of A/Switzerland/9715293/2013 ca or 0.5 ml of L-15 21 days apart. The ferrets were challenged with 106 TCID50 in 1 ml of influenza A/Switzerland/9715293/2013 (H3N2) (A) or A/California/07/2009 (H1N1pdm09) (B) virus. Ferrets from each group were sacrificed on days 2 (D2) and 4 (D4) post-infection and viral load in their lungs and nasal turbinates (NT) determined and expressed as log10 TCID50/g. Horizontal bars represent mean titers and symbols represent titers from individual ferrets. * p<0.05.
The H1N1pdm09 virus that was used for heterosubtypic challenge replicated to high titer in the NT (mean 107.8 and 106.5 TCID50/g at 2 and 4 dpc, respectively) and to modest to moderate titer (mean 102.9 and 104.3 TCID50/g at 2 and 4 dpc) in the lungs (Fig 6B). Both the S-FLU and ca vaccine viruses provided modest reduction (104.6 and 103.4 TCID50/g, respectively) in H1N1pdm09 titers in the NT at 2 dpc and further reduction (102.3 and 102.8, respectively) at 4 dpc though only the S-FLU group on day 4 was significantly different from the mock-immunized group (p<0.05). A statistically significant reduction in lung virus titers was observed on day 4 post-challenge, only in animals that had received the H3N2 ca virus vaccine (p=0.002).
Effect of H3N2 S-FLU vaccine on transmission in ferrets
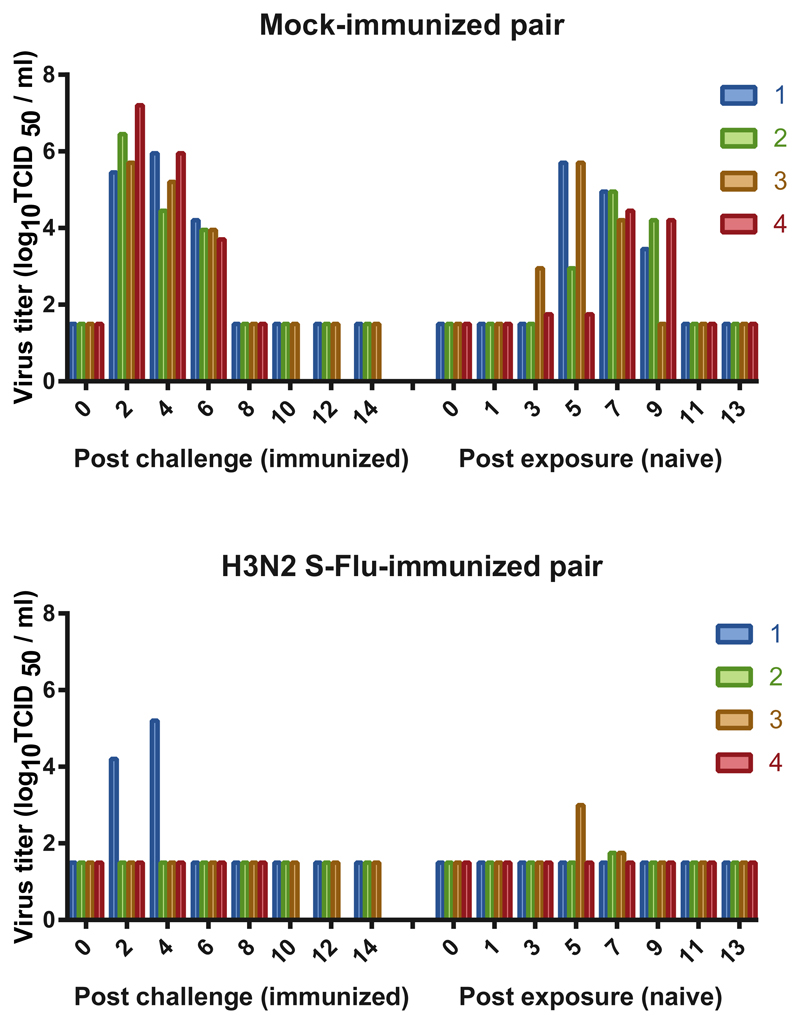
We next determined whether immunization with H3N2 S-FLU would prevent transmission of the heterologous H1N1pdm09 challenge virus. Four ferrets each were vaccinated intranasally with two doses of H3N2 S-FLU and 4 ferrets were mock vaccinated; the ferrets were challenged intranasally with influenza A/California/07/2009 (H1N1pdm09) 21 days after the second dose of vaccine and were placed in transmission cages. The following day, an unvaccinated naïve ferret was introduced adjacent to each infected ferret. The experimentally infected and respiratory contact ferrets were followed with nasal washes and serology to determine whether the H1N1pdm09 virus transmits by the respiratory route from experimentally infected to airborne contact ferrets. One ferret each in the mock-immunized and H3N2-S-FLU vaccinated group reached their humane endpoints 9 days post-challenge from an intercurrent infection in the animal house. Unfortunately, we were not able to identify the etiology of the intercurrent infection that caused weight loss in ferrets. None of the ferrets were found dead. They were euthanized in accordance with our approved animal study protocol due to weight loss. The virus inoculum was not contaminated with bacteria and the virus dose was confirmed to be correct.
All four of the mock-immunized ferrets were infected with H1N1pdm09 and transmitted to their airborne contacts (Fig 7). Infection with and transmission of the H1N1pdm09 virus was greatly reduced in frequency and viral load in the animals that were immunized with H3N2 S-FLU vaccine and their airborne contacts. Challenge virus was detected in nasal washes of 1 of 3 H3N2 S-FLU-vaccinated ferrets but all 3 seroconverted. Virus was detected in 2 of 3 of the corresponding airborne contact animals at low titer for a limited period but neither of the contact animals seroconverted. Thus, the H3N2 S-FLU vaccine severely restricted shedding of the H1N1pdm09 challenge virus, though infection did occur. Although airborne transmission occurred, the intensity of the transmitted infection to airborne contact ferrets was markedly reduced with low titer, short duration viral shedding and no seroconversion in airborne contact ferrets.
Figure 7. Ferrets immunized with H3N2 vaccine were protected against challenge infection with H1N1pdm09 virus and the transmission to naïve animals was restricted.
Four ferrets were vaccinated intranasally with two doses H3N2 S-FLU and 4 ferrets were mock vaccinated. Twenty one days after the second immunization the ferrets were challenged intranasally with influenza A/California/07/2009 (H1N1pdm09) and the ferrets were placed in transmission cages. The following day, an unvaccinated naïve ferret was introduced adjacent to each infected ferret. Nasal washes were collected every other day for 14 days and virus titers in the experimentally infected and airborne contact ferrets are presented. Each bar represents a single ferret. One ferret each in the mock-immunized and H3N2-S-FLU vaccinated group reached their humane endpoints 9 days post-challenge from an intercurrent infection in the animal house (etiology not identified).
Discussion
There is strong epidemiological evidence for an association between a cross-reactive T cell response and heterologous protection between group 1 and 2 influenza A viruses in humans (22, 23). In the first of these studies the association with the T cell response was predominantly with reduced fever and symptoms (22), whereas in the second the T cell response to NP was associated with reduced viral shedding in symptomatic volunteers (23). In these studies the correlation was with the T cell response in peripheral blood that had likely been induced by prior natural infection. This evidence, combined with a long history of animal studies demonstrating the protective effect of T cells induced by live influenza virus infection (24) provides the rationale for developing a safe and BPIV that induces a strong T cell response in the human lung. Here we compared the outcome of heterosubtypic influenza virus challenge after S-FLU vaccination in both pigs and ferrets. H3N2 S-FLU immunization of pigs had a minimal effect on H1N1pdm09 replication after challenge but a significant effect on pathology. By contrast in the ferret the same vaccine preparation induced sterile immunity to the matched H3N2 challenge, and reduced replication and aerosol transmission to naïve recipients following H1N1pdm09 challenge. Our results also show that aerosol delivery of H3N2 S-FLU vaccine is safe and induced strong local lung immune responses and TRM in BAL and lung tissues of pigs.
In earlier experiments in ferrets and mice in which there was a complete mismatch between the immunizing H1N1 or H5N1 (group 1) S-FLU and challenge H3N2 and H7N9 (group 2) we observed a significant reduction in replication of the infectious challenge virus (3, 5). In the present study we used another mismatched immunization and challenge combination and confirmed a significant effect on replication of the challenge virus in ferrets but not pigs. A possible reason for the difference between pigs and ferrets might be that the H3N2 S-FLU used here is coated with the clade 3C.3a H3 haemagglutinin. This H3 is exquisitely specific for alpha2-6 sialic acid, but has low affinity (25) and although the pig respiratory tract expresses both alpha2-3 and 2-6 (26), it is possible that the binding of H3 to the pig respiratory tract is poor. Experiments with S-FLU coated in different HAs may resolve this. Another possibility is that although H3N2 S-FLU induced a strong local response against the immunizing and challenge viruses, this was insufficient to prevent replication of the challenge virus. We speculate that a higher dose of vaccine might be required as earlier work in pigs immunized with attenuated influenza showed a reduction in challenge virus replication despite mismatching (27). Further work to examine whether a higher dose of vaccine is required to fully protect the lungs of large animals need to be performed.
In other experiments in small animals the effect of fully heterosubtypic immunization was similar to what we observe in pigs. For example a single-replication cycle H1N1 (group 1) BPIV based on the partial deletion of the M2 gene (28) induced sterilising immunity against matched challenge and protected mice against death from heterosubtypic H3N2 (group 2) challenge, but did not prevent viral replication in NT and lung. The protective effect was associated with the induction of crossreactive T cells but not antibody, and a reduced inflammatory neutrophil infiltrate in the lung. In ferrets with partial matching of a single cycle live attenuated virus, where vaccine (H1N1, group 1) and challenge (H5N1, group 1) were selected from the same genetic group, protection was associated with the induction of crossreactive antibody to the conserved group 1 haemagglutinin stem and the N1 neuraminidase in addition to crossreactive T cells, and challenge viral replication was reduced (29). These results suggest that protective immune responses to live attenuated or single cycle viruses may be cumulative, and partial matching between vaccine and challenge within the same genetic group can add incremental protective value through the induction of crossreactive antibodies, as strongly suggested by the epidemiology of human infections (30). Unfortunately the group of origin of future pandemic or even seasonal viruses cannot be predicted.
It is increasingly evident that local immune responses and particularly lung TRM play a major role in protection against influenza viruses in mice (9, 10, 31). Pulmonary TRM in the BAL and lung tissues, have greater protective capacity than circulating memory CD8 T cells (9, 32, 33). BAL TRM are associated with reduced symptoms and viral load in respiratory syncytial virus infection in humans (34). Here for the first time we show that we can distinguish TRM in pigs as has been shown in mice following intravenous administration of CD3 antibody. We demonstrate that more than 90% of BAL cells are inaccessible to intravascular antibody as well as a proportion of the lung tissue cells. Aerosol immunization with H3N2 S-FLU induces a strong immune response of these cells, which may not reduce viral replication, but may be able to induce a beneficial reduction in local inflammation, through the release of immuno-modulating cytokines (35–37).
In summary our data show that the same vaccine has different protective efficacy in pigs and ferrets. In the absence of antibody, lung T cell immunity can consistently reduce disease severity, but does not always abolish viral replication. We suggest that candidate BPIV should be tested in more than one species. The pig maybe a relevant large animal model because it is a natural host for influenza viruses and has very similar respiratory anatomy to humans.
Supplementary Material
Acknowledgements
We thank Peter Beverley for helpful discussion and critical reading of the manuscript. We are grateful to the animal staff at the Pirbright Institute and at the Animal and Plant Health Agency for excellent animal care.
This work was funded by “Bill & Melinda Gates Foundation” grant OPP1148786, the Biotechnology and Biological Sciences Research Council grant BBS/E/I/00007031 and sLoLa grant BB/L001330/1, the Townsend-Jeantet Prize Charitable Trust (registered charity No 1011770). KS and YM were supported by the Intramural Research Program of NIAID, NIH. The challenge swine influenza strain was isolated and characterised under Defra project SV3041 ‘Monitoring of influenza A viruses in the UK pig’.
Footnotes
Abbreviations used in this article: IAV, influenza A virus; HA, haemagglutinin; MDCK, Madin-Darby canine kidney; BAL, broncho-alveolar lavage; RPMI, Roswell Park Memorial Institute; ELISPOT, Enzyme Linked ImmunoSpot assay.
Disclosure
AT is named on a European patent (publication no. EP2758525 A2, published July 30, 2014) concerning the use of S-FLU as a vaccine, which is owned jointly by the University of Oxford and the Townsend - Jeantet Charitable Trust (Registered Charity Number 1011770). The other authors declare no competing financial interests.
References
- 1.Paules CI, Marston HD, Eisinger RW, Baltimore D, Fauci AS. The Pathway to a Universal Influenza Vaccine. Immunity. 2017;47:599–603. doi: 10.1016/j.immuni.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Nogales A, Baker SF, Domm W, Martinez-Sobrido L. Development and applications of single-cycle infectious influenza A virus (sciIAV) Virus research. 2016;216:26–40. doi: 10.1016/j.virusres.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powell TJ, Silk JD, Sharps J, Fodor E, Townsend AR. Pseudotyped influenza A virus as a vaccine for the induction of heterotypic immunity. Journal of virology. 2012;86:13397–13406. doi: 10.1128/JVI.01820-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend AR, Gotch FM, Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985;42:457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- 5.Baz M, Boonnak K, Paskel M, Santos C, Powell T, Townsend A, Subbarao K. Nonreplicating Influenza A Virus Vaccines Confer Broad Protection against Lethal Challenge. MBio. 2015;6 doi: 10.1128/mBio.01487-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan SB, Hemmink JD, Porter E, Harley H, Holly H, Aramouni M, Everett HE, Brookes S, Bailey M, Townsend AM, Charleston B, et al. Aerosol Delivery of a Candidate Universal Influenza Vaccine Reduces Viral Load in Pigs Challenged with Pandemic H1N1 Virus. Journal of immunology (Baltimore, Md : 1950) 2016;196:5014–5023. doi: 10.4049/jimmunol.1502632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. Journal of immunology (Baltimore, Md : 1950) 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, Southern PJ, Masopust D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- 13.Vidana B, Martinez J, Martinez-Orellana P, Garcia Migura L, Montoya M, Martorell J, Majo N. Heterogeneous pathological outcomes after experimental pH1N1 influenza infection in ferrets correlate with viral replication and host immune responses in the lung. Veterinary research. 2014;45:85. doi: 10.1186/s13567-014-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauger PC, Vincent AL, Loving CL, Henningson JN, Lager KM, Janke BH, Kehrli ME, Jr, Roth JA. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet Pathol. 2012;49:900–912. doi: 10.1177/0300985812439724. [DOI] [PubMed] [Google Scholar]
- 15.Lakdawala SS, Lamirande EW, Suguitan AL, Jr, Wang W, Santos CP, Vogel L, Matsuoka Y, Lindsley WG, Jin H, Subbarao K. Eurasian-origin gene segments contribute to the transmissibility, aerosol release, and morphology of the 2009 pandemic H1N1 influenza virus. PLoS pathogens. 2011;7:e1002443. doi: 10.1371/journal.ppat.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang KY, Rijal P, Schimanski L, Powell TJ, Lin TY, McCauley JW, Daniels RS, Townsend AR. Focused antibody response to influenza linked to antigenic drift. The Journal of clinical investigation. 2015;125:2631–2645. doi: 10.1172/JCI81104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brookes SM, Nunez A, Choudhury B, Matrosovich M, Essen SC, Clifford D, Slomka MJ, Kuntz-Simon G, Garcon F, Nash B, Hanna A, et al. Replication, pathogenesis and transmission of pandemic (H1N1) 2009 virus in non-immune pigs. PloS one. 2010;5:e9068. doi: 10.1371/journal.pone.0009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerner W, Talker SC, Koinig HC, Sedlak C, Mair KH, Saalmuller A. Phenotypic and functional differentiation of porcine alphabeta T cells: current knowledge and available tools. Molecular immunology. 2015;66:3–13. doi: 10.1016/j.molimm.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nature protocols. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronan EO, Lee LN, Tchilian EZ, Beverley PC. Nasal associated lymphoid tissue (NALT) contributes little to protection against aerosol challenge with Mycobacterium tuberculosis after immunisation with a recombinant adenoviral vaccine. Vaccine. 2010;28:5179–5184. doi: 10.1016/j.vaccine.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 21.Moore IN, Lamirande EW, Paskel M, Donahue D, Kenney H, Qin J, Subbarao K. Severity of clinical disease and pathology in ferrets experimentally infected with influenza viruses is influenced by inoculum volume. Journal of virology. 2014;88:13879–13891. doi: 10.1128/JVI.02341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nature medicine. 2013;19:1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 23.Hayward AC, Wang L, Goonetilleke N, Fragaszy EB, Bermingham A, Copas A, Dukes O, Millett ER, Nazareth I, Nguyen-Van-Tam JS, Watson JM, et al. Natural T Cell-mediated Protection against Seasonal and Pandemic Influenza. Results of the Flu Watch Cohort Study. Am J Respir Crit Care Med. 2015;191:1422–1431. doi: 10.1164/rccm.201411-1988OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty PC, Kelso A. Toward a broadly protective influenza vaccine. The Journal of clinical investigation. 2008;118:3273–3275. doi: 10.1172/JCI37232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin YP, Xiong X, Wharton SA, Martin SR, Coombs PJ, Vachieri SG, Christodoulou E, Walker PA, Liu J, Skehel JJ, Gamblin SJ, et al. Evolution of the receptor binding properties of the influenza A(H3N2) hemagglutinin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21474–21479. doi: 10.1073/pnas.1218841110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelli RK, Kuchipudi SV, White GA, Perez BB, Dunham SP, Chang KC. Comparative distribution of human and avian type sialic acid influenza receptors in the pig. BMC veterinary research. 2010;6:4. doi: 10.1186/1746-6148-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loving CL, Lager KM, Vincent AL, Brockmeier SL, Gauger PC, Anderson TK, Kitikoon P, Perez DR, Kehrli ME., Jr Efficacy in pigs of inactivated and live attenuated influenza virus vaccines against infection and transmission of an emerging H3N2 similar to the 2011-2012 H3N2v. Journal of virology. 2013;87:9895–9903. doi: 10.1128/JVI.01038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarawar S, Hatta Y, Watanabe S, Dias P, Neumann G, Kawaoka Y, Bilsel P. M2SR, a novel live single replication influenza virus vaccine, provides effective heterosubtypic protection in mice. Vaccine. 2016;34:5090–5098. doi: 10.1016/j.vaccine.2016.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatta Y, Boltz D, Sarawar S, Kawaoka Y, Neumann G, Bilsel P. M2SR, a novel live influenza vaccine, protects mice and ferrets against highly pathogenic avian influenza. Vaccine. 2017;35:4177–4183. doi: 10.1016/j.vaccine.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science (New York, N.Y.) 2016;354:722–726. doi: 10.1126/science.aag1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau YF, Wright AR, Subbarao K. The contribution of systemic and pulmonary immune effectors to vaccine-induced protection from H5N1 influenza virus infection. Journal of virology. 2012;86:5089–5098. doi: 10.1128/JVI.07205-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nature medicine. 2015;21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nature reviews Immunology. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 34.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, Almond M, Wong EH, Sykes A, Maybeno M, Del Rosario J, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nature Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinen PP, de Boer-Luijtze EA, Bianchi AT. Respiratory and systemic humoral and cellular immune responses of pigs to a heterosubtypic influenza A virus infection. The Journal of general virology. 2001;82:2697–2707. doi: 10.1099/0022-1317-82-11-2697. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nature medicine. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.