Abstract
Background
There is increasing evidence for shared genetic susceptibility between schizophrenia and bipolar disorder. While genetic variants only convey subtle increases in risk individually, their combination into a polygenic risk score constitutes a strong disease predictor.
Aim
To investigate whether schizophrenia and bipolar disorder polygenic risk scores can distinguish people with broadly-defined psychosis and their unaffected relatives from controls.
Methods
Using the latest Psychiatric Genomics Consortium data, we calculated schizophrenia and bipolar disorder polygenic risk scores in 1,168 people with psychosis, 552 unaffected relatives and 1,472 controls.
Results
Patients with broadly-defined psychosis had dramatic increases in schizophrenia and bipolar polygenic risk scores, as did their relatives, albeit to a lesser degree. However, the accuracy of predictive models was modest.
Conclusions
Although polygenic risk scores are not ready for clinical use, it is hoped that as they are refined they could help towards risk reduction advice and early interventions for psychosis.
Declaration of interest
None
Keywords: Bipolar disorder, polygenic, prediction, psychotic disorders, polygenic risk scores, schizophrenia
Introduction
Psychotic disorders affect approximately 4% of the general population 1. Epidemiological and genetic studies show that they have high heritability 2,3. A Psychiatric Genomics Consortium mega-analysis of genome-wide association studies (GWAS) for schizophrenia identified more than a hundred common single nucleotide polymorphisms (SNPs) with small individual effects conferring susceptibility to the disorder 4. A similar mega-analysis for bipolar disorder, albeit with a more modest sample size, identified common risk variants specific to bipolar disorder and some shared with schizophrenia 5. Genetic epidemiology studies have shown that when compared to controls, first degree relatives of people with schizophrenia have increased risk for bipolar disorder and first degree relatives of people with bipolar disorder have increased risk for schizophrenia 6. GWAS have now provided molecular evidence for this common genetic architecture between schizophrenia and bipolar disorder 5,7–11. Psychotic disorders are highly polygenic with thousands of contributing common genetic variants 12,13. While each individual variant has a very low predictive power, their combination into a polygenic risk score (PRS) represents a stronger predictor of disease 8,14–20. Our primary aim was to evaluate whether polygenic risk scores specific for schizophrenia or bipolar disorder, could discriminate case-control status in our sample of patients with broadly-defined psychosis. Our secondary aim was to investigate whether polygenic risk scores were different in the unaffected relatives of patients with broadly defined psychotic disorder compared to controls.
Methods and Materials
Sample description
Samples were collected at research centres across Europe and Australia. Our study included cases with a range of psychotic disorders (1,168), unaffected relatives of patients (552) and healthy controls with no personal or family history of psychosis (1,472) (Table 1). The sample presented here was included in previous genome wide association studies seeking to identify loci for schizophrenia or psychosis. Details of sample overlap are in the supplement 4,9,21. In order to avoid any inflation of the polygenic risk score effect size, in each analysis we included only participants that were unrelated. This was achieved by random exclusion of related participants.
Table 1.
Demographics in the cases, relatives and controls
| Cases | Relatives | Controls | ||
|---|---|---|---|---|
| Age, years: mean(s.d) | 33.8 (10.2) | 44.8 (15.5) | 40.2 (14.3) | |
| Gender, female: n(%) | 386 (33) | 343 (62) | 763 (52) | |
| Schizophrenia | 733 (62.8) | |||
| Schizoaffective | 59 (5.1) | |||
| Bipolar disorder | 109 (9.3) | |||
| Cases | Brief psychotic disorder | 43 (3.7) | ||
|
sub-diagnosis groups N (%) |
Delusional disorder | 19 (1.6) | ||
| Psychosis drug induced | 7 (0.6) | |||
| Schizophreniform disorder | 94 (8) | |||
| Psychotic disorder NOS | 104 (8.9) | |||
| Total | 1,168 | 552 | 1,472 | |
All participants provided written informed consent, and the study was approved by the respective ethical committees at each one of the participating centres.
Of the 1,168 cases in this study, 733 met criteria for schizophrenia (62.8%), 59 for schizoaffective disorder (5.1%), 104 for psychotic disorder not otherwise specified (8.9%), 94 for schizophreniform disorder (8%), 43 for brief psychotic disorder (3.7%), 19 for delusional disorder (1.6%), 7 for substance-induced psychosis (0.6%) and 109 for bipolar disorder with psychotic features (9.3%) (Table 1).
Additional details are provided in Table S1 and S2 of the Supplement.
DNA Preparation, Genotyping and Imputation
Genomic DNA obtained from blood was sent to the Wellcome Trust Sanger Institute (Cambridge, United Kingdom). Samples were genotyped with the Genome-wide Human SNP Array 6.0 at Affymetrix Services Laboratory as part of the Wellcome Trust Case Control Consortium round 2 project (https://www.wtccc.org.uk/). Thereafter the data quality control, imputation and statistical analyses were conducted by KL, JT, SC and EB at University College London. DNA Preparation, Genotyping and Imputation are described in more details in the supplement and in Bramon et al (2014)9.
Phenotype definition
Participants were excluded from the study if they had either a history of neurologic disease or head injury resulting in loss of consciousness lasting more than five minutes. DSM-IV 22 diagnosis was ascertained using a structured clinical interview with one of the following three instruments: The Schedule for Affective Disorders and Schizophrenia, The Structured Clinical Interview for DSM Disorders, or the Schedules for Clinical Assessment in Neuropsychiatry 23–25.
Population Structure Analysis
To investigate the genetic structure in the data, we performed principal component analysis (PCA) using EIGENSOFT version 3.0 on a pruned set of single nucleotide polymorphisms (SNPs) 26. We applied the following SNP pruning filters on 695,193 SNPs, which remained after quality control: a 10% minor allele frequency, 10-3 Hardy-Weinberg equilibrium deviation threshold, and all SNPs within a 1,500 SNP window had to have r2 below 0.2 (window shift of 150 used). Thus, a subset of 71,677 SNPs was selected for principal component analysis 26,27 and three ancestry covariate vectors were obtained 9. Plots can be found in Figure S1 in the supplement.
Polygenic Risk Scores calculation
We calculated the polygenic risk scores separately for schizophrenia and for bipolar disorder in all our study participants following established methodology 8,28,29. Odds ratios of allelic association tests were obtained from the most recent Psychiatric Genomics Consortium mega-analysis of genome-wide association studies for schizophrenia 4 and for bipolar disorder 5, excluding all samples overlapping with the current study. For schizophrenia, the used discovery sample included 31,658 cases and 42,022 controls, and for bipolar disorder, it included 7,481 cases and 9,250 controls 4,5. In each discovery samples, SNPs were selected at 10 significance thresholds (PT < 5×10-08, 1×10-06, 1×10-04, 1×10-03, 0.01, 0.05, 0.1, 0.2, 0.5, 1). LD pruning was used to identify SNPs in linkage equilibrium with each other. The number of SNPs included at each p-value threshold is shown in the supplement Table S4. In order to obtain polygenic risk scores in each individual, for each SNP the number of risk alleles carried by the individual (0, 1, 2) was multiplied by the log of the odds ratio of allelic association test. The polygenic risk score was then calculated adding up the values obtained for each SNP.
Statistical analysis
We used logistic regression, with the first three population structure principal components and the centre of ascertainment of the samples as covariates to test whether the polygenic risk scores were predictive of case-control or relative-control status in our study. The proportion of the variance explained by the polygenic risk score was calculated as Nagelkerke’s pseudo-R2, by comparing a full model (PRS plus covariates) to a reference model (covariates only). The R package pROC 30 was used to calculate the area under the receiver operator characteristic curve (AUC) in both the full and reference models.
In the primary analysis, schizophrenia PRS and bipolar disorder PRS were compared between 1,168 cases and 1,472 controls. In the secondary analysis, we split the 1,168 cases with broadly defined psychosis into three subcategories, depending on the DSM diagnosis: schizophrenia/schizoaffective disorder, bipolar disorder and all other psychotic disorders. We then compared both schizophrenia and bipolar disorder PRS between 552 unaffected relatives and healthy controls. See table S5 for a breakdown of these secondary analysis sub-groups. In order to divide cases and controls into decile categories, we calculated Z standardised polygenic risk scores, using the mean and standard deviation of controls in each centre.
Results
Analysis of polygenic risk scores in psychotic disorders
We calculated polygenic risk scores for schizophrenia and bipolar disorder in 1,472 controls and 1,168 people diagnosed with a range of psychotic disorders. Density plots of schizophrenia and bipolar disorder PRS are shown in the supplementary material (Figure S2).
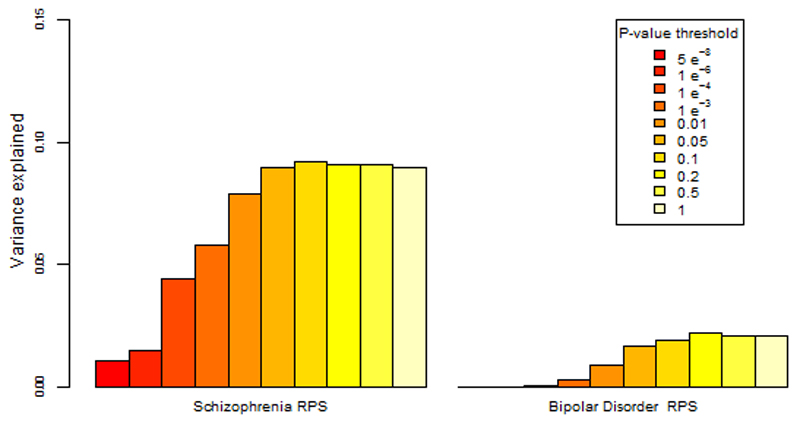
Using logistic regression, we found highly significant differences for both schizophrenia and bipolar disorder polygenic risk scores (PRS) between cases with psychosis and controls (Table 2 and Table S6). The difference was greater for increasingly liberal P-value thresholds (Table 2 and Table S6). Compared to the bipolar disorder PRS, the schizophrenia PRS had a better ability to discriminate between cases and controls.
Table 2.
Comparison of Schizophrenia and bipolar disorder polygenic risk scores (PRS) between patients with psychotic disorders and controls
| Polygenic Risk Score P-value Thresholds | |||||
|---|---|---|---|---|---|
| 5×10-08 | 1×10-04 | 0.05 | 1 | ||
|
Schizophrenia Polygenic Risk Scores |
P-value | 1.3×10-06 | 6.8×10-21 | 7.6×10-40 | 5.7×10-40 |
| Variance explained | 1.1% | 4.4% | 9% | 9% | |
|
Bipolar disorder Polygenic Risk Scores |
P-value | 0.6 | 0.25 | 2.8×10-09 | 5.7×10-11 |
| Variance explained | <0.1% | <0.1% | 1.7% | 2.1% | |
Schizophrenia polygenic risk scores (SZ PRS) and bipolar disorder polygenic risk scores (BD PRS) were calculated using as reference, respectively, the outcome of the schizophrenia and bipolar disorder mega-analyses conducted by the Psychiatric Genomics Consortium (PGC). We then compared the scores between 1,168 cases and 1,472 controls using standard logistic regression at ten different P value thresholds (PT 5×10-08, 1×10-06, 1×10-04, 1×10-03, 0.01, 0.05, 0.1, 0.2, 0.5, 1). Regression models included the first three ancestry based principal components and a cohort indicator as covariates. For clarity, here we report P values and the variance explained in disease risk as measured by Nagelkerke’s pseudo R2 at four p value thresholds (PT 5×10-08, 1×10-04, 0.05, 1). Results at each one of the ten different thresholds are available in Table S6.
The proportion of the variance in psychosis risk explained by the schizophrenia PRS increased with progressively more inclusive P value thresholds, reaching a plateau of 9% variance explained at the 0.05 P value threshold (Nagelkerke’s pseudo-R2 = 9 %; p= 7.6x10-40) (Table 2, Table S6 and Figure 1). At the same P value threshold the variance explained by the bipolar disorder PRS was only 1.7% (PT = 0.05, Nagelkerke’s pseudo-R2 = 1.7%) (Table 2, Table S6 and Figure 1). Results for all the P value thresholds used are reported in figure 1 and in the supplementary material (Table S6).
Figure 1. Percentage of the variance in disease risk explained by the schizophrenia and the bipolar disorder polygenic risk scores (PRS).
The proportion of variance explained (calculated as Nagelkerke’s pseudo R2) was computed by comparison of the full model (either schizophrenia based or BD based PRS plus covariates) to the reduced model (covariates only). As per standard procedures (Ripke et al, 2014), ten different P value thresholds (PT) were used to select risk alleles used in the computation of polygenic risk scores. The variance explained at each P value threshold (5×10-08, 1×10-06, 1×10-04, 1×10-03, 0.01, 0.05, 0.1, 0.2, 0.5 and 1) is shown (legend above plot). Significance testing results are available in Table S6.
Given that 68% of our cases had a diagnosis of schizophrenia/schizoaffective disorder, to rule out the possibility that the results obtained were driven by this subgroup, we tested whether the schizophrenia and bipolar disorder PRS were able to discriminate between cases and controls in each of the three diagnostic subcategories included in our study (schizophrenia/schizoaffective disorder combined, bipolar disorder or other psychotic disorders). We demonstrated that even if the discriminative ability of the schizophrenia PRS was highest in the schizophrenia/schizoaffective disorder subcategory, it was also able to discriminate cases with either bipolar disorder or other psychotic disorders from controls with highly significant group differences. At PT = 0.05 the variance in case-control status explained by the schizophrenia PRS (Nagelkerke’s pseudo R2) in the bipolar disorder and other psychotic disorders subcategory was 3.4%, providing evidence that our results were not only driven by schizophrenia/schizoaffective disorder subcategory (Table 3 and Table S7).
Table 3.
Schizophrenia and bipolar disorder polygenic risk scores (PRS) in the three diagnostic subgroups and in unaffected relatives versus controls.
| Clinical sub-groups | Schizophrenia PRS |
Bipolar disorder PRS |
|
|---|---|---|---|
| PT = 0.05 | PT = 0.05 | ||
| P-value | 6.1×10-39 | 9.2×10-08 | |
| Schizophrenia/Schizoaffective (n=792) versus controls (n=1,472) |
Variance explained | 10.3% | 1.6% |
| P-value | 6.2×10-06 | 6.5×10-03 | |
| Bipolar disorder (n=109) versus controls (n=1,058) |
Variance explained | 3.4% | 1.2% |
| P-value | 1.2×10-08 | 1.2×10-03 | |
| Other psychotic disorders (n=267) versus Controls (n=1,429) |
Variance explained | 3.3% | 1% |
| Relatives (n=552) versus Controls (n=1,221) |
P-value | 1.2×10-04 | 2.1×10-02 |
Significance of the case-control PRS difference was analysed by standard logistic regression using different P value thresholds (PT 5×10-08, 1×10-04, 0.05 and 1). Here, P values and Nagelkerke’s R2 obtained at PT = 0.05 are reported. Results at each one of the four different PT are available in Table S7. Logistic regression included the first three ancestry based principal components and a cohort indicator as covariates. We report the proportion of the phenotypic variance explained by the risk polygenic score as measured by Nagelkerke’s pseudo R2
To evaluate the accuracy of the schizophrenia and bipolar disorder PRS in the detection of broadly defined psychotic disorders, we calculated the area under the curve of the receiver operator characteristic curves (AUC). For the model containing only covariates (cohort and three population structure principal components) the AUC was 0.63. Adding the schizophrenia PRS to the model increased the AUC to 0.7, whereas adding the bipolar PRS increased it to 0.65 (Figure S3).
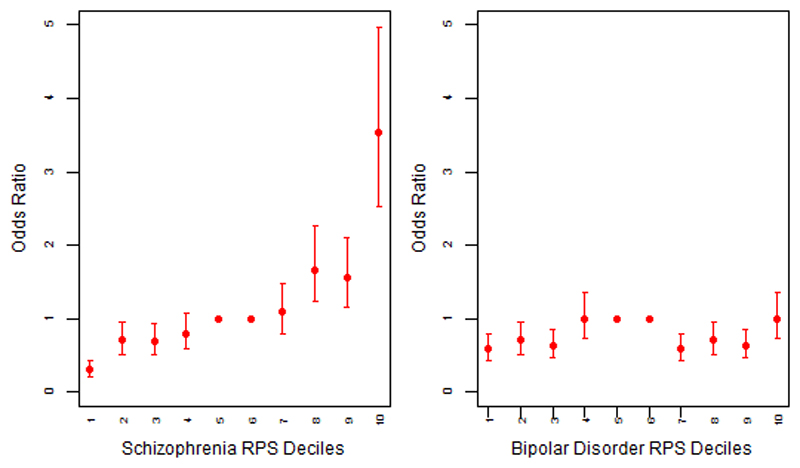
We then divided our sample into deciles based on schizophrenia and bipolar disorder PRS and calculated the odds ratios (OR) for affected status for each decile using as reference the central risk deciles (5th and 6th). As expected, we observed an increase in the case to control ratio in progressively higher decile categories (Figure 2 and Table S8). Similarly, the odds of having broadly defined psychosis increased progressively across PRS deciles. Compared to individuals in the central deciles (5th and 6th), those at the tenth and highest decile had an odds ratio for psychosis of 3.53 (95% CI 2.53 to 4.97) for schizophrenia PRS (Figure 3 and Table S9). For the bipolar PRS no difference was found between central and highest deciles (OR=1, 95% CI: 0.73 to 1.35) (Figure 3 and Table S9).
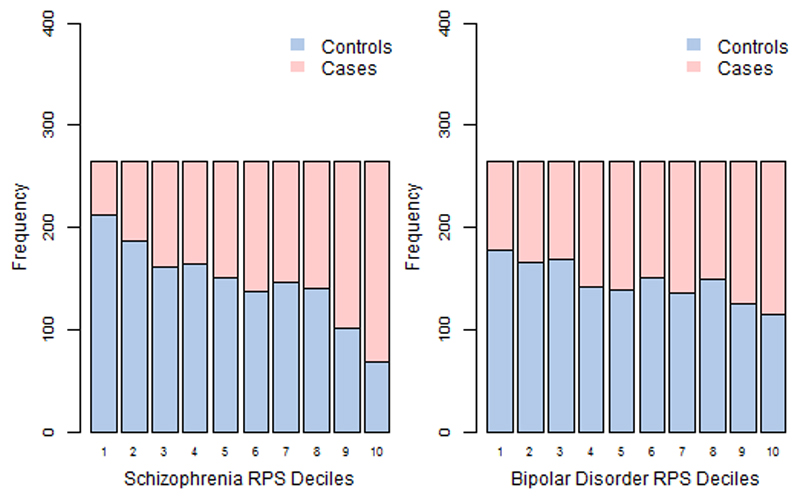
Figure 2. Case and control distribution in the risk polygenic score (PRS) deciles.
The Y-axis corresponds to the number of individuals in each PRS decile. The threshold used to calculate PRS was PT = 0.05. Based on their PRS, samples were allocated to deciles (decile 1 = lowest PRS, 10 = highest PRS).
The figure shows that for especially for schizophrenia PRS the effect is concentrated in the tails of the distribution (deciles 1-2 and 9-10). There is very little difference between the deciles 4 to 7 in the middle, as is expected from a normal distribution.
Figure 3. Odds ratio for broadly defined psychosis by risk polygenic score (PRS).
The threshold used for selecting risk alleles was PT = 0.05. Based on PRS samples were allocated to deciles (decile 1 = lowest PRS, 10 = highest PRS). A dummy variable was created to compare the central deciles 5 and 6, used as reference to the others. Odds Ratio (OR) and 95% confidence intervals (CI) were estimated using logistic regression including ethnicity principal components and cohort indicator as covariates.
The points represent the odds ratios. The bars represent the lower and upper confidence interval of the odds ratios.
Analysis of polygenic risk scores in the unaffected relatives of people with psychosis
Given the established heritability of psychotic disorders, we evaluated whether schizophrenia and bipolar disorder PRS could discriminate between unaffected relatives, who had never experienced any psychotic symptoms and healthy controls (Figure S4). Compared to controls, unaffected relatives had significantly higher PRS both for schizophrenia (P= 1.2×10-4) and bipolar disorder (P=2.1×10-2). Analyses at p value threshold of 0.05 are shown in Table 3 and full details are in Table S7.
Discussion
In this study, we have shown that polygenic risk scores specific for schizophrenia or for bipolar disorder obtained from a large international cohort are also associated with broadly-defined psychosis in an independent sample. Compared to controls, patients with a range of psychotic disorders have significantly higher PRS for both schizophrenia and for bipolar disorder. The schizophrenia and bipolar disorder PRS explained respectively 9% and 2% of the variance in psychosis risk, which is substantial for a single variable.
The PRS for schizophrenia had a much better performance than the PRS for bipolar disorder and this could be due to several factors. Firstly, the schizophrenia PRS contains a more accurate measure of genetic susceptibility, since it is derived from a much larger discovery sample than the bipolar PRS 5,31. The last Psychiatric Genomics Consortium schizophrenia meta-analysis provided evidence that increasing the size of discovery samples leads to a significant increase in the variance explained by PRS 8,31. Secondly, our cases with a range of psychotic disorders included a majority of patients with schizophrenia and schizoaffective disorder (68%), which drives these performance results. However, our secondary analyses subdividing in three diagnostic categories, also showed a better performance for the schizophrenia PRS in discriminating cases with bipolar disorder and other psychotic disorders from controls. Therefore, the use of larger discovery sample sizes appears to be the best way forward to further enhance the accuracy of PRS 8.
Genome wide association studies have provided evidence for genetic overlap between schizophrenia and bipolar disorder 5,7–11,14.Our findings add evidence to the hypothesis of shared genetic architecture across the psychosis spectrum, supporting a continuum model for the aetiology of these disorders 32,33. The bipolar patients included in this study had type I bipolar disorder with a history of psychotic symptoms at some point in their illness. Therefore, in our sample it was not possible to make any comparison of schizophrenia and bipolar PRS in patients with bipolar with and without psychotic features. A study just published showed the existence of a gradient of schizophrenia PRS across bipolar disorder subtypes (bipolar disorder type I with psychosis> bipolar disorder type I without psychosis>bipolar disorder type II) 34.
Given the heritability and familial aggregation patterns in schizophrenia and bipolar disorder, we expected unaffected relatives to have higher PRS than the general population 35–37. In a recent study, Bigdeli et al showed that 217 healthy first degree relatives of patients with schizophrenia and healthy controls could be distinguished by schizophrenia PRS 37. We replicated their findings using an independent sample with 552 unaffected relatives of patients diagnosed with a wide range of psychotic disorders. Furthermore, we showed that the bipolar disorder PRS is significantly higher amongst healthy relatives compared to controls.
Strengths and limitations of polygenic risk scores
Even if the schizophrenia and bipolar PRS can discriminate cases from controls, their accuracy is currently modest, as indicated by the area under the receiver operator characteristic curve (AUC) of 0.7 and 0.65 for schizophrenia and bipolar disorder respectively. The AUC is an estimate of diagnostic accuracy which equals to 0.5 when a diagnostic test is no better than chance and reaches 1 if the test could discriminate patients from controls to perfection 38,39. Typically an AUC of 0.7 is considered to have moderate discriminatory power and only when reaching 0.9 it is deemed to have high discriminatory power 40,41. For example, the models used in general practice to estimate cardiovascular disease risk and to offer preventative interventions have reached AUC in the range of 0.74 to 0.85 42,43. In the case of psychotic disorders, currently the moderate accuracy precludes the use of schizophrenia and bipolar PRS as a diagnostic or prognostic tool in clinical practice.
Current genetic findings explain only about a third of the genetic variance of these disorders. The so called ‘missing heritability’ may reside in further common variants yet to be identified, rare mutations, copy number variants (CNVs) and gene-gene interactions 12. As larger samples are being collected through international efforts, additional common and rare genetic variants will be identified and the performance of polygenic risk scores is expected to improve 17,44.
In the future polygenic risk scores may also incorporate socio-environmental factors as well as gene-gene and gene-environment interactions, thus eventually enabling their use in clinical practice for risk reduction advice as it is happening in cardiovascular disease 45–53. There is growing interest in the potential of polygenic risk scores in public health campaigns to reduce environmental risks and to facilitate access to early treatment for psychosis 54. Finally, polygenic risk scores constitute a powerful research tool, that combined with large epidemiological studies of environmental risks are advancing our understanding of the aetiology of psychotic disorders.
Supplementary Material
Acknowledgements
We would like to thank all the patients, relatives and controls who took part in this research, as well as the clinical staff who facilitated their involvement.
This work was funded by the Medical Research Council (G0901310), the Wellcome Trust (grants 085475/B/08/Z, 085475/Z/08/Z) the European Union’s Seventh Framework Programme for research, technological development and demonstration (grant 602450). This study was also supported by the NIHR Biomedical Research Centre at University College London (mental health theme) and by the NIHR Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry- Kings College London. We thank Tristan Clark and the UCL Computer Science Cluster team for their ongoing support.
Further support: NHIR Academic Clinical fellowship awarded to Maria Stella Calafato. Elvira Bramon acknowledges research funding from: BMA Margaret Temple grants 2016 and 2006, MRC- Korean Health Industry Development Institute Partnering Award (MC_PC_16014), MRC New Investigator Award and a MRC Centenary Award (G0901310), National Institute of Health Research UK post-doctoral fellowship, the Psychiatry Research Trust, the Schizophrenia Research Fund, the Brain and Behaviour Research foundation’s NARSAD Young Investigator Awards 2005, 2008, Wellcome Trust Research Training Fellowship and the NIHR Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry Kings College London. The Brain and Behaviour Research foundation’s (NARSAD’s) Young Investigator Award (Grant 22604, awarded to C. Iyegbe). The BMA Margaret Temple grant 2016 to Johan Thygesen. European Research Council Marie Curie award to A Díez-Revuelta.
The infrastructure for the GROUP consortium is funded through the Geestkracht programme of the Dutch Health Research Council (ZON-MW, grant number 10-000-1001), and matching funds from participating pharmaceutical companies (Lundbeck, AstraZeneca, Eli Lilly, Janssen Cilag) and universities and mental health care organizations (Amsterdam: Academic Psychiatric Centre of the Academic Medical Center and the mental health institutions: GGZ Ingeest, Arkin, Dijk en Duin, GGZ Rivierduinen, Erasmus Medical Centre, GGZ Noord Holland Noord. Maastricht: Maastricht University Medical Centre and the mental health institutions: GGZ Eindhoven en de kempen, GGZ Breburg, GGZ Oost-Brabant, Vincent van Gogh voor Geestelijke Gezondheid, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem. Groningen: University Medical Center Groningen and the mental health institutions: Lentis, GGZ Friesland, GGZ Drenthe, Dimence, Mediant, GGNet Warnsveld, Yulius Dordrecht and Parnassia psycho-medical center (The Hague). Utrecht: University Medical Center Utrecht and the mental health institutions Altrecht, GGZ Centraal, Riagg Amersfoort and Delta).
The sample from Spain was collected at the Hospital Universitario Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant support: Carlos III Health Institute PI020499, PI050427, PI060507, Plan Nacional de Drugs Research Grant 2005- Orden sco/3246/2004, SENY Fundació Research Grant CI 2005-0308007 and Fundación Marqués de Valdecilla API07/011. We wish to acknowledge Biobanco HUMV-IDIVAL for hosting and managing blood samples and IDIVAL Neuroimaging Unit for imaging acquirement and analysis. We wish to thank the PAFIP researchers who helped with data collection and the participants and their families for participating in the study. The present data were obtained at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant support: MINECO Exp.: SAF2013-46292-R
Appendix
Co-authors who are members of the Psychosis Endophenotypes International Consortium (PEIC):
Maria J. Arranz1,2, Steven Bakker3, Stephan Bender4,5, Elvira Bramon6,2, Wiepke Cahn3, David Collier7,2, Benedicto Crespo-Facorro8,9, Marta Di Forti2, Jeremy Hall10, Mei-Hua Hall11, Conrad Iyegbe2, Assen Jablensky12, René S. Kahn3, Luba Kalaydjieva13, Eugenia Kravariti2, Stephen M Lawrie10, Cathryn M. Lewis2, Kuang Lin2,14, Don H. Linszen15, Ignacio Mata16,9, Colm McDonald17, Andrew M McIntosh10,18, Robin M. Murray2, Roel A. Ophoff19, Marco Picchioni2, John Powell2, Dan Rujescu20,21, Timothea Toulopoulou2,22,23, Jim Van Os24,2, Muriel Walshe6,2, Matthias Weisbrod25,5, and Durk Wiersma26.
PEIC affiliations:
1 Fundació de Docència i Recerca Mútua de Terrassa, Universitat de Barcelona, Catalonia, Spain.
2 Institute of Psychiatry, Psychology and Neuroscience, King’s College London, De Crespigny Park, London SE5 8AF, UK
3 University Medical Center Utrecht, Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, The Netherlands.
4 Child and Adolescent Psychiatry, University of Technology Dresden, Fetscherstrasse 74, 01307 Dresden, Germany.
5 General Psychiatry, Vossstraße 4, 69115 Heidelberg, Germany.
6 Division of Psychiatry & Institute of Cognitive Neuroscience, University College London, UK.
7 Discovery Neuroscience Research, Lilly, UK.
8 University Hospital Marqués de Valdecilla, IDIVAL, Department of Psychiatry, School of Medicine, University of Cantabria, Santander, Spain.
9 CIBERSAM, Centro Investigación Biomédica en Red Salud Mental, Madrid, Spain.
10 College of Biomedical and Life Sciences, Cardiff University, CF24 4HQ Cardiff, UK.
11 Mclean Hospital, Harvard Medical School, Belmont MA, USA
12 Centre for Clinical Research in Neuropsychiatry, The University of Western Australia, Perth, Australia
13 Western Australian Institute for Medical Research and Centre for Medical Research, The University of Western Australia, Perth, Australia
14 Nuffield Department of Population Health, University of Oxford, Ocford, UK
15 Academic Medical Centre University of Amsterdam, Department of Psychiatry, Amsterdam The Netherlands
16 Fundacion Argibide, Pamplona, Spain.
17 The Centre for Neuroimaging &Cognitive Genomics (NICOG) and NCBES Galway Neuroscience Centre, National University of Ireland Galway, Galway Ireland
18 Centre for Cognitive Ageing and Cognitive Epidemiology, University of Edinburgh, UK
19 UCLA Center for Neurobehavioral Genetics, 695 Charles E. Young Drive South, Los Angeles CA 90095, USA.
20 University of Munich, Dept. of Psychiatry, Munich, Germany
21 University of Halle, Dept. of Psychiatry, Halle, Germany.
22 Department of Psychology, Bilkent University, Main Campus, Bilkent, Ankara, Turkey
23 The State Key Laboratory of Brain and Cognitive Sciences and the Department of Psychology, The University of Hong Kong, Hong Kong, China
24 Maastricht University Medical Centre, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht, The Netherlands
25 General Psychiatry and Psychotherapy, SRH Klinikum Karlsbad-Langensteinbach, Guttmannstrasse 1 76307 Karlsbad, Germany
26 University Medical Center Groningen, Department of Psychiatry, University of Groningen, The Netherlands
Co-authors who are members of the Genetic Risk and Outcome of Psychosis (GROUP) consortium:
Richard Bruggeman, MD, PhD, Department of Psychiatry, University Medical Center Groningen, University of Groningen; Wiepke Cahn, MD, PhD, Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht; Lieuwe de Haan, MD, PhD, Department of Psychiatry, Academic Medical Center, University of Amsterdam; René S. Kahn, MD, PhD, Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, Utrecht, the Netherlands; Carin Meijer, PhD, Department of Psychiatry, Academic Medical Center, University of Amsterdam; Inez Myin-Germeys, PhD, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht University Medical Center; Jim van Os, MD, PhD, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht University Medical Center, Maastricht, the Netherlands, and King’s College London, King’s Health Partners, Department of Psychosis Studies, Institute of Psychiatry, London, England; and Agna A. Bartels-Velthuis, PhD, Department of Psychiatry, University Medical Center Groningen, University.
Membership of Wellcome Trust Case Control Consortium 2 (WTCCC2)
Management Committee
Peter Donnelly (Chair), Ines Barroso (Deputy Chair), Jenefer M Blackwell, Elvira Bramon, Matthew A Brown, Juan P Casas, Aiden Corvin, Panos Deloukas, Audrey Duncanson, Janusz Jankowski, Hugh S Markus, Christopher G Mathew, Colin NA Palmer, Robert Plomin, Anna Rautanen, Stephen J Sawcer, Richard C Trembath, Ananth C Viswanathan, Nicholas W Wood
Data and Analysis Group
Chris C A Spencer, Gavin Band, Céline Bellenguez, Colin Freeman, Garrett Hellenthal, Eleni Giannoulatou, Matti Pirinen, Richard Pearson, Amy Strange, Zhan Su, Damjan Vukcevic, Peter Donnelly
DNA, Genotyping, Data QC and Informatics Group
Cordelia Langford, Sarah E Hunt, Sarah Edkins, Rhian Gwilliam, Hannah Blackburn, Suzannah J Bumpstead, Serge Dronov, Matthew Gillman, Emma Gray, Naomi Hammond, Alagurevathi Jayakumar, Owen T McCann, Jennifer Liddle, Simon C Potter, Radhi Ravindrarajah, Michelle Ricketts, Avazeh Tashakkori-Ghanbaria, Matthew Waller, Paul Weston, Sara Widaa, Pamela Whittaker, Ines Barroso, Panos Deloukas.
Publications Committee
Christopher G Mathew (Chair), Jenefer M Blackwell, Matthew A Brown, Aiden Corvin, Mark I McCarthy, Chris C A Spencer
Footnotes
Author contributions
Dr Maria Stella Calafato was the main analysts and drafted/revised the manuscript. Dr Elvira Bramon, Dr Johan H Thygesen, Dr Siri Ranlund, Eirini Zartaloudi, Dr Álvaro Díez-Revuelta contributed to drafting/revising the manuscript. Dr Wiepke Cahn, Dr Benedicto Crespo-Facorro, Dr Marta Di Forti, GROUP**, Dr Mei-Hua Hall, Dr Conrad Iyegbe, Dr Assen Jablensky, Dr Rene Kahn, Dr Luba Kalaydjieva, Dr Eugenia Kravariti, Dr Kuang Lin, Dr Colm McDonald, Dr Andrew M McIntosh, Dr Andrew McQuillin, PEIC**, Dr Marco Picchioni, Dr Dan Rujescu, Dr Madiha Shaikh, Dr Timothea Toulopoulou, Dr Jim Van Os, Dr Evangelos Vassos, Dr Muriel Walshe, WTCCC2^, Dr John Powell, Dr Cathryn M. Lewis, Dr Robin M Murray and Dr Elvira Bramon oversaw collection and performed initial analysis of the samples. All authors contributed to conception, design and interpretation of the data, revised the draft and approved the final version to be published.
Financial Disclosures
Prof. Robin Murray: Honoraria for lectures from Janssen, Lundbeck, Lilly, Otsuka, Sunovian
All other authors declare that they have no financial interests or potential conflicts of interest
References
- 1.Bogren M, Mattisson C, Isberg PE, Nettelbladt P. How common are psychotic and bipolar disorders? A 50-year follow-up of the Lundby population. Nord J Psychiatry. 2009;63:336–46. doi: 10.1080/08039480903009118. [DOI] [PubMed] [Google Scholar]
- 2.Iyegbe C, Campbell D, Butler A, Ajnakina O, Sham P. The emerging molecular architecture of schizophrenia, polygenic risk scores and the clinical implications for GxE research. Soc Psychiatry Psychiatr Epidemiol. 2014;49:169–82. doi: 10.1007/s00127-014-0823-2. [DOI] [PubMed] [Google Scholar]
- 3.Gratten J, Wray NR, Keller MC, Visscher PM. Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat Neurosci. 2014;17:782–90. doi: 10.1038/nn.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripke S, Neale BM, Corvin A, Walter JTR, Schizophrenia Working Group of the Psychiatric Genomics Consortium Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sklar P, Ripke S, Scott L, Andreassen O, Cichon S. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011;43:977–83. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–9. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruderfer DM, Fanous aH, Ripke S, McQuillin a, Amdur RL, Gejman PV, et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014;19:1017–24. doi: 10.1038/mp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bramon E, Pirinen M, Strange A, Lin K, Freeman C, Bellenguez C, et al. A genome-wide association analysis of a broad psychosis phenotype identifies three loci for further investigation. Biol Psychiatry. 2014;75:386–97. doi: 10.1016/j.biopsych.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neale BM, Sklar P. Genetic analysis of schizophrenia and bipolar disorder reveals polygenicity but also suggests new directions for molecular interrogation. Curr Opin Neurobiol. 2015;30:131–8. doi: 10.1016/j.conb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Cardno AG, Owen MJ. Genetic relationships between schizophrenia, bipolar disorder, and schizoaffective disorder. Schizophr Bull. 2014;40:504–15. doi: 10.1093/schbul/sbu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison PJ. Recent genetic findings in schizophrenia and their therapeutic relevance. J Psychopharmacol. 2015;29:85–96. doi: 10.1177/0269881114553647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science (80- ) 2015;349:1489–94. doi: 10.1126/science.aaa8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group C, Consortium PG Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derks EM, Vorstman JaS, Ripke S, Kahn RS, Ophoff Ra. Investigation of the genetic association between quantitative measures of psychosis and schizophrenia: a polygenic risk score analysis. PLoS One. 2012;7:e37852. doi: 10.1371/journal.pone.0037852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu D, Mathews CA, Scharf JM, Neale BM, Davis LK, Gamazon ER, et al. Cross-disorder genome-wide analyses suggest a complex genetic relationship between tourette’s syndrome and OCD. Am J Psychiatry. 2015;172:82–93. doi: 10.1176/appi.ajp.2014.13101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, Middeldorp CM. Research review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry. 2014;55:1068–87. doi: 10.1111/jcpp.12295. [DOI] [PubMed] [Google Scholar]
- 18.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wray NR, Visscher PM. Narrowing the boundaries of the genetic architecture of schizophrenia. Schizophr Bull. 2010;36:14–23. doi: 10.1093/schbul/sbp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassos E, Di Forti M, Coleman J, Iyegbe C, Prata D, Euesden J, et al. An Examination of Polygenic Score Risk Prediction in Individuals with First Episode Psychosis. Biol Psychiatry. 2016;0:135–45. doi: 10.1016/j.biopsych.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, te. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.Wing JK, Babor T, Brugha T, Burke J, Cooper JE, Giel R, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–93. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 24.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–44. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 26.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:2074–93. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick Na, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 28.Euesden J, Lewis CM, O’Reilly PF. PRSice: Polygenic Risk Score software. Bioinformatics. 2014;31:1466–8. doi: 10.1093/bioinformatics/btu848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans DM, Visscher PM, Wray NR. Harnessing the information contained within genome-wide association studies to improve individual prediction of complex disease risk. Hum Mol Genet. 2009;18:3525–31. doi: 10.1093/hmg/ddp295. [DOI] [PubMed] [Google Scholar]
- 30.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans Pa, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendler KS, Walsh D. Schizophreniform disorder, delusional disorder and psychotic disorder not otherwise specified: clinical features, outcome and familial psychopathology. Acta Psychiatr Scand. 1995;91:370–8. doi: 10.1111/j.1600-0447.1995.tb09796.x. [DOI] [PubMed] [Google Scholar]
- 33.Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and intermediate phenotypes of the schizophrenia-bipolar disorder boundary. Neurosci Biobehav Rev. 2010;34:897–921. doi: 10.1016/j.neubiorev.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Allardyce J, Leonenko G, Hamshere M, Pardiñas AF, Forty L, Knott S, et al. Association between schizophrenia-related polygenic liability and the occurrence and level of mood-incongruent psychotic symptoms in bipolar disorder. JAMA Psychiatry. 2018;75:28–35. doi: 10.1001/jamapsychiatry.2017.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doherty JL, Owen MJ. Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Med. 2014;6:29. doi: 10.1186/gm546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bigdeli TB, Ripke S, Bacanu SA, Lee SH, Wray NR, Gejman PV, et al. Genome-wide association study reveals greater polygenic loading for schizophrenia in cases with a family history of illness. Am J Med Genet Part B Neuropsychiatr Genet. 2016;171:276–89. doi: 10.1002/ajmg.b.32402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigdeli TB, Bacanu S-A, Webb BT, Walsh D, O’Neill FA, Fanous AH, et al. Molecular validation of the schizophrenia spectrum. Schizophr Bull. 2014;40:60–5. doi: 10.1093/schbul/sbt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–7. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 39.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp J Intern Med. 2013;4:627–35. [PMC free article] [PubMed] [Google Scholar]
- 40.Grzybowski M, Younger JG. Statistical Methodology: III. Receiver Operating Characteristic (ROC) Curves. Acad Emerg Med. 1997;4:818–26. doi: 10.1111/j.1553-2712.1997.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 41.Swets JA. Measuring the accuracy of diagnostic systems. Science (80- ) 1988;240:1285–93. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 42.Cook NR. Statistical evaluation of prognostic versus diagnostic models: Beyond the ROC curve. Clin Chem. 2008;54:17–23. doi: 10.1373/clinchem.2007.096529. [DOI] [PubMed] [Google Scholar]
- 43.Harrison SL, Ding J, Tang EYH, Siervo M, Robinson L, Jagger C, et al. Cardiovascular disease risk models and longitudinal changes in cognition: A systematic review. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2016;37:2315–81. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mak TSH, Kwan JSH, Campbell DD, Sham PC. Local True Discovery Rate Weighted Polygenic Scores Using GWAS Summary Data. Behav Genet. 2016;46:573–82. doi: 10.1007/s10519-015-9770-2. [DOI] [PubMed] [Google Scholar]
- 47.Kirkbride JB. The emerging molecular architecture of schizophrenia, polygenic risk scores and the clinical implications for gXe research. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1531–4. doi: 10.1007/s00127-014-0961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr Bull. 2016;42:1262–9. doi: 10.1093/schbul/sbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morgan C, Charalambides M, Hutchinson G, Murray RM. Migration, ethnicity, and psychosis: Toward a sociodevelopmental model. Schizophr Bull. 2010;36:655–64. doi: 10.1093/schbul/sbq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Os J, et al. Identifying Gene-Environment Interactions in Schizophrenia: Contemporary Challenges for Integrated, Large-scale Investigations. Schizophr Bull. 2014;40:729–36. doi: 10.1093/schbul/sbu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agerbo E, Sullivan PF, Vilhjálmsson BJ, Pedersen CB, Mors O, Børglum AD, et al. Polygenic Risk Score, Parental Socioeconomic Status, Family History of Psychiatric Disorders, and the Risk for Schizophrenia: A Danish Population-Based Study and Meta-analysis. JAMA psychiatry. 2015;72:635–41. doi: 10.1001/jamapsychiatry.2015.0346. [DOI] [PubMed] [Google Scholar]
- 52.Mcgrath JJ, Mortensen PB, Visscher PM, Wray NR. Where GWAS and epidemiology meet: Opportunities for the simultaneous study of genetic and environmental risk factors in schizophrenia. Schizophr Bull. 2013;39:955–9. doi: 10.1093/schbul/sbt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tzeng JY, Zhang D, Pongpanich M, Smith C, McCarthy MI, Sale MM, et al. Studying gene and gene-environment effects of uncommon and common variants on continuous traits: A marker-set approach using gene-trait similarity regression. Am J Hum Genet. 2011;89:277–88. doi: 10.1016/j.ajhg.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinkhuyzen aaE, Wray NR. Novel directions for G × E analysis in psychiatry. Epidemiol Psychiatr Sci. 2015;24:12–9. doi: 10.1017/S2045796014000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.