Abstract
Background
Fractional anisotropy (FA) in the uncinate fasciculus and the cingulum may be biomarkers for bipolar disorder and may even be distinctly affected in different subtypes of bipolar disorder, an area in need of further research.
Aim
This study aims to establish if FA in the uncinate fasciculus and cingulum shows differences between healthy controls (HC), patients with bipolar disorder type I (BD-I) and type II (BD-II), and their unaffected siblings.
Methods
FA measures from the uncinate fasciculus, cingulum body and parahippocampal cingulum were compared through tractography methods in 40 HC, 32 BD-I, 34 BD-II, 17 siblings of BD-I and 14 siblings of BD-II.
Results
The main effects were found in both the right and left uncinate with BD-I showing significantly lower FA than both BD-II and HC. BD-II participants did not differ from HC. Siblings showed similar effects in the left UF. In a subsequent complementary analysis, we investigated the association between FA in the uncinate fasciculus and polygenic risk for bipolar and psychosis in a large cohort (n= 570) of healthy subjects. However, we found no significant association.
Conclusions
FA in the uncinate fasciculus differs significantly between BD-I compared to BD-II and HC. This supports the hypothesis of differences in the physiological sub-tract between BD subtypes. Similar results were found in unaffected siblings, suggesting the potential for this biomarker to represent an endophenotype for BD-I. However FA in the uncinate fasciculus seems unrelated to polygenic risk for BD or psychosis.
Bipolar disorder (BD) can be subdivided into bipolar type I (BD-I) and bipolar type II (BD-II) based on the intensity, duration or presence of psychotic symptoms during episodes of ‘high’ mood (1). Therefore, BD-II has been regarded as a milder form of BD-I, with both subtypes sharing the same neurophysiological underpinnings; however, recent research has shown clear divergences in their clinical presentation and epidemiology (2) and their genetic basis (3), suggestive of a more fundamental difference between subtypes. Taking both subtypes together, BD is highly heritable and influenced by the additive effect of a large number of genes (4). Current theoretical models point at inefficient connectivity between prefrontal brain areas and subcortical structures associated with emotion and cognition as the basis of the extreme mood episodes present in BD (5–7). This hypothesis finds support from several studies reporting differences in MRI derived white matter microstructure measures between participants with bipolar and healthy controls (HC) (8–13). Among white matter tracts studied, the uncinate fasciculus (UF) and the cingulum bundle are suggested to be at the core of the emotion regulation circuitry (12, 14), and several studies have reported reduced fractional anisotropy (FA) in both these tracts in BD participants compared to healthy controls (7, 9, 15–17). The few studies so far comparing white matter microstructure between BD-I and BD-II have shown differences in FA across subtypes (18–20). Our group (15) has previously used tractography to focus on the UF and showed reduced FA in BD-I compared to BD-II and healthy participants, along with behavioural and BOLD responses to an emotion paradigm suggestive of emotion regulation deficits in BD-I, but not in BD-II. White matter microstructure in the UF was thus posited as a potential biomarker which could explain the phenotypic differences between bipolar disorder subtypes. Unaffected relatives of BD patients have also shown reduced FA (21) in whole brain analyses, supporting the idea of this representing a potential endophenotype for the disorder.
In the present study, we aimed to confirm our previous results of a reduced FA in the UF of BD-I compared to BD-II and HC using a larger sample, and to extend this comparison into the cingulum bundle. We restricted our analyses to these tracts based on previous literature and the proposed role of these in emotion regulation due to their anatomical location. We were also interested in exploring whether FA reductions in tracts showing differences between BD and HC are also present in non-affected siblings of bipolar patients, indicative of a familial aggregation; and examining whether FA in these tracts is associated with polygenic risk for BD (22) and/or psychosis (23).
Method
Participants
The total sample included 137 participants: 66 patients with BD (32 BD-I and 34 BD-II), 31 of their unaffected siblings (17 siblings of BD-I (Sib-I) and 14 siblings of BD-II (Sib-II)) and 40 HC. All patients were recruited from the National Centre for Mental Health (http://www.ncmh.info) and the Bipolar Disorder Research Network (http://bdrn.org). These were well-characterized patients previously diagnosed by trained researchers using standardised clinical interviews. A clinically trained and experienced researcher (XC) further interviewed participants using the Mini-International Neuropsychiatric Interview (MINI) (24) to confirm diagnosis and suitability for inclusion. Unaffected siblings were contacted via recruited patients, and only one sibling was included from each family. HC were recruited from the community via advertisement. Both HC and siblings were also interviewed using the MINI to verify suitability for inclusion. Participants were invited if aged above 35 to minimise risk of siblings and HC developing BD or psychosis in the future. All subjects passed institutional MRI safety screening and had no history of neurological disorders or brain injuries. BD participants had been euthymic – defined as ‘absence of significant mood episodes or changes in treatment received’ – for at least two months prior to scanning. Exclusion criteria for all participants included presence of alcohol/substance dependence within the past 12 months. To avoid confounding diagnosis with other psychotic syndromes (eg. schizophrenia or schizoaffective), participants with BD were excluded if reported any positive history of delusions or hallucinations outside a mood episode. In the case of unaffected siblings, those were excluded in case of reporting any personal history of mood disorder or psychosis. HC participants were excluded in case of any personal history of any mental disorders or family history of bipolar or psychosis in first-degree relatives.
All participants gave written informed consent prior to inclusion in the study, completed the Hamilton Depression Rating Scale (HDRS, (25)), the Young Mania Rating Scale (YMRS, (26)) and the National Adult Reading Test (NART, (27)) on the day of the scan. The study was approved by the local National Health Service-Research Ethics Committee.
Polygenic risk analysis sample
Based on our results a secondary analysis of FA in the UF was performed looking separately at polygenic risk scores (PRS) for BD (22) and psychosis (23) in a subgroup of subjects (n=661; mean age = 19.7; 81% male) from the Avon Longitudinal Study of Parents and Children (ALSPAC). ALSPAC is a population-based birth cohort of 14,062 live births – with expected dates of delivery 1st April 1991 to 31st December 1992 – of which 13,988 children were alive at 1 year of age. Data within ALSPAC has been reported extensively (28, 29) and the study website contains details of all the data that is available through a fully searchable data dictionary (http://www.alspac.bris.ac.uk/alspac/researchers/data-access/data-dictionary/).
Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local National Health Service-Research Ethics Committee.
MRI data acquisition
MRI imaging for both samples (case-control and ALSPAC) was performed on the same GE HDx 3T scanner (GE Healthcare, Milwaukee WI) at Cardiff University Brain Research Imaging Centre (CUBRIC). A T1-weighted brain scan was acquired for co-registration using an axial 3D fast spoiled gradient recalled (FSPGR) sequence (TR/TE/TI = 8/3/ 450 ms; Flip Angle = 200; acquisition matrix= 256(AP)x192(LR)x172(SI), 1mm isotropic voxels), followed by a diffusion tensor imaging (DTI) sequence with a twice-refocused spin-echo echo-planar parallel to AC-PC plane. Acquisition was peripherally gated to the cardiac cycle. Data were obtained from 60 slices of 2.4mm thickness, FOV= 230mm, matrix size 96×96, TE= 87ms and parallel imaging (ASSET factor=2), b-values= 0 and 1200 s/mm2, encoding diffusion along 30 isotropically distributed directions and three non-diffusion-weighted scans according to an optimized gradient vector scheme (30). Part of the ALSPAC sample (n=219) was acquired as 60 directions, of which the optimal 30 directions were selected (31) together with the first 3 b0 images, to make this dataset equivalent to the above and allowing joint processing of all data.
DTI data processing
DTI data were processed using ExploreDTI version 4.8.3 (32). First T1 structural data were downsampled to 1.5x1.5x1.5 mm resolution. Eddy current and subject motion correction were performed with an affine registration to the non-diffusion-weighted images (33). EPI correction of the DTI data was performed, warping the data to the downsampled T1 FSPGR (34). RESTORE (35) and RESDORE (36) corrections were run, together with free water correction (37). Whole brain tractography was performed with a damped Richardson-Lucy algorithm (38). Termination criteria were an angle threshold>45°, fODF peak<0.05 and FA<0.2.
For the case-control sample, fibre tracts were obtained through an automated tractography pipeline (39), informed by manual tractography performed by SF. As our method of segmenting the cingulum does not include the majority of the parahippocampal part of the cingulum bundle (PHC), this was calculated separately. Each automatically reconstructed tract was visually inspected in ExploreDTI and edited where necessary, to reach the same quality as manual tractography. During this process, the researcher (SF) was kept blind to participant’s group allocation. For an example of tracts see Appendix 1. Where it was not possible to successfully reconstruct a tract, this was excluded. The final numbers included were: UF left n=131; UF right n=137; cingulum body (CB) right and left n=137; PHC left n=135; PHC right n=134.
For the ALSPAC PRS group an automated tractography script model was used to segment the UF. After validation of the automatic tractography model, tracts flagged up due to small number of streamlines or low FA values were manually checked, and deleted when tracts were unsuccessfully reconstructed. Final numbers were UF left n=652; UF right n=658.
In both cases, FA values were extracted for the tracts of interest, by calculating the average of FA measures at each vertex of each streamline in the tract.
Statistical analysis
Demographic and clinical variables were compared across groups using ANOVAs except YMRS and HAMD for which suitable non-parametric tests (Kruskal-Wallis) were used. Gender distribution across groups was compared using a Chi-square test. Clinical descriptors (not available in all participants) were compared between BD-I and BD-II using t-tests. FA was compared by means of two separated ANOVAs, one including BD-I, BD-II and HC, and a second including SIB-I, SIB-II and HC. In both cases Fisher’s Least Significant Difference (LSD) post hoc testing was applied where appropriate. Bonferroni was used to correct for multiple comparisons.
Polygenic risk analysis
Quality controlled (QC) genotype data were received from the University of Bristol. Briefly, a total of 9,912 subjects from the ALSPAC study were genotyped using the Illumina HumanHap550 quad genome-wide SNP genotyping platform (Illumina Inc., San Diego, CA, USA) by 23andMe subcontracting the Wellcome Trust Sanger Institute (Cambridge, UK) and the Laboratory Corporation of America (Burlington, NC, USA). Individuals were removed if they had undetermined X chromosome heterozygosity, abnormal heterozygosity, cryptic relatedness up to third-degree relatives using identity by descent, genotyping completeness less than 97%, and non-European ethnicity admixture detected by a multidimensional scaling analysis seeded with HapMap 2 individuals. Markers with minor allele frequency (MAF) below 0.01, complete genotyping less than 95%, and an exact test of Hardy–Weinberg equilibrium (p<5E-07) were removed. After quality control, 8,365 unrelated individuals and 500,527 genotyped single nucleotide polymorphisms (SNPs) were available for analysis. Autosomal chromosomes were imputed using the reference panel HRCv1.1 (hrc.r1.1.2016) (40) using a mixed population panel. Phasing was done using Eagle v2.3 (41) and imputation done using Mimimac3 (42). Imputed data were converted to best guess genotypes using plink 1.9 (43) with multiallelic sites, variants with an exact test of Hardy–Weinberg equilibrium (p<1E-06) and MAF < 0.01 were removed.
Polygenic risk scores were calculated according to the International Schizophrenia Consortium method (22). Two polygenic scores were generated; one for BD (22) and one for a combined BD+SCZ cases vs controls (23), which can be considered a proxy for psychosis, and can therefore be more predictive of BD-I than BD-II in our sample (44). Training data for BD were taken from the Cross-Disorder Group of the Psychiatric Genomics Consortium (45) with 6,990 BD cases and 4,820 controls. For BD+SCZ, data from Ruderfer et al. (2014) on 19,779 cases (10,410 BD and 9,369 SCZ) and 19,423 non-overlapping controls were used as training data (23). Scores were generated in plink (43) (using --score) using 6 nested progressive p-value thresholds (PT) of respectively 0.00001, 0.0001, 0.01, 0.1, 0.3 and 0.5. SNPs with MAF < 0.1 and imputation quality < 0.9 were removed. Linkage disequilibrium (LD) independent SNPs were retained using informative pruning in plink (43)(--clump to remove SNPs with LD > 0.1). 570 individuals had both polygenic risk scores and brain imaging phenotypes available. Linear regression was performed separately for each PRS threshold and left and right FA values, using PRS as the explanatory variable and adjusting for age and gender as covariates.
Results
Demographics and clinical description
Groups did not differ with regards to age, gender distribution or performance in the NART (p>.1). As expected, both bipolar groups showed higher scores in the HDRS and YMRS than the other groups (both p<0.01), although scores remained well below clinical thresholds (Table 1).
Table 1.
Demographic characteristics and psychometric scores across groups
| HC n=40 | BD-I n= 32 | BD-II n= 34 | Sib-I n= 17 | Sib-II n= 14 | Group comparison | |
|---|---|---|---|---|---|---|
| Females, n (%) | 24 (60%) | 22 (68%) | 19 (56%) | 9 (53%) | 9 (64%) | χ2 (4) = 1.710, p>.1 |
| Age, mean (sd) | 43.5 (4.9) | 45.0 (6.2) | 42.8 (7.0) | 47.2 (5.5) | 43.6 (8.0) | F(4,132)=1.763, p>.1 |
| NARTa, mean (sd) | 36.8 (7.0) | 35.2 (8.3) | 34.8 (6.9) | 36.1 (5.5) | 35.4 (7.9) | F(4,121)=0.348, p>.1 |
| YMRS, mean (sd) | 0.45 (0.8) | 2.72 (2.4) | 2.65 (3.0) | 0.53 (0.7) | 0.86 (1.1) | χ2 (4) = 46.885, p<0.01 |
| HDRS, mean (sd) | 0.48 (0.9) | 3.50 (3.1) | 3.85 (3.7) | 1.00 (1.2) | 1.57 (1.9) | χ2 (4) = 26.854, p<0.01 |
Number of correct responses in the NART is reported.
NART: National Adult Reading Test; YMRS: Young Mania Rating Scale; HDRS: Hamilton Depression Rating Scale; sd: standard deviation; HC: Healthy Controls; BD-I: bipolar disorder type I; BD-II: bipolar disorder type II; Sib-I: unaffected siblings of bipolar type I participants; Sib-II: unaffected siblings of bipolar type II participants.
On average BD participants in our study experienced their first mood episode at age 19, despite first being diagnosed as bipolar aged 31; those variables did not differ between BD-I and BD-II groups (t(50)= 0.21, p>.1; and t(54)= 1.24, p>.1, respectively). Only 9 participants in our clinical sample (14%) were free of medication at the time of this study, whereas the majority (n=36, 65%) were taking a combination of at least 2 different class of drugs (see Table 2 for details). One third of the participants with bipolar disorder (n=22; 12 BD-I, 10 BD-II) had history of at least one anxiety-related comorbid diagnosis, panic disorder with/without agoraphobia being the most prevalent (31% BD-I, 35% BD-II), followed by obsessive-compulsive disorder (13% BD-I, 12% BD-II), health anxiety, eating disorder and generalised anxiety disorder were present at a much lower rate (3% of the total sample, n=2). Due to the strict inclusion criteria, no other diagnoses were present in this sample. Only 2 siblings presented with mental health history, referring past history of generalised anxiety disorder (n=1) and health anxiety (n=1). Following our inclusion criteria, control participants had no history of any psychiatric disorder.
Table 2.
Clinical characterisation of participants with BD-I and BD-I
| BD-I n=32 | BD-II n=34 | Group comparison | |
|---|---|---|---|
| Age first episode, mean (sd) | 19 (5.8) | 19 (7.0) | t (50) = 0.21, p>.1 |
| Age diagnosis, mean (sd) | 30 (6.9) | 34 (9.5) | t (54) = 1.24, p>.1 |
| Co-diagnosis of anxiety disorder, n (%) | 12 (38%) | 10 (29%) | χ2 (1) = 0.49, p>.1 |
| Medication* | |||
| Non-medicated, n (%) | 1 (3%) | 8 (24%) | χ2 (1) = 5.60, p<.05 |
| Antidepressants, n (%) | 16 (50%) | 15 (44%) | χ2 (1) = 3.65, p>.1 |
| Lithium, n (%) | 8 (25%) | 7 (21%) | χ2 (1) = 2.49, p>.1 |
| Anticonvulsants, n (%) | 16 (50%) | 15 (44%) | χ2 (1) = 3.65, p>.1 |
| Antipsychotics, n (%) | 20 (63%) | 7 (21%) | χ2 (1) = 12.88, p<.001 |
| Combination of above, n (%) | 22 (69%) | 14 (41%) | χ2 (1) = 5.82, p<.05 |
Medication data from 1 BD-I participant was inaccessible.
Fractional anisotropy between-groups analyses
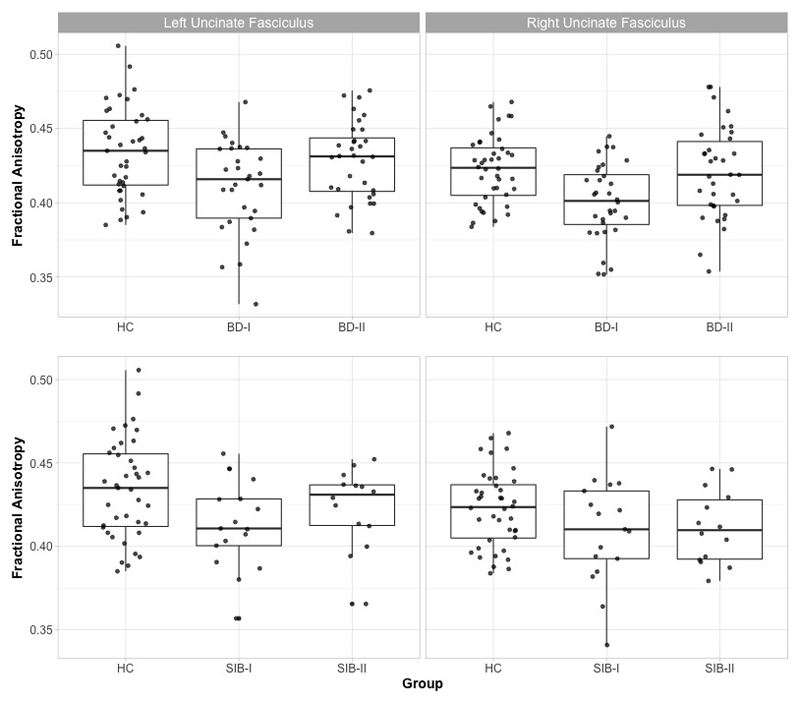
ANOVAs comparing FA across patients and controls showed a significant group effect in left and right UF (F(2,97)= 5.87, p=0.004; F(2,103)= 7.22, p=0.001; respectively). These comparisons survived Bonferroni correction. Post hoc analysis showed that in both cases the effect was driven by BD-I participants showing reduced FA compared to both HC (p=0.001 for both left and right) and BD-II (p=0.019, and p=0.003, respectively left and right). Whereas there was no difference between BD-II and HC (p>0.1) (Figure 1). To exclude an effect of age in these results, these analyses were repeated with age as covariate. Results remained almost identical.
Figure 1.
ANOVAs including FA in CB or PHC showed no significant group effect (left CB F(2,103)= 2.53, p=0.08; right CB F(2,103)= 1.37; left PHC F(2,101)= 0.011; right PHC F(2,101)= 1.30; all other p>0.1) (Mean FA and standard deviation for all tracts across groups are presented in Appendix 2).
Since this study contained a number of participants (BD-I n= 15, BD-II n= 14, HC n=18) included in our previously reported results (15), we re-ran the tests using only newly recruited participants. Results mainly replicated those listed above, with both left and right UF being significantly different between groups, a result which survived Bonferroni correction. Post-hoc testing again showed BD-I FA < HC FA (Appendix 3).
Based on the results above, ANOVAs for FA in left and right UF including unaffected siblings and HC were run. Results showed a significant group effect in the left UF (F(2,67)= 3.47, p=0.037) but not in the right (F(2,68)= 2.03, p>0.1). Post hoc pairwise comparisons showed that the left UF effect was again driven by Sib-I showing reduced FA compared to HC (p=0.012). In this case, though, no other post-hoc comparison was significant, and the main ANOVA’s group effect did not survive Bonferroni correction. For completeness, we run the ANOVAs for FA in the CB and PHC; but again none of these resulted in a significant group effect: left CB F(2,68)= 0.38; right CB F(2,68)= 1.35; left PHC F(2,68)= 0.32; right PHC F(2,67)= 1.65; all p>0.1. (Figure 2).
Finally, post hoc paired sample t-tests comparing BD/sibling pairs showed no significant difference in the UF between BD-I vs Sib-I (left, t(12)= 0.172, p>0.1; right, t(13)= 1.286, p>0.1) or BD-II vs Sib-II (left, t(10)= 0.448, p>.01; right, t(10)= 0.784, p>0.1).
Medication effects on BD-I v BD-II results
Point-biserial correlations between each medication class status (i.e. taking v not-taking) and FA in the UF showed a significant positive association between Lithium status and FA in the left UF (r=0.37, p=.004) and negative between anticonvulsant status and right UF (r=-0.26, p=0.036). There were trends between Lithium status and FA in the right UF (r=0.24, p=0.055), and combination of drugs treatment status and FA in the left UF (r=0.24, p=0.069). No other association approached significance. Regression analyses were then run to ascertain whether clinical group allocation (i.e. BD-I v BD-II) still explained a significant amount of variance after accounting for FA variance explained by those medication variables. Clinical group allocation (BD-I v BD-II) still explained a significant amount of FA in the left UF after accounting for variance explained by Lithium status (R2change= 0.103, F(1,57)= 7.73, p=0.007), and the same was found for the right UF (R2change= 0.124, F(1,62)= 9.42, p=0.003). Similarly, clinical group significantly predicted FA in the right UF once accounting for variance associated with anticonvulsant status (R2change= 0.101, F(1,62)= 7.57, p=0.008), and in the left UF once accounting for variance explained by combined drugs treatment (R2change= 0.149, F(1,57)= 10.72, p=0.002).
Association between FA in the UF and polygenic risk for bipolar disorder and psychosis
There was no significant correlation between polygenic risk for BD or psychosis at any of the six PT used and FA in the left or right UF. In all cases R2 values remain very low (<0.01, Appendix 4).
Discussion
This study contributes to the gradual unravelling of the neuropathophysiology of BD. Our main aim was to test our hypothesis of reduced FA in the UF in patients with BD-I, but not BD-II. We also aimed to extend this research into the cingulum, where we expected the same pattern of effects. The results supported the former, but not the latter. Interestingly, white matter microstructure differences in the UF were partially mirrored in unaffected siblings, suggesting this as a potential endophenotype specifically for BD-I. Despite the potential genetic basis of this difference suggested by our results in siblings, we did not find any association between FA in the UF and polygenic scoring for BD or psychosis in a larger independent population sample.
Our results confirmed previously reported decreased FA in the UF of participants with BD (10, 11, 19, 46). We also confirmed previous findings from our group (15) in a larger sample; showing this to be the case for BD-I, but not BD-II. Rather than showing an intermediate position between BD-I and HC, FA in the UF for BD-II behaved indistinguishably from HC, while significantly differing from BD-I. This result indicates a potential distinct pathophysiological mechanism between bipolar subtypes, rather than supporting the hypothesis of a simple difference in symptom severity, and merits further investigation. Interestingly, previous research has found a similar pattern of white matter deficits in frontal and parietal regions between BD-I and SCZ patients (46). In accordance with the diagnostic criteria, the patients recruited for this study with a BD-II diagnosis did not experience psychosis during episodes of high mood and in our sample most people with BD-II also denied experiencing psychotic symptoms during depression. The majority of our participants with BD-I did report experiencing psychotic symptoms during mania. Thus, this difference in FA between BD subtypes in the UF could reflect vulnerability to psychosis, rather than mood symptoms. Future research should address this question, looking at FA in the UF as a potential crosscutting biomarker for psychosis following the RDoC perspective (47).
Our results did not concur with previous findings in the cingulum indicating reduced FA in BD compared to HC (9, 12, 19, 20), even when combining all BD participants in a single group (Appendix 5). This could be explained by methodological differences. Most previous findings for the cingulum were based on voxel-wise comparisons (8, 19, 20) or used a very different tract mapping methodology (9, 12) than here. To our knowledge, only two previous studies used equivalent methods of fiber tracking to the one reported here (10, 13), one of which also failed to observe significant group effects for the cingulum (10). The advantage of tractography over voxel-wise methods is its power to detect effects in pre-defined tracts of interest rather than representing an exploratory approach across the whole brain, requiring more correction for multiple comparisons. Due to our strong a-priori hypotheses about the UF, CB and PHC, tractography was selected as the most adequate approach. In any case, our results should call into question the relevance of FA in the cingulum as a potential biomarker for BD.
Interestingly, our results are also suggestive of reduced FA in the left UF for Sib-I compared to HC, but not for Sib-II. No significant group effect was found in the right UF despite post-hoc testing showing a trend toward lower FA, again between Sib-I and HC (p=0.08). We also found that both sibling groups did not differ from their affected relatives with regards to FA in the UF. These findings are consistent with previous research reporting siblings of patients with BD showing intermediate FA values between patients and controls in various white matter regions (48), and therefore placing FA in the UF as a potential endophenotype for this disorder. This is the first time that this result incorporating patients and their siblings has been reported using tractography on a-priori selected tracts of interest rather than based on a whole-brain voxel-wise exploratory approach. Importantly, our sibling groups were selected on the basis of an absence of any personal history of mood disorders and psychosis, and their age (age >35) which indicates lower probability of developing BD in the future (49); as a consequence, our sibling groups can be considered at a higher familial risk but yet resilient. This strengthens the hypothesis of lower FA in the UF representing an endophenotype for BD-I, likely to be present as a premorbid risk marker independently of mental state and psychopathological history, and endorses future research in this area.
Considering the results so far discussed and previous literature showing FA to be heritable (50), one could have predicted that common genetic variance associated with bipolar disorder or psychosis would correlate with FA in the UF, which surprisingly was not the case. Among currently available GWAS studies for BD, BD-I is over-represented compared to BD-II (51) and therefore we predicted that any BD polygenic score based on this data would mainly represent BD-I risk. Also, as we advocated earlier, if FA in the UF reflects vulnerability for psychosis rather than mood disorder, one would expect the polygenic score for psychosis (23) to also correlate with FA in the UF. However, our results did not confirm this notion, calling into question whether the familial effects reported above are driven by common genetic factors. Further replication in larger samples and with more suited polygenic scores than the ones currently available (i.e. specific for BD- I/BD-II) would however be required before any firm conclusions can be drawn.
Our study has some limitations that should be noted. First our BD groups include participants used in a previous study reporting FA differences in UF (15). It is important to notice, though, that these two samples were acquired in the same MRI machine and using exactly the same acquisition sequence, and all data were (re-)processed together for this study. We also replicated our analyses after excluding those participants who took part in the previous study, and obtained very similar results, although with decreased statistical power (Appendix 3). Second, due to our stringent inclusion criteria (i.e. lack of any history of mood disorder or psychosis, age >35 and only one sibling per family) our sample size for unaffected siblings is modest, which has limited our power to detect significant effects. Despite the limitation in sample size, the use of such a group of siblings strengthens our conclusion regarding FA in the UF as a potential endophenotype for BD-I. Finally, the potential confounding effect of medication is a common problem in studies recruiting patients with chronic illnesses like bipolar disorder. Despite that, we have shown that differences in FA in the UF found between BD-I and BD-II were not fully explained by medication status. Moreover, it has been suggested that medication effects are smaller than originally thought but could increase type II errors due to normalization effect over brain function and structure (52). With this in mind, we believe that our main results regarding differences between patients and controls are not secondary to medication.
In conclusion, we showed the microstructure of white matter in the UF to be compromised in BD-I participants, but not in BD-II, compared to HC. A similar effect, albeit reduced, was seen in unaffected siblings of BD-I, indicating familiality and postulating FA in the UF as a potential endophenotype for the disorder.
Supplementary Material
Acknowledgements
This research project was funded through a 2010 NARSAD Young Investigator Award (ref: 17319) to Dr Xavier Caseras. Sonya Foley is funded by CUBRIC and the school of Psychology, Cardiff University. Dr Katherine E Tansey is supported by the Wellcome Trust (grant reference: WT105613/Z/14/Z) and the College of Biomedical and Life Sciences at Cardiff University. Judith Harrison is supported by the Welsh Clinical Academic Training Scheme. Matthew Bracher-Smith is funded by a PhD studentship through the MRC Centre for Neuropsychiatric Genetics & Genomics. Dr. Greg Parker was funded through a Wellcome Trust New Investigator Award (096646/Z/11/Z) to Prof Derek K. Jones.
We are grateful to the National Centre for Mental Health (NCMH) and the Bipolar Disorder Research Network for their support with recruitment.
We are extremely grateful to all the families who took part in the ALSPAC study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council and Wellcome (Grant ref: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and they will serve as guarantors for the contents of this paper. GWAS data were generated by Sample Logistics and Genotyping Facilities at Wellcome Sanger Institute and LabCorp (Laboratory Corportation of America) using support from 23andMe.
Many thanks to Dr. Sonya Bells for the use of her uncinate fasciculus model for the ALSPAC cohort.
Funding
This research project was funded through a 2010 NARSAD Young Investigator Award (ref: 17319) to Dr Xavier Caseras. Sonya Foley is funded by CUBRIC and the school of Psychology, Cardiff University. Sonya Foley was previously funded by an ISSF grant to Cardiff University School of Medicine. Dr Katherine E Tansey is supported by the Wellcome Trust (grant reference: WT105613/Z/14/Z) and the College of Biomedical and Life Sciences at Cardiff University. Dr Judith Harrison is supported by the Welsh Clinical Academic Training Scheme. Matthew Bracher-Smith is funded by a PhD studentship through the MRC Centre for Neuropsychiatric Genetics & Genomics. Dr. Greg Parker was funded through a Wellcome Trust New Investigator Award (096646/Z/11/Z) to Prof Derek K. Jones.
Biographies
Further Author information
Sonya F Foley MSc, Cardiff University Brain Research Imaging Centre, Cardiff University, UK
Contribution: Recruitment, data acquisition, data analysis and interpretation, drafting and revising article
Matthew Bracher-Smith BSc, MRC Centre for Neuropsychiatric Genetics and Genomics, Institute of Psychological Medicine and Clinical Neurosciences, Cardiff University, UK
Contribution: Data analysis, contributions to article
Katherine E Tansey PhD, Core Bioinformatics and Statistics Team, College of Biomedical and Life Sciences, Cardiff University, Cardiff, UK
Contribution: Genetics expertise and data analysis and interpretation, contributions to article
Judith Harrison MBChB, MRC Centre for Neuropsychiatric Genetics and Genomics, Institute of Psychological Medicine and Clinical Neurosciences, Cardiff University, UK
Contribution: Data analysis, contributions to article
Greg D Parker PhD, Cardiff University Brain Research Imaging Centre, Cardiff University, UK
Contribution: DTI data processing expertise and methods provision, contributions to article
Xavier Caseras PhD, MRC Centre for Neuropsychiatric Genetics and Genomics, Institute of Psychological Medicine and Clinical Neurosciences, Cardiff University, UK
Contribution: Conception and design of study, recruitment, data acquisition, data interpretation, drafting and revising article, final approval of article
Footnotes
Declaration of interest: The authors have no financial disclosure or conflict of interest to declare.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- 2.Baek JH, Park DY, Choi J, Kim JS, Choi JS, Ha K, et al. Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. Journal of affective disorders. 2011;131(1):59–67. doi: 10.1016/j.jad.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Charney A, Ruderfer D, Stahl E, Moran J, Chambert K, Belliveau R, et al. Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Translational Psychiatry. 2017;7(1):e993. doi: 10.1038/tp.2016.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett JH, Smoller JW. The genetics of bipolar disorder. Neuroscience. 2009;164(1):331–43. doi: 10.1016/j.neuroscience.2009.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambilla P, Bellani M, Yeh P-H, Soares JC, Tansella M. White matter connectivity in bipolar disorder. International Review of Psychiatry. 2009;21(4):380–6. doi: 10.1080/09540260902962172. [DOI] [PubMed] [Google Scholar]
- 6.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in cognitive sciences. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. American Journal of Psychiatry. 2014;171(8):829–43. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedetti F, Yeh P-H, Bellani M, Radaelli D, Nicoletti MA, Poletti S, et al. Disruption of White Matter Integrity in Bipolar Depression as a Possible Structural Marker of Illness. Biological Psychiatry. 2011;69(4):309–17. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Sarrazin S, Poupon C, Linke J, Wessa M, Phillips M, Delavest M, et al. A multicenter tractography study of deep white matter tracts in bipolar I disorder: psychotic features and interhemispheric disconnectivity. JAMA psychiatry. 2014;71(4):388–96. doi: 10.1001/jamapsychiatry.2013.4513. [DOI] [PubMed] [Google Scholar]
- 10.Lin F, Weng S, Xie B, Wu G, Lei H. Abnormal frontal cortex white matter connections in bipolar disorder: A DTI tractography study. Journal of Affective Disorders. 2011;131(1–3):299–306. doi: 10.1016/j.jad.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh AM, Maniega SM, Lymer GKS, McKirdy J, Hall J, Sussmann JED, et al. White Matter Tractography in Bipolar Disorder and Schizophrenia. Biological Psychiatry. 2008;64(12):1088–92. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Jackowski M, Kalmar JH, Chepenik LG, Tie K, Qiu M, et al. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. The British Journal of Psychiatry. 2008;193(2):126–9. doi: 10.1192/bjp.bp.107.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Versace A, Andreazza AC, Young L, Fournier JC, Almeida JR, Stiffler RS, et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Molecular psychiatry. 2014;19(2):200–8. doi: 10.1038/mp.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum White Matter in Young Women at Risk of Depression: The Effect of Family History and Anhedonia. Biological Psychiatry. 2012;72(4):296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Caseras X, Murphy K, Lawrence NS, Fuentes-Claramonte P, Watts J, Jones DK, et al. Emotion regulation deficits in euthymic bipolar I versus bipolar II disorder: a functional and diffusion-tensor imaging study. Bipolar disorders. 2015;17(5):461–70. doi: 10.1111/bdi.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emsell L, Chaddock C, Forde N, Van Hecke W, Barker GJ, Leemans A, et al. White matter microstructural abnormalities in families multiply affected with bipolar I disorder: a diffusion tensor tractography study. Psychological Medicine. 2014;44(10):2139–50. doi: 10.1017/S0033291713002845. [DOI] [PubMed] [Google Scholar]
- 17.Maller JJ, Thaveenthiran P, Thomson RH, McQueen S, Fitzgerald PB. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. Journal of Affective Disorders. 2014;169:118–27. doi: 10.1016/j.jad.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Ambrosi E, Chiapponi C, Sani G, Manfredi G, Piras F, Caltagirone C, et al. White matter microstructural characteristics in Bipolar I and Bipolar II Disorder: A diffusion tensor imaging study. Journal of Affective Disorders. 2016;189:176–83. doi: 10.1016/j.jad.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Ha TH, Her JY, Kim JH, Chang JS, Cho HS, Ha K. Similarities and differences of white matter connectivity and water diffusivity in bipolar I and II disorder. Neuroscience Letters. 2011;505(2):150–4. doi: 10.1016/j.neulet.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Liu J-X, Chen Y-S, Hsieh J-C, Su T-P, Yeh T-C, Chen L-F. Differences in white matter abnormalities between bipolar I and II disorders. Journal of affective disorders. 2010;127(1):309–15. doi: 10.1016/j.jad.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Sprooten E, Sussmann JE, Clugston A, Peel A, McKirdy J, Moorhead TWJ, et al. White matter integrity in individuals at high genetic risk of bipolar disorder. Biological psychiatry. 2011;70(4):350–6. doi: 10.1016/j.biopsych.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 22.International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia that overlaps with bipolar disorder. Nature. 2009;460(7256):748. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruderfer DM, Fanous AH, Ripke S, McQuillin A, Amdur RL, Gejman PV, et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Molecular psychiatry. 2014;19(9):1017–24. doi: 10.1038/mp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European psychiatry. 1997;12(5):224–31. [Google Scholar]
- 25.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133(5):429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 27.Nelson HE, Willison J. National Adult Reading Test (NART) Nfer-Nelson; Windsor: 1991. [Google Scholar]
- 28.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. International journal of epidemiology. 2012;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort profile: the ‘children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. International journal of epidemiology. 2013;42(1):111–27. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones D, Horsfield M, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42 [PubMed] [Google Scholar]
- 31.Cook PA, Symms M, Boulby PA, Alexander DC. Optimal acquisition orders of diffusion-weighted MRI measurements. Journal of magnetic resonance imaging. 2007;25(5):1051–8. doi: 10.1002/jmri.20905. [DOI] [PubMed] [Google Scholar]
- 32.Leemans A, Jeurissen B, Sijbers J, Jones D, editors. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. 17th Annual Meeting of Intl Soc Mag Reson Med; 2009. [Google Scholar]
- 33.Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine. 2009;61(6):1336–49. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- 34.Wu M, Chang L-C, Walker L, Lemaitre H, Barnett AS, Marenco S, et al., editors. Comparison of EPI distortion correction methods in diffusion tensor MRI using a novel framework. International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang LC, Jones DK, Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magnetic resonance in medicine. 2005;53(5):1088–95. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- 36.Parker G, Marshall D, Rosin P, Drage N, Richmond S, Jones D, editors. RESDORE: robust estimation in spherical deconvolution by outlier rejection. Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City, Utah, USA. 2013. [Google Scholar]
- 37.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine. 2009;62(3):717–30. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- 38.Dell'Acqua F, Scifo P, Rizzo G, Catani M, Simmons A, Scotti G, et al. A modified damped Richardson–Lucy algorithm to reduce isotropic background effects in spherical deconvolution. Neuroimage. 2010;49(2):1446–58. doi: 10.1016/j.neuroimage.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Parker G, Rosin PL, Marshall D. Automated Segmentation of Diffusion Weighted MRI Tractography. 2012 [Google Scholar]
- 40.Haplotype Reference Consortium. A reference panel of 64,976 haplotypes for genotype imputation. Nature genetics. 2016;48(10):1279–83. doi: 10.1038/ng.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loh P-R, Danecek P, Palamara PF, Fuchsberger C, Schoenherr S, Forer L, et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nature genetics. 2016;48(11):1443–8. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nature genetics. 2016;48(10):1284–7. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4(1):7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craddock N, O'Donovan MC, Owen MJ. Genes for Schizophrenia and Bipolar Disorder? Implications for Psychiatric Nosology. Schizophrenia Bulletin. 2006;32(1):9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet. 2013;381(9875):1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar disorders. 2009;11(1):11–8. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 47.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am Psychiatric Assoc. 2010 doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 48.Sprooten E, Brumbaugh MS, Knowles EE, McKay DR, Lewis J, Barrett J, et al. Reduced white matter integrity in sibling pairs discordant for bipolar disorder. American Journal of Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellivier F, Golmard J-L, Rietschel M, Schulze TG, Malafosse A, Preisig M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. American Journal of Psychiatry. 2003;160(5):999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- 50.Kochunov P, Jahanshad N, Marcus D, Winkler A, Sprooten E, Nichols TE, et al. Heritability of fractional anisotropy in human white matter: A comparison of Human Connectome Project and ENIGMA-DTI data. NeuroImage. 2015;111:300–11. doi: 10.1016/j.neuroimage.2015.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature genetics. 2011;43(10):977–83. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar disorders. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.