Abstract
The chemokine CXCL17 is associated with the innate response in mucosal tissues but is poorly characterized. Similarly, the G protein-coupled receptor GPR35, expressed by monocytes and mast cells has been implicated in the immune response, although its precise role is ill-defined. A recent manuscript reported that GPR35 was able to signal in response to CXCL17, which we set out to confirm in this study.
GPR35 was readily expressed using transfection systems, but failed to signal in response to CXCL17 in assays of β-arrestin recruitment, inositol phosphate production, calcium flux and receptor endocytosis. Similarly, in chemotaxis assays, GPR35 did not confirm sensitivity to a range of CXCL17 concentrations above that observed in the parental cell line. We subsequently employed a real time chemotaxis assay (TAXIScan) to investigate the migratory responses of human monocytes and the monocytic cell line, THP-1 to a gradient of CXCL17. Freshly isolated human monocytes displayed no obvious migration to CXCL17. Resting THP-1 cells showed a trend towards directional migration along a CXCL17 gradient, which was significantly enhanced by overnight incubation with the prostaglandin PGE2. However, pre-treatment of PGE2-treated THP-1 cells with the well characterized GPR35 antagonist ML145 did not significantly impair their migratory responses to CXCL17 gradient. CXCL17 was susceptible to cleavage with chymase although this had little effect its ability to recruit THP-1 cells.
We therefore conclude that GPR35 is unlikely to be a bona fide receptor for CXCL17 and that THP-1 cells express an as yet unidentified receptor for CXCL17.
Introduction
Intensive efforts by the chemokine research community over the last two decades have identified a family of around 45 such proteins in the human, noted for their ability to induce the directional migration, i.e. chemotaxis, of leukocytes (1). Considerable progress has been made regarding our understanding of this family and how the signals they induce via specific G protein-coupled receptors (GPCRs) shape the immune responses of the host (2). In the case of the chemokine receptors CCR5 and CXCR4, this knowledge has been successfully translated into medicines with clinical efficacy in the treatment of HIV infection, the treatment of WHIM (Warts, Hypogammaglobulinemia, Immunodeficiency, and Myelokathexis) syndrome and the mobilization of stem cells (3–5). Despite this progress, within the chemokine family there still remain a small number of “orphan” chemokines for which no specific GPCR partners have been identified. These include the CXC chemokines CXCL14 (6, 7) and CXCL17 (8).
CXCL17 was first described in the literature as a monocyte recruiting chemokine (8) and its overexpression has been shown to promote the growth of a variety of tumors in vivo (9, 10). In humans, CXCL17 appears to have roles in both homeostatic and inflammatory settings. Its expression is restricted to mucosal sites including the small intestine, trachea and lung where it is associated with a broad spectrum antimicrobial function, albeit when at micromolar concentrations of chemokine (11). Notably, CXCL17 was undetectable in the bronchial alveolar lavage (BAL) of healthy subjects but expressed at significant levels in the BAL of patients suffering from idiopathic pulmonary fibrosis (IPF) (11). This prompted the authors of that study to speculate that CXCL17 plays a role in microbial killing within the IPF lung (often associated with infection in advanced stages of the disease) or is involved with the associated remodelling via the recruitment of myeloid cells. Consistent with this later hypothesis, the same group went on to generate a CXCL17-deficient mouse model which was notable for the reduced levels of macrophages observed in the lung under homeostatic conditions (12).
GPR35 was originally identified in the laboratory of O’Dowd (13) as an open reading frame predicted to encode a GPCR. Subsequent demonstration that it is expressed by various cells of the immune system has led to the suggestion that it may have potential as a therapeutic target in inflammatory disease (14, 15). In human, two distinct GPR35 isoforms, known as GPR35a and GPR35b, are expressed with GPR35b differing from GPR35a by the presence of additional 31 amino acids at the N-terminus (16), analogous to the two N-terminally spliced isoforms of the chemokine receptor CXCR3 (17). Endogenous ligands identified as activating GPR35 include the tryptophan metabolite kynurenic acid (18) and various lysophosphatidic acids (19), although the millimolar concentrations of the former ligand needed to induce signalling at human GPR35 has led to questions about the physiological relevance of the original finding (20). Amongst synthetic compounds, the asthma medications cromolyn disodium (21) and lodoxamide (22) which serve to stabilize mast cells have also been shown to be agonists of GPR35, implicating GPR35 in the allergic response.
Recently, Maravillas-Montero and colleagues described CXCL17 as a GPR35 ligand with nanomolar activity in both chemotaxis and intracellular calcium flux assays (23). Here we describe an investigation into the potency and specify of CXCL17 as a GPR35 ligand using a battery of in vitro assays in both GPR35 transfected cell lines, human monocytes and the human myeloid cell line THP-1.
Materials and Methods
Materials
Materials were purchased from Thermofisher (Paisley, UK) unless otherwise described. Recombinant human CXCL17 generated in E. coli. was purchased from R&D Systems (Abingdon, UK) and was the 96 amino acid species comprised of amino acids Leu24-Leu119. A plasmid containing an insert encoding a 3xHA-tagged GPR35a cDNA (Cat #GPR035TN00) was purchased from the cDNA Resource Center (Bloomsburg University, PA, USA) and is an N-terminal 3xHA-tagged variant of the GPR35 plasmid which was derived by PCR from the clone used by Maravillas-Montero et al. (23). The insert was re-sequenced by MWG-Eurofins (Ebersberg, Germany) and found to be authentic. Other cDNAs encoding human GPR35a and GPR35b, as well as the mouse and rat orthologs of GPR35 have been detailed previously (20). The GPR35 antagonist ML145 was purchased from Biotechne (Oxford, UK) and was originally identified as a GPR35 inhibitor by a group from the Sanford-Burnham Center for Chemical Genomics using an arrestin recruitment assay (AID: 2079) (24). The murine anti-HA monoclonal antibody (HA.11) was purchased from Cambridge Bioscience Ltd (Cambridge, UK). CCL2, CCL15 and CCL23 were purchased from Peprotech EC (London, UK)
Cell culture
The mouse pre-B cell line L1.2 and THP-1 cells were cultured in RPMI 1640 (Sigma-Aldrich, Dorset, UK) containing 10% foetal bovine serum, penicillin/streptomycin, Minimum Essential Medium Non-essential Amino acids, sodium pyruvate and 2-mercaptoethanol and incubated at 37°C, 5% CO2. Cells were maintained in this medium at a concentration of 0.5 x 106 cells/ml. Human embryonic kidney (HEK) 293T cells were maintained in Dulbecco’s Modification of Eagle’s Medium supplemented with 0.292 g/l l-glutamine, 10% (v/v) fetal bovine serum, and 1% penicillin/streptomycin mixture. Peripheral blood was taken from healthy normal subjects according to a protocol approved by a local ethics committee. Monocytes were isolated by negative selection using the RosetteSep kit (StemCell Technologies, Grenoble, France).
Cell transfections
This protocol was essentially that as previously described (25). Briefly, 1.0 - 1.5 x 107 cells/ml were used for each transfection in a volume of 800µl of RPMI. This was transferred to a 0.4 cm gap electroporation cuvette after which 50µl of a 10 mg/ml solution of baker’s yeast tRNA (Sigma-Aldrich) was added to the cuvette. 1µg of the GPR35 plasmid per 1 x 106 cells was added to the cuvette and the cells subjected to electroporation at 330V, 950 μF. After allowing the cells to recover for 20 minutes at room temperature, they were transferred to a flask containing complete medium and incubated for 18 hours to 24 hours before receptor expression was examined by flow cytometry prior to experimentation. For transient transfections, sodium butyrate (Sigma-Aldrich,) was added to a final concentration of 10 mM to enhance receptor expression. To generate the L1.2 clone 23 stably expressing GPR35, cells were transfected as before and selected after 48hr of culture by the addition of 1mg/ml G418. Surviving cells were expanded and cloned by limiting dilution. Clone 23 was identified by flow cytometry as expressing suitable amounts of GPR35 on its surface. Transient transfections using HEK293T cells were performed using polyethylenimine, with experiments carried out 48 hours after transfection.
Flow cytometry
Briefly, 0.5 x 106 of transfected cells were used for each staining procedure. Cell pellets were resuspended in 100 µl of FACS buffer (PBS, 0.25% BSA, 0.05% NaN3) containing primary antibody at a concentration of 10μg/ml and incubated for 5 minutes on ice. The cells were then washed with 500 µl FACS buffer and pelleted by centrifugation (1500g) for 30 seconds. The supernatant was discarded and the cell pellet was resuspended in 100 µl FACS buffer containing Fluorescein isothiocyanate (FITC)-conjugated secondary antibody and incubated at 4°C for 5 minutes. The cells were then washed with 500 µl FACS buffer and centrifuged briefly as before for 30 seconds. The supernatant was discarded and the cell pellets were resuspended in 500 µl staining buffer containing TO-PRO3 at a dilution of 1:10,000. The samples were read on a FACSCalibur flow cytometer (Becton Dickinson, Oxford, UK).
Modified Boyden chamber chemotaxis assays
Assays were performed essentially as described by Vaidehi et al (25) using 96-well disposable chemotaxis plates (Neuro Probe Inc., Gaitherburg, USA). Cells were allowed to migrate in response to chemokines for 5 hours at 37°C, 5% CO2 after which Cell Titer-Glo® (Promega, UK) was used to measure the responses via luminescence were read from a plate reader as before (26). Data from these assays are shown as chemotactic indices following division by the basal migratory responses to buffer alone. In some experiments, prior to use in the assay, cells were pre-incubated for 15 minutes at room temperature in buffer containing DMSO (vehicle) or a 1μM final concentration of the GPR35 antagonist ML145.
Endocytosis assay
L1.2 cells expressing 3xHA-GPR35 were resuspended in RPMI containing 0.1% BSA at 2 x 106 cells/ml. To 25 µl volumes of cells were added 25 µl of the same buffer containing either 20 µM lodoxamide, 10 µM lodoxamide, 2 µM lodoxamide or 2 µM CXCL17. Controls contained the vehicle DMSO. For each data point the cells were incubated for 15 minutes at 4°C (to measure basal GPR35 expression in the absence of endocytosis) and 37°C (to measure agonist-driven endocytosis of the receptor). GPR35 remaining on the cell surface after treatment was quantified by flow cytometry using the anti-HA antibody as described above.
Real-time chemotaxis assays (TAXIScan)
TAXIScan apparatus for the real time visualization of leukocyte migration was assembled and used according to a published protocol (27). Monocytes or THP-1 cells were allowed to migrate for 1 hour at 37°C along gradients generated by the addition of 1µl of either 1µM CXCL17, 10µM CXCL17 or 1µM CCL2. Cells were also allowed to migrate in the absence of a stimulus to obtain basal chemotaxis measurements. In some experiments, prior to use, cells were pre-incubated for 15 minutes at room temperature in buffer containing DMSO (vehicle) or 1μM final concentration of the GPR35 antagonist ML145. Sequential image data were captured every minute as individual jpegs which were subsequently processed with ImageJ (National Institutes of Health), equipped with the manual tracking (Fabrice Cordelieres, Institut Curie, Orsay (France) and chemotaxis tool plugins (Ibidi, Martinsried, Germany).
Individual experiments consisted of triplicate or quadruplicate for each stimulus and data illustrated are collated from an equal number of experiments as highlighted in the appropriate figure legend. The total numbers of cells tracked under each condition are shown in the top right hand corner of each plot. For each individual cell, the forward migration index parallel to the gradient (FMI‖) was calculated using the chemotaxis tool plugin. FMI‖ is defined as the distance travelled by the cell in the y axis (i.e. along the chemokine gradient) divided by the accumulated distance travelled and is a reliable measure of migration along a chemoattractant gradient (28).
GPR35-β-arrestin-2 interaction assays
The bioluminescence resonance energy transfer (BRET)–based β-arrestin-2 recruitment assays were performed using HEK293T cells transfected transiently to co-express forms of human, mouse or rat GPR35 along with β-arrestin-2, as described previously (20).
Inositol phosphate assays
Chimeric G protein α subunits were as described in Jenkins et al., (20) and following expression in HEK293T cells along with the described forms of GPR35, inositol phosphate measurements were performed as previously described (20).
Intracellular Calcium Flux Assays
These were performed as previously described (29) using cells that were loaded with the dye FURA-2 AM. Real time data were recovered using a fluorimeter (LS-50B, Perkin-Elmer, Beaconsfield, UK). Data are expressed as the relative ratio of fluorescence emitted at 510 nm after sequential stimulation at 340 and 380 nm.
Chemokine Digestion and SDS-PAGE analysis
20µg of CXCL17 was resuspended in a buffer composed of 100mM Tris-HCl (pH 7.8) and 10mM CaCl2. The aliquot was divided into two with one aliquot receiving the addition of 0.1-0.4µg of recombinant human chymase (Sigma Aldrich, Poole. UK). Both tubes were incubated for 18 hours at 37°C before being stored at -20°C until further use. SDS PAGE was carried out using precast NuPAGE 4-12% Bis-Tris Protein Gels. Samples were mixed with 2x Tris-Glycine SDS Sample Buffer and heated to 95 °C for 10 min prior to loading alongside SeeBlue Plus2 pre-stained protein standards. Gels were run at 150 V for 50 min and then fixed in 25% isopropanol (IPA) and 10% acetic acid (HOAc) for 30 min with agitation. Fixed gels were stained with 0.25% Coomassie G-250 (Bio-Rad Laboratories, Watford, UK) in 25% IPA and 10% HOAc for 2 h after which they were destained with 10% HOAc overnight. Images were acquired with myECL Imager (Thermo Fisher Scientific).
Statistical Analysis
Data analysis was carried out using GraphPad Prism version 6.0 (GraphPAD Software, SanDiego, USA). Experiments were assessed an appropriate statistical test noted in the figure legend. In all tests, p<0.05 was considered as statistically significant.
Results
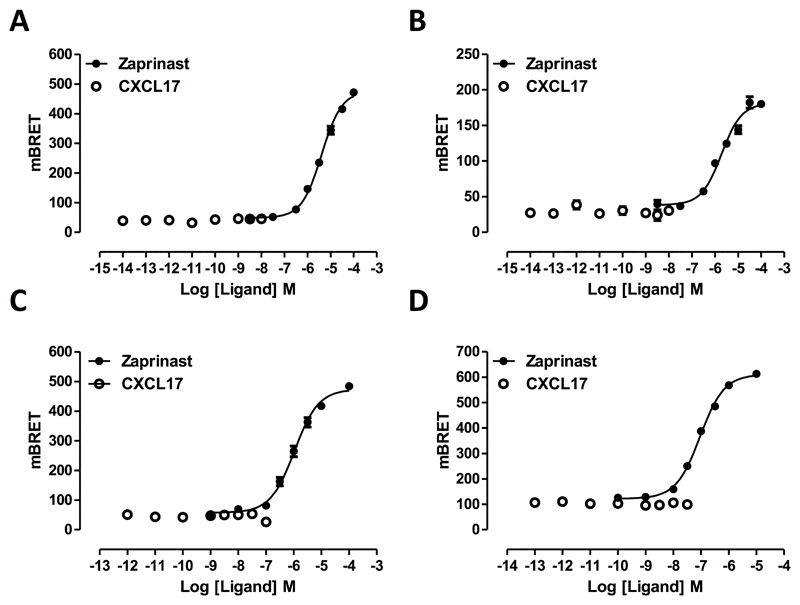
The most widely used approach to demonstrate agonist ligand function at GPR35 has been to measure induced recruitment to the receptor of a β-arrestin isoform (30–33). Following co-transfection into HEK293T cells of the short isoform of human GPR35 (GPR35a), tagged at the C-terminus with enhanced Yellow Fluorescent Protein (eYFP), along with a C-terminally Renilla luciferase-tagged form of β-arrestin-2, the well characterized synthetic GPR35 agonist zaprinast (22, 30, 32, 34) promoted interactions between the receptor and the arrestin in a concentration-dependent fashion (Figure 1A) with pEC50 = 5.46 +/- 0.04 (mean +/- S.E.M., n = 3). In contrast, at concentrations up to 100 nM, CXCL17 was entirely without effect (Figure 1A). Human GPR35 exists as two isoforms with the longer version possessing an extended N-terminal domain comprising an additional 31 amino acids (16). As binding of chemokines to their cognate receptors often involves key interactions within the receptor N-terminal domain (35, 36) we next tested whether this longer isoform of human GPR35 (GPR35b) would recruit β-arrestin-2 in a CXCL17-dependent manner. The chemokine was also entirely inactive in these experiments (Figure 1B) whereas, once again, zaprinast was an effective agonist (Figure 1B) with pEC50 = 5.77 +/- 0.10 (mean +/- S.E.M, n =3). To assess whether CXCL17 might have effects at rodent orthologs of GPR35, experiments akin to those described above were performed using either mouse (Figure 1C) or rat GPR35 (Figure 1D). In these species only a single isoform has been identified and this corresponds to human GPR35a (18). However, once more CXCL17 was inactive, whilst at each rodent ortholog zaprinast was an effective agonist. As reported previously (32) zaprinast was more than 10 fold more potent at rat GPR35 (pEC50 = 7.04 +/- 0.03) than the mouse (pEC50 = 5.98 +/- 0.05) form.
Figure 1. Zaprinast, but not CXCL17, is able to promote interactions between β-arrestin-2 and isoforms and species orthologs of GPR35.
Following co-expression of C-terminally eYFP-tagged forms of human GPR35a (A), human GPR35B (B), mouse (C) or rat (D) GPR35 and Renilla luciferase-tagged β-arrestin-2 the indicated concentrations of the GPR35 agonist zaprinast (filled symbols) or CXCL17 (open symbols) were added and induced interactions between GPCR35 and β-arrestin-2 assessed 5 minutes later by measuring bioluminescence resonance energy transfer.
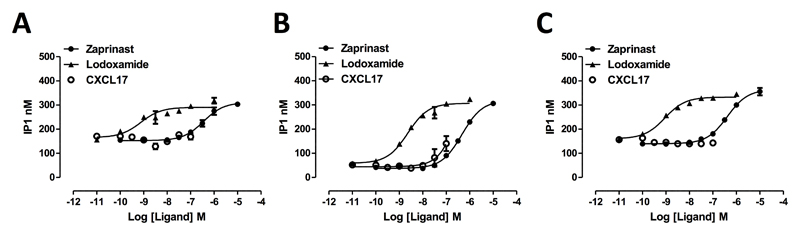
Interaction with a β-arrestin reflects initial steps in desensitization and internalization of a GPCR rather than the canonical route(s) of G protein-mediated signal transduction. To assess whether, although unable to promote interactions with a β-arrestin, CXCL17 might still activate GPR35 but act as an entirely G protein-biased (37) agonist, we co-expressed human GPR35a alongside chimeric G protein α subunits (38, 39) consisting of the backbone sequence of the G protein Gq, which allows downstream activation of phospholipase β1 and the hydrolysis of the membrane phospholipid phosphatidylinositol 4,5 bisphosphate, but in which the C-terminal 5 amino acids are replaced by the corresponding residues from either G13 or Gi1/2, as these are the receptor-interacting residues of the G proteins that GPR35 has previously been shown to be able to couple to (20). When employing the Gq-G13 chimera measures of inositol phosphate production showed both zaprinast (pEC50 = 6.57 +/- 0.05) (Figure 2A) and the high potency agonist lodoxamide (22) (pEC50 = 9.13 +/- 0.24) (Figure 2A) were able to increase levels of inositol phosphates in a concentration-dependent manner. Once more CXCL17 was unable to mimic these effects (Figure 2A). Equivalent results were obtained when using the Gq-Gi1/2 chimera (Figure 2B) except at the very highest concentration of CXCL17 used (100 nM) where a very minor increase in inositol phosphate production compared to the increase produced by either zaprinast (pEC50 = 6.33 +/- 0.03) or lodoxamide (pEC50 = 8.64 +/- 0.08) was detected (Figure 2B). However, when using GPR35b in concert with the Gq-Gi1/2 chimera no response to CXCL17 could be detected (Figure 2C) whereas both zaprinast (pEC50 = 6.39 +/- 0.05) and lodoxamide (pEC50 = 9.05 +/- 0.07) remained efficacious.
Figure 2. CXCL17 is unable to promote G protein-mediated signalling via isoforms of human GPR35.
Either the short, GPR35a, (A, B) or the N-terminally extended, GPR35b, (C) isoforms of human GPR35 were expressed in HEK293 cells along with the chimeric G protein α subunits Gq-G13 (A) or Gq-Gi1/2 (B, C). Following incubation with the indicated concentrations of zaprinast (filled circles), lodoxamide (filled triangles) or CXCL17 (open symbols) for 30 minutes levels of inositol phosphates were measured.
Overall, these studies indicate that at least in heterologous transfected cell systems CXCL17 in unable to act as an agonist for either the long or short isoforms of human GPR35 or to function at the corresponding rat and mouse orthologues.
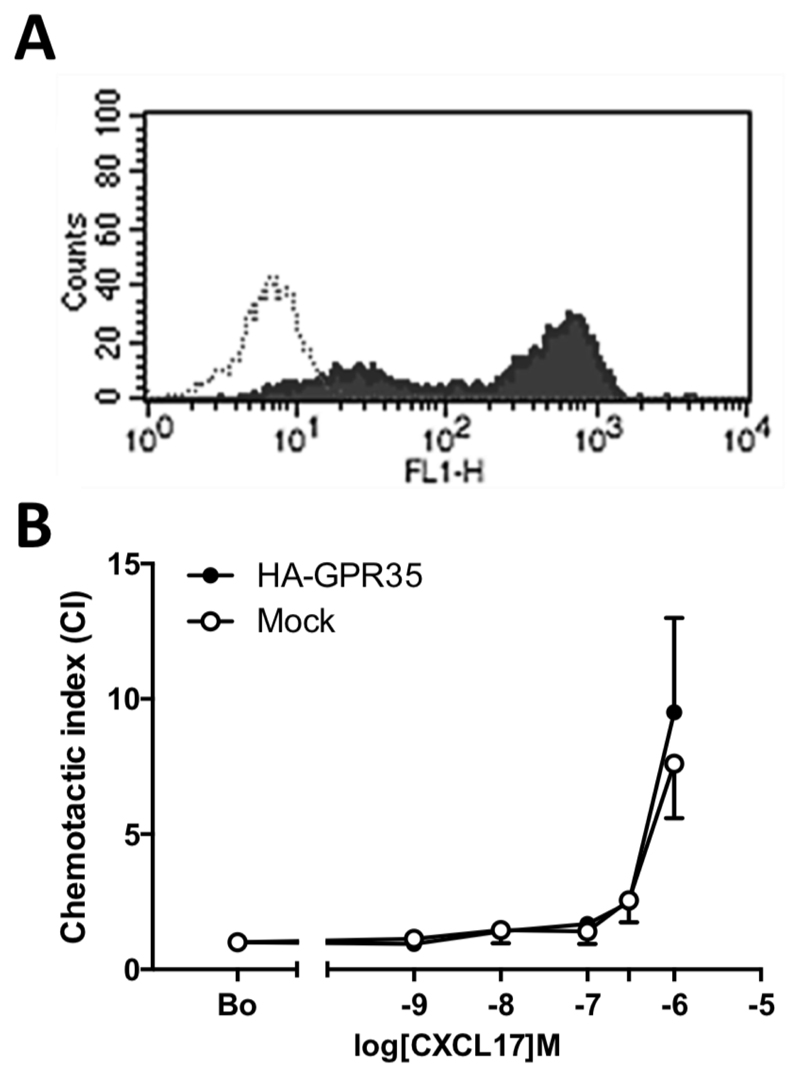
Since the mouse pre-B cell line Ba/F3 had been shown previously to be successfully transfected with GPR35 we switched to express the receptor in the mouse pre-B cell line L1.2. which we have previously used to good effect in expressing chemokine receptors (25). L1.2 cells were transiently transfected with a 3XHA-GPR35 plasmid and flow cytometric analysis using an anti-HA antibody found the construct to be very well expressed on the cell surface (Figure 3A). In modified Boyden chamber assays, there was little difference in the chemotactic responses between naïve and 3XHA-GPR35 transfectants (Figure 3B). Both GPR35 transfectants and naïve L1.2 cells showed migratory responses to 1µM CXCL17 which were not significantly different from each other. No chemotaxis at CXCL17 concentrations of 100nM and below was observed, in keeping with the pharmacological data observed in HEK293T cells (Figures 1 and 2).
Figure 3. GPR35 is readily expressed in L1.2 cells but does not mediate chemotactic responses to CXCL17.
Panel represents typical staining profiles obtained for L1.2 cells transiently transfected with the 3xHA GPR35 plasmid. Staining with isotype control is shown as an open histogram, whilst anti-HA staining is shown as a filled histogram. Panel B shows the migratory responses of GPR35 expressing L1.2 cells and naïve L1.2 cells to increasing CXCL17 concentrations in a modified Boyden Chamber. Data are shown as the mean ± SEM from four experiments.
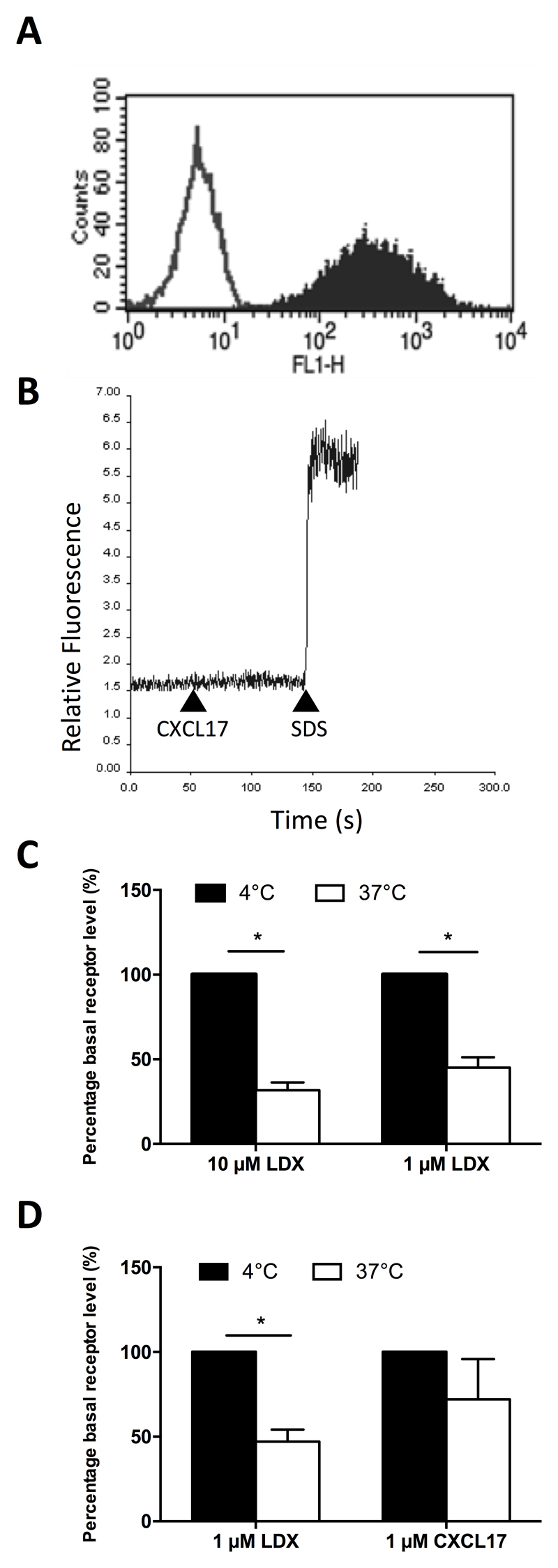
To make our assays as robust as possible, we generated an L1.2 clone designated clone 23 which stably expressed GPR35 at high levels (Figure 4A). We then used cells of this clone in assays of chemotaxis, calcium flux and receptor endocytosis. No significant levels of chemotaxis in response to 1 μM CXCL17 were observed in this line above those observed in the absence of a stimulus (data not shown). Likewise, no calcium flux responses to 100nM CXCL17 were observed with this line, despite loading of the cells with Fura-2 as denoted by the response to cell lysis with SDS (Figure 4B). Significant endocytosis of GPR35 in response to 1 μM and 10μM concentrations of the high potency GPR35 agonist lodoxamide (22) was observed when cells were incubated at 37°C but not at 4 °C, suggestive of clathrin-mediated endocytosis (Figure 4C). Directly comparing 1 μM concentrations of lodoxamide and CXCL17 in the same assay, lodoxamide was able to induce significant endocytosis of GPR35, whilst CXCL17 was without activity (Figure 4D).
Figure 4. GPR35 is endocytosed by lodoxamide but not by CXCL17.
Panel A shows Anti-HA staining of clone 23, an L1.2 line stably expressing 3xHA-GPR35 (solid histogram) with isotype control staining shown as an open histogram. Panel B shows a lack of intracellular calcium flux in response to 100nM CXCL17 in clone 23 cells. 0.1% SDS was used as a positive control to lyse cells and show successful loading with the Fura-2 dye. The data shown are from a single experiment representative of four experiments. Panel C shows 3xHA-GPR35 internalization in clone 23 following incubation with different lodoxamide (LDX) concentrations at 4°C or 37°C for 15 minutes. (n=3). Panel D shows 3xHA-GPR35 internalization in response to 1 µM lodoxamide or 1 µM CXCL17 incubated at 4°C or 37C for 15 minutes (n=4). Data are shown as the mean ± SEM for the number of experiments shown in brackets. Statistical differences between controls were confirmed by a Student’s t-test (* p<0.05).
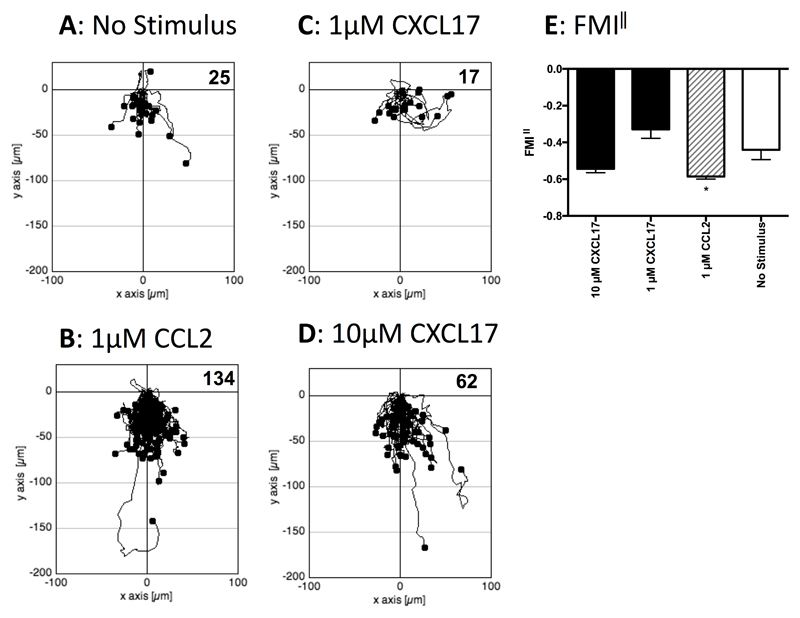
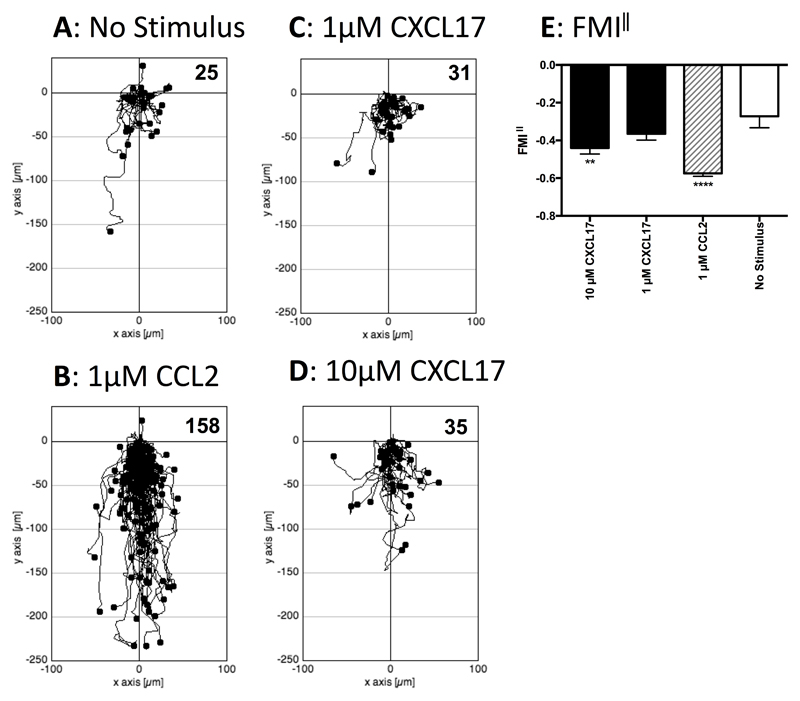
As it was possible that cells that express GPR35 endogenously also express other proteins lacking in HEK293T cells that allows CXCL17 to act as a GPR35 agonist, we turned our attention to the study of human THP-1 cells, which were previously shown to be responsive to CXCL17 (23). Initial experiments employed a real-time assay of cell migration (TAXIScan) with resting cells. THP-1 cells were introduced into the chamber and left to migrate for 1 hour without exposure to a stimulus or exposed to gradients formed from the introduction of a 1μl volume of 1μM CCL2, 1μM CXCL17 or 10μM CXCL17. Images were taken at 1 minute intervals and the tracks of the cells from several pooled experiments are shown in Figure 5. Whilst little basal migration was observed in the absence of a stimulus (Figure 5A), many more cells were observed to migrate in response to 1μM CCL2 (Figure 5B). Although little cell migration above basal was observed in response to 1μM CXCL17 (Figure 5C), approximately 3 times more cells migrated in response to 10μM CXCL17 (Figure 5D). Subsequent analysis of the forward migration index along the chemokine gradient (FMI‖) showed a significant response to 1μM CCL2 and a trend towards significant migration towards 10μM CXCL17 when compared to the basal levels of migration (Figure 5E). We subsequently repeated the TAXIScan analysis using THP-1 cells that had been cultured overnight in the presence of PGE2 (Figure 6), since this treatment has been reported previously to enhance chemotactic responses of these cells to CXCL17 (23). In comparison to the basal migration (Figure 6A) we observed robust migration of cells to 1μM CCL2 with a 6-fold increase in the number of migrating cells (Figure 6B). Responses to 1μM CXCL17 and 10μM CXCL17 were more modest, although the migration towards 10μM CXCL17 appeared above that observed under basal conditions (Figures 6C & D). This was confirmed when the FMI‖ components of migration were analysed, with the responses to 1μM CCL2 and 10μM CXCL17 were significantly greater than the basal response (Figure 6E). Thus, incubation of THP-1 cells with PGE2 increased their capacity to respond by chemotaxis to gradients of CCL2 and of CXCL17.
Figure 5. Analysis of the migration of THP-1 cells along chemokine gradients.
Panels A-D show the paths travelled by individual cells in response to the stimuli indicated. The figures show data pooled from 3 experiments, with 3 movies analysed per condition. Numbers of cells tracked for each condition are shown in the top right-hand corner of the panels. Panel E shows the Mean FMI‖ ± SEM of the data shown in panels A-D. Statistical differences between no stimulus and the indicated stimuli were confirmed by a one-way ANOVA with Bonferroni’s multiple comparisons test (* p<0.05).
Figure 6. Analysis of the migration of PGE2 treated THP-1 cells along chemokine gradients.
Panels A-D show the paths travelled by individual cells in response to the stimuli indicated. The figures show data pooled from 3 experiments, with 3 movies analysed per condition. Numbers of cells tracked for each condition are shown in the top right hand corner of the panels. Panel E shows the Mean FMI‖ ± SEM of the data shown in panels A-D. Statistical differences between no stimulus and the indicated stimuli were confirmed by a one-way ANOVA with Bonferroni’s multiple comparisons test (** p<0.01, **** p<0.0001).
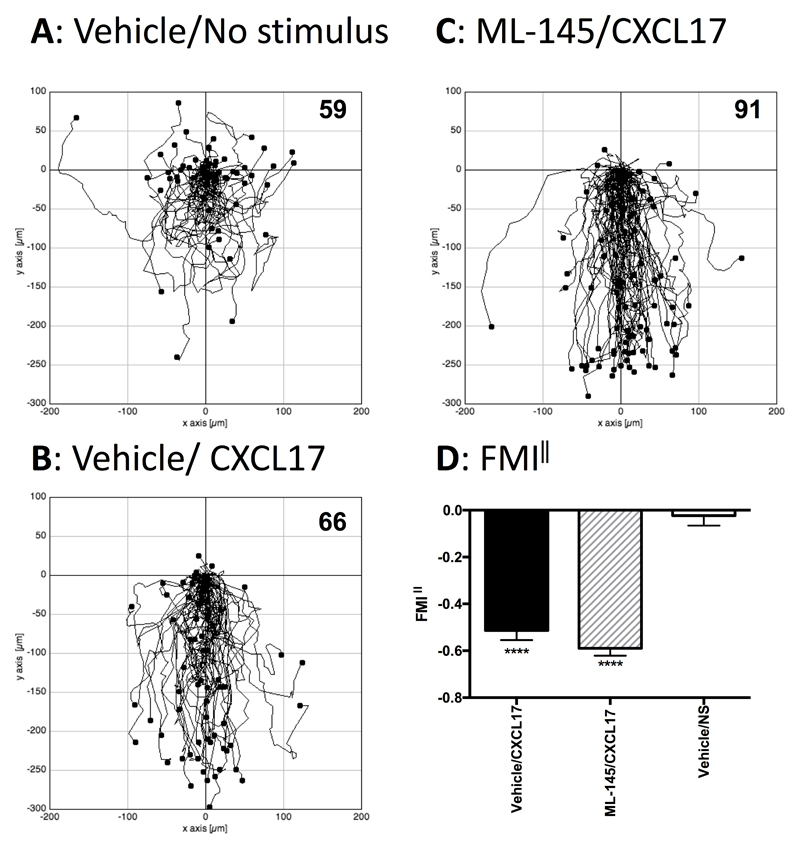
Having established that PGE2-treated THP-1 cells could respond to CXCL17 in chemotaxis assays, the question remained if this was mediated via GPR35, since Maravillas-Montero and colleagues had shown that such treatment induces an increase in the levels of GPR35 transcripts (23). We therefore examined whether or not the migratory responses of PGE2-treated THP-1 cells were sensitive to ML145, a potent, selective antagonist of human GPR35 with an IC50 value against EC80 concentrations of various GPR35 agonists in the region of 20 nM (32). PGE2-treated THP-1 cells were incubated in buffer containing 1 μM ML145 or an identical concentration of the DMSO vehicle, after which they were used directly in TAXIScan assays (Figure 7). In keeping with our earlier analysis, whilst little directional migration was observed in the absence of a stimulus, significant numbers of cells were observed to migrate in response to 1μM CXCL17 (Figures 7A-C). Pre-exposure to the GPR35 antagonist ML145 had no inhibitory effect on ability of the cells to response to 1μM CXCL17, which was confirmed by subsequent analysis of FMI‖ component (Figure 7D). Thus, we conclude that whilst PGE2-treated THP-1 cells are able to migrate along gradients of CXCL17, this is unlikely to be mediated by GPR35.
Figure 7. Analysis of the migration of PGE2-treated THP-1 cells along CXCL17 gradients following pretreatment with the GPR35 antagonist ML145.
Panels A-C show the paths travelled by individual cells in response to the stimuli indicated. Panels A and B show the responses of cells pre-treated with the vehicle DMSO, whilst data in Panel C show the responses of cells pre-treated with the GPR35 antagonist ML145 at a final concentration of 1μM. Data were pooled from three experiments, with 4 movies analysed per condition in each experiment. The total number of cells tracked for each condition are shown in the top right hand corner of the panels. Panel D shows the Mean FMI‖ ± SEM of the data shown in panels A-C. Statistical differences between non stimulus and the indicated stimuli were confirmed by a one-way ANOVA with Bonferroni’s multiple comparisons test (**** p<0.0001).
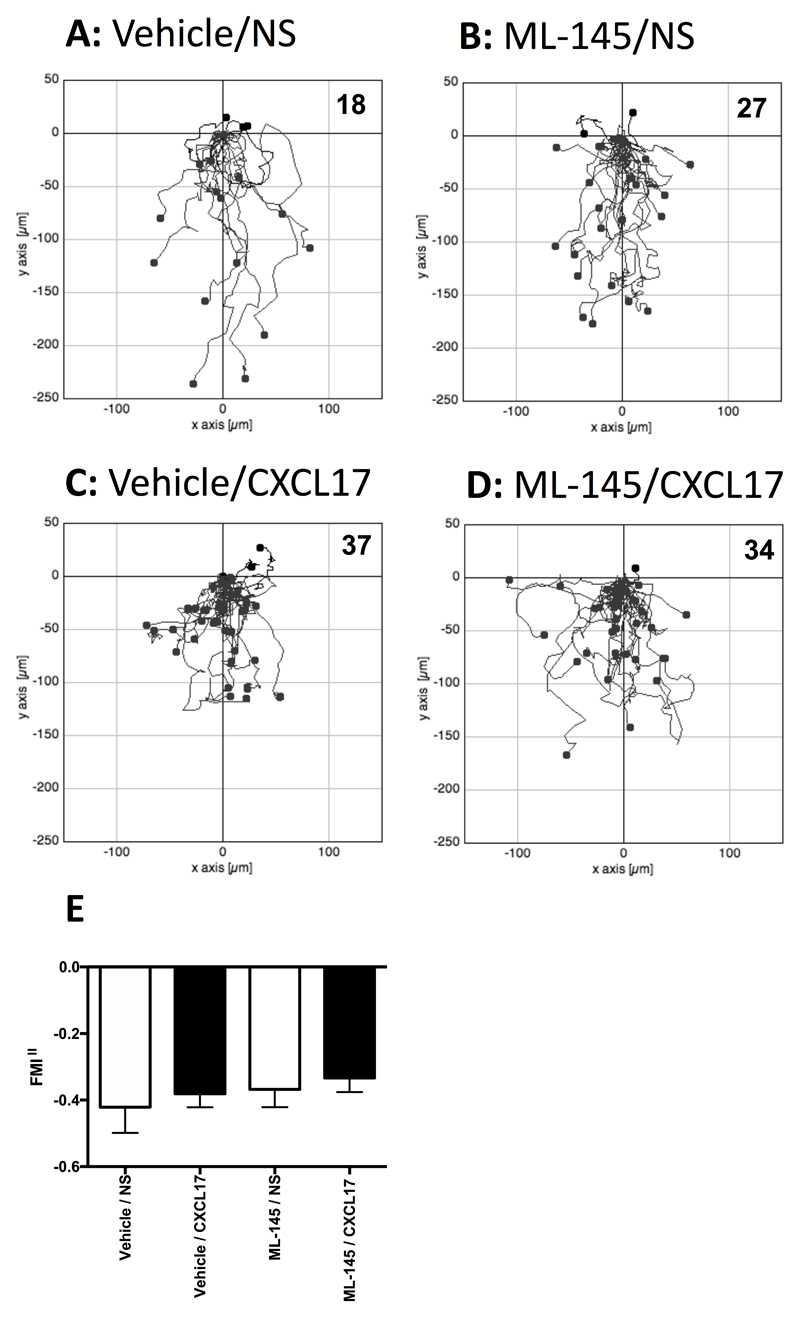
Since Pisabarro and colleagues had previously shown that CXCL17 was chemotactic for CD14+ PBMCs (8), we isolated primary human monocytes and pre-treated them with either vehicle (DMSO) or a final concentration of 1 μM ML145 prior to TAXIScan assays using CXCL17 as an attractant (Figure 8). No significant migration in response to CXCL17 was observed above that seen in the lack of a stimulus (Figure 8A& 8C) which was unaffected by pre-treatment with ML-145 (Figure 8B & 8D).
Figure 8. Analysis of the migration of monocytes along CXCL17 gradients following pretreatment with the GPR35 antagonist ML145.
Panels A-D show the paths travelled by individual cells in response to the stimuli indicated. Panels A and B show the responses of cells pre-treated with the vehicle DMSO, whilst data in Panels C and D show the responses of cells pre-treated with the GPR35 antagonist ML145 at a final concentration of 1μM. Data were pooled from four experiments, with 3 movies analysed per condition in each experiment. The total number of cells tracked for each condition are shown in the top right hand corner of the panels. Panel E shows the Mean FMI‖ ± SEM of the data shown in panels A-D.
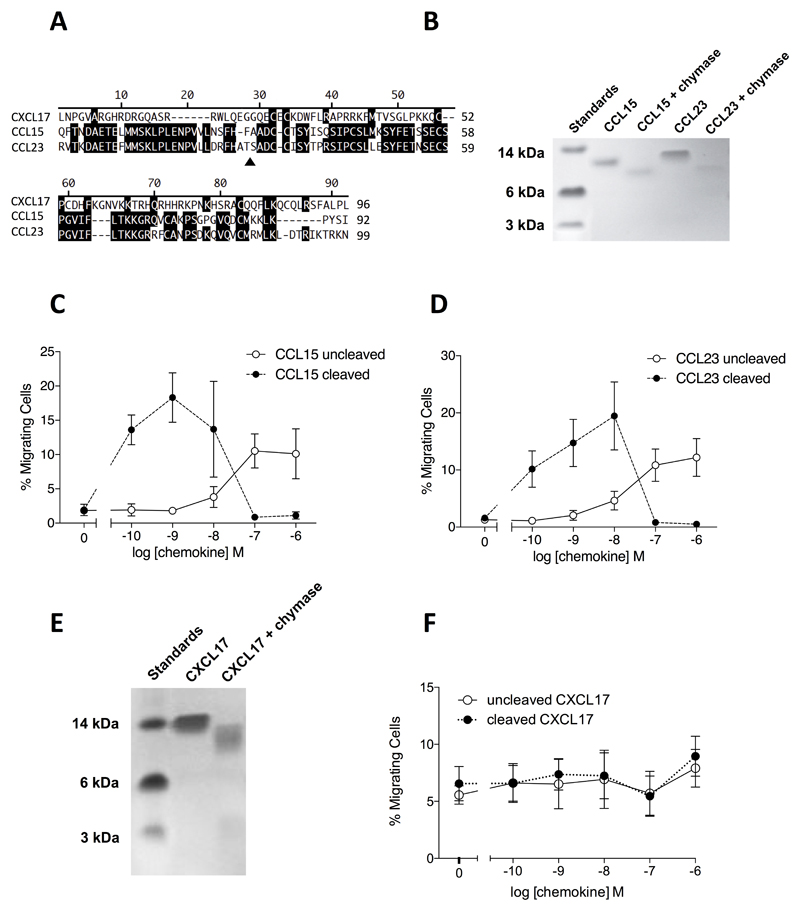
CXCL17 is unusual amongst chemokines in that the mature protein (devoid of the signal peptide) contains an extended N-terminus, as also observed with the CC-chemokines CCL15 and CCL23 (Figure 9A). Since the potency of CCL15 and CCL23 is significantly increased by N-terminal cleavage (40) we assessed whether this was also true for CXCL17. Mast cell chymase was chosen as a potential proteolytic activator of CXCL17 given the mucosal expression pattern of CXCL17 (11). In agreement with a previous report (40), CCL15 and CCL23 were cleaved following incubation with recombinant human chymase (Figure 9B) which lead to increased potency in chemotaxis assays employing CCR1 transfectants (Figure 9C & 9D). CXCL17 was also a substrate for chymase (Figure 9E), although cleavage failed to influence the potency or efficacy of the chemokine in chemotaxis assays employing PGE2-treated THP-1 cells, across a range of CXCL17 concentrations (Figure 9F).
Figure 9. CXCL17 undergoes cleavage by mast cell chymase with little effect upon potency or efficacy in chemotaxis assays.
Panel A shows an alignment of the mature forms of human CXCL17, CCL15 and CCL23. The site of chymase cleavage of CCL15 and CCL23 is shown by a filled triangle. Panel B shows the cleavage of CCL15 and CCL23 following an 18hr incubation with chymase. Proteins were separated by SDS-PAGE and visualized by Coomassie blue staining (n=3). Panels C and D show the relative potency and efficacy of cleaved and uncleaved CCL15 (Panel C) and CCL23 (Panel D) in Boyden chamber chemotaxis assays using CCR1 transfectants (n=3). Panel E shows the cleavage of CXCL17 an 18hr incubation with chymase. Proteins were separated by SDS-PAGE and visualized by Coomassie blue staining (n=3). Panel F shows the relative potency and efficacy of cleaved and uncleaved CXCL17 in Boyden chamber chemotaxis assays using PGE2 treated THP-1 cells (n=4).
Discussion
CXCL17 was the last of the CXC chemokines to be identified by genomic analysis and was originally reported to be chemotactic for dendritic cells and monocytes (8). The gene encoding CXCL17 residues on chromosome 19q13.2, distinct from the cluster on chromosome 4q13.3 where the majority of the CXC chemokines reside (1). In keeping with its discrete chromosomal location, the functions of this chemokine are poorly understood, although its expression appears to be restricted to mucosal tissues (11). Mice deficient in CXCL17 have been reported to have significantly reduced numbers of macrophages in the lung during homeostasis, whilst injection of CXCL17 i.p. results in increased recruitment of macrophages to the peritoneum (12). Thus, in both loss of function and gain of function rodent studies, CXCL17 appears to recruit macrophages. The role of elevated levels of CXCL17 reported in the lungs of patients with IPF should serve as a driver for research in this area (11). IPF is a progressive, debilitating disease and is a unmet clinical need, with patients having an average life expectancy of only 3 years from diagnosis (41). Since pulmonary macrophages are thought to drive the pathology (42), the identification of the receptor for CXCL17 might provide a potential therapeutic target and the recent report that GPR35 is a CXCL17 receptor (23) therefore deserves close scrutiny.
GPR35 mRNA has been reported to be expressed in the lungs, stomach, small intestine, colon, and spleens of both humans and mice (18) and also by leukocytes including basophils, eosinophils, mast cells (21) and iNKT cells (43). As such, GPR35 has attracted attention as a therapeutic target for the treatment of disorders including hypertension, ulcerative colitis and asthma (15). The role of GPR35 in this latter disease is of particular interest, since a number of medications prescribed to stabilize mast cells (including lodoxamide, bufrolin, amlexanox and pemirolast) have been found by Mackenzie and colleagues to activate GPR35 with robust efficacy (22). Using HEK293T transfectants expressing various species orthologs of GPR35 or either the short and longer isoforms of human GPR35, we were unable to show any activity of CXCL17 at the receptor in assays of GPR35 activation including β-arrestin 2 recruitment or IP1 production (Figures 1 and 2). Whilst our manuscript was in revision, Park et al reported that GPR35 expressed in HEK293 T cells was unable to be activated by CXCL17 as deduced by an AP-TGFα shedding assay, supportive of our data (44).
Using the L1.2 cell line as a background in which to successfully express an HA-tagged variant of GPR35, we observed migration of GPR35 transfectants at a singular concentration of 1μM CXCL17 which trended towards significance when compared to basal migration. However, the responses of the parental cell line were of a similar potency and efficacy, suggesting that this weak response was not mediated by the GPR35 introduced into the cells. Presumably, this endogenous CXCL17 receptor is able to recognize the human CXCL17 used in this study, which is 71% identical to the mouse orthologue. In contrast to a previous report (23), we were unable to observe intracellular calcium flux in L1.2 transfectants stably expressing 3xHA-GPR35 in response to a 100nM concentration of CXCL17. The reasons for this are unclear. It is possible that the L1.2 cell line, although like Ba/F3 a mouse B cell line, does not provide a cellular background in which GPR35 can efficiently couple to the intracellular signalling machinery. This would be in contrast to the many other chemokine receptors ourselves and others have successfully expressed in the L1.2 cell line. Similarly, the N-terminal 3xHA tag used by ourselves but not by Maravillas-Montero and colleagues, has the potential to interfere with chemokine binding and presentation by GPR35, although again, we and others have expressed a variety of epitope-tagged chemokine receptors with little adverse effect on receptor function.
Using the TAXIScan system to assess cell migration in real time, THP-1 cells trended towards significant levels of migration along gradients generated by the addition of 1μM CXCL17 to the TAXIScan chamber. This was markedly potentiated by overnight culture with PGE2, in agreement with the findings of Maravillas-Montero and colleagues, (23). A previous manuscript reported that rat CXCL17 expressed in HEK293 cells undergoes additional N-terminal cleavage, generating an N-terminally truncated species with increased efficacy in chemotaxis assays (45). Given that CXCL17 is associated with mucosal tissues and that mast cell chymase has been shown to cleave the N-termini of the chemokines CCL15 and CCL23 and increase their potency and efficacy (40) we tested the postulate that chymase might enhance the potency and efficacy of CXCL17 for PGE2, -treated THP-1 cells. However, despite obvious cleavage of CXCL17 by mast cell chymase, we failed to see any significant effect of cleavage upon the potency or efficacy of CXCL17 in Boyden chamber chemotaxis assays. It remains to be seen whether other proteases are able to cleave CXCL17 into a more potent, active form.
Freshly isolated monocytes were unresponsive to CXCL17 in TAXIScan assays, in keeping with the need to treat the THP-1 cells with PGE2 to observe robust migration. However, this is in contrast to a previous report in which CD14+ cells from a mixture of PBMCs were reported to be recruited by 250 nM -1μM concentrations of CXCL17 (8). It should be noted that the recombinant CXCL17 used in that study was generated in house and unlike the CXCL17 used in this study, contained a His tag, which may have influenced receptor binding. Curiously, the authors of that study reported that PGE2 treatment of monocytes significantly reduced the responses of cells to their recombinant CXCL17, in stark contrast to the observations of ourselves and Maravillas-Montero and colleagues. McCully et al have previously shown that PGE2 is an essential component of the epidermis conditioned media, responsible for increasing T cell responses to the chemokine CCL1 and subsequent homing of these cells to the skin (46). The effects of PGE2 in that experimental system are mediated by the EP4 receptor and result in elevated intracellular cAMP levels which can be mimicked by culture of the cells with forskolin. In those studies, in combination with metabolites of vitamin D3, PGE2 had the effect of increasing the cell surface expression of CCR8, the cognate receptor for CCL1 (46).
It is tempting to speculate that in our system, the overnight culture of THP-1 cells with PGE2 results in elevated levels of a CXCL17 receptor and increased chemotactic responsiveness to CXCL17. Indeed, this was the postulate of Maravilla-Montero and colleagues when they showed that GPR35 mRNA levels were elevated by such treatment. However, in that study, the authors failed to directly show that the induction of GPR35 was directly responsible for the increased responsiveness to CXCL17 (23). Here, using a well-characterized antagonist of GPR35 which has nanomolar potency at GPR35 we were able to show that chemotaxis of PGE2-treated THP-1 cells towards a source of CXCL17 was unimpeded by GPR35 blockade. These data are also in agreement with those of Park et al, who in a recent study using the alternative small molecule GPR35 antagonist CID2745687, showed that THP-1 cell migration to CXCL17 was independent of GPR35 (44).Taken together with our transfectant data which was overwhelmingly negative in terms of finding CXCL17 activity at GPR35, we must therefore conclude that GPR35 is not a bona fide receptor for CXCL17 and that this chemokine remains an orphan. As such, efforts to identify the CXCL17 receptor should be resumed with intensity.
Footnotes
We are grateful to the Biotechnology and Biological Sciences Research Council, grant number [BB/P000649/1] (GM) and to Asthma UK, grant number [AUK-BC-2015-01] (JEP) for financial support.
References
- 1.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, Mantovani A, et al. International Union of Pharmacology. LXXXIX. Update on the Extended Family of Chemokine Receptors and Introducing a New Nomenclature for Atypical Chemokine Receptors. Pharmacological Reviews. 2013;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilliam BL, Riedel DJ, Redfield RR. Clinical use of CCR5 inhibitors in HIV and beyond. J Transl Med. 2011;9(Suppl 1):S9. doi: 10.1186/1479-5876-9-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, Kwatemaa N, Starling J, Fleisher TA, Priel DAL, Merideth MA, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308–2316. doi: 10.1182/blood-2013-09-527226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahaseth H, Kaufman J. Optimizing stem cell collection through CXCR4 antagonists. Front Biosci (Schol Ed) 2012;4:611–619. doi: 10.2741/s288. [DOI] [PubMed] [Google Scholar]
- 6.Hromas R, Broxmeyer HE, Kim C, Nakshatri H, Christopherson K, Azam M, Hou Y-H. Cloning of BRAK, a Novel Divergent CXC Chemokine Preferentially Expressed in Normal versus Malignant Cells. Biochem Biophys Res Commun. 1999;255:703–706. doi: 10.1006/bbrc.1999.0257. [DOI] [PubMed] [Google Scholar]
- 7.Kurth I, Willimann K, Schaerli P, Hunziker T, Clark-Lewis I, Moser B. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J Exp Med. 2001;194:855–861. doi: 10.1084/jem.194.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisabarro MT, Leung B, Kwong M, Corpuz R, Frantz GD, Chiang N, Vandlen R, Diehl LJ, Skelton N, Kim HS, Eaton D, et al. Cutting edge: novel human dendritic cell- and monocyte-attracting chemokine-like protein identified by fold recognition methods. J Immunol. 2006;176:2069–2073. doi: 10.4049/jimmunol.176.4.2069. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein EJ, Head R, Griggs DW, Sun D, Evans RJ, Swearingen ML, Westlin MM, Mazzarella R. VCC-1, a novel chemokine, promotes tumor growth. Biochem Biophys Res Commun. 2006;350:74–81. doi: 10.1016/j.bbrc.2006.08.194. [DOI] [PubMed] [Google Scholar]
- 10.Matsui A, Yokoo H, Negishi Y, Endo-Takahashi Y, Chun NAL, Kadouchi I, Suzuki R, Maruyama K, Aramaki Y, Semba K, Kobayashi E, et al. CXCL17 expression by tumor cells recruits CD11b+Gr1 high F4/80- cells and promotes tumor progression. PLoS ONE. 2012;7:e44080. doi: 10.1371/journal.pone.0044080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkhardt AM, Tai KP, Flores-Guiterrez JP, Vilches-Cisneros N, Kamdar K, Barbosa-Quintana O, Valle-Rios R, Hevezi PA, Zuniga J, Selman M, Ouellette AJ, et al. CXCL17 Is a Mucosal Chemokine Elevated in Idiopathic Pulmonary Fibrosis That Exhibits Broad Antimicrobial Activity. The Journal of Immunology. 2012;188:6399–6406. doi: 10.4049/jimmunol.1102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkhardt AM, Maravillas-Montero JL, Carnevale CD, Vilches-Cisneros N, Flores JP, Hevezi PA, Zlotnik A. CXCL17 Is a Major Chemotactic Factor for Lung Macrophages. The Journal of Immunology. 2014;193:1468–1474. doi: 10.4049/jimmunol.1400551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Dowd BF, Nguyen T, Marchese A, Cheng R, Lynch KR, Heng HH, Kolakowski LF, George SR. Discovery of three novel G-protein-coupled receptor genes. Genomics. 1998;47:310–313. doi: 10.1006/geno.1998.5095. [DOI] [PubMed] [Google Scholar]
- 14.Shore DM, Reggio PH. The therapeutic potential of orphan GPCRs, GPR35 and GPR55. Front Pharmacol. 2015;6:1–22. doi: 10.3389/fphar.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Divorty N, Mackenzie AE, Nicklin SA, Milligan G. G protein-coupled receptor 35: an emerging target in inflammatory and cardiovascular disease. Front Pharmacol. 2015;6:41. doi: 10.3389/fphar.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okumura S-I, Baba H, Kumada T, Nanmoku K, Nakajima H, Nakane Y, Hioki K, Ikenaka K. Cloning of a G-protein-coupled receptor that shows an activity to transform NIH3T3 cells and is expressed in gastric cancer cells. Cancer Sci. 2004;95:131–135. doi: 10.1111/j.1349-7006.2004.tb03193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J. Kynurenic Acid as a Ligand for Orphan G Protein-coupled Receptor GPR35. J Biol Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 19.Oka S, Ota R, Shima M, Yamashita A, Sugiura T. GPR35 is a novel lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2010;395:232–237. doi: 10.1016/j.bbrc.2010.03.169. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins L, Alvarez-Curto E, Campbell K, de Munnik S, Canals M, Schlyer S, Milligan G. Agonist activation of the G protein-coupled receptor GPR35 involves transmembrane domain III and is transduced via Gα13 and β-arrestin-2. British Journal of Pharmacology. 2011;162:733–748. doi: 10.1111/j.1476-5381.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Lu JY-L, Wu X, Summer S, Whoriskey J, Saris C, Reagan JD. G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology. 2010;86:1–5. doi: 10.1159/000314164. [DOI] [PubMed] [Google Scholar]
- 22.MacKenzie AE, Caltabiano G, Kent TC, Jenkins L, McCallum JE, Hudson BD, Nicklin SA, Fawcett L, Markwick R, Charlton SJ, Milligan G. The Antiallergic Mast Cell Stabilizers Lodoxamide and Bufrolin as the First High and Equipotent Agonists of Human and Rat GPR35. Mol Pharmacol. 2013;85:91–104. doi: 10.1124/mol.113.089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maravillas-Montero JL, Burkhardt AM, Hevezi PA, Carnevale CD, Smit MJ, Zlotnik A. Cutting edge: GPR35/CXCR8 is the receptor of the mucosal chemokine CXCL17. The Journal of Immunology. 2015;194:29–33. doi: 10.4049/jimmunol.1401704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heynen-Genel S, Dahl R, Shi S, Sauer M, Hariharan S, Sergienko E, Dad S, Chung TD, Stonich D, Su Y, Caron M, et al. Selective GPR35 Antagonists - Probes 1 & 2. 2010 [PubMed] [Google Scholar]
- 25.Vaidehi N, Pease JE, Horuk R. Modeling small molecule-compound binding to G-protein-coupled receptors. Meth Enzymol. 2009;460:263–288. doi: 10.1016/S0076-6879(09)05213-6. [DOI] [PubMed] [Google Scholar]
- 26.Viney JM, Andrew DP, Phillips RM, Meiser A, Patel P, Lennartz-Walker M, Cousins DJ, Barton NP, Hall DA, Pease JE. Distinct conformations of the chemokine receptor CCR4 with implications for its targeting in allergy. The Journal of Immunology. 2014;192:3419–3427. doi: 10.4049/jimmunol.1300232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanegasaki S, Nomura Y, Nitta N, Akiyama S, Tamatani T, Goshoh Y, Yoshida T, Sato T, Kikuchi Y. A novel optical assay system for the quantitative measurement of chemotaxis. J Immunol Methods. 2003;282:1–11. doi: 10.1016/j.jim.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Zengel P, Nguyen-Hoang A, Schildhammer C, Zantl R, Kahl V, Horn E. μ-Slide Chemotaxis: A new chamber for long-term chemotaxis studies. BMC Cell Biology. 2011;12:21. doi: 10.1186/1471-2121-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox JM, Najarro P, Smith GL, Struyf S, Proost P, Pease JE. Structure/function relationships of CCR8 agonists and antagonists. Amino-terminal extension of CCL1 by a single amino acid generates a partial agonist. J Biol Chem. 2006;281:36652–36661. doi: 10.1074/jbc.M605584200. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins L, Brea J, Smith NJ, Hudson BD, Reilly G, Bryant NJ, Castro M, Loza M-I, Milligan G. Identification of novel species-selective agonists of the G-protein-coupled receptor GPR35 that promote recruitment of β-arrestin-2 and activate Gα13. Biochem J. 2010;432:451–459. doi: 10.1042/BJ20101287. [DOI] [PubMed] [Google Scholar]
- 31.Deng H, Hu H, Ling S, Ferrie AM, Fang Y. Discovery of Natural Phenols as G Protein-Coupled Receptor-35 (GPR35) Agonists. ACS Med Chem Lett. 2012;3:165–169. doi: 10.1021/ml2003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins L, Harries N, Lappin JE, MacKenzie AE, Neetoo-Isseljee Z, Southern C, McIver EG, Nicklin SA, Taylor DL, Milligan G. Antagonists of GPR35 Display High Species Ortholog Selectivity and Varying Modes of Action. Journal of Pharmacology and Experimental Therapeutics. 2012;343:683–695. doi: 10.1124/jpet.112.198945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng H, Hu H, Fang Y. Multiple tyrosine metabolites are GPR35 agonists. Sci Rep. 2012;2:373. doi: 10.1038/srep00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taniguchi Y, Tonai-Kachi H, Shinjo K. Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Letters. 2006;580:5003–5008. doi: 10.1016/j.febslet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 35.Monteclaro FS, Charo IF. The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1alpha receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. J Biol Chem. 1996;271:19084–19092. doi: 10.1074/jbc.271.32.19084. [DOI] [PubMed] [Google Scholar]
- 36.Pease JE, Wang J, Ponath PD, Murphy PM. The N-terminal extracellular segments of the chemokine receptors CCR1 and CCR3 are determinants for MIP-1alpha and eotaxin binding, respectively, but a second domain is essential for efficient receptor activation. J Biol Chem. 1998;273:19972–19976. doi: 10.1074/jbc.273.32.19972. [DOI] [PubMed] [Google Scholar]
- 37.Steen A, Larsen O, Thiele S, Rosenkilde MM. Biased and g protein-independent signaling of chemokine receptors. Front Immunol. 2014;5:277. doi: 10.3389/fimmu.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coward P, Chan SD, Wada HG, Humphries GM, Conklin BR. Chimeric G proteins allow a high-throughput signaling assay of Gi-coupled receptors. Anal Biochem. 1999;270:242–248. doi: 10.1006/abio.1999.4061. [DOI] [PubMed] [Google Scholar]
- 39.Kostenis E, Waelbroeck M, Milligan G. Techniques: promiscuous Galpha proteins in basic research and drug discovery. Trends Pharmacol Sci. 2005;26:595–602. doi: 10.1016/j.tips.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Berahovich RD, Miao Z, Wang Y, Premack B, Howard MC, Schall TJ. Proteolytic Activation of Alternative CCR1 Ligands in Inflammation. J Immunol. 2005;174:7341–7351. doi: 10.4049/jimmunol.174.11.7341. [DOI] [PubMed] [Google Scholar]
- 41.Daccord C, Maher TM. Recent advances in understanding idiopathic pulmonary fibrosis. F1000Res. 2016;5:1046. doi: 10.12688/f1000research.8209.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrne AJ, Maher TM, Lloyd CM. Pulmonary Macrophages: A New Therapeutic Pathway in Fibrosing Lung Disease? Trends in Molecular Medicine. 2016;22:303–316. doi: 10.1016/j.molmed.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Fallarini S, Magliulo L, Paoletti T, de Lalla C, Lombardi G. Expression of functional GPR35 in human iNKT cells. Biochem Biophys Res Commun. 2010;398:420–425. doi: 10.1016/j.bbrc.2010.06.091. [DOI] [PubMed] [Google Scholar]
- 44.Park S-J, Lee S-J, Nam S-Y, Im D-S. GPR35 mediates lodoxamide-induced migration inhibitory response but not CXCL17-induced migration stimulatory response in THP-1 cells; Is GPR35 a receptor for CXCL17? British Journal of Pharmacology. 2017;175:154–161. doi: 10.1111/bph.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee W-Y, Wang C-J, Lin T-Y, Hsiao C-L, Luo C-W. CXCL17, an orphan chemokine, acts as a novel angiogenic and anti-inflammatory factor. American Journal of Physiology-Endocrinology and Metabolism. 2013;304:E32–E40. doi: 10.1152/ajpendo.00083.2012. [DOI] [PubMed] [Google Scholar]
- 46.McCully ML, Collins PJ, Hughes TR, Thomas CP, Billen J, O'Donnell VB, Moser B. Skin Metabolites Define a New Paradigm in the Localization of Skin Tropic Memory T Cells. The Journal of Immunology. 2015;195:96–104. doi: 10.4049/jimmunol.1402961. [DOI] [PMC free article] [PubMed] [Google Scholar]