Abstract
Ozone is a strong oxidant and a key stress elicitor. The immediate and longer-term impacts of ozone in species with both de novo and storage volatile emissions such a tobacco (Nicotiana tabacum) that has terpene-containing glandular trichomes on leaf surface are poorly understood. In this study, we exposed N. tabacum ‘Wisconsin’ leaves to acute ozone doses of 0 (control), 400, 600, 800, and 1000 ppb for 30 min and studied the effects of ozone exposure on ozone uptake, gas-exchange characteristics, and emissions of lipoxygenase pathway volatiles, and mono- and sesquiterpenes. Foliage emissions of LOX volatiles were quantitatively related to the severity of ozone exposure, but the stress dose vs. emission relationship was weaker for terpenoids. Analysis of leaf terpene contents and composition indicated that several monoterpenes and sesquiterpenes were not stored in leaves and were de novo synthesized upon ozone exposure. The highest degree of elicitation rate for each compound was observed immediately after ozone treatment and it declined considerably through recovery. Leaf ozone uptake was dominated by non-stomatal deposition, and the emissions of total LOX volatiles, and mono- and sesquiterpenes emission were positively correlated with non-stomatal ozone deposition. Overall, this study demonstrates remarkably high ozone resistance of the studied tobacco cultivar and indicated that ozone effects on volatile emissions primarily reflected modifications in the release of stored volatiles and upon reaction of ozone with leaf surface structure.
Keywords: Acute ozone stress, de novo emission, elicitation rate, glandular trichome, LOX volatiles, monoterpenes, non-stomatal ozone deposition, sesquiterpenes, solvent extract, stomatal ozone uptake, trichome permeability
1. Introduction
Ozone (O3) is a phytotoxic gas that leads to major losses of vegetation productivity worldwide (Fares et al., 2010b). In the troposphere, ozone formation is controlled by concentrations of NO2 and reactive volatile organic compounds in the presence of sunlight (Fowler et al., 2009; Karnosky et al., 2005). Currently, tropospheric ozone concentration over most of the terrestrial surface is between 20 and 45 ppb (Sicard et al., 2017), but in local pollution hotspots, much higher concentrations can be observed, e.g., in major industrial cities in China, the ozone concentration is ca. 70 ppb (Yuan et al., 2015) and in industrial cities in India, it is ca. 50-60 ppb (Sharma et al., 2013). However, even the current average levels of tropospheric ozone are capable of causing detrimental effects on certain sensitive crop plants, such as wheat, maize, and soybean (Rai and Agrawal, 2014).
Plants act as a sink for ozone by two ways (1) stomatal uptake, and (2) non-stomatal deposition (Altimir et al., 2006; Fares et al., 2010b). During stomatal uptake, ozone diffuses through the stomata into leaf intercellular air spaces along the ozone gradient from the ambient air to leaf intercellular air space (Laisk et al., 1989). Stomatal uptake is the primary passage for ozone into the leaf mesophyll cells (Fares et al., 2008). Once taken up, ozone as a strong oxidant can result in a major oxidative stress in plants (Calfapietra et al., 2013; Schwanz et al., 1996).
There is still a large uncertainty in how plant stress severity scales with ozone uptake. In particular, counterintuitively, less physiological damage has been observed in species with greater ozone uptake; especially, in species with greater volatile emissions (Fares et al., 2010b; Kanagendran et al., 2018). Yet, many model species used also have significant volatile-containing surface-structures, in particular glandular trichomes, and it is currently unclear whether the greater ozone tolerance of species with greater ozone uptake is associated with ozone detoxification inside the leaves or on the leaf surface (Jardine et al., 2012; Jud et al., 2016).
Nicotiana tabacum L. is an annual herb that has terpene-filled glandular trichomes on leaf surface and has therefore been used as a model species for ozone-plant surface reactions (Jud et al., 2016). There have been several studies looking at the physiological impact of ozone on N. tabacum (Beauchamp et al., 2005; Cheng and Sun, 2013; Eltayeb et al., 2006; Heidenreich et al., 2006; Janzik et al., 2005; Mancini et al., 2006; Pasqualini et al., 2009; Samuel et al., 2005; Silva et al., 2012), but the majority of studies have mainly focused on short-term immediate stress responses rather than considered the full response from exposure through the recovery. There is also a lack of knowledge of how the emissions of the lipoxygenase pathway (LOX volatiles, also called green leaf volatiles), mono- and sesquiterpenes are regulated upon acute ozone doses from exposure through recovery and their relationship with foliage surface ozone uptake and elicitation rate.
There is a significant variability in ozone sensitivity among N. tabacum cultivars with N. tabacum ‘Bel W3’ being more sensitive to ozone than many other crop plants, and therefore it is often used as an ozone bioindicator (Krupa et al., 2001; Schrauder et al., 1998). In particular, the study of Heiden et al. (1999) compared ozone-induced volatile emissions among the ozone-resistant cultivar ‘Bel B’ and ozone-sensitive cultivar ‘Bel W3’ and found that the elicitation of LOX volatiles and volatile isoprenoid release depended strongly both on the duration of ozone exposure and cultivar. However, it is unclear how more resistant cultivars with potentially a greater share of surface deposition flux respond to acute ozone exposure and how immediate stress responses and gene expression level elicitation responses correlate with the ozone dose.
In particular, elicitation of de novo volatile emissions by activation of a gene expression level response upon ozone exposure is expected to increase through the recovery phase, while the release of volatiles from leaf surface is expected to decrease during the recovery. Thus, time kinetics of volatile responses upon ozone exposure can provide important insight into development of oxidative stress vis-à-vis defence responses and explain non-intuitive ozone dose vs. physiological plant responses in different tobacco cultivars.
In this study, we used short-term acute (400-1000 ppb) ozone exposures and studied the changes in photosynthetic characteristics, emissions of LOX volatiles, and mono- and sesquiterpenes, stomatal ozone uptake rates, non-stomatal ozone deposition rates, and stress- dependent elicitation rates of each volatile through recovery phase in the ozone-resistant N. tabacum ‘Wisconsin’. In addition, terpenoid content and composition in untreated leaves were also studied to confirm the capacity for terpenoid storage by leaves and allow separation of stored and de novo synthesized terpene sources. The objectives of this study were to (a) assess the relationships between ozone concentration and changes in foliage photosynthetic characteristics and emissions of LOX volatiles and mono- and sesquiterpenes from ozone exposure through recovery, and (b) distribute leaf ozone uptake between stomatal ozone uptake and surface deposition and relate the different components of ozone flux to the degree of elicitation of different volatiles. Different stressed-induced mechanisms varying from gene expression to allocation of primary intermediates can be responsible for stress-dependent changes in volatile emission.
We hypothesized that acute ozone exposure to foliage of this N. tabacum ozone-resistant cultivar will lead to: (a) moderate reductions in gas exchange characteristics, (b) greater non-stomatal deposition rate of ozone than stomatal uptake rate, (c) alteration in LOX volatile, and mono- and sesquiterpene emission rates primarily due to changes in the constitutive emissions, most likely associated with changes in trichome surface permeability, and (d) changes in stress-dependent elicitation rates of LOX volatile, mono- and sesquiterpene emissions.
2. Materials and Methods
2.1. Plant material and growth conditions
Tobacco (N. tabacum ‘Wisconsin’) seeds were sown in 1 L plastic pots filled with commercial potting soil (Biolan Oy, Kekkilä group, Finland). Four weeks after germination, seedlings were transplanted into 3 L plastic pots filled with the same potting soil. The plants were kept under the light intensity of 400-500 µmol m-2 s-1 provided by metal halide lamps (HPI-T Plus 400 W, Philips) for 12 h photoperiod, and relative humidity of 60%; and day/night temperature of 24/18 °C were maintained for the whole experimental period. Plants were watered daily to soil field capacity. In addition to the nutrients provided by the soil, a liquid fertilizer (Baltic Agro, Lithuania; NPK content ratio: 5:5:6; and micronutrients B (0.01%), Cu (0.03%), Fe (0.06%), Mn (0.028%), and Zn (0.007%) was applied for optimum growth conditions. Once a week, 70 ml of diluted liquid fertilizer (ca. 0.5 % solution) was applied to each plant till the end of this study.
2.2. Experimental design and data collection
The experiment was conducted with the leaves of 10-12 weeks old plants. Fully mature similar-sized leaves from similar canopy positions and a similar leaf order were used for the experiment. For each of the five ozone exposure concentrations, five arbitrarily selected leaves from a single plant were used to measure gas exchange and volatile emission rates at five recovery time points, and stomatal ozone uptake and non-stomatal ozone deposition during ozone exposures. Each leaf from each plant was separately treated with the same ozone concentration and sampled at a single recovery time point. All exposures were replicated with three individual plants including control measurements. Therefore, in total, 75 leaves (5 recovery time points x 3 plants for each exposure x 5 exposures including 0, 400, 600, 800, and 1000 ppm ozone) from 15 plants were used. In addition, there were six untreated leaves from six plants used for the estimation of contents and composition of foliage terpenoids by solvent extract analysis. This experimental design does not allow for direct assessment of leaf-to-leaf variability in emission responses, but treated leaves were measured only once to avoid possible leaf damage and release of surface volatiles when the leaves have to be removed, inserted and sealed in leaf chamber repeatedly [see (Niinemets et al., 2011) for discussion of the caveats of plant volatile measurements].
2.3. Experimental setup and ozone exposure
A custom-made cylindrical double-walled glass chamber (1.2 L) with stainless steel bottom was used for ozone treatments, gas exchange measurements and volatile sampling. As the leaf was the experimental unit in this study, individual leaves were placed in the 1.2 L glass chamber for ozone treatment. The chamber temperature was maintained constant at 25 °C by circulating thermostatted water among the walls of the chamber. Light was provided by four 50 W halogen lamps. A thermistor (NTC thermistor, model ACC-001, RTI Electronics, Inc., St. Anaheim, CA, USA) was used to monitor the air temperature inside the chamber, and leaf temperature was measured by a thermocouple attached to the lower side of the leaf surface. Ambient air was filtered through activated charcoal and supplied to the chamber in the form of pure and ozone-free air. A fan inserted inside the chamber provided constant high turbulence air mixing. The chamber ports could be switched between reference and measurement modes in order to take gas-exchange measurements (see Copolovici and Niinemets (2010) for further details of the glass chamber set up). CO2 and H2O concentrations of the chamber in- and outlet were measured with an infra-red dual-channel gas analyser operated in differential mode (CIRAS II, PP-systems, Amesbury, MA, USA).
Ozone was generated with a Certizon C100 ozoniser (Erwin Sander Elektroapparatenbau GmbH, Germany). Arbitrarily selected mature leaves were exposed to 400, 600, 800, and 1000 ppb ozone for 30 minutes, whereas untreated leaves were used as control. The ozone concentration in the inlet and outlet of the chamber was monitored using a UV photometric ozone detector (Model 49i, Thermo Fisher Scientific, Franklin MA, USA).
2.4. Gas exchange measurements, volatile sampling, and gas chromatography - mass spectrometry (GC-MS) analyses
After leaf enclosure, standard environmental conditions were established: light intensity at leaf surface of 700 μmol m-2 s-1; leaf temperature of 25 °C; ambient CO2 concentration of 380-400 μmol mol-1; and relative air humidity of 50–60%. Once the foliage gas-exchange rates had reached a steady state, usually after ca. 15 min of leaf enclosure, foliage gas exchange rates were recorded and volatiles were collected (control). Then the leaf was exposed to ozone for 30 min, and the measurements were repeated at 0.5, 3, 10, 24 and 48 h after the exposure. Net assimilation rate (A), and stomatal conductance to water vapour (gs) were calculated according to von Caemmerer and Farquhar (1981).
Volatile samples were collected onto multi-bed stainless steel adsorbent cartridges connected to a portable suction pump 210-1003 MTX (SKC Inc., Houston, TX, USA) providing a constant suction rate of 200 ml min−1. A detailed description of the method and adsorbent cartridges can be found in Kännaste et al. (2014) and Niinemets et al. (2010). An air sample was taken before the leaf enclosure to estimate the chamber volatile background without the leaf. Adsorbent cartridges were analysed with a combined Shimadzu TD20 automated cartridge desorber and Shimadzu 2010 Plus GC–MS system (Shimadzu Corporation, Kyoto, Japan) for LOX volatiles, monoterpenes, and sesquiterpenes (Kännaste et al. (2014) and Toome et al. (2010) for further details about GC-MS analysis and compound identification), and the emission rates were calculated according to Niinemets et al. (2011).
2.5. Quantitative estimation of foliage terpene contents
Terpene contents were quantitatively estimated from untreated leaves. For the chemical analyses of foliage terpene contents, six fresh leaf samples (120 ± 30 mg) were randomly chosen from individual plants and weighed into a 2 ml tube with 2.8 mm stainless-steel beads (Bertin Technologies, Aix-en-Provence, France). Tubes with fresh samples were instantly frozen in liquid N2 and then 1.5 ml GC-grade hexane (Sigma-Aldrich, St. Louis, MO, USA) was added into each tube. The mixture was then immediately homogenized with a homogenizer (Precellys 24, Bertin Technologies, Aix-en-Provence, France) at 6500 g for 30 seconds at 25°C. The homogenized plant material was incubated in a Thermo-Shaker (TS-100C, Biosan, Riga, Latvia) for 3 h at 1000 rpm at 25°C. After incubation, samples were centrifuged (Universal 320R, Hettich, Tuttlingen, Germany) for 30 min at 13000 g at 25°C. An aliquot of 1 ml supernatant was pipetted into a 2 ml GC-MS glass vial (Sigma-Aldrich, St. Louis, MO, USA) for GC-MS analysis. A detailed description of GC-MS analysis and quantitative estimation of foliage terpene contents can be found in Kännaste et al. (2013).
2.6. Chlorophyll florescence measurements
Dark-adapted chlorophyll fluorescence yield (10-min darkening, Fv/Fm) of each ozone-treated leaf was estimated with a PAM fluorimeter (Walz IMAG-MIN/B, Walz GmbH, Effeltrich, Germany) after gas exchange measurements and volatile sampling were completed. A saturating pulse intensity of 7000 µmol m-2 s-1 was used.
2.7. Estimation of ozone uptake by individual leaves
Average stomatal ozone uptake rate and non-stomatal ozone deposition rate for the exposure period by each individual leaf at each dose of ozone was calculated from measurements of chamber in- and outgoing ozone concentrations. First, total ozone uptake flux (ΦO3,Tot) was calculated as the product of chamber flow rate and difference in ozone concentrations between chamber in- and outlets.
Average stomatal ozone uptake rate (ΦO3,S) was calculated as the product of average chamber O3 concentration and average stomatal conductance for ozone (gO3). The latter was estimated as the 30 min. average stomatal conductance to water vapour divided by the ratio of the binary diffusion coefficients for ozone and water vapour (2.03) assuming that the intercellular ozone concentration was zero (Laisk et al., 1989). This assumption might overestimate ozone uptake as ozone concentrations in the leaf interior can reach small non-zero values (Moldau and Bichele, 2002). The ratio of water vapor and ozone diffusivities was calculated using an ozone diffusion coefficient in air of 1.267·10-5 m2 s-1 at 22.84 °C (Ivanov et al., 2007) and a water vapor diffusion coefficient in air of 2.569·10-5 m2 s-1 at the same temperature as defined by Chapman and Enskog (Li et al., 2017; Niinemets and Reichstein, 2003). In fact, as the ratio of the collision integrals for both water vapor and O3 changes little with temperature (calculated according to Tucker and Nelken 1982), temperature effects on this ratio were negligible (the change in the ratio was less than 0.1% between 25 °C used for leaf measurements and 22.84 °C).
Cuticular ozone uptake into the leaf interior was excluded because compared with stomata, the cuticle is highly impermeable to ozone (Kerstiens and Lendzian, 1989). Ozone deposition flux was estimated as the difference between ΦO3,Tot and ΦO3,S. A detailed description of quantifying stomatal ozone flux and non-stomatal ozone deposition can be found in Winner (1994). Stomatal ozone uptake rate and non-stomatal ozone deposition rate were calculated for each ozone exposure.
2.8. Data analyses
Measurements were replicated thrice with each ozone concentration. Generalized linear model (GLM) with maximum likelihood model fitting was used to test for individual and interactive effects of ozone and the time of recovery on the emissions of total LOX volatiles, total monoterpenes, total sesquiterpenes, individual mono- and sesquiterpenes, and elicitation rate of each compound. The elicitation rates of log-transformed LOX volatiles, and mono- and sesquiterpene emission rates were calculated using the data fitted to a model selected based on the emission pattern of each compound (Beauchamp et al., 2005). Here we used the sigmoidal model in the form of
In this case, elicitation rate of each compound emission was calculated at recovery times 0.5, 10, and 48 h, compared to the emission of corresponding compounds from control leaves. Here, y0 is the initial level of emission (emission of control) at time x0 (0 h for control emission), a is the saturation level of emission (emission of treatment sample at each recovery point), x is the recovery time (such as 0.5, 10, and 48 h), and (1/b) is the elicitation rate. Elicitation rate of log-transformed emission data at each recovery point was calculated for each replicate and then, the mean (± SE) elicitation rate was estimated.
Emission data were log-transformed after checking for normality of distribution of data and homogeneity of variances, in order to minimize the inequality of variances for Wald chi-square test in GLM. Correlation analyses of net assimilation rate, intercellular CO2 concentration, and stomatal conductance to water vapour vs. ozone doses at different times through recovery were carried out. In addition, correlations of total LOX volatile, total monoterpene, and total sesquiterpene emission rates with stomatal ozone uptake rate and non-stomatal ozone deposition rate at different recovery periods were also examined by non-parametric Spearman correlations.
Elicitation rate calculation was carried out using SigmaPlot Version 12.5 (Systat Software Inc, San Jose, CA, USA). All other statistical analyses were conducted using SPSS Version 24 (IBM SPSS, Chicago, IL, WA). All statistical effects were considered significant at P < 0.05.
3. Results
3.1. Effects of ozone stress on foliage photosynthetic characteristics
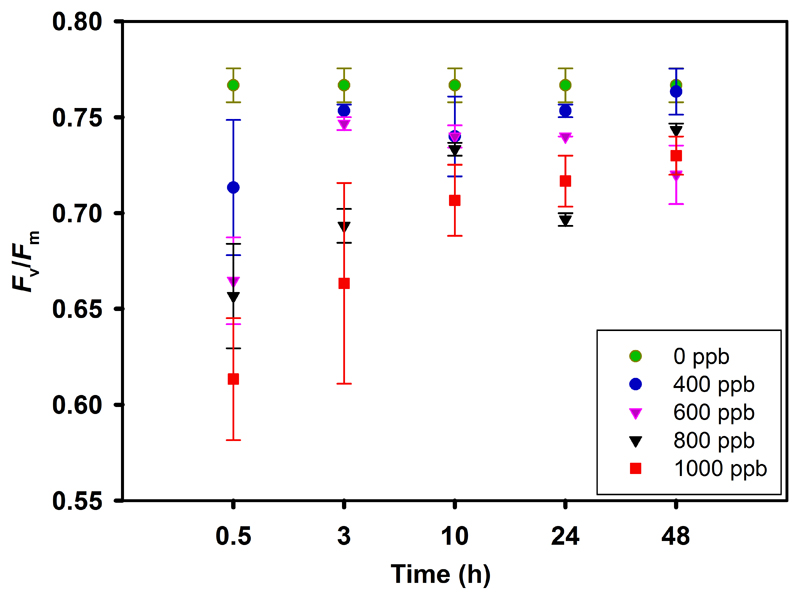
The maximum dark-adapted photosystem II (PSII) quantum yield (Fv/Fm) significantly decreased in leaves exposed to 600-1000 ppb ozone; however, 400 ppb exposure of ozone caused only a marginal decline in Fv/Fm (Fig. 1). Fv/Fm in 0.5 h after the exposure was negatively correlated with ozone concentration (r2 = 0.73, P < 0.001, data not shown). For leaves exposed to 400 ppb, Fv/Fm recovered almost fully in 48 h after exposure, but the recovery was only partial for leaves exposed to 600-1000 ppb ozone (Fig. 1).
Fig. 1.
Dark-adapted (10 min darkening) average (±SE) maximum fluorescence yield of photosystem II (PSII; Fv/Fm) of mature leaves of 10-12 weeks old Nicotiana tabacum ‘Wisconsin’ at 0.5, 3, 10, 24, and 48 hours of recovery following the exposure with ozone concentrations of 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber at 25 °C. All measurements were replicated at least thrice.
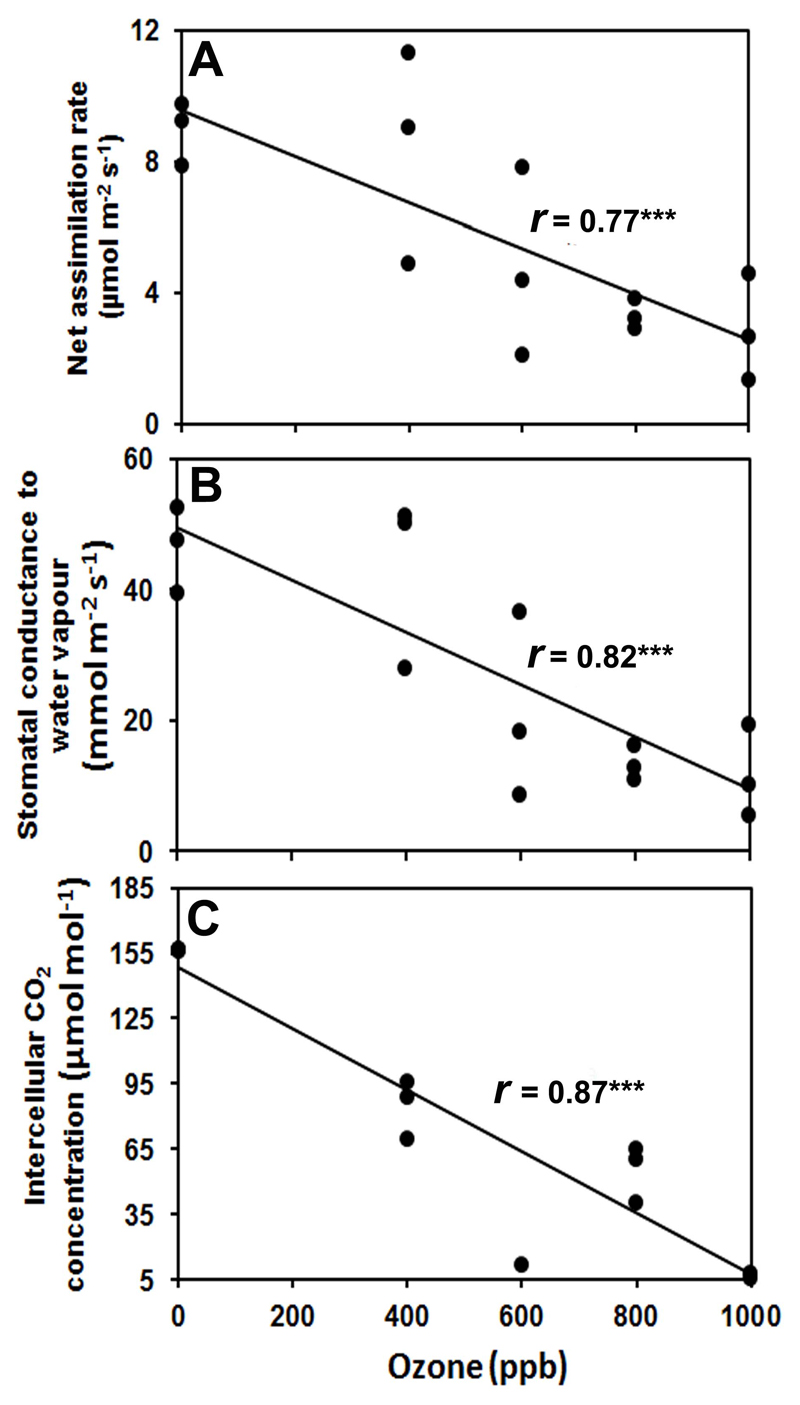
Exposure of ozone led to a statistically significant reduction in net assimilation rate (A, Fig. 2A), stomatal conductance to water vapour (gs, Fig. 2B), and intercellular CO2 concentration (Ci, Fig. 2C) for all exposure concentrations. However, net assimilation rate, stomatal conductance to water vapour, and intercellular CO2 concentration completely recovered after 48 h of recovery for all treatments (Supplementary Fig. S1).
Fig. 2.
Changes in (A) leaf net assimilation rate (A), (B) stomatal conductance to water vapour (gs), and (C) intercellular CO2 concentration (Ci) of mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’ in relation to ozone concentration during exposure. The measurements were taken at 0.5 h after ozone exposure at 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber 25 °C. All measurements were replicated at least thrice. Gas exchange measurements were conducted at leaf temperature of 25 °C, quantum flux density of 700 μmol m-2 s-1 at leaf surface, ambient CO2 concentration of 380-400 μmol mol-1, and relative air humidity of 50–60%. Each data point corresponds to an individual replicate measurement. Data were fitted by Spearman correlations. Statistical significance of regressions is indicated as: *** -P < 0.001.
3.2. Emission of foliar LOX volatiles in relation to ozone dose and time of recovery
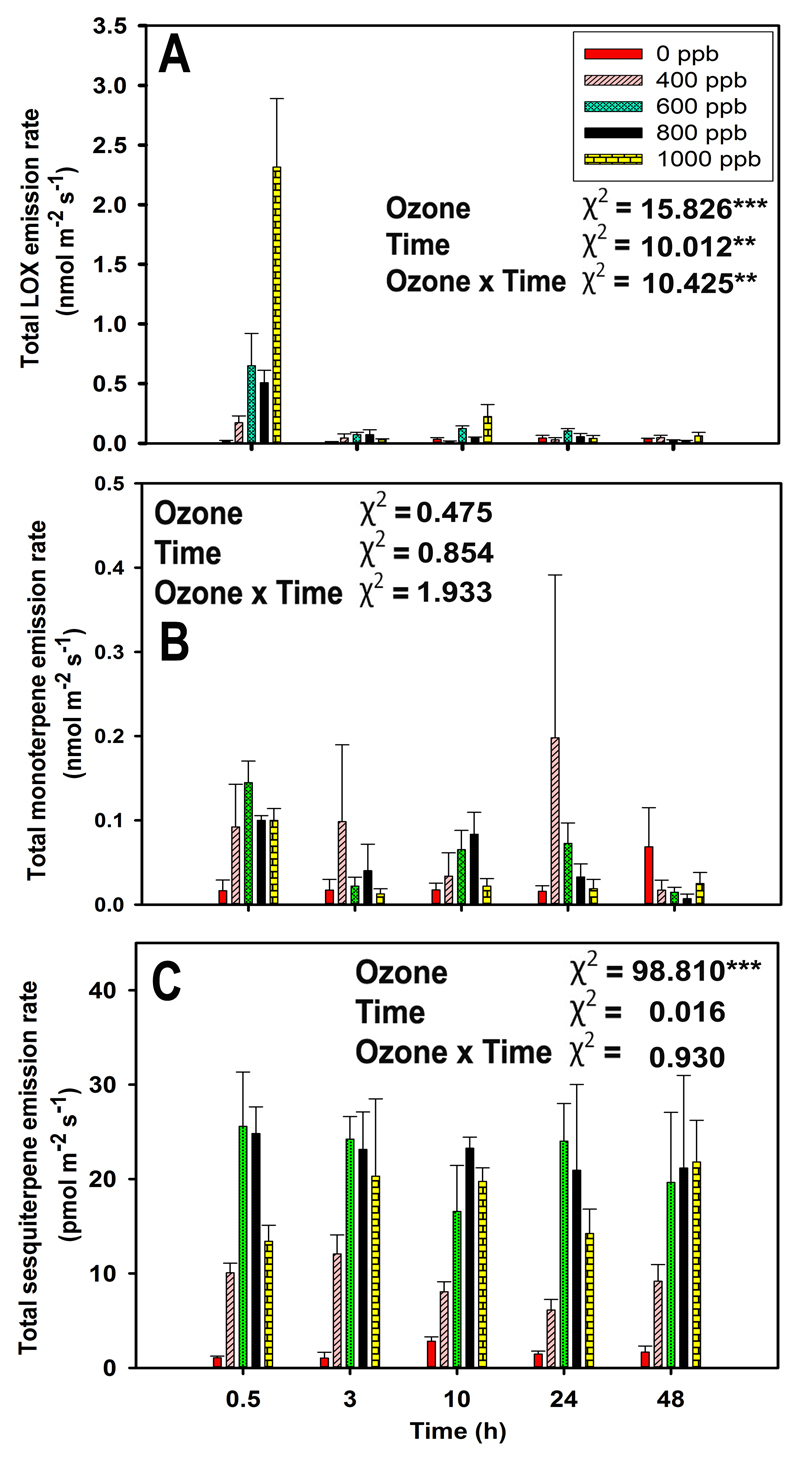
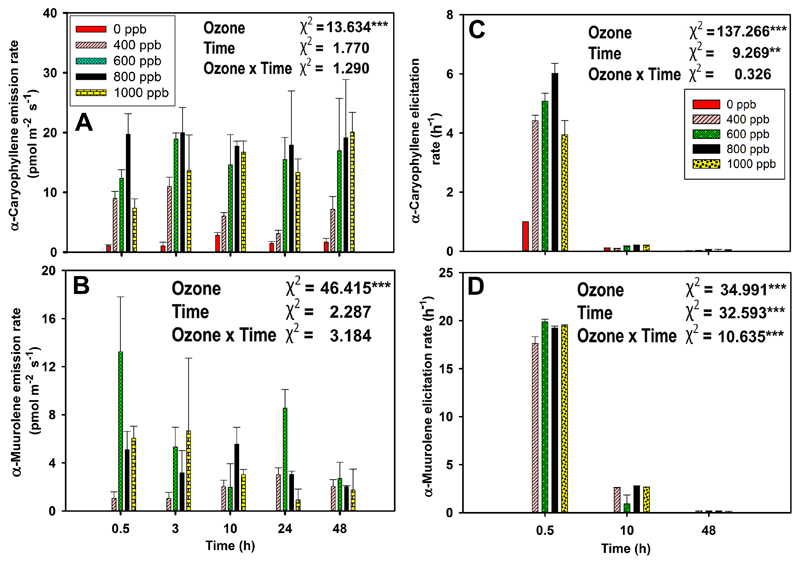
In non-treated leaves, LOX pathway volatiles were emitted at a very low level (average ± SE = 0.03 ± 0.01 nmol m-2 s-1). Ozone treatment strongly enhanced the emission of LOX volatiles after ozone exposure (Fig. 3A and Fig. 4A, B). The total emission of LOX volatiles was the highest immediately after ozone exposure and decayed over the recovery phase to pre-stress level (Fig. 3A, P < 0.001 for ozone treatments and P < 0.01 for the recovery time). Statistically significant ozone concentration effect reflected the highest rate of total LOX volatile emission at 1000 ppb ozone, ca. 160-fold higher rate compared with the control, followed by the intermediate rates at 600 and 800 ppb, and minor enhancement at 400 ppb (Fig. 3A).
Fig. 3.
Average (+SE) emission rates of total lipoxygenase pathway volatiles (LOX or green leaf volatiles, A), total monoterpenes (B), and total sesquiterpenes (C) in mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’ at 0.5, 3, 10, 24, and 48 hours of recovery after ozone exposure at 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber at 25 °C. Data are averages of three independent replicates. Individual effects of ozone, recovery time (Time), and their interactions on emission rates were tested by GLM with maximum likelihood model fitting. Wald chi-square (χ2) test statistics and statistical significance are indicated as: ** - P < 0.01, *** - P < 0.001.
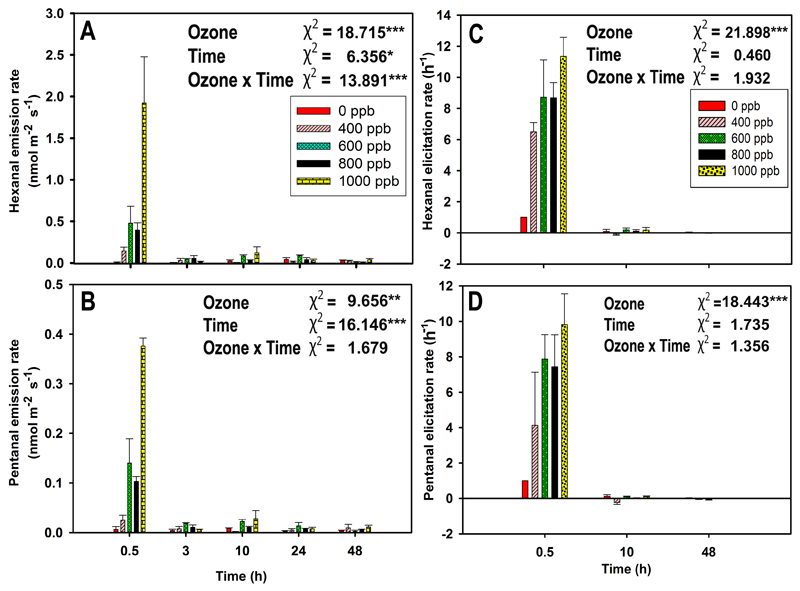
Fig. 4.
Average (+SE) emission rates of individual lipoxygenase pathway volatiles (LOX) hexanal (A), and pentanal (B) and average (+SE) elicitation rate of hexanal (C) and pentanal (D) in mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’ at different recovery times (0.5, 3, 10, 24, and 48 hours for emission rate and 0.5, 10, 48 hours for elicitation rate) after ozone exposure at 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber at 25 °C. The elicitation rate of emissions is defined as the rate of change of emission (positive values indicate an increase of the rate and negative values a decrease of the rate). Data are averages of three independent replicates. Individual effects of ozone, recovery time (Time), and their interactions on emission rates were tested by GLM with maximum likelihood model fitting. Wald chi-square (χ2) test statistics and statistical significance are indicated as: * - P < 0.05, ** - P < 0.01, *** - P < 0.001.
The ozone-elicited LOX volatile emissions were dominated by the saturated aldehydes hexanal and pentanal throughout the time of recovery, and as total LOX volatile emissions, the emissions were the greatest at 0.5 h after the ozone exposure. In this case, ozone and recovery time had a statistically significant impact on LOX volatile emission rates (Fig. 3A and Fig. 4A, B). In this study, the emissions of classic LOX volatiles, (Z)-3-hexenol, and (E)-2-hexenal were observed in trace levels only at 1000 ppb ozone dose (data not shown).
3.3. Emission responses of foliar mono- and sesquiterpenes in dependence of ozone exposure and time of recovery
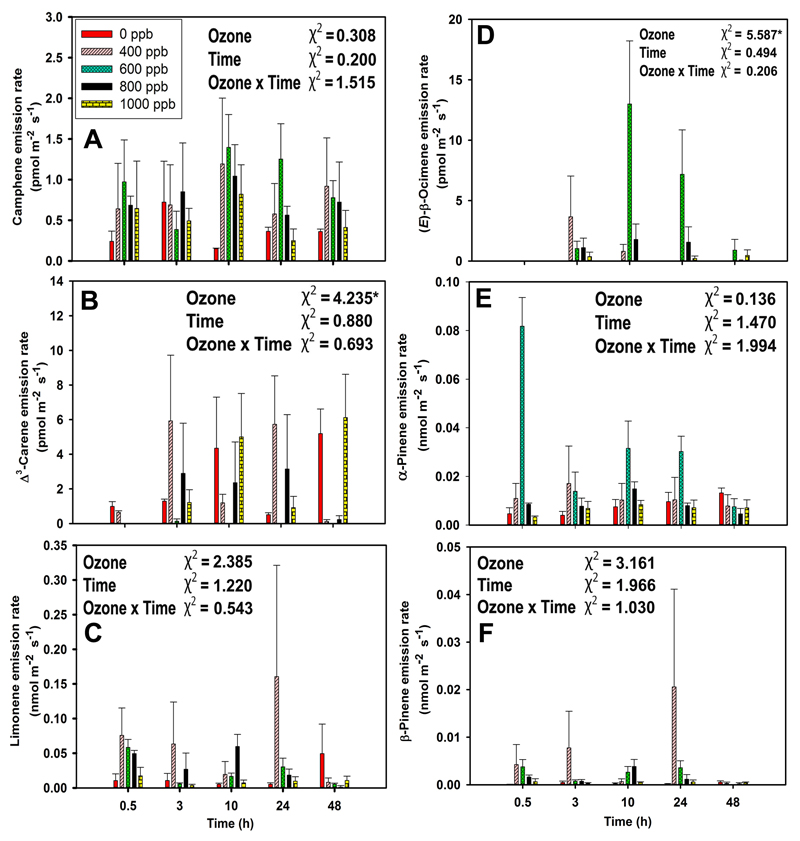
Non-treated leaves of N. tabacum were low-level emitters of monoterpenes (average± SE rate of emission of 0.02 ± 0.01 nmol m-2 s-1) and sesquiterpenes (1.66 ± 0.25 pmol m-2 s-1) (Fig. 3B, C). In control leaves, the blend of emitted monoterpenes was dominated by limonene, followed by α-pinene, β-pinene, Δ3-carene, and camphene (Fig. 5), whereas α-caryophyllene was observed as the main sesquiterpene (Fig. 7A). In addition, the monoterpene (E)-β-ocimene (Fig. 5D), and the sesquiterpene α-muurolene were emitted upon ozone exposure (Fig. 7B).
Fig. 5.
Average (+SE) emission rates of key monoterpenes (A-F) from mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’ at 0.5, 3, 10, 24, and 48 hours of recovery after ozone exposure at 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber at 25 °C. Individual effects of ozone, recovery time (Time), and their interactions on emission rates were tested by GLM with maximum likelihood model fitting. Wald chi-square (χ2) test statistics and statistical significance are indicated as: * - P < 0.05.
Fig. 7.
Average (+SE) emission rates of α-caryophyllene (A) and α-muurolene (B) and average (+SE) elicitation rate of α-caryophyllene (C) and α-muurolene (D) in mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’ at different recovery times (0.5, 3, 10, 24, and 48 hours for emission rate and 0.5, 10, 48 hours for elicitation rate) after ozone exposure at 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber at 25 °C. The elicitation rate of emissions is defined as the rate of change of emission (positive values indicate an increase of the rate and negative values a decrease of the rate). Data are averages of three independent replicates. Individual effects of ozone, recovery time (Time), and their interactions on emission rates were tested by GLM with maximum likelihood model fitting. Wald chi-square (χ2) test statistics and statistical significance are indicated as: ** - P < 0.01, *** - P < 0.001.
The effect of ozone concentration on monoterpene emissions was non-linear with the highest emissions initially observed for 600 ppb exposure at 0.5 h since the exposure (Fig. 3B). The concentration effect was more clearcut for sesquiterpene emissions till 600 ppb of exposure, then the emission decreased with increasing ozone concentration (Fig. 3C). Monoterpene emissions of ozone-treated leaves remained similar through the recovery, except at the end of the recovery at 48 h when the emissions of treated leaves had decreased below the values observed for control leaves (Fig. 3B). In contrast, no such decline was observed for sesquiterpenes (Fig. 3C).
The time-courses of individual monoterpene emissions broadly reflected the variation in total monoterpene emissions, except Δ3-carene, where ozone concentration and time had a minor impact on the emissions (Fig. 5B). In addition, there were some differences in reaching the peak emissions and modified emissions patterns through recovery for different monoterpenes. In particular, β-pinene and limonene emissions tended to peak in the leaves exposed to 400 ppb ozone at 24 h after exposure (Fig. 5C, F), but α-pinene emissions peaked at 600 ppb ozone exposure and at 0.5 h since the exposure (Fig. 5E). The emission of the characteristic stress-induced monoterpene, (E)-β-ocimene, was first observed after 3 h since ozone treatment, and the emissions peaked for 600 ppb ozone at 10 h since the exposure (Fig. 5D).
In the case of sesquiterpenes, ozone concentration and time of recovery affected α-caryophyllene emissions similarly to total sesquiterpene emissions (Fig. 3C, 7A). In contrast, there were no emission responses of α-muurolene observed from control leaves and the highest α-muurolene emissions were observed for 600 ppb ozone treatment at 0.5 h since the exposure, and the emissions decreased slightly through the recovery phase (Fig. 7B). Furthermore, we have observed γ-cadinene and γ-muurolene in trace levels in response to 600-1000 ppb ozone exposures (data not shown).
3.4. Quantitative estimation of terpene content in Nicotiana tabacum leaves
Four monoterpenes, Δ3-carene, limonene, α-pinene, and β-pinene were stored in the tissues of untreated leaves (Table 1). Δ3-Carene was the most abundant monoterpene observed in the leaf tissues followed by α-pinene, limonene, and β-pinene. Sesquiterpenes, α-caryophyllene and α-muurolene were not observed at a detectable level in leaf tissues (Table 1).
Table 1.
Average (±SE) values of terpene contents in untreated leaves of Nicotiana tabacum ‘Wisconsin’. Data are averages of six independent replicates.
| Foliage terpene contents (nmol g−1 DM) | ||
|---|---|---|
| No | Compound | Mean ± SE |
| 1 | Camphene | nd |
| 2 | Δ3-Carene | 43 ± 7 |
| 3 | Limonene | 16 ± 5 |
| 4 | (E)-β-Ocimene | nd |
| 5 | α-Pinene | 20 ± 3 |
| 6 | β-Pinene | 10 ± 3 |
| 7 | α-Caryophyllene | nd |
| 8 | α-Muurolene | nd |
3.5. Elicitation rate of emissions of LOX volatiles, mono- and sesquiterpenes
At the initial enhancement, hexanal emission was ca. five times higher than pentanal emission upon ozone stress, but hexanal elicitation rate was almost equal to pentanal elicitation rate. Furthermore, at 48 h of recovery, hexanal emission rate was three times higher than pentanal emission rate, but its elicitation rate was two times lower than pentanal elicitation rate (Figs. 4A-D). Ozone had a statistically significant impact (P < 0.001) on the elicitation rates of hexanal and pentanal (Fig. 4 C-D).
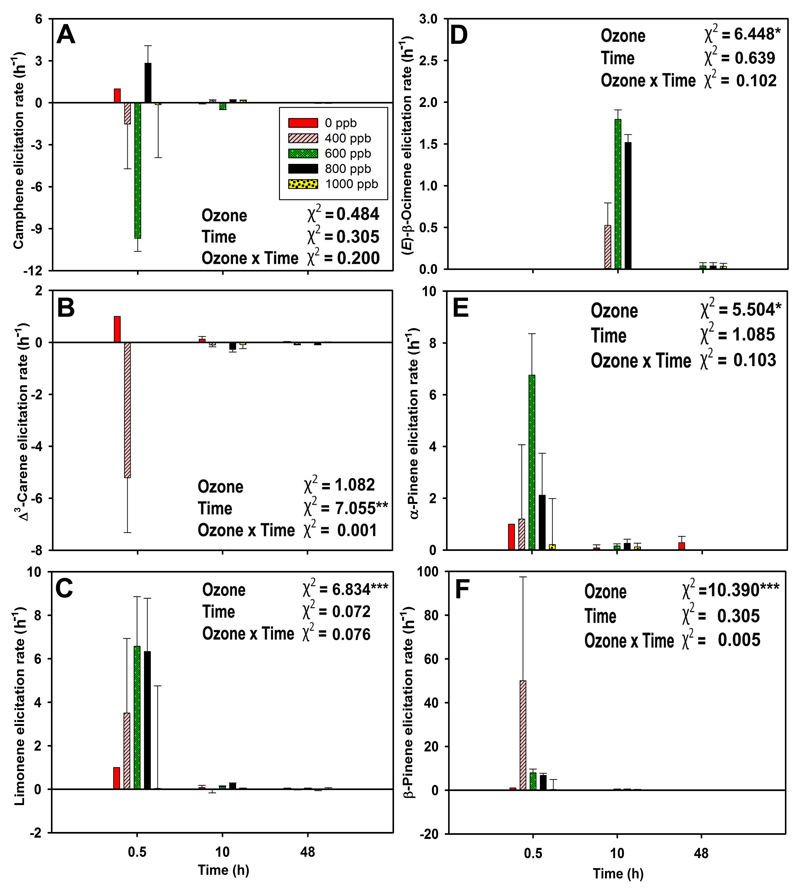
β-Pinene was the monoterpene elicited to the greatest degree, followed by limonene, α-pinene, and (E)-β-ocimene, although limonene was the highest emitted monoterpene. The lowest elicitation rate was observed for camphene. Δ3-carene emission rate was ca. four times higher than camphene emission rate but the elicitation rate of both compounds was almost equal (Fig. 5 and Fig. 6). Ozone had a statistically significant impact on elicitation rates of limonene, (E)-β-ocimene, α-pinene, and β-pinene (Fig. 6A-F). The elicitation rate for (E)-β-ocimene was positive through the entire recovery period (Fig. 6D).
Fig. 6.
Average (+SE) elicitation rate of monoterpenes in mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’ at 0.5, 10, and 48 hours of recovery after ozone exposure at 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber at 25 °C. The elicitation rate of emissions is defined as the rate of change of emission (positive values indicate an increase of the rate and negative values a decrease of the rate). Data are averages of three independent replicates. Individual effects of ozone, recovery time (Time), and their interactions on emission rates were tested by GLM with maximum likelihood model fitting. Wald chi-square (χ2) test statistics and statistical significance are indicated as: * - P < 0.05, ** - P < 0.01, *** - P < 0.001.
α-Muurolene was the most strongly elicited sesquiterpene, followed by α-caryophyllene. For both sesquiterpenes, ozone and recovery time significantly affected the elicitation rates (Fig. 7C and 7D). At 0.5 h of recovery, α-muurolene elicitation rate was ca. four times higher than α-caryophyllene elicitation rate, although the emission rate of α-caryophyllene was ca. two times higher than that of α-muurolene. At 48 h of recovery, the elicitation rate of both compounds reached the lowest level (Fig. 7A-D).
3.6. Emissions of LOX volatiles, mono- and sesquiterpenes in relation to stomatal ozone uptake and non-stomatal ozone deposition rates
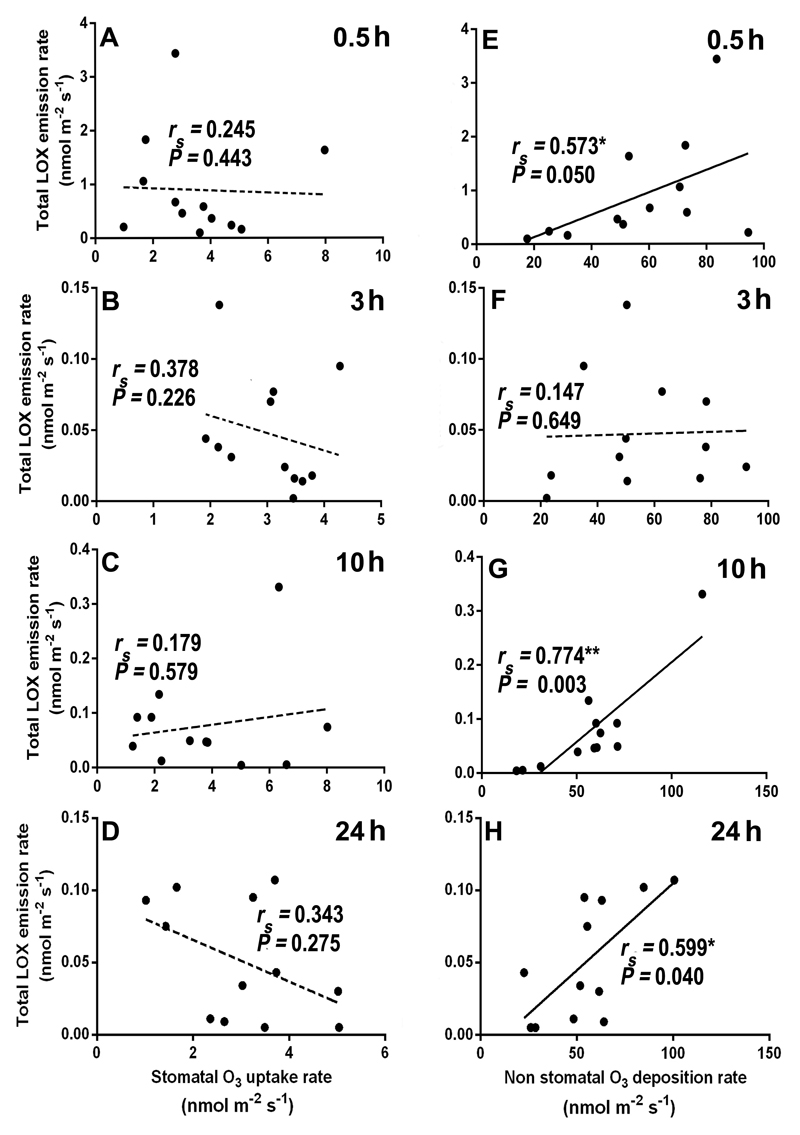
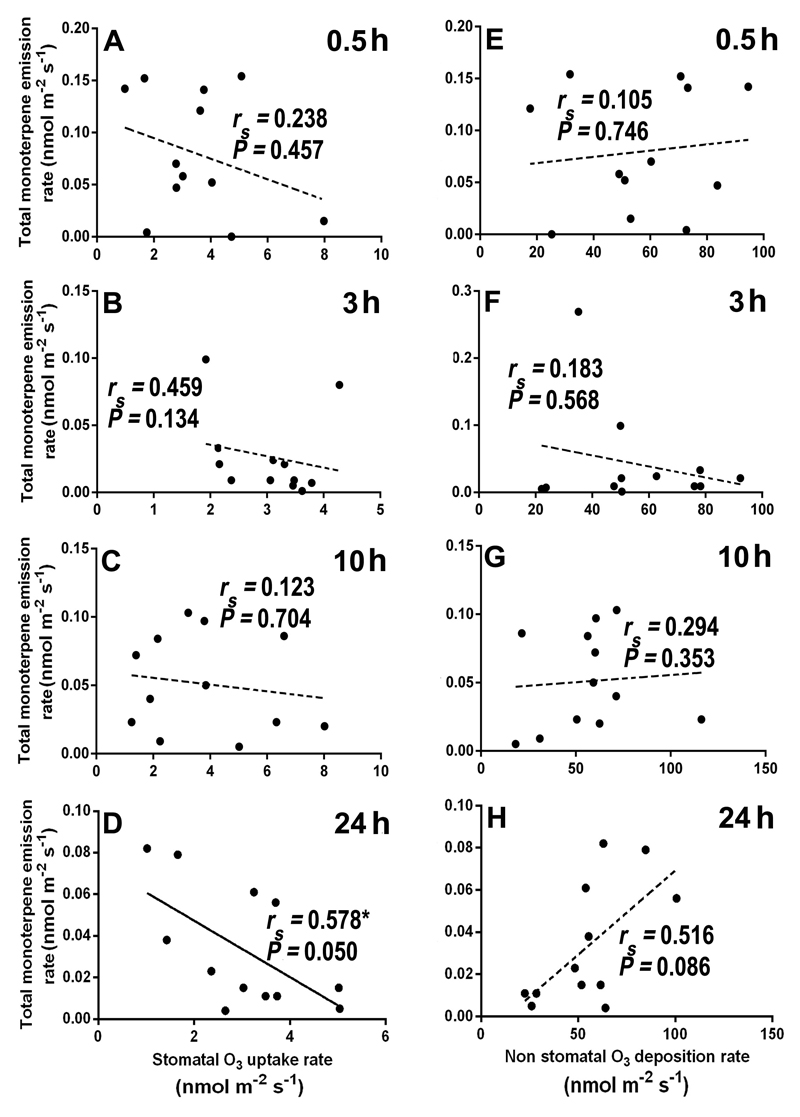
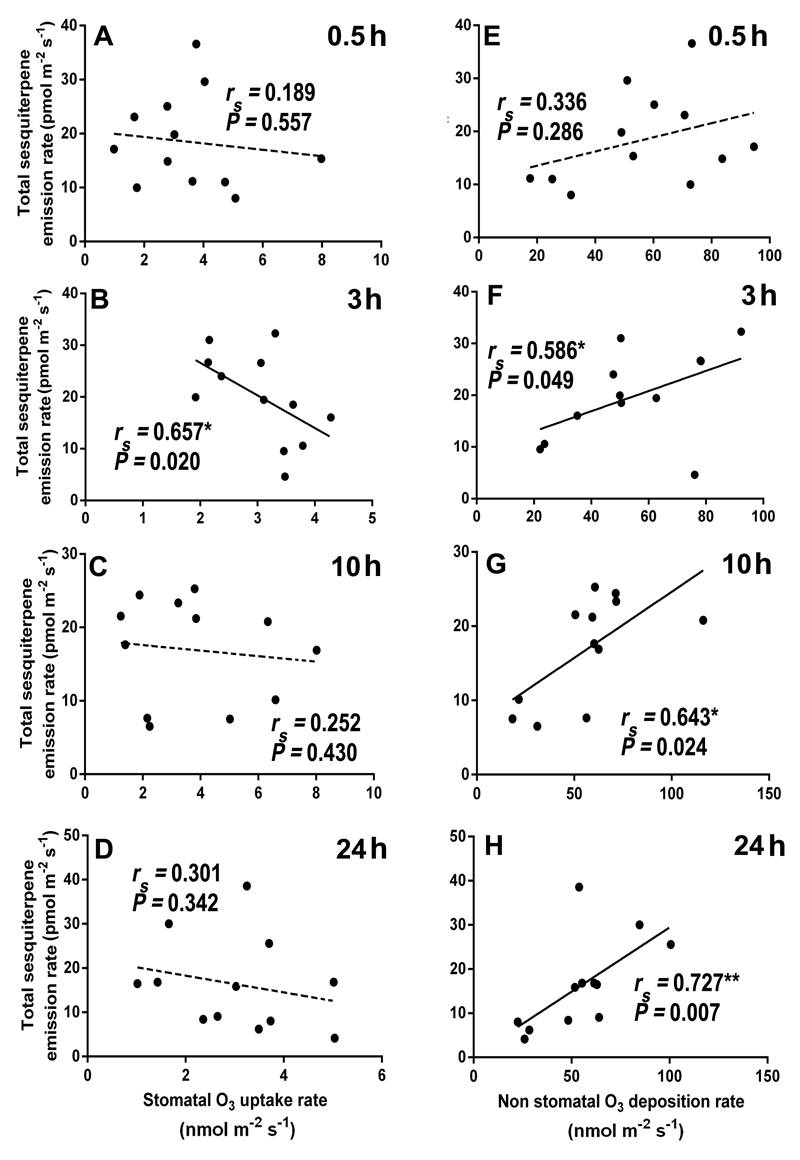
Overall, total LOX volatile emission rate was poorly correlated with stomatal ozone uptake rate (Figs. 8A-D), but the correlations with non-stomatal ozone deposition rate (Fig. 8E-H) were positive and statistically significant at 0.5, 10, and 24 h since the exposure (Fig. 8E, 8G, and 8H). In the case of total monoterpenes, a statistically significant negative correlation between stomatal ozone uptake rate (Fig. 9D), and a marginally significant positive correlation between non-stomatal ozone deposition rate (Fig. 9H) and monoterpene emission were observed at 24 h of recovery. For sesquiterpenes, the total emission rate was negatively correlated with stomatal ozone uptake rate at all recovery times (Fig. 10A-D), and positively and significantly correlated with ozone deposition rate at 3, 10 and 24 h of recovery (Fig. 10F, G, and H).
Fig. 8.
Correlations of total LOX emission rates with stomatal ozone uptake rate (A-D) and non-stomatal ozone deposition rate (E-H) at different times (0.5, 3, 10, and 24 h) during recovery in mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’. Each data point indicates an individual replicate. Data were fitted by Spearman correlation. Non-significant regressions (P > 0.05) are shown by dashed lines. Statistical significance is indicated as: * -P < 0.05, ** - P < 0.01.
Fig. 9.
Correlations of total monoterpene emission rates with stomatal ozone uptake rate (A-D) and non-stomatal ozone deposition rate (E-H) at different times (0.5, 3, 10, and 24 h) through recovery in mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’. Data were fitted by Spearman correlation. Non-significant regressions (P > 0.05) are shown by dashed lines. Statistical significance is indicated as: * -P < 0.05.
Fig. 10.
Correlations of total sesquiterpene emission rates with stomatal ozone uptake rate (A-D) and non-stomatal ozone deposition rate (E-H) at different times (0.5, 3, 10, and 24 h) during recovery in mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’. Data were fitted by Spearman correlation. Non-significant regressions (P > 0.05) are shown by dashed lines. Statistical significance is indicated as: * -P < 0.05, **- P < 0.01.
4. Discussion
4.1. Changes in leaf photosynthetic characteristics upon acute ozone stress
Acute ozone concentrations caused a sharp decline in the maximum dark-adapted photosystem II quantum yield (Fv/Fm), especially at the exposures of 800 and 1000 ppb (Fig. 1). A reduction in Fv/Fm is typically associated with sustained photoinhibition due to damage at PSII (Demmig-Adams and Adams, 2006; Osmond et al., 1999). In fact, studies have demonstrated that exposure of leaves to elevated ozone results in increasingly deactivated PSII centres, thereby reducing the photochemical capacity of PSII (Guidi and Degl’Innocenti, 2008). Photoinhibitory effects of ozone are primarily caused by the overall net reduction of D1 core protein of PSII (Adir et al., 2003; Singh et al., 2009).
There is also evidence that elevated ozone inhibits photosynthesis in a time-dependent manner due to suppression of Calvin cycle (Guidi and Degl’Innocenti, 2008). In potato (Solanum tuberosum) leaves, elevated ozone inhibited the activity and reduced the quantity of ribulose-1,5-bisphosphate carboxylase/oxygenase leading to reduced rate of photosynthesis (Dann and Pell, 1989) as was also observed in our study (Fig. 2A). However, the reductions in photosynthesis rate and intercellular CO2 concentration were associated with the decline of stomatal conductance to water vapour (Fig. 2A-C). Although net assimilation rate and stomatal conductance typically decrease in parallel through abiotic stresses (Copolovici et al., 2012; Kask et al., 2016, Kanagendran et al., 2018), this result indicates that the inhibitory effect of elevated ozone on net assimilation was at least partly associated with stomatal closure.
The reductions in both net assimilation rate, stomatal conductance to water vapour, and intercellular CO2 concentration were strongly correlated with ozone concentration during the exposure (Figs. 2A-C), indicating a dose-dependent response as observed in several studies (Akhtar et al., 2010; Degl'Innocenti et al., 2002; Guidi et al., 2001). Despite high treatment ozone concentrations, the recovery of photosynthetic characteristics at 48 h since ozone exposure indicates the high degree of ozone resistance of N. tabacum ’Wisconsin’ used in our study (Supplementary Fig. S1). However, from an ecological perspective, we note that the recovery times of 24-48 h are too long for a stress factor such as ozone that has a diurnal cycle. Another exposure cycle in the following day would not allow for such recovery to take place.
4.2. Effect of acute ozone exposure on the emission of lipoxygenase pathway (LOX) volatiles
Multiple lipoxygenases are constitutively active in plant cells, and once their substrate, polyunsaturated fatty acids become available, LOX volatiles are rapidly released from membranes (Feussner and Wasternack, 2002). Typically, the release of polyunsaturated fatty acids from membranes and concomitant release of LOX volatiles are considered to be an acute stress response associated with major membrane-level damage (Jiang et al., 2017; Jiang et al., 2016). However, the situation with ozone might be more complicated as ozone itself can cause oxidative degradation of membrane lipids as well as oxidize cuticular lipids on leaf surface (Senser et al., 1987; Slimestad et al., 1995).
In fact, the typical LOX volatiles emitted upon acute abiotic stress are (E)-2-hexenal, (Z)-3-hexenol, 1-hexanol, and (Z)-3-hexenyl acetate (Copolovici et al., 2012; Copolovici and Niinemets, 2010; Heiden et al., 2003). Indeed, Heiden et al. (1999) reported emissions of these classical LOX volatiles upon acute ozone fumigation of 175 ppb for 5 h and 150 ppb for 11 h from ozone-sensitive N. tabacum ‘Bel W3’ and ozone-resistant N. tabacum ‘Bel B’. In our study, however, the emissions of (E)-2-hexenal, (Z)-3-hexenol were observed in trace level at the highest ozone doses, and there were no emission of 1-hexanol, and (Z)-3-hexenyl acetate upon elevated ozone. This suggests that the impact of ozone exposure on LOX volatiles depends on ozone concentration and exposure duration used and also varies among cultivars with different ozone sensitivity. In our study, we observed that the oxygenated volatile emissions were dominated by the saturated aldehyde hexanal followed by the saturated aldehyde pentanal and that these emissions were related to ozone concentration in a dose-dependent manner right after ozone exposure at 0.5 h (Fig. 3A and Figs. 4A-B). The formation of hexanal is partly attributed to the breakdown of membrane lipids by lipoxygenase and hydroperoxide lyase enzymes (Heiden et al., 2003) and thus, the emission of hexanal following ozone fumigation may be associated with the direct effect of the lipoxygenase cleavage of fatty acids in the leaf membranes exposed to ozone (Paoletti, 2006). Similar to our study, (Carriero et al., 2016) observed that the dominant LOX volatile was hexanal emitted following short-term acute ozone exposures in silver birch (Betula pendula). All these reports suggest that hexanal is a ubiquitous LOX volatile product emitted in relation to acute ozone stress. Nevertheless, we cannot rule out alternative biochemical reactions leading to saturated aldehyde formation (Wildt et al., 2003) or direct reactions of ozone with leaf surface lipids or elicitation of LOX volatile production within leaf surface structures such as secretory cells feeding leaf glandular trichomes. At least the rapid cessation of LOX volatile emissions (Fig. 3A and Figs. 4A-B) suggests that even the highest ozone exposures did not result in a major cellular damage, consistent with almost full recovery of leaf photosynthetic characteristics (Figs. 1-2 and Supplementary Fig. S1).
4.3. Effect of acute ozone exposure on the emission of mono- and sesquiterpenes
Mainly due to the presence of glandular trichomes, N. tabacum is a constitutive low-level terpene emitter under non-stressed conditions. Relatively low levels of terpene emissions observed for non-stressed leaves are likely associated with a limited storage capacity for monoterpenes in glandular trichomes in tobacco (Ohara et al., 2003) (Table 1), compared with less volatile diterpene storage in trichomes (Cui et al., 2011).
Upon ozone exposure, mono- and sesquiterpene emissions were generally enhanced, but the responses varied in time for different ozone doses (Figs. 3B-C, 5, and 7A-B). Although total monoterpene emission was enhanced by ozone, it peaked at ozone concentrations of 400-600 ppb at different times during recovery and ultimately decreased to levels below that observed in control leaves at 48 h after exposure (Fig. 3B). In contrast, sesquiterpene emissions were more exposure dose dependent and weakly decreased over the recovery period (Fig. 3C). This difference among the two terpenoid classes is puzzling and might be associated with different metabolic pathways responsible for synthesis of these volatiles. In the case of monoterpenes, the synthesis occurs through the plastidial 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway (MEP/DOXP pathway), whereas sesquiterpene synthesis occurs through mevalonate-dependent pathway in cytosol (Pazouki and Niinemets, 2016; Rajabi Memari et al., 2013; Rosenkranz and Schnitzler, 2013). In the mesophyll cells, MEP/DOXP pathway activity is strongly dependent on immediate availability of NADPH and ATP generated by photosynthetic electron transport (Niinemets et al., 2002; Rasulov et al., 2009), and thus, modifications in the activity of photosynthesis are expected to impact the substrate availability for monoterpene synthesis. In contrast, no such photosynthetic control is expected on the rate of sesquiterpene synthesis. Indeed, elevated ozone, 800-1000 ppb, significantly inhibited photosynthesis and in turn, it decreased emission rates of monoterpenes compared to the control treatment, suggesting that such a substrate-level control might be possible. However, monoterpene emission was actually initially enhanced for lower ozone concentrations and the substrate-level control does also not explain the decline of monoterpene emission, especially at 48 h when photosynthesis had almost fully recovered (cf. Figs. 1, 2, 3B, and Supplementary Fig. S1). Such a discrepancy might be associated with the circumstance that under ozone stress, a higher proportion of MEP/DOXP pathway products might have been allocated to the replacement of non-volatile larger isoprenoids, such as chlorophyll and carotenoids, oxidized upon ozone exposure (Pazouki et al., 2016). In addition, as the stress might alter the allocation of photosynthates between carbon storage and biosynthesis of new chemicals (Fichtner et al., 1994), full recovery of photosynthesis right after stress does not always guarantee that photosynthetic products will be high enough to support the rate of biosynthesis of secondary compounds.
An alternative explanation to the observed discrepancy between changes in photosynthesis and monoterpene emissions is that monoterpenes were primarily emitted from terpene pools stored in glandular trichomes (Table 1). Ozone exposure can enhance trichome emissions via damage of trichome surface, thereby enhancing terpene permeability (Jud et al., 2016). On the other hand, ozone itself can oxidize the terpenes released at a constant rate from trichomes (Pinto et al., 2007), and this could explain the decreased emissions at the highest ozone concentrations (Fig. 3B). Furthermore, once damaged, discharge of terpene pools can be responsible for the monoterpene emission rates such that they reached to values lower than those observed in control leaves. In the case of sesquiterpenes, changes in the emissions can also be explained by ozone effects on trichome surface permeability, albeit that sesquiterpenes are much less volatile than monoterpenes (Copolovici and Niinemets, 2015; Copolovici and Niinemets, 2005), and thus emptying the pools is expected to take much longer than those for monoterpenes. However, this explanation still does not explain why there was an apparent dose dependence for sesquiterpenes and not for monoterpenes.
4.4. Contribution of terpenoids synthesized de novo and from storage pool to the terpenoid emission
Quantitative estimation of terpene contents in tobacco leaves demonstrated that Δ3-carene was the most abundant monoterpene stored in fresh leaf tissues followed by α-pinene, limonene, and β-pinene. However, the blend of monoterpenes emitted by both ozone-treated and untreated leaves was dominated by limonene, followed by α-pinene, and β-pinene (Fig. 5). Therefore, a major portion of the emission of limonene is likely synthesized de novo, but camphene and (E)-β-ocimene were fully de novo synthesized in this study. Similarly, as there were no traces of α-caryophyllene and α-muurolene found in the leaf tissues, those two sesquiterpenes were ultimately de novo synthesized (Table 1 and Figs 7A, B).
4.5. Ozone-dependent modification of the blend of emitted terpenoids and time kinetics of elicitation rates of LOX volatiles and terpenoids
Several studies have demonstrated that upregulation of terminal enzymes following a stress was responsible for the enhanced emission of volatile isoprenoids (Frank et al., 2009; Pateraki and Kanellis, 2010; Singh and Johnson-Flanagan, 1998). In a study with the ozone-sensitive tobacco cultivar ‘Bel W3’, Beauchamp et al. (2005) further demonstrated significant emissions of methyl salicylate (MeSA), suggesting a major physiological stress response upon ozone exposure in this sensitive cultivar. In our study, emissions of six monoterpenes, two sesquiterpenes and no traces of MeSA were observed. Lack of MeSA emission together with the lack of characteristic LOX volatiles (see above), further suggests that the observed modifications in terpenoid emissions, particularly Δ3-carene, α-pinene, and β-pinene, in this ozone-resistant cultivar primarily reflected changes in the constitutive emissions, most likely associated with changes in trichome surface permeability, further supported by correlation analysis and solvent extract analysis (Figs. 8-10 and Table 1).
Nevertheless, lack of storage capacity and characteristic emission response observed suggested elicitation of de novo synthesis of (E)-β-ocimene upon ozone exposures. In particular, relatively low-level emissions of (E)-β-ocimene were detected at later stages during recovery with the emissions first observed at 3 h, and peaking at 10 h since the exposure (Fig. 5D), suggesting that ozone exposure did activate defence responses. (E)-β-Ocimene has been considered an iconic stress monoterpene, and its emissions have been shown to be elicited upon biotic and abiotic stresses including exposure to high doses of ozone in Phaseolus vulgaris (Li et al., 2017), cold and heat shock in Solanum lycopersicum (Copolovici et al., 2012; Pazouki et al., 2016), feeding by larvae of beet armyworm (Spodoptera exigua) in Medicago truncatula (Navia-Giné et al., 2009), and feeding by larvae of common white wave (Cabera pusaria) in leaves of Alnus glutinosa (Copolovici et al., 2011).
Sesquiterpenes, including α-caryophyllene are also commonly considered stress-inducible (Lung et al., 2016), but in our study, it was α-muurolene, emission of which was lacking in control leaves and was induced during the recovery phase (Fig. 7B). Thus, this evidence suggests that ozone treatment could have changed the expression of several terpene synthase genes in our study, leading to a certain modification in the bend of emitted volatiles, albeit the influence of de novo gene expression on total emission was likely minor in our study.
A great variability of emission rates of each compound, despite almost similar elicitation rates can be explained by several factors governing the underlying mechanism, such as; (1) ozone exposure can trigger an array of reactions and mechanisms varying from elicitation of gene expression to protein turnover, signal transduction and all these process are time consuming. At the onset of ozone treatment, LOX volatile elicitation rate is primarily determined by the rate of free fatty acid break-down, resulting in an initial emission burst; higher emission rates of mono- and sesquiterpenes, and concomitantly higher elicitation rate can further partly rely on the rapid increase of trichome permeability and thus, the release of stored terpenes. On the other hand, the depletion of terpene contents in the storage structures leads to declining elicitation rates, ultimately even to negative values; (2) expression of an array of genes in LOX, MEP, and MVA pathways is elicited after some hours of stress application and have a substantial impact on emission responses along with substrate level control (Pazouki et al., 2016), especially at 10 h of elicitation in this study. However, at 48 h of recovery, the elicitation rate became negative for most of the compounds, reflecting lower emission rates than in controls; (3) physiological and physico-chemical limitations within the leaf mesophyll, such as the availability of ATP, NADPH, glyceraldehyde 3-phosphate, maximum activity of rate-controlling enzymes, and compound gas-phase partial pressure, and aqueous- and lipid-phase concentrations that control the volatile diffusion at the leaf–atmosphere interface (Niinemets and Reichstein, 2003 for a review). While physiological limitations can affect the maximum synthesis rate of volatiles, physico-chemical limitations on de novo synthesized volatiles, except for lipophilic compounds such as non-oxygenated terpenes, can have a substantial influence on the time-lags between synthesis and emission.
4.6. Emission responses of stress volatiles in response to stomatal ozone uptake and non-stomatal ozone deposition
Ozone uptake by the leaves from the ambient atmosphere occurs by two means, by stomatal uptake and by surface deposition. These means of uptake have fundamentally different physiological impacts. In addition, once taken up, the ultimate damaging effect of ozone depends on the extent to which ozone is scavenged by water-soluble antioxidants within the aqueous phase in cell walls and by volatile antioxidants within leaf gas-phase as well as by lipid-soluble volatile and non-volatile antioxidants. Among these volatile antioxidants (Bouvier-Brown et al., 2009; Goldstein et al., 2004; Kurpius and Goldstein, 2003), mono- and sesquiterpenes are particularly reactive with ozone (Fares et al., 2010a).
Analysis of the distribution of ozone flux between stomatal uptake and surface deposition indicated that stomatal absorption of ozone was much lower than the non-stomatal deposition for N. tabacum ‘Wisconsin’ (Figs. 8-10). We argue that the high surface deposition flux reflects presence of terpene-filled glandular trichomes on leaf surface, and accordingly direct reaction of ozone with unsaturated terpenoids, e.g., unsaturated semi-volatile compounds such as cis-abienol (C20H34O) present on N. tabacum leaf surface that can act as an efficient sink for ozone and powerful chemical barrier against stomatal ozone uptake at the leaf surface (Jud et al., 2016), but of course, other terpenoids including mono- and sesquiterpenes can contribute to ozone detoxification. Furthermore, non-stomatal deposition itself due to volatiles produced by plant not only occurs at the leaf surface, but also can occur within the leaf boundary layer and even outside the boundary layer (Altimir et al., 2006; Fares et al., 2008).
Comparisons of the correlations of total emission rates of LOX volatiles, mono- and sesquiterpenes, with stomatal ozone uptake and with non-stomatal ozone deposition indicated that the correlations were stronger with non-stomatal deposition (Figs. 8A-D, Figs. 9A-D, and Figs. 10A-D vs. Figs. 8E-H, Figs. 9E-H and Figs. 10E-H). Counterintuitively, in several cases, total emission rates of mono- and sesquiterpenes were negatively correlated with stomatal ozone uptake. There can be several explanations for these negative correlations. First, when ozone enters the leaf mesophyll cells, it can rapidly react with water-soluble antioxidants such as putrescine and apoplastic ascorbate and therefore, the amount of ozone molecules trigging stress is typically much lower than that entering through stomata (Laisk et al., 1989). Second, volatile isoprenoids can react directly with ozone inside the leaves or with ozone-induced reactive oxygen species (ROS) (Loreto and Fares, 2007), resulting in lower rates of emission of mono- and sesquiterpenes than their biosynthesis rates. Lack of the correlation immediately after the exposure suggests that terpene reactions with ozone inside the leaves unlikely played a role in these negative correlations. Yet, there is typically a secondary rise of ROS, several hours after initial stress impact (Beauchamp et al., 2005; Jiang et al., 2017; Li et al., 2017), and it is plausible that the correlations observed 3-24 h since the ozone exposure reflected isoprenoid reactions with the endogenously generated ROS.
The positive correlations among non-stomatal ozone deposition and total LOX volatile, and mono- and sesquiterpene emissions (Fig. 8E-H, Fig. 9E-H, and Fig. 10E-H) are wholly consistent with the hypothesis of the surface release of volatiles from glandular trichomes or impacted secretory cells. These results collectively suggest that a significant part of volatile emission in response to ozone exposure was released from leaf surface.
5. Conclusions
Ozone is believed to affect plants in a dose-dependent manner, i.e. the effects depend both on ozone concentration above the threshold value (concentration eliciting a physiological response, typically a low value of 40 ppb is assumed) and the duration of the ozone exposure (Beauchamp et al., 2005; Niinemets et al., 2010), but as our study demonstrates the ozone dose responses can be complicated due to surface reactions. Taken together, these results from one stress cycle, with a long time for recovery indicate that acute ozone exposure led to a significant reduction of foliar gas-exchange characteristics, but the reductions in all these characteristics were almost fully reversible even at the highest ozone concentrations underscoring the high ozone resistance of N. tabacum ‘Wisconsin’. The emission rate of LOX volatiles was dose-dependent, whereas the dose dependencies for terpenes, especially for monoterpenes were weaker, but de novo elicitation of terpenoid synthesis was moderate, again corroborating the high ozone resistance of this genotype.
In this study, most of the ozone flux to the leaf was due to non-stomatal ozone deposition, and non-stomatal ozone deposition scaled with the release of terpenes, suggesting that terpenoid emission responses to ozone mostly reflected modification of terpene release from trichomes on leaf surface. The results further suggest that the surprisingly high ozone-resistance of N. tabacum ‘Wisconsin’ is likely due to surface reactions of volatiles released upon ozone exposure. This study demonstrated that counterintuitively, in this ozone-resistant tobacco cultivar, the quantity of ozone uptake through stomata is not proportional to the volatile emission rates. Overall, there was a substantial variability of emission rates of each compound upon similar ozone uptake, suggesting that several genetic, physiological, and physiochemical factors control elicitation and emission of volatile compounds upon acute ozone stress in a complex manner. Further studies are required to understand the complex linkages between elicitation and emission due to genetic, physiological and physiochemical factors in multiple tobacco cultivars with varying ozone surface and stomatal deposition fluxes.
Supplementary Material
Changes in (A-D) leaf net assimilation rate (A), (E-H) stomatal conductance to water vapour (gs), and (I-L) intercellular CO2 concentration (Ci) of mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’ in relation to ozone concentration during exposure. The measurements were taken at 0.5, 3, 10, 24, and 48 h after ozone exposure at 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber 25 °C. All measurements were replicated at least thrice. Gas exchange measurements were conducted at leaf temperature of 25 °C, quantum flux density of 700 μmol m-2 s-1 at leaf surface, ambient CO2 concentration of 380-400 μmol mol-1, and relative humidity of 50–60%. Each data point corresponds to an individual replicate measurement. Data were fitted by Spearman correlations. Statistical significance of regressions is indicated as: * -P < 0.05, *** -P < 0.001.
Highlights.
-
➢
Ozone-driven isoprenoid emissions scaled with surface ozone uptake, indicating that isoprenoids were released from trichomes.
-
➢
Greater non-stomatal ozone uptake due to ozone detoxification on the leaf surface underlies the greater ozone tolerance.
Acknowledgments
This study has funded by grants from the European Commission through the European Research Council (advanced grant 322603, SIP-VOL+), and the European Regional Development Fund (Center of Excellence EcolChange) and the Estonian Ministry of Science and Education (institutional grant IUT-8-3).
Abbreviations
- BVOC
biogenic volatile organic compound
- GLM
generalized linear model
- GLV
green leaf volatiles
- LOX volatiles
lipoxygenase pathway volatiles
- MEP/DOXP pathway
2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway
- MVA pathway
mevalonate pathway
References
- Adir N, Zer H, Shochat S, Ohad I. Photoinhibition – a historical perspective. Photosynthesis Research. 2003;76:343–370. doi: 10.1023/A:1024969518145. [DOI] [PubMed] [Google Scholar]
- Akhtar N, Yamaguchi M, Inada H, Hoshino D, Kondo T, Fukami M, Funada R, Izuta T. Effects of ozone on growth, yield and leaf gas exchange rates of four Bangladeshi cultivars of rice (Oryza sativa L.) Environmental Pollution. 2010;158:2970–2976. doi: 10.1016/j.envpol.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Altimir N, Kolari P, Tuovinen JP, Vesala T, Bäck J, Suni T, Kulmala M, Hari P. Foliage surface ozone deposition: a role for surface moisture? Biogeosciences. 2006;3:209–228. [Google Scholar]
- Beauchamp J, Wisthaler A, Hansel A, Kleist E, Miebach M, Niinemets Ü, Schurr U, Wildt J. Ozone induced emissions of biogenic VOC from tobacco: relations between ozone uptake and emission of LOX products. Plant, Cell and Environment. 2005;28:1334–1343. [Google Scholar]
- Bouvier-Brown NC, Goldstein AH, Gilman JB, Kuster WC, de Gouw JA. In-situ ambient quantification of monoterpenes, sesquiterpenes, and related oxygenated compounds during BEARPEX 2007: implications for gas- and particle-phase chemistry. Atmospheric Chemistry and Physics. 2009;9:5505–5518. [Google Scholar]
- Calfapietra C, Pallozzi E, Lusini I, Velikova V. Modification of BVOC emissions by changes in atmospheric [CO2] and air pollution. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Berlin: Springer; 2013. pp. 253–284. [Google Scholar]
- Carriero G, Brunetti C, Fares S, Hayes F, Hoshika Y, Mills G, Tattini M, Paoletti E. BVOC responses to realistic nitrogen fertilization and ozone exposure in silver birch. Environmental Pollution. 2016;213:988–995. doi: 10.1016/j.envpol.2015.12.047. [DOI] [PubMed] [Google Scholar]
- Cheng J-F, Sun E-J. Factors affecting ozone sensitivity of tobacco Bel-W3 seedlings. Botanical Studies. 2013;54:1–7. doi: 10.1186/1999-3110-54-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Pazouki L, Niinemets Ü. Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cold and heat shock treatments. Journal of Plant Physiology. 2012;169:664–672. doi: 10.1016/j.jplph.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Kännaste A, Remmel T, Vislap V, Niinemets Ü. Volatile emissions from Alnus glutionosa induced by herbivory are quantitatively related to the extent of damage. Journal of Chemical Ecology. 2011;37:18–28. doi: 10.1007/s10886-010-9897-9. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Flooding induced emissions of volatile signalling compounds in three tree species with differing waterlogging tolerance. Plant, Cell and Environment. 2010;33:1582–1594. doi: 10.1111/j.1365-3040.2010.02166.x. [DOI] [PubMed] [Google Scholar]
- Copolovici L, Niinemets Ü. Temperature dependencies of Henry’s law constants for different plant sesquiterpenes. Chemosphere. 2015;138:751–757. doi: 10.1016/j.chemosphere.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici LO, Niinemets Ü. Temperature dependencies of Henry’s law constants and octanol/water partition coefficients for key plant volatile monoterpenoids. Chemosphere. 2005;61:1390–1400. doi: 10.1016/j.chemosphere.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Cui H, Zhang S-T, Yang H-J, Ji H, Wang X-J. Gene expression profile analysis of tobacco leaf trichomes. BMC Plant Biology. 2011;11:1–10. doi: 10.1186/1471-2229-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann MS, Pell EJ. Decline of activity and quantity of ribulose bisphosphate carboxylase/oxygenase and net photosynthesis in ozone-treated potato foliage. Plant Physiology. 1989;91:427–432. doi: 10.1104/pp.91.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degl'Innocenti E, Guidi L, Soldatini GF. Characterisation of the photosynthetic response of tobacco leaves to ozone: CO2 assimilation and chlorophyll fluorescence. Journal of Plant Physiology. 2002;159:845–853. [Google Scholar]
- Demmig-Adams B, Adams WW. Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation: Tansley review. New Phytologist. 2006;172:11–21. doi: 10.1111/j.1469-8137.2006.01835.x. [DOI] [PubMed] [Google Scholar]
- Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Morishima I, Shibahara T, Inanaga S, Tanaka K. Enhanced tolerance to ozone and drought stresses in transgenic tobacco overexpressing dehydroascorbate reductase in cytosol. Physiologia Plantarum. 2006;127:57–65. [Google Scholar]
- Fares S, Goldstein A, Loreto F. Determinants of ozone fluxes and metrics for ozone risk assessment in plants. J Exp Bot. 2010a;61:629–633. doi: 10.1093/jxb/erp336. [DOI] [PubMed] [Google Scholar]
- Fares S, Loreto F, Kleist E, Wildt J. Stomatal uptake and stomatal deposition of ozone in isoprene and monoterpene emitting plants. Plant Biology. 2008;10:44–54. doi: 10.1055/s-2007-965257. [DOI] [PubMed] [Google Scholar]
- Fares S, Park J-H, Ormeno E, Gentner DR, McKay M, Loreto F, Karlik J, Goldstein AH. Ozone uptake by citrus trees exposed to a range of ozone concentrations. Atmospheric Environment. 2010b;44:3404–3412. [Google Scholar]
- Feussner I, Wasternack C. The lipoxygenase pathway. Annual Review of Plant Biology. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- Fichtner K, Koch GW, Mooney HA. Photosynthesis, storage, and allocation. In: Schulze ED, Caldwell MM, editors. Ecophysiology of photosynthesis. Springer Verlag; Berlin: 1994. pp. 133–146. [Google Scholar]
- Fowler D, Pilegaard K, Sutton MA, Ambus P, Raivonen M, Duyzer J, Simpson D, Fagerli H, Fuzzi S, Schjoerring JK, Granier C, et al. Atmospheric composition change: ecosystems - atmosphere interactions. Atmospheric Environment. 2009;43:5193–5267. [Google Scholar]
- Frank G, Pressman E, Ophir R, Althan L, Shaked R, Freedman M, Shen S, Firon N. Transcriptional profiling of maturing tomato (Solanum lycopersicum L.) microspores reveals the involvement of heat shock proteins, ROS scavengers, hormones, and sugars in the heat stress response. Journal of Experimental Botany. 2009;60:3891–3908. doi: 10.1093/jxb/erp234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AH, McKay M, Kurpius MR, Schade GW, Lee A, Holzinger R, Rasmussen RA. Forest thinning experiment confirms ozone deposition to forest canopy is dominated by reaction with biogenic VOCs. Geophysical Research Letters. 2004;31:1–4. [Google Scholar]
- Guidi L, Degl’Innocenti E. Ozone effects on high light-induced photoinhibition in Phaseolus vulgaris. Plant Science. 2008;174:590–596. [Google Scholar]
- Guidi L, Nali C, Lorenzini G, Filippi F, Soldatini GF. Effect of chronic ozone fumigation on the photosynthetic process of poplar clones showing different sensitivity. Environmental Pollution. 2001;113:245–254. doi: 10.1016/s0269-7491(00)00194-9. [DOI] [PubMed] [Google Scholar]
- Heiden AC, Hoffmann T, Kahl J, Kley D, Klockow D, Langebartels C, Mehlhorn H, Sandermann H, Schraudner M, Schuh G, Wildt J. Emission of volatile organic compounds from ozone-exposed plants. Journal of Applied Ecology. 1999;9:1160–1167. [Google Scholar]
- Heiden AC, Kobel K, Langebartels C, Schuh-Thomas G, Wildt J. Emissions of oxygenated volatile organic compounds from plants. Part I: Emissions from lipoxygenase activity. Journal of Atmospheric Chemistry. 2003;45:143–172. [Google Scholar]
- Heidenreich B, Bieber E, Sandermann H, Ernst D. Identification of a new member of the WRKY family in tobacco. Involved in ozone-induced gene regulation? Acta Physiologiae Plantarum. 2006;28:117–125. [Google Scholar]
- Ivanov AV, Trakhtenberg S, Bertram AK, Gershenzon YM, Molina MJ. OH, HO2, and ozone gaseous diffusion coefficients. Journal of Physical Chemistry A. 2007;111:1632–1637. doi: 10.1021/jp066558w. [DOI] [PubMed] [Google Scholar]
- Janzik I, Preiskowski S, Kneifel H. Ozone has dramatic effects on the regulation of the prechorismate pathway in tobacco (Nicotiana tabacum L. cv. Bel W3) Planta. 2005;223:20–27. doi: 10.1007/s00425-005-0060-8. [DOI] [PubMed] [Google Scholar]
- Jardine KJ, Monson RK, Abrell L, Saleska SR, Arneth A, Jardine A, Ishida FY, Maria A, Serrano Y, Artaxo P, Karl T, et al. Within-plant isoprene oxidation confirmed by direct emissions of oxidation products methyl vinyl ketone and methacrolein. Global Change Biology. 2012;18:973–984. [Google Scholar]
- Jiang Y, Ye J, Li S, Niinemets Ü. Dose-dependent biphasic emissions of methyl jasmonate-induced biogenic volatiles in cucumber (Cucumis sativus) Journal of Experimental Botany. 2017;68:4679–4694. doi: 10.1093/jxb/erx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YF, Ye JY, Veromann LL, Niinemets Ü. Scaling of photosynthesis and constitutive and induced volatile emissions with severity of leaf infection by rust fungus (Melampsora larici-populina) in Populus balsamifera var. suaveolens. Tree Physiology. 2016;36:856–872. doi: 10.1093/treephys/tpw035. [DOI] [PubMed] [Google Scholar]
- Jud W, Fischer L, Canaval E, Wohlfahrt G, Tissier A, Hansel A. Plant surface reactions: an opportunistic ozone defence mechanism impacting atmospheric chemistry. Atmospheric Chemistry and Physics. 2016;16:277–292. [Google Scholar]
- Kanagendran A, Pazouki L, Niinemets Ü. Differential regulation of volatile emission from Eucalyptus globulus leaves upon single and combined ozone and wounding treatments through recovery and relationships with ozone uptake. Environmental and Experimental Botany. 2018;145:21–38. doi: 10.1016/j.envexpbot.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kännaste A, Copolovici L, Niinemets Ü. Gas chromatography–mass spectrometry method for determination of biogenic volatile organic compounds emitted by plants. In: Rodríguez-Concepción M, editor. Plant Isoprenoids: Methods and Protocols. New York, NY: Springer New York; 2014. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- Kännaste A, Copolovici L, Pazouki L, Suhhorutšenko M, Niinemets Ü. Highly variable chemical signatures over short spatial distances among Scots pine (Pinus sylvestris) populations. Tree Physiology. 2013;33:374–387. doi: 10.1093/treephys/tpt013. [DOI] [PubMed] [Google Scholar]
- Karnosky DF, Pregitzer KS, Zak DR, Kubiske ME, Hendrey GR, Weinstein D, Nosal M, Percy KE. Scaling ozone responses of forest trees to the ecosystem level in a changing climate. Plant, Cell and Environment. 2005;28:965–981. [Google Scholar]
- Kask K, Kännaste A, Talts E, Copolovici L, Niinemets Ü. How specialized volatiles respond to chronic and short-term physiological and shock heat stress in Brassica nigra. Plant, Cell and Environment. 2016;39:2027–2042. doi: 10.1111/pce.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstiens G, Lendzian KJ. Interactions between ozone and plant cuticles. I. ozone deposition and permeability. New Phytologist. 1989;112:13–19. [Google Scholar]
- Krupa S, McGrath MT, Andersen CP, Booker FL, Burkey KO, Chappelka AH, Chevone BI, Pell EJ, Zilinskas BA. Ambient Ozone and Plant Health. Plant Disease. 2001;85:4–12. doi: 10.1094/PDIS.2001.85.1.4. [DOI] [PubMed] [Google Scholar]
- Kurpius MR, Goldstein AH. Gas-phase chemistry dominates O3 loss to a forest, implying a source of aerosols and hydroxyl radicals to the atmosphere. Geophysical Research Letters. 2003;30:1–4. [Google Scholar]
- Laisk A, Kull O, Moldau H. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiology. 1989;90:1163–1167. doi: 10.1104/pp.90.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Harley PC, Niinemets Ü. Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant, Cell and Environment. 2017;40:1984–2003. doi: 10.1111/pce.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Fares S. Is ozone flux inside leaves only a damage indicator? clues from volatile isoprenoid studies. Plant Physiology. 2007;143:1096–1100. doi: 10.1104/pp.106.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung I, Soran ML, Opris O, Trusca MRC, Niinemets Ü, Copolovici L. Induction of stress volatiles and changes in essential oil content and composition upon microwave exposure in the aromatic plant Ocimum basilicum. Science of the Total Environment. 2016;569:489–495. doi: 10.1016/j.scitotenv.2016.06.147. [DOI] [PubMed] [Google Scholar]
- Mancini A, Buschini A, Restivo FM, Rossi C, Poli P. Oxidative stress as DNA damage in different transgenic tobacco plants. Plant Science. 2006;170:845–852. [Google Scholar]
- Moldau H, Bichele I. Plasmalemma protection by the apoplast as assessed from above-zero ozone concentrations in leaf intercellular air spaces. Planta. 2002;214:484–487. doi: 10.1007/s00425-001-0678-0. [DOI] [PubMed] [Google Scholar]
- Navia-Giné WG, Yuan JS, Mauromoustakos A, Murphy JB, Chen F, Korth KL. Medicago truncatula (E)-β-ocimene synthase is induced by insect herbivory with corresponding increases in emission of volatile ocimene. Plant Physiology and Biochemistry. 2009;47:416–425. doi: 10.1016/j.plaphy.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Copolovici L, Hüve K. High within-canopy variation in isoprene emission potentials in temperate trees: Implications for predicting canopy-scale isoprene fluxes. Biogeosciences. 2010;115 [Google Scholar]
- Niinemets Ü, Hauff K, Bertin N, Tenhunen JD, Steinbrecher R, Seufert G. Monoterpene emissions in relation to foliar photosynthetic and structural variables in Mediterranean evergreen Quercus species. New Phytologist. 2002;153:243–256. [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther AB, Kesselmeier J, et al. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences. 2011;8:2209–2246. [Google Scholar]
- Niinemets Ü, Reichstein M. Controls on the emission of plant volatiles through stomata: sensitivity or insensitivity of the emission rates to stomatal closure explained. Journal of Geophysical Research-Atmospheres. 2003;108:4208. doi:4210.1029/2002JD002620. [Google Scholar]
- Ohara K, Ujihara T, Endo T, Sato F, Yazaki K. Limonene production in tobacco with Perilla limonene synthase cDNA. Journal of Experimental Botany. 2003;54:2635–2642. doi: 10.1093/jxb/erg300. [DOI] [PubMed] [Google Scholar]
- Osmond CB, Anderson JM, Ball MC, Egerton JG. Compromising efficiency: the molecular ecology of light-resource utilization in plants. In: Press MC, Scholes JD, Barker MG, editors. Physiological plant ecology; The 39th Symposium of the British Ecological Society held at the University of York; 7-9 September 1998; Oxford: Blackwell Science; 1999. pp. 1–24. [Google Scholar]
- Paoletti E. Impact of ozone on Mediterranean forests: A review. Environmental Pollution. 2006;144:463–474. doi: 10.1016/j.envpol.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Pasqualini S, Meier S, Gehring C, Madeo L, Fornaciari M, Romano B, Ederli L. Ozone and nitric oxide induce cGMP-dependent and -independent transcription of defence genes in tobacco. New Phytologist. 2009;181:860–870. doi: 10.1111/j.1469-8137.2008.02711.x. [DOI] [PubMed] [Google Scholar]
- Pateraki I, Kanellis AK. Stress and developmental responses of terpenoid biosynthetic genes in Cistus creticus subsp. creticus. Plant Cell Reports. 2010;29:629–641. doi: 10.1007/s00299-010-0849-1. [DOI] [PubMed] [Google Scholar]
- Pazouki L, Kanagendran A, Li S, Kännaste A, Rajabi Memari H, Bichele R, Niinemets Ü. Mono- and sesquiterpene release from tomato (Solanum lycopersicum) leaves upon mild and severe heat stress and through recovery: From gene expression to emission responses. Environmental and Experimental Botany. 2016;132:1–15. doi: 10.1016/j.envexpbot.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazouki L, Niinemets U. Multi-substrate terpene synthases: Their occurrence and physiological significance. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.01019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto DM, Nerg AM, Holopainen JK. The role of ozone-reactive compounds, terpenes, and green leaf volatiles (GLVs), in the orientation of Cotesia plutellae. Journal of Chemical Ecology. 2007;33:2218–2228. doi: 10.1007/s10886-007-9376-0. [DOI] [PubMed] [Google Scholar]
- Rai R, Agrawal M. Assessment of competitive ability of two Indian wheat cultivars under ambient O3 at different developmental stages. Environmental Science and Pollution Research. 2014;21:1039–1053. doi: 10.1007/s11356-013-1981-6. [DOI] [PubMed] [Google Scholar]
- Rajabi Memari H, Pazouki L, Niinemets Ü. The biochemistry and molecular biology of volatile messengers in trees. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Berlin: Springer; 2013. pp. 47–93. [Google Scholar]
- Rasulov B, Copolovici L, Laisk A, Niinemets Ü. Postillumination isoprene emission: In vivo measurements of dimethylallyldiphosphate pool size and isoprene synthase kinetics in aspen leaves. Plant Physiology. 2009;149:1609–1618. doi: 10.1104/pp.108.133512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz M, Schnitzler J-P. Genetic engineering of BVOC emissions from trees. In: Niinemets Ü, Monson RK, editors. Biology, controls and models of tree volatile organic compound emissions. Berlin: Springer; 2013. pp. 95–118. [Google Scholar]
- Samuel MA, Walia A, Mansfield SD, Ellis BE. Overexpression of SIPK in tobacco enhances ozone-induced ethylene formation and blocks ozone-induced SA accumulation. Journal of Experimental Botany. 2005;56:2195–2201. doi: 10.1093/jxb/eri219. [DOI] [PubMed] [Google Scholar]
- Schrauder M, Moeder W, Wiese C, Van Camp W, Inzé D, Langebartels C, Sandermann H., Jr Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant Journal. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Schwanz P, Häberle K-H, Polle A. Interactive effects of elevated CO2, ozone and drought stress on the activities of antioxidative enzymes in needles of Norway spruce trees (Picea abies [L.] Karsten) grown with luxurious N-supply. Journal of Plant Physiology. 1996;148:351–355. [Google Scholar]
- Senser M, Höpker K-A, Peuker A, Glashagen B. Wirkungen extremer Ozonkonzentrationen auf Koniferen. (Effect of extreme ozone concentrations on conifers) Allgemeine Forst Zeitschrift. 1987:709–714. 27/28/29. [Google Scholar]
- Sharma P, Kuniyal JC, Chand K, Guleria RP, Dhyani PP, Chauhan C. Surface ozone concentration and its behaviour with aerosols in the northwestern Himalaya, India. Atmospheric Environment. 2013;71:44–53. [Google Scholar]
- Sicard P, Anav A, De Marco A, Paoletti E. Projected global tropospheric ozone impacts on vegetation under different emission and climate scenarios. Atmospheric Chemistry and Physics Discussion. 2017;2017:1–34. [Google Scholar]
- Silva DT, Meirelles ST, Moraes RM. Relationship between ozone, meteorological conditions, gas exchange and leaf injury in Nicotiana tabacum Bel-W3 in a sub-tropical region. Atmospheric Environment. 2012;60:211–216. [Google Scholar]
- Singh E, Tiwari S, Agrawal M. Effects of elevated ozone on photosynthesis and stomatal conductance of two soybean varieties: a case study to assess impacts of one component of predicted global climate change. Plant Biology. 2009;11:101–108. doi: 10.1111/j.1438-8677.2009.00263.x. [DOI] [PubMed] [Google Scholar]
- Singh M, Johnson-Flanagan A. Co-ordination of photosynthetic gene expression during low-temperature acclimation and development in Brassica napus cv. Jet Neuf leaves. Plant Science. 1998;135:171–181. [Google Scholar]
- Slimestad R, Gjelsvik S, Gragl-Nielsen O. Detection of effects of ozone on birch, Betula pendula Roth., by chemomerical evaluation of concentrations of lipid components in leaves. Analytica Chimica Acta. 1995;304:209–216. [Google Scholar]
- Toome M, Randjärv P, Copolovici L, Niinemets Ü, Heinsoo K, Luik A, Noe SM. Leaf rust induced volatile organic compounds signalling in willow during the infection. Planta. 2010;232:235–243. doi: 10.1007/s00425-010-1169-y. [DOI] [PubMed] [Google Scholar]
- Tucker WA, Nelken LH. Diffusion coefficients in air and water. In: Lyman WJ, R WF, R DH, editors. Handbook of chemical property estimation methods: Environmental Behaviour of Organic Compounds. McGraw-Hill; New York, NY, USA: 1982. pp. 17/11–17/25. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Wildt J, Kobel K, Schuh-Thomas G, Heiden AC. Emissions of oxygenated volatile organic compounds from plants - part II: Emissions of saturated aldehydes. Journal of Atmospheric Chemistry. 2003;45:173–196. [Google Scholar]
- Winner WE. Mechanistic analysis of plant responses to air pollution. Ecological Applications. 1994;4:651–661. [Google Scholar]
- Yuan XY, Calatayud V, Jiang LJ, Manning WJ, Hayes F, Tian Y, Feng ZZ. Assessing the effects of ambient ozone in China on snap bean genotypes by using ethylenediurea (EDU) Environmental Pollution. 2015;205:199–208. doi: 10.1016/j.envpol.2015.05.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes in (A-D) leaf net assimilation rate (A), (E-H) stomatal conductance to water vapour (gs), and (I-L) intercellular CO2 concentration (Ci) of mature leaves of 10-12 weeks old N. tabacum ‘Wisconsin’ in relation to ozone concentration during exposure. The measurements were taken at 0.5, 3, 10, 24, and 48 h after ozone exposure at 0, 400, 600, 800, and 1000 ppb for 30 min in a custom-made cylindrical double-walled glass chamber 25 °C. All measurements were replicated at least thrice. Gas exchange measurements were conducted at leaf temperature of 25 °C, quantum flux density of 700 μmol m-2 s-1 at leaf surface, ambient CO2 concentration of 380-400 μmol mol-1, and relative humidity of 50–60%. Each data point corresponds to an individual replicate measurement. Data were fitted by Spearman correlations. Statistical significance of regressions is indicated as: * -P < 0.05, *** -P < 0.001.