Abstract
Antibiotic-associated diarrhoea is amongst the most common adverse events related to antibiotic use. Most cases are mild, but Clostridium difficile infection causes a spectrum of disease ranging from occasional diarrhoea to colitis, toxic megacolon, and potentially death. Recent developments in our understanding of the biology of the gut microbiota have given new insights into the pathogenesis of these conditions, as well as revealing a role for manipulation of the gut microbiota as a novel therapeutic approach. This CME review will give an overview of assessment of these conditions, before particularly focusing on the rapidly-developing area of their treatment.
Keywords: Clostridium difficile, antibiotic, diarrhoea, probiotic, microbiota, faecal microbiota transplantation
Introduction
As well as being a potential reservoir for pathogenic bacteria, the gut also has an extensive ecosystem (~1011 bacteria per gram of intestinal content) of microorganisms with no overt pathogenicity. It is now appreciated that this ecosystem (often called the ‘gut microbiota’) performs roles essential for the maintenance of host health, including short chain fatty acid and bile acid metabolism1. Antibiotic-associated diarrhoea (AAD) is now understood to represent an imbalance of the gut microbiota resulting from antibiotic use, with several mechanisms appearing to contribute to the disease process.
Most cases of AAD are mild and self-limiting, and are associated with negative stool culture results. Given the widespread use of antibiotics, it is unsurprising that this condition is so common, affecting 5-39% of people treated with antibiotics2. One key mechanism of AAD appears to be changes in the gut microbiota causing decreased short chain fatty acid absorption, resulting in osmotic diarrhoea3.
However, one particular form of AAD – Clostridium difficile infection (CDI) – may cause more severe gastrointestinal disease. Clostridium difficile (now also referred to as Clostridioides difficile) is a Gram positive, spore-forming anaerobic bacillus. Spores of C. difficile can survive for long periods on inanimate objects (resisting heat, acid and antibiotics) - a major reason why this bacterium can cause such problems within healthcare settings. C. difficile is spread via the faeco-oral route, and causes disease in humans through the production of two protein exotoxins (toxin A and toxin B) which are cytotoxic to colonic epithelial cells4. The host’s adaptive immune response following exposure to C. difficile also appears important, with high IgG antitoxin level production being protective4. Antibiotic use is the major risk factor for CDI, causing antibiotic-related loss of gut microbial communities that protect against gut infection, facilitating germination and vegetative growth of the organism when it enters the gut of vulnerable people. Risk factors for CDI are summarised in Table 1. The range of clinical disease that may occur in CDI is wide; whilst diarrhoea and fever occur in almost all cases, the most severe cases are characterised by colitis, toxic megacolon (dilatation of the colon, with the risk of perforation), multi-organ failure, or even death.
Table 1. Risk factors for Clostridium difficile infection.
| Risk factor | Details |
|---|---|
| Antibiotics | Almost all antibiotics may increase vulnerability to CDI, but cephalosporins, fluoroquinolones, clindamycin and certain penicillins (e.g. co-amoxiclav) increase risk to the greatest extent. |
| Acid-suppressant medications | Both PPI and H2-receptor antagonists appear to increase risk (risk particularly increased with PPI). |
| Age | Rates tenfold higher in those >65 years than those younger. |
| Hospitalisation |
|
| Immunosuppression |
Rates of CDI (and associated morbidity/ mortality) peaked in the UK approximately 10 years ago (with ~70000 cases per year - ~4000 of which were fatal - attributed to CDI in 20075). Rates have since rapidly decreased. There are currently ~12000 cases per year in the UK5, with the sharp recent decline in incidence in the UK attributed to infection prevention and control interventions, including antibiotic stewardship6.
Diagnosis
Typical AAD is a clinical diagnosis, based on the relationship between recent antibiotic use and the development of diarrhoea, and the exclusion of potential alternative causes of diarrhoea.
Diagnosis of CDI may be difficult for several reasons. Firstly, the currently available tests lack ideal sensitivity and specificity. Secondly, C. difficile may either cause asymptomatic colonisation or active gastrointestinal infection, and conventional tests do not clearly differentiate between these two states. As such, guidelines recommend only testing for CDI where patients have diarrhoea and grounds for suspecting an infective aetiology7. Tests for CDI are summarised in Table 2.
Table 2. Tests available for Clostridium difficile infection.
Given the variability of sensitivity and specificity of these tests, a two-stage testing algorithm is widely-applied. In the UK, toxin A/B EIA testing is combined with GDH or PCR testing as the initial screen7.
| Test | Details |
|---|---|
| Glutamate dehydrogenase (GDH) enzyme immunoassay (EIA) | GDH is a protein present in all isolates of C. difficile. This test is sensitive, quick and relatively cheap, but lacks high specificity for toxigenic forms of C. difficile. It is therefore often used as an initial screening test. |
| Toxin A and B EIA | This test has high specificity but variable degrees of sensitivity. |
| Toxin A and B polymerase chain reaction (PCR) | Whilst the PCR test is specific for genes encoding toxins produced by C. difficile, a positive result does not help distinguish active toxin production from asymptomatic carriage of toxigenic C. difficile. |
| Cell culture cytotoxicity assay | This is still widely-recognised as the reference standard test, although is rarely performed in most clinical laboratories. |
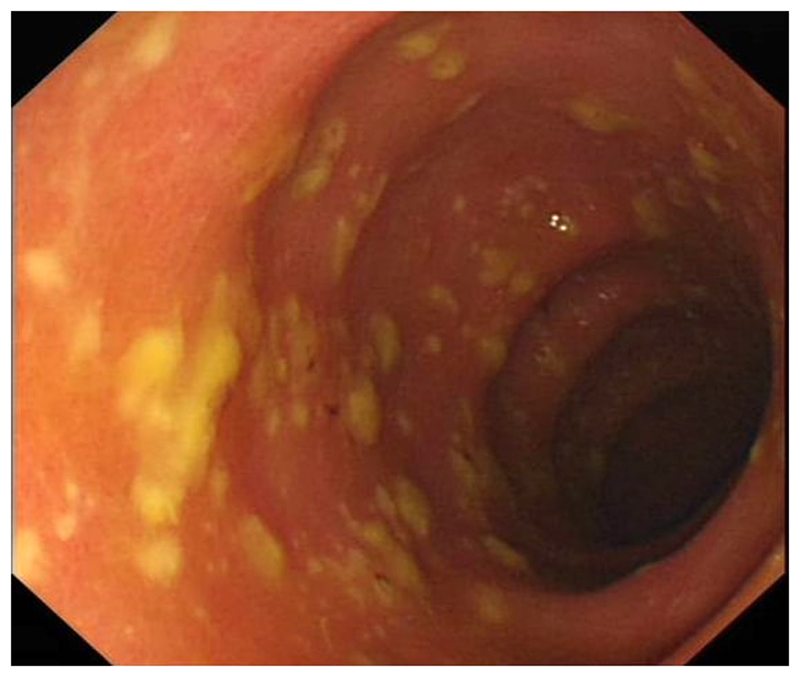
Several other investigations may also be useful in the assessment of people with CDI. Leucocytosis (particularly neutrophilia) is common, and may be very marked. Abdominal radiograph is the preferred test for assessing for toxic megacolon, whilst abdominal CT is sensitive for the presence of CDI-related colitis. Lower gastrointestinal endoscopy may show only oedema or erythema, or may show the classical finding of pseudomembranes, i.e. raised yellowish plaques that may be intermittently scattered throughout affected colonic mucosa (Figure 1).
Figure a. Pseudomembranous colitis.
Where clinicians have a strong clinical suspicion of CDI, empirical therapy should be considered regardless of the laboratory result; this is because with a high pre-test probability, the negative predictive value of the tests currently available is insufficiently high to exclude disease.
Treatment
In all cases of AAD, initial care includes removal of precipitating factors (i.e. cessation of antibiotics). Whilst this will resolve the majority of cases of typical AAD (and some cases of CDI), most cases of CDI will require more specific intervention. In treating CDI, a severity assessment is important for establishing the most appropriate treatment. The version described in the current Public Health England (PHE) guidelines8 is that most commonly used, i.e.:
White cell count > 15 x 109/l.
Acutely rising creatinine (e.g. >50% increase above baseline).
Temperature > 38.5°C.
Severe colitis (either from clinical abdominal signs or from radiological investigations).
Conventional treatment of CDI
Until recently, the antibiotics metronidazole and vancomycin were the only pharmacological options for the treatment of CDI. Metronidazole may be administered orally or intravenously, whilst vancomycin may be given orally or per rectum for this indication. Vancomycin is not used intravenously to treat CDI, since it has very limited penetration into the gut mucosa. Intravenous immunoglobulin (IVIg) may also have a role in treating severe disease. Until recently, these treatments were effective for most cases of CDI, with surgical intervention (colectomy) reserved for the small number of severe cases refractory to medical therapy8.
However, over the past decade, CDI has become more difficult to treat with conventional therapies for several reasons. Firstly, there has been the emergence of increasing rates of CDI treatment failure with metronidazole, with rates of non-response now quoted at >20%9; this is one of the reasons why patients with CDI and markers of severity should be treated with vancomycin in preference to metronidazole8. Secondly, rates of recurrent CDI (i.e. return of disease within a short period after stopping antibiotic therapy) are also increasing9. Thirdly, there has been the emergence of hypervirulent strains of C. difficile – particularly the NAP1/027 strain, associated with secretion of large quantities of toxin and poor response to conventional antibiotics10. As such, novel approaches for therapy for CDI have been urgently sought.
New antibiotics: Fidaxomicin
Fidaxomicin is a novel macrocyclic antibiotic, administered orally, with a narrow spectrum of activity. Although fidaxomicin has been shown in clinical trials to have efficacy in treating recurrent CDI11 – and has consequently been adapted into clinical guidelines8 - there are still a number of concerns regarding its use. These include its apparently limited efficacy against the NAP1/027 strain, the lack of evidence regarding its use in CDI with severe colitis, and its considerable expense (600 times more expensive than a course of metronidazole).
Manipulation of the gut microbiota: probiotics and faecal microbiota transplantation
Given that all forms of AAD represent a perturbation of the gut microbiota, restoration of the usual commensal microbiota is an appealing means of treating the condition.
One approach to achieve this is through the use of probiotics, i.e. the delivery of live microorganisms (such as via a suspension/ drink) into the host. There are limited data regarding the use of probiotics to treat AAD/ CDI, with relevant studies suggesting a possible modest benefit in treating non-severe CDI. However, significant uncertainties still exist about the specific organisms to include in the formulation, the dose to be used etc., and probiotics are therefore not recommended at present.
Another approach has been the use of faecal microbiota transplantation (FMT), i.e. taking a stool sample from a healthy donor (with presumed normal gut microbiota), processing this in a laboratory into a liquidised bacterial suspension, and delivering it into the gut of affected people. Whilst a number of case reports/ small case series have described this treatment for over 60 years, the first major randomised trial investigating its efficacy was in 201312. In this study, patients with recurrent CDI were randomised to receive either bowel lavage followed by FMT (via nasoduodenal tube), vancomycin alone, or vancomycin with bowel lavage. The study was stopped early at a planned interim analysis on ethical grounds, as 81% of patients (n=13/16) had recovered in the FMT arm, compared to 31% (n=4/13) receiving vancomycin alone, and 23% (n=3/13) receiving vancomycin and bowel lavage. Two of the three patients not responding to an initial FMT were successfully treated with a second. There were no major adverse effects. A subsequent randomised trial demonstrated that FMT was superior to vancomycin in treating recurrent CDI when administered colonoscopically13.
These and other studies have helped establish optimal practical aspects related to FMT administration (Table 3). FMT appears generally safe with mild, self-limiting, gastrointestinal and constitutional symptoms being the most common complications described. However, there have been a small number of potential transmissions of infection, perforations associated with its administration, and even deaths related to its use14. As a result of these clinical studies (coupled with other studies demonstrating its cost effectiveness), FMT has now been accepted as an approved treatment for recurrent/ refractory CDI by NICE15 and in PHE guidelines8. Whilst FMT is now available as treatment for CDI within a growing number of UK centres, the expense of establishing a service, and ongoing discussions about the regulation and governance of FMT within the UK, have impacted upon more rapid adoption16.
Table 3. Practical aspects of FMT administration. (Adapted from 20,21).
| Criteria | Details | ||
|---|---|---|---|
| Donor selection | Screening questionnaire | People > 60 years of age, those with recent antibiotic use (within six weeks), those with significant risk factors for blood-borne viruses, recent constitutional illness, and/ or recent travel to countries with high rates of infectious diarrhoea are typically excluded from further assessment. | |
| Medical questionnaire | People with personal/ family history of disorders associated with perturbation of the gut microbiota (including inflammatory bowel disease, irritable bowel syndrome, metabolic syndrome, autoimmune disorders and/ or gastrointestinal malignancy) are typically excluded from further assessment. | ||
| Laboratory testing, repeated at regular intervals, typically includes: | Blood screening |
|
|
| Stool screening |
|
||
| Choice of recipients | Indications |
|
|
| Contraindications |
|
||
| Transplant preparation |
|
||
| Transplant delivery |
|
||
Given the obvious potential drawbacks of FMT (including its unpalatable nature and the invasive means of administration), there is great interest in refining FMT from its current state to a more acceptable pill or drink. ‘Capsulised’ FMT is now starting to become available (in which faecal slurry or freeze-dried stool is placed into capsules), and has recently been demonstrated to be of similar efficacy to colonoscopic FMT in a large randomised trial17.
Other novel approaches
A number of novel approaches for the treatment of CDI are at various stages of development; these are summarised in Table 4.
Table 4. Novel approaches for the treatment of CDI.
| Approach | Details | |
|---|---|---|
| Antimicrobials | Antibiotics | A number of novel antibiotics are in clinical trials. |
| Ribaxamase | This is a beta-lactamase that may be co-administered with systemic broad-spectrum antibiotics, with the aim of degrading the antibiotics in the gut before they can affect the gut microbiota and create a propensity to CDI. | |
| Immunisation | Passive immunisation | Co-administration of bezlotoxumab (an anti-toxin B antibody) together with conventional antibiotic therapy has been shown to reduce the rate of recurrence of CDI compared to antibiotic therapy alone22. |
| Active immunisation | Anti-toxin vaccines are now in clinical trials. However, early data suggest reduced seroconversion in older people, those most at risk of CDI. | |
| Microbiome manipulation | Non-toxigenic C. difficile23 |
|
Conclusion
AAD/ CDI remains a common problem. Whilst many cases are mild, a significant number – particularly of CDI – can be difficult to treat, and potentially life-threatening. Developments in our understanding of the importance of the gut microbiota, and the marked efficacy of FMT in clinical trials, have raised the profile of CDI research and the search for more refined, targeted therapies.
Key points.
Antibiotic use may result in diarrhoea through different mechanisms, most importantly: i. osmotic diarrhoea (through the loss of gut bacteria that absorb short chain fatty acids), and ii. colonisation and overgrowth of toxin-secreting Clostridium difficile.
Since typical laboratory tests for the presence of C. difficile do not have high sensitivity and specificity, a combination of tests should be used when making the diagnosis of CDI.
CDI has recently become harder to treat with conventional antibiotics for several reasons, including increased treatment failure with metronidazole, rising rates of CDI recurrence, and hypervirulent strains of C. difficile (e.g. NAP1/027).
Fidaxomicin is a new antibiotic that has considerable efficacy in treating recurrent CDI, though there is uncertainty as to its use in particular situations, including treating the NAP1/027 strain.
Randomised clinical trial evidence demonstrates that faecal microbiota transplant (FMT) is more effective than vancomycin for the treatment of recurrent/ refractory CDI, and this treatment is now approved in guidelines.
SAQs.
-
Which of the following is most consistent with a case of severe Clostridium difficile infection?
Neutrophil count of 16 x 109/l (1.5-7.0).
Platelet count of 548 x 109/l (150-450).
Serum alanine aminotransferase of 74 U/l (5-35).
Serum potassium of 2.8 mmol/l (3.5-4.9).
Temperature of 37.9°C.
Answer: ‘a’.
A variety of scoring systems to evaluate the severity of CDI are described. The most commonly used in the UK is the current PHE guideline 8:- White cell count > 15 x 109/l.
- Acutely rising creatinine (e.g. >50% increase above baseline).
- Temperature > 38.5°C.
- Severe colitis (either from clinical abdominal signs or from radiological investigations).
This patient’s neutrophil count of 16 x 109/l means that ‘a’ is the correct answer. None of the other factors have consistently been identified as markers of severity of CDI. Vancomycin should be used in preference to metronidazole in patients with severe CDI, because of the relatively high rates of metronidazole failure, and slower clinical response to metronidazole compared with oral vancomycin8.
-
A patient with recurrent Clostridium difficile infection requires antibiotic therapy. Which of the following is the most appropriate choice?
- Amikacin
- Cefalexin
- Clindamycin
- Fidaxomicin
- Levofloxacin
Answer: ‘d’.
Fidaxomicin is a novel macrocyclic antibiotic that has been shown in trials to have efficacy in treating CDI. The other antibiotics listed are associated with an increased risk of CDI, and would not be an appropriate treatment.
-
Which of the following would be the strongest contraindication to faecal microbiota transplantation (FMT) for a patient with recurrent Clostridium difficile infection?
- Current use of infliximab for ulcerative colitis
- Faecal incontinence
- HIV infection with CD4 count of 212 cell/mm3
- Life-threatening nut allergy
- Renal transplant one year earlier
Answer: ‘d’.
Faecal incontinence is not a contraindication to FMT. Experience of the use of FMT in treating recurrent FMT in those with immunosuppression is more limited than in immunocompetent patients, but it still appears relatively safe and effective 18. Given that it is impossible to fully control the donor’s diet, life-threatening nut allergy would be a contraindication to FMT.
Footnotes
Author contribution: BHM wrote the text, and HRTW reviewed and edited it.
Conflict of interests: The authors have no conflicts of interest to declare.
References
- 1.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 16(5):292–307. doi: 10.1159/000016879. doi:16879. [DOI] [PubMed] [Google Scholar]
- 3.Högenauer C, Hammer HF, Krejs GJ, Reisinger EC. Mechanisms and management of antibiotic-associated diarrhea. [Accessed March 20, 2017];Clin Infect Dis. 1998 27(4):702–710. doi: 10.1086/514958. [DOI] [PubMed] [Google Scholar]
- 4.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7(7):526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 5.About Public Health England. [Accessed March 20, 2017]; www.gov.uk/phe.
- 6.King A, Mullish BH, Williams HRT, Aylin P. Comparative epidemiology of Clostridium difficile infection: England and the USA. Int J Qual Heal Care. 2017;29(6) doi: 10.1093/intqhc/mzx120. [DOI] [PubMed] [Google Scholar]
- 7.UPDATED GUIDANCE ON THE DIAGNOSIS AND REPORTING OF CLOSTRIDIUM DIFFICILE. [Accessed March 20, 2017]; https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215135/dh_133016.pdf.
- 8.Health England P. Updated guidance on the management and treatment of Clostridium difficile infection. [Accessed March 20, 2017];2013 http://www.gov.uk/phe.
- 9.Kelly CP, LaMont JT. Clostridium difficile — More Difficult Than Ever. N Engl J Med. 2008;359(18):1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 10.Martin JSH, Monaghan TM, Wilcox MH. Clostridium difficile infection: epidemiology, diagnosis and understanding transmission. Nat Rev Gastroenterol Hepatol. 2016;13(4):206–216. doi: 10.1038/nrgastro.2016.25. [DOI] [PubMed] [Google Scholar]
- 11.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus Vancomycin for Clostridium difficile Infection. N Engl J Med. 2011;364(5):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 12.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N Engl J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 13.Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–843. doi: 10.1111/apt.13144. [DOI] [PubMed] [Google Scholar]
- 14.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117–127. doi: 10.1016/j.jhin.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 15.Faecal microbiota tr Faecal microbiota transplant for recurrent ansplant for recurrent Clostridium difficile infection Clostridium difficile infection Interventional procedure guidance. [Accessed March 20, 2017];2014 https://www.nice.org.uk/guidance/ipg485/resources/faecal-microbiota-transplant-for-recurrent-clostridium-difficile-infection-1899869993554885.
- 16.Mullish BH, Williams HRT. Obstacles to establishing an NHS faecal transplant programme. [Accessed March 20, 2017];BMJ. 2015 351:h6043. doi: 10.1136/bmj.h6043. [DOI] [PubMed] [Google Scholar]
- 17.Kao D, Roach B, Silva M, et al. Effect of Oral Capsule– vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection. JAMA. 2017;318(20):1985. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized Frozen Preparation for Transplantation of Fecal Microbiota for Recurrent Clostridium difficile Infection. Am J Gastroenterol. 2012;107(5):761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 20.Mullish BH, Marchesi JR, Thursz MR, Williams HRT. Microbiome manipulation with faecal microbiome transplantation as a therapeutic strategy in Clostridium difficile infection. QJM. 2015;108(5) doi: 10.1093/qjmed/hcu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 2017;376(4):305–317. doi: 10.1056/NEJMoa1602615. [DOI] [PubMed] [Google Scholar]
- 23.Gerding DN, Meyer T, Lee C, et al. Administration of Spores of Nontoxigenic Clostridium difficile Strain M3 for Prevention of Recurrent C difficile Infection. JAMA. 2015;313(17):1719. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]