Abstract
The 3D culture of human mesenchymal stem cells (hMSCs) represents a more physiological environment than classical 2D culture and has been used to enhance the MSC secretome or extend cell survival after transplantation. Here we describe a simple and affordable method to generate 3D spheroids of hMSCs by seeding them at high density in a low-binding 96-well plate.
Spheroids of hMSCs cultured in low-binding 96-well plates can be used to study the basic biology of the cells and to generate conditioned media or spheroids to be used in transplantation therapeutic approaches. These MSCs or their secretome can be used as a regenerative therapy and for tissue repair across multiple disease areas, including neurodegeneration.
In comparison to other methods (hanging drop, use of gels or biomaterials, magnetic levitation, etc.), the method described here is simple and affordable with no need to use specialized equipment, expensive materials or complex reagents.
Keywords: Low-binding plate, Spheroid, 3D culture, Human mesenchymal stem cells, High density
Background
Mesenchymal stem cells (MSCs) are an attractive candidate for the development of novel regenerative therapies for diseases such as stroke or amyotrophic lateral sclerosis (Chen et al., 2001; Bang et al., 2005; Boido et al., 2014). Their versatility makes the optimization and standardization of techniques essential to ensure MSC therapies can provide as much benefit as possible. One possible way to maximize the therapeutic potential (e.g., enhanced secretion of anti-inflammatory mediators) of MSCs is to culture them in 3D (Bartosh et al., 2010). Cells do not normally grow in monolayers in physiological conditions, therefore culturing them in 3D provides a more realistic environment, and increases secretion of certain factors such as vascular endothelial growth factor (VEGF) or granulocyte-colony stimulating factor (GCSF), amongst others (Caplan and Correa, 2011; Redondo-Castro et al., 2018). Some of these factors exert beneficial actions leading to an enhanced repair response (Torres-Espín et al., 2013; Kalladka and Muir, 2014) and by modulating the inflammatory component (Bernardo and Fibbe, 2013; Mathew et al., 2017).
Several methods have been developed to generate spheroids including magnetic levitation (Haisler et al., 2013); nanoparticles (Daquinag et al., 2013), hanging drop techniques (Bartosh et al., 2010; Murphy et al., 2014), suspension methods (Carpenedo et al., 2007) and hydrogels (Laschke et al., 2013; Tseng et al., 2017). Some of these methods, despite being effective, are time consuming or expensive as they require complex reagents or equipment (Cha et al., 2017). For this reason, we have been culturing spheroids using a very simple method (Redondo-Castro et al., 2018) that only requires a low-binding 96-well plate combined with a high-density suspension of cells.
With this method, we are able to obtain mature spheroids in a few days, with a very high rate of efficiency and reproducibility. Moreover, phenotypic characterization of spheroids shows that this method could be really useful for researchers developing cell therapies (either cell suspensions for transplants or generating cell-derived products such as conditioned media), as well as in other research fields.
Materials and Reagents
-
Cell culture plasticware
T75 and/or T25 flasks (Corning, catalog numbers: 430641U for T75 and 3056 for T25)
Plates low cell binding, 96 wells, round bottom (Thermo Fisher Scientific, Nunc™, catalog number: 145399)
Centrifuge tubes (15 ml; 50 ml, Corning, catalog numbers: 430790; 430828)
Cryovials (STARLAB, catalog number: E3110-6122)
Plastic stripettes (5 ml; 10 ml; 25 ml, Corning, Costar®, catalog numbers: 4487; 4488; 4489)
Pipette tips (TipOne, STARLAB, catalog numbers: S1111-3700; S1111-1706; S1111-6701)
Non-adherent microfuge tubes (Eppendorf, catalog number: 0030108116)
-
Reagents
DMEM low glucose (Sigma-Aldrich, catalog number: D6046)
Fetal bovine serum (FBS, Thermo Fisher Scientific, Gibco™, catalog number: 10500064)
Gelatin, Analar (BDH, catalog number: 440454B)
L-Glutamine, 200 mM (Sigma-Aldrich, catalog number: G7513)
MesenPRO RS™ Medium (Thermo Fisher Scientific, Gibco™, catalog number: 12746012)
PBS, without calcium and magnesium (Sigma-Aldrich, catalog number: D8537)
Penicillin-streptomycin (P/S), 10,000 units penicillin and 10 mg streptomycin per ml (Sigma-Aldrich, catalog number: P0781)
Trypsin/EDTA 10x (Sigma-Aldrich, catalog number: T4174)
Triton X-100 (Sigma-Aldrich, catalog number: T8787)
Trypan blue solution (Sigma-Aldrich, catalog number: T8154, 0.4% [w/v] solution)
Paraformaldehyde (Sigma-Aldrich, catalog number: P6148)
Methanol (Fisher Scientific, CAS: 67-56-1)
Fish skin gelatin (Sigma-Aldrich, catalog number:G7041)
-
Antibodies:
Mouse anti-fibrillin (used at 1/200, Merck, catalog number: MAB1919)
Rabbit anti-fibronectin (used at 1/200, Sigma-Aldrich, catalog number: F3648)
Donkey anti-mouse 680 nm (used at 1/400, LI-COR, catalog number: 926-68072)
Donkey anti-rabbit 488 nM (used at 1/500, Thermo Fisher Scientific, Invitrogen™, catalog number: R37118)
DAPI (used at 1/100,000,Thermo Fisher Scientific, Invitrogen™, catalog number: D1306)
Gelatin solution (see Recipes)
MesenPRO RS™ Medium (see Recipes)
DMEM low glucose (see Recipes)
Trypsin (see Recipes)
Equipment
Water bath, 37 °C (Grant JB Nova)
CO2 Incubator (Eppendorf, New Brunswick™, model: Galaxy® 170 S)
Glass hemocytometer (Brand)
Laminar flow hood (ENVAIR, model: Envair Eco Safe Basic Plus)
Inverted microscope (Olympus, model: CKX31)
Moticam 2300 camera coupled to Motic Images Plus 2.0 ML software (Motic, model: Moticam 2300)
Cell culture centrifuge (Sigma Laborzentrifugen, model: 3-16KL)
Aspirator (dry vacuum pump/compressor, Welch Vacuum - Gardner Denver, model: 2511)
Autoclave (Prestige Medical, model: Classic 2100 Extended, catalog number: 210004UK)
Software
Motic Images Plus 2.0 ML software, Motic®
Procedure
-
Preparing the cells
Culture the cells in flasks (cells may come from a frozen vial or from a previously established culture. Cells identity can be confirmed by using specific markers, detailed in Dominici et al., 2006 or Redondo-Castro et al., 2017). Recommended initial densities are around 2,500 cells/cm2, but numbers can be adjusted (depending on the donors and their proliferation properties). Ensure cells are evenly distributed and incubate at 37 °C in MesenPRO RS™ Medium (or any other suitable culture media for MSCs).
When cells are being cultured (from a previous passage sub-culture or from the frozen vial) and reach 70-80% confluency, they are ready to be sub-cultured or used to generate spheroids. One passage normally takes one week, but this time can change depending on the donor; proliferation of cells from some donors can be as slow as three weeks between passages. Cells should be used up to passage 6, and the medium should be changed every 3-6 days (slower growing cells require less changes of media).
Warm gelatin solution in a water bath (~37 °C), and add enough gelatin solution to cover the entire surface of the flask/well. Ideally, leave them in an incubator overnight (37 °C). Aspirate the gelatin and rinse wash with warm or room temperature PBS. Flasks and plates coated with gelatin can be kept in the incubator until cells are ready (ideally this step needs to be done on the same day, but can be done the day before if needed. Flasks can be kept in the incubator ON, at 37 °C).
Once cells are ready, aspirate media and wash cells twice with warm PBS. Then add the solution containing trypsin, diluted 1/10 in PBS, from the 10x stock [final concentration of trypsin is 0.5% (w/v)]. Use the minimum volume necessary to cover the flask surface (normally 1-2 ml for a T25 or 5 ml for a T75, volumes may vary). It should be warm, as trypsin works optimally at 37°C. Return flasks to the incubator for 5 min, checking them every 2 min for detachment of cells.
Once detached, transfer the solution containing cells and trypsin to a falcon tube. Add the double of the volume of DMEM low glucose (containing FBS, check recipe section. MesenPRO RS™ medium can be used at this step, but DMEM is normally cheaper) to the flask, to wash out the remaining cells in the flask (if you added 5 ml of trypsin, add then 10 ml of media to stop the reaction). Transfer the whole volume to the tube and centrifuge at 770 x g for 5 min.
Discard the supernatant and resuspend the cells in 1-2 ml of media. Count the viable cells (using trypan blue) in a glass hemocytometer and divide the cells into new flasks or seed them to generate spheroids if they are in the right passage (P5-P6).
-
Spheroid formation
Resuspend the pellet in MesenPRO RS™ medium (14,000-60,000 cells per well [Figure 1C] do not need further modifications of this protocol–suspension volumes or incubation days), and add 50 μl of cell suspension to each well of the low cell binding plate. Variations of density can be used, according to specific requirements. Mixing and resuspending the cell suspension during the plating can help obtaining more reproducible spheroid sizes across the plate.
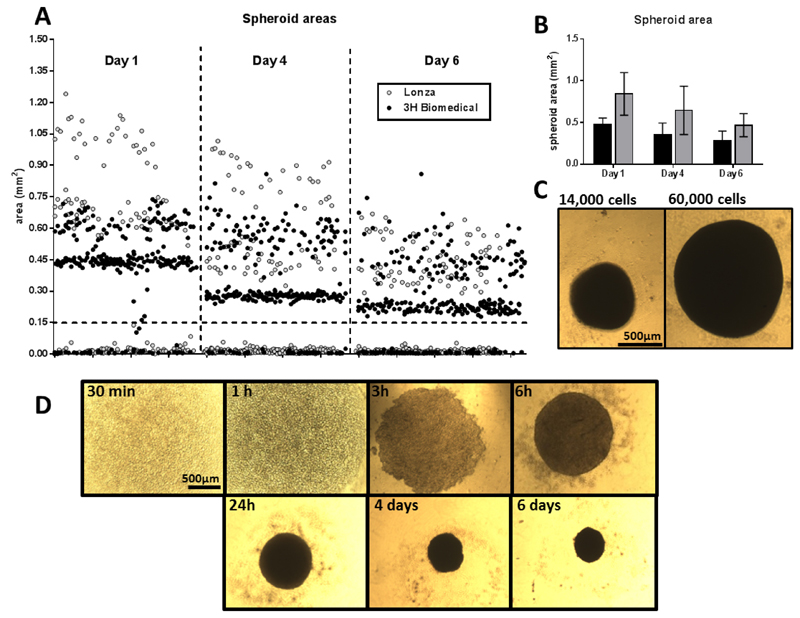
Spheroids will begin to appear in less than 24 h (Figures 1A and 1D). Just few hours after seeding, most of the wells should contain small spheroids that will progressively converge into one large spheroid. After 5 days in culture, spheroids are visible by eye and are stable enough for experimental investigation (Figure 2). Cells from different donors may grow faster or slower (Figures 1A and 1B), therefore culture times may need to be adjusted. If longer periods of culture are needed, or big numbers of cells are used, media can be changed by slowly removing the medium without disrupting the spheroid. Small preparations (14,000-25,000 cells) do not need media change during those 5-6 days.
-
Treating and collecting spheroids
Treatments can be added directly to the media, without need of moving the spheroids. Make sure to adjust volumes and concentrations. Be sure that your incubator has water to avoid evaporation which could lead to changes in the final volume of media surrounding the spheroid and give rise to inaccuracies in the results.
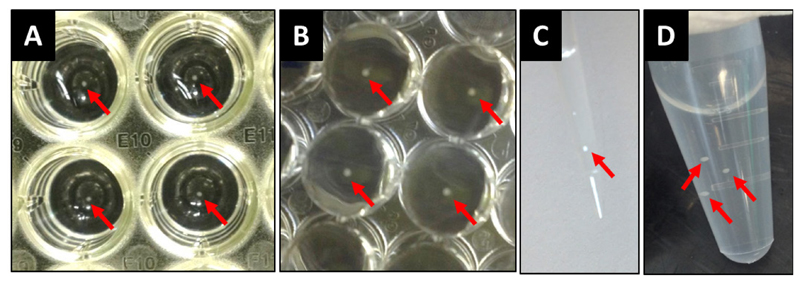
Supernatants can be easily collected with a plastic pipette tip (200 μl or 1 ml size will be suitable), just taking care to avoid the spheroid. Collect spheroids individually or together with the supernatants and transfer them to a tube using a plastic pipette tip (Figure 2).
-
Immunostaining of spheroids
Transfer spheroids to non-adherent microfuge tubes containing 4% paraformaldehyde in PBS containing 1% Triton X-100 at 4 °C for 4 h or overnight with gentle rocking (any tube rotator can be used here, use ~500 μl to ensure proper fixation of the spheroids).
Wash spheroids in 0.1% Triton X-100 in PBS, at least a couple of times (~15 min/wash; all washes require ~500 μl to ensure efficient washing; no centrifugation is needed, wait 1 min before aspirating the liquid so the spheroids will sediment into the bottom of the tube).
Dehydrate by exposure to an increasing series of methanol (10%, 20%, 50%, 75% and 95%) in PBS solution for 15-20 min each at 4 °C(~500 μl of each concentration should be enough).
Incubate spheroids in 100% methanol at 4° C for 4 h or overnight with gentle rocking.
Rehydrate spheroids (involve the same decreasing methanol series).
Block unspecific staining by incubating spheroids in 3% fish skin gelatin (FSG) in 0.1% Triton X-100/PBS solution at 4 °C for 10 min-1 h to overnight with gentle rocking.
Incubate with primary antibody diluted in 0.1% Triton X-100/PBS + 3% FSG, overnight at 4°C with gentle rocking. Optimal concentration of antibodies should be tested by users. We present an example using fibronectin and fibrillin-1, but other proteins can be detected by this method.
Wash spheroids three times in 0.1% Triton X-100 each for 30 min at room temperature.
Incubate spheroids with secondary antibody in 0.1% Triton X-100/PBS for 3-24 h at 4°C with gentle rocking. DAPI or other staining steps can be added at this point.
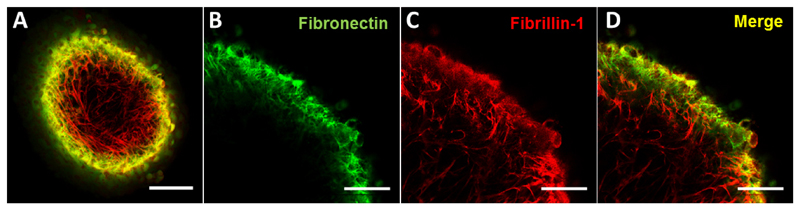
Wash three times using 0.1% Triton X-100/PBS. Spheroids are ready to be imaged (e.g., by fluorescence or confocal microscopy, see examples in Figure 3). We imaged the spheroid by using dipping confocal lenses whilst the spheroid was immersed in PBS solution, but different approaches can be used and every researcher needs to adapt this step to their own microscopes and resources.
Figure 1. Formation of spheroids.
A. Area of spheroids from 2 different human donors (in white dots for the cells purchased from Lonza and black dots for the cells purchased from 3H Biomedical), at days 1, 4 and 6 after cell seeding. Notice the difference in size between donors, the progressive reduction on size and the exclusion criteria for all structures smaller than 0.15 mm2 (indicated with a dotted line). B. Average area of spheroids from the same donors at the same time points; donor from 3H Biomedical in black, donor from Lonza in grey. C. Representative images of spheroids formed with 14,000 and 60,000 cells, after 5 days. Scale bar = 500 μm. D. Time course of the formation of a spheroid (20,000 cells) up to 6 days in vitro. Notice the reduction on size. Scale bar = 500 μm.
Figure 2. Working with spheroids.
Spheroids can be seen in the wells from above (A) and below (B) the plate (5 days spheroids in the picture, 20,000 cells). Spheroids can be easily aspirated with a pipette tip (C) and transferred into centrifuge tubes (D). Spheroids are indicated with arrows. As a reference, the wells from (A) and (B) are from a 96-well plate.
Figure 3. Example of immunofluorescent staining of spheroids.
Confocal images of an immunostained spheroid (A, scale bar = 100 μm), and details at higher magnification (scale bars = 50 μm) of fibronectin (B) and fibrillin-1 staining (C), as well as the merge image (D).
Data analysis
Measuring spheroids: spheroid growth can be monitored by taking photos and measuring their maximum sectional area using a camera attached to the inverted microscope. Bright field images are sufficient to achieve reliable measurements. All structures smaller than 0.15 mm2 are not considered spheroids and excluded from further analysis (Figures 1A and 1B). Further details on this process can be found in (Redondo-Castro et al., 2018).
Biological measurements: supernatants can be directly used in different assays, such as ELISAs, or Western blots. Spheroids can be lysed to use them in molecular biology assays (ELISAs, Western blots, PCR, etc.) or processed for histological or immunocytochemistry protocols (Figure 4).
Conditioned media: supernatants can be used as conditioned medium for cell and in vivo treatments.
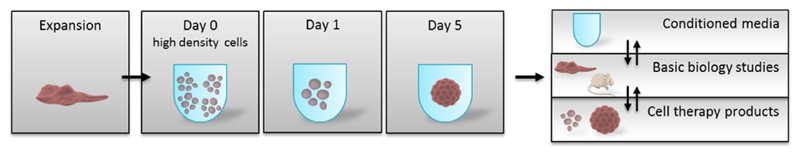
Figure 4. Spheroid formation and applications.
Scheme depicting the main stages of spheroid formation and some of the main applications.
Notes
Cells from different donors may behave differently (Figure 1). Some donors require longer incubation times between passages or different incubation times before spheroids form. Adjust the times to your donor characteristics.
As the cells are contained in a small volume of media, it is important that evaporation is not an issue, as that can affect the concentrations of treatments and therefore cell behavior. Ensure your incubator tray always contains water. It is a good idea to use all the outer wells as an extra reservoir of liquid containing PBS and 1-2% of P/S.
Immunofluorescence protocols may require some further optimization by the user, as some epitopes may be more accessible than others (especially the ones on the surface of the spheroid). So we recommend optimization of the concentrations and timings of incubations to ensure proper penetration and staining of the right targets.
Recipes
-
Gelatin solution
Dissolve gelatin (0.1%) in distilled water and autoclave the solution
Store the solution at 4 °C and use within two weeks
-
MesenPRO RS™ Medium
Defrost the MesenPro supplement (it is sold with the media) and add it to a 500 ml bottle
Add 5 ml of P/S
Store the medium in 50 ml aliquots and keep at 4 °C
Add 5 ml of fresh glutamine, just before feeding the cells (glutamine in media can last up to 14 days)
-
DMEM low glucose
Add 50 ml of FBS and 5 ml of P/S to a 500 ml bottle of media
Store the media at 4 °C if not use immediately
Add 5 ml of fresh glutamine, just before adding it to the cells (glutamine in media can last up to 14 days)
-
Trypsin
Dilute trypsin/EDTA10x to 1x in PBS, just before use
Acknowledgments
The work was supported with funds from the Stroke Association (grant TSA 2017/03, UK),the Engineering and Physical Sciences Research Council (EPSRC, UK), the Medical Research Council Centre for Doctoral Training (MRC-CDT) in Regenerative Medicine studentship grant EP/L014904/1, and the Manchester Regenerative Medicine Network (MaRMN).
Footnotes
Author contributions statements: All authors contributed to the writing, editing and testing of this protocol.
Conflicts of interest statement: The authors declare that there are no conflicts of interest or competing interests.
References
- 1.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 2.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, Claypool K, Lee RH, Choi H, Prockop DJ. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107(31):13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Boido M, Piras A, Valsecchi V, Spigolon G, Mareschi K, Ferrero I, Vizzini A, Temi S, Mazzini L, Fagioli F, Vercelli A. Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2014;16(8):1059–1072. doi: 10.1016/j.jcyt.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;25(9):2224–2234. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 7.Cha JM, Park H, Shin EK, Sung JH, Kim O, Jung W, Bang OY, Kim J. A novel cylindrical microwell featuring inverted-pyramidal opening for efficient cell spheroid formation without cell loss. Biofabrication. 2017;9(3) doi: 10.1088/1758-5090/aa8111. 035006. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 9.Daquinag AC, Souza GR, Kolonin MG. Adipose tissue engineering in threedimensional levitation tissue culture system based on magnetic nanoparticles. Tissue Eng Part C Methods. 2013;19(5):336–344. doi: 10.1089/ten.tec.2012.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 11.Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR. Three-dimensional cell culturing by magnetic levitation. Nat Protoc. 2013;8(10):1940–1949. doi: 10.1038/nprot.2013.125. [DOI] [PubMed] [Google Scholar]
- 12.Kalladka D, Muir KW. Brain repair: cell therapy in stroke. Stem Cells Cloning. 2014;7:31–44. doi: 10.2147/SCCAA.S38003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laschke MW, Schank TE, Scheuer C, Kleer S, Schuler S, Metzger W, Eglin D, Alini M, Menger MD. Three-dimensional spheroids of adipose-derived mesenchymal stem cells are potent initiators of blood vessel formation in porous polyurethane scaffolds. Acta Biomater. 2013;9(6):6876–6884. doi: 10.1016/j.actbio.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Mathew B, Poston JN, Dreixler JC, Torres L, Lopez J, Zelkha R, Balyasnikova I, Lesniak MS, Roth S. Bone-marrow mesenchymal stem-cell administration significantly improves outcome after retinal ischemia in rats. Graefes Arch Clin Exp Ophthalmol. 2017;255(8):1581–1592. doi: 10.1007/s00417-017-3690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy KC, Fang SY, Leach JK. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014;357(1):91–99. doi: 10.1007/s00441-014-1830-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redondo-Castro E, Cunningham CJ, Miller J, Brown H, Allan SM, Pinteaux E. Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Res Ther. 2018;9(1):11. doi: 10.1186/s13287-017-0753-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8(1):79. doi: 10.1186/s13287-017-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres-Espin A, Hernandez J, Navarro X. Gene expression changes in the injured spinal cord following transplantation of mesenchymal stem cells or olfactory ensheathing cells. PLoS One. 2013;8(10):e76141. doi: 10.1371/journal.pone.0076141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng TC, Wong CW, Hsieh FY, Hsu SH. Biomaterial substrate-mediated multicellular spheroid formation and their applications in tissue engineering. Biotechnol J. 2017;12(12) doi: 10.1002/biot.201700064. [DOI] [PubMed] [Google Scholar]