Abstract
Aims
Chronic central serous chorioretinopathy (CSCR) is poorly understood. Fluid accumulates in the subretinal space and retinal pigment epitheliopathy and neurosensory atrophy may develop. Permanent vision loss occurs in approximately one third of cases. There are no effective treatments for CSCR. Recent studies have shown the mineralocorticoid receptor antagonist, eplerenone, to be effective in resolving subretinal fluid and improving visual acuity. This trial aims to compare the safety and efficacy of eplerenone in patients with CSCR in a double-masked randomised placebo-controlled trial.
Methods
Patients are randomised 1:1 to receive eplerenone with usual care or placebo with usual care for 12 months; 25 mg/day for 1 week, then 50 mg/day up to 12 months (unless discontinued for safety or resolution of CSCR). Key eligibility criteria are: age 18-60 years, one eye with CSCR for ≥4 months duration, best corrected visual acuity (BCVA) >53 and <86 letters and no previous treatment. The primary outcome is BCVA at 12 months. Secondary outcomes include resolution of subretinal fluid, development of macular atrophy, subfoveal choroidal thickness, changes in low luminance visual acuity, health-related quality of life and safety.
Conclusions
Recruitment is complete but was slower than expected. We maintained the eligibility criteria to ensure participants had ‘true’ CSCR and recruited additional centres. Effective distribution of the investigational medicinal product (IMP) was achieved by implementing a database to manage ordering and accountability of IMP packs. The results will provide adequately-powered evidence to inform clinical decisions about using eplerenone to treat patients with CSCR.
Keywords: Central serous chorioretinopathy, central serous retinopathy, eplerenone, placebo, randomised controlled trial, masked, sub-retinal fluid, mineralocorticoid receptor antagonist, investigational medicinal product
Introduction
Central serous chorioretinopathy (CSCR) is a poorly understood eye disease. Fluid that accumulates under the retina causing a neurosensory retinal detachment, is a sign of pigment epitheliopathy, which can lead to permanent vision loss in up to a third of cases [1]; some resolve spontaneously but others persist for years, recur or affect the second eye [2]. Spontaneous resolution typically occurs within three months of onset [2], hence persistent or recurring subretinal fluid beyond three months is defined as chronic. The incidence is 10 per 100 000 men and 2 per 100 000 women [2]. The cause is unknown although CSCR can occur in families and we recently identified the first genetic determinants [3].
There are no proven treatments and little progress has been made in understanding CSCR [2]. The current treatment of choice is photodynamic laser therapy (PDT) but there are few definitive randomised controlled trials (RCTs) supporting its use and most of the studies are small [4]. One RCT reported half-dose verteporfin PDT to have benefits in an acute CSCR population but the effects of PDT in chronic CSCR have not been definitively studied in a placebo-controlled RCT [5]. PDT carries a risk of retinal scarring, atrophy or choroidal ischaemia. Since CSCR often resolves spontaneously [6], ophthalmologists are reluctant to use PDT. Some patients are treated with anti-vascular endothelial growth factor (anti-VEGF) therapy but evidence to support this treatment is equivocal [7]. Most patients with chronic CSCR have no active treatment and up to a third may have permanent visual loss [1].
In a rat model of CSCR, choroidal vasodilation and subretinal fluid (a feature of CSCR) were induced by aldosterone, a mineralocorticoid receptor (MR) activator [8]. Blocking this pathway prevented choroidal thickening. Subsequently, two patients with non-resolved chronic CSCR were treated with oral eplerenone, a specific MR antagonist, for five weeks. Their retinal detachment and choroidal vasodilation resolved, and the associated visual acuity improvements were maintained for 5 months after stopping eplerenone [8]. These results have prompted investigation of MR blockade as a therapy to reverse CSCR.
In a subsequent small cohort of patients with chronic CSCR of at least four months duration, a significant reduction in central macular thickness, subretinal fluid level, and an improvement in visual acuity was observed in some patients [9]. A double-masked RCT concluded that eplerenone was safe in patients with CSCR but was not beneficial [10], an unsurprising result given the small sample size and short-term intervention. The biology underpinning treatment with eplerenone combined with the absence of high quality evidence provided a strong rationale to conduct a long-term double-masked RCT to test the efficacy of eplerenone in patients with chronic CSCR.
Objectives
The objectives of the VICI trial are:
-
(a)
To evaluate whether best corrected visual acuity (BCVA) following eplerenone treatment with usual care is superior to placebo with usual care.
-
(b)
To evaluate: resolution of subretinal fluid (SRF); safety; patient-reported visual function; the response of the choroid and retinal pigment epithelium (RPE) to treatment; low luminance visual acuity.
-
(c)
(To generate a biobank from treatment naïve CSCR patients for future mechanistic studies.
Subjects and methods
Trial design
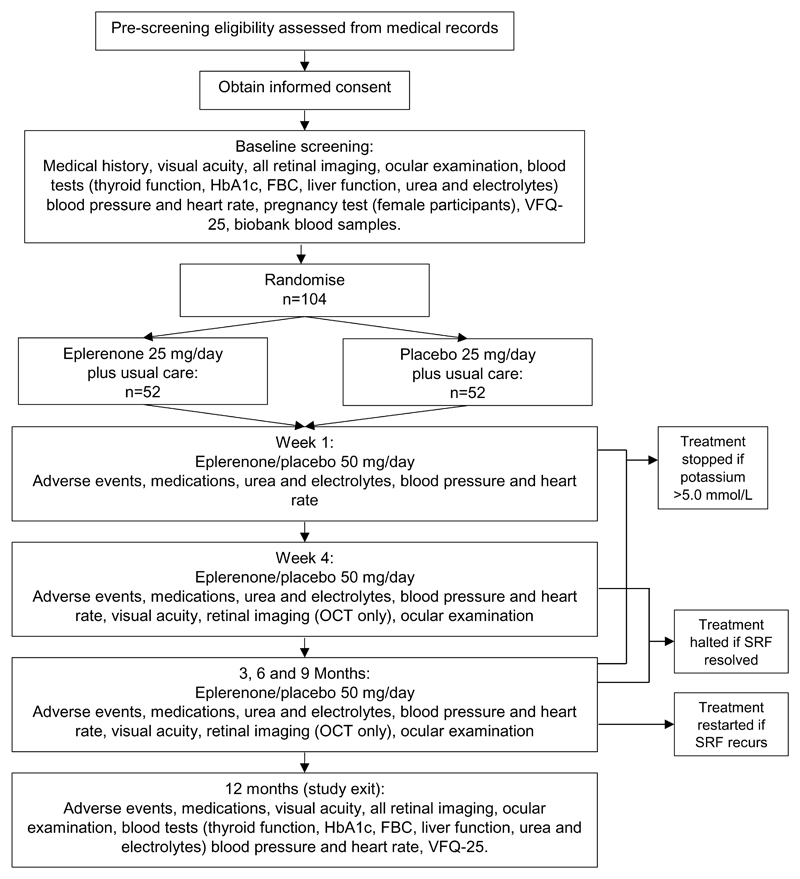
The VICI trial is a multicentre, individually randomised (1:1), double-masked, placebo-controlled parallel group RCT. Eligible patients who give written informed consent will be randomised to eplerenone treatment with usual care or placebo with usual care for a period of 12 months. Recruitment has taken place in 22 sites and was projected to take 12 months. Figure 1 shows the study schema.
Figure 1. Trial Schema.
Schema showing the recruitment pathway, follow-up schedule and assessments.
Usual care usually comprises observation without any intervention. The protocol recommends that such treatments should only be offered if BCVA deteriorates by ≥ 15 letters from baseline, an established criterion [11, 12]. Investigators are discouraged from offering alternative therapies; if used, information about alternative therapies is collected.
All participating sites are secondary or tertiary care NHS Trusts based in the United Kingdom (UK). The trial has been approved by the Wales Research Ethics Committee (ref 16 / WA / 0069) and the Medicines and Healthcare products Regulatory Agency (MHRA). The principles of Good Clinical Practice will be adhered to throughout in accordance with the Declaration of Helsinki.
Study population
Inclusion criteria
-
1.
≥ 18 and ≤ 60 years old;
-
2.
CSCR ≥ 4 months duration in one eye, defined as: subfoveal presence of sub-retinal fluid (SRF) on optical coherence tomography (OCT) AND characteristic appearance of CSCR on fundus fluorescein angiogram (FFA) and indocyanine green angiography (ICGA) AND a patient history and examination consistent with CSCR having been present for ≥ 4 months;
-
3.
A female participant must: (a) have a negative pregnancy test and be prepared to use effective contraception during participation in the trial and 3 months after, or (b) be surgically sterile or (c) be post-menopausal for > 12 months;
-
4.
Able to provide written informed consent.
The following inclusion criteria apply to the study eye:
-
5.
An early treatment diabetic retinopathy study (ETDRS),[13, 14], BCVA score of >53 and <86 letters.
-
6.
Clear ocular media and adequate pupillary dilatation to permit photography.
Patient-level exclusion criteria:
-
1.
Hyperkalaemia (serum potassium >5.0 mmol/L);
-
2.
Hepatic or renal impairment (patients with severe renal insufficiency (estimated glomerular filtration rate (eGFR) <30 ml per minute per 1.73 m2) or patients with severe hepatic insufficiency (Child-Pugh Class C; see Supplementary Information 1 for definitions);
-
3.
Pregnancy or breast feeding;
-
4.
Known allergy to fluorescein or indocyanine green;
-
5.
Receiving concomitant medications (see Supplementary Information 2 for details);
-
6.
Hypersensitivity or allergy to eplerenone or any of its excipients;
-
7.
Hereditary galactose intolerance, Lapp lactase deficiency or glucose-galactose malabsorption;
-
8.
Aspirin >75 mg per day.
The following exclusion criteria apply to the study eye:
-
9.
Choroidal neovascularisation;
-
10.
Previous/current treatment with eplerenone or previous/current treatment with PDT, anti-vascular endothelial growth factor (anti-VEGF) therapy, intra-ocular steroid use or thermal laser therapy for CSCR;
-
11.
Presence of any other disease which could cause retinal fluid or SRF to accumulate (e.g. diabetic retinopathy, polypoidal choroidal vasculopathy, domed shaped maculopathy or choroidal haemangioma) or affect visual acuity;
-
12.
Myopia >6 dioptres.
CSCR can be bilateral at presentation or may develop in the contralateral eye during the study. Treatment is given orally and any effect is through systemic absorption. Therefore, eye-specific outcomes such as BCVA and low luminance visual acuity (LLVA), and FA, ICGA and OCT parameters, are measured in both eyes of participants throughout the trial.
Patients with CSCR are identified and approached according to local site procedures. All potential participants receive an invitation letter and participant information leaflet describing the study and most have > 24 hours to consider whether to participate. A member of the local site research team answers any questions, confirms eligibility and takes written informed consent if the patient decides to participate. The principal investigator or a delegated clinician confirms eligibility before randomisation. Details of all patients approached and reason(s) for non-participation are documented.
Investigational medicinal products (IMPs)
The IMP in this trial is either a) eplerenone (Zentiva; Guilford, UK) at 25 mg per day, increased to 50 mg per day after one week, plus usual care, or b) placebo capsules, plus usual care. The placebo is lactose which was chosen because it is present in the licensed medication. IMP is continued until there is evidence of complete resolution of SRF or until 12 months after baseline. If SRF recurs after resolution during the follow-up period, participants re-start IMP and follow the same dose escalation procedure.
Over-encapsulated gelatin capsules mask the IMP (Newcastle Specials Pharmacy Production Unit; Newcastle-upon-Tyne, UK). Capsules are packaged in plastic bottles (10 capsules of 25 mg eplerenone/placebo per bottle; 36 capsules of 50 mg eplerenone/placebo per bottle), distributed to sites by the manufacturing pharmacy and stored in site pharmacies at room temperature.
Safety criteria and IMP cessation
Serum potassium is measured at each follow-up time-point because hyperkalaemia is a known side effect of eplerenone. Participants switch from 25 mg/day to 50 mg/day at week 1, providing serum potassium is ≤ 5.0 mmol/L. If serum potassium exceeds 5.0 mmol/L at any time-point, the participant stops taking the study drug and hyperkalaemia is recorded as an adverse event. Such participants are invited to continue with follow-up visits up to 12 months.
Safety reporting
Data on adverse events and reactions are collected throughout the follow-up period, by asking participants at each follow-up visit. The local research team also reviews a participant’s medical records for hospital admissions, if a participant fails to attend. Each participant’s GP is notified of their participation, with a request to inform the local research team about any suspected adverse event or reaction.
The data are recorded on case report forms (CRFs). All serious adverse events (SAEs) are reported to the Clinical Trials and Evaluation Unit (CTEU) Bristol within 24 hours of the local site team becoming aware. Causality of SAEs is decided by the treating clinician. CTEU Bristol reports all SAEs to the Sponsor within 24 hours, and to the MHRA and the data monitoring and safety committee (DMSC) annually and biannually, respectively. Reporting of any suspected unexpected serious adverse reaction (SUSAR) to the MHRA, research ethics committee and DMSC is expedited (maximum of 7 days in the event of death and 15 days for all other SUSARs).
Adherence to medication
Adherence is monitored by the CTEU Bristol from data submitted by sites and reported to the trial oversight committees. The risk of non-adherence is mitigated by: regular follow-up visits (at least every 3 months) when participants are asked whether they have missed a treatment; prescribing a limited amount of IMP at each visit; requiring participants to return unused IMP capsules; recording the number of capsules returned (double-counted by nurse and pharmacist). If the observed number of capsules returned at a visit is > 5 more than expected, the local research team is advised to maintain closer contact with the participant to encourage adherence. Reasons for non-adherence will be explored and documented.
Outcomes
Primary outcome
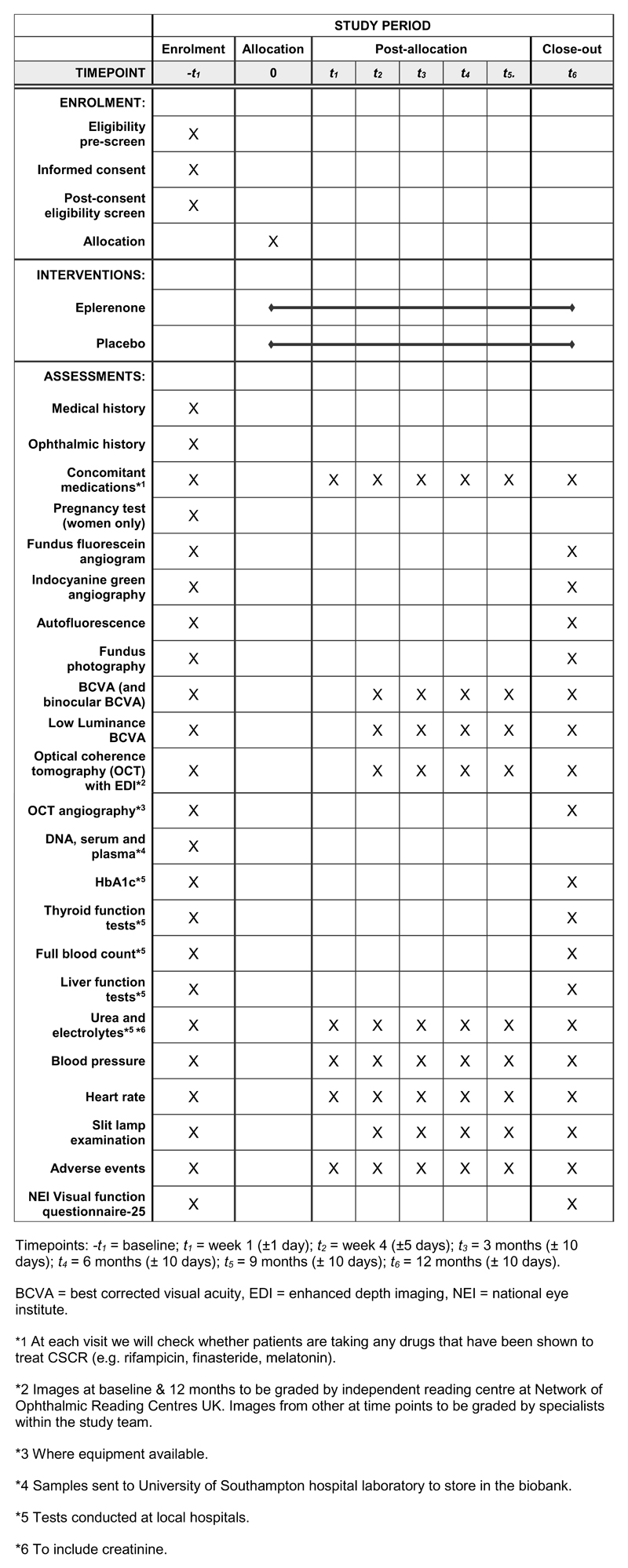
The primary outcome is the BCVA at the 12 month visit [13, 14]. BCVA is assessed at baseline, 4 weeks, 3, 6, 9 and 12 months post-randomisation.
Secondary outcomes
-
a)
LLVA, measured as for BCVA, immediately afterwards, by adding a 2-log neutral density filter.
-
b)
Central Subfield Retinal Thickness (CSRT), measured by OCT at 12 months.
-
c)
SRF thickness as measured by OCT, vertically at the thickest point or sub-foveally if SRF is not thickest at the fovea.
-
d)
Systemic and ocular adverse events at any time during follow-up.
-
e)
Development of macular atrophy of the RPE, defined as hypoautofluorescence at 12 months. The area of subfoveal and total hypo-autofluorescence measured at baseline and 12 months, and atrophy assessed by measuring homogenous autofluorescence using Heidelberg Spectralis software (Franklin, Massachusetts, US).
-
f)
Subfoveal choroidal thickness: one measurement at the fovea and one at the thickest macular point (in microns), measured by enhanced depth imaging OCT at 12 months.
-
g)
Reduced choroidal permeability at 12 months, measured from ICGA, and graded as yes, no or cannot grade. Comparison of 12 month images to baseline will qualitatively assess changes, graded as better, worse, completely resolved or cannot grade.
-
h)
Time to resolution of SRF.
-
i)
Complete, partial (decrease in CSRT >25% of from baseline due to resolution of SRF) or no resolution of SRF (change in CSRT ≤ ± 25% from baseline) at each time point of the study. Recurrence is defined as new SRF in a study eye after complete resolution of SRF.
-
j)
Patient-reported visual function using the Visual Functioning Questionnaire-25, version 2000 (VFQ-25) at 12 months.
-
k)
Classification of study eyes by each FFA phenotype, e.g. smoke stack, ink-blot, chronic epitheliopathy.
-
l)
Classification of study eyes as early (complete or partial resolution of sub-foveal SRF by 3 months from baseline), late (complete or partial resolution of sub-foveal SRF after 6 months from baseline), or non-responder.
-
m)
Incident CSCR in the fellow eye, measured by OCT, FFA, ICGA or autofluorescence (AF).
Heidelberg imaging equipment is mandatory to minimise inconsistency in images. Figure 2 shows the schedule of assessment of outcomes and investigations.
Figure 2. SPIRIT diagram of trial timepoints and data collection schedule.
Trial timepoints and data collection schedule.
Randomisation
Participants are randomised within four weeks of the screening visit by the ophthalmologist or research nurse via a secure internet-based randomisation system (GeneSYS, CTEU Bristol, UK) [15]. Randomised allocations were generated before recruiting the first participant and supplied to the manufacturing pharmacy to label the bottles of IMP with unique bottle numbers. Allocations are concealed until a participants’ identity and eligibility are captured in the trial database.
Features to minimise bias
The trial is placebo-controlled. Bottles of IMP are allocated to participants by the unique bottle number. Bottles are labelled identically except for the unique number. Visual acuity examiners and imaging technicians have no information about outcomes or adverse events from any previous visit when carrying out tests, minimising the risk of biasing measurements or unmasking. The interviewer who administers the VFQ-25 booklets is masked. All retinal images are graded by masked, trained and quality assured independent graders in the Network of Ophthalmic Reading Centres UK (NetWORC UK) [16]. We will report retention for each outcome, including reasons for attrition or exclusions from the analyses.
IMP database
Allocation of bottles of study drug is managed via a secure, password-protected, internet-based IMP database with site and role-restricted access. Different users (local site research teams, site pharmacists, and trial management staff) access role-specific modules of the database to place orders, monitor local stock levels, etc. Further details are available in Supplementary Information 3.
Unmasking
The treating investigator can request unmasking but only in the event of a medical emergency for which knowledge of the allocation will affect the patient’s care. The chief investigator or co-lead investigator has the final decision and unilateral right to unmask the allocation.
If required, unmasking can be performed by the CTEU Bristol or a local site pharmacist, using the IMP database. Local site pharmacists have sealed code-break envelopes as a backup option in the event of internet failure, which will be collected and inspected at the end of the trial for signs of tampering. Any unmasking will be recorded and reported at the end of the trial.
Biobank
At baseline, eligible consented participants are asked to donate 30 mL of blood. Donating a blood sample is optional. Samples are sent to a biobank at the University of Southampton, UK. The blood is processed, with aliquots stored at -80°C as whole blood, plasma and serum. The samples will inform future mechanistic studies about CSCR.
Sample size
A sample size of 45 patients in each group is sufficient to detect a difference of five letters in BCVA between the eplerenone and placebo groups with 90% power and 5% significance (2-tailed), assuming:
- a)
-
b)
correlation between baseline and any follow up BCVA is 0.5,
-
c)
minimum of 2 follow up assessments/participant,
-
d)
correlation between BCVA on follow-up visits is 0.8.
The target sample size is 104, allowing for ≤14% dropout over the 12 month period.
Plan for statistical analysis
Outcomes measured at multiple time points (e.g. BCVA) will be compared between study eyes in the two treatment groups using mixed models for repeated measures, adjusting for baseline, allowing all patients with data to be included in the analysis. Continuous outcomes may be transformed, if necessary. Interactions between treatment and time will be examined. If an interaction is statistically significant (p<0.05), changes in treatment effect with time will be reported. If an interaction is not statistically significant an overall treatment effect will be reported. Treatment effects at 12 months will be reported with 95% confidence intervals. Cross-overs will be documented. With the exception of adverse events, the analyses will be according to the intention-to-treat. Non-adherence to medication will also be reported; depending on the extent, the statistical analysis plan may include additional analyses to investigate the interaction between adherence and treatment. A secondary analysis will include primary outcome data from both eyes, with each eye being designated as having CSCR or not at each visit, estimating the interaction of treatment and CSCR status.
Additional analyses of the overall trial cohort will investigate associations between final visual acuity and a) patient’s age and b) granular/confluent hypoautofluorescence in the macula at randomisation.
No subgroup analyses are planned. However, depending on the level of adherence observed, and the availability of OCT angiography at baseline or final visit, two subgroup analyses may be described in the statistical analysis plan and carried out, testing the following interactions: a) good/poor adherence and treatment; b) presence/absence of new vessels and treatment.
Trial management and monitoring
Preparation of study documents, site initiation and training, day-to-day running of the trial and monitoring of sites according to the central monitoring plan has been/is being managed by CTEU Bristol. A trial management group (TMG) (chief investigator, co-lead investigator, trial managers and key collaborators) is overseeing the trial and meets regularly to review milestones. A DMSC meets biannually to review accruing data. A trial steering committee (TSC) oversees the overall trial, receives reports and recommendations from the DMSC and TMG and has ultimate responsibility for any decision about continuation of the trial. The trial oversight committees are described in the acknowledgments section.
Protocol amendments
Version 4.0 was used when recruitment started (14/12/2016) and version 5.0 of the protocol (26/01/2017) is currently in use. The only changes between these versions were to remove fasting blood glucose from the baseline assessment and to include fundus photography at baseline and 12 months.
Discussion
Recruitment started on 14/12/2016 and ended on 28/02/2018. Recruitment has been challenging, primarily due to: a) the detailed eligibility criteria; and b) the use of placebo as the comparator.
-
a)
Predominant reasons for ineligibility have been age >60 years or BCVA score ≥86 letters. The TMG decided not to increase the upper age limit as CSCR can be difficult to diagnose and has a similar pathology to macular degeneration, which is more prevalent in older patients. Including patients in whom the underlying cause of vision loss might not be CSCR could dilute the treatment effect and risk harm from eplerenone treatment for no benefit. With respect to the BCVA threshold, improvement of BCVA at screening compared to presentation due to over-refraction with a plus lens has made many patients ineligible. The upper eligible BCVA score was originally 78 letters but increased to 85 letters before the first participant was recruited. We have not increased the upper BCVA limit further because of the risk of a ceiling effect.
-
b)
The placebo comparator was challenging because some patients preferred to receive PDT (some participating sites are tertiary referral centres for PDT). The rarity of the condition meant that we needed to include sites that offer PDT to meet our recruitment projection. We have encountered logistical challenges with distributing IMP bottles. Sufficient IMP bottles have been produced for 104 participants, plus a limited supply of surplus stock. Careful distribution of IMP during the recruitment phase has ensured all 22 sites are adequately stocked for both potential and randomised participants. Requiring IMP as two doses has further complicated distribution. Fewer 25 mg bottles have been manufactured, as they are only prescribed at baseline or when restarting treatment when disease recurs, which has required frequent re-distribution of 25 mg bottles from lower to higher recruiting sites. Over-production could be more cost-effective than managing and redistributing the IMP stock, depending on the costs of manufacturing the IMP.
Another consideration in this trial has been the shelf-life of the IMP, which was reduced by over-encapsulation from 24 months to 18 months. We manufactured the IMP ready for the original start of recruitment and delays in trial set-up resulted in IMP expiring before use, which has had cost implications. To optimise the management and accountability of the IMP we designed an internet-based, role-restricted, IMP management database (Supplementary Information 3).
Monitoring adherence to the intervention is an important consideration for the management of this trial as participants are responsible for administering the IMP at home. The impact of non-adherence on the trial is two-fold: dilution of the treatment effect; potential to undermine safety monitoring processes (e.g. advice to continue taking the IMP based on serum potassium results). Adherence to the intervention is closely monitored as described in the Subjects and Methods section. The trial is relatively low risk with regards to the safety considerations of administering the IMP at home (e.g. overdosing). Eplerenone has a short half-life, low toxicity and is non-addictive. There are no known cases of overdosing from eplerenone and the most likely manifestations of an overdose are anticipated to be hyperkalaemia or hypotension, clinical indicators of which are being monitored at follow-up visits, with participants being withdrawn if necessary.
The results of this trial will fill a gap in the knowledge regarding the efficacy and safety of eplerenone for the treatment of CSCR in the longer term. This will include data on the rate of disease resolution and subsequent recurrence with eplerenone, something which is as yet unknown in this population. As patients with CSCR have limited therapeutic options, further evidence on the actions of eplerenone treatment for CSCR would be welcomed and could help inform future treatment decisions.
Data collection for this trial is ongoing. After publication of the trial results, the anonymised data will be made available upon reasonable request to the Sponsor institution, University Hospital Southampton NHS Foundation Trust. A statement on data sharing is included in the protocol [19].
Supplementary Material
Supplementary information is available at Eye’s website.
Acknowledgements
This project is funded by the Efficacy and Mechanism Evaluation (EME) Programme, an MRC and NIHR partnership (ref: 13/94/15). The views expressed in this publication are those of the author(s) and not necessarily those of the MRC, NHS, NIHR or the Department of Health.
The EME Programme is funded by the MRC and NIHR, with contributions from the CSO in Scotland and NISCHR in Wales and the HSC R&D Division, Public Health Agency in Northern Ireland.
The trial is sponsored by University Hospital Southampton NHS Foundation Trust, Southampton, UK.
This study was designed and delivered in collaboration with the Clinical Trials and Evaluation Unit (CTEU), a UKCRC registered clinical trials unit which, as part of the Bristol Trials Centre, is in receipt of National Institute for Health Research CTU support funding.
The authors would like to acknowledge the Macular Society and Fight for Sight for their feedback on the patient information leaflet and the DMSC and TSC for their oversight of the trial. The independent DMSC is formed of a Chairperson, two consultant ophthalmologists and one consultant cardiologist. The independent TSC is formed of a Chairperson, two consultant ophthalmologists, a consultant cardiologist, an ophthalmic statistician and patient and public involvement representatives; other TSC members with observer status represent the trial management team and the Sponsor.
Footnotes
Conflicts of interest
AW, LC, LE, CAR, AC, SE, and BCR have no conflicts of interest. FBC is an inventor on a patent protecting the use of mineralocorticoid receptor antagonists in CSCR. SS has received research grants, travel grants and speaker fees from Novartis, Bayer, Allergan, Roche, Heidelberg Engineering, Optos. AL has received travel grants and speaker fees from Bayer and Roche.
Author contributions
AL conceived the trial; AL, SS, BCR, AC, CR obtained funding; AL, SS, BCR and CR designed the trial; AW, LE and LC managed the trial with input from AL, SS, BCR and CR; UC, SE and FBC provided expert input; AC and AL set up the biobank; AW wrote the first draft of the manuscript. All authors reviewed the manuscript and amended/approved the final version.
References
- 1.Loo RH, Scott IU, Flynn HW, Jr, Gass JD, Murray TG, Lewis ML, et al. Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina. 2002;22(1):19–24. doi: 10.1097/00006982-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond) 2010;24(12):1743–56. doi: 10.1038/eye.2010.130. [DOI] [PubMed] [Google Scholar]
- 3.Schubert C, Pryds A, Zeng S, Xie Y, Freund KB, Spaide RF, et al. Cadherin 5 is regulated by corticosteroids and associated with central serous chorioretinopathy. Hum Mutat. 2014;35(7):859–67. doi: 10.1002/humu.22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erikitola OC, Crosby-Nwaobi R, Lotery AJ, Sivaprasad S. Photodynamic therapy for central serous chorioretinopathy. Eye (Lond) 2014;28(8):944–57. doi: 10.1038/eye.2014.134. Epub 2014/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmology. 2008;115(10):1756–65. doi: 10.1016/j.ophtha.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Salehi M, Wenick AS, Law HA, Evans JR, Gehlbach P. Interventions for central serous chorioretinopathy: a network meta-analysis. Cochrane Database Syst Rev. 2015;12:CD011841. doi: 10.1002/14651858.CD011841.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung YR, Seo EJ, Lew HM, Lee KH. Lack of positive effect of intravitreal bevacizumab in central serous chorioretinopathy: meta-analysis and review. Eye (Lond) 2013;27(12):1339–46. doi: 10.1038/eye.2013.236. Epub 2013/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao M, Celerier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672–9. doi: 10.1172/Jci61427. WOS:000306044600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy A Pilot Study. Retina-J Ret Vit Dis. 2013;33(10):2096–102. doi: 10.1097/IAE.0b013e318297a07a. WOS:000330237900014. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz R, Habot-Wilner Z, Martinez MR, Nutman A, Goldenberg D, Cohen S, et al. Eplerenone for chronic central serous chorioretinopathy-a randomized controlled prospective study. Acta ophthalmologica. 2017 doi: 10.1111/aos.13491. Epub 2017/06/28. [DOI] [PubMed] [Google Scholar]
- 11.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65 e5. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Beck RW, Maguire MG, Bressler NM, Glassman AR, Lindblad AS, Ferris FL. Visual acuity as an outcome measure in clinical trials of retinal diseases. Ophthalmology. 2007;114(10):1804–9. doi: 10.1016/j.ophtha.2007.06.047. Epub 2007/10/03. [DOI] [PubMed] [Google Scholar]
- 14.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–6. Epub 1982/07/01. [PubMed] [Google Scholar]
- 15.Hutton D, Smith N, Cappel-Porter H, Saw C, Rogers CA. Development of the “GeneSYS” database system to support trial data capture and conduct. Trials. 2013 [Internet]. 29/11/2013. [66]. [Google Scholar]
- 16.UK n. Network of ophthalmic reading centres UK. Available from: http://www.networcuk.com/
- 17.Reeves BC, Wood JM, Hill AR. Reliability of high- and low-contrast letter charts. Ophthalmic Physiol Opt. 1993;13(1):17–26. doi: 10.1111/j.1475-1313.1993.tb00421.x. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 18.Becker R, Teichler G, Graf M. Reproducibility of visual acuity assessment in normal and low visual acuity. Strabismus. 2007;15(1):3–6. doi: 10.1080/09273970601172435. Epub 2007/05/25. [DOI] [PubMed] [Google Scholar]
- 19.Clinical efficacy and mechanistic evaluation of Eplerenone for central serous chorio-retinopathy the VICI study 2016. Available from: https://njl-admin.nihr.ac.uk/document/download/2009989.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.