Abstract
Objective
Right hemisphere stroke may cause an ipsilesional attention bias and left hemispatial neglect. Computerized time-limited tasks are more sensitive than conventional paper-pencil tests in detecting these spatial attention deficits. However, their frequency in the acute stage of stroke, the neuroanatomical basis and functional relevance for patients’ everyday life are unclear.
Method
A realistic visual search task is introduced, in which eye movements are recorded while the patient searches for paperclips among different everyday objects on a computer display. The “Desk task” performance of 34 acute right hemisphere stroke patients was compared to established paper-pencil tests for neglect and the Posner reaction time task, and finally correlated to structural brain lesions.
Results
Most of the patients, even those without clinical neglect signs and with normal paper-pencil test performance, exhibited a clear ipsilesional attention bias in the Desk task. This bias was highly correlated to the left-right asymmetry in the Posner task and to neglect-related functional impairment scores. Lesion-symptom mapping revealed task-specific differences: deficits in the Desk task were associated with lesions of the superior temporal gyrus, contralesional unawareness in the Posner task with ventral frontal cortex lesions and paper-pencil cancellation bias with damage to the inferior parietal lobe. Neglect behavior was further associated with distinct fronto-parietal white matter tract disconnections (ILF, SLF, Arcuate).
Conclusions
Results from the novel Desk task indicate a functional relevance of spatial attention deficits in right brain damaged patients, even if they are “subclinical”. This should be considered especially in patients without obvious clinical neglect signs.
Keywords: Spatial Attention, Neglect, Stroke, Eye Movements
Hemispatial neglect constitutes a major cognitive syndrome that frequently occurs after right hemisphere stroke (Parton, Malhotra, & Husain, 2004; Ringman, Saver, Woolson, Clarke, & Adams, 2004). Patients show strong lateralized (but also non-lateralized) spatial attention deficits: they are unaware of objects, people and even their own body parts in the contralesional left half of space (Husain & Rorden, 2003; Robertson & Halligan, 1999). Due to the resulting impairments in the activities of daily living, hemispatial neglect counts among the major predictors for poor functional outcome following stroke (Jehkonen et al., 2000; Katz, Hartman-Maeir, Ring, & Soroker, 1999).
One, if not the main, core symptom of spatial neglect is an ipsilesional spatial attention bias leading to an unawareness of stimuli in contralesional space (Driver & Vuilleumier, 2001; Robertson & Halligan, 1999). An attentional priority map in the brain that is imbalanced due to the unilateral brain damage has been proposed as the pathophysiological basis (Pouget & Driver, 2000). Following this hypothesis, the damaged brain favors objects in ipsilesional hemispace in the competition for attention, whereas contralesional objects must be either extremely salient or relevant, i.e. of higher attentional priority, to be noticed (Bays, Singh-Curry, Gorgoraptis, Driver, & Husain, 2010; Fecteau & Munoz, 2006).
The spatial attention bias can be assessed by different measures. In clinical practice usually batteries of different paper-and-pencil tests are used at the bedside to diagnose the heterogeneous neglect syndrome that cannot be detected by a single test in all patients (Azouvi et al., 2006; Parton et al., 2004; Wilson, Cockburn, & Halligan, 1987). The individual paper-and-pencil tests differ with respect to their sensitivity and retest-reliability in acute and chronic stroke patients (Azouvi et al., 2002; Machner, Mah, Gorgoraptis, & Husain, 2012). Furthermore, several studies have shown that computerized reaction time tasks are more sensitive than standard paper-and-pencil tests especially in the detection of lateralized spatial attention deficits in patients with mild or remitted neglect (Bonato, Priftis, Marenzi, Umilta, & Zorzi, 2010; Deouell, Sacher, & Soroker, 2005; Erez, Katz, Ring, & Soroker, 2009; Rengachary, d'Avossa, Sapir, Shulman, & Corbetta, 2009; Schendel & Robertson, 2002). However, most of these studies did not relate the abstract neuropsychological test performance to clinical impairments or neglect-related functional disability and therefore could not provide information on the relevance of a mild lateralized attention deficit for patients’ activities of daily living. Furthermore, they lacked detailed neuroanatomical information such as the exact size and location of brain lesions. We know from different studies, using the lesion-symptom mapping approach including novel meta-analyses, that there is not only one critical lesion site that determines whether a patient develops spatial neglect or not (Chechlacz, Rotshtein, & Humphreys, 2012; Molenberghs, Sale, & Mattingley, 2012; Verdon, Schwartz, Lovblad, Hauert, & Vuilleumier, 2010; Vuilleumier, 2013). In line with the heterogeneity of the behavioral neglect symptoms there is a variation in the brain lesions that explain different impairments of this multicomponent syndrome.
In our study, we confronted acute right hemisphere stroke patients with a real-life search situation using a novel, computerized visual search task including eye movement recordings - the Desk task. Patients were stratified into one of three groups based on their neglect-related functional disability as assessed by the Catherine Bergego Scale: no neglect, moderate or severe neglect. Their performance in the Desk task was compared to the performance in an established battery of paper-and-pencil tests for neglect and the well-known Posner reaction time task (Posner, Walker, Friedrich, & Rafal, 1984). Finally, patients’ behavioral data were related to neuroanatomical data of structural brain damage using voxel-based lesion symptom mapping.
The main questions were:
-
(i)
Is the naturalistic Desk task equally or even more sensitive than established computerized or paper-and-pencil tests in detecting neglect behavior in acute right hemisphere stroke patients?
-
(ii)
Is there a common or distinct neural basis for lateralized deficits in right hemisphere stroke patients among the different tasks for spatial attention?
-
(iii)
What is the clinical and functional relevance of an ipsilesional spatial attention bias in the everyday life of right brain damaged patients?
Methods
Participants
The study has been approved by the local Ethics Committee of the University of Lübeck (AZ 12-064). Written informed consent according to the Declaration of Helsinki was obtained from all participants. The patients were in-patients at the Department of Neurology, University Hospital Schleswig-Holstein, Campus Lübeck, who had suffered a first-time right hemisphere stroke as proven by cranial magnetic resonance imaging. Patients were screened for visual field defects (e.g. homonymous hemianopia) and visual extinction by finger perimetry at the bedside. For the assessment of primary afferent visual field defects, each of the four quadrants were stimulated by peripheral finger wiggling two times in a random order and the patients were asked to verbally respond by indicating the side of the stimulus. If a stimulus was missed both times on unilateral stimulation, a visual field defect was documented. Those patients with quadrantanopia or hemianopia were excluded from the study and did not undergo further testing. In case of intact visual fields, a bilateral stimulation with peripheral finger wiggling in the upper or lower quadrants was performed (again two runs each) and visual extinction was documented if the contralesional stimulus was missed at least once on bilateral stimulation. Visual extinction, as opposed to primary visual fields defects, was no exclusion criterion.
Patients were further clinically examined and assessed by use of the following scales: the NIH Stroke Scale, the modified Rankin Scale, the Barthel Index as a general measure of functional independence and the Catherine Bergego Scale (CBS) as a more specific measure of neglect-related functional disability (Azouvi et al., 2003). The CBS was based on direct observation of the patient’s functioning in 10 real-life situations as assessed by the occupational therapist and the stroke nurse of the patient. For each of ten items there is a score between 0 (no neglect) and 3 (severe neglect), the maximum total score sums up to 30 points. If one or more items could not be assessed in a patient, we applied an equation by Chen et al. to correct for the missing data without falsely reducing the overall neglect score in the patient (Chen, Hreha, Fortis, Goedert, & Barrett, 2012).
Based on the degree of neglect-related functional disability as reflected by the Catherine Bergego Scale (CBS) the patients were assigned to one of three subgroups: (i) no neglect (CBS score = 0), (ii) moderate neglect (CBS score from 1 to 15) or (iii) severe neglect (CBS score from 16 to 30).
Eleven healthy subjects with no neurological or ophthalmological disease constituted the control study group.
Demographical, clinical and functional characteristics of the participants are presented in Table 1 separately for the four study groups. There was no significant difference between the study groups with respect to age (F(1,41)=0.167, p>0.9), gender (Chi2=7.18, p>0.06) and time since lesion (F(2,31)=0.679, p>0.5).
Table 1. Clinical characteristics of the study groups.
| Healthy Controls | No Neglect | Moderate Neglect | Severe Neglect | |
|---|---|---|---|---|
| Number of subjects | 11 | 10 | 12 | 12 |
| Demographic | ||||
| Age (years) | 69 ±4 | 71 ±3 | 68 ±3 | 69 ±3 |
| Sex (n female) | 8 | 7 | 5 | 3 |
| Clinical | ||||
| Time since stroke (d) | - | 4 ±1 | 4 ±1 | 5 ±1 |
| Type of stroke | ||||
| - Ischaemic (n) | - | 9 | 10 | 11 |
| - Haemorrhagic (n) | - | 1 | 2 | 1 |
| Visual extinction (n) | 0 | 0 | 7 | 11 |
| Functional | ||||
| Catherine Bergego Scale | 0 | 0 | 8±1 | 24±1 |
| mRankin Score | 0 | 2.4 ±0.4 | 3.1 ±0.4 | 4.4 ±0.2 |
| NIH-Stroke Scale | 0 | 3 ±1 | 5 ±1 | 11 ±1 |
| Barthel Index (%) | 100 | 69 ±10 | 53 ±11 | 13 ±5 |
Mean ±sem.
Computerized tests for spatial attention
All participants underwent two computerized tests for spatial attention: (i) the Desk task, a novel naturalistic visual search task in an everyday-life situation and (ii) a reaction time task as a variant of the classic Posner cueing paradigm (Posner et al., 1984).
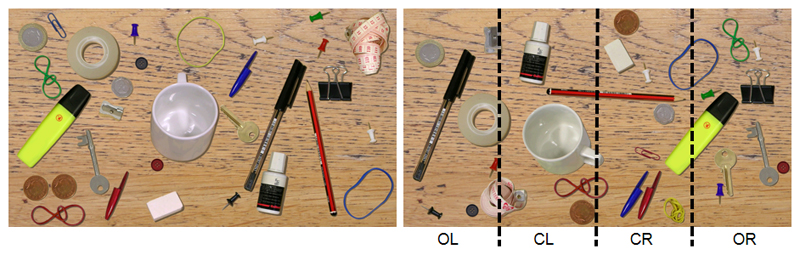
For the Desk task, participants were seated in front of a 24” widescreen TFT monitor (Samsung SyncMaster 2443BW with a resolution of 1920 x 1200 pixel and a refresh rate of 60 Hz). At an eye-to-screen distance of 60 cm the display covered a visual field of 48° x 30°. Each trial started with a central fixation cross presented on a black background, followed by the presentation of a naturalistic image of a desk scene (Figure 1). On the desk there were 30 different everyday objects, e.g. a pen, a coin, a key. Patients were instructed to search for a paperclip that could be either red or blue. As soon as they found the target, they should press a response button. Afterwards, in order to control for false alarms, they were asked to report the colour of the target detected. Then the examiner manually started the next trial. Each trial was finished after a maximum of 12 seconds or earlier by a button press upon target detection. Hence, the duration of each individual trial varied depending on the participant’s speed of search, but no stimulus image was presented longer than the maximum duration of 12 seconds. There were a total of 100 different desk scene images that were always presented in the same order. In 80 % of the images there was a target present, 20% were no-target trials. With respect to its horizontal x-position on the screen, the target was located with an equal probability within one of four columns (outmost left, left of centre, right of centre or outmost right).
Figure 1. Desk task stimulus.
Left: An example of the naturalistic image of a desk (left picture) that was presented to the participants, who were instructed to search for a paperclip and report its colour (either red or blue).
Right: Offline data analysis was performed separately for four different horizontal locations (columns) on the screen in which the target could have been presented: outmost left (OL), centre left (CL), centre right (CR), outmost right (OR). In this example the target (red paperclip) was placed in the CR column.
For the trials where a target was present, we analyzed the detection rate, i.e. the percentage of target-present trials where the participant pressed the response button to signal target detection and provided the correct colour of the paper-clip. The search duration was the time until the button was pressed or the maximum duration of a single trial (12 seconds) in cases where the target was not found.
In 32 out of 34 patients and in 6 out of the 11 age-matched control subjects we recorded eye movements throughout the Desk task using a contact-free remote eye tracker running at 50 Hz (SMI RED-X, Teltow, Germany). From the eye movement data we derived the horizontal fixation distribution including the parameters Centre of Fixation and Field of Exploration.
The Posner reaction time task is able to assess three components of visual attention that are typically impaired in hemispatial neglect (Posner et al., 1984; Rengachary, He, Shulman, & Corbetta, 2011): (i) A lateralized deficit in visual perception and attention, i.e. a relative response time (RT) delay or lower accuracy for targets presented in the contralesional, as compared to the ipsilesional visual field (side/visual field effect), (ii) a deficit of reorienting spatial attention, i.e. a relative delay in responding to targets at unattended, as compared to attended locations (validity effect) and (iii) a deficit in disengaging attention from the ipsilesional field reflected by specific difficulties in responding to the unattended targets in the contralesional field (disengagement deficit).
For our Posner paradigm, patients were seated in front of a notebook (HP Pavilion dv8-1190eg) with an 18.4” TFT wide screen monitor of 1920x1080 pixel resolution. The display showed a dark-grey background with a light-grey fixation cross at the centre and two light-grey square frames (size 1.5°), positioned on the horizontal meridian at either side with an eccentricity of 3.5° from the centre. The cue consisted of one of the rectangle frames turning yellow for 300 ms. After a delay of 150 or 500 ms (stimulus onset asynchrony, SOA), the target (a light grey asterisk) appeared within one of the two frames and remained present until a response was made or 2 seconds had elapsed. An interval of 1 second separated trials. In 50% of the trials, the target appeared at the location indicated by the cue (valid condition), while on the other 50% of the trials, it appeared at the opposite location (invalid condition). Hence, the peripheral cues were exogenous and “uninformative” with an equal probability of targets occurring in the cued or uncued box (Chica, Bartolomeo, & Valero-Cabre, 2011). Patients were instructed to press a response button with their right index finger as soon as the target appeared.
There were two experimental blocks of 40 trials each (10 valid left, 10 valid right, 10 invalid left and 10 invalid right). There was a brief period of rest between the two blocks. We analyzed reaction time and the number of target responses (detection rate). Trials in which the participant missed the target were assigned the longest possible RT (2000 ms) (see also (Rengachary et al., 2009)).
Paper-and-pencil tests
Patients were investigated using a paper-and-pencil test battery for spatial neglect (Machner, Dorr, et al., 2012; Machner, Konemund, Sprenger, von der Gablentz, & Helmchen, 2014), originally adapted from the French “Batterie d’évaluation de la négligence spatial” (Azouvi et al., 2006). In detail, it consisted of the following tests: Line bisection, Star cancellation and Text reading task from the German version of the Behavioral Inattention Test (Wilson et al., 1987), the Bells cancellation test (Gauthier, Dehaut, & Joanette, 1989) and a Figure copying task (Ogden, 1985).
Besides the standard evaluation of the cancellation task performance (number of targets cancelled on the right and on the left) we used the software by Rorden and Karnath (Rorden & Karnath, 2010) to assess the Center of Cancellation (CoC) as a quantitative marker of the focus of attention in horizontal space (Binder, Marshall, Lazar, Benjamin, & Mohr, 1992; Gainotti, Perri, & Cappa, 2002).
Lesion analyses
Using the MRIcron software (http://people.cas.sc.edu/rorden/mricron/), the lesion of every stroke patient was first delineated directly on the individual FLAIR image where the acute stroke lesion is best visible within the first days (Rorden & Brett, 2000). Since T1 images achieve the best normalisation results, the FLAIR image with the delineated lesion was co-registered to the T1 scan and then normalised to the Montreal Neurological Institute (MNI) space (Seghier, Ramlackhansingh, Crinion, Leff, & Price, 2008). Notably, we had to exclude 2 patients (one “no neglect” and one “severe neglect” patient) from the lesion analyses due to insufficient quality of the magnetic resonance images for normalization processing.
Using MRIcron, the individual MRI lesion volume was determined for each patient and the lesion overlap images per group were created. Furthermore, a subtraction analysis was performed to visualise regions that were damaged more frequently in neglect than in no-neglect patients. To evaluate and denominate the affected regions with respect to grey matter and white matter fibre tracts we overlaid the maps on the Automated Anatomical Labelling atlas (Tzourio-Mazoyer et al., 2002) and on the JHU white matter tractography atlas (Hua et al., 2008).
To investigate the relationship between lesion location and behavioral deficits (clinical scores, paper-and-pencil and computerized test performances) voxel-based lesion-symptom mapping analyses were performed using the t-test statistics of the Non-Parametric Mapping software NPM that comes with the MRIcron package (Rorden, Karnath, & Bonilha, 2007). Only significant voxels damaged in at least 20% of subjects that survived the correction for multiple comparisons using false discovery rate (FDR) are reported and presented.
In order to assess the relevance of disconnected white matter tracts, we mapped the lesion from each patient onto tractography reconstructions of white matter pathways obtained from a group of healthy controls (Rojkova et al., 2016) using free available software from the BCBtoolkit (http://www.toolkit.bcblab.com) (Foulon et al., 2018).
In detail, “disconnectome maps” were calculated by using a set of 10 healthy controls (Rojkova et al., 2016) diffusion weighted imaging datasets and tracking fibers passing through each patient’s lesion. For each participant tractography was estimated as indicated in a previous work by Thiebaut de Schotten and colleagues (Thiebaut de Schotten et al., 2011). Patients' lesions in the MNI152 space are registered to each control native space using affine and diffeomorphic deformations (Avants et al., 2011; Klein et al., 2009) and subsequently used as seed for the tractography in Trackvis (http://www.nmr.mgh.harvard.edu/~rpwang/dtk). Tractographies from the lesions were transformed in visitation maps (Thiebaut de Schotten et al., 2011), binarised and brought to the MNI152 using the inverse of precedent deformations. Finally, a percentage overlap map was produced by summing at each point in MNI space the normalized visitation map of each healthy subject. Hence, in the resulting disconnectome map, the value in each voxel take into account the interindividual variability of tract reconstructions in controls, and indicate a probability of disconnection from 0 to 100% for a given lesion (Thiebaut de Schotten et al., 2015). For the disconnectomes of our patients, the default threshold of >50% probability of disconnection was chosen.
In the last step, we quantified the severity of the disconnection by measuring the probability of different tracts relevant for visuospatial attention to be disconnected in each patient by using the “Tractotron” software from the BCB toolkit (Foulon et al., 2018; Thiebaut de Schotten et al., 2014). In the subsequent statistical analyses using SPSS, we analyzed the impact of white matter tract disconnection on behavioral parameters such as, for instance, the exploration bias in cancellation tasks and visual search (CoC - center of cancellation; CoF - center of fixation).
Statistics
Statistical analyses were performed with SPSS (22.0.0.2; IBM Corp., Somer, NY, USA). Data are reported as Mean ± Standard Error of the Mean (SEM), error bars in the figures show the SEM. In order to analyze the test performances either one-factor ANOVAs (paper-and-pencil tests, clinical data) or ANOVAs with repeated measures (computerized tests) were performed, using GROUP as between-subject factor and target POSITION and cue VALIDITY in the Posner paradigm or target POSITION in the Desk paradigm as the within-subject factors. In some comparisons the sphericity requirement was violated. Therefore, we report F-values with Greenhouse-Geisser correction but report degrees of freedom (df) uncorrected in order to show the factorial analysis design. Significance levels of post-hoc t-tests were Bonferroni corrected for multiple testing; the statistical significance level was set at p < 0.05. Correlation analyses were performed using the non-parametric Spearman-Rho’s coefficient because most of the data (e.g. clinical scores) was either not normally distributed or not on a continuous scale.
Results
Desk task
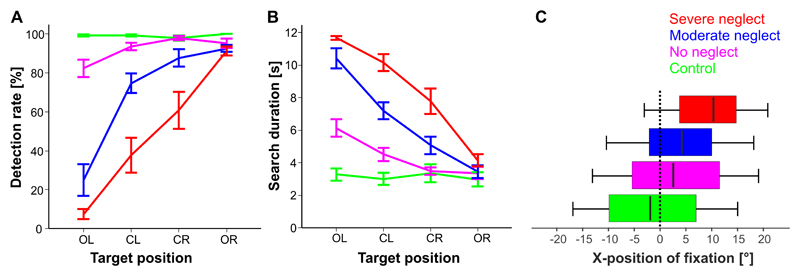
The results are depicted in Figure 2 (A and B) separately for the 4 study groups and the 4 possible horizontal target positions on the screen (outmost left column “OL”, centre left “CL”, centre right “CR”, outmost right “OR”).
Figure 2. Desk task performance.
Detection rate (A) and search duration (B) are depicted separately for the four study groups and the horizontal x-position (column), where the target was presented on the screen: outmost left (OL), centre left (CL), centre right (CR) or outmost right (OR).
(C) Horizontal fixation distribution is illustrated as a boxplot function of fixations (cumulative time of gaze samples) in relation to the horizontal location where they landed on the screen, separately for the four study groups. The median band represents the Center of Fixation (CoF), where 50% of all gaze samples were left and 50% right of its x-position. Boxes represent the horizontal range where 25% (left end) or 75% (right end) of the fixations fell into. The ends of the whiskers reflect the leftmost and rightmost 2.5% of horizontal positions of fixations. After averaging over the participants of each group 95% of all gaze samples fell into that range, thus they represent the mean Field of Exploration for each study group.
For the parameter detection rate the ANOVA revealed main effects for POSITION (F(3,41)=80.4, p<0.001), GROUP (F(3,41)=47.5, p<0.001) and for the interaction POSITION*GROUP (F(9,123)=21.4, p<0.001). Post-hoc analyses revealed the following differences:
-
(i)
In the “no neglect” group, the detection rate for the outmost left target position was significantly lower than for the center right target position (d=15.6 ±0.05 %, p=0.039). In the outmost left target (OL) position, “no neglect” patients had lower detection rates than healthy controls (d=16 ±4 %, p=0.024).
-
(ii)
In the “severe neglect“ group, there were continuously decreasing detection rates from the outmost right target position to the outmost left (p always <0.001). A similar pattern was evident in the “moderate neglect” group (p always <0.05 for CR to CL and CL to OL) with the exception of OR to CR (n.s.).
For the parameter search duration the ANOVA revealed main effects for POSITION (F(3,41)=87.2, p<0.001), GROUP (F(3,41)=38.4, p<0.001) and for the interaction POSITION*GROUP (F(9,123)=21.3, p<0.001). Post-hoc analyses revealed the following differences:
-
(i)
The “no neglect” group showed significantly longer search durations for targets in the outmost left column of the screen (OL) than for targets in the other three columns (p always <0.01). For targets in the OL position, “no neglect” patients had significantly longer search durations than healthy controls (d=2.9 ±0.7 sec, p=0.002).
-
(ii)
Both the severe and the moderate neglect group showed a typical attentional gradient with increasing search durations for target positions along the horizontal axis from right to left (p always <0.01).
-
(iii)
Comparing the search durations between all study groups for those targets in the outmost right column of the screen yielded no significant differences, i.e. even severe neglect patients were as fast as healthy controls.
From the eye movement recordings we derived the horizontal fixation distribution by relating the cumulative time of all gaze samples to the x-positions on the screen where they landed.
Fig. 2C depicts the results as a Boxplot function, where the median represents the Center of Fixation (CoF) and the range of the whiskers the Field of Exploration.
The one-factor ANOVA on the Center of Fixation (CoF) showed a significant main effect for GROUP (F(3,37)=26.9, p<0.001). Post-hoc analyses revealed that the center of fixation in the “no neglect” group (3.5 ±0.7°) was significantly rightward deviated (d=5.7 ±1.4°, p<0.01) as compared to the healthy controls (-2.2 ±0.5°), and was not significant different from the spatial exploration bias of “moderate neglect” patients (4.3 ±0.9°). The “severe neglect” group, however, showed the greatest rightward shift of exploratory eye movements with a CoF at 10.0° (±0.9°), as compared to all the other study groups (p always <0.001).
The oneway ANOVA on the Field of Exploration revealed a significant effect for GROUP (F(3,37)=6.9, p<0.01). Post-hoc analyses showed that only the “severe neglect” group had a significantly reduced field of exploration (23.8 ±1.2°) as compared to healthy controls (31.4 ±1.4°, p=0.009) and “no neglect” patients (30.9 ±1.0°, p=0.005), while the other three groups including the “moderate neglect” (28.4 ±1.4°) patients did not differ from each other.
Posner paradigm
Due to the 2x2 task’s design we analyzed detection rates and reaction times for the following four conditions: (i) both target and cue appeared on the left (POSITION: left, VALIDITY: valid); (ii) the target appeared on the left, the cue on the right (POSITION: left, VALIDITY: invalid); (iii) the target appeared on the right, the cue on the left (POSITION: right, VALIDITY: invalid); (iv) both target and cue appeared on the right (POSITION: right, VALIDITY: valid).
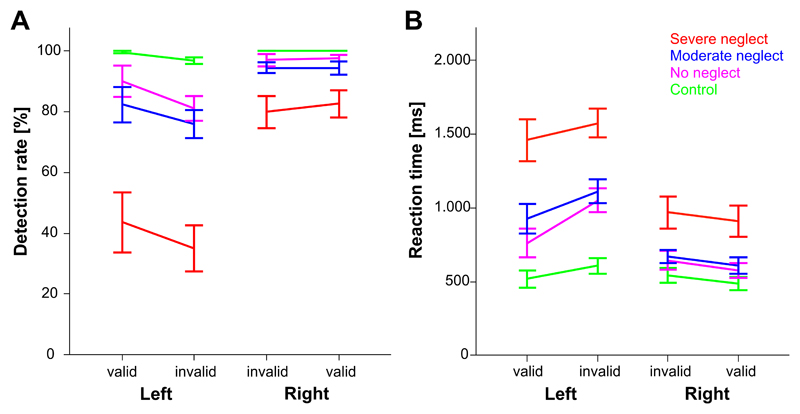
Fig. 3 depicts the results for the detection rate and reaction time of the four study groups separately for the four different target-cue conditions.
Figure 3. Posner task performance.
Detection rate (A) and reaction time (B) are depicted separately for the four study groups, the position/visual hemifield where the target appeared (Left or Right) and the validity of the preceding cue (valid or invalid).
For the dependent variable detection rate the ANOVA revealed main effects for GROUP (F(3,41)=18.4, p<0.001), POSITION (F(1,41)=74.4, p<0.001) and VALIDITY (F(1,41)=21.3, p<0.001). Significant interactions were found for GROUP*POSITION (F(3,41)=18.1, p<0.001) and POSITION*VALIDITY (F(1,41)=11.5, p=0.002). Post-hoc analyses yielded the following differences:
-
(i)
Severe neglect patients had lower detection rates than all the other study groups, this was pronounced for targets in the left (p always <0.001) but also evident for targets in the right hemifield (p always <0.01).
-
(ii)
The “no neglect” patients detected fewer targets on the left than on the right side (d=11.8 ±4.3 %, p=0.01). This hemifield effect was very similar to the “moderate neglect” group (d=15.3 ±4.0 %, p<0.001). The “severe neglect” group exhibited the greatest hemifield difference (d=41.9 ±4.0 %, p<0.001).
-
(iii)
In the patient groups, the validity effect of the cue depended on the hemifield in which the target was subsequently presented. Thus, only for targets on the left but not on the right, an invalid cue led to a significant decrease in the detection rate (“no neglect”: p=0.007, “moderate neglect”: p=0.031, “severe neglect”: p=0.005). However, a POSITION x VALIDITY ANOVA, separately performed for each patient group (within-group analysis), did not reveal a significant interaction and specific disengagement deficit (p always > 0.23).
-
(iv)
Healthy controls’ detection rates did not differ between left and right targets or whether these had been preceded by valid or invalid cues.
The ANOVA and the post-hoc analyses on the variable reaction time (RT) yielded similar results as for the detection rate. There were main effects for GROUP (F(3,41)=14.7, p<0.001), POSITION (F(1,41)=135.8, p<0.001) and VALIDITY (F(1,41)=62.5, p<0.001). Significant interactions were found for GROUP*POSITION (F(3,41)=15.9, p<0.001) and POSITION*VALIDITY (F(1,41)=14.0, p=0.001).
Post-hoc analyses yielded the following differences:
-
(i)
In contrast to healthy controls, the “no neglect” patients showed higher reaction times for targets on the left than on the right (p<0.001). This hemifield effect was also evident in the “moderate neglect” group (p<0.001) and the “severe neglect” group (p<0.001). Notably, severe neglect patients had higher reaction times than all the other study groups, pronounced for targets in the left hemifield (p<0.001) but also for targets in the right hemifield (p always <0.021).
-
(ii)
In the patient groups (p always <0.031), the validity influenced the reaction times in both hemifields, i.e. invalid cues led to an increase of the reaction times for targets in either hemifield (deficit of reorienting). Healthy controls showed such a validity effect only for targets in the right hemifield (p=0.031; left hemifield p=0.101).
-
(iii)
Compared to controls, the no neglect group (and the other two patient groups) had significantly slower reaction times for unattended (invalidly cued) targets in the contralesional hemifield (p<0.003). However, when performing the POSITION x VALIDITY ANOVA separately for each patient group (within-group analysis), there was again no significant interaction that would indicate a specific disengagement deficit (p always > 0.15).
Paper-and-pencil tests
The mean results of the four study groups for the different paper-and-pencil tests are presented in Table 2.
Table 2. Paper-and-pencil test performance of the different study groups.
| Healthy Controls | No Neglect | Moderate Neglect | Severe Neglect | |
|---|---|---|---|---|
| Bells Test | ||||
| Cancellations left | 15.9 ±0.3 | 13.6 ±1.0 | 5.9 ±1.3 | 1.1 ±0.7 |
| Cancellations right | 16.4 ±0.2 | 14.1 ±1.0 | 13.4 ±0.9 | 7.3 ±1.6 |
| Center of Cancellation (CoC) | 0.01 ±0.01 | 0.03 ±0.01 | 0.32 ±0.07 | 0.77 ±0.07 |
| Star Cancellation | ||||
| Cancellations left | 27.0 ±0.0 | 24.0 ±0.9 | 14.3 ±3.4 | 4.7 ±2.5 |
| Cancellations right | 26.9 ±0.1 | 25.4 ±0.8 | 22.3 ±1.6 | 14.8 ±2.3 |
| Center of Cancellation (CoC) | 0.0 ±0 | 0.02 ±0.01 | 0.30 ±0.10 | 0.68 ±0.10 |
| Line Bisection | ||||
| Rightward deviation from the centre [mm] | 1.9 ±1.4 | 7.1 ±2.3 | 14.8 ±5.0 | 21.7 ±3.2 |
| Text Reading | ||||
| Errors [n] | 0 ±0 | 0 ±0 | 33 ±13 | 68 ±14 |
| Figure copying | ||||
| Ogden Scene (Score) | 0 ±0 | 1.3 ±0.5 | 2.7 ±0.4 | 4.0 ±0 |
Mean ±sem.
The ANOVAs performed for the different parameters of each test consistently yielded a significant main effect for GROUP (p always <0.01). The post-hoc analyses revealed the following pattern for the different tests:
-
(i)
The “no neglect”-group did not differ significantly from healthy controls in Bells CoC, Bells cancellations left, Stars CoC, Stars cancellations left, Line bisection, Text reading errors and the Ogden score.
-
(ii)
The “moderate neglect”-group showed worse test performance than healthy controls in Bells CoC (p<0.01), Bells cancellations left (p<0.01), Stars CoC (p<0.05), Stars cancellations left (p<0.01), Line bisection (trend, p<0.1), Ogden score (p<0.01), but not in Text reading errors (p=0.15).
-
(iii)
The “severe neglect”-group performed worse than controls in Line bisection (p<0.01) and Reading errors (p<0.01), and even worse than the “moderate neglect”-group with respect to the Bells CoC (p<0.01), Bells cancellations left (p<0.01), Stars CoC (p<0.01), Stars cancellations left (p<0.05) and the Ogden score (p<0.05).
Lateralization indices and correlation of behavioral parameters
In order to obtain one marker for the lateralized deficit in each of the computerized attention tasks, we calculated a Lateralization Index (LI) for both the Desk and the Posner task. By using the following equation, we subtracted the detection rate for targets in the left hemifield from the one in the right hemifield and divided by their sum:
Thereby, we received a Lateralization Index for each patient in each task, which ranged from -1 (strongest leftward bias) over 0 (no bias) to +1 (strongest rightward bias). This marker resembles the CoC in the Bells test and the CoF in the Desk task.
The highest correlation between the CBS score as a marker of neglect-related functional disability and all the different paper-and-pencil test parameters was for the CoC from the Bells test (r=0.904, p<0.001). Among the computerized task parameters, the mean Search Duration in the Desk task showed the highest correlation with the CBS score (r=0.791, p<0.001), followed by the Desk’s Lateralization Index (r=0.772, p<0.001). But also the oculomotor parameter CoF from the Desk task (r=0.705), the Lateralization Index in the Posner task (r=0.594) and its mean Reaction Time (r=0.473) were significantly correlated with the CBS score (p always <0.001).
The lateralized deficit in the computerized tasks correlated highly with the respective general speed of performance in either task (Desk’s LI with Search Duration: r=0.852, p<0.001; Posner’s LI with Reaction Time: r=0.869, p<0.001). Between the different tasks, the LI in the Desk task correlated significantly with the LI in the Posner task (r=0.699, p<0.001) and with the CoC in the Bell’s task (r=0.804, p<0.001).
Lesion overlaps and voxel-wise lesion symptom mapping
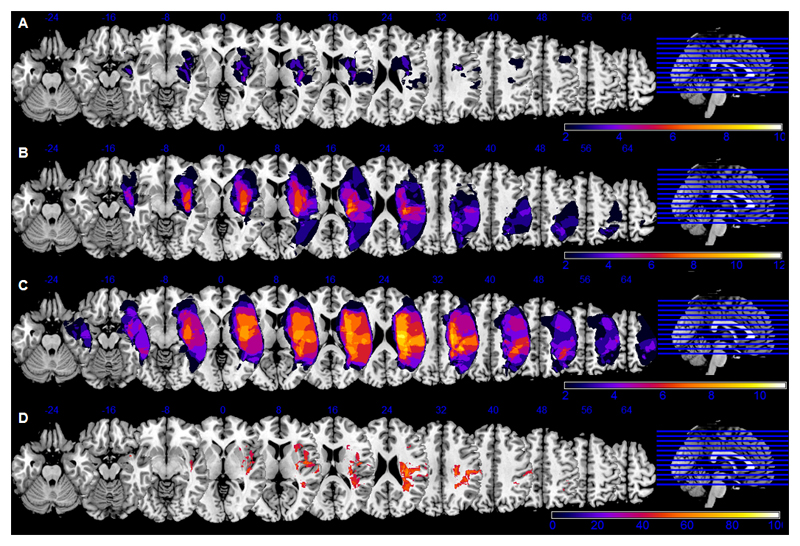
Fig. 4 (A-C) depicts the overlap analyses of stroke lesions separately for the three patient groups. The rather heterogenous “no neglect” group (mean lesion size = 28.0 ±27.5 cm3) had on average smaller brain lesions than the “severe neglect” group (158.6 ±24.9 cm3; p=0.004), whereas the “moderate neglect” group (75.4 ±23.8 cm3) did not differ significantly from the others. Furthermore, lesion volume correlated significantly with the neglect-related behavioural impairments as assessed by functional scales (CBS score: r=0.570, p=0.001), paper-and-pencil tests (CoC Bells: 0.619, p<0.001) and computerized tasks (Desk’s LI: r=0.462, p=0.008; Desk’s CoF: r=0.485, p=0.007; Posner’s LI: r=0.509, p=0.003).
Figure 4. Lesion overlaps and subtraction analysis.
Lesion overlap analyses were performed separately for “no neglect” (A), “moderate neglect” (B) and “severe neglect” (C) patients. The absolute number of overlapping lesions in a specific voxel/area is indicated by the color bar (min. n=2 patients show damage in this voxel, max. = all patients in the group have damage in this voxel).
The subtraction analysis (D) show brain regions that were at least 40% (percentage color coded up to max = 100%) more frequently damaged in patients with clinically manifest neglect than in “no neglect” patients: the insula and inferior frontal gyrus, the supramarginal gyrus and the fronto-parietal white matter.
The Montreal Neurological Institute (MNI) Z coordinates are presented above each transverse section.
The subtraction analysis (Fig. 4D), i.e. “no neglect” patients subtracted from the sum of “moderate” and “severe neglect” patients, revealed the following regions that were more frequently involved in patients with clinical manifest neglect: the ventral frontal cortex (including insula and inferior frontal gyrus), the inferior parietal lobe (supramarginal gyrus) and the fronto-parietal white matter (superior longitudinal fasciculus).
Next, a voxel-wise lesion symptom mapping was performed for the following behavioral parameters: CBS score, CoC Bells, Deviation in Line bisection; Reaction time (mean) and Lateralization Index in the Posner task; Search duration (mean), Lateralization Index and the Center of Fixation (CoF) in the Desk task. Figures 5 and 6 illustrate the major results.
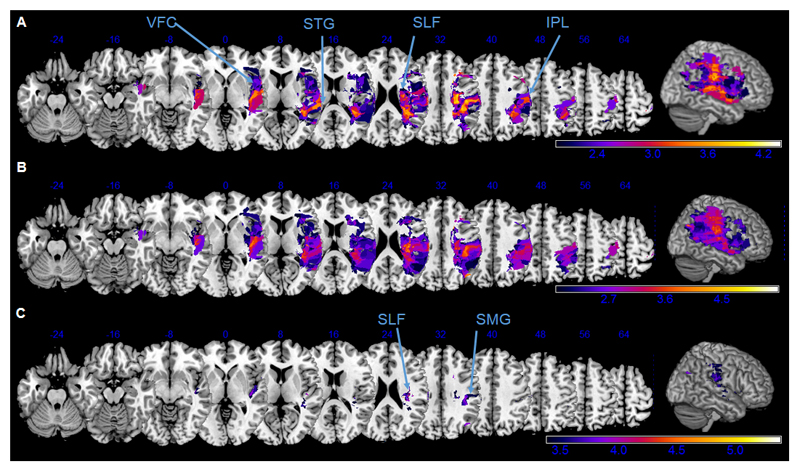
Figure 5. Voxel-wise lesion symptom mapping of CBS score and Bell’s CoC.
Neuronal correlates of the neglect related functional disability (CBS score, A) and the spatial attention bias in the Bell’s cancellation task (CoC, B and C), as revealed by a voxel-based lesion-symptom mapping. Z-values are shown color-coded, FDR-corrected at p<0.05 (A, B) or p<0.01 (C). Only voxels that were damaged in at least 20% of the subjects were analysed. Z coordinates above each transverse slice are given in MNI space. Detailed description in the main text.
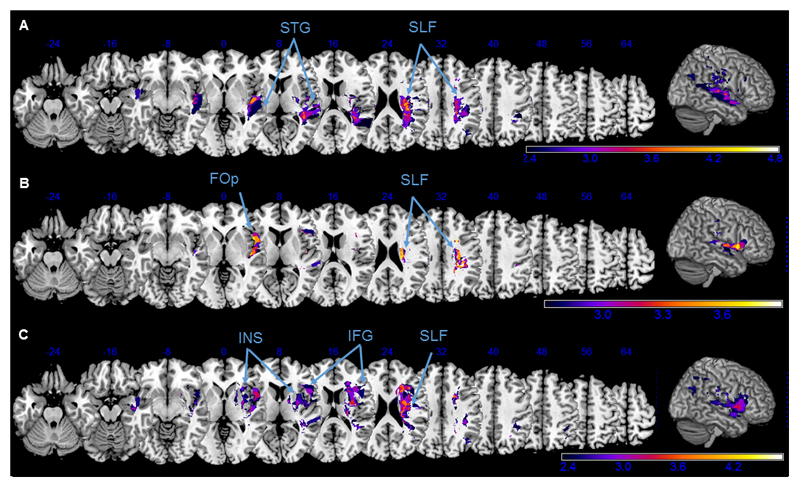
Figure 6. Voxel-wise lesion symptom mapping of computerized task parameters.
Anatomical correlates of the spatial attention deficits in the computerized tasks, revealed by a voxel-based lesion-symptom mapping for the following behavioral parameters: Search Duration (A) and Centre of Fixation (B) in the Desk task and Lateralization Index in the Posner task (C). Z-values are shown color-coded, FDR-corrected p < 0.05. Only voxels that were damaged in at least 20% of the subjects were analysed. Z coordinates above each transverse slice are given in MNI space. Detailed description in the main text.
The brain regions that were more frequently damaged in patients with higher neglect related functional disability (CBS score, Fig. 5A) were the ventral frontal cortex (VFC), the superior temporal gyrus (STG) and the inferior parietal lobe (IPL), and the white matter tracts along the superior longitudinal fasciculus (SLF). Interestingly, these were almost identical to the regions that were associated with a stronger rightward bias in the Bells cancellation task (CoC, Fig. 5B). Lesioned voxels in the supramarginal gyrus (SMG) and along the SLF (Fig. 5C) survived even the stricter p<0.01 level of significance, indicating a critical role of those structures in the Bells cancellation performance.
In the VLSM analysis of Line deviation (bisection error) there were no voxels that attained the level of statistical significance.
The VLSM for the different computerized task parameters (Fig. 6) revealed an interesting dissociation of their individual neuronal bases, despite the high correlation of their behavioral results as reported in the previous section.
Among the Desk task parameters, an increase of the mean search duration was associated with damage to the STG (Fig. 6A). While the VLSM of the Desk’s Lateralization Index (i.e. difference in detecting targets in the left versus the right visual hemifield) yielded only a few significant voxels along the SLF (not illustrated), the rightward oculomotor bias during the accompanying visual exploration (CoF) was significantly related to lesions affecting the frontal operculum (Fig. 6B).
In the VLSM analysis of mean reaction time in the Posner task, there were no significant clusters of voxels that attained statistical significance. However, missing left sided targets during the Posner task (Lateralization index) was significantly associated with lesions of the insula (INS) and the inferior frontal gyrus (IFG, Fig. 6C).
For all the test parameters reported above, voxels in the deep fronto-parietal white matter along the SLF consistently attained statistical significance (Fig. 6A-C).
We also ran all the VLSM analyses with “lesion volume” as an additional regressor/covariate but due to the relatively small and heterogenous stroke cohort, these analyses did not reveal voxels that survived the statistical threshold of FDR p<0.05.
White matter tract disconnections
In order to further analyze the impact of disconnections of the different white matter tracts for neglect behavior and the spatial attention bias, we applied the Disconnectome and Tractotron software from the BCB toolkit to our patients’ lesion data (see Method section).
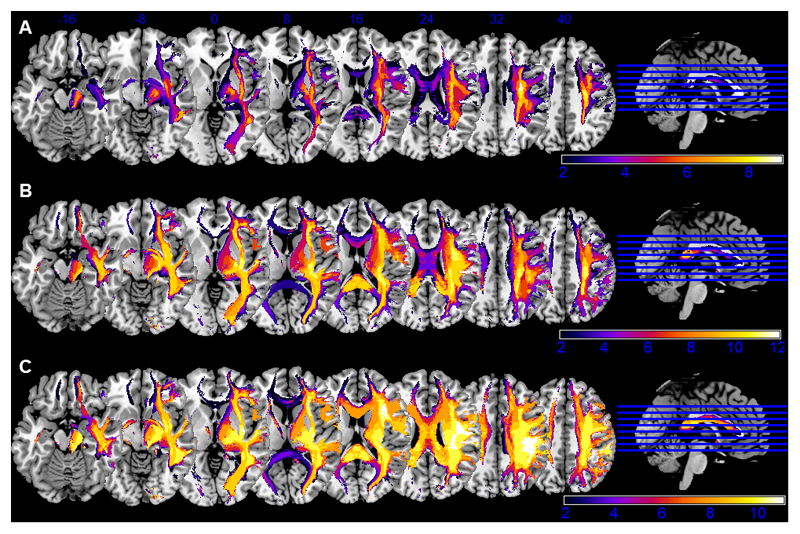
The disconnectome maps, showing those tracts that are disconnected with a probability of >50% by the patient’s lesion, are visualized as group-wise overlaps for the three groups of different neglect severities in Figure 7. In both neglect patient groups, the disconnectomes indicate severe white matter disconnections for the fronto-parietal tracts (SLF I-III) in the right hemisphere as well as affection of the corpus callosum. However, the disconnectome maps of patients without clinical signs of neglect (see Fig. 7a) also comprised these fronto-parietal white matter tracts, even if their involvement appears less severe.
Figure 7. Overlap of disconnectome maps.
The patients’ individual disconnectomes (visualizing those tracts that are disconnected by the lesion with >50% probability) are presented as overlaps for the different study groups (A: no neglect, B: moderate neglect, C: severe neglect). The absolute number of overlapping disconnectomes in a specific area is indicated by the color bar. The upper line shows blue Z coordinates of the slices in MNI space.
We therefore applied the Tractotron software to our patients’ lesion and behavioral data, in order to analyze the association of structural disconnections and behavioral deficits more precisely. Table 3 provides group results with respect to the relation of neglect severity and disconnections of several white matter tracts that have been previously associated with spatial neglect (Bartolomeo, Thiebaut de Schotten, & Doricchi, 2007; Doricchi, Thiebaut de Schotten, Tomaiuolo, & Bartolomeo, 2008). It shows that almost all the patients with moderate or severe neglect had a disruption of white matter tracts including the posterior segment of the arcuate fasciculus (Arcuate), the inferior fronto-occipital fasciculus (IFOF), the inferior longitudinal fasciculus (ILF) and the three branches of the superior longitudinal fasciculus (SLF I, II, III). However, the majority of the right hemisphere stroke patients without any clinical signs of neglect also showed a disruption of the SLF II, III and the IFOF, whereas >50% of these patients had no lesions along the temporo-parietal part of the arcuate and the SLF I (Table 3).
Table 3. Disconnections of right hemisphere white matter tracts in the different study groups.
| No Neglect |
Moderate Neglect |
Severe Neglect |
|
|---|---|---|---|
| Arcuate, posterior segment | 44 | 83 | 100 |
| Inferior fronto-occipital fasciculus | 78 | 92 | 91 |
| Inferior longitudinal fasciculus | 56 | 83 | 91 |
| Superior longitudinal fasciculus I | 44 | 67 | 91 |
| Superior longitudinal fasciculus II | 89 | 92 | 100 |
| Superior longitudinal fasciculus III | 89 | 100 | 100 |
Percentage (%) of patients within each study group whose brain lesions disconnected the tracts of interest with a probability of >50%.
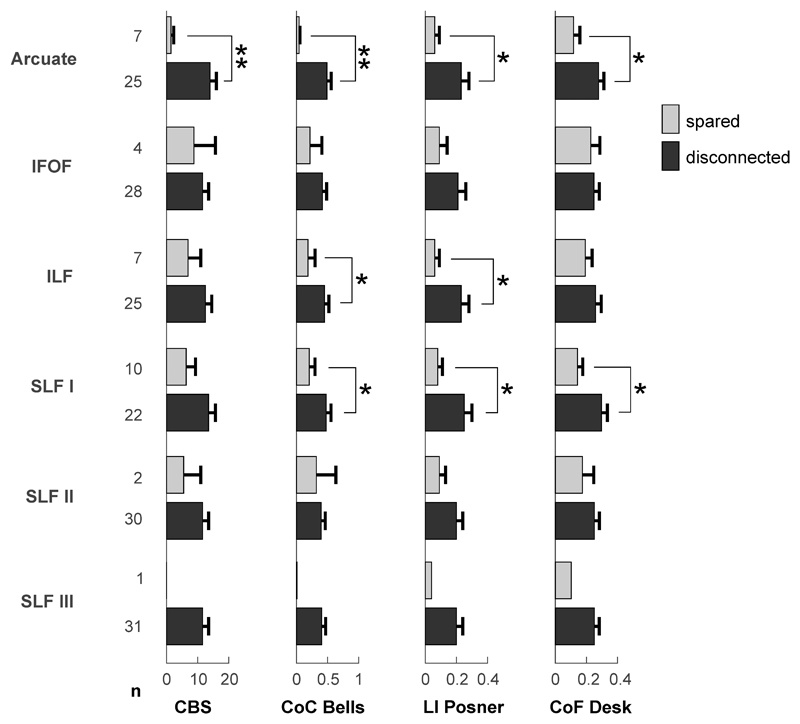
In the next step, we therefore investigated the patients’ performance in different behavioral tests as a function of white matter tract involvement by their brain lesions. Using the results from the Tractotron analysis, patients were divided into those whose lesions spared a particular tract and those whose lesions disconnected the tract with at least 50% probability. Then, their mean results in different behavioral test parameters as revealed by the previous VLSM analyses (see section 3.5) were compared between a sparing and disconnection of relevant tracts (Fig. 8). The posterior segment of the arcuate fasciculus appeared relevant for all the behavioral parameters indicating a spatial attention bias. To specify, sparing of this tract was associated with normal or nearly normal performance in the Bells test (CoC) and both computerized tests (Posner’s lateralization index, Desk CoF) as well as with a lack of clinical neglect signs (CBG), whereas disruption of the tract was correlated with a strong ipsilesional attention bias. Similar results were found for the ILF for the parameters Bells CoC and Posner lateralization index. Surprisingly, only the disconnection of branch I of the SLF was significantly associated with the bevioural test deficits, while patients with or without SLF II disconnection had similar performances. The SLF III was disrupted in all but one patient of our stroke cohort, which prevented a sufficient statistical comparison. Nonetheless, this group of SLF III disconnected patients involved also those patients with near to normal performance on spatial attention tests and the CBG scoring.
Figure 8. Behavioral test performances in dependence of white matter tract affection.
The behavioral test results of the CBG (Catherine Bergego Scale score), CoC Bells (center of cancellation in the Bells test), LI Posner (lateralization index in the Posner task) and CoF Desk (center of fixation in the Desk task) are depicted as a mean performance of patients without (light gray) or with (dark gray) disconnection of different white matter tracts of interest. The number of patients (n) with or without disconnection of the according tract is provided on the left of the y-axis. Error bars show SEM. * p < 0.05, ** p <0.01, Mann-Whitney-U test.
Discussion
Sensitivity of the realistic Desk task for spatial attention deficits
Using a novel computerized task that simulated a real-life visual search situation, we have shown that acute right hemisphere stroke patients both with and without clinical signs of spatial neglect exhibit a functionally relevant ipsilesional spatial attention bias that leads to misses of contralesional targets and a rightward shift of exploratory eye movements.
In acute right brain damaged patients who showed normal performance in the paper-and-pencil test battery for spatial neglect (“no neglect” group), the lateralized deficit was evident in the novel visual search task as in the Posner reaction time task. In the Desk task, the “no neglect” patients showed the typical pattern of a declining attentional gradient for target locations from right to left, though on a milder level than in those patients with moderate or severe neglect. The equivalent in the Posner task was the decreased detection rate and increased reaction time for contralesional targets (Posner et al., 1984; Rengachary et al., 2011).
It is known that time-limited tasks and tasks of higher attentional demands (Bonato et al., 2010; Deouell et al., 2005; Olk, Harvey, & Gilchrist, 2002; Posner et al., 1984; Rapcsak, Verfaellie, Fleet, & Heilman, 1989; Rengachary et al., 2009; van Kessel, van Nes, Brouwer, Geurts, & Fasotti, 2010) are able to disclose a “hidden” contralesional attention deficit which patients with mild or recovered neglect can compensate for during simple paper-and-pencil tests without time constraints (see reviews by: (Bonato, 2012; Schendel & Robertson, 2002)). Furthermore, different studies previously showed that the ipsilesional deviation of exploratory eye movements is a typical sign of spatial neglect (Behrmann, Ebert, & Black, 2004; Hornak, 1992; Karnath, Niemeier, & Dichgans, 1998; Ptak, Golay, Muri, & Schnider, 2009) and that this oculomotor bias even seems to be especially sensitive for the detection of mild neglect in the chronic stage (Pflugshaupt et al., 2004).
We could show, however, that this is not only true for tasks that test reflexive shifts of spatial attention towards rapidly presented abstract stimuli but also for a realistic visual search task, where goal-directed shifts had to be performed towards everyday objects in a naturalistic visual environment. To summarize, our novel realistic visual search task was as sensitive as the established Posner task for contralesional attention deficit in acute right hemisphere stroke patients and certainly more sensitive than the conventional paper-and-pencil test battery that classified some of the affected patients as “normal”.
The neural basis of the ipsilesional attention bias
The common neural basis of the ipsilesional attention bias may be the previously proposed attentional priority map, imbalanced due to unilateral brain damage and preferring ipsilesional over contralesional stimuli (Fecteau & Munoz, 2006; Pouget & Driver, 2000; Serences & Yantis, 2006). Although neurons along the intraparietal sulcus (IPS) exhibit necessary features such as multisensory processing and the ability to encode spatial location of objects in multiple egocentric frames of reference (Pouget & Driver, 2000; Ptak & Fellrath, 2013), the IPS is certainly not the only region that hosts an attentional priority map and that can explain all the facets of the multi-component neglect syndrome.
In line with a network theory of spatial attention processing and previous lesion-symptom studies on neglect including meta-analyses (Chechlacz et al., 2012; Corbetta & Shulman, 2011; Fecteau & Munoz, 2006; Molenberghs et al., 2012; Serences & Yantis, 2006; Verdon et al., 2010), our lesion overlaps and voxel-wise lesion symptom mapping analyses did not yield a single critical cortical region responsible for the ipsilesional attention bias, but pointed to several regions involved in different processes of spatial attention.
The subtraction analysis between neglect and no neglect patients as well as the VLSM analysis of neglect-related functional disability (CBS score) revealed common regions typically associated with neglect behavior (Doricchi et al., 2008; Mort et al., 2003; Verdon et al., 2010): the ventral frontal cortex (including insula and inferior frontal gyrus), the inferior parietal lobe (particularly the supramarginal gyrus) and the fronto-parietal white matter.
The VLSM analyses of the different computerized and paper-pencil test parameters, however, revealed several dissociations. The ipsilesional attention bias, as reflected by the “Center of Fixation” in the Desk task or the “Lateralization Index” in the Posner task, was specifically correlated with ventral frontal cortex lesions (insula, inferior frontal gyrus, frontal operculum). This is in line with previous observations and the assumed role of the VFC in combining information from the ventral and dorsal attention networks (Rengachary et al., 2011).
In contrast, the paper-and-pencil test equivalent, i.e. the rightward shifted Center of Cancellation in the Bells test, was highly associated with inferior parietal lesions affecting the supramarginal gyrus. This underlines the role of the parietal lobe as an integrator of multisensory information from objects in space on its way to a motor response, i.e. sensory-motor transformation such as required for visual search and cancellation (Pouget & Driver, 2000; Ptak & Fellrath, 2013).
An increased mean search duration in the Desk task was associated with lesions involving the superior temporal gyrus (STG). While its “crucial” role for the development for neglect behavior has been extensively debated and remains controversial (Karnath, Ferber, & Himmelbach, 2001; Mort et al., 2003), a previous study using inhibitory transcranial magnetic stimulation over the right STG showed disturbances of visual search performance in difficult feature search after the stimulation (Ellison, Schindler, Pattison, & Milner, 2004). Hence, the STG plays some role in visual search for objects in space and discrimination of targets from distractors. However, neither our lesion-symptom mapping on the neglect-related functional disability nor the subtraction analysis on clinically manifest neglect versus no-neglect patients detected a critical involvement of the STG.
Beside the different cortical structures mentioned above, it was voxels in the deep fronto-parietal white matter that consistently attained statistical significance in classical nonparametric VLSM analyses of almost all the reported test parameters. We applied novel tools of white matter analyses (Foulon et al., 2018) in order to disentangle whether this really indicates a critical role of the tracts for spatial attention processes or whether this just reflects the usual anatomy of vascular supply in patients with infarction of the middle cerebral artery. First, disconnectome maps indicated a profound affection of fronto-parietal white matter tracts and the corpus callosum in those patients with moderate to severe neglect, which would support the view of spatial neglect as a disconnection syndrome (Bartolomeo et al., 2007; Doricchi et al., 2008; Hattori et al., 2017). In line of this proposal, analyzing the impact of individual white matter tract disconnections on behavioral parameters revealed a significant association between the disconnection of the arcuate fasciculus (the temporo-parietal, posterior segment) and the first branch of the superior longitudinal fasciculus (SLF I) and also partly of the inferior longitudinal fasciculus. In contrast, we found no correlation of SLF II disruption and spatial attention tests performances. Moreover, despite involvement of the SLF III in almost all brain lesions of our stroke patients, only a part of them had a manifest spatial neglect syndrome. This does not exclude a relevant role of these two SLF branches for spatial attention (Bartolomeo et al., 2007; Thiebaut de Schotten et al., 2014), but argues against the view of SLF disconnections as a necessary prerequisite for spatial neglect development. It might also indicate the common vascular supply of the middle cerebral artery, of which large territorial infarctions, that typically lead to the full-blown picture of spatial neglect due to multifocal cortical damage (Gottesman et al., 2008; Ringman et al., 2004), overlap in central parts of the right hemisphere involving those white matter tracts. In line with these previous observations, our lesion-symptom mapping analyses including correlations of lesion size and behavioral neglect parameters revealed that larger brain lesions were associated with more severe neglect. It still remains a matter of debate and hard to disentangle, however, whether the disrupted fronto-parietal white matter tracts in right hemisphere stroke patients with large brain lesions and severe neglect are responsible for the cognitive deficit or just epiphenomena in terms of “collateral damage”, while lesions of (multiple or remote) cortical sites are deemed causal for neglect. Future studies on this specific issue will have to respect the multifaceted nature of neglect and its different components (Molenberghs et al., 2012; Verdon et al., 2010), the potential consistent errors of lesion-symptom inferences from data with high-dimensional structure (Mah, Husain, Rees, & Nachev, 2014) and the fact that brain lesions have local and remote effects that impact functionally and structurally connected circuits in the brain (Foulon et al., 2018).
Clinical and functional relevance of “subclinical” lateralized attention deficits
What is the functional relevance of the ipsilesional attention bias detected by the computerized tests, especially if it so mild (“subclinical”) that it is missed by the established paper-and-pencil test batteries and clinical examination for neglect? Does our realistic visual search task really bear a diagnostic advantage for care providers in testing stroke patients for spatial attention deficits?
As described above, the time-limited computerized Desk task disclosed a spatial attention bias in right hemisphere stroke patients without clinical signs of neglect that was not detected by the established paper-and-pencil tests. Hence, its sensitivity for lateralized spatial attention deficits in these patients was higher than for the conventional bedside tests. The question remains, however, whether this mild bias is of functional relevance for the patients. Among the different paper-and-pencil tests, cancellation tasks (such as the Bells test in our study or the Mesulam cancellation task in previous studies (Azouvi et al., 2002; Rengachary et al., 2009; Rorden & Karnath, 2010)) have proven not only very sensitive for spatial neglect, they correlate especially well with the functional impairments of the patients. In fact, it was not a parameter from the computerized tasks that showed the highest correlation with the Catherine Bergego Scale (CBS) score in our stroke cohort, but the Center of Cancellation from the Bells test. Hence, one could argue that time-limited computerized tasks disclose a mild spatial attention bias that, nonetheless, is not functionally relevant for the patients in their everyday life. However, while the CBS may be a clinically feasible and relevant score of neglect-related functional disability in the basic activities of daily living, such as eating from a plate, getting dressed and moving on the ward or rehabilitation unit, it certainly does not cover more demanding activities such as orienting in traffic or shopping in the supermarket. These situations of higher attentional demand may be better reflected by more complex and time-limited tasks with numerous objects competing for attention. This assumption is supported by a case report of Deouell and colleagues (Deouell et al., 2005). They describe a right hemisphere stroke patient who fully recovered from initial neglect according to his Behavioral Inattention Test performance (almost the highest score) and who showed normal visual fields on perimetry. After receiving back his driver’s license, however, he was involved in nine car accidents always affecting the left side of his car. In this patient, the authors uncovered a significant slowing of responses to left-sided as compared to right-sided events in a computerized, dynamic reaction time task. Since our Desk task directly tests a realistic visual search in a naturalistic environment of everyday objects, and not the latencies of responses to rapidly presented abstract objects, it provides a concrete information on an important everyday life function. Deficits in this task can then be extrapolated to other situations of similar or higher attentional demands that patients encounter in their everyday life.
Conclusions and limitations
We have to emphasize that, in contrast to previous studies that investigated computerized reaction time tasks in neglect patients in a subacute or chronic stage (Bays et al., 2010; Deouell et al., 2005; Rengachary et al., 2011), our stroke cohort was tested in a very acute stage of stroke (range 1 - 13 days post-stroke, mean 4 days). While brain lesions in the MRI scans at that time (and consecutively our lesion-symptom analyses) may be influenced by edema due to early disturbances of cerebro-vascular autoregulation, the behavioral data are less affected by individual compensational processes and reflect the pure attentional deficits and sometimes full blown clinical picture of hemispatial neglect. Although compensational processes lead to a remission of neglect symptoms in most of the acute patients, a significant proportion of right hemisphere stroke patients will exhibit spatial attention deficits and especially the ipsilesional bias continuing into their chronic stage (Jehkonen et al., 2000; Rengachary et al., 2011; Ringman et al., 2004). Since this bias can be extremely functionally relevant in more demanding everyday life situations as demonstrated by the Desk task, care providers in and outside rehabilitation units should consider these deficits especially in those right hemisphere stroke patients who are otherwise less impaired and may have originally been diagnosed as “no neglect” patients.
Public Significance Statements.
Introducing a realistic visual search task with everyday objects (Desk scene), we demonstrate a functionally relevant ipsilesional attention bias in right hemisphere stroke patients, that was evident even in those patients without spatial neglect on clinical assessment and established paper-and-pencil tests. We highlight the distinct neuroanatomical basis of this attention bias depending on the different tests used and discuss its functional relevance for patients and the consequences for care providers.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (grants MA5332/1-1 and MA5332/3-1 to BM) and the Wellcome Trust (grants 091806 and 106926 to PMB).
References
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouvi P, Bartolomeo P, Beis JM, Perennou D, Pradat-Diehl P, Rousseaux M. A battery of tests for the quantitative assessment of unilateral neglect. Restorative Neurology and Neuroscience. 2006;24(4–6):273–285. [PubMed] [Google Scholar]
- Azouvi P, Olivier S, de MG, Samuel C, Louis-Dreyfus A, Tesio L. Behavioral assessment of unilateral neglect: study of the psychometric properties of the Catherine Bergego Scale. Archives of Physical Medicine and Rehabilitation. 2003;84(1):51–57. doi: 10.1053/apmr.2003.50062. [DOI] [PubMed] [Google Scholar]
- Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, Beis JM, et al. Rousseaux M. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. Journal of Neurology, Neurosurgery and Psychiatry. 2002;73(2):160–166. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17(11):2479–2490. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Bays PM, Singh-Curry V, Gorgoraptis N, Driver J, Husain M. Integration of goal- and stimulus-related visual signals revealed by damage to human parietal cortex. Journal of Neuroscience. 2010;30(17):5968–5978. doi: 10.1523/JNEUROSCI.0997-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Ebert P, Black SE. Hemispatial neglect and visual search: a large scale analysis. Cortex. 2004;40(2):247–263. doi: 10.1016/s0010-9452(08)70120-5. [DOI] [PubMed] [Google Scholar]
- Binder J, Marshall R, Lazar R, Benjamin J, Mohr JP. Distinct syndromes of hemineglect. Arch Neurol. 1992;49(11):1187–1194. doi: 10.1001/archneur.1992.00530350109026. [DOI] [PubMed] [Google Scholar]
- Bonato M. Neglect and extinction depend greatly on task demands: a review. Front Hum Neurosci. 2012;6:195. doi: 10.3389/fnhum.2012.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato M, Priftis K, Marenzi R, Umilta C, Zorzi M. Increased attentional demands impair contralesional space awareness following stroke. Neuropsychologia. 2010;48(13):3934–3940. doi: 10.1016/j.neuropsychologia.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Chechlacz M, Rotshtein P, Humphreys GW. Neuroanatomical Dissections of Unilateral Visual Neglect Symptoms: ALE Meta-Analysis of Lesion-Symptom Mapping. Front Hum Neurosci. 2012;6:230. doi: 10.3389/fnhum.2012.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Hreha K, Fortis P, Goedert KM, Barrett AM. Functional assessment of spatial neglect: a review of the Catherine Bergego scale and an introduction of the Kessler foundation neglect assessment process. Top Stroke Rehabil. 2012;19(5):423–435. doi: 10.1310/tsr1905-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chica AB, Bartolomeo P, Valero-Cabre A. Dorsal and ventral parietal contributions to spatial orienting in the human brain. J Neurosci. 2011;31(22):8143–8149. doi: 10.1523/JNEUROSCI.5463-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Spatial neglect and attention networks. Annual Review of Neuroscience. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deouell LY, Sacher Y, Soroker N. Assessment of spatial attention after brain damage with a dynamic reaction time test. Journal of the International Neuropsychological Society. 2005;11(6):697–707. doi: 10.1017/S13556177050824. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P. White matter (dis)connections and gray matter (dys)functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex. 2008;44(8):983–995. doi: 10.1016/j.cortex.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. 2001;79(1–2):39–88. doi: 10.1016/s0010-0277(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Ellison A, Schindler I, Pattison LL, Milner AD. An exploration of the role of the superior temporal gyrus in visual search and spatial perception using TMS. Brain. 2004;127(Pt 10):2307–2315. doi: 10.1093/brain/awh244. [DOI] [PubMed] [Google Scholar]
- Erez AB, Katz N, Ring H, Soroker N. Assessment of spatial neglect using computerised feature and conjunction visual search tasks. Neuropsychol Rehabil. 2009;19(5):677–695. doi: 10.1080/09602010802711160. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends in Cognitive Sciences. 2006;10(8):382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Foulon C, Cerliani L, Kinkingnéhun S, Levy R, Rosso C, Urbanski M, et al. Thiebaut de Schotten M. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience. 2018;7(3):1–17. doi: 10.1093/gigascience/giy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G, Perri R, Cappa A. Left hand movements and right hemisphere activation in unilateral spatial neglect: a test of the interhemispheric imbalance hypothesis. Neuropsychologia. 2002;40(8):1350–1355. doi: 10.1016/s0028-3932(01)00211-1. [DOI] [PubMed] [Google Scholar]
- Gauthier L, Dehaut F, Joanette Y. The Bells test. J Clin Exp Neuropsychol. 1989;11:49–54. [Google Scholar]
- Gottesman RF, Kleinman JT, Davis C, Heidler-Gary J, Newhart M, Kannan V, Hillis AE. Unilateral neglect is more severe and common in older patients with right hemispheric stroke. Neurology. 2008;71(18):1439–1444. doi: 10.1212/01.wnl.0000327888.48230.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Ito K, Nakazawa C, Numasawa Y, Watanabe M, Aoki S, et al. Yokota T. Structural connectivity in spatial attention network: reconstruction from left hemispatial neglect. Brain Imaging Behav. 2017 doi: 10.1007/s11682-017-9698-7. [DOI] [PubMed] [Google Scholar]
- Hornak J. Ocular exploration in the dark by patients with visual neglect. Neuropsychologia. 1992;30(6):547–552. doi: 10.1016/0028-3932(92)90057-s. [DOI] [PubMed] [Google Scholar]
- Hua K, Zhang JY, Wakana S, Jiang HY, Li X, Reich DS, et al. Mori S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nature Reviews Neuroscience. 2003;4(1):26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Jehkonen M, Ahonen JP, Dastidar P, Koivisto AM, Laippala P, Vilkki J, Molnar G. Visual neglect as a predictor of functional outcome one year after stroke. Acta Neurol Scand. 2000;101(3):195–201. doi: 10.1034/j.1600-0404.2000.101003195.x. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411(6840):950–953. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Niemeier M, Dichgans J. Space exploration in neglect. Brain. 1998;121(Pt 12):2357–2367. doi: 10.1093/brain/121.12.2357. [DOI] [PubMed] [Google Scholar]
- Katz N, Hartman-Maeir A, Ring H, Soroker N. Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Archives of Physical Medicine and Rehabilitation. 1999;80(4):379–384. doi: 10.1016/s0003-9993(99)90273-3. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner B, Dorr M, Sprenger A, von der GJ, Heide W, Barth E, Helmchen C. Impact of dynamic bottom-up features and top-down control on the visual exploration of moving real-world scenes in hemispatial neglect. Neuropsychologia. 2012;50(10):2415–2425. doi: 10.1016/j.neuropsychologia.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Machner B, Konemund I, Sprenger A, von der Gablentz J, Helmchen C. Randomized controlled trial on hemifield eye patching and optokinetic stimulation in acute spatial neglect. Stroke. 2014;45(8):2465–2468. doi: 10.1161/STROKEAHA.114.006059. [DOI] [PubMed] [Google Scholar]
- Machner B, Mah YH, Gorgoraptis N, Husain M. How reliable is repeated testing for hemispatial neglect? Implications for clinical follow-up and treatment trials. Journal of Neurology, Neurosurgery and Psychiatry. 2012;83(10):1032–1034. doi: 10.1136/jnnp-2012-303296. [DOI] [PubMed] [Google Scholar]
- Mah YH, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain. 2014;137(Pt 9):2522–2531. doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Sale MV, Mattingley JB. Is there a critical lesion site for unilateral spatial neglect? A meta-analysis using activation likelihood estimation. Front Hum Neurosci. 2012;6:78. doi: 10.3389/fnhum.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual neglect. Brain. 2003;126(Pt 9):1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Ogden JA. Contralesional neglect of constructed visual images in right and left brain-damaged patients. Neuropsychologia. 1985;23(2):273–277. doi: 10.1016/0028-3932(85)90112-5. [DOI] [PubMed] [Google Scholar]
- Olk B, Harvey M, Gilchrist ID. First saccades reveal biases in recovered neglect. Neurocase. 2002;8(4):306–313. doi: 10.1076/neur.8.3.306.16191. [DOI] [PubMed] [Google Scholar]
- Parton A, Malhotra P, Husain M. Hemispatial neglect. J Neurol Neurosurg Psychiatry. 2004;75(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- Pflugshaupt T, Bopp SA, Heinemann D, Mosimann UP, von Wartburg R, Nyffeler T, et al. Muri RM. Residual oculomotor and exploratory deficits in patients with recovered hemineglect. Neuropsychologia. 2004;42(9):1203–1211. doi: 10.1016/j.neuropsychologia.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4(7):1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Driver J. Relating unilateral neglect to the neural coding of space. Current Opinion in Neurobiology. 2000;10(2):242–249. doi: 10.1016/s0959-4388(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Ptak R, Fellrath J. Spatial neglect and the neural coding of attentional priority. Neurosci Biobehav Rev. 2013;37(4):705–722. doi: 10.1016/j.neubiorev.2013.01.026. [DOI] [PubMed] [Google Scholar]
- Ptak R, Golay L, Muri RM, Schnider A. Looking left with left neglect: the role of spatial attention when active vision selects local image features for fixation. Cortex. 2009;45(10):1156–1166. doi: 10.1016/j.cortex.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Verfaellie M, Fleet WS, Heilman KM. Selective attention in hemispatial neglect. Arch Neurol. 1989;46(2):178–182. doi: 10.1001/archneur.1989.00520380082018. [DOI] [PubMed] [Google Scholar]
- Rengachary J, d'Avossa G, Sapir A, Shulman GL, Corbetta M. Is the posner reaction time test more accurate than clinical tests in detecting left neglect in acute and chronic stroke? Archives of Physical Medicine and Rehabilitation. 2009;90(12):2081–2088. doi: 10.1016/j.apmr.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengachary J, He BJ, Shulman GL, Corbetta M. A behavioral analysis of spatial neglect and its recovery after stroke. Front Hum Neurosci. 2011;5:29. doi: 10.3389/fnhum.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63(3):468–474. doi: 10.1212/01.wnl.0000133011.10689.ce. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Halligan PW. Spatial neglect: a clinical handbook for diagnosis and treatment. Hove: Psychology Press; 1999. [Google Scholar]
- Rojkova K, Volle E, Urbanski M, Humbert F, Dell'Acqua F, Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct Funct. 2016;221(3):1751–1766. doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioural Neurology. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. A simple measure of neglect severity. Neuropsychologia. 2010;48(9):2758–2763. doi: 10.1016/j.neuropsychologia.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. Journal of Cognitive Neuroscience. 2007;19(7):1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Schendel KL, Robertson LC. Using reaction time to assess patients with unilateral neglect and extinction. Journal of Clinical and Experimental Neuropsychology. 2002;24(7):941–950. doi: 10.1076/jcen.24.7.941.8390. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Ramlackhansingh A, Crinion J, Leff AP, Price CJ. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. Neuroimage. 2008;41(4):1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10(1):38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M. A lateralized brain network for visuospatial attention. Nat Neurosci. 2011;14(10):1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Ratiu P, Leslie A, Howells H, Cabanis E, et al. Catani M. From Phineas Gage and Monsieur Leborgne to H.M.: Revisiting Disconnection Syndromes. Cereb Cortex. 2015;25(12):4812–4827. doi: 10.1093/cercor/bhv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Tomaiuolo F, Aiello M, Merola S, Silvetti M, Lecce F, et al. Doricchi F. Damage to white matter pathways in subacute and chronic spatial neglect: a group study and 2 single-case studies with complete virtual "in vivo" tractography dissection. Cereb Cortex. 2014;24(3):691–706. doi: 10.1093/cercor/bhs351. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Kessel ME, van Nes IJ, Brouwer WH, Geurts AC, Fasotti L. Visuospatial asymmetry and non-spatial attention in subacute stroke patients with and without neglect. Cortex. 2010;46(5):602–612. doi: 10.1016/j.cortex.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133(Pt 3):880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Mapping the functional neuroanatomy of spatial neglect and human parietal lobe functions: progress and challenges. Ann N Y Acad Sci. 2013;1296:50–74. doi: 10.1111/nyas.12161. [DOI] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Halligan P. Development of a behavioral test of visuospatial neglect. Archives of Physical Medicine and Rehabilitation. 1987;68(2):98–102. [PubMed] [Google Scholar]