Abstract
Objective
To assess differences in cognition functions and gross brain structure in children 7-years after an episode of severe acute malnutrition (SAM), compared to other Malawian children.
Design
Prospective longitudinal cohort assessing school grade achieved and results of five computer-based (CANTAB) tests, covering three cognitive domains. A subset underwent brain MRI scans which were reviewed using a standardised checklist of gross abnormalities and compared to a reference population of Malawian children.
Setting
Blantyre, Malawi
Subjects
Children discharged from SAM treatment in 2006 and 2007 (n=320; median age 9.3 years) were compared to controls: siblings closest in age to the SAM survivors and age/sex – matched community children.
Results
SAM survivors were significantly more likely to be in a lower grade at school than controls (adjusted OR 0.4, 95% CI 0.3to 0.6, p<0.0001); and had consistently poorer scores in all CANTAB cognitive tests. Adjusting for HIV and socio-economic status diminished statistically significant differences. There were no significant difference in odds of brain abnormalities and sinusitis between SAM survivors (n=49) and reference children (OR 1.11, 95% CI 0.61 to 2.03, p=0.73).
Conclusion
Despite apparent preservation in gross brain structure, persistent impaired school achievement is likely to be detrimental to individual attainment and economic well-being. Understanding the multifactorial causes of lower school achievement is therefore needed to design interventions for SAM survivors to thrive in adulthood. The cognitive and potential economic implications of SAM need further emphasis to better advocate for SAM prevention and early treatment.
Keywords: Severe acute malnutrition, acute malnutrition, long-term outcomes, post-discharge, cognitive function, brain structure, Malawi
Introduction
More than 200 million children aged under five years worldwide fail to reach their full developmental potential (1). It has long been recognised that social and environmental factors, including nutrition, have a strong influence on cognitive, language and socio-emotional development (2, 3). Recent focus on the importance of early-life exposures has resulted in strong global advocacy movements such as “Scaling Up Nutrition” (SUN) which highlights the long term impacts of the “1st 1000 days of life”(4). SUN’s main focus is on chronic childhood malnutrition resulting in stunting (low height-for-age): this has well documented adverse consequences for individual, population and societal development (5). In contrast, the links between acute malnutrition, which is also a major global public health problem, and development have been less well described.
Severe acute malnutrition (SAM) affects at least 17 million children under five worldwide (6). Infants and children in the first 2 years of life are most vulnerable due to a high basal metabolic rate, increased nutritional requirements due to rapid physical growth, and increased risk of infections (7). Reducing mortality from SAM is still a priority, however with SAM survival rates increasing, the long-term outcomes also need consideration (8).
A number of studies have explored potential effects of SAM on brain function and structure, but many of these use old case definitions of SAM, short time scales and diverse, complex testing tools which measure a variety of different outcomes (9–11). One pivotal review of studies linking SAM and mental development between 1956 and 1994 concluded that school-age children who suffered from early childhood undernutrition generally had poorer IQ levels, cognitive function, school achievement, and greater behavioural problems than matched controls and, to a lesser extent, siblings (12). However, no consistent, specific cognitive deficit was found across the studies reviewed.
A more recent review of 15 studies which included publications from large cohorts in Mauritius and Barbados (13–15), found consistent associations between SAM and various cognitive impairments including short term memory, problem solving, IQ, cognitive processing, working memory and academic skills. However, again there were no studies using current anthropometric definitions of SAM (15, 16). Moreover, these studies used different and often complex assessment tools which are unsuitable to assess cognitive outcomes in large scale, field-based, multi-outcome epidemiological studies.
A relatively quick and simple approach is the Cambridge Neuropsychological Testing Automated Battery (“CANTAB”) (17), which utilises touch-screen technology to measure cognitive function in a series of tests. CANTAB tests were recently successfully used in a trial to assess the impact of school feeding on cognitive function in Malawi (18).
Besides functional changes associated with SAM, there is also interest in possible changes in underlying brain structure. Indeed, early studies using computerized tomography (CT) showed that SAM was associated with acute brain changes (19), some of which resolved after nutritional rehabilitation (20). Similarly, more recent magnetic resonance imaging (MRI) studies during an episode of SAM showed structural changes including dilated ventricles, cerebral atrophy and periventricular white matter change (21, 22); some of these features had resolved at 90 days, but it is unknown whether any longer-term changes remain in SAM survivors.
Our study aimed to assess multiple aspects of cognition in the years following treatment for an episode of SAM, including: school achievement, cognitive function as assessed by CANTAB computer-based testing, and brain structure as assessed by MRI scan. As SAM survival increases, this evidence on its long-term outcomes is much needed, not only to shape better short-term interventions but also to better advocate for prevention strategies.
Methods
This was a longitudinal cohort study which prospectively followed-up survivors of SAM 7-years post-discharge from treatment in Blantyre, Malawi, to examine cognitive function and other health outcomes. Sibling and age/sex -matched community controls were recruited for comparison.
Study setting and subjects
Full details of the cohort, as well as additional methods and results on other outcomes have been described elsewhere (8, 23, 24). In brief, the cohort originally included all patients admitted to the nutrition ward for treatment of SAM in Queen Elizabeth Central Hospital, Blantyre, Malawi, from 12th July 2006 to 9th March 2007 (1024 children). The median age of the children at admission was 21.5 months (interquartile range 15-32 months). Results of survival and anthropometry at the baseline study and the 1-year follow-up have been described previously (25, 26). Sibling controls were defined as those closest in age to the SAM survivor (“case child”), between the ages of 6 and 15.9 years; community controls were defined as a child living in the same community, of the same sex, and within 12 months of age of the case child, randomly selected by spinning a bottle at the case child’s home to select a random direction, then enquiring door-to-door to find the first eligible child. Children who had ever been treated for acute malnutrition were excluded from the control group. Informed written consent was obtained from the child’s parent or guardian; assent was required from the children themselves. One control child per case child undertook CANTAB testing; wherever possible, community controls were prioritized over sibling controls in order to maximise age-matching.
For the MRI scans, only SAM survivors were scanned and results from the recent “Brain Imaging in Normal Kids” (BRINK) study in Blantyre, Malawi were used as a reference group in place of controls (27).
Variables
Cognitive function
cognitive function was assessed by reported school achievement and using the “Cambridge Neuropsychological Testing Automated Battery” (CANTAB)(17). School achievement was assessed by current/highest school grade as, in Malawi, graduation to the next grade is dependent on passing exams rather than dictated by a child’s age. CANTAB is a widely used, well-validated tool suitable for children aged 4 years and above, with various tests covering three cognitive domains: visual memory, visual attention, and working memory/planning (28). We used a subset of tests, selected to examine a range of cognitive functions, and also to allow us to compare our results to a previous CANTAB study in Malawi (18). Test do not require the ability to read any numerical or alphabetical values and are described in Table 1.
Table 1.
description of tests in the CANTAB assessment, presented in the order of administration used in this study
| Test | Cognitive domain | Description |
|---|---|---|
|
Working memory / planning | Largely used to familiarise the child with the computer touch screen, the child must touch a series of crosses (x) when they appear on the screen. |
|
Visual Memory | Boxes are displayed on the screen with different shapes inside them. Each shape is displayed randomly for a number of seconds and then removed. The child needs to remember which shape is in each box. More boxes are added to increase test complexity as the child progresses. |
|
Visual Memory | Random characters are displayed on the screen one after the other. At the end of the sequence each of the characters is then displayed besides another character that was not displayed. The child needs to remember which was displayed. |
|
Visual attention | Two circles are displayed on the screen, one big and the other little. The child needs to touch on one of the circles, this is followed by a confirmation of whether it is correct or not. When the “rule” changes and the other circle is correct. The child must learn and then adapt. |
|
Visual attention | A continuation of BLC: Two objects are displayed on the screen inside boxes. At first, the participant has to guess which object is correct. If they get it correct they have to press that object continuously. When the “rule” changes, they will get a message that the object is incorrect and must adapt. |
Brain structure (MRI)
Brain structure was assessed by a brain MRI scan on a sub-set of participants. The subset of SAM survivors selected for MRI was dictated by the availability of the MRI machine and the child who had a study appointment on that day. If there was more than one child appointment on that day, priority was given to the older child as they were more likely to remain still for the duration of the scan (no sedation was used). The scan was conducted following the BRINK study protocol, using a 0.35T GE Signa Ovation scanner (Sag T1 FLAIR, Ax T2 FRSE, Cor T2 FRSE, Ax DWI scans)(27). Scans were reviewed by a consultant radiologist using the BRINK standard list of possible abnormalities. When abnormalities were noted, children were referred to a child neurologist for further assessment and treated as needed.
Sample Size
Sample size was predetermined by the cohort size and survival. Community controls were more difficult to recruit than were cases because they had no previous personal connection with the study team and were restricted to the number of eligible children in the family and community. The achieved sample size was expected to be powered at 90% to detect a z-score difference of 0.5 between the cases and controls for height-for-age, the main study outcome (8). The sample size required to detect differences in CANTAB outcomes was not known due to lack of previous data. However, the achieved sample size was similar to that of a previous nutrition study with CANTAB outcomes in Malawi, where 418 children were recruited at baseline and test outcomes were compared for 100 vs 90 children at 1-year follow-up (18). We aimed for a much larger sub-sample size for MRI scans than has previously been done in SAM studies, however financial constraints were also a key consideration (21). Our sample size was half the size of the number scanned in the BRINK trial, which was studying the general population (27).
Analysis
Multivariable ordered logistic regression was used to assess differences in school grade between SAM survivors and controls, adjusted for a priori potential confounders (age, sex, HIV status and socioeconomic status (SES)). An analysis additionally adjusting for height-for-age z-score (HAZ) is also presented, given the known association between stunting and school achievement. Simple and multivariable linear regression was used to assess difference in CANTAB test scores between SAM survivors and controls; as well as the association with height-for-age z-score, wealth quintile, and severity of SAM at admission. The test “IED total stages completed” was analysed using ordered logistic regression as this is an ordered, categorical, outcome variable. Differences in the prevalence of apparent brain abnormalities between SAM survivors and the Malawian reference population were assessed using simple logistic regression. Association between brain abnormalities and potential confounders within the group of SAM survivors was also assessed in the same manner. All analyses were conducted with Stata (Release 14. College Station, TX: StataCorp LP).
Results
Cognitive function
Using ordered logistic regression, we found SAM survivors were significantly more likely to be in a lower school grade than age-matched community controls, either with or without adjustment for age, sex, HIV status, HAZ, and SES (Table 2).
Table 2.
results of ordered logistic regression analysis comparing school grade achieved for SAM survivors vs controls (reference)
| Mean (SD) for SAM survivors (n=315) | Mean (SD) for community controls (n=178) | Unadjusted odds ratio (95% CI), p value | Adjusted odds ratio‡ (95% CI), p value | Adjusted odds ratioβ inc. stunting (95% CI), p value | |
|---|---|---|---|---|---|
| School grade achieved (SAM survivors vs controls(ref)) | 2.5 (1.3) | 3.1 (1.6) | 0.50 (0.4 to 0.7), p<0.0001 | 0.40 (0.3 to 0.6) p<0.0001 | 0.54 (0.35 to 0.81) p=0.003 |
adjusted for age, sex, HIV status and socioeconomic status.
Adjusted for height-for-age z-score, age, sex, HIV status and socioeconomic status. Community controls are age and sex matched.
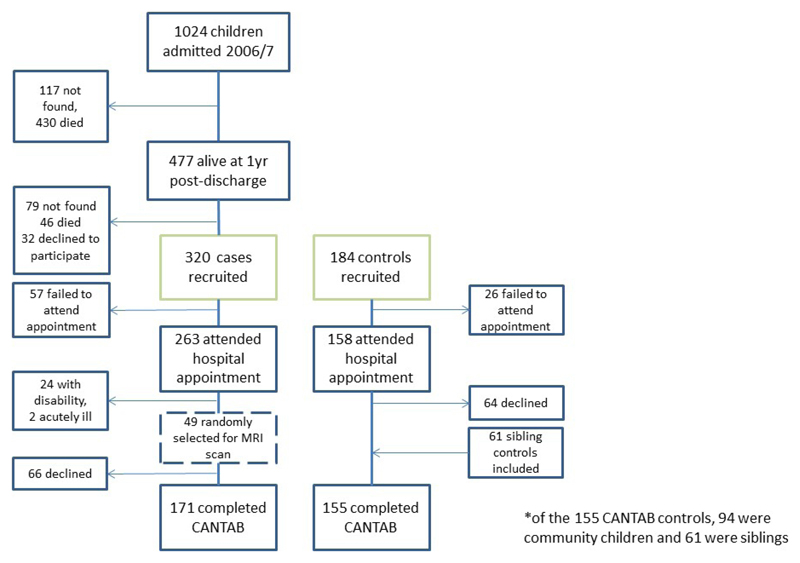
For CANTAB cognitive testing, 171 SAM survivors and 155 controls completed the tests (94 were community controls and 61 were sibling controls) (Figure 1). Inter-group comparison showed SAM survivors had on average worse scores in all eight test outcomes (Table 3). This was statistically significant for BLC (visual attention), PRM (visual memory), and IED (visual attention) when adjusted for age only. However, only BLC was statistically significantly different after adjusting for sex, HIV and SES. No outcomes were statistically significantly different after additional adjusting for HAZ. Note the large standard deviations for most outcomes suggests that a greater sample size would be necessary to detect any potential (smaller) difference. For results disaggregated by sibling and community controls, see Supplemental Table 1.
Figure 1. Recruitment flow diagram for brain structure and cognitive function outcomes.
Table 3.
results of regression analysis comparing outcomes of CANTAB tests of SAM survivors to controls
| CANTAB outcomes | SAM Survivors (n=171) | Controls (n=155) | SAM survivors vs controls Only adjusted for age |
SAM survivors vs controls Adjusted for age, sex, HIV, SES |
SAM survivors vs controls Adjusted for HAZ, age, sex, HIV, SES |
|||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Difference (CI) | P value | Difference (CI) | P value | Difference (CI) | P value | |
| BLC % correct | 94.0 (13.1) | 97.8 (5.4) | -4.02 (-6.5, -1.6) | 0.001* | -3.35 (-6.1, -0.6) | 0.02* | -1.78 (-4.1, 0.5) | 0.13 |
| IED total errors (adjusted) | 93.7 (81.5) | 75.3 (77.3) | 18.34 (-1.1, 37.7) | 0.06 | 6.79 (-13.5, 27.1) | 0.51 | 3.75 (-16.8, 24.3) | 0.72 |
| MOT mean error | 10.0 (2.8) | 9.8 (2.9) | 0.15 (-0.5, 0.8) | 0.64 | 0.31 (-0.4, 1.0) | 0.37 | 0.29 (-0.4, 1.0) | 0.41 |
| MOT mean latency (millisecs) | 1347 (502) | 1257 (423) | 86.4 (-16.0, 188.8) | 0.09 | 52.9 (-60.0, 166.0) | 0.36 | 22.3 (-90.2, 134.8) | 0.70 |
| PAL total errors (adjusted) | 111.1 (70.4) | 96.3 (71.7) | 13.9 (-1.5, 29.4) | 0.08 | 8.04 (-8.8, 24.9) | 0.35 | 5.57 (-11.4, 22.5) | 0.52 |
| PAL total errors (6 shapes, adjusted) | 31.2 (19.7) | 28.4 (20.8) | 2.61 (-1.8, 7.1) | 0.25 | 1.23 (-3.6, 6.1) | 0.62 | 0.59 (-4.3, 5.5) | 0.81 |
| PRM % correct | 63.6 (16.0) | 69.5 (16.3) | -4.36 (-8.0, -0.71) | 0.02* | -3.73 (-7.6, 0.1) | 0.06 | -3.6 (-7.5, 0.3) | 0.07 |
| IED total stages completed (ordered logistic‡) | 5.77 (3.5) | 6.5 (3.3) | -0.46 (-0.9,-0.02) | 0.04* | -0.32 (-0.8, 0.2) | 0.21 | -0.24 (-0.8, 0.3) | 0.35 |
HAZ= height-for-age z-score. SES= socioeconomic status. BLC= big/little circle; MOT=motor screening test; PAL= paired associated learning; PRM= pattern recognition memory; IED= Intra/ Extradimensional Set Shift. Linear regress used unless otherwise stated‡; *indicates significant difference (p<0.05). Test outcomes which quantify the number of total errors have been adjusted for incomplete tests as subjects who fail at earlier stages of the test have fewer opportunities to make errors.
As stunting is known to be associated with poorer cognitive outcomes, we also assessed the performance of the CANTAB tool by regressing outcomes against height-for-age z-score (HAZ) (Table 4). Results show that for every unit increase in HAZ, mean latency for MOT test was 66 milliseconds quicker (p=0.009), after adjusting for age, sex, HIV status and SES. Other test outcomes were not significantly associated with HAZ after adjustment. Associations between test results and wealth quintiles can be found in Supplemental Table 2. There was no significant associations between severity of oedema at admission (Grade 1-3), nor mid-upper arm circumference (MUAC) at admission in non-oedematous cases, with CANTAB outcomes (supplemental Table 3) suggesting no evidence of a “dose-effect” of SAM severity at admission. However sample size for this sub-analysis is small.
Table 4.
Association between CANTAB cognitive testing outcomes and height-for-age z-score (HAZ) for whole sample
| CANTAB outcomes | Unadjusted regression of CANTAB outcomes on HAZ | Adjusted (age, sex, HIV, SES) regression of CANTAB outcomes on HAZ | ||
|---|---|---|---|---|
| Unit Difference (CI) | P value | Unit Difference (CI) | P value | |
| BLC % correct | 0·80 (-0·18, 1·79) | 0·11 | 0·94 (-0·10, 1·97) | 0·08 |
| IED total errors | -0·61 (-10·0, 8·80) | 0·90 | 2·05 (-7·25, 11·35) | 0·66 |
| MOT mean error | -0·24 (-0·52, 0·05) | 0·11 | -0·24 (-0·5, 0·07) | 0·13 |
| MOT mean latency (millisecs) | -60·5 (-107, -13·2) | 0·01* | -66·0 (-115, -16·7) | 0·009* |
| PAL total errors | -0·27 (-7·5, 6·9) | 0·94 | 0·53 (-6·9, 8·0) | 0·88 |
| PAL total errors (6 shapes) | -0·21 (-2·3, 1·8) | 0·84 | 0·06 (-2·1, 2·2) | 0·95 |
| PRM % correct | 0·65 (-1·15, 2·45) | 0·48 | 0·48 (-1·25, 2·22) | 0·58 |
Linear regression of CANTAB outcomes against height-for-age z-score (HAZ) for whole sample (n=326).
indicated significant difference (p<0·05)
Brain structure (MRI)
49 survivors of SAM underwent brain MRI scans (Figure 1). 51% (25/49) had an MRI scan with an abnormality, the majority of which was sinusitis (43%; 21/49). Other abnormalities detected included gliosis (8%; 4/49) and chronic stroke (2%; 1/49) (see full descriptions in Table 5). Results were similar to those found in BRINK study reference population where 46% (44/96) of children had an MRI scan with an abnormality; similarly, the majority of the abnormalities were sinusitis (29%; 28/96); 17% (16/96) of children had an abnormal brain structure (27).
Table 5.
Summary of MRI brain scan abnormalities detected in survivors of SAM
| MRI finding | n | Sex | Age (years) | HIV status |
|---|---|---|---|---|
| Pan sinusitis | 10 | 5 Female 5 Male |
Mean 9.9 range 8-15 |
8 negative 2 positive |
| Spheno-ethmoidal sinusitis | 3 | 1 Female 3 Male |
Mean 10 range 8-13 |
1 negative 2 positive |
| Sphenoid and maxillary sinusitis | 2 | Female | Mean 8.5 range 8-9 |
Negative |
| Ethmoid and maxillary sinusitis | 3 | 2 Female 1 Male |
Mean 9.6 range 8-12 |
1 negative 2 positive |
| Frontal sinusitis | 1 | Male | 8 years | Negative |
| Maxillary sinusitis | 1 | Male | 7 years | Negative |
| Gliosis in subcortical white matter of frontal lobes | 1 | Male | 7 years | Negative |
| Gliosis of cerebellum and pan sinusitis | 1 | Female | 10 years | Positive |
| Gliosis of cerebellum | 1 | Female | 10 years | Negative |
| Peritrigonal gliosis | 1 | Female | 8 years | Negative |
| Chronic stroke of the left putamen and caudate head | 1 | Female | 11 years | Positive |
| Summary – all abnormalities | 25 |
13 Female 11 Male |
Mean 9.1 range 7-15 |
17 negative 8 positive |
The odds ratio of having any brain abnormality including sinusitis was 1.23 (95% CI 0.62 to 2.44) for survivors of SAM compared to the BRINK controls (p=0.55); when eliminating sinusitis, odds ratio for abnormal brain structures for SAM survivors compared to BRINK controls was 0.57 (95% CI 0.20 to 1.60) (p=0.30). There was also no significant association between brain abnormalities and other potential risk factors, including HIV status, age, sex, and SES.
Discussion
SAM survivors were more likely to be in a lower grade at school than community control children. SAM was also associated with consistently poorer scores in all CANTAB cognitive tests, significantly so in areas of visual attention and visual memory. Although adjusting for HIV and SES diminished most of the statistically significant differences. SAM survivors did not have increased odds of gross structural brain abnormalities compared to “normal” Malawian children.
Our observation of long-term cognitive deficits in SAM survivors adds to other abnormalities including stunting, underweight, lack of lean mass and diminished muscle strength previously reported in this group (8). The absence of long-term structural brain changes (despite functional impairments) compared to the national comparison group, also concurs with results from previous studies which found that abnormalities observed during and shortly after SAM did not persist after treatment (20, 21). Head circumferences of SAM survivors in this cohort also did not differ significantly to community controls 7-years post-SAM (51.1cm vs 52.1cm respectively; p=0·12)(8). Although brain structure of SAM survivors did not appear to differ from normal Malawian children, it is important to note the high rate of incidental MRI abnormalities in both SAM survivors and the reference group when compared to US populations (27). Sinusitis was especially prevalent, and although this is not uncommon among radiology scans in children (29), its presence may highlight a high background burden of infection and chronic inflammation, and has found to be associated with exposure to indoor cooking smoke (27, 30).
For CANTAB cognitive testing, SAM survivors scored significantly worse in areas of visual memory and visual attention. SAM survivors had worse mean scores in all tests; this could be an indication that they also perform worse on average in school exams, which may explain the significantly lower school grade achieved. There is also evidence that children who are stunted can be entered into school later, kept in lower grades at school, or socially interacted with in less advance ways, because they appear or behave younger than their contemporaries, which would also affect SAM survivors (31, 32).
After adjusting for HIV and SES, differences in CANTAB scores were not statistically significant, except for the BLC visual attention test. This suggests that much of the cognitive impairment in the SAM survivors is due to the confounding effects of HIV and SES, or that these variables lie on the casual pathway. In practical terms, whether directly or indirectly related to the nutritional insult, an episode of SAM still indicates a child who is at risk of poor cognitive function. The implications of HIV are another clear indication of the “vicious cycle” of infection and malnutrition (33). The effect of survivor bias in the SAM survivors and the evolutionary adaptation of “brain sparing” development may also explain the diminished effect size after adjustment, between cases and controls (34). It is interesting that HAZ is not statistically associated with CANTAB scores. However this may be explained by the high prevalence of stunting across the whole sample, including controls (mean -1.6 (SD 1.2) z-scores).
A false negative in the adjusted CANTAB results should also be considered due to potentially suboptimal sample size. For the great majority of our cohort, this was the first time they have used a computer therefore the learning curve associated with this likely added ‘noise’ to the resulting data. This may explain the large standard deviations present across the test scores, necessitating a larger sample size. For example, for PAL total errors, a post-hoc sample size calculation suggests that, with 5% significance and 80% power, a sample size of 362 in each group would be needed to demonstrate a statistically significant difference (35).
To understand how our population performed against children in other contexts, we compared CANTAB results in our study against others in the literature (Table 6) (18, 28). Nkhoma et al., used CANTAB to test cognitive improvements following a school feeding program in Malawi (18). One of the 13 CANTAB outcomes presented was significantly improved between the feeding group and controls (IED Pre-ED errors reduced p=0.02). If we compare our SAM survivors and controls between the ages of 6 and 8 years to Nkhoma’s control group results at baseline, we see some relatively large differences in scores, though no clear trend as to which children did better or worse, perhaps, again, results of large standard deviations. When compared with published data from UK school children of the same age (28), we find that UK scores are generally better than those in Malawi. With UK children very used to computers relative to this cohort of Malawi children, this is not unexpected. The outlier (MOT) could be due to chance or due to differences in coaching since it is the first test in the battery.
Table 6.
presentation of mean scores for CANTAB tests for children in other studies in Malawi and UK, and SAM survivors (aged 6-8 only)
| CANTAB outcome | Malawian children Nkhoma et al. Mean (SD) (n=111, aged 6-8 years) | UK children by Luciana et al. Mean (SD) (n= 198, aged 6-8 only) | Malawian children Lelijveld et al., (aged 6-8 only) Mean (SD) | |
|---|---|---|---|---|
| SAM survivors (n=35) |
Controls (n=29) |
|||
| MOT mean errors | N/A | 22.0 (17.0) | 9.45 (2.4) | 11.1 (2.8) |
| MOT mean latency | N/A | 873.7 (188.0) | 1431.5 (605.9) | 1242.5 (465.3) |
| PAL total errors (adjusted) | 74.8 (17.9) | N/A | 117.1 (77.8) | 99 (82.8) |
| PRM % correct | N/A | 83 (10.7) | 59.2 (14.7) | 68.6 (17.2) |
| IED Total Errors (adjusted) | 157 (69.9) | N/A | 99.6 (82.5) | 67.5 (58.8) |
| IED Stage completed | 3.4 (3.2) | 7.66 (2.1) | 5.6 (3.7) | 6.8 (3.0) |
BLC= big/little circle; MOT=motor screening test; PAL= paired associated learning; PRM= pattern recognition memory; IED= Intra/ Extradimensional Set Shift. Mean CANTAB values for 6-8 year olds in other Malawian school children(18), in school children in the UK(28), and in Malawian SAM survivors and controls for this study (Lelijveld et al.,). N/A indicates that results were not presented for these test outcomes.
We acknowledge some limitations of our study: “healthy survivor” bias is vital to note and will likely have affected all our study outcomes, as many of our original cohort of SAM children died soon after admission or in the year after. Only 352/1024 of those originally admitted were still alive for follow-up at this 7-year stage. Those whose brain structure and cognitive function was most affected by SAM are also those who have likely died and thus our results are therefore likely to be an underestimate of the adverse impact of SAM on cognition and related outcomes at the population level.
Secondly, we do not have data on prenatal nutritional status, or birth weight. These could be potential confounders and SAM may be a symptom of other underlying problems rather than directly causing impairments itself.
For MRI scans, selection bias may have played a part since children or carers who were nervous of the scan process where less likely to consent to a scan. We also acknowledge that subtle but clinically important changes in brain volume would not necessarily have been identified in our study: future work may quantify brain volume to explore this issue in more detail.
Finally, this SAM population differs to cohorts treated today and hence results are not directly generalizable. Whereas all of our children were initially treated as inpatients, today’s programmes focus on early identification and treatment through “community management of acute malnutrition (CMAM).” They also use WHO-growth standards for admission whereas, in 2006, we used NCHS growth references, per national protocols (36).
Balancing these limitations, our study also has many strengths. It is one of very few that has looked in detail at such a wide range of outcomes following SAM. It is also rare to get a follow-up period of this length post-SAM. Most importantly, we have generated baseline data for relatively novel assessment tools, such as CANTAB, which can be used to inform the design of future studies. Ideally, these will be intervention studies that seek to support children affected by SAM to not only survive, but thrive.
In conclusion, based on school achievement and trends in CANTAB cognitive test results, SAM survivors likely have impaired cognitive function, especially in visual memory and visual attention, compared to controls, 7-years post-discharge from treatment. However, there was no evidence of gross alterations in brain structure using MRI scans. Whether the cause of impaired school achievement and cognitive function is biological or social warrants further exploration given the apparent preservation of brain structure, although this should not detract from the practical importance of poorer school achievement. The use of CANTAB as a novel cognitive testing tool was popular and feasible in this field setting however these results suggest that sample size in future studies, especially in computer-naïve contexts, may need to be larger (>300 per group) to detect significant inter-group differences.
Supplementary Material
Acknowledgements
first and foremost, we gratefully acknowledge all of the children and their families who took part in the study. We also recognise important support from The Department of Paediatrics and Child Health, Queen Elizabeth Central Hospital & The Malawi College of Medicine who hosted the study. In particular, the staff working on the MOYO nutrition ward and the excellent work of the study data collection team. We also thank James Medcalf, Esther Gondwe, and Diana Kayaye for their support throughout the study.
Funding support: this research was funded by The Wellcome Trust through an “Enhancement Award” (grant number 101113/Z/13/A) and the “Southern Africa Consortium for Research Excellence” (SACORE) consortium.
Footnotes
Authorship: NL, MK, RSH, MJN, AS and JW designed the research; NL, MK, AAJ, EC, MM, SDK conducted the research; NL, MG and MK analysed the data; NL wrote the paper and had responsibility for final content. All authors read and approved the final manuscript.
Conflict of Interest: All authors declare no conflicts of interest.
Ethical Standard Disclosure: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Malawi College of Medicine Research and Ethics Committee (COMREC) (reference P·02/13/1342); and University College London Research Ethics Committee (reference 4683/001). Written informed consent was obtained from all subject’s legal guardians and verbal assent was obtained from minors
References
- 1.Walker SP, Wachs TD, Gardner JM, et al. Child development: risk factors for adverse outcomes in developing countries. The lancet. 2007;369(9556):145–57. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 2.Grantham-McGregor SM, Pollitt E, Wachs TD, et al. Summary of the scientific evidence on the nature and determinants of child development and their implications for programmatic interventions with young children. Food and Nutrition Bulletin. 1999;20(1):4–6. [Google Scholar]
- 3.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. The Lancet. 2011;378(9799):1325–38. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 4.Scaling Up Nutrition (SUN) Available: http://scalingupnutrition.org/about
- 5.WHO. Childhood Stunting: Context, Causes and Consequences. WHO Conceptual framework. 2013 [Available from: http://www.who.int/nutrition/events/2013_ChildhoodStunting_colloquium_14Oct_ConceptualFramework_colour.pdf.
- 6.UNICEF, WHO, World_Bank_Group. Joint Child Malnutrition Estimates: Levels and Trends in Child Malnutrition. 2017.
- 7.Waterlow JC. Protein-Energy Malnutrition (reprint of original 1992 version, with new supplementary material) Smith-Gordon; 2006. [Google Scholar]
- 8.Lelijveld N, Seal A, Wells JC, et al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study. The Lancet Global Health. 2016 doi: 10.1016/S2214-109X(16)30133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winick M, Brasel JA. Early malnutrition and subsequent brain development. Ann N Y Acad Sci. 1977;300:280–2. doi: 10.1111/j.1749-6632.1977.tb19328.x. [DOI] [PubMed] [Google Scholar]
- 10.Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72(4):267–84. doi: 10.1111/nure.12102. [DOI] [PubMed] [Google Scholar]
- 11.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85(2):614S–20. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 12.Grantham-McGregor S. A review of studies of the effect of severe malnutrition on mental development. The Journal of nutrition. 1995;125(8 Suppl):2233S–8S. doi: 10.1093/jn/125.suppl_8.2233S. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Raine A, Venables PH, et al. Malnutrition at age 3 years and lower cognitive ability at age 11 years: independence from psychosocial adversity. Archives of pediatrics & adolescent medicine. 2003;157(6):593–600. doi: 10.1001/archpedi.157.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waber DP, Bryce CP, Girard JM, et al. Impaired IQ and academic skills in adults who experienced moderate to severe infantile malnutrition: a 40-year study. Nutritional neuroscience. 2014;17(2):58–64. doi: 10.1179/1476830513Y.0000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyheneix M. Impact of severe acute malnutrition on cognition and behaviour: a systematic review. London School of Hygiene and Tropical Medicine; 2015. [Google Scholar]
- 16.WHO, UNICEF. WHO child growth standards and the identification of severe acute malnutrition in infants and children. A joint statement by the World Health Organization and the United Nations Children's Fund. 2009 (2009) [Available from: http://www.who.int/nutrition/publications/severemalnutrition/9789241598163/en/index.html. [PubMed]
- 17.Cambridge Neuropsychological Test Automated Battery (CANTAB) [Available from: http://www.cambridgecognition.com/cantab/
- 18.Nkhoma OW, Duffy ME, Cory-Slechta DA, et al. Early-stage primary school children attending a school in the Malawian School Feeding Program (SFP) have better reversal learning and lean muscle mass growth than those attending a non-SFP school. The Journal of nutrition. 2013;143(8):1324–30. doi: 10.3945/jn.112.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeyinka AO, Akinyinka OO, Falade AG. Computerized tomography measures of brain slice area and ventricular sizes in protein energy malnutrition: a preliminary study. West Afr J Med. 1996;15(4):232–6. [PubMed] [Google Scholar]
- 20.Househam KC. Computed tomography of the brain in kwashiorkor: a follow up study. Arch Dis Child. 1991;66(5):623–6. doi: 10.1136/adc.66.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Sherif AM, Babrs G, Ismail A. Cranial magnetic resonance imaging (MRI) changes in severely malnourished children before and after treatment. Life Science Journal. 2012;9(3):589–92. [Google Scholar]
- 22.Atalabi OM, Lagunju IA, Tongo OO, et al. Cranial magnetic resonance imaging findings in kwashiorkor. International Journal of Neuroscience. 2010;120(1):23–7. doi: 10.3109/00207450903315727. [DOI] [PubMed] [Google Scholar]
- 23.Lelijveld N. Long-term Effects of Severe Acute Malnutrition on Growth, Body Composition and Function; A prospective cohort study in Malawi. University College London: 2016. [Google Scholar]
- 24.Lelijveld N, Kerac M, Seal A, et al. Long-term effects of severe acute malnutrition on lung function in Malawian children: a cohort study. European Respiratory Journal. 2017;49(4) doi: 10.1183/13993003.01301-2016. 1601301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerac M, Bunn J, Seal A, et al. Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double-blind efficacy randomised controlled trial in Malawi. The Lancet. 2009;374(9684):136–44. doi: 10.1016/S0140-6736(09)60884-9. [DOI] [PubMed] [Google Scholar]
- 26.Kerac M, Bunn J, Chagaluka G, et al. Follow-Up of Post-Discharge Growth and Mortality after Treatment for Severe Acute Malnutrition (FuSAM Study): A Prospective Cohort Study. PloS one. 2014;9(6):e96030. doi: 10.1371/journal.pone.0096030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potchen M, Kampondeni S, Mallewa M, et al. Brain imaging in normal kids: a community-based MRI study in Malawian children. Tropical Medicine & International Health. 2013;18(4):398–402. doi: 10.1111/tmi.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luciana M, Nelson CA. Assessment of neuropsychological function through use of the Cambridge Neuropsychological Testing Automated Battery: performance in 4- to 12-year-old children. Developmental neuropsychology. 2002;22(3):595–624. doi: 10.1207/S15326942DN2203_3. [DOI] [PubMed] [Google Scholar]
- 29.Von Kalle T, Fabig-Moritz C, Heumann H, et al. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren. © Georg Thieme Verlag KG; 2012. editors. Incidental findings in paranasal sinuses and mastoid cells: a cross-sectional magnetic resonance imaging (MRI) study in a pediatric radiology department. [DOI] [PubMed] [Google Scholar]
- 30.Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends in immunology. 2016;37(6):386–98. doi: 10.1016/j.it.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown JL, Pollitt E. Malnutrition, poverty and intellectual development. Scientific American. 1996;274(2):38–43. doi: 10.1038/scientificamerican0296-38. [DOI] [PubMed] [Google Scholar]
- 32.Alderman H, Hoogeveen H, Rossi M. Preschool nutrition and subsequent schooling attainment: longitudinal evidence from Tanzania. Economic Development and Cultural Change. 2009;57(2):239–60. [Google Scholar]
- 33.Scrimshaw NS, Taylor CE, Gordon JE, et al. Interactions of nutrition and infection. 1968 [PubMed] [Google Scholar]
- 34.Barker D. Developmental origins of adult health and disease. Journal of Epidemiology & Community Health. 2004;58(2):114–5. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dean A, Sullivan K, Soe M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 3.01. [updated 2013/04/06]; [Available from: www.OpenEpi.com.
- 36.Bhutta ZA, Berkley JA, Bandsma RHJ, et al. Severe childhood malnutrition. Nat Rev Dis Primers. 2017;3:17067. doi: 10.1038/nrdp.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.