Abstract
The present study aimed to investigate the influence of housing conditions on contextual fear memory malleability. Male Wistar rats were housed in enriched, standard, or impoverished conditions after weaning and remained in these conditions throughout the entire experiment. After six weeks into those housing conditions, all animals underwent a 3-day protocol including contextual fear conditioning (day 1), memory reactivation followed by systemic administration of midazolam or vehicle (day 2), and a retention test (day 3). Percentage freezing was used as a behavioral measure of contextual fear. There was no evidence for an effect of housing conditions on the sensitivity of contextual fear memory to amnestic effects of post-reactivation midazolam administration, and no indication for amnestic effects of post-reactivation midazolam overall (including in the standard group). The inability to replicate previous demonstrations of post-reactivation amnesia using the same protocol underscores the subtle nature of post-reactivation pharmacological memory interference. Notably, impoverished housing resulted in a decrease in contextual freezing during contextual fear conditioning, reactivation and retention testing, compared to enriched and standard housing conditions. This observation warrants caution when interpreting the results from experiments regarding effects of housing on fear memory processes, particularly when freezing is used as a measure of fear.
Keywords: Impoverished housing, Enriched housing, Contextual fear conditioning, Freezing, Post-reactivation amnesia
1. Introduction
According to the European Directive on the protection of animals used for scientific purposes (2010/63/EU), all laboratory animals should be socially housed and provided with appropriate (physical) environmental enrichment, with the main goal of enhancing animal welfare and reducing stress-induced behavior. Environmental enrichment can be achieved by providing an expanded surface area and by introducing materials that extend opportunities for physical exercise, play, exploration, and environmental control [1,2]. In other (non-EU) labs and in commercial breeding sites, where other standards apply, different housing conditions may be adopted. In these cases, rodents are often group-housed without enrichment, or sometimes even housed individually. In light of published results showing effects of housing on behavioral and neurobiological plasticity, those differences in housing conditions between labs (and over time) have raised questions regarding their consequences for the comparability and replicability of findings emerging from different labs [3] (but see [4]).
The aim of the current experiment was to investigate the effect of housing conditions during rearing on several aspects of contextual fear memory in rats. This was inspired by failed efforts to replicate previous findings from other labs on pharmacological induction of post-reactivation amnesia (‘reconsolidation blockade’) for contextual fear memories (Schroyens et al., in prep). After an extensive series of 24 conceptual and 4 exact replication attempts, we carried out a thorough comparison of rearing conditions at a number of the labs and breeders involved. Based on the results of that comparison, we hypothesized that differences in the animals’ housing conditions prior to fear conditioning may influence fear memory malleability and hence determine whether post-reactivation amnesia can be obtained. In support of this hypothesis, there is a vast amount of research in rodents showing effects of enriched housing (EH) and impoverished housing (IH) conditions on learning and memory generally.
Although there is a wide variety in enrichment procedures used across experiments (i.e., different timing, duration, amount and type of EH), EH has generally been found to enhance neurogenesis, long-term potentiation (LTP), and synaptic density (relative to standard housing, SH) [5–7]. In line with those neurobiological changes, EH has been shown to result in superior learning and memory on several laboratory tasks including Morris water maze performance and novel object recognition [2]. Upon exposure to a novel environment, EH rats generally show decreased locomotor activity and faster habituation to the context, which has been interpreted as EH-induced improvements in contextual processing. Interestingly, several researchers have also explored the effects of EH on fear memory acquisition. The majority of those studies found enhanced conditioned freezing to the training context in EH rodents when using a cued fear conditioning procedure [5,7–11] (but see [12,13]), but not after contextual fear conditioning [8,14]. In addition, it has been shown that EH rats are faster in processing contextual information and have a greater ability to discriminate between similar contexts, compared to SH rats [8,15].
In line with the general tendency of EH to result in enhanced learning and memory, impoverished housing (IH), usually implemented through post-weaning individual housing, has been found to elicit mostly opposite results. It has been found that IH rats exhibit decreased hippocampal neurogenesis and LTP, which are, in turn, associated with a general reduction in learning and memory (e.g., water maze, radial maze) [16]. Furthermore, IH has been shown to induce an anxiogenic profile in the elevated-plus maze [17,18]. In the open field test (OFT), IH has repeatedly been shown to result in enhanced locomotor activity and slower habituation [19,20] (but see [21]). This enhanced locomotor activity during exposure to a novel context has been attributed to several phenomena, including a deficit in behavioral inhibition, increased arousal, or an increased urge for exploration [16]. Finally, and again opposite to what has been found in EH animals, IH has been shown to induce selective deficits in contextual fear learning when using a cued fear conditioning procedure [3,18,19,21]. Interestingly, the neurobiological and behavioral effects of post-weaning individual housing have inspired several researchers to use this developmental manipulation for modeling certain aspects of neuropsychiatric disorders such as schizophrenia, anxiety disorders, and depression [18].
To conclude, existing literature indicates that housing conditions during rearing, be it enriched or impoverished, can impact subsequent learning and memory in rodents. We hypothesized that these alterations in learning and/or memory retrieval might influence a consolidated memory trace’s sensitivity to interference and thus explain the discrepant findings between labs regarding pharmacological reconsolidation interference. Therefore, the aim of the current experiment was to investigate the influence of housing conditions on contextual fear memory malleability in rats. To this end, rats were introduced to different housing conditions (enriched, standard, or impoverished) post-weaning and subjected to a contextual fear conditioning protocol 6 weeks later. Freezing was used as a behavioral readout of contextual fear. Locomotor activity in a novel environment was assessed exploratorily after completion of the fear conditioning procedure.
2. Materials and methods
2.1. Preregistration
The experimental procedures and statistical analyses were pre-registered on the Open Science Framework (OSF) (https://osf.io/g92v8). Raw data files are also available on OSF [22–24].
2.2. Subjects
Fifty male Wistar rats (PND 22 at time of arrival in the lab; ordered from Centro de Medicina Comparada, Esperanza, Santa Fe, Argentina) were maintained on a 12 h light–dark cycle (lights on at 7:00 am), with a room temperature of 21–22 °C. Food and water were available ad libitum. The experimental protocol was approved by the KU Leuven animal ethics committee (in accordance with the Belgian Royal Decree of 29/05/2013 and European Directive 2010/63/EU), and by the animal care and use committee of the Facultad de Ciencias Químicas, Universidad Nacional de Córdoba (Resolution number 742) which is in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All experiments were conducted at the Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Argentina, between 9.30 a.m. and 5:00 p.m.
2.3. Handling
Animals were weighed three times per week, and cages were refreshed regularly. Before the start of the contextual fear conditioning procedure, all rats were handled briefly on three subsequent days. The last handling session took place 1–2 days before conditioning.
2.4. Post-weaning housing conditions
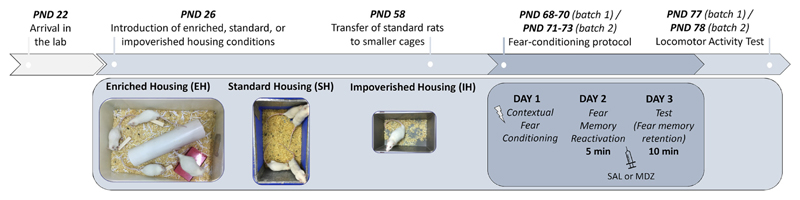
Animals were randomly subdivided into three housing conditions: enriched housing (EH, n = 16), standard housing (SH, n = 18), or impoverished housing (IH, n = 16). Upon arrival in the lab (PND 22), rats were allowed to acclimatize for 4 days, during which they were housed in 60 (L) × 40 (W) × 18.5 cm (H) cages of 4 (EH), 9 (SH), or 8 (IH) rats. At PND 26, cage enrichment was introduced in the EH condition and rats of the IH condition were transferred to individual cages. Animals remained in these housing conditions until the end of the experiment, except for the rats in the SH condition, which were transferred to smaller cages (43 × 28.5 × 18.5 cm) of four rats per cage at PND 58. This was done for practical reasons, and in order to exactly replicate successful previous experiments on post-reactivation amnesia that had been performed in the same lab [25–27]. See Table 1 for a detailed overview of the housing conditions and Fig. 1 for pictures of the cages.
Table 1.
Overview of the housing conditions. (*) Two of these rats (1 of each cage) were used for pilot testing after PND 58 (data not shown). Note that although the surface area per rat was larger for IH than for the other housing conditions, the total space to move for the IH animals was compromised due to the low height of the cages.
| Properties | Enriched housing (EH) (n = 16) PND 26 – end |
Standard housing (SH) (n = 18*) |
Impoverished housing (IH) (n = 16) PND 26 – end |
||
|---|---|---|---|---|---|
| PND 26 – PND 58 | PND 58 – end | ||||
| # rats/cage | 4 | 9* | 4 | 1 | |
| Cage size | 60 × 40 × 18.5 cm | 60 × 40 × 18.5 cm | 43 × 28.5 × 18.5 cm | 27 × 17 × 11.5 cm | |
| Surface area/rat | 600 cm2 | 267 cm2 | 306 cm2 | 459 cm2 | |
| Bedding material | Yes | Yes | Yes | Limited amount (V = 250 ml, covering 85% of the floor) | |
| Enrichment | 3 tunnels, paper shred, and several toys that were changed regularly | No | No | No | |
Fig. 1.
Experimental design. After around six weeks in their respective housing conditions, rats underwent a contextual fear conditioning procedure. Immediately after the reactivation session (day 2), saline (SAL) or midazolam (MDZ) was administered. One week after the end of the conditioning protocol, half of the rats (4 from each group) were exposed to a novel context to assess locomotor activity. Standard rats were first housed in large (non-enriched) cages of 9 rats (equal in size to the EH cage depicted in the left picture) and then transferred to smaller cages of 4 rats per cage at PND 58 (middle picture).
Enriched cages contained 3 tunnels (two on the floor and one hanging from the top grid), and a pile of shredded paper in one corner. In addition, EH rats received a number of toys that were changed 3 times per week for novelty. These toys included 2 artificial bones, 4 wooden blocks, 2 balls, 2 kid toys in the shape of a flower, 6 bottle caps, and 2 small plastic cylinders. IH rats were housed in a separate room (i.e., apart from SH and EH rats). Note that the amount of enrichment that was used in the current experiment is rather limited compared to the (variety of) protocols that have been used in some studies. This relatively modest environmental variability was adopted in light of the relatively limited variations in rearing conditions that were observed between relevant labs and/or breeders. As in most isolation-rearing protocols, rats were physically isolated but could still hear and smell each other.
2.5. Drug administration
Midazolam (MDZ, Gobbi Novag S.A., Argentina) was diluted in sterile saline (SAL, 0.9%, w/v) at a concentration of 3 mg/ml. MDZ or SAL was injected intraperitoneally (IP) immediately after fear memory reactivation at a volume of 1 ml/kg. For each housing condition, rats were semi-randomly assigned to the MDZ or SAL group (n = 8 per group), with the restriction that each home cage contained 2 SAL rats and 2 MDZ rats. These parameters (amnestic drug, dose, mode and time of administration, …) were identical to the ones used in prior experiments showing successful induction of amnesia [25–27].
2.6. Procedure
See Fig. 1 for a schematic overview of the procedure.
2.7. Contextual fear conditioning and testing
2.7.1. Apparatus
The conditioning chamber (20 × 23 × 20 cm) contained a grid floor of 10 parallel stainless-steel bars spaced 1.5 cm apart (center to center) and with a diameter of 4 mm each. A scrambled shocker (Ugo Basile Biological Research Apparatus) was used for shock administration. The chamber was placed in a room illuminated by a white fluorescent light located on the ceiling. Ventilation fans and shock scramblers provided background noise. The chamber was cleaned with alcohol (80% in water) before and/or after each session. The fear conditioning procedure was identical to the one used in previous successful experiments [25–27].
2.7.2. Fear conditioning
After a pre-shock period of 3 min, 3 scrambled foot shocks (0.5 mA, 3 s, ITI = 30 s) were administered. After the final shock, animals remained in the context for 50 s.
2.7.3. Reactivation session
One day after training, rats were re-exposed to the training context for 5 min, without shock delivery. Immediately after the reactivation session, MDZ or SAL was administered IP in an adjacent room.
2.7.4. Test session
One day after the reactivation session, rats were again exposed to the training context for 10 min to assess fear memory retention (without shock delivery).
2.7.5. Behavioral scoring
Percentage of time the animals spent freezing (a defensive response characterized by complete immobility apart from movements associated with breathing) was used as a behavioral measure of contextual fear. Freezing was manually scored from videos for each behavioral session (pre- and post-shock, reactivation, test) by a rater blinded to experimental conditions. The amount of freezing is expressed as a percentage of the total scoring period. Percentage freezing per minute was calculated as well in order to assess temporal patterns of contextual fear.
2.8. Locomotor activity test
About one week after contextual fear conditioning (and after about 7 weeks in their respective housing conditions), a test for locomotor activity was performed for exploratory reasons. Half of the rats (n = 24, 4 rats from each group) were exposed for 30 min to a novel context, which consisted of a plastic transparent box (43 (L) × 30 (W) × 31 (H) cm) in a dimly lit room. Four identical boxes were placed inside the room, so 4 that rats could be tested simultaneously. Distance traveled and % movement (in 30 min and in 5-min time bins) were recorded using Ethovision XT (version 11.5, Noldus, Netherlands). The software’s default criteria, i.e., start velocity of 2 cm/s and stop velocity of 1.75 cm/s, were used to calculate % movement.
2.9. Planned statistical analyses
Graphs report means and SDs. All preregistered analyses are reported (see also Supplement A). T-tests and (one-way/repeated-measures/mixed) ANOVAs were used for the analyses. Depending on the research question, (some of) the following factors were included in the analysis: Treatment (SAL vs. MDZ), Housing (EH vs. SH or IH vs. SH or EH, SH, IH), Session (reactivation vs. test), and Time (min 1, min 2, …., min 10 of the test session). For each analysis that included % freezing during test, we evaluated freezing during the first five minutes of the test session and during the complete 10-min session. P-values lower than 0.05 were regarded as significant and significant ANOVAs were followed up by Newman-Keuls post-hoc tests. If Mauchly’s test indicated a violation of the sphericity assumption, Greenhouse-Geisser corrections were applied. JASP was used for statistical analyses [28], and R was used for creation of the graphs [29].
Grubb’s tests (GraphPad Software website) were used to detect outliers during reactivation (per housing condition separately) and rats that showed less than 25% freezing during reactivation were excluded. The latter predefined criterion aimed to exclude rats that did not sufficiently acquire the context-shock association, because a lack of acquisition arguably prevents the assessment of subsequent memory interference. Statistical analyses regarding (the influence of housing conditions on) the amnestic effect of MDZ were performed on the subject sample thus selected (i.e., excluding animals that showed exceedingly low freezing on day 2) as well as on the complete dataset (i.e., without exclusions) (3.2.1, 3.2.1), in agreement with the preregistration. For the effects of housing on % freezing during training (pre-shock, post-shock) and reactivation (3.2.3, 3.2.4), we only report the results of analyses performed on the complete dataset, since effects on baseline freezing and fear memory acquisition are of main interest here; it would therefore be inappropriate to exclude animals that did not sufficiently acquire the context-shock association for these latter analyses (see Supplement A for an overview of all performed analyses).
Bayesian analogues were performed exploratorily in order to quantify evidence for either hypothesis (H0 or HA). The default Cauchy prior width of r = .707 was used (JASP, based on the BayesFactor package in R). An overview of these analyses can be found in Supplement B.
2.10. Additional statistical analyses
In order to control for baseline differences in freezing between housing conditions, mixed ANOVAs with factors Session (baseline vs. reactivation or reactivation vs. test) and Housing (EH, SH, IH) were performed and difference scores (freezing during test - freezing during baseline) were calculated. The locomotor activity test and accompanying analyses were also performed exploratorily. These analyses were not included in the preregistration of the study and were planned after seeing the main contextual fear conditioning data.
3. Results
3.1. Housing conditions influence body weight
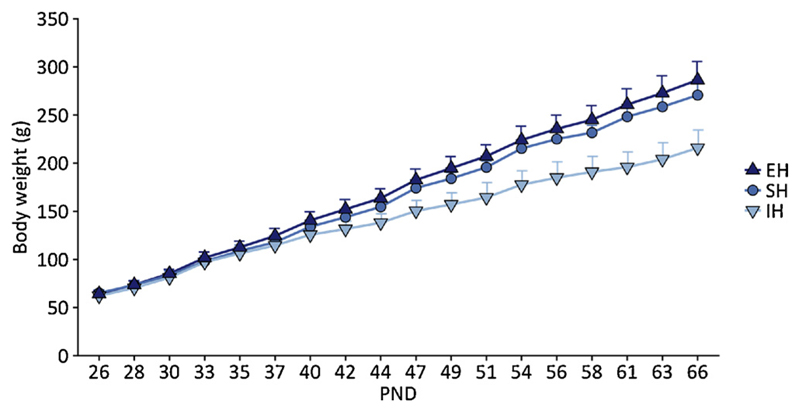
Enriched housing (EH) and impoverished housing (IH) both affected body weight, as compared to standard housing (SH) (N = 48). While EH rats showed increased weight gain throughout development, IH rats showed decreased weight gain, compared to SH rats (Fig. 2). The mixed ANOVA showed a main effect of Housing (F(2, 45) = 56.80, p < .001, η2p = .716) and Age (F(1.934, 87.049) = 4664.16, p < .001, η2p = .990), and a Housing by Age interaction (F(3.869, 87.049) = 68.46, p < .001, η2p = .753). Body weight at PND 66 (a few days before conditioning) differed significantly between housing conditions (F(2, 45) = 76.90, p < .001, η2p = .774), and post-hoc comparisons showed that all housing conditions differed significantly from each other.
Fig. 2.
Housing conditions influence body weight.
3.2. The influence of housing conditions on contextual fear memory
3.2.1. No amnestic effect of MDZ in the standard housing group
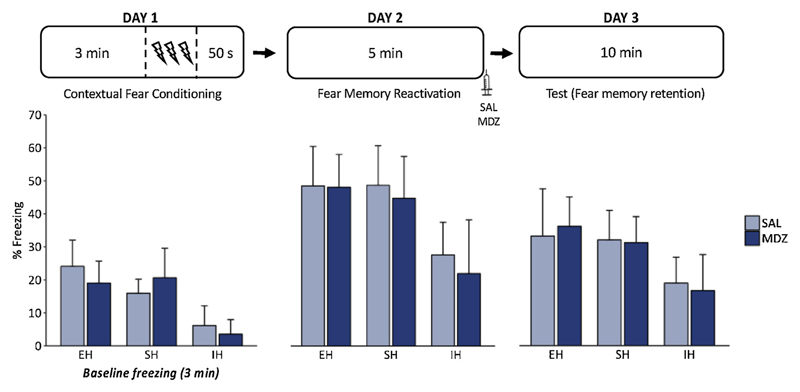
Due to deviations from normality in the SH-SAL group, non-parametric Mann-Whitney U tests were conducted to investigate treatment effects on freezing during the test session. A one-sided t-test (SAL > MDZ) showed that post-reactivation MDZ administration did not induce an attenuation of freezing across the 10-min test session in SH rats (p = .360, rank biserial correlation = .125) (Fig. 3). When considering the first 5 min of the test session only, there was a significant difference between SAL- and MDZ-treated rats (p = .041, rank biserial correlation = .531). Although preregistered and statistically significant, the difference between MDZ rats (M = 23.88, SD = 5.59) and SAL rats (M = 29.46, SD = 9.02) should not be taken as strong evidence for MDZ-induced amnesia, particularly when considering that there already was a (non-significant) difference in freezing during the reactivation session (i.e., prior to drug administration) between MDZ rats (M = 45.46, SD = 12.88) and SAL rats (M = 49.46, SD = 12.17) (p = .636, rank biserial correlation = .156). Note that a Bayesian analysis provides only anecdotal evidence for an amnestic effect of MDZ (MDZ < SAL) in the first 5 min of the test (BF-0 = 1.536). In combination with the fact that no significant difference in freezing was observed across the full 10-min session (with Bayesian analysis yielding anecdotal evidence for the absence of an amnestic effect of MDZ across the complete test session, BF-0 = .487), these results imply a failure to clearly replicate previous findings in the same lab. An overview of temporal patterns in % freezing (in 1-min bins) can be found in Supplement C.
Fig. 3.
Post-reactivation MDZ administration did not induce amnesia in either housing condition. For the retention test, the average % freezing during the complete 10-min session is represented. All rats are included in these graphs. EH = enriched housing (n = 16), SH = standard housing (n = 16), IH = impoverished housing (n = 16), SAL = saline, MDZ = midazolam.
3.2.2. The influence of housing conditions on the amnestic effect of MDZ
Results of the planned statistical analyses that aimed to assess the influence of housing conditions on memory malleability – for EH vs. SH and IH vs. SH separately – are reported in the following paragraphs (3.2.2, 3.2.4). Note that the absence of an amnestic effect in the SH group did not allow us to investigate whether enriched or impoverished housing could induce resistance to memory interference. Facilitating effects of housing conditions on fear memory interference, on the other hand, could still be evaluated.
According to predefined exclusion criteria, 8 rats (3 IH-SAL, 5 IH-MDZ) were excluded due to freezing levels of < 25% during the reactivation session. Note that all excluded rats belonged to the IH group. Similar to what was observed in the SH group, post-reactivation MDZ administration did not affect freezing during the test session in either EH or IH rats. Indeed, the ANOVAs showed that there were no interactions between Housing (EH vs. SH and IH vs. SH) and Treatment (SAL vs. MDZ), confirming that housing conditions (either enriched or impoverished) did not affect sensitivity to the amnestic treatment. This conclusion holds for all analyses, considering the first 5 min of the test or the complete 10-min session, and also when including the full sample (i.e., without exclusion of rats showing < 25% freezing during reactivation). A complete overview containing results from all statistical analyses performed can be found in Supplement A.
The ANOVA on freezing during reactivation showed a significant difference between groups (IH-SAL, IH-MDZ, SH-SAL, SH-MDZ) (F (3,28) = 8.078, p < .001, η2p = .464) when all animals were included in the analysis (for rationale, see Section 2.9). In order to control for the difference in freezing during reactivation, the factor ‘Session’ (reactivation vs. test) was included in the ANOVA. The absence of a Housing (IH vs. SH) × Treatment × Session interaction confirms that IH did not affect fear memory malleability (F(1,28) = .200, p = .658, η2p = .007 for the first 5 min of the test session; F(1,28) = .001, p = .970, η2p = 0 for the complete 10-min session). Unexpectedly, this analysis did reveal a (marginally) significant Session × Housing interaction (F(1,28) = 7.448, p = .011, η2p = .210 for the first 5 min of the test session; F(1,28) = 3.887, p = .059, η2p = .122 for the complete 10-min session), on which we will elaborate in part 3.2.4 and in the discussion section.
3.2.3. Baseline freezing was attenuated in IH rats and unaffected in EH rats, relative to SH rats
All rats were included (N = 48) for the purpose of the analyses reported below (3.2.3–3.2.4; for rationale, see Section 2.9). Baseline freezing (i.e., during the 3-min pre-shock period) differed between housing conditions (F(2,45) = 27.70, p < .001, η2p = .552). Post-hoc comparisons revealed that IH rats (M = 4.861, SD = 5.265) showed significantly less freezing compared to SH (M = 18.299, SD = 7.159) and EH rats (M = 21.562, SD = 7.537). The same pattern was observed for post-shock freezing (F(2,45) = 10.42, p < .001, η2p = .317). As clarified in the following paragraphs, this baseline difference in freezing hampers the interpretation of housing effects on fear memory retention on subsequent testing days.
3.2.4. The effect of housing conditions on the acquisition and retention of contextual fear memory
Since baseline differences in freezing confounded the significant effect of Housing on freezing during reactivation (F(2,45) = 18.97, p < .001, η2p = .457), alternative analyses were performed exploratorily. Visual inspection of average freezing data suggests increases from baseline (day 1) to reactivation (day 2), implying successful acquisition in all groups. The explorative ANOVA with factors Session (baseline vs. reactivation) and Housing (EH, SH, IH) suggested that there were no differences in the increase in freezing between housing conditions (Session × Housing: F(2,45) = 1.941, p = .155, η2p = .079; main effect of Session: F(1,45) = 173.258, p < .001, = .794).
The decrease in freezing from reactivation (day 2) to test (day 3) shows that freezing decreased in all housing conditions (F(2,45) = 117.073, p < .001, η2p = .722 for the first 5 min of the test session; F(2,45) = 56.295, p < .001, η2p = .556 for the complete 10-min session). Note that the latter ANOVA was performed exploratively. The significant Housing (IH vs. SH) by Session (reactivation vs. test) interaction (planned analysis, see 3.2.2) suggested that this decrease was smaller in the IH group, suggesting a possible impairment of extinction in the IH group. However, although the inclusion of the factor ‘Session’ in the ANOVA partly takes into account differences in freezing during reactivation, the lower scores in the IH group may still account for the respectively attenuated decrease in freezing from day 2 to day 3, since there simply is less room for a decline in freezing in this group. One common approach to control for baseline freezing is by using difference scores (freezing during reactivation/test minus freezing during baseline). However, when applied to our sample, difference scores do not allow to completely level out group differences during reactivation (M = 29.16, SD = 12.90 for the SH rats; M = 20.31, SD = 14.97 for the IH rats). Implications of these findings are elaborated upon in the discussion section.
3.3. No evidence for an effect of housing during the general locomotor activity test
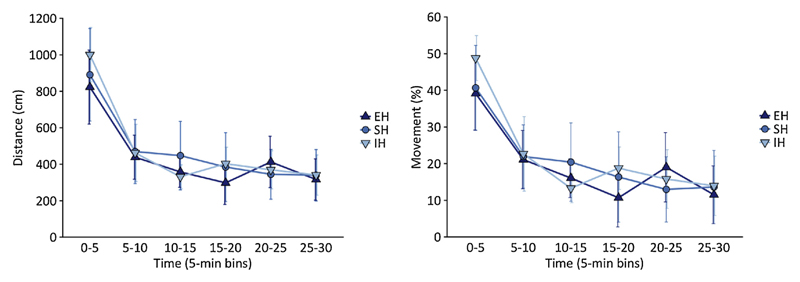
Four rats were excluded (2 EH and 2 SH) because they could not be tracked by the software throughout the entire session, resulting in samples sizes of 6 EH rats, 6 SH rats, and 8 IH rats. The ANOVAs with factor Housing (EH, SH, IH) showed that there was no difference in distance traveled or % movement during the 30-min activity test between housing conditions (F(2,17) = .354, p = .707, η2p = .040 and F (2,17) = .338, p = .718, η2p = .038, respectively). In addition, there was no housing effect on habituation to the novel context, as shown by the absence of significant Housing × Time (six 5-min time bins) interactions (for distance traveled: F(10,85) = 1.377, p = .205, η2p = .139 and for % movement: F(10,85) = 1.689, p = .097, η2p = .166) (Fig. 4).
Fig. 4.
No evidence for an effect of housing conditions on distance travelled (left) or % movement (right) during a 30-min locomotor activity test. EH = enriched housing, SH = standard housing, IH = impoverished housing.
4. Discussion
The aim of the current study was to investigate whether housing conditions influence fear memory malleability. To this end, we adopted a previously-used protocol [25–27] and manipulated housing conditions (standard, enriched, impoverished). In the standard housing group, all parameters were kept as similar as possible to previous publications.
Despite the use of the same protocol as in successful reports, we failed to find clear evidence for an amnestic effect of post-reactivation midazolam (MDZ) administration in the standard housing group. Given that previous studies report large effect sizes (d = 2.65–4.77, estimated from reported graphs) [25–27], our sample size of 16 rats (8/group) should have been sufficient to detect an effect of MDZ. The failure to replicate amnestic effects of MDZ on contextual fear memory is in line with other recent results obtained in Leuven and elsewhere (Schroyens et al., in prep), while in contrast with published findings from other labs [30–40] and successful replications in the Córdoba lab where the current experiment was carried out [25–27].
A notable difference between the current experiment and successful studies is that all rats (including those that received post-reactivation saline) showed a decrease in freezing from reactivation (day 2, 50% freezing) to the test session (day 3, around 30% freezing) (see Fig. 3). This decrease was not observed in the control groups of previous experiments in which amnesia was obtained [25–27], while freezing levels during training and reactivation were very comparable between the present study and other reports (suggesting similar fear memory acquisition). Importantly, the presence of a considerable decrease in freezing in the saline rats is problematic for observing amnestic effects of MDZ. The previously-mentioned successful studies reported similar decreases in the MDZ group (i.e., from around 50% to around 30%). Therefore, in the current study, the decrease in freezing from reactivation to test in saline rats might have prevented detection of MDZ-induced amnestic effects.
Alternatively, the present failure to observe differences between SAL and MDZ might indicate that the reactivation session in the current study, rather than inducing memory destabilization, induced a different memory-related process that evoked a decrease in fear memory expression. It has been shown the duration of the reactivation session determines whether mere memory retrieval (no prediction error, PE), destabilization (small/single PE) or extinction (large/multiple PE) will take place [31,41,42]. In addition, an intermediate reactivation duration (i.e., in between those inducing destabilization and extinction) has been shown to cause a decrease in fear that is insensitive to amnestic treatment, similar to what is observed in the current study [41,42]. Although the reactivation duration was the same as in prior successful studies, the parameters might have not been optimal to achieve destabilization in this specific group of rats. Since previous studies showed that MDZ administration after extinction learning interferes with its consolidation [31], and we did not observe an effect of post-reactivation MDZ whatsoever, it seems unlikely that extinction was induced in these animals, unless these animals were less sensitive to MDZ. Note that the decrease in freezing from reactivation to test is observed in almost all animals (except for 1 SH-SAL rat), suggesting that the absence of an amnestic effect cannot be attributed to individual differences canceling each other out (i.e., some rats experiencing extinction, while others destabilize; see Supplement D).
There are some methodological differences between the current and prior successful studies that have been performed in the same lab. First, animals were ordered from a commercial supplier for the present study, whereas the animals used in previous experiments were bred in-house. This implies a different living environment for the rats during the pre-weaning stage, and the necessity of transportation from the breeding site to the lab where the experiment was carried out for the present study, which likely is a stressful experience. Pre-natal stressors or altered mother-pup relationships have been shown to impact brain development and behavior in rodents [43]. Amnesia after post-reactivation MDZ administration has been observed in another lab in Córdoba, Argentina when adult rats were ordered from the same supplier as in the current experiment, possibly ruling out a role of genetic differences [35]. Second, the study was carried out by another experimenter. In any case, the discrepant results between this and prior studies that used the same protocol in the same lab, indicates that the induction of post-reactivation amnesia is subtle and might depend on prior experiences of the rats. In fact, this hypothesis was to be addressed in this experiment. While the current results illustrate that neither enriched nor impoverished housing enhanced memory malleability compared to standard housing, the absence of an amnestic effect in the standard group did not allow us to investigate whether housing conditions can induce resistance to memory interference.
Apart from the effect of housing conditions on memory malleability, their influence on contextual fear expression during training (pre-shock, post-shock) and on fear memory retention was investigated. While enriched housing did not yield noticeable effects relative to standard housing, impoverished housing induced lower contextual freezing during all testing phases (see Fig. 3). Importantly, the attenuation of freezing in impoverished rats was already observed during the 3-min pre-shock period.
The difference in baseline freezing between housing conditions complicates the interpretation of housing effects on contextual fear expression on day 2 and 3. More specifically, when not considering baseline differences, one might wrongfully interpret the difference in freezing on day 2 as evidence for an impairing effect of impoverishment on contextual fear memory acquisition. Likewise, the significant Housing (IH vs. SH) by Session (reactivation vs. test) interaction, although at first sight indicative of an impairment in contextual fear extinction, may be confounded by inherent differences in freezing as well (see 3.2.4). We further showed that the use of difference scores (freezing during test minus freezing during baseline), a common approach to control for baseline differences in contextual freezing, cannot completely eliminate this confound. It is therefore crucial to take into consideration the tendency of impoverished rats to exhibit less freezing when interpreting these data. As explained in the next paragraphs, baseline differences in freezing can pose critical issues for the investigation of housing effects on contextual fear memory when freezing is used as index of fear.
The presence of a certain degree of baseline freezing in our SH rats allowed us to investigate the influence of housing conditions on contextual freezing prior to any manipulation (e.g., shock administration). This is in stark contrast with many published studies investigating effects of impoverished housing on (contextual) fear memory retention, which observe baseline freezing levels of 0–5% or do not report baseline freezing. This (near) absence of baseline freezing hampers the assessment of differences between housing conditions during this period, since there is no room for freezing to decline in the manipulated group, compared to the control group. Therefore, any failure to observe baseline differences in these cases should not be regarded as conclusive evidence for the absence of housing effects on contextual freezing per se, especially given the results of the current experiment.
Since it has been established that impoverished rats show hyperactivity during exposure to a novel context (usually quantified as increased distance traveled in an open field), studies in which freezing is used as the main outcome measure typically try to control for activity confounds in different ways, e.g., by assessing housing effects on post-shock freezing. However, freezing levels immediately after the shock are difficult to interpret. First of all, freezing during this – relatively short – period reflects the unconditioned response towards the shock, which can in itself also be influenced by housing conditions. Second, in our sample, post-shock freezing was not correlated with baseline freezing for each housing group separately, indicating that low pre-shock freezing does not correspond with low post-shock freezing on an individual level. A second measure that has previously been included to assess activity confounds is the analysis of the rats’ behavior (e.g., distance traveled) in a novel, distinct context. Note that we also included such a measurement after completion of the conditioning protocol. While this test session did not reveal any differences in distance traveled or % movement between housing conditions, we did, on the other hand, find differences when looking at manual recordings of baseline freezing during the conditioning session. Overall, the point is that in studies in which baseline freezing cannot be assessed, it may not be possible to fully rule out that differences in (changes of) contextual fear expression between housing conditions are due to inherent group differences in the tendency to freeze. A similar issue probably holds for cued fear conditioning (see arguments in [44]), and might also affect any neurobehavioral procedure in which animals need to be housed individually.
One could reasonably argue that the effect of impoverishment on contextual freezing during tests could have been influenced by differences in shock sensitivity between housing conditions. For example, rats with lower weight (in this case, the impoverished rats) might have had heightened sensitivity to the shocks due to their lower resistance, resulting in a more aversive learning experience. However, the present data do not suggest such a housing effect on shock sensitivity. First, the difference in contextual freezing is already present before shock administration and remains stable during the post-shock period (and during subsequent testing, see Supplement C). Second, the impoverished rats show lower freezing during testing compared to the other housing conditions in which rats weighed more (i.e., an effect opposite to what one might expect based on their respective weights).
Why do adult rats that have been reared in isolation show lower contextual freezing compared to their standard-housed counterparts? Intuitively, the amount of freezing upon encountering a novel environment may represent the result of a behavioral competition between the urge to explore a new environment (manifested in increased movement), versus the expression of anxiety-related responses upon this novel encounter (manifested increased freezing). On one hand, chronic mild stress induced by impoverished housing might have inoculated IH rats against subsequent stressful experiences, such as exposure to a novel environment, resulting in the observed decrement in freezing. However, decreased anxiety as a consequence of impoverished housing has generally not been observed using behavioral tests [45]. Impoverished rats in the current experiment did not show any other anxiety-related signs, such as escape behavior, during initial exposure to the conditioning context. On the other hand, decreased freezing in impoverished rats could be attributed to increased hyperreactivity of isolation-reared rats, a well-established effect that might have over-ruled their tendency to freeze. While hyperactivity in isolated rats was first interpreted as a deficit in inhibitory control of behavior, it has also been shown that impoverished rats show increased exploratory tendencies [16]. For example, it has been shown that impoverished rats show increased preference for a novel environment, independent of their higher activity levels [46]. It therefore seems that decreased freezing scores in impoverished rats may rather reflect their higher motivation for exploration competing with the freezing response.
As mentioned before, enriched housing did not affect contextual freezing during any of the testing phases. Note that the implementation of ‘enrichment’ varies considerably in the literature. For example, while most studies include a running wheel in the enriched cages [5,7,8,10,11], we only provided tunnels, toys, and an increased surface area per rat for the enriched animals. This relatively modest approach was adopted to be in line with actual rearing environments used in European breeding facilities. Most published studies that addressed enrichment effects on contextual fear in rodents have used a cued fear conditioning paradigm, and they mainly found enhancing effects of enrichment on conditioned contextual freezing [5,7–9, but see 10,11]. When using a contextual fear conditioning paradigm, it has been shown that enrichment has no effect on [8] or impairs [14] conditioned contextual freezing. Other studies revealed that enrichment enhanced performance after contextual fear conditioning when more challenging tasks were adopted (e.g., brief pre-shock period, immature rats, contextual discrimination) [8,15,47,48]. These studies found no effect of enrichment on conditioned freezing in the training context, indicating that more sensitive measures may be required to detect the subtle effects of enrichment on contextual processing. In this regard, the absence of enrichment effects on fear memory retention in the current experiment should not be considered as evidence for enrichment not affecting fear memory malleability.
5. Conclusions
Two main conclusions can be drawn from the current findings. First, we failed to obtain any evidence for the induction of amnesia of a contextual fear memory by post-reactivation administration of MDZ in rats. This failure to replicate previous studies using the same protocol suggests that pharmacological post-reactivation memory manipulations may depend on subtle differences in the animals’ experiences prior to the experiment. Second, while there were no effects of enrichment on contextual freezing on any of the testing days, the current results show that impoverished rats had a general tendency to show less contextual freezing, compared to socially housed, non-enriched rats. The effect of impoverishment on (baseline) freezing levels may complicate the unequivocal interpretation of previous findings regarding effects of impoverished housing on several aspects of fear memory, particularly if freezing was used as a measure of contextual fear.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bbr.2018.10.040.
Acknowledgements
Special thanks to Pablo Espejo and Vanesa Ortiz for their help and advice concerning the experiment and for their support during the first author’s unforgettable stay in Córdoba, Argentina. We also want to thank Amy Milton, Emma Cahill and George Vousden, for the inspiring discussions, the sharing of their ideas, and the kind welcome in Cambridge. Thanks also to Zsuzsanna Vegh and Pierre Hansquine for their help with the analyses of the locomotor activity test. Thanks to Janvier Labs and Charles River Laboratories to provide us with all the necessary information concerning the housing conditions in their facilities.
Funding sources
This work was supported by a Consolidator Grant of the European Research Council (ERC) [T. Beckers, grant number 648176]; and a Doctoral Fellowship of the Research Foundation – Flanders (FWO) [N. Schroyens, grant number 1114018N].
Footnotes
Declarations of interest
None.
References
- [1].European Parliament and council of the European Union. Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. 2010 https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063.
- [2].Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats—Behavioural and neurochemical aspects. Behav Brain Res. 2011;222:246–264. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- [3].Kulesskaya N, Rauvala H, Voikar V. Evaluation of social and physical enrichment in modulation of behavioural phenotype in C57BL/6J female mice. PLoS One. 2011;6:e24755. doi: 10.1371/journal.pone.0024755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Würbel H, Garner JP. Refinement of rodent research through environmental enrichment and systematic randomization, National Centre for the Replacement. Refine Reduct Anim Res. 2007;9 [Google Scholar]
- [5].Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- [7].Rampon C, Tang Y-P, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:7. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- [8].Barbelivien A, Herbeaux K, Oberling P, Kelche C, Galani R, Majchrzak M. Environmental enrichment increases responding to contextual cues but decreases overall conditioned fear in the rat. Behav Brain Res. 2006;169:231–238. doi: 10.1016/j.bbr.2006.01.012. [DOI] [PubMed] [Google Scholar]
- [9].Hunter AS. Impaired extinction of fear conditioning after REM deprivation is magnified by rearing in an enriched environment. Neurobiol Learn Mem. 2015;122:11–18. doi: 10.1016/j.nlm.2015.01.003. [DOI] [PubMed] [Google Scholar]
- [10].Tang Y-P, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41:779–790. doi: 10.1016/S0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- [11].Zanardi A, Ferrari R, Leo G, Maskos U, Changeux J-P, Zoli M. Loss of high-affinity nicotinic receptors increases the vulnerability to excitotoxic lesion and decreases the positive effects of an enriched environment. FASEB J. 2007;21:4028–4037. doi: 10.1096/fj.07-8260com. [DOI] [PubMed] [Google Scholar]
- [12].Mitra R, Sapolsky RM. Effects of enrichment predominate over those of chronic stress on fear-related behavior in male rats. Stress. 2009;12:305–312. doi: 10.1080/10253890802379955. [DOI] [PubMed] [Google Scholar]
- [13].Pietropaolo S, Feldon J, Alleva E, Cirulli F, Yee BK. The role of voluntary exercise in enriched rearing: a behavioral analysis. Behav Neurosci. 2006;120:787–803. doi: 10.1037/0735-7044.120.4.787. [DOI] [PubMed] [Google Scholar]
- [14].Imanaka A, Morinobu S, Toki S, Yamawaki S. Importance of early environment in the development of post-traumatic stress disorder-like behaviors. Behav Brain Res. 2006;173:129–137. doi: 10.1016/j.bbr.2006.06.012. [DOI] [PubMed] [Google Scholar]
- [15].Woodcock EA, Richardson R. Effects of environmental enrichment on rate of contextual processing and discriminative ability in adult rats. Neurobiol Learn Mem. 2000;73:1–10. doi: 10.1006/nlme.1999.3911. [DOI] [PubMed] [Google Scholar]
- [16].Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- [17].Lapiz MDS, Fulford A, Muchimapura S, Mason R, Parker T, Marsden CA. Influence of postweaning social isolation in the rat on brain development, conditioned behavior, and neurotransmission. Neurosci Behav Physiol. 2003;33:13–29. doi: 10.1023/a:1021171129766. [DOI] [PubMed] [Google Scholar]
- [18].Weiss IC, Pryce CR, Jongen-Rêlo A-L, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- [19].Võikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- [20].Weiss IC, Feldon J, Domeney AM. Isolation rearing-induced disruption of pre-pulse inhibition: Further evidence for fragility of the response. Behav Pharmacol. 1999;10:139–149. doi: 10.1097/00008877-199907000-00011. [DOI] [PubMed] [Google Scholar]
- [21].Gresack JE, Risbrough VB, Scott CN, Coste S, Stenzel-Poore M, Geyer MA, Powell SB. Isolation rearing-induced deficits in contextual fear learning do not require CRF2 receptors. Behav Brain Res. 2010;209:80–84. doi: 10.1016/j.bbr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schroyens N. Locomotor Activity Test.xlsx. 2018 [dataset] https://osf.io/8k6uh/
- [23].Schroyens N. NSARG01-freezing.csv. 2018 [dataset] https://osf.io/y7hqj/
- [24].Schroyens N. NSARG01-weights.csv. 2018 [dataset] https://osf.io/8k4gd/
- [25].Espejo PJ, Ortiz V, Martijena ID, Molina VA. GABAergic signaling within the Basolateral Amygdala Complex modulates resistance to the labilization/reconsolidation process. Neurobiol Learn Mem. 2017;144:166–173. doi: 10.1016/j.nlm.2017.06.004. [DOI] [PubMed] [Google Scholar]
- [26].Espejo PJ, Ortiz V, Martijena ID, Molina VA. Stress-induced resistance to the fear memory labilization/reconsolidation process. Involvement of the basolateral amygdala complex. Neuropharmacology. 2016;109:349–356. doi: 10.1016/j.neuropharm.2016.06.033. [DOI] [PubMed] [Google Scholar]
- [27].Ortiz V, Giachero M, Espejo PJ, Molina VA, Martijena ID. The effect of midazolam and propranolol on fear memory reconsolidation in ethanol-withdrawn rats: Influence of d-cycloserine. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].JASP team. JASP (version 0.8.5.1) 2017 https://jasp-stats.org.
- [29].R Foundation. R (version 3.3.2) 2016 https://www.r-project.org.
- [30].Akagi K, Yamada M, Saitoh A, Oka J-I, Yamada M. Post-reexposure administration of riluzole attenuates the reconsolidation of conditioned fear memory in rats. Neuropharmacology. 2018;131:1–10. doi: 10.1016/j.neuropharm.2017.12.009. [DOI] [PubMed] [Google Scholar]
- [31].Alfei JM, Ferrer Monti RI, Molina VA, Bueno AM, Urcelay GP. Prediction error and trace dominance determine the fate of fear memories after post-training manipulations. Learn Mem. 2015;22:385–400. doi: 10.1101/lm.038513.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bustos SG, Giachero M, Maldonado H, Molina VA. Previous stress attenuates the susceptibility to midazolam's disruptive effect on fear memory reconsolidation: Influence of pre-reactivation D-cycloserine administration. Neuropsychopharmacology. 2010;35:1097–1108. doi: 10.1038/npp.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bustos SG, Maldonado H, Molina VA. Disruptive effect of midazolam on fear memory reconsolidation: decisive influence of reactivation time span and memory age. Neuropsychopharmacology. 2009;34:446–457. doi: 10.1038/npp.2008.75. [DOI] [PubMed] [Google Scholar]
- [34].Bustos SG, Maldonado H, Molina VA. Midazolam disrupts fear memory reconsolidation. Neuroscience. 2006;139:831–842. doi: 10.1016/j.neuroscience.2005.12.064. [DOI] [PubMed] [Google Scholar]
- [35].Ferrer Monti RI, Alfei JM, Mugnaini M, Bueno AM, Beckers T, Urcelay GP, Molina VA. A comparison of behavioral and pharmacological interventions to attenuate reactivated fear memories. Learn Mem. 2017;24:369–374. doi: 10.1101/lm.045385.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ferrer Monti RI, Giachero M, Alfei JM, Bueno AM, Cuadra G, Molina VA. An appetitive experience after fear memory destabilization attenuates fear retention: involvement GluN2B-NMDA receptors in the Basolateral Amygdala Complex. Learn Mem. 2016;23:465–478. doi: 10.1101/lm.042564.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pineyro ME, Ferrer Monti RI, Alfei JM, Bueno AM, Urcelay GP. Memory destabilization is critical for the success of the reactivation-extinction procedure. Learn Mem. 2013;21:785–793. doi: 10.1101/lm.032714.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Saitoh A, Akagi K, Oka J-I, Yamada M. Post-reexposure administration of d-cycloserine facilitates reconsolidation of contextual conditioned fear memory in rats. J Neural Transm. 2017;124:583–587. doi: 10.1007/s00702-017-1704-0. [DOI] [PubMed] [Google Scholar]
- [39].Stern CAJ, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ. On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology. 2012;37:2132–2142. doi: 10.1038/npp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang S, Cranney J. The role of GABA and anxiety in the reconsolidation of conditioned fear. Behav Neurosci. 2008;122:1295–1305. doi: 10.1037/a0013273. [DOI] [PubMed] [Google Scholar]
- [41].Merlo E, Milton AL, Everitt BJ. A novel retrieval-dependent memory process revealed by the arrest of ERK1/2 activation in the basolateral amygdala. J Neurosci. 2018;38:3199–3207. doi: 10.1523/JNEUROSCI.3273-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cassini LF, Flavell CR, Amaral OB, Lee JLC. On the transition from reconsolidation to extinction of contextual fear memories. Learn Mem. 2017;24:392–399. doi: 10.1101/lm.045724.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weinstock M. Prenatal stressors in rodents: effects on behavior. Neurobiol Stress. 2016;6:3–13. doi: 10.1016/j.ynstr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jacobs NS, Cushman JD, Fanselow MS. The accurate measurement of fear memory in Pavlovian conditioning: resolving the baseline issue. J Neurosci Methods. 2010;190:235–239. doi: 10.1016/j.jneumeth.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [46].Sahakian BJ, Robbins TW, Iversen SD. The effects of isolation rearing on exploration in the rat. Anim Learn Behav. 1977;5:193–198. doi: 10.3758/BF03214077. [DOI] [Google Scholar]
- [47].Woodcock EA, Richardson R. Effects of multisensory environmental stimulation on contextual conditioning in the developing rat. Neurobiol Learn Mem. 2000;74:89–104. doi: 10.1006/nlme.1999.3949. [DOI] [PubMed] [Google Scholar]
- [48].Clemenson GD, Lee SW, Deng W, Barrera VR, Iwamoto KS, Fanselow MS, Gage FH. Enrichment rescues contextual discrimination deficit associated with immediate shock. Hippocampus. 2015;25:385–392. doi: 10.1002/hipo.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.