Abstract
The human mitochondrial outer membrane protein voltage-dependent anion channel isoform 2 (hVDAC2) is a β-barrel metabolite flux channel that is indispensable for cell survival. It is well established that physical forces imposed on a transmembrane protein by its surrounding lipid environment decide protein structure and stability. Yet, how the mitochondrial membrane and protein-lipid interplay together regulate hVDAC2 stability is unknown. Here, we combine experimental biophysical investigations of protein stability with all-atom molecular dynamics simulations to study the effect of the most abundant mitochondrial phosphocholine (PC) lipids on hVDAC2. We demonstrate experimentally that increasing the PC lipid acyl chain length from diC14:0 to diC18:0-PC has a nonlinear effect on the β-barrel. We show that protein stability is highest in diC16:0-PC, which exhibits a negative mismatch with the hVDAC2 barrel. Our simulations also reveal that structural rigidity of hVDAC2 is highest under optimal negative mismatch provided by diC16:0-PC bilayers. Further, we validate our observations by altering the physical properties of PC membranes indirectly using cholesterol. We propose that VDAC plasticity and stability in the mitochondrial outer membrane are modulated by physical properties of the bilayer.
Introduction
Cellular biomembranes possess a dynamic phospholipid bilayer, harboring membrane proteins that carry out vital functions for cell survival. In humans, although transmembrane proteins are largely helical in nature, transmembrane β-barrels are present almost exclusively in the outer mitochondrial membrane (OMM) (1). The OMM possesses a distinct composition of phospholipids with trace amounts of cardiolipin and cholesterol. The physicochemical nature of these lipids influence membrane protein energetics (2). Therefore, lipid-dependent regulation forms a vital component of membrane protein function, stability, and oligomerization (3,4). Previous studies have identified the role of bilayer lateral pressure and bilayer stress, membrane asymmetry, protein-lipid hydrophobic mismatch, and curvature stress on the oligomerization of transmembrane helices (4–6), in providing a signaling platform, and also causing unfavorable aggregation (7,8). Similar studies on transmembrane β-barrels are limited (1,7) and have suggested that the rigidity of β-barrel proteins do not allow for considerable structural plasticity in a mismatched bilayer (9,10).
We asked if the OMM modulates the energetics of its most abundant β-barrel channel, namely the human voltage-dependent anion channel (hVDAC). Humans have three VDAC isoforms that adopt 19-stranded β-barrel structures. They additionally possess a flexible N-terminal voltage-sensor helix that docks within the folded barrel (11–13). hVDAC1 and hVDAC2 are vital for metabolite and nutrient flux (13–15). In particular, the hVDAC2 isoform has gained considerable recent interest owing to its antiapoptotic properties, its potential involvement in forming the permeability transition pore, and its relevance to neurodegeneration and cardiomyopathies (13,15–17). Cholesterol is believed to affect VDAC dynamics (18–20). VDAC oligomerization and gating are also influenced by the surrounding lipid (21–24). Although several studies focus on the role of VDACs in apoptosis and their cholesterol and cardiolipin dependence, no study has explicitly addressed the effect of phospholipids on VDAC stability.
The OMM is enriched with phospholipids, with >50% of the lipid content being phosphocholine (PC). The interplay and inter-regulation of hVDACs and PC lipids has been postulated but not explored in detail. The crystal structures of VDACs suggest that the hydrophobic thickness of this β-barrel is ~2.34 nm (25–28). When compared with documented values of hydrophobic thickness of lipid bilayers (~2.6 nm for di14:0 PC, ~2.9 nm for di16:0 PC, and ~3.2 nm for di18:0 PC (29)), an optimal hydrophobic match for VDACs is provided by di14:0 PC. However, 14-C lipids constitute <0.1% of the OMM. Hence, the molecular basis of VDAC-lipid interplay in the OMM calls for a detailed study.
Here, we combine experimental measurements of protein stability with all-atom molecular dynamics simulations (MDSs) of hVDAC2 to understand the effect of acyl chain length on this transmembrane β-barrel. Surprisingly, we find that the diacyl 16:0 phosphocholine (diC16:0-PC, DPPC) system confers the highest stability to hVDAC2 by providing an optimal negative mismatch with the transmembrane region of the barrel. The addition of cholesterol lowers hVDAC2 stability in diC16:0-PC lipids indirectly by increasing the membrane thickness. We propose that cells can regulate the stability of hVDAC2 in the mitochondrial outer membrane by altering the physical characteristics of the lipid bilayer.
Materials and Methods
Lipids
All lipids and detergents were procured from Avanti Polar Lipids, Alabaster, AL. The 12C detergent used was n-dodecylphosphocholine (DPC), and the saturated long-chain lipids used were 1,2-dimyristoyl-sn-glycero-3-phosphocholine (diC14:0-PC, DMPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (diC16:0-PC, DPPC), and 1,2-distearoyl-sn-glycero-3-phosphocholine (diC18:0-PC, DSPC).
Unfolded protein stock
Purified protein powder (expressed in Escherichia coli and purified without any additional tag, using reported methods (30); see Fig. S1 for purification profile and sodium dodecyl sulfate polyacrylamide gel electrophoresis image) was dissolved in 6 M guanidinium chloride and 10 mM dithiothreitol (DTT) at 60°C for 5 min. Samples were centrifuged for 1 h at 4°C to remove trace amounts of undissolved protein, and the supernatant was used for folding. The protein concentration was adjusted to 250 μM. A molar extinction coefficient of 36,900 M−1 cm−1 calculated at 280 nm was used for determining the concentrations.
Protein folding in PC bicelles
The folded hVDAC2 full-length protein was prepared by two methods: indirect folding (hVDAC2 was folded in micelles, followed by reconstitution hVDAC2-micelle assembly into bicelles) or direct folding (folding of hVDAC2 into preformed bicelles). Details of the indirect folding method are provided in the Supporting Materials and Methods. The thermal parameters measured for hVDAC2 from both preparations were similar. Hence, all the experiments were performed by hVDAC2 directly folded in preformed bicelles.
Bicelles of q = 1 were prepared by mixing 20 mM of the respective long-chain lipid with 20 mM DPC as the short-chain lipid. Bicelles were prepared in 0.9 volume of buffer A containing 10 mM DTT by subjecting the lipid suspension to repeated freeze-thaw cycles using liquid nitrogen and 60°C heating block (42°C for DMPC bicelles) till the solution became transparent. Once formed, the bicelles were prechilled at 4°C and used for hVDAC2 reconstitution.
Direct reconstitution of protein in bicelles was achieved by a 10-fold dilution of 250 μM of 0.1 volume of the unfolded protein stock prepared in guanidinium chloride into 0.9 volumes of the prechilled bicelles. The bicelle-protein assembly was subjected to three rounds of heating (35°C), cooling (4°C), and vortexing (30 s) cycles and then allowed to equilibrate overnight at 4°C by gentle mixing at 15 rotations per minute. This sample was further diluted fivefold in buffer A (50 mM sodium phosphate (pH 7.2), 100 mM NaCl) and subjected to centrifugation at 13,500 rotations per minute, 4°C for 1 h to remove trace amounts of aggregated protein. Folding of hVDAC2 was verified using the fluorescence emission spectra in bicelles and single-channel-gating measurements in a planar bilayer membrane using an electrophysiology set-up, using reported methods (30,31) (Fig. S2). The final folding mixture had 5 μM protein, 4 mM DPC, and 4 mM long-chain lipid to achieve a bicelle q = 1.0 in buffer A containing 2 mM DTT.
Cholesterol doping in PC bicelles
The different long-chain PC lipids (DMPC, DPPC, DSPC) in chloroform were doped with different percents (w/v) of cholesterol (0.02, 0.03, and 0.04% with respect to the long-chain lipid) and dried under a stream of nitrogen, followed by lyophilization. Bicelles of q = 1.0 having varying percentage of cholesterol were prepared as described above, with DPC as the short-chain lipid. A negligible amount of lipid was lost in the case of DSPC:DPC bicelles with 0.02% cholesterol. Occasionally, protein folding efficiency was affected in the 0.04% cholesterol-doped condition. The conversion between percent w/v and mole percent is presented in Table S2.
Differential scanning microcalorimetry
Bicelles are known to exhibit complex phases that are temperature dependent. To verify whether this interfered in our measurements, bicelles of different q (0.5, 0.75, and 1.0) and varying chain lengths (14-C, 16-C, 18-C) were prepared in buffer A. DPC was used as the short-chain lipid. The enthalpic transitions of these bicelles were monitored from 4 to 80 or 120°C at a ramp rate of 1°C/min on a MicroCal VP-DSC microcalorimeter. A 1 s filtering period and high gain mode were used to check the transition temperature of all the bicelle preparations.
Thermal denaturation measurements of hVDAC2
Thermal denaturation of folded hVDAC2 in various bicelle conditions was carried out on a JASCO (Easton, MD) J-815 circular dichroism (CD) spectropolarimeter. Wavelength scans were obtained at 4°C using a quartz cuvette of 1 mm pathlength and acquisition settings of 0.5 nm data pitch, 100 nm/min scan speed, 1 s data integration time, and 1 nm bandwidth. Data were averaged over three accumulations. Thermal denaturation was monitored at 215 nm from 4 to 95°C at 1°C intervals, with 1°C/min ramp rate, 1 nm bandwidth, and 1 s data integration time. Each experiment was repeated two to three times with independent protein preparations to check for reproducibility. After correction for buffer (and empty bicelle) contribution, data were smoothed using the means-movement method. The fraction unfolded (fU) data were calculated using the following formula:
Here, θObs is the observed molar ellipticity at 215 nm (ME215) at a given temperature, and θF and θUF are the ME215 values for the folded protein at 4°C and unfolded protein at 95°C, respectively. All fU data were fitted to a two-state equation for thermal denaturation (32) to derive the Tm (the midpoint of thermal denaturation) and ΔHapp (cooperativity of the unfolding transition; the enthalpy measured is an apparent value because the protein shows irreversible unfolding). Values obtained from two to three independent experiments were averaged to obtain the mean Tm and ΔHapp the SD.
Isothermal unfolding kinetics of hVDAC2
Folded hVDAC2 in bicelles was subjected to isothermal unfolding with time, and the process was monitored using far-ultraviolet (UV) CD at 215 nm. Data were acquired from 40 to 95°C at 2°C intervals using a 1 s data integration time, 1 s data pitch, and 1 nm bandwidth. The first (rapid) transition was fitted to a single exponential decay function using reported methods (30) to derive the rate of protein unfolding (kU) in each q of all bicelle preparations. The natural logarithm values of the rates (lnkU) were plotted against 1000/T, where T is the temperature in K. The Arrhenius plot was fitted to a linear function to derive the activation energy (Eact) using the formula slope = −Eact/R, where R is the gas constant (1.987 cal K−1 mol−1).
All-atom MD Simulations
The hVDAC2 structure was generated using iterative threading assembly refinement (I-TASSER) (33) and used as the input file for all the simulations. The alignment of hVDAC2 in the lipid membrane was generated using PyMOL v1.5.0.5 by superposing the I-TASSER-generated hVDAC2 structure onto the zebrafish VDAC2 structure (Protein Data Bank: 4BUM) obtained from the Orientation of Proteins in Membranes database (25). The Bilayer Builder tool in the CHARMM-GUI web server (34) was used to generate the assembled hVDAC2-lipid moiety and the input files required for simulation.
One hVDAC2 molecule was inserted into an assembled lipid bilayer that was generated with or without cholesterol (1). For the condition without cholesterol, four bilayer types were generated using PC lipids of varying hydrocarbon chain length, as follows: 1) 14-C, DMPC; 2) 16-C, DPPC; 3) 18-C, DSPC; and 4) equal mixture of DPPC and DSPC (2). For the condition with cholesterol, DPPC bilayers were generated with increasing cholesterol content (1.25, 5, 12.5, and 25% mole fraction cholesterol), corresponding to 1, 4, 10, and 20 cholesterol molecules per leaflet. Cholesterol was introduced in the bilayer by replacing the equivalent number of DPPC molecules.
In all cases, the protein-lipid complex was hydrated using a 2.0 nm water layer on either side of the bilayer, and 0.1 M NaCl was used to neutralize the charges. The final box dimension for each system was 8.5 × 8.5 × 8.5 nm3. A CHARMM-36 force field and NPT (constant particle number, pressure, and temperature) ensemble with zero external surface tension was used to generate the input assembly. The system contained one protein, 80 lipid molecules per leaflet, 19 Na+ and 20 Cl− ions, and ~9000–11,000 water molecules. In the DPPC/DSPC mixed bilayer system, an equal number of DPPC and DSPC molecules were retained in each leaflet. In the cholesterol-doped DPPC bilayer system, cholesterol molecules were distributed evenly in the upper and lower leaflets at the start of the simulation.
All simulations were carried out using GROMACS v5.0.4 (35) using reported methods (36). For systems without cholesterol, two 200 ns simulations were carried out using independent starting VDAC-lipid assemblies. For the DPPC-cholesterol assemblies, one 100 ns simulation was run using independent starting VDAC-lipid assemblies. The temperatures used for the final equilibration and production steps were maintained above the phase-transition temperature of each lipid (Table S1). For the mixed DPPC/DSPC system, the numerical average of the individual DPPC and DSPC phase-transition temperatures was used for the simulation. The temperature used for DPPC was retained for the DPPC-cholesterol system as well.
After energy minimization, six steps of equilibration were carried out, wherein restraints were reduced in a stepwise manner (37). At the end, a production run with zero restraints was executed to generate the final trajectory. Analysis of the trajectory was carried out using GROMACS v5.0.4, VMD v1.9.3, or PyMOL. GridMAT-MD_V2.0 or MEMBPLUGIN v1.1 (38) was used to calculate the average lipid thickness map, area per lipid (APL), and lipid order parameter (Scd). Here, the lipid ordered parameter is defined as the angle of rotation of acyl chains from its bilayer normal axis and can be calculated using the formula (39)
where θ is the angle between the C-H bond vector to the bilayer normal axis.
Data from the first 5 ns of the simulation was excluded for RMSF calculation. Lipid physical parameters including Scd, APL, and bilayer thickness were calculated using 50 successive frames between 10 and 200 ns of the simulation data. The change in lipid thickness along the trajectory was calculated from the distance between center of mass of all the phosphate atoms present in the two lipid leaflets.
Results and Discussion
Intrinsic protein-lipid interactions and protein adaptation can be studied biophysically by varying the protein/lipid ratios and lipid characteristics (e.g., headgroup, chain length, saturation). Here, we specifically address the effect of PC lipids, which are the most abundant OMM lipids, on hVDAC2. To deduce protein stability, we characterize hVDAC2 biophysically by measuring the protein response to temperature in PC and cholesterol-doped PC membranes. Further, we present all-atom MDSs results of hVDAC2 dynamics in PC and doped-PC bilayers. Our results show that an optimal negative mismatch imposed by the PC bilayer stabilizes the hVDAC2 β-barrel.
hVDAC2-di16:0-PC negative mismatch stabilizes the β-barrel
To address the effect of diacyl chain length on hVDAC2, we prepared isotropic PC bicelles of q = 1.0 in diC14:0-PC (DMPC), diC16:0-PC (DPPC), and diC18:0-PC (DSPC) as the long-chain lipid. Although the physical properties of bicelles are different from lipid bilayers (40), they are useful membrane mimetics that support the folding of hVDAC2. Of the three lipids, DPPC (~36%), DSPC (~20%), and the unsaturated analogs of DSPC (C18:1-PC, ~18%; C18:2-PC, ~16.6%) are abundant in the OMM, constituting >90% of the total PC content (41,42). We used DPC (monoacyl C12:0-PC (43)) as the short-chain lipid. The far-UV CD spectrum of hVDAC2 folded in bicelles shows a negative maximum at ~215 nm, which is characteristic of a β-rich structure. The spectra are similar in all three lipids (DMPC, DPPC, and DSPC) (Fig. 1 A, left, BM spectra), suggesting that β-barrel formation is supported in all three diacyl chain PCs. We additionally verified β-barrel formation using its fluorescence properties and ion-channel-gating characteristics (see Fig. S2).
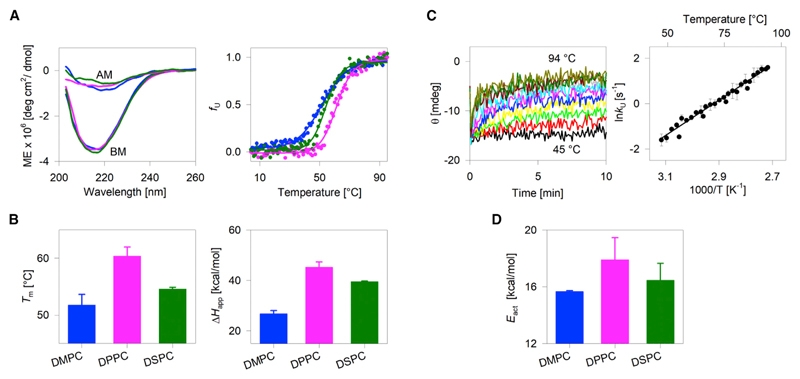
Figure 1.
Effect of lipid-diacyl-chain length on hVDAC2 stability. (A, left) Representative far-UV CD wavelength scans of folded hVDAC2 at 4°C. BM, before thermal denaturation; AM, after thermal denaturation. (A, right) Dependence of the unfolded protein fraction (fU) on temperature, derived from far-UV CD thermal unfolding at 215 nm. Fits of the data from DMPC (blue), DPPC (pink), and DSPC (green) bicelles to a two-state thermal denaturation model are in solid lines. (B) Comparison of the Tm and ΔHapp derived from the thermal unfolding measurements reveals that stability is highest in DPPC. Error bars represent the SD calculated from three independent experiments. The significance of the differences was measured for DMPC and DSPC with respect to DPPC using t-test. In the case of Tm, p-values are 0.0005 for DMPC-DPPC and 0.0012 for DSPC-DPPC. For ΔHapp, p-values are 0.0002 for DMPC-DPPC and 0.01 for DSPC-DPPC. (C, left) Representative isotherms for the unfolding kinetics of hVDAC2 monitored in DMPC using far-UV CD (215 nm) at various temperatures from 46 to 94°C at 2°C intervals. The fit of each isotherm to a single exponential function provided the unfolding rate (kU) at that temperature. (C, right) A representative Arrhenius plot (in DMPC bicelles) obtained by plotting the ln kU against temperature. Fit (solid line) of the data to the Arrhenius equation yielded the activation energy (Eact). (D) Dependence of Eact of hVDAC2 on the acyl-chain length. Error bars represent the SD calculated from two independent experiments, with each experiment containing ~25 independently measured rates. Overall, the hVDAC2 stability from Tm, ΔHapp, and Eact is highest in DPPC. The complete data are presented in Figs. S3 and S4. To see this figure in color, go online.
We used temperature as the perturbant to monitor hVDAC2 stability. Upon heating, hVDAC2 undergoes coupled unfolding and aggregation (30,31). These aggregates contribute marginally to the measured ellipticity at 215 nm (Fig. 1 A, left, after melting [AM] spectra). Hence, the thermal denaturation monitored using far-UV CD measures the combined process of barrel unfolding and aggregation. We followed the unfolding and aggregation processes by monitoring the loss in secondary structure content (reduction in ellipticity at 215 nm) using far-UV CD. The thermal transition of empty bicelles was assessed independently using microcalorimetry to ensure that the lipid phase transition temperature was different from protein unfolding (Fig. S3). We also verified that the transition temperature of our bicelle preparations matches previous reports (44).
Fig. 1 summarizes our results from thermal denaturation studies. hVDAC2 unfolds cooperatively beyond ~25–50°C in the different bicelle preparations (Fig. 1 A, right; Fig. S4). The data were fitted to a two-state thermal denaturation function using reported methods (32) to derive the midpoint temperature of unfolding and aggregation (Tm) and the apparent unfolding and aggregation enthalpy (ΔHapp; representing cooperativity of the unfolding process) in each lipid. Notably, DPPC, with a 16-C diacyl chain, emerges as the most thermostable lipidic condition for hVDAC2. Here, the two major measures of protein stability (Tm and ΔHapp) are significantly high only in DPPC (Fig. 1, A and B). DMPC, which has a 14-C diacyl chain, emerges as the lowest thermostable lipidic condition for hVDAC2, whereas the barrel exhibits moderate stability in DSPC. A nonlinear variation in hVDAC2 stability is therefore seen with a linear increase in diacyl PC chain length.
Additionally, we measured the activation energy barrier (Eact) separating the folded and aggregated states of hVDAC2. Here, the rate of hVDAC2 unfolding and aggregation is measured using far-UV CD by monitoring the rate of loss in θ215 at different temperatures (Fig. 1 C, left). The plot of the coupled unfolding and aggregation rate at specific temperatures correlates linearly with the temperature (Fig. 1 C, right), and the Eact is derived from the Arrhenius equation. In line with the thermal parameters, the Eact is also the highest in DPPC:DPC bicelles (Figs. 1 D and S4).
Interestingly, in DPPC bilayers, where hVDAC2 stability is highest, the β-barrel exhibits a negative mismatch to the hydrophobic bilayer (see Fig. S5). This is also evident when we compare the transmembrane domain span of VDACs (hydrophobic thickness of ~2.3 ± 0.11 nm (25–28)) with the physical properties of the DPPC bilayer. The diacyl chains of DPPC provide a hydrophobic span of ~2.9 nm. In contrast, the membrane span of the 14-C DMPC is ~2.6 nm (29). Although DMPC provides a better match to the hydrophobic face of hVDAC2, our experiments reveal that the barrel exhibits lowered stability in this lipid. Therefore, we find that a degree of specificity exists between hVDAC2 and its surrounding lipid, with negative mismatch optimally stabilizing the barrel.
hVDAC2 structural rigidity highest in optimal negative mismatch provided by DPPC bilayer
To understand the molecular basis of hVDAC2 stability in bilayer mismatch conditions and to validate our conclusions, we carried out all-atom MDSs of hVDAC2 in PC bilayers. First, we modeled the structure of hVDAC2 based on the available structures of hVDAC1 and zebrafish VDAC2. The modeled β-barrel was then assembled in a lipid bilayer (~80 lipid molecules in each leaflet). The lipids used were diC14:0-PC (DMPC), diC16:0-PC (DPPC), diC18:0-PC (DSPC), and an equimolar mixture of diC16:0-PC and diC18:0-PC (DPPC-DSPC; DPDS). The protein-lipid assembly was first equilibrated by energy minimization. Each equilibrated assembly was used to perform independent 200 ns all-atom MDSs at a temperature that was at least 1°C above the reported phase transition temperature of the lipid (36).
Fig. 2, A and B compare the root mean-square deviation (RMSD), difference in per-residue root mean-square fluctuation (ΔRMSF), and total RMSF in DMPC (14-C), DPPC (16-C), and DSPC (18-C). Notably, these values are lowest in the DPPC system, which indicates that hVDAC2 is less dynamic in DPPC. The dynamicity is lowered for both the strand and loop residues (Fig. S6); therefore, DPPC modulates the RMSF of the complete hVDAC2 barrel. The values are high in both DMPC and DSPC, suggesting that the structural plasticity of hVDAC2 is modulated nonlinearly with changes in bilayer thickness. We reach a similar conclusion upon mapping the protein dynamics on hVDAC2 structure (see Figs. S7–S9).
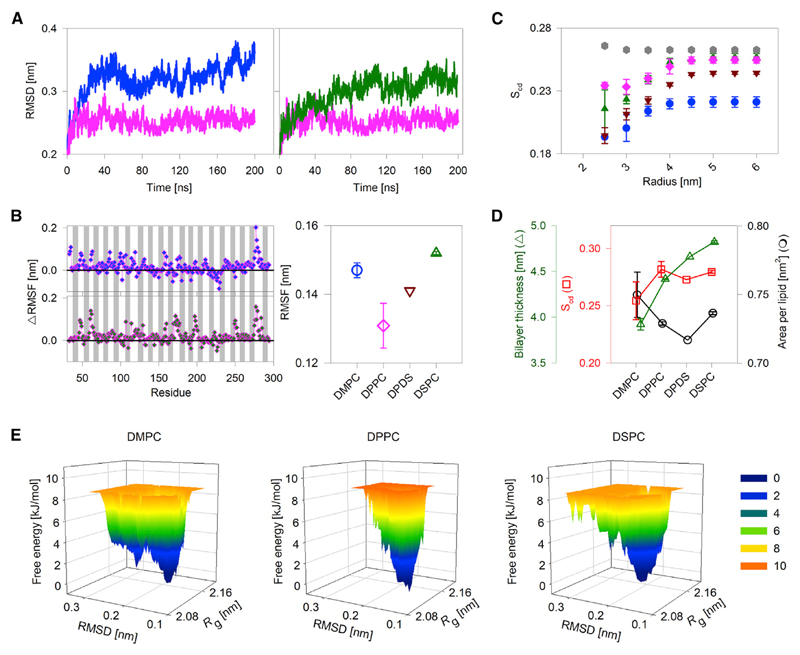
Figure 2.
hVDAC2-lipid parameters derived from all-atom MDSs. (A) A comparison of the RMSD in DMPC (blue), DPPC (pink), and DSPC (green) for the transmembrane region of hVDAC2. The RMSD is lowest in DPPC. (B) A comparison of per-residue ΔRMSF (left) and overall RMSF of the transmembrane region (right) in PC bilayers. ΔRMSF was calculated for each residue as RMSFDMPC – RMSFDPPC (left, top) and RMSFDSPC – RMSFDPPC (left, bottom). The 19 β-strands are indicated by gray bars, and loop regions are shown as white spaces (α1 is omitted). Data for DPPC + DSPC (DPDS) is in brown. The RMSF is lowest in DPPC. (C and D) Variation in lipid physical properties such as bilayer order parameter (Scd), area per lipid (APL), and bilayer thickness in the presence of hVDAC2. (C) Bilayer Scd was calculated for 50 frames from 10 to 200 ns trajectory are plotted for each acyl carbon of DMPC (blue circle), DPPC (pink diamond), DSPC (green upward triangle), and DPDS (brown inverted triangle) protein-lipid bilayer systems. Also included as control is the Scd for DPDS bilayer without protein (gray hexagons), wherein no change in the Scd is seen. The magnitude of increase in Scd is lowest in DPPC; DPPC also shows the highest Scd near the protein. Also note that the presence of protein decreases the overall Scd (compare gray hexagons with brown inverted triangles at 6 nm). (D) Average Scd, APL, and bilayer thickness derived from 50 frames of the 10 to 200 ns trajectory for the four lipid conditions shows a nonlinear change in Scd, APL, and bilayer thickness with a linear increase in the acyl-chain length. All error bars are from two independent 200 ns simulations. (E) A representative FEL plotted with respect to the radius of gyration (Rg) and RMSD of hVDAC2 in DMPC, DPPC, and DSPC bilayers. In DPPC, hVDAC2 samples a limited number of compact conformations (see the lower Rg values) and also lower RMSD values along the trajectory when compared to other lipids. Numbers beside the color scale correspond to the free energy in kJ/mol. A lower free-energy value corresponds to a more stable system. Additional data and analyses are presented in Figs. S5–S16. To see this figure in color, go online.
The free-energy landscape (FEL) represents various conformational states present in a protein molecule. We plotted the FEL with respect to changes in the radius of gyration Rg (representing the compactness of a molecule; the lower the Rg, the greater the compactness) and RMSD (representing the overall structural deviation from the original structure). A narrow FEL with lower RMSD and Rg indicates that the complex samples a limited number of compact conformations along the trajectory and leads to the formation of a barrel that is buried. We obtain a narrow FEL only for the hVDAC2-DPPC system, suggesting that the system dynamicity is low here, and it attains an energy-minimized stable state. On the other hand, the FEL is high for DMPC and DSPC systems, suggesting increased dynamicity in the system. Overall, our observation from MDS is in excellent agreement with our experiments and confirms that hVDAC2-DPPC systems are optimally stabilized. The structural plasticity increases as the diacyl chain is either shortened to 14-C in DMPC or lengthened to 18-C in DSPC. We conclude that hVDAC2 barrel stability is modulated nonlinearly with bilayer thickness.
hVDAC2 dynamics depends on its lipid environment. In turn, the physical properties of the lipids can be affected in the presence of hVDAC2. Hence, we analyzed lipid alterations occurring because of the hVDAC2 molecule. We calculated the distance dependence of the lipid order parameter (Scd) at 2.5–6.0 nm from the center of hVDAC2 pore using a 0.5 nm gap size. The analysis shows that the order parameter is lowered considerably near the protein, as it introduces perturbation in the lipid bilayer (Fig. 2 C). Notably, the Scd at a distance >4.0 nm is still lower than the Scd calculated from simulations of bilayers lacking protein (for example, the Scd of DPDS at 6.0 nm is ~0.26 and ~0.24 without and with hVDAC2, respectively; Fig. 2 C). Therefore, the incorporation of hVDAC2 alters the physical properties of the PC bilayer.
Interestingly, the acyl-chain ordering in the protein vicinity is highest for DPPC. Further, the overall Scd, APL, and bilayer thickness are expected to change linearly with 2-C increase in the acyl chain. However, we find that the lipid physical properties vary nonlinearly between DMPC, DPPC, and DSPC (Fig. 2 D), supporting our inference that the hVDAC2 barrel distinctively affects the bilayer characteristics. Additionally, in DPPC, in which hVDAC2 is stabilized by optimal negative mismatch, alteration of lipid physical properties is lowest. In other words, the hVDAC2 barrel is accommodated in DPPC with minimal changes in the local physical properties of the lipid molecules.
We also observe prominent membrane thinning in the vicinity of strands β1–β3, β7–β9, and β17–β19 in all lipidic conditions (see Figs. S10–S16). These strands comprise known homo- and hetero-oligomerization zones for VDACs (16,45,46), suggesting that intrinsic weakening of protein-lipid interactions, driven by increase in polarity of residues in the primary sequence, may facilitate VDAC association with its binding partners.
Bilayer physical properties influence hVDAC2-lipid interplay
Next, we asked whether it is the bilayer physical property that regulates hVDAC2 stability or whether our observation was specific to di16:0-PC. To address this, we used cholesterol. Cholesterol alters the physical properties and dynamics of the bilayer, such as bilayer thickness, fluidity, melting temperature of lipids and membrane microviscosity, and lipid bilayer packing (47). First, we folded the protein in DMPC, DPPC, and DSPC bicelles containing preincorporated cholesterol. We varied the cholesterol content from 0.02 to 0.04% (with respect to long-chain lipid); this corresponds to 13–26 mol% cholesterol for a bicelle of q = 1.0. The conditions of diacyl PC bicelles containing cholesterol wherein we were successfully able to fold hVDAC2 are listed in Table S2. DSPC bicelles with higher cholesterol content did not support the folding of hVDAC2, likely because of the increase in lipid ordering and negative mismatch. Hence, our results from cholesterol-doped DSPC are limited.
Fig. 3, A and B summarize our results from hVDAC2-PC-cholesterol systems. We obtain comparable far-UV CD spectra for hVDAC2 in the absence and presence of increasing cholesterol content in the PC membrane (Fig. 3 A, left). Further, cooperative unfolding and aggregation of hVDAC2 is observed in all conditions (Fig. 3 A, right; also Fig. S17). A comparison of the thermal parameters derived from the denaturation measurements show that increasing the cholesterol content has an overall destabilizing effect on hVDAC2 in cholesterol-doped DPPC bicelles (Fig. 3 B). Largely, the Tm is lowered as the cholesterol doping increases. Also interesting is to note that with increasing cholesterol content, hVDAC2 Tm increases in DMPC bicelles (Fig. 3 B, left). This observation contrasts with the results we obtained for nondoped conditions (see Fig. 1). The ΔHapp is largely similar in all conditions, suggesting that cholesterol primarily influences the stability of only the folded state of hVDAC2.
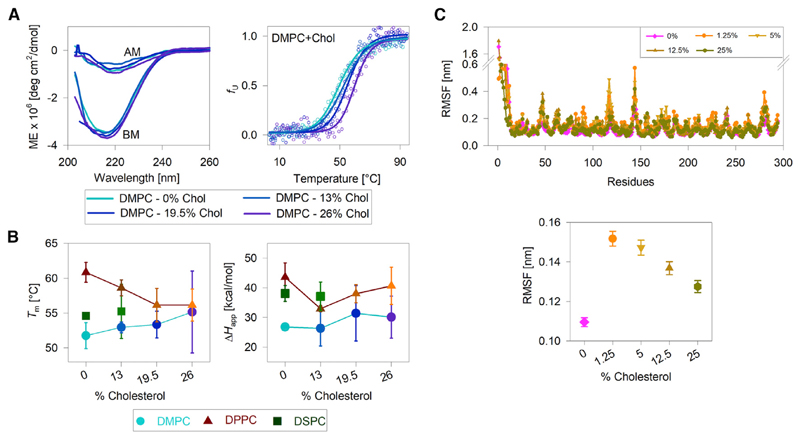
Figure 3.
Effect of bilayer thickness and acyl-chain ordering on hVDAC2 stability. (A, left) Representative far-UV CD wavelength scans of folded hVDAC2 at 4°C in DMPC bicelles doped with increasing mole percent of cholesterol (0–26%). BM, before thermal denaturation; AM, after thermal denaturation. (A, right) Dependence of the unfolded protein fraction (fU) on temperature, derived from far-UV CD thermal unfolding at 215 nm (see foot of the figure for color code). Fits of the data to a two-state thermal denaturation model to derive Tm and ΔHapp are in solid lines. Increasing the cholesterol content increases hVDAC2 thermal stability in DMPC bicelles. (B) A comparison of the Tm and ΔHapp derived in all three bicelles with and without cholesterol (see foot of the figure for color/symbol code). With increasing cholesterol content, the Tm increases in DMPC and decreases in DPPC. The ΔHapp shows a modest nonlinear variation with increasing cholesterol content, suggesting that the PC lipid modulates hVDAC2 stability without affecting the unfolding cooperativity. Error bars represent the SD from two to three independent experiments. For 26% cholesterol samples, errors in Tm are high because of difficulties in bicelle preparation. DSPC bicelles containing >13% cholesterol could not be prepared. (C) A comparison of per-residue RMSF (top) and overall RMSF (bottom) of the transmembrane region of hVDAC2 in DPPC bilayers doped with increasing mole percent of cholesterol. The RMSF is lowest in DPPC without additional cholesterol. Note that the % cholesterol in (B) and (C) cannot be compared directly because they represent mole percent in (B), obtained with respect to the concentration of the long-chain lipid, and mole fraction in (C). See Materials and Methods and Table S2 for details. The complete data and additional results are presented in Figs. S17–S26. To see this figure in color, go online.
Cholesterol increases bilayer thickness by increasing acyl chain ordering (47). Our results (Fig. 3, A and B) allow us to conclude that the bilayer thickness, which is modulated by cholesterol, regulates the stability of folded hVDAC2. Moreover, cholesterol-induced changes in protein stability is dependent on the acyl-chain length, suggesting that the optimal negative mismatch and membrane rigidity are foremost contributing factors to hVDAC2 stability. We reach a similar conclusion from our MDS results from hVDAC2-DPPC-cholesterol systems, wherein barrel dynamicity is lowest in 0% cholesterol (Figs. 3 C and S18–S26). We conclude that bilayer physical characteristics influence hVDAC2 stability, which can be varied through lipid-cholesterol interactions. It is also noteworthy that the vicinities of β7–β9 and β16–β18 retain the ability to cause bilayer thinning in cholesterol systems, reaffirming our inference that the lipid distribution is unaffected in this region of hVDAC2.
Conclusions
It is estimated that ~40–55% of the total OMM lipids are PCs (41,42), with 16:0-PC accounting for >35% and 18:0-PC for ~20% of the total OMM PC content (41). Further, VDACs are the most abundant OMM proteins. Hence, VDACs reside in a physiological environment rich in 16:0-PC and 18:0-PC. Our studies show that this negative mismatch provided by the OMM PCs indeed stabilizes hVDAC2. Although transmembrane helices adapt to the hydrophobic mismatch through conformational changes (9,10,48), bacterial transmembrane β-barrels are considered as rigid bodies that undergo structural deformation only under excessive mismatch or extreme lateral pressure (9,49). For example, E. coli OmpF binding to di(C14:1)PC is highest owing to the intrinsic hydrophobic match, and OmpF distorts bilayers of longer chains to achieve the hydrophobic matching (9). In interesting contrast, our study reveals that mitochondrial hVDAC2 is both sensitive to incremental changes in the diacyl-chain length and modulates its stability under conditions of bilayer match versus mismatch (Fig. 4). This biophysical response appears to be linked directly to the physical characteristics of the bilayer. Additionally, we find that unlike bacterial transmembrane β-barrels that exhibit characteristics of rigid structures (9), the hVDAC2 barrel undergoes deformation in all lipid-chain lengths; the scaffold deformation is lowest in DPPC. Hence, hVDAC2 is likely to be less rigid than its counterparts in the bacterial outer membrane.
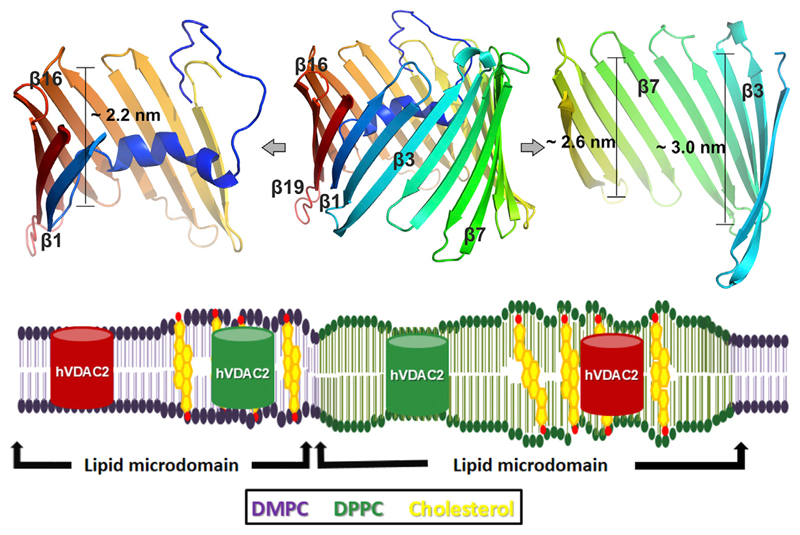
Figure 4.
Interaction dynamics of the asymmetric scaffold of hVDAC2 barrel with physical properties of the surrounding bilayer. (Upper panel) A ribbon diagram highlighting the asymmetric scaffold of the hVDAC2 barrel. The complete barrel is shown in the middle. Strands β2–β6 (right) at the N-terminal region of hVDAC2 have a transmembrane span of ~3.0 nm, which decreases to ~2.6 nm at the middle of the barrel near β9–β12. Strands β14–β18 at the C-terminal region of hVDAC2 (left) have a dimension of ~2.2 nm and are expected to exhibit the highest negative mismatch with DPPC. Note that the N-helix docks in the vicinity of these strands and can contribute to the stability of the C-terminal region. (Lower panel) A cartoon representation of how hVDAC2 stability can be modulated by bilayer mismatch in various lipid microdomains in the presence of cholesterol. An optimal bilayer mismatch achieved with DMPC-cholesterol or DPPC bilayers can increase hVDAC2 stability (green barrel). Suboptimal mismatch may lower hVDAC2 stability (red barrel) and can modulate barrel oligomerization in the mitochondrial outer membrane. To see this figure in color, go online.
Because mammalian mitochondria possess 16-C and 18-C PC lipids (42), we propose, based on our findings, that optimal mismatch conditions induced by PC chain length promotes hVDAC2 stability. Intraprotein and protein-lipid interactions, which are highest in DPPC membranes (see Figs. 1 and 2), along with asymmetry in the transmembrane region of the hVDAC2 barrel scaffold (Fig. 4, upper panel), could be important contributors to its measured stability in 16-C membranes. The ability of VDACs to induce local membrane deformation and thinning, as well as reduction in total lipid number in the vicinity of β7–9 and β17–β19-β1–β3 in all PCs (see Figs. S10 and S11), suggests that VDAC oligomerization interfaces are intrinsically available in the hVDAC2 barrel. Indeed, β7–β10 is a known zone for BAK (Bcl-2 homologous antagonist killer) binding (16) and VDAC oligomerization (11,50); the second zone (β17–β19, β1–β3) is needed for homodimer formation (28,51).
It is conceivable that mitochondria might be able to elegantly regulate VDAC stability and function by varying the generic bilayer physical properties. To our knowledge, this is the first observation of a negative mismatch stabilizing a human membrane protein barrel. Further studies in this direction could provide molecular insight on whether lipid sorting in the OMM allows VDACs to switch between homeostasis and apoptotic states.
Supplementary Material
Supporting Materials and Methods, 26 figures, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)31220-7.
Acknowledgments
We thank Udit Aswal and Svetlana Maurya for technical assistance with the MDS.
S.R.S thanks IISER Bhopal for research fellowship. R.M. is a Wellcome Trust-DBT India Alliance Intermediate Fellow. This work was supported by the Wellcome Trust-DBT India Alliance award number IA/I/14/1/501305 to R.M.
Footnotes
Author Contributions
R.M. designed the research. S.R.S. and P.Z. performed the stability measurements. S.R.S. performed the simulations. All authors analyzed the data and wrote the manuscript.
References
- 1.Schuler MH, Di Bartolomeo F, et al. Becker T. Phosphatidylcholine affects the role of the sorting and assembly machinery in the biogenesis of mitochondrial β-barrel proteins. J Biol Chem. 2015;290:26523–26532. doi: 10.1074/jbc.M115.687921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laganowsky A, Reading E, et al. Robinson CV. Membrane proteins bind lipids selectively to modulate their structure and function. Nature. 2014;510:172–175. doi: 10.1038/nature13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AG. Lipid-protein interactions. Biochem Soc Trans. 2011;39:761–766. doi: 10.1042/BST0390761. [DOI] [PubMed] [Google Scholar]
- 4.Lee AG. Biological membranes: the importance of molecular detail. Trends Biochem Sci. 2011;36:493–500. doi: 10.1016/j.tibs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Andersen OS, Koeppe RE., II Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 6.Nicolson GL. The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta. 2014;1838:1451–1466. doi: 10.1016/j.bbamem.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Ferrer I. Altered mitochondria, energy metabolism, voltage-dependent anion channel, and lipid rafts converge to exhaust neurons in Alzheimer’s disease. J Bioenerg Biomembr. 2009;41:425–431. doi: 10.1007/s10863-009-9243-5. [DOI] [PubMed] [Google Scholar]
- 8.Halbleib K, Pesek K, et al. Ernst R. Activation of the unfolded protein response by lipid bilayer stress. Mol Cell. 2017;67:673–684.e8. doi: 10.1016/j.molcel.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 9.O’Keeffe AH, East JM, Lee AG. Selectivity in lipid binding to the bacterial outer membrane protein OmpF. Biophys J. 2000;79:2066–2074. doi: 10.1016/S0006-3495(00)76454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Messina A, Reina S, et al. De Pinto V. VDAC isoforms in mammals. Biochim Biophys Acta. 2012;1818:1466–1476. doi: 10.1016/j.bbamem.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Zachariae U, Schneider R, et al. Lange A. β-Barrel mobility underlies closure of the voltage-dependent anion channel. Structure. 2012;20:1540–1549. doi: 10.1016/j.str.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurya SR, Mahalakshmi R. Mitochondrial VDAC2 and cell homeostasis: highlighting hidden structural features and unique functionalities. Biol Rev Camb Philos Soc. 2017;92:1843–1858. doi: 10.1111/brv.12311. [DOI] [PubMed] [Google Scholar]
- 14.Smilansky A, Dangoor L, et al. Shoshan-Barmatz V. The voltage-dependent anion channel 1 mediates amyloid β toxicity and represents a potential target for Alzheimer disease therapy. J Biol Chem. 2015;290:30670–30683. doi: 10.1074/jbc.M115.691493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naghdi S, Hajnóczky G. VDAC2-specific cellular functions and the underlying structure. Biochim Biophys Acta. 2016;1863:2503–2514. doi: 10.1016/j.bbamcr.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naghdi S, Várnai P, Hajnóczky G. Motifs of VDAC2 required for mitochondrial Bak import and tBid-induced apoptosis. Proc Natl Acad Sci USA. 2015;112:E5590–E5599. doi: 10.1073/pnas.1510574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maurya SR, Mahalakshmi R. VDAC-2: mitochondrial outer membrane regulator masquerading as a channel? FEBS J. 2016;283:1831–1836. doi: 10.1111/febs.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiser BP, Salari R, et al. Brannigan G. Computational investigation of cholesterol binding sites on mitochondrial VDAC. J Phys Chem B. 2014;118:9852–9860. doi: 10.1021/jp504516a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattin Z, Schneider R, et al. Lange A. Solid-state NMR, electrophysiology and molecular dynamics characterization of human VDAC2. J Biomol NMR. 2015;61:311–320. doi: 10.1007/s10858-014-9876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budelier MM, Cheng WWL, et al. Evers AS. Photoaffinity labeling with cholesterol analogues precisely maps a cholesterol-binding site in voltage-dependent anion channel-1. J Biol Chem. 2017;292:9294–9304. doi: 10.1074/jbc.M116.773069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rostovtseva TK, Kazemi N, et al. Bezrukov SM. Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes. J Biol Chem. 2006;281:37496–37506. doi: 10.1074/jbc.M602548200. [DOI] [PubMed] [Google Scholar]
- 22.Rostovtseva TK, Bezrukov SM. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr. 2008;40:163–170. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betaneli V, Petrov EP, Schwille P. The role of lipids in VDAC oligomerization. Biophys J. 2012;102:523–531. doi: 10.1016/j.bpj.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eddy MT, Ong TC, et al. Griffin RG. Lipid dynamics and protein-lipid interactions in 2D crystals formed with the β-barrel integral membrane protein VDAC1. J Am Chem Soc. 2012;134:6375–6387. doi: 10.1021/ja300347v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lomize MA, Lomize AL, et al. Mosberg HI. OPM: orientations of proteins in membranes database. Bioinformatics. 2006;22:623–625. doi: 10.1093/bioinformatics/btk023. [DOI] [PubMed] [Google Scholar]
- 26.Hiller S, Garces RG, et al. Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ujwal R, Cascio D, et al. Abramson J. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schredelseker J, Paz A, et al. Abramson J. High resolution structure and double electron-electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J Biol Chem. 2014;289:12566–12577. doi: 10.1074/jbc.M113.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kučerka N, Nieh MP, Katsaras J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim Biophys Acta. 2011;1808:2761–2771. doi: 10.1016/j.bbamem.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Maurya SR, Mahalakshmi R. Modulation of human mitochondrial voltage-dependent anion channel 2 (hVDAC-2) structural stability by cysteine-assisted barrel-lipid interactions. J Biol Chem. 2013;288:25584–25592. doi: 10.1074/jbc.M113.493692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurya SR, Mahalakshmi R. N-helix and cysteines inter-regulate human mitochondrial VDAC-2 function and biochemistry. J Biol Chem. 2015;290:30240–30252. doi: 10.1074/jbc.M115.693978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1:2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jo S, Kim T, et al. Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 35.Van Der Spoel D, Lindahl E, et al. Berendsen HJ. GROMACS: fast, flexible, and free. J Comput Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Cheng X, et al. Im W. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J Chem Theory Comput. 2016;12:405–413. doi: 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng X, Jo S, et al. Im W. CHARMM-GUI micelle builder for pure/mixed micelle and protein/micelle complex systems. J Chem Inf Model. 2013;53:2171–2180. doi: 10.1021/ci4002684. [DOI] [PubMed] [Google Scholar]
- 38.Guixà-González R, Rodriguez-Espigares I, et al. Selent J. MEMBPLUGIN: studying membrane complexity in VMD. Bioinformatics. 2014;30:1478–1480. doi: 10.1093/bioinformatics/btu037. [DOI] [PubMed] [Google Scholar]
- 39.Heller H, Schaefer M, Schulten K. Molecular-dynamics simulation of a bilayer of 200 lipids in the gel and in the liquid-crystal phases. J Phys Chem. 1993;97:8343–8360. [Google Scholar]
- 40.Caldwell TA, Baoukina S, et al. Columbus L. Low- q bicelles are mixed micelles. J Phys Chem Lett. 2018;9:4469–4473. doi: 10.1021/acs.jpclett.8b02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ardail D, Privat JP, et al. Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J Biol Chem. 1990;265:18797–18802. [PubMed] [Google Scholar]
- 42.Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Chaturvedi D, Mahalakshmi R. Position-specific contribution of interface tryptophans on membrane protein energetics. Biochim Biophys Acta Biomembr. 2018;1860:451–457. doi: 10.1016/j.bbamem.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silvius JR. Thermotropic phase transitions of pure lipids in model membranes and their modifications by membrane proteins. In: Jost PC, Griffith OH, editors. Lipid-Protein Interactions. John Wiley & Sons, Inc.; 1982. pp. 239–281. [Google Scholar]
- 45.Geula S, Naveed H, et al. Shoshan-Barmatz V. Structure-based analysis of VDAC1 protein: defining oligomer contact sites. J Biol Chem. 2012;287:2179–2190. doi: 10.1074/jbc.M111.268920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maurya SR, Mahalakshmi R. Control of human VDAC-2 scaffold dynamics by interfacial tryptophans is position specific. Biochim Biophys Acta. 2016;1858:2993–3004. doi: 10.1016/j.bbamem.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiu SW, Jakobsson E, et al. Scott HL. Cholesterol-induced modifications in lipid bilayers: a simulation study. Biophys J. 2002;83:1842–1853. doi: 10.1016/S0006-3495(02)73949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaiser HJ, Orłowski A, et al. Simons K. Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proc Natl Acad Sci USA. 2011;108:16628–16633. doi: 10.1073/pnas.1103742108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong H, Tamm LK. Elastic coupling of integral membrane protein stability to lipid bilayer forces. Proc Natl Acad Sci USA. 2004;101:4065–4070. doi: 10.1073/pnas.0400358101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lella M, Mahalakshmi R. Direct structural annotation of membrane protein aggregation loci using peptide-based reverse mapping. J Phys Chem Lett. 2018;9:2967–2971. doi: 10.1021/acs.jpclett.8b00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosaka T, Okazaki M, et al. Shirouzu M. Crystal structural characterization reveals novel oligomeric interactions of human voltage-dependent anion channel 1. Protein Sci. 2017;26:1749–1758. doi: 10.1002/pro.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.