Abstract
Neurodegeneration has been reported in young animals after exposure to all the commonly used general anesthetics (GA). The brain may be particularly vulnerable to anesthetic toxicity during peak synaptogenesis (in gestation and infancy). Human studies of long-term neurodevelopmental outcome following GA in early childhood report contradictory findings. This review assesses the strengths and deficiencies in human research methodologies to inform future studies. We identified 76 studies, published between 1990 and 2017, of long-term neurodevelopmental outcome following early childhood or in utero GA exposure: 49 retrospective, 9 ambi-directional, 17 prospective cohort studies and one randomized controlled trial (RCT). Forty-nine studies were explicitly concerned with anesthetic-induced neurotoxicity (AIN). Full texts were appraised for methodological challenges and possible solutions. Major challenges identified included: delineating effects of anesthesia from surgery; defining the timing and duration of exposure; selection of a surgical cohort and intervention; addressing multiple confounding life course factors; detecting modest neurotoxic effects with small sample sizes (median 131 children, IQR: 50-372); selection of sensitive neurodevelopmental outcomes at appropriate ages for different developmental domains; insufficient length of follow-up (median age: 6 years, IQR: 2-12) and sample attrition. We discuss potential solutions to these challenges. Further adequately powered, multi-center, prospective RCT of AIN in children are required. However, we believe that the inherent methodological challenges of studying AIN necessitate the parallel use of well-designed observational cohort studies.
Introduction
General anesthesia (GA) has long been considered a safe means of enabling pediatric surgery, unpleasant procedures or medical imaging. However, concerns have accumulated that fetuses, babies and young children exposed to GA may experience long-lasting neurotoxic effects1. Approximately 200,0002 of the 4 million3 children <6 years of age in the United Kingdom undergo GA annually (5%), making the risk of anesthetic-induced neurotoxicity (AIN) a critical public health issue.
Pre-clinical studies demonstrate that exposure to all commonly used intravenous and inhalational anesthetic agents is associated with altered brain development in immature animals including non-human primates4,5. Single long exposures6 as well as multiple exposures7 adversely affect neurodevelopment. The duration and timing of exposure influences the neurotoxic potential of general anesthetic agents. The brain is thought to be particularly vulnerable during the period of synaptogenesis4. In humans this ‘vulnerable time-window’ is reportedly between the third trimester and 2-3 years of age6,8–11.
Human observational studies of AIN are heterogeneous in their methodologies and offer contrary conclusions. Studies of single brief GA for minor procedures are generally reassuring but worse long-term neurodevelopmental outcome has been reported following prolonged/repeated exposure1. Pooled effect estimates from observational studies indicate at least a modest risk of impaired neurodevelopment following GA for surgery in childhood12,13. To date, only one ongoing randomized controlled trial (RCT) of awake-spinal versus sevoflurane GA for herniorrhaphy before 60 weeks post-menstrual age has reported secondary outcomes14. The GAS trial reassuringly finds equivalent cognitive scores between groups at two years old. However, more comprehensive cognitive assessment in later childhood could still detect AIN.
Rising numbers of original studies and an exponential increase in review articles on pediatric anesthetic neurotoxicity over the past 10 years (Figure 1) have prompted regulatory and professional bodies to release precautionary statements concerning pediatric GA. The United States Food and Drug Administration cautions against lengthy/repeated GA or sedation in the third trimester and children <3 years old15. Guidance from the United Kingdom and Ireland16, and a statement from European bodies17, advocate avoiding unnecessary GA but recommend no changes to clinical practice.
Figure 1.
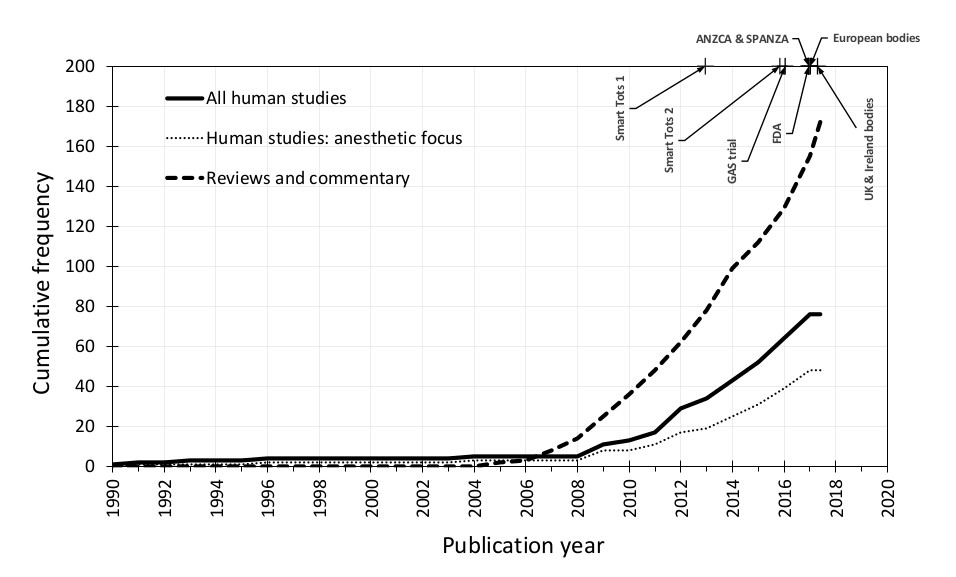
Cumulative number of human observational studies and randomized controlled trials of neurodevelopment following general anesthesia exposure at age <6 years (thick black line); those specifically designed to study anesthetic-induced neurotoxicity (AIN; dotted line). We place this in the context of the number of commentaries and review articles (dashed line) and milestone statements and publications concerning AIN. Smart Tots 1: Smart Tots consensus statement on the use of anesthetics and sedatives in children 2012122; Smart Tots 2: consensus statement on the use of anesthetic and sedative drugs in infants and toddlers 2015123; GAS trial: GAS randomized controlled trial secondary outcomes published 201614; FDA: U.S. Food and Drug Administration safety communication 201615; ANZCA & SPANZA: joint warning from the Australian and New Zealand College of Anaesthetists and the Society for Paediatric Anaesthesia in New Zealand and Australia 2016124; European bodies: consensus statement of the European Society of Anaesthesiology, the European Society for Paediatric Anaesthesiology, the European Association of Cardiothoracic Anaesthesiology and the European Safe Tots Anaesthesia Research Initiative 201717; UK & Ireland bodies: joint professional guidance on the use of general anesthesia in young children 201716.
There has been much discussion of the limitations of the existing human evidence-base for AIN. Therefore, to inform the design of future clinical studies, we identified and reviewed the seventy-six clinical studies of long-term neurodevelopmental outcome following early childhood or in utero GA exposure that were published between 1990 and April 2018 (Appendix 1) to identify particular challenges encountered in performing these types of study as well as feasible, pragmatic methodological solutions. We sought methods used to: isolate the effects of GA from surgery/disease; characterize anesthetic exposure and surgical intervention; address confounding; detect marginal neurotoxic effects and define what the implications of the research are for clinical practice. These are summarized in Table 1.
Table 1.
Challenges and potential solutions in human studies of anesthetic-induced neurotoxicity. GA: general anesthesia; MAC: minimum alveolar concentration.
| Challenges | Solutions |
|---|---|
| Measuring direct neurotoxic effects of GA | |
| Defining the GA exposure | |
| Selection of surgical cohort and procedure | |
| Addressing multiple confounding factors |
|
| Detecting modest neurotoxic effects |
|
| Measurement of neurodevelopmental outcome | |
| Length of follow-up and sample attrition |
|
Delineating the neurotoxic effect of anesthesia
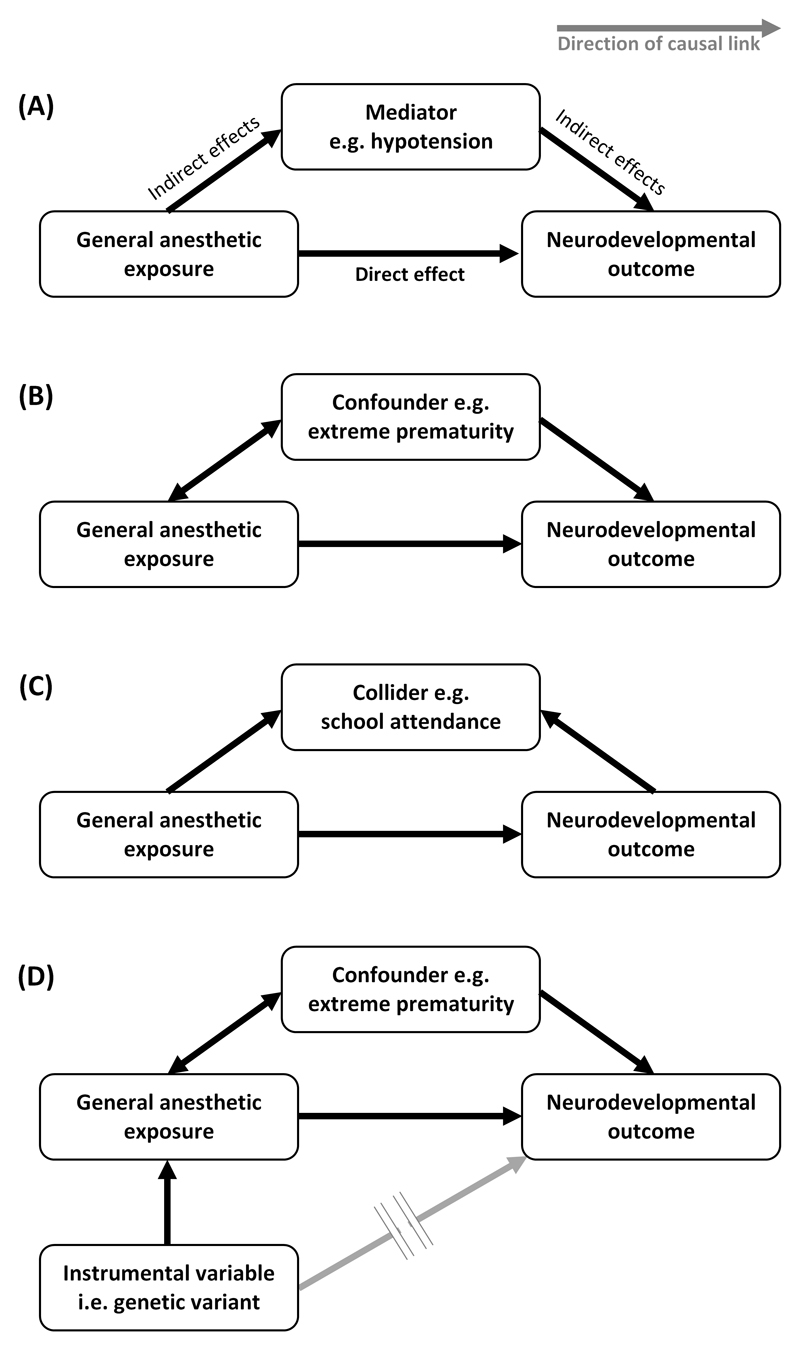
Perhaps the greatest challenge to studying AIN is in separating direct toxic effects of GA on the brain from indirect effects of anesthesia (disturbance of normal physiology e.g. hypoxia, hyperoxia, hypotension and hypothermia)18, of surgery (stress response19 and systemic inflammation) and the peri-operative course (complications, pain20, artificial or inadequate nutrition21). We illustrate this concept in Figure 2A.
Figure 2.
Key concepts in the epidemiology of anesthetic-induced neurotoxicity (see text for detailed explanation). Arrows represent the direction of causality between variables. (A) Impaired neurodevelopmental outcome may result from direct neurotoxic effects of general anesthesia (the effect of interest) and/or indirect effects which lie on different causal pathways which operate through mediator variables. (B) Confounding variables are associated with the anesthetic exposure and also influence neurodevelopmental outcome, but do not lie on a causal pathway between anesthesia and neurodevelopment. If confounders are not balanced through randomized study design or accounted for in statistical analyses then the estimated direct neurotoxic effect of general anesthesia is biased. (C) Collider variables are a common effect of general anesthesia exposure and neurodevelopmental outcome. Statistical adjustment for a collider variable which has been mistaken for a confounder can introduce collider-stratification bias. (D) Mendelian randomization is a novel study design for unbiased causal inference in observational studies which exploits the random allocation of genetic material during human reproduction to set-up a natural analogy to a randomized controlled trial. It utilizes genetic variants which are selected to be associated with general anesthetic exposure (but importantly, not directly with impaired neurodevelopment) as instrumental variables.
All but two studies14,22 make comparisons between GA and surgery groups, with or without control, and therefore cannot distinguish anesthesia-induced from surgery-induced effects. Although methodologically ideal, a 2 × 2 factorial design (anesthesia yes/no × surgery yes/no) to determine the effect of anesthesia on neurodevelopment would be logistically and ethically challenging in children or animals and arguably not possible.
A pragmatic non-randomized study might compare (a) GA without surgery e.g. undergoing imaging, endoscopic or interventional procedures, (b) GA with surgery and (c) no GA or surgery23. Careful choice of the category (a) children would be required. For example, children undergoing neuroimaging may have co-morbidities which are independent risk factors for poor neurodevelopmental outcome23. Category (c) controls could be non-hospitalized siblings/classmates or hospitalized non-surgical children. It is important that children who undergo additional surgeries in later childhood are not excluded from either the intervention or control groups to avoid selection biases24.
Spinal anesthesia in immature rats has been shown to not accelerate neuronal apoptosis nor cause neurobehavioral abnormality25. An ideal randomized study, therefore, could compare (a) GA for surgery, (b) awake-regional anesthesia for surgery and (c) no anesthesia or surgery controls. The GAS trial14 adopted a similar strategy, with children undergoing GA/surgery or intended to undergo awake-spinal anesthesia for inguinal herniorrhaphy. In reality, this approach restricts the sample to children undergoing infra-umbilical procedures for which awake-neuraxial anesthesia is a feasible alternative to GA and may therefore limit external generalizability to other patient groups. Careful control or adjustment for differential incidence of deranged physiology between GA and awake-regional anesthesia groups (e.g. significant hypotension more common in the former26) is required to avoid biasing results. Furthermore, children with inadequate blocks or who do not tolerate awake-regional anesthesia may require sedation or conversion to GA (18% in the GAS trial but may be up to 80%27), which may defeat the purpose of the study design. However, per protocol analyses of non-inferiority or equivalence trials where there is cross-over of patients between exposure categories would still test whether GA was harmful to child neurodevelopment.
The toxic exposure to general anesthesia
Although brain structure and function develop throughout childhood, a period of peak synaptogenesis in early childhood has strong implications for later cognition, language, and social behaviour6,28. Exposure during this ‘vulnerable time-window’ of brain development ought to be the focus of anesthetic research. Although its timing is well defined in animal species, with the overwhelming majority of studies performed on postnatal day seven in rats11,24, human AIN studies have quoted a heterogeneous range of definitions e.g. “third trimester to 2 years”8, “third trimester to 6 weeks”9, “0 to 36 months”29, “early gestation through to infancy”10 or “birth to 2-3 years”30. The concept of a single vulnerable time-window may be an oversimplification since there are significant regional differences in the timing and pace of peak synaptogenesis24,31 which are reflected in discordant results for different domains of neurodevelopment32–35. Furthermore, the age of the neuron as opposed to the age of the child can determine vulnerability to anesthetics36,37. At present it seems pragmatic to investigate GA exposures up to three years of age.
Since most of the studies (n=49; 64.5%) employ retrospective observational designs and many were not designed to investigate AIN per se (n=27, 35.5%)38–48, data concerning anesthetic exposure is often limited. Some investigators make assumptions which, if incorrect, could undermine their study e.g. babies are presumed to undergo GA for minor procedures which may have been conducted under regional anesthesia10; or circumcision is presumed to be performed without GA in the perinatal period but under GA for older children in another study36. Whether randomized or non-randomized prospective or retrospective designs, AIN studies need to strive to accurately ascertain the exposure of each child to avoid underestimating the true effect of GA (false negative results).
A dose-response relationship has been detected with increasing numbers of co-administered anesthetic agents30 and been sought by comparing single versus multiple anesthetic exposures49,50. However, as dose and duration of GA vary widely between procedures, these are poor surrogates for cumulative dose of anesthetic drug exposure32. Furthermore, inaccurate reporting of composite procedures, e.g. adenoidectomy / tonsillectomy / myringotomy, may lead to misclassification of children to the multiple exposure group51. Children requiring repeated procedures may have confounding reasons for poor neurodevelopmental outcome which may not be captured in the study dataset. Ideally, dose-response analyses ought to use a prospectively determined duration of anesthesia in minutes for specified drugs, or dose in age-adjusted minimum alveolar concentration (MAC)-hours for inhalational agents52–54 or cumulative mg/kg for intravenous anesthesia55. This level of detail may be more achievable with electronic anesthetic record keeping recording systems.
Choice of intervention
In observational studies, selection of participants in terms of their diagnosis/disease and surgical procedure ought to minimize ‘confounding by indication’ – a scenario in which the disease or the surgery itself is an independent risk factor for poor neurodevelopmental outcome. Studies of neurosurgical and cardiothoracic surgical cohorts39,56, as well as children operated on with major congenital or chromosomal abnormalities21,57 are classically affected. However, studies of GA for neuroimaging23, some otorhinolaryngology procedures (e.g. adenotonsillectomy for obstructive sleep apnea associated with learning difficulty49,58 or myringotomy and grommet insertion associated with speech/language delay59), pyloromyotomy associated with significant hyperbilirubinemia24 or nutritional inadequacy48, gastroschisis34, craniosyntosis18 and cancer surgery60 may be similarly compromised.
When selecting study participants, a balance ought to be struck between the risk of confounding by indication and being as inclusive as possible to maximize external validity. A healthy, elective surgical cohort undergoing relatively minor surgery would be ideal61. Inguinal herniorraphy14,20,62 or surgery for solitary urogenital problems63 (e.g. circumcision or hypospadias repair) are common and have no known independent association with poor neurodevelopmental outcome. Particular care should be exercised if it is necessary to pool multiple surgical procedures to increase statistical power64.
Anesthetic agents readily cross the placental barrier, which has previously permitted studies in children born to occupationally exposed mothers65 and children born by Cesarean under GA22,66,67. These studies may not demonstrate AIN because of the poorly defined, chronic low-dose occupational exposure or the relatively brief exposure at Cesarean delivery. Studying AIN in the context of (a) GA Cesarean versus (b) neuraxial anesthetic Cesarean and (c) spontaneous vertex delivery is also fraught with difficulty. Results may be confounded by opioids used for labor analgesia which may cause neonatal respiratory depression or the use of labor epidural analgesia which may reduce stress response in the control group22. The indication for Cesarean intervention, as well as an increased frequency of prematurity, complications of pregnancy and perinatal insults in the intervention groups may also confound results. Studying intrauterine surgery to correct fetal abnormalities would offer a longer well-defined general anesthetic drug exposure, but no such work has been published.
Addressing confounding
The association between GA and neurodevelopmental outcome is heavily confounded by factors throughout the life course (Figure 2B; Table 2). Properly conducted RCT should evenly distribute known/measured and unknown/unmeasured confounders across groups at randomization, thereby overcoming confounder bias.
Table 2.
Potential confounders of the association between anesthesia and neurodevelopment which have been measured in human anesthetic-induced neurotoxicity studies. ACTH: adrenocorticotropic hormone; ADHD: attention-deficit/hyperactivity disorder; ASA: American Society of Anesthesiologists; IL-6: interleukin-6; FiO2: fractional concentration of inspired oxygen; MRI: magnetic resonance imaging; PaO2: partial pressure of oxygen in arterial blood; TGA: transposition of the great arteries; TOF: tetralogy of Fallot; VSD: ventricular septal defect; ZIP code: zone improvement plan code.
| Child demographics | |
| Age / year of birth9,32,47,49,50,53,65,79,85,91,111–113 | Language14,33 |
| Gender9,10,18,22–24,28,32,34,36,39,40,42,49–51,53,56,60,61,64,65,67,79,85,111–115 | Month / quarter of birth (accounts for school entry cohorts)28,60 |
| Race / ethnicity10,18,19,23,32,36,40,79,114 | Year of birth cohort (accounts for changes in assessment tool)10,28 |
| Socio-economic characteristics | |
| Socio-economic status39–41,49,53,56,91,113,115 | Received income support9 |
| Housing class61 | Involved in child welfare system9 |
| Household / maternal income9,32,36,42,60,114 | Insurance system: eligibility status, provider49,67 |
| Years / level of education8,10,20,22,24,34,40,42,50,51,56,60,61,63–65,107,111,114 | Geographical location e.g. ZIP code / postal code49,78,92,115 |
| Occupation65,78 | Urbanity / rurality of residence9,28,67 |
| Family composition | |
| Parental living arrangements: co-habiting, living with one parent/guardian32,60,61 | Number of siblings60 |
| Parental marital status67,97,115 | Birth order61,115 |
| Parental death97 | Sibships49 |
| Other parent / guardian factors | |
| Maternal intelligence quotient51,54,116 | Race / nationality67 |
| Age at birth of (first) child8,9,20,22,24,28,64,67,115 | Language spoken at home40,43,44,52,71,111 |
| Maternal parity67 | Maternal smoking: never, pre- or in pregnancy51 |
| Medical history49 | Maternal alcohol consumption: never, pre- or in pregnancy51,88 |
| Parental stress107 | Developmental delay, mental disability, psychiatric disorder, autism or ADHD61 |
| Childhood influences | |
| Mentoring by older siblings61 | Problems at school63 |
| Sports participation102 | Childhood trauma63 |
| Pregnancy and peri-partum | |
| Intrauterine growth retardation21,78 | Labor analgesia: epidural, spinal, opioid, nitrous oxide |
| Prematurity / gestational age at birth9,14,18,20–23,28,30,32,34,36,39,40,42,50–54,56,57,60,61,63,67,84,107,111,116,117 | Mode of delivery: normal vaginal, assisted vaginal, Cesarean22,112 |
| Birthweight (centile) / small or large for gestational age 8–10,20–24,32,34,36,38–41,50,52,54,56,57,63,64,67,84,85,92,102,111,112,116 | Urgency of assisted or operative delivery |
| Induction of labour22 | Multiple birth cohort10 |
| Prolonged labor | Intrauterine fetal distress |
| Maternal complications of pregnancy, labor or delivery22,67,78,79 | Intrauterine or birth asphyxia / resuscitation at delivery42,78,92,97,103,107 |
| 5- or 10-minute Apgar scores10,22,42,60,67,84 | |
| Peri-natal fetal morbidity | |
| Respiratory distress syndrome or other neonatal respiratory disoder67,78,92 | Fetal and neonatal hemorrhage, hemolytic disease of the newborn or other hematological condition78 |
| Endocrine and metabolic disturbances78,92 | Perinatal infection78,92 |
| Perinatal jaundice67,78 | Disorders of digestive system78 |
| Past or peri-operative neurological status | |
| Microcephaly39,107 / head circumference39,40 | Pre-operative neurodevelopmental scores18 |
| MRI brain maturity score54 | Hand dominance53 |
| MRI intracranial volume32 | Abnormal neurological examination53,107 |
| Number of sedated MRI117 | Mental / psychiatric disorder or disability97 50,54,88 |
| Meningitis10 | Neurosurgery71 |
| Seizures10,118 | Head trauma +/- loss of consciousness53 |
| Traumatic or ischemic central nervous system injury63,88,116–118, brain malformation107, intra-cerebral haemorrhage10, severe cystic periventricular leukomalacia10, cerebral oedema67, stroke42, cerebral palsy18 or other neurological disease / injury14,30,61,92 | |
| Secondary care interaction | |
| Number / duration of admissions35,36,38–40,42,44,52,54,102,107 | Followed by cardiologist or cardiovascular service28 |
| Number of outpatient clinic attendances36 | |
| Anesthetic and surgical factors | |
| Indication for general anesthesia: type of surgery, imaging or examination23,30,92 | Age at general anesthesia18,23,30,39–42,44,55,107 |
| Diagnosis e.g. anatomical defect in cardiac surgery, site of craniocyntosis suture18, type of cleft lip and palate8 | Weight at general anesthesia39,40,55,67,113 |
| Surgical centre10,49 | Bispectal index at end of surgery19 |
| Surgical approach: open, minimally invasive107 | Hemodynamic and respiratory instability during anesthetic88 |
| Complications of surgery30 | |
| Other child medical history | |
| Genotype: genetic syndrome / genetic polymorphisms / chromosomal abnormality / predisposition to neurodevelopmental disorders19,21,38–44,49,52,54,55,61,71,103,116,119,120 | Composite morbidity scores e.g. Johns Hopkins Resource Utilization Band9, John Hopkins Adjusted Diagnosis Group112,114, ASA physical status >269 |
| Phenotype: (major or multiple) congenital anomalies / birth defects / dysmorphic syndrome14,18,20,21,30,34,36,42,49,54,63,64,67,78,92,102,107,116–119 | History of fetal intervention (radiotherapy, brachytherapy, pharmacotherapy, chemotherapy)28 |
| Other co-morbidities likely to influence neurodevelopment: cardiac disease30,61, Hirschsprung’s disease42,51, retinoblastoma42,51, Sturge-Weber syndrome121, severe renal disorders61, endocrine disease92, jaundice23,36,67, chronic respiratory disease e.g. bronchopulmonary dysplasia10,30, strabismus surgery68, cardiac surgery41 or other significant health conditions requiring surgery18 | Physical disabilities28 |
| Past and peri-operative critical care admissions | |
| Number / duration of admissions19,23,30,40,42,44,47,107,116 | Cardio-pulmonary arrest42,54,116 |
| Composite scores e.g. Parmelee Post-natal Complication Scale35 | Nutrition: oral, artificial supplement, entirely artificial38 |
| Invasive or non-invasive ventilation: use of, duration14,23,32,40,44,102,117 | Total parenteral nutrition: use of, prolonged use102 |
| Oxygen requirement e.g. pre-operative FiO242 | Necrotizing enterocolitis10,117 |
| Lowest PaO255,107 | Gut perforation21 |
| Pre- / post-natal steroids or respiratory distress10,19,117 | Infections: number117, complicated by sepsis10,21,102 |
| Hypotension42,117 | Lowest temperature47 |
| Blood loss and coagulopathy18 | Other peri-operative complications: thromboembolic42, haemorrhagic42, hypoxia42, acidosis42, hypocalcaemia42 seizure42 |
| Volume of blood components given18 | |
| Duration / cumulative doses of other drugs | |
| Opioids40,54,116 | Chloral hydrate55 |
| Benzodiazepines40,54,55,116 | Anti-epileptic drugs53,88 |
| Ketamine55 | Substance abuse88 |
| Cardiac surgery | |
| Prenatal diagnosis39,42,55 | Diameter ascending aorta39 |
| Palliative or corrective surgery44,107 | Indexed shunt diameter39 |
| Congenital lesion: cyanotic or acyanotic107, 1- or 2-ventricle circulation38,39,42,54,55; anatomical defect e.g. TOF, TGA or VSD41 | Patent ductus arteriosus (distinguish surgically closed) 10,117 |
| Composite scores e.g. Society of Thoracic Surgeons mortality category38, Aristotle score42, Risk Adjustment for Congenital Heart Surgery55 | Prostaglandin-dependant42 |
| Somatic38 and cerebral regional38,54,116 SaO2: pre-, intra- and 72 hours post-operatively | Extra-corporeal membranous oxygenation: use, duration38,47 |
| Inotropes: duration, mean score39,40,42,54,55 | Cardio-pulmonary bypass: use, duration19,38–40,54,107,116 |
| Duration of open sternum40 | Deep hypothermic circulatory arrest: use, duration38,40–42,54,107 |
| Post-operative catheterization or re-operation42 | Aortic cross-clamp: use, duration47,55 |
| EPO or aprotinin administration54,116 | Selective cerebral perfusion time39 |
| Anti-coagulant or anti-platelet drug at discharge42 | Afterload reduction time39 |
| Hematocrit: intra- / post-operatively18 lowest on cardio-pulmonary bypass52, at end of bypass, after haemodilution40 | |
| Other biochemical measurements | |
| Hypothalamo-pituitary axis e.g. baseline ACTH19 | Worst lactate or base excess intra- and post-operatively40,42 |
| Adrenaline level | Inflammation e.g. IL-6 at end of surgery19 |
Observational studies of AIN must control (via restriction, stratification or regression adjustment) for differences in known/measured confounders between groups to avoid extensive bias. However, data concerning pregnancy/peri-partum factors (e.g. prematurity, fetal acidosis, birth asphyxia) and perioperative factors (e.g. temperature, hypo/hyperoxia, hemodynamics, adverse events) are often unknown, especially in retrospective studies. Some factors which ought to be adjusted for, e.g. American Society of Anesthesiologists physical status, are not routinely recorded for non-exposed children and smaller studies may make no attempt to adjust for confounders at all48,68–72. By definition, unknown/unmeasured confounders cannot be controlled for but their potential impacts on the results of observational studies can be simulated statistically73.
Adjustment for multiple potential confounders in observational studies is performed with the intention of reducing confounder bias. However, care must be exercised to avoid ‘overadjustment’74 – whereby this very process decreases precision or paradoxically increases net bias though several mechanisms. Firstly, attempting to control for increasing numbers of variables reduces the precision of the neurotoxic effect estimates generated by statistical models. Wide (imprecise) confidence intervals around the effect estimates may mask any evidence of AIN, leading to false negative conclusions. The second mechanism concerns ‘intermediate variables’, which are distinguished from confounders by lying on the causal pathway between exposure and outcome. For example, we might speculate that AIN is mediated via hypotension (Figure 2A). In the case of multiple causal pathways between exposure and outcome, then mistakenly controlling for hypotension (or some descending proxy thereof such as volume of crystalloid or amount of vasoactive drug administered) would produce a null-biased result i.e. falsely reducing the apparent strength of any neurotoxic effect estimate. Worse still, if the only causal path between GA exposure and impaired neurodevelopment were mediated through hypotension, then mistakenly controlling for this intermediate variable (or it’s proxies) ought to entirely nullify any neurotoxic effect estimate, again producing falsely reassuring conclusions. The third mechanism involves ‘collider variables’, which are defined as a common effect of the exposure and outcome (Figure 2C). Mistaken control for this common effect induces a spurious (non-causal) association between GA exposure and neurodevelopmental outcome through which confounding can flow, paradoxically inducing bias (termed ‘collider-stratification bias’) into the neurotoxic effect estimate where none previously existed. An illustrative example comes from studies of prenatal pollutant exposure and long-term child neurodevelopment in which the pollutants also cause fetal loss75. Since outcome can only be determined in live-born children, if investigators condition on live birth status (in this case by restriction to live-born children as is typical in pediatric cohort studies), bias arising from common causes of fetal death and long-term neurodevelopmental outcome (i.e. confounders of the association between fetal death and neurodevelopment) is induced.
Collectively, the pitfalls of multivariable analysis necessitate thoughtful selection of potential confounders, which may be assisted by drawing a ‘directed acyclic graph’76 – a visual representation of the assumed associations between exposure, outcome and other measured/unmeasured variables using unidirectional arrows to represent the direction of causality (and temporality). These graphs distill the causal model underlying the epidemiological problem, informing the choice of confounding, intermediate and collider variables which would be required to build a statistical model to test for an unbiased relationship between GA and neurodevelopmental outcome. The aforementioned pitfalls of multiple confounder adjustment also necessitate cautious ‘stepwise’ modelling whereby potential confounders are sequentially added to the developing statistical model and its output scrutinized at each step for paradoxical effects. A sudden reversal of the effect estimate following the stepwise incorporation of the latest potential confounder, for example, may prompt a re-evaluation of the causal assumptions regarding that variable and whether it may operate as a collider as opposed to a confounder in the causal model. It would be dangerous to simply attempt to simultaneously adjust for all measured child characteristics in a non-randomized AIN study.
Conventional techniques for confounder adjustment include various regression models (e.g. linear, logistic, Poisson or Cox proportional hazards modelling)9,18,19,24,29,32,39,55,64,77,78 and matching techniques. Group/frequency matching ensures that the proportions of subjects with given characteristics are the same in each group50,79. Individual/pair matching ensures that pairs of children, one from each group, share similar characteristics28,49. Results from matched pairs are less confounded but require larger sample sizes to achieve the same precision.
More innovative approaches may help uncover associations. Propensity score analysis is a pragmatic choice of method to reduce the complexity and computational burden of statistical models which attempt to control for a multitude of potential confounding variables in a non-randomized study. It reduces the dimensionality of the dataset from a large collection of variables to a single propensity score, which is generated by a regression model from those variables that are thought to influence membership to the GA group in the study. The propensity score assigned to each child would take a value between zero and one and represent the estimated probability of GA group membership, conditional on the values of those variables thought to influence GA versus non-GA group membership. The propensity score can then be adjusted for as an independent variable in a regression model (as opposed to entering the collection of known/measured confounders). Alternatively, one can match individual children between GA and non-GA groups who have similar likelihoods of GA group membership (i.e. similar propensity scores) such that known/measured confounders are balanced across the two groups10,20,36,53,56,78. These ‘propensity-adjusted’ or ‘propensity-matched’ estimates of neurotoxic effect on neurodevelopment ought to be unbiased by known/measured confounders.
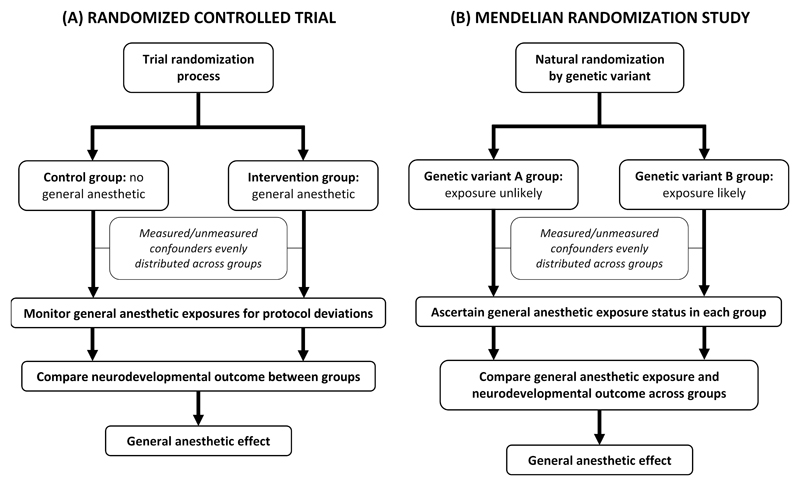
Mendelian randomization is an advance in observational epidemiology which overcomes confounding by both known/measured and unknown/unmeasured factors. It can provide unbiased evidence for causal relations between a modifiable exposure and patient outcome80,81. Instead of the traditional exposure variable (i.e. GA/surgery), it considers ‘instrumental variables’ (Figure 2D). These are either one or a combination of multiple genetic variants (i.e. alleles or single nucleotide polymorphisms) that are randomly allocated to children at meiosis in human reproduction and are selected on that basis that they robustly predict GA exposure without directly influencing neurodevelopmental outcome (except via the GA exposure itself). Candidate genetic variants are typically identified from large genome-wide association studies but could conceivably be associated with certain disease states (increasing the propensity for GA to facilitate procedures, medical imaging or surgery) or with suxamethonium apnea or malignant hyperpyrexia (reducing the propensity for GA where there is an established child or family history). Random natural assortment of genetic material ensures that instrumental variable status is independent of factors which confound the association between the traditional exposure variable (GA/surgery) and neurodevelopmental outcome. Once child outcomes are compared based on the instrumental variable (rather than GA exposure) then inter-group differences in GA exposure and neurodevelopment ought to reflect true, unconfounded causal relationships between GA/surgery and neurodevelopmental outcome (Figure 3). We believe that the Mendelian randomization approach to detecting AIN may be especially feasible using a ‘two sample’ Mendelian randomization in which data linking the chosen genetic variants to GA exposure need not come from the same sample as data which links GA exposure to neurodevelopment. No observational studies of AIN published to date have used Mendelian randomization. However, it offers the potential to elucidate an unconfounded link between anesthesia and neurodevelopment using what is an efficient natural analogy to an RCT.
Figure 3.
Contrasting the conduct of (A) randomized controlled trials and (B) Mendelian randomization studies. See text for full explanation.
As an illustrative example, the effect of prenatal alcohol exposure on child academic achievement has been studied recently using the Mendelian randomization approach82,83. Here, researchers have exploited genetic variation in the alcohol dehydrogenase gene as an instrument for in utero alcohol exposure. Mothers with the rare allele metabolize alcohol faster, resulting in more rapid production of ethanol metabolites which cause unpleasant symptoms. These mothers are shown to consume less alcohol. Investigators demonstrate that the instrumental variable, unlike alcohol consumption, is unrelated to potential confounders of the association between prenatal alcohol exposure and academic achievement such as socio-economic status. Whereas traditional regression analyses based on an alcohol consumption exposure variable have returned ambiguous results, presumably due to residual confounding (e.g. maternal wine consumption being protective for child educational attainment), the instrumental variable analyses demonstrate robust positive effects on child educational achievement in children whose mothers were induced by their genotype to abstinence or lower alcohol consumption in pregnancy.
Twin or sibling studies attempt to eliminate confounding by genetic and environmental factors e.g. uterine environment, parental education, parenting style, home/family environment, neighborhood, educational and socio-economic factors29,49,57,62. In a monozygotic concordant-discordant design, participants in each group share the same genetics and family-level environmental factors57. Differences in neurodevelopmental outcome across groups would then reflect the toxic effect of GA/surgery.
Longitudinal study designs, where neurodevelopment is repeatedly assessed over time, allows children to serve as their own controls42,44,47. This approach mitigates confounding by static confounders e.g. genetics and socio-economic status.
Finally, other approaches may dispense with control groups altogether. One could focus on the interaction between GA and age at exposure i.e. compare children who undergo early versus late surgery9,28,36,63,84,85. Associations would not be confounded by diagnosis and surgery/anesthetic factors since all subjects could be similarly exposed. However, this approach mandates that surgery can be postponed, which is not always feasible.
Detecting modest neurotoxic effects
In utero or early childhood exposure to a range of neurotoxicants (e.g. metals, organic solvents, pesticides) can adversely affect neurobehavioral development86,87. Ethanol, like anesthetic agents, acts at gamma-aminobutyric acid (GABA) and N-methyl-D-aspartic acid (NMDA) receptors and causes neuronal apoptosis in the developing brain88. Robust detrimental associations between heavy and binge prenatal alcohol exposure and adverse child neurodevelopment are established89,90. However, studies of light-to-moderate prenatal alcohol exposure have suffered from residual confounding and have reported inconsistent conclusions even with sample sizes in the order 10,000 children. We can presume that large samples will similarly be required to reliably detect any long-term neurotoxic effects following childhood GA – an effect which may also be comparable or small relative to the effects of confounding factors9,28,51,55,60,91. Large samples are also required to permit adjustment or matching techniques to account for confounding. Existing AIN studies vary in size between 15 and 125,000 subjects with a median 131 children (interquartile range; IQR: 50-372) so are often likely to be underpowered and potentially falsely reassuring.
Besides pursuing larger sample sizes, comparing exposed to unexposed children in 1:4 ratio to maximize statistical power60,78,92, avoiding short-duration interventions (e.g. maternal GA for Cesarean delivery or myringotomy and grommet insertion), studying exposure during the ‘vulnerable time-window’ of brain development and using sensitive outcome measures are strategies which may increase the likelihood of detecting neurotoxic effects of GA.
Neurodevelopmental outcome
The neurodevelopmental outcome measures reported in the literature are varied and encompass (a) intelligence/cognition, (b) academic achievement, (c) development/behavior and (d) neuropsychiatric diagnoses i.e. attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD) and learning disability (LD)93. Prospective evaluation in multiple domains of development using a battery of sensitive, validated outcomes and trained, blinded assessors is the gold standard. However, the risk of detecting spurious associations increases with multiple outcomes. It is therefore wise to caution against the over-interpretation of solitary detrimental associations in the context of a panel of otherwise reassuring results.
Measures of intelligence/cognition are thought to remain stable throughout the life course unless disrupted by severe disease93,94. However, assessment is not feasible until basic cognitive skills are achieved by 4-6 years old93,95. Age-normalized intelligence scores permit comparisons of outcome at different ages and enable referencing to population scores72.
Academic achievement in standardized national tests reflects intelligence / cognition96, but is muddied by multiple external factors e.g. self-esteem and lifestyle factors93. School grade performance in children with dyslexia or dyspraxia may be boosted by extra help in school, mitigating any negative effect on academic achievement61. Although standardized national tests are administered at population level, which makes them a feasible outcome for large population studies, not all children participate e.g. private schools or non-entry due to learning difficulty8. Investigating academic achievement does however confer the pragmatic advantage that parents/guardians are likely to be highly invested in their child’s school performance20.
Child development evolves in surges and plateaus, referenced to well-defined developmental milestones expected at certain ages, which permits outcome assessment even at the youngest ages93. The reliability of subjective developmental/behavioral data collected through parental survey is questionable: developmental delay in language/speech, mathematics and reading domains may not be noticed until challenged in school; behavioral problems may not manifest until children communicate and interact with their peers in school32,78,92,97. An ideal AIN study should use trained, blinded assessors (e.g. pediatric neuropsychologists) to measure outcome using a comprehensive battery of developmental assessments. Scores generated by this method of outcome assessment are objective and highly sensitive to subtle neurotoxic effects that may be difficult to detect clinically32. The use of such comprehensive neurodevelopmental assessments is most feasible in smaller studies which prospectively assess outcome20, but it is also available in some retrospective datasets51. The Bayley Scales of Infant Development32,62 is the most extensively used example98, but the latest third version may overestimate development in certain groups99,100, and caution is required if comparisons are made with scores from previous iterations101.
Neuropsychiatric diagnoses for developmental/behavioral disorders are multifactorial in origin (including genetic predisposition), with a heterogeneous and changing clinical presentation over time93. Children may spontaneously ‘catch-up’49 or benefit from supportive interventions in childhood85,93. Neuropsychiatric diagnoses are almost exclusively parameterized as binary outcomes (e.g. from International Classification of Diseases, 9th Revision diagnosis codes, school or healthcare records) as opposed to ‘risk scores’. These binary outcomes are likely to be too crude/insensitive to detect any subtle effects of anesthetic exposure23. Non-diagnosis (especially before the group communication/interaction and higher cognitive demands placed on schoolchildren32,92), under-reporting and incorrect diagnosis coding in databases is likely to introduce misclassification bias. Studying LD confers particular advantages though: a high incidence (5-10%) and recording in large educational databases93.
Post-operative follow-up and sample attrition
The time interval between anesthesia and first neurodevelopmental assessment must be sufficiently long to distinguish long-term neurotoxic effects from short-term post-operative cognitive-behavioral changes (i.e. ≥6 months36). It must also allow sufficient latency for marginal neurodevelopmental deficits to manifest in domains of development which emerge, differentiate and are amenable to thorough neuropsychological testing at older ages e.g. cognitive skills such as language/speech/reading, mathematics, memory, and executive functioning from late childhood14,29,54. Furthermore, neurodevelopmental evaluation in schoolchildren is known to be more robust and predictive for adulthood than when measured at in preschool children because of the variability in young children’s developmental trajectories14,21,34,52,54. There has been concern that multiple life course factors may dilute any differences in outcome between exposed and unexposed children after such long follow-up. However, subtle associations between starting school in January versus December in educational achievement and intelligence quotient scores have been detected in large cohorts as late as 18 years old60. Existing studies of AIN follow-up children until a median age of 6 years (IQR: 2-12).
Prolonged follow-up makes retrospective or ambi-directional (meaning retrospective ascertainment of exposure but prospective measurement of outcome) studies29,62,102,103 efficient compared to prospective randomized and non-randomized designs. But it also makes sample attrition (e.g. due to withdrawal, death, migration, moving schools or healthcare provider) a significant problem e.g. 50% of initially enrolled children completing assessment at two years in one study42. Most observational studies report a ‘complete case analysis’, in which any children with missing data are disregarded8,18,20,21,54,97,102. The amount of missing data and reasons for this are frequently omitted. As well as a suffering a reduction in precision, their results may be biased when neurodevelopmental outcome data are missing non-randomly51,104. For example, if GA slowed child neurodevelopment then exposed children may be lost to follow-up if they were unable or reluctant to engage in intelligence testing. Effect estimates would then underestimate the true effect of GA in the complete case analysis.
Even research funded to intensively follow-up children in prospective randomized or non-randomized studies will have missing data. Statistical methods can be used to permit unbiased analyses without excluding affected cases104. Choice of method depends on the probable mechanism of data loss. Multiple imputation is a popular technique used when data are believed to be missing at random. Missing data are inferred from a rich observed dataset to construct multiple plausible datasets, which are pooled to produce a result which reflects the uncertainty in the imputed data. Data which is missing not at random can only be addressed through experiments which test the sensitivity of results to different mechanisms of data loss.
Interpreting results in clinical practice
Despite considerable interest and anxiety, there is at present no conclusive evidence or consensus that GA harms the developing brain. Childhood GA typically comprises single short exposures and is likely to carry low risk14,29,105. However, if GA is thought to pose long-term neurodevelopmental risks, then the impacts on clinical practice could be far-reaching.
In considering the current clinical implications it should be noted that the evidence-base is comprised mainly of retrospective observational studies, whose subjects were anesthetized in the 1970s - 1990s, since when there have been widespread changes in practice. Pediatric anesthesia may have become safer24 as isoflurane/sevoflurane and intravenous anesthesia have replaced the ‘Liverpool technique’ (muscle relaxation and nitrous oxide for neonatal procedures), halothane, enflurane and methoxyflurane22; and our profession became more conscious of optimal fluid management, adopted obligatory multi-parameter monitoring incorporating pulse oximetry and capnography; and there have been changes in who is delivering anesthetic care to children56.
Nonetheless if the evidence-base becomes stronger, then surgeons, physicians and general practitioners will require a new appreciation of the neurotoxic risks of anesthesia to inform clinical decision making and the consent process. Important topics for discussion with children, parents or guardians would include which elective procedures could be deferred, the associated risks of delay, alternative anesthetic management (e.g. alternative anesthetic agents or regional techniques) and possible mitigating or protective strategies62.
Withholding general anesthetic drugs during neonatal surgery (e.g. the ‘Liverpool technique’) may not be an option today and is certainly unethical in later childhood. Painful stimulation and the associated strong stress response are also thought to impair neurodevelopment20,106.
Modifiable factors certainly include optimizing perioperative physiology, good perioperative analgesia, psychosocial support and avoidance of unpleasant experiences or prolonged hospitalization. Determining which general anesthetic drugs and techniques might carry the lowest risk will require researchers to accurately quantify the duration, cumulative dose and interactions of specific agents29. Whether time to allow remodeling/repair between sequential GA can mitigate neurotoxic damage could be investigated9. Neuroprotection afforded by strict maintenance of physiological parameters, pharmacotherapies, preconditioning and novel neurogenesis techniques are being researched38,107. Maintaining cerebral glucose and oxygen delivery by minimizing cardiopulmonary bypass and deep hypothermic circulatory arrest times may play a role in pediatric cardiac surgery38,44.
Most GA is provided for healthy elective cases. Here, the physical or psychosocial harms of deferring or cancelling surgery or procedures would need careful weighing against the risk and impact of potential neurodevelopmental impairment on the individual, especially for repeated or prolonged anesthesia. For example, impaired wound healing and cosmesis, concerns about impaired speech/language development and social stigma may preclude deferral of surgery in cleft lip and palate84. The current level of concern about neurotoxicity would not preclude the provision of GA for emergency surgery or Cesarean delivery.
High-risk groups for poor developmental outcome (e.g. multiple prolonged GA) may require follow-up neurodevelopmental screening with the option of referral for early school intervention programs to attempt to mitigate any harms and improve developmental acquisition and school performance108.
Conclusion
Despite growing international concern that GA in childhood leads to long-term neurodevelopmental impairment, delineating GA-induced effects from those of surgery remains a significant challenge in the study of AIN. Deficiencies of existing research also include inconsistent exposure definitions, selection of cohorts with independent risk factors for impaired neurodevelopment, extensive confounding, the need to detect subtle neurotoxic effects, blunt neurodevelopmental assessment tools and sample attrition over the long-term follow-up required.
RCT represent the gold standard tool in the present climate of clinical equipoise14. However, randomizing children to GA-surgery versus regional anesthesia-surgery versus no anesthesia-no surgery poses significant ethical and logistical challenges, particularly if prolonged or repeated GA is to be studied. This coupled with the large sample sizes and prolonged follow-up required to detect neurotoxic effects necessitates the design of more efficient, sophisticated observational studies1,29,109 and has driven calls for the adoption of surrogate indices such as neuroimaging and biomarker techniques to evaluate neuronal inflammation and apoptosis110.
Large observational studies can produce more precise, more timely results which are not constrained to studying single short GA exposures. We advocate prospective or ambi-directional cohort studies which accurately ascertain GA exposure, rigorously control for confounders and prospectively follow-up neurodevelopment into adolescence. They will also permit researchers to elucidate the role of potential mediators and effect modifiers of any neurotoxic effect to inform strategies to mitigate the potential neurotoxic risks of GA in early childhood.
In parallel there is a need for ongoing animal work to characterize the mechanisms of AIN, the relative neurotoxic potentials of different anesthetic agents at different stages of development and modifiable factors to reduce AIN. These animal studies will need to more carefully control physiological parameters, anesthetic dosing and more closely mimic the surgical insult if their findings are to be generalizable to human pediatric anesthesia.
Given the inherent challenges of studying AIN, we must acknowledge that it may never be possible to demonstrate AIN in conventional clinical trials. Ultimately, multiple complementary approaches are required to accumulate sufficient evidence to inform a consensus opinion on the neurotoxic potential of GA – currently the single greatest issue in modern pediatric anesthetic practice.
Supplementary Material
Acknowledgments
Financial disclosures: GJW and HG are NIHR funded.
Footnotes
Conflicts of interest: GJW, AEP and HG have no conflicts of interest.
References
- 1.Andropoulos DB, Greene MF. Anesthesia and developing brains—implications of the FDA warning. New England Journal of Medicine. 2017;376(10):905–907. doi: 10.1056/NEJMp1700196. [DOI] [PubMed] [Google Scholar]
- 2.Sury M, Palmer J, Cook T, Pandit J. The state of UK anaesthesia: a survey of National Health Service activity in 2013. British Journal of Anaesthesia. 2014;113(4):575–584. doi: 10.1093/bja/aeu292. [DOI] [PubMed] [Google Scholar]
- 3.Humby P. Overview of the UK population: February 2016. Overview of the UK population, its size, characteristics and the causes of population change including national and regional variation: Office for National Statistics. 2016 [Google Scholar]
- 4.Jevtovic-Todorovic V. Exposure of Developing Brain to General AnesthesiaWhat Is the Animal Evidence? Anesthesiology: The Journal of the American Society of Anesthesiologists. 2017 [Google Scholar]
- 5.Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nature Reviews Neuroscience. 2016;17(11):705. doi: 10.1038/nrn.2016.128. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksson A, Pontén E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-d-aspartate and γ-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2007;107(3):427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 7.Zou X, Patterson TA, Sadovova N, et al. Potential neurotoxicity of ketamine in the developing rat brain. Toxicological sciences. 2009;108(1):149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clausen NG, Pedersen DA, Pedersen JK, et al. Oral Clefts and Academic Performance in Adolescence: The Impact of Anesthesia-Related Neurotoxicity, Timing of Surgery, and Type of Oral Clefts. Cleft Palate-Craniofacial Journal. 2016;54(4):371–380. doi: 10.1597/15-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham MR, Brownell M, Chateau DG, Dragan RD, Burchill C, Fransoo RR. Neurodevelopmental Assessment in Kindergarten in Children Exposed to General Anesthesia before the Age of 4 Years. Anesthesiology. 2016;125(4):667–677. doi: 10.1097/ALN.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 10.Morriss FH, Jr, Saha S, Bell EF, et al. Surgery and neurodevelopmental outcome of very low-birth-weight infants. JAMA Pediatrics. 2014;168(8):746–754. doi: 10.1001/jamapediatrics.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walters JL, Paule MG. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicology and teratology. 2017;60:2–23. doi: 10.1016/j.ntt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 12.DiMaggio C, Sun LS, Ing C, Li G. Pediatric anesthesia and neurodevelopmental impairments: a Bayesian meta-analysis. Journal of Neurosurgical Anesthesiology. 2012;24(4):376–381. doi: 10.1097/ANA.0b013e31826a038d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Du L, Du Z, Jiang H, Han D, Li Q. Association between childhood exposure to single general anesthesia and neurodevelopment: a systematic review and meta-analysis of cohort study. Journal of Anesthesia. 2015;29(5):749–757. doi: 10.1007/s00540-015-2030-z. [DOI] [PubMed] [Google Scholar]
- 14.Davidson A, Disma N, Graaff J, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet (london, england) 2016;387(10015):239–250. doi: 10.1016/S0140-6736(15)00608-X. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/219/CN-01134219/frame.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration. Drug safety communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. 2016 [Google Scholar]
- 16.Association of Paediatric Anaesthetists of Great Britain and Ireland, Royal College of Anaesthetists, Association of Anaesthetists of Great Britain and Ireland, The College of Anaesthetists of Ireland. Joint professional guidance on the use of general anaesthesia in young children. 2017 [Google Scholar]
- 17.Hansen TG. Use of anesthetics in young children: Consensus statement of the European Society of Anaesthesiology (ESA), the European Society for Paediatric Anaesthesiology (ESPA), the European Association of Cardiothoracic Anaesthesiology (EACTA), and the European Safe Tots Anaesthesia Research Initiative (EuroSTAR) Pediatric Anesthesia. 2017;27(6):558–559. doi: 10.1111/pan.13160. [DOI] [PubMed] [Google Scholar]
- 18.Naumann HL, Haberkern CM, Pietila KE, et al. Duration of exposure to cranial vault surgery: associations with neurodevelopment among children with single-suture craniosynostosis. Pediatric Anesthesia. 2012;22(11):1053–1061. doi: 10.1111/j.1460-9592.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naguib AN, Winch PD, Tobias JD, et al. Neurodevelopmental outcome after cardiac surgery utilizing cardiopulmonary bypass in children. Saudi journal of anaesthesia. 2015;9(1):12–18. doi: 10.4103/1658-354X.146255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen TG, Pedersen JK, Henneberg SW, et al. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114(5):1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 21.Elsinga RM, Roze E, Van Braeckel KN, Hulscher JB, Bos AF. Motor and cognitive outcome at school age of children with surgically treated intestinal obstructions in the neonatal period. Early Human Development. 2013;89(3):181–185. doi: 10.1016/j.earlhumdev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Sprung J, Flick RP, Wilder RT, et al. Anesthesia for cesarean delivery and learning disabilities in a population-based birth cohort. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2009;111(2):302–310. doi: 10.1097/ALN.0b013e3181adf481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nestor KA, Zeidan M, Boncore E, et al. Neurodevelopmental outcomes in infants undergoing general anesthesia. Journal of Pediatric Surgery. 2017;52(6):895–900. doi: 10.1016/j.jpedsurg.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Hansen TG, Pedersen JK, Henneberg SW, Morton NS, Christensen K. Educational outcome in adolescence following pyloric stenosis repair before 3 months of age: a nationwide cohort study. Pediatric Anesthesia. 2013;23(10):883–890. doi: 10.1111/pan.12225. [DOI] [PubMed] [Google Scholar]
- 25.Yahalom B, Athiraman U, Soriano SG, et al. Spinal Anesthesia in Infant RatsDevelopment of a Model and Assessment of Neurologic Outcomes. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2011;114(6):1325–1335. doi: 10.1097/ALN.0b013e31821b5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCann ME, Withington DE, Arnup SJ, et al. Differences in Blood Pressure in Infants After General Anesthesia Compared to Awake Regional Anesthesia (GAS Study-A Prospective Randomized Trial) Anesthesia & Analgesia. 2017;125(3):837–845. doi: 10.1213/ANE.0000000000001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somri M, Tome R, Yanovski B, et al. Combined spinal–epidural anesthesia in major abdominal surgery in high-risk neonates and infants. Pediatric Anesthesia. 2007;17(11):1059–1065. doi: 10.1111/j.1460-9592.2007.02278.x. [DOI] [PubMed] [Google Scholar]
- 28.O’Leary JD, Janus M, Duku E, et al. A population-based study evaluating the association between surgery in early life and child development at primary school entry. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2016;125(2):272–279. doi: 10.1097/ALN.0000000000001200. [DOI] [PubMed] [Google Scholar]
- 29.Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. Jama. 2016;315(21):2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chemaly M, El-Rajab MA, Ziade FM, Naja ZM. Effect of one anesthetic exposure on long-term behavioral changes in children. Journal of Clinical Anesthesia. 2014;26(7):551–556. doi: 10.1016/j.jclinane.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of comparative Neurology. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Ing C, DiMaggio C, Whitehouse A, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130(3):e476–485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 33.Ing CH, DiMaggio CJ, Whitehouse AJ, et al. Neurodevelopmental outcomes after initial childhood anesthetic exposure between ages 3 and 10 years. Journal of Neurosurgical Anesthesiology. 2014;26(4):377–386. doi: 10.1097/ANA.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 34.Lap CC, Bolhuis SW, Van Braeckel KN, et al. Functional outcome at school age of children born with gastroschisis. Early human development. 2017;106:47–52. doi: 10.1016/j.earlhumdev.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Ludman L, Spitz L, Landsdown R. Developmental progress of newborns undergoing neonatal surgery. Journal of pediatric surgery. 1990;25(5):469–471. doi: 10.1016/0022-3468(90)90552-k. [DOI] [PubMed] [Google Scholar]
- 36.Ing C, Sun M, Olfson M, et al. Age at Exposure to Surgery and Anesthesia in Children and Association With Mental Disorder Diagnosis. Anesthesia & Analgesia. 2017;125(6):1988–1998. doi: 10.1213/ANE.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofacer RD, Deng M, Ward CG, et al. Cell age–specific vulnerability of neurons to anesthetic toxicity. Annals of neurology. 2013;73(6):695–704. doi: 10.1002/ana.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman GM, Brosig CL, Bear LM, Tweddell JS, Mussatto KA. Effect of Intercurrent Operation and Cerebral Oxygenation on Developmental Trajectory in Congenital Heart Disease. Annals of Thoracic Surgery. 2016;101(2):708–716. doi: 10.1016/j.athoracsur.2015.08.059. [DOI] [PubMed] [Google Scholar]
- 39.Hansen JH, Rotermann I, Logoteta J, et al. Neurodevelopmental outcome in hypoplastic left heart syndrome: Impact of perioperative cerebral tissue oxygenation of the Norwood procedure. The Journal of thoracic and cardiovascular surgery. 2016;151(5):1358–1366. doi: 10.1016/j.jtcvs.2016.02.035. [DOI] [PubMed] [Google Scholar]
- 40.Gaynor JW, Ittenbach RF, Gerdes M, et al. Neurodevelopmental outcomes in preschool survivors of the Fontan procedure. The Journal of thoracic and cardiovascular surgery. 2014;147(4):1276–1283. e1275. doi: 10.1016/j.jtcvs.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng HH, Wypij D, Laussen PC, et al. Cerebral blood flow velocity and neurodevelopmental outcome in infants undergoing surgery for congenital heart disease. The Annals of thoracic surgery. 2014;98(1):125–132. doi: 10.1016/j.athoracsur.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sananes R, Manlhiot C, Kelly E, et al. Neurodevelopmental outcomes after open heart operations before 3 months of age. The Annals of thoracic surgery. 2012;93(5):1577–1583. doi: 10.1016/j.athoracsur.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Long SH, Galea MP, Eldridge BJ, Harris SR. Performance of 2-year-old children after early surgery for congenital heart disease on the Bayley Scales of Infant and Toddler Development. Early human development. 2012;88(8):603–607. doi: 10.1016/j.earlhumdev.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Long SH, Harris SR, Eldridge BJ, Galea MP. Gross motor development is delayed following early cardiac surgery. Cardiology in the Young. 2012;22(5):574–582. doi: 10.1017/S1047951112000121. [DOI] [PubMed] [Google Scholar]
- 45.Minutillo C, Rao SC, Pirie S, McMichael J, Dickinson JE. Growth and developmental outcomes of infants with gastroschisis at one year of age: a retrospective study. Journal of pediatric surgery. 2013;48(8):1688–1696. doi: 10.1016/j.jpedsurg.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 46.Rocha G, Azevedo I, Pinto JC, Guimarães H. Follow-up of the survivors of congenital diaphragmatic hernia. Early human development. 2012;88(4):255–258. doi: 10.1016/j.earlhumdev.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Fan X-C, Ye M, Li D-Z, Shi Y, Xu Y. Cognitive function in congenital heart disease after cardiac surgery with extracorporeal circulation. World Journal of Pediatrics. 2010;6(3):268–270. doi: 10.1007/s12519-010-0017-2. [DOI] [PubMed] [Google Scholar]
- 48.Walker K, Halliday R, Holland AJ, Karskens C, Badawi N. Early developmental outcome of infants with infantile hypertrophic pyloric stenosis. Journal of pediatric surgery. 2010;45(12):2369–2372. doi: 10.1016/j.jpedsurg.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 49.Dimaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesthesia and Analgesia. 2011;113(5):1143–1151. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128(5):e1053–1061. doi: 10.1542/peds.2011-0351. [Erratum appears in Pediatrics. 2012 Mar;129(3):595] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Heer IJ, Tiemeier H, Hoeks SE, Weber F. Intelligence quotient scores at the age of 6 years in children anaesthetised before the age of 5 years. Anaesthesia. 2017;72(1):57–62. doi: 10.1111/anae.13687. [DOI] [PubMed] [Google Scholar]
- 52.Diaz LK, Gaynor JW, Koh SJ, et al. Increasing cumulative exposure to volatile anesthetic agents is associated with poorer neurodevelopmental outcomes in children with hypoplastic left heart syndrome. Journal of Thoracic & Cardiovascular Surgery. 2016;152(2):482–489. doi: 10.1016/j.jtcvs.2016.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and Brain Structure Following Early Childhood Surgery With Anesthesia. Pediatrics. 2015;136(1):e1–12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andropoulos DB, Ahmad HB, Haq T, et al. The association between brain injury, perioperative anesthetic exposure, and 12-month neurodevelopmental outcomes after neonatal cardiac surgery: a retrospective cohort study. Pediatric Anesthesia. 2014;24(3):266–274. doi: 10.1111/pan.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia Guerra G, Robertson CM, Alton GY, et al. Neurotoxicity of sedative and analgesia drugs in young infants with congenital heart disease: 4-year follow-up. Pediatric Anesthesia. 2014;24(3):257–265. doi: 10.1111/pan.12257. [DOI] [PubMed] [Google Scholar]
- 56.Hu D, Flick RP, Zaccariello MJ, et al. Association between Exposure of Young Children to Procedures Requiring General Anesthesia and Learning and Behavioral Outcomes in a Population-based Birth Cohort. Anesthesiology. 2017;127(2):227–240. doi: 10.1097/ALN.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Research & Human Genetics: the Official Journal of the International Society for Twin Studies. 2009;12(3):246–253. doi: 10.1375/twin.12.3.246. [DOI] [PubMed] [Google Scholar]
- 58.Kurnatowski P, Putyński L, Łapienis M, Kowalska B. Neurocognitive abilities in children with adenotonsillar hypertrophy. International journal of pediatric otorhinolaryngology. 2006;70(3):419–424. doi: 10.1016/j.ijporl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Kacmarynski DS, Levine SC, Pearson SE, Maisel RH. Complications of otitis media before placement of tympanostomy tubes in children. Archives of Otolaryngology–Head & Neck Surgery. 2004;130(3):289–292. doi: 10.1001/archotol.130.3.289. [DOI] [PubMed] [Google Scholar]
- 60.Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F. Association of Anesthesia and Surgery During Childhood With Long-term Academic Performance. JAMA Pediatrics. 2017;171(1):e163470. doi: 10.1001/jamapediatrics.2016.3470. [DOI] [PubMed] [Google Scholar]
- 61.Bong CL, Allen JC, Kim JT. The effects of exposure to general anesthesia in infancy on academic performance at age 12. Anesthesia & Analgesia. 2013;117(6):1419–1428. doi: 10.1213/ANE.0b013e318299a7c2. [DOI] [PubMed] [Google Scholar]
- 62.Sun LS, Li G, DiMaggio CJ, et al. Feasibility and pilot study of the Pediatric Anesthesia NeuroDevelopment Assessment (PANDA) project. Journal of Neurosurgical Anesthesiology. 2012;24(4):382–388. doi: 10.1097/ANA.0b013e31826a0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalkman CJ, Peelen L, Moons KG, et al. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110(4):805–812. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 64.Hansen TG, Pedersen JK, Henneberg SW, Morton NS, Christensen K. Neurosurgical conditions and procedures in infancy are associated with mortality and academic performances in adolescence: a nationwide cohort study. Pediatric Anesthesia. 2015;25(2):186–192. doi: 10.1111/pan.12533. [DOI] [PubMed] [Google Scholar]
- 65.Ratzon NZ, Ornoy A, Pardo A, Rachel M, Hatch M. Developmental evaluation of children born to mothers occupationally exposed to waste anesthetic gases. Birth Defects Research. 2004;70(7):476–482. doi: 10.1002/bdra.20044. [DOI] [PubMed] [Google Scholar]
- 66.Hattori R, Desimaru M, Nagayama I, Inoue K. Autistic and developmental disorders after general anaesthetic delivery. The Lancet. 1991;337(8753):1357–1358. doi: 10.1016/0140-6736(91)93045-b. [DOI] [PubMed] [Google Scholar]
- 67.Chien L-N, Lin H-C, Shao Y-HJ, Chiou S-T, Chiou H-Y. Risk of autism associated with general anesthesia during cesarean delivery: a population-based birth-cohort analysis. Journal of autism and developmental disorders. 2015;45(4):932–942. doi: 10.1007/s10803-014-2247-y. [DOI] [PubMed] [Google Scholar]
- 68.Yazar S, Hewitt AW, Forward H, et al. Early anesthesia exposure and the effect on visual acuity, refractive error, and retinal nerve fiber layer thickness of young adults. The Journal of pediatrics. 2016;169:256–259. e251. doi: 10.1016/j.jpeds.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 69.Bakri MH, Ismail EA, Ali MS, Elsedfy GO, Sayed TA, Ibrahim A. Behavioral and emotional effects of repeated general anesthesia in young children. Saudi journal of anaesthesia. 2015;9(2):161. doi: 10.4103/1658-354X.152843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan J, Li YR, Zhang Y, Lu Y, Jiang H. Repeated exposure to anesthetic ketamine can negatively impact neurodevelopment in infants: a prospective preliminary clinical study. Journal of Child Neurology. 2014;29(10):1333–1338. doi: 10.1177/0883073813517508. [DOI] [PubMed] [Google Scholar]
- 71.Walker K, Badawi N, Halliday R, et al. Early developmental outcomes following major noncardiac and cardiac surgery in term infants: a population-based study. The Journal of pediatrics. 2012;161(4):748–752. e741. doi: 10.1016/j.jpeds.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 72.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology: The Journal of the American Society of Anesthesiologists. 2012;117(3):494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 73.Carnegie NB, Harada M, Hill JL. Assessing sensitivity to unmeasured confounding using a simulated potential confounder. Journal of Research on Educational Effectiveness. 2016;9(3):395–420. [Google Scholar]
- 74.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology (Cambridge, Mass) 2009;20(4):488. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. JIjoe. 2015;44(1):345–354. doi: 10.1093/ije/dyu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC medical research methodology. 2008;8(1):70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. Journal of Pediatrics. 2012;160(3):409–414. doi: 10.1016/j.jpeds.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Ko WR, Liaw YP, Huang JY, et al. Exposure to general anesthesia in early life and the risk of attention deficit/hyperactivity disorder development: a nationwide, retrospective matched-cohort study. Pediatric Anesthesia. 2014;24(7):741–748. doi: 10.1111/pan.12371. [DOI] [PubMed] [Google Scholar]
- 79.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. Journal of Neurosurgical Anesthesiology. 2009;21(4):286–291. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gupta V, Walia G, Sachdeva M. ‘Mendelian randomization’: an approach for exploring causal relations in epidemiology. Public health. 2017;145:113–119. doi: 10.1016/j.puhe.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 81.Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Statistical methods in medical research. 2007;16(4):309–330. doi: 10.1177/0962280206077743. [DOI] [PubMed] [Google Scholar]
- 82.von Hinke Kessler Scholder S, Wehby GL, Lewis S, Zuccolo LJTEJ. Alcohol exposure in utero and child academic achievement. 2014;124(576):634–667. doi: 10.1111/ecoj.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuccolo L, Lewis SJ, Davey Smith G, et al. Prenatal alcohol exposure and offspring cognition and school performance. A ‘Mendelian randomization’natural experiment. 2013;42(5):1358–1370. doi: 10.1093/ije/dyt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petrackova I, Zach J, Borsky J, et al. Early and late operation of cleft lip and intelligence quotient and psychosocial development in 3-7 years. Early Human Development. 2015;91(2):149–152. doi: 10.1016/j.earlhumdev.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 85.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110(4):796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. The Lancet Neurology. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. The Lancet. 2006;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 88.Taghon TA, Masunga AN, Small RH, Kashou NH. A comparison of functional magnetic resonance imaging findings in children with and without a history of early exposure to general anesthesia. Pediatric Anesthesia. 2015;25(3):239–246. doi: 10.1111/pan.12606. [DOI] [PubMed] [Google Scholar]
- 89.Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcoholism: Clinical and Experimental Research. 2014;38(1):214–226. doi: 10.1111/acer.12214. [DOI] [PubMed] [Google Scholar]
- 90.Testa M, Quigley BM, Eiden RD. The effects of prenatal alcohol exposure on infant mental development: a meta-analytical review. Alcohol and Alcoholism. 2003;38(4):295–304. doi: 10.1093/alcalc/agg087. [DOI] [PubMed] [Google Scholar]
- 91.Conrad AL, Goodwin JW, Choi J, Block RI, Nopoulos P. The Relationship of Exposure to Anesthesia on Outcomes in Children With Isolated Oral Clefts. Journal of Child Neurology. 2017;32(3):308–315. doi: 10.1177/0883073816681257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ko WR, Huang JY, Chiang YC, et al. Risk of autistic disorder after exposure to general anaesthesia and surgery: a nationwide, retrospective matched cohort study. European Journal of Anaesthesiology. 2015;32(5):303–310. doi: 10.1097/EJA.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 93.Clausen NG, Kähler S, Hansen T. Systematic review of the neurocognitive outcomes used in studies of paediatric anaesthesia neurotoxicity. British Journal of Anaesthesia. 2018 doi: 10.1016/j.bja.2017.11.107. [DOI] [PubMed] [Google Scholar]
- 94.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nature reviews neuroscience. 2010;11(3):201. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- 95.Beers SR, Rofey DL, McIntyre KA. Neurodevelopmental assessment after anesthesia in childhood: review of the literature and recommendations. Anesthesia & Analgesia. 2014;119(3):661–669. doi: 10.1213/ANE.0000000000000326. [DOI] [PubMed] [Google Scholar]
- 96.Naglieri JA, Bornstein BT. Intelligence and achievement: Just how correlated are they? Journal of Psychoeducational Assessment. 2003;21(3):244–260. [Google Scholar]
- 97.Fan Q, Cai Y, Chen K, Li W. Prognostic study of sevoflurane-based general anesthesia on cognitive function in children. Journal of Anesthesia. 2013;27(4):493–499. doi: 10.1007/s00540-013-1566-z. [DOI] [PubMed] [Google Scholar]
- 98.Albers CA, Grieve AJ. Test review: Bayley, N.(2006). Bayley scales of infant and toddler development–third edition. San Antonio, TX: Harcourt assessment. Journal of Psychoeducational Assessment. 2007;25(2):180–190. [Google Scholar]
- 99.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Archives of pediatrics & adolescent medicine. 2010;164(4):352–356. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 100.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 101.Moore T, Johnson S, Haider S, Hennessy E, Marlow N. Relationship between test scores using the second and third editions of the Bayley Scales in extremely preterm children. The Journal of pediatrics. 2012;160(4):553–558. doi: 10.1016/j.jpeds.2011.09.047. [DOI] [PubMed] [Google Scholar]
- 102.Harmsen WJ, Aarsen FJ, van der Cammen-van Zijp MHM, et al. Developmental problems in patients with oesophageal atresia: a longitudinal follow-up study. Archives of Disease in Childhood Fetal & Neonatal Edition. 2017;102(3):F214–F219. doi: 10.1136/archdischild-2015-309976. [DOI] [PubMed] [Google Scholar]
- 103.Doberschuetz N, Dewitz R, Rolle U, Schlosser R, Allendorf A. Follow-Up of Children with Gastrointestinal Malformations and Postnatal Surgery and Anesthesia: Evaluation at Two Years of Age. Neonatology. 2016;110(1):8–13. doi: 10.1159/000443873. [DOI] [PubMed] [Google Scholar]
- 104.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davidson AJ, Morton NS, Arnup SJ, et al. Apnea after Awake Regional and General Anesthesia in Infants: The General Anesthesia Compared to Spinal Anesthesia Study--Comparing Apnea and Neurodevelopmental Outcomes, a Randomized Controlled Trial. Anesthesiology. 2015;123(1):38–54. doi: 10.1097/ALN.0000000000000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anand K. Consensus statement for the prevention and management of pain in the newborn. Archives of pediatrics & adolescent medicine. 2001;155(2):173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 107.Majnemer A, Limperopoulos C, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C. A new look at outcomes of infants with congenital heart disease. Pediatric neurology. 2009;40(3):197–204. doi: 10.1016/j.pediatrneurol.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 108.Anderson LM, Shinn C, Fullilove MT, et al. The effectiveness of early childhood development programs: A systematic review. American journal of preventive medicine. 2003;24(3):32–46. doi: 10.1016/s0749-3797(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 109.Gleich SJ, Flick R, Hu D, et al. Neurodevelopment of children exposed to anesthesia: design of the Mayo Anesthesia Safety in Kids (MASK) study. Contemporary clinical trials. 2015;41:45–54. doi: 10.1016/j.cct.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bilotta F, Evered LA, Gruenbaum SE. Neurotoxicity of anesthetic drugs: an update. Current Opinion in Anaesthesiology. 2017;30(4):452–457. doi: 10.1097/ACO.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 111.Poor Zamany Nejat Kermany M, Roodneshin F, Ahmadi Dizgah N, Gerami E, Riahi E. Early childhood exposure to short periods of sevoflurane is not associated with later, lasting cognitive deficits. Pediatric Anesthesia. 2016;26(10):1018–1025. doi: 10.1111/pan.12969. [DOI] [PubMed] [Google Scholar]
- 112.Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Paper presented at: Mayo Clinic Proceedings; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stratmann G, Lee J, Sall JW, et al. Effect of general anesthesia in infancy on long-term recognition memory in humans and rats. Neuropsychopharmacology. 2014;39(10):2275. doi: 10.1038/npp.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ing C, Hegarty MK, Perkins JW, et al. Duration of general anaesthetic exposure in early childhood and long-term language and cognitive ability. British Journal of Anaesthesia. 2017;119(3):532–540. doi: 10.1093/bja/aew413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ludman L, Spitz L, Lansdown R. Intellectual development at 3 years of age of children who underwent major neonatal surgery. Journal of Pediatric Surgery. 1993;28(2):130–134. doi: 10.1016/s0022-3468(05)80257-x. [DOI] [PubMed] [Google Scholar]
- 116.Andropoulos DB, Easley RB, Brady K, et al. Changing expectations for neurological outcomes after the neonatal arterial switch operation. The Annals of thoracic surgery. 2012;94(4):1250–1256. doi: 10.1016/j.athoracsur.2012.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gano D, Andersen SK, Glass HC, et al. Impaired cognitive performance in premature newborns with two or more surgeries prior to term-equivalent age. Pediatric Research. 2015;78(3):323–329. doi: 10.1038/pr.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seltzer L, Swartz MF, Kwon J, et al. Neurodevelopmental outcomes after neonatal cardiac surgery: Role of cortical isoelectric activity. Journal of Thoracic & Cardiovascular Surgery. 2016;151(4):1137–1142. doi: 10.1016/j.jtcvs.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 119.Birajdar S, Rao S, McMichael J. Neurodevelopmental outcomes of neonates undergoing surgery under general anesthesia for malrotation of intestines. Early Human Development. 2017;109:32–36. doi: 10.1016/j.earlhumdev.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Guerra GG, Robertson CM, Alton GY, et al. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy. Pediatric Anesthesia. 2011;21(9):932–941. doi: 10.1111/j.1460-9592.2011.03581.x. [DOI] [PubMed] [Google Scholar]
- 121.Terushkin V, Brauer J, Bernstein L, Geronemus R. Effect of General Anesthesia on Neurodevelopmental Abnormalities in Children Undergoing Treatment of Vascular Anomalies With Laser Surgery: A Retrospective Review. Dermatologic Surgery. 2017;43(4):534–540. doi: 10.1097/DSS.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 122.Smart Tots. Consensus statement on the use of anesthetics and sedatives in children. 2012 [Google Scholar]
- 123.Smart Tots. Consensus statement on the use of anesthetic and sedative drugs in infants and toddlers. 2015 doi: 10.1016/j.jopan.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 124.Australian and New Zealand College of Anaesthetists, Society for Paediatric Anaesthesia in New Zealand and Australia. Warnings: Young children, pregnant women. 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.