Abstract
Changes of intracellular Ca2+ concentration regulate many aspects of cardiac myocyte function. About 99% of the cytoplasmic calcium in cardiac myocytes is bound to buffers and their properties will therefore have a major influence on Ca2+ signalling. This article considers the fundamental properties and identities of the buffers and how to measure them. It reviews the effects of buffering on the systolic Ca2+ transient and how this may change physiologically as well as in heart failure and both atrial and ventricular arrhythmias. It is concluded that the consequences of this strong buffering may be more significant than currently appreciated and a fuller understanding is needed for proper understanding of cardiac calcium cycling and contractility.
Keywords: calcium, buffer, heart failure, arrhythmia
Introduction
The importance of changes of intracellular calcium concentration in cardiac function needs little introduction; see 1, 2 for reviews. The systolic rise of ionized cytoplasmic calcium concentration ([Ca2+]i) activates contraction and regulates many sarcolemmal ion currents and thereby the electrophysiology of the cell; abnormal Ca2+ handling is implicated in the genesis of arrhythmias. Calcium is also a major factor in the control of gene expression. Work using fluorescent Ca2+ indicators has demonstrated how alterations of Ca2+ fluxes into the cytoplasm underlie changes of contractility in health and disease. It is, however, often overlooked that only about 1% of cytoplasmic Ca2+ is free, with the remainder being bound to cytoplasmic buffers 3. Therefore the properties of these buffers will potentially play as large a role as do Ca2+ fluxes in determining the size and kinetics of changes of [Ca2+]i.
Here we review recent progress in characterizing cytoplasmic buffers and their effects on the physiology of cardiac muscle as well as disease mechanisms. Importantly, we also highlight the numerous areas where more work is required.
Properties of intracellular Ca2+ buffers
Chemistry and kinetics of Ca2+ binding
Calcium (Ca2+) has several chemical features that make it a ubiquitous second messenger 4, 5. It forms a chemically active divalent cation in aqueous solution with an ionic radius larger than the other common divalent ion (Mg2+) resulting in higher affinity binding. The fact that its intracellular concentration is much lower than extracellular permits large changes of concentration because of sarcolemmal fluxes. Ca2+ binding alters the tertiary structure of proteins with consequences for their catalytic activity. This is a reciprocal interaction as binding also resists changes of the free concentration of an ion by acting as a buffer.
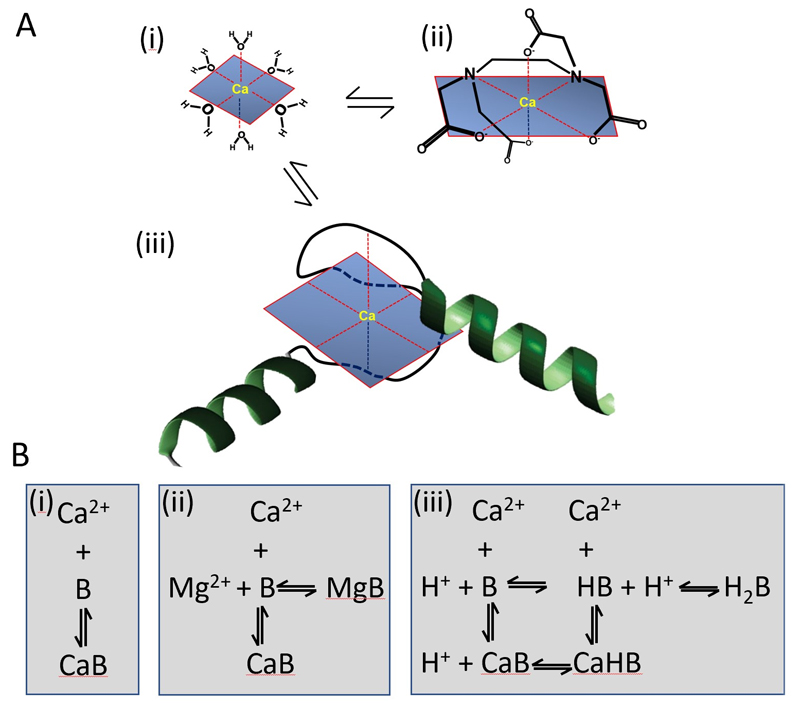
The formation of several coordinate bonds between single Ca2+ ions and ligands is known as chelation. The electrons for these bonds typically come from nitrogen or oxygen atoms, replacing water molecules in the solvation sphere of the Ca2+ ion with a series of bonds (usually 6) in a claw-like or chelation arrangement (Fig 1A). For example, EDTA is an organic chelator designed to bind divalent cations with very high affinity. The EF hand, a helix-loop-helix configuration, is the most common Ca2+ binding motif in proteins 6. EF hand sites generally occur in pairs and their affinity for Ca2+ and Mg2+ depends on the amino acids used to form the coordinate bonds as well as the surrounding protein environment. For example, calmodulin has four EF hand domains: (i) a high affinity site that is normally bound at resting Ca2+ concentrations with a dissociation constant (Kd) of approximately 100 nM, (ii) two further binding sites on the C-terminal with Kd values of approximately 300 nM 7. The 4th binding site has the lowest affinity (Kd = 10 µM) and therefore binds negligible Ca2+ within the physiological range (0.1-1 µM). It may have a role in controlling enzymes located near sarcoplasmic reticulum Ca2+ release sites in cardiac cells where [Ca2+]i rises to approximately 100 µM 7, 8.
Fig. 1.
A. Schematics showing Ca2+ in various environments: (i) water; (ii) bound to EDTA; (iii) bound to an EF hand. B. Reaction schemes for Ca2+ binding to various buffers: (i) simple binding; (ii) competition with Mg2+; (iii) competition with protons.
The speed of the chelation reaction depends on its complexity (Table 1). The fastest binding occurs with small molecules (MW <1000) such as BAPTA or Ca2+ indicators (e.g. Fura-2 or Fluo-3), with a forward rate constant that approaches the diffusion-controlled limit (minimally 108 M-1 s-1) 17. Ca2+ binding to the EF hand structure of the regulatory site of troponin C is slightly slower9. With even more complex reaction schemes (Fig 1B) that involve displacement of ions (e.g. H+ or Mg2+) before Ca2+ binding, the kinetics slow considerably. For example, Ca2+ binding to the chelator EGTA requires dissociation of protons from intermediate forms of the ligand, reducing the overall forward rate constant (Table 1) 18. Different forms of the EF hand motif such as the Mg2+ sites of myosin, TnC and parvalbumin, have high relative affinities for Mg2+ that result in significant Mg2+ bound under physiological conditions19. The need for Mg2+ to dissociate as part of the equilibration results in a low apparent rate constant of Ca2+ binding.
Table 1. Concentration and properties of the major cellular buffers in ventricular myocytes.
| Concentration | Kd | On rate | |
|---|---|---|---|
| μmol/L cell cytoplasm | μM | M-1.s-1 | |
| Ca buffers | |||
| Troponin C (regulatory) 9, 10 | 70 | 0.6 | 3.27x107 |
| SERCA11 | 47 | 0.6 | †1.00 x108 |
| Calmodulin3 | 24 | 7 | 3.4 x107 |
| Sarcolemma (low)12 | 42 | 13 | †1.0 x108 |
| Sarcolemma (high) 12 | 15 | 0.3 | †1.0 x108 |
| Ca/Mg buffers | |||
| Troponin C (Mg) 9, 13 | 140 | 0.0195 | 3.3 x103 |
| Myosin9 | 140 | 4.62 | 9.6 x104 |
| ATP14 | 5000 | 1200 | 1.9 x106 |
| Histidyl dipeptides15, 16 | 20000 | 1000 | 6.1 x106 |
| Chemical probes | |||
| BAPTA17 | 0.178 | 5.0 x108 | |
| EGTA18 (pH7.2) | 0.180 | 2.3 x106 | |
The cellular buffers are organized in two groups: top, Ca buffers; middle Ca/Mg buffers. The properties of two chemical agents (BAPTA & EGTA) are shown below. For the cellular buffers, the first two columns show the buffer concentration and its Kd. The third column gives the value of the on rate constant for Ca2+ binding to the buffer. This has been calculated under standard conditions (initial free [Ca2+] 100 nM, addition of 10 μM Ca2+ to 10 μM buffer). The value for calmodulin is an approximation to one binding site 11. For the Mg/Ca buffers the Kd and rate constant have been corrected for the apparent value in the presence of a cellular Mg2+ of 0.5 mM.
Indicates rate constant was estimated based on diffusion limit.
Ca2+ binding sites in cardiac muscle
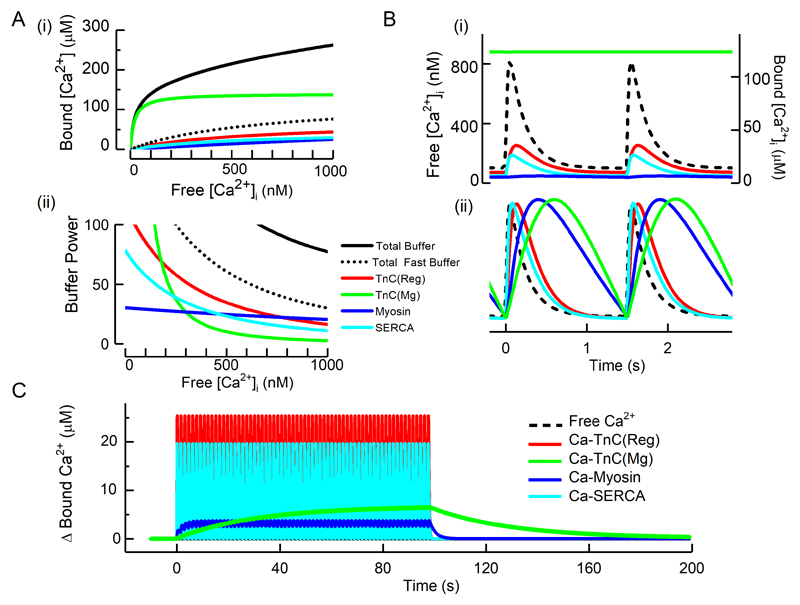
Based on previous work 3, 11, 20, Table 1 lists the major Ca2+ binding ligands, their estimated cytoplasmic concentrations, and dissociation constants (Kd) alongside estimates of the rate constants of Ca2+ binding. The ligands which bind appreciable amounts of Mg2+ under physiological conditions are grouped separately. The steady state Ca2+ binding for several buffers as a function of [Ca2+] is shown in Fig 2A (i). The two major contributors to buffering are Troponin C (TnC) and SERCA.
Fig 2.
Properties of cellular cardiac Ca2+ buffers. A (i) Steady state Ca2+ buffering showing the dependence of bound on free Ca2+. The coloured curves show the contributions of the two Ca2+ binding sites (regulatory and Mg) of TnC, myosin and SERCA. The solid black line shows the total, calculated as the sum of these components and the others listed in Table 1. The dotted black line represents the sum of the fast buffers (top part of Table 1). (ii) Buffer power (calculated as in equation 2) as a function of [Ca2+]i. B. Time course of change of bound [Ca2+] in response to systolic Ca2+ transients applied at 1.5 Hz. (i) Absolute levels of [Ca2+]. The dashed line shows free [Ca2+] and the coloured traces the concentration of the Ca2+-bound form of the various ligands. (ii) normalized concentrations to emphasise kinetics. C. The change of bound Ca2+ in response to a series of Ca2+ transients (not shown) applied at 1.5 Hz.
Troponin C
TnC has two classes of Ca2+ binding sites (Fig 2A): (i) a single, lower affinity, “regulatory” site that modulates myofibril activation and thence force; (ii) two high affinity sites that can also bind Mg2+, the “Mg2+ sites”. There is ample evidence that the affinity for Ca2+ of the regulatory site changes in various situations. For example acidification decreases the binding of Ca2+ 21. Work using a fluorescent TnC showed that phosphorylation of Troponin I (TnI), as occurs during beta adrenergic stimulation, shifts the relationship between fluorescence and [Ca2+]i to higher [Ca2+]i indicating decreased Ca2+ affinity. A similar approach has shown that troponin and tropomyosin mutations affect Ca2+ affinity 22 and such mutations have been directly shown to affect Ca2+ buffering23, 24. It should, however, be noted that there are many circumstances (see below for discussion of heart failure) where the only available data is of a shift in the relationship between [Ca2+]i and force. It is often not certain whether this shift results from a direct effect on Ca2+ binding or a subsequent step in the contraction mechanism 25. For example, caffeine shifts the relationship to lower [Ca2+]i but this effect is not accompanied by increased Ca2+ binding 26. Finally, much less is known about the properties of the Mg2+ site on troponin than the regulatory one. One issue, which also applies to other cellular buffers, is that studies of Ca2+ binding are generally carried out in vitro using artificial solutions as opposed to cytoplasm. Given that many cellular constituents may affect the properties of this important buffer, it is important to characterize Ca2+ binding to the Mg2+ sites and investigate the factors that affect it. These sites can be mutated and normal contraction requires only one of the two Mg2+ sites 27. It would be interesting to know the effects on cardiac function and Ca2+ cycling of the expected large decrease of Ca2+ buffering.
SERCA
The inclusion of the SR Ca2+ pump (SERCA) as a buffer emphasises that it has two roles in decreasing cytoplasmic [Ca2+]i 28. Initial buffering by binding is followed by active sequestration. In rabbit ventricle, systole involves an increase of approximately 60 µM total Ca2+ resulting in a rise of free [Ca2+]i of about 0.6 µM. Due to the affinity of SERCA binding sites, ~30 µM binds immediately and, with a peak uptake rate of ~200 µM/s, only about 2 pump cycles are required to sequester the Ca2+ associated with a Ca2+ transient. This emphasises the importance of the initial binding/buffering by SERCA in addition to its turn-over in determining the rate of decay of the cytoplasmic Ca2+ transient 29.
Other ligands
One important distinction is whether the buffers are immobile, or can diffuse. Table 1 gives values for the fixed sarcolemmal binding sites. The highly diffusible ATP binds Mg2+ and Ca2+ with moderately fast kinetics but, although present at 5 mM, its low affinity results in only a modest contribution to buffering. Other diffusible ligands include creatine phosphate and histidyl dipeptides (HDPs) which also bind Ca2+ and Mg2+. In heart, the predominant forms of this latter group of compounds include homocarnosine and anserine with a total concentration of approximately 20 mM15. The affinities of Ca2+ and Mg2+ for these HDPs are lower than that of ATP and together they constitute the bulk of the diffusible Ca2+ buffers. One feature of diffusible Ca2+ buffers is their ability to increase the apparent diffusion coefficient of Ca2+ through diffusion of the Ca-bound form 30. The HDPs are also weak acids and contribute to the pH buffer power of the cytosol, thereby linking intracellular pH and Ca2+ buffering (see below).
Buffer kinetics
The importance of the different kinetics of the major buffers is illustrated in Fig 2B(i). The amount of Ca2+ bound to the regulatory site of TnC lags slightly behind free [Ca2+]. The lag is much greater for the slower buffers (here the Mg2+ sites of myosin and TnC) and this is emphasised in the normalized data of Fig. 2B(ii). During a train of stimuli (Fig 2C) the slow kinetics of these buffers results in a beat-to-beat increase of bound Ca2+. Even at 1.5 Hz, these two slow sites together accumulate a total of about 10 µM Ca2+ and, at higher rates, when diastolic [Ca2+]i increases, greater binding is to be expected.
Measurement of Ca2+ buffering
As discussed above, buffering depends on the summed effects of a variety of Ca2+ binding molecules. It is often convenient to approximate this with a composite buffer value described by a single dissociation constant and ligand concentration. The simplest method is by titration. Solaro et al 31 studied isolated cardiac myofilaments and calculated that about 22 µmole of Ca2+ per kg heart is required to produce 50% maximum contraction. This was accompanied by a rise of free Ca2+ of about 1.4 µM indicating that the myofilaments alone can bind more than 90% of the total Ca2+. A subsequent approach, using cardiac homogenates, found that to raise free [Ca2+] to 1 µM required 72 µmol/kg total Ca2+ 32. Hove-Madsen and Bers 33 performed similar experiments using permeabilized cells. This removed complications of extracellular components and allowed study of mitochondrial and SR buffering separately from cytoplasmic. They found that cytoplasmic buffering could be described by a Kd of 0.42 µM, plus a much lower affinity component (Kd = 79 µM).
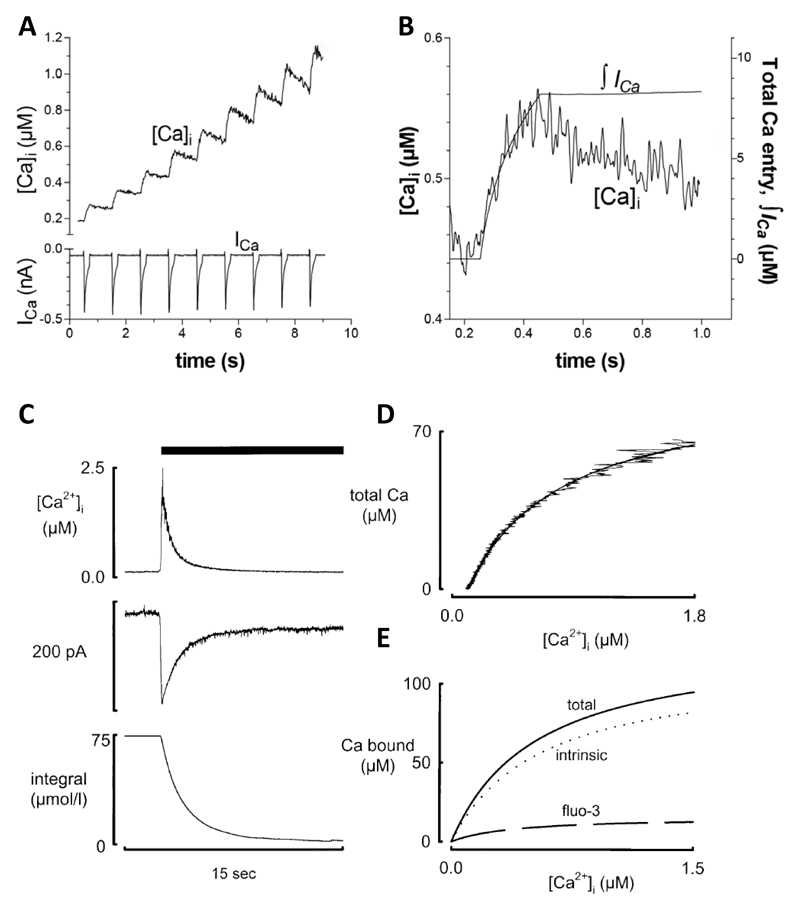
The methods described above involve destruction of the cell membrane. It is also important to be able to measure buffering under physiological conditions. This was first done by depolarizing ventricular myocytes and measuring the total entry of calcium through the L-type Ca2+ current 20, 34 under conditions in which Ca2+ removal mechanisms were inhibited (see Fig 3A&B). Berlin et al compared Ca2+ entry with the rise of [Ca2+]i giving a Kd of 0.96 µM and maximum buffer capacity of 123 µmol/l 34. A related method compared the entry of Ca2+ through NCX with [Ca2+]i as estimated indirectly from changes of cell length 37. A limitation of the method of Berlin et al is that it requires the irreversible SERCA inhibitor thapsigargin, precluding repeated measurements before and after other interventions. An alternative approach uses rapid application of caffeine to release calcium from the SR resulting in an abrupt increase of [Ca2+]i which then decays as sodium calcium exchange (NCX) removes Ca2+ from the cell. Integrating the NCX current gives a measure of the change of total Ca2+ concentration which is compared continuously with the change of free [Ca2+]i to characterize buffers 36 (Fig 3C-E). The caffeine response typically decays with a time constant of 1-2 s 38 so this cannot detect slower buffers. In ferret ventricular myocytes, this method gave a Kd of 0.59 µM with a maximum capacity of 114 µmol/l cell equivalent to 175 µmol/l cytoplasm36 and, in rat ventricular myocytes, a Kd of 0.49 µM and maximum capacity of 149 µmol/l cytoplasm39. This is stronger Ca2+ buffering than that found by Berlin et al 34. This may be partly due to the fact that the caffeine method includes buffering by SERCA since thapsigargin is not present. Consistent with this, addition of thapsigargin has been shown to decrease buffer power 40.
Fig 3.
Measurement of buffering in intact cells. A. Comparison of the effects of Ca2+ influx through the L-type Ca2+ channel (bottom) with the resulting increase of [Ca2+]i. B. Relationship between the integral of the L-type Ca2+ current and the change of [Ca2+]i. Ca2+ removal by SR, mitochondria, NCX and PMCA were inhibited with thapsigargin, a mitochondrial uncoupler, Na-free solution and elevated external Ca2+ concentration respectively. Figure reproduced from35 from an original paper 34. C. Determination of Ca2+ buffering from the caffeine-evoked release of Ca2+ from the SR. Traces show (from top to bottom): [Ca2+]i, NCX current, integral of current. Caffeine (10 mM) was applied as shown by the bar. D. Relationship between total Ca2+ (estimated from the integral of NCX current) and [Ca2+]i. E. Separation of buffering into total, the contribution from the Ca-sensitive indicator (fluo-3) and the calculated intrinsic buffering of the cytoplasm. Reproduced from 36.
Buffering and the systolic Ca2+ transient
Alterations of buffering power affect the systolic Ca2+ transient and thence contraction. Incorporation of Ca-sensitive indicators has the side effect of increasing Ca2+ buffering and this decreases systolic and increases diastolic force as well as slowing the rate of mechanical relaxation 41, 42. Subsequent work found a decrease of both the amplitude and rate constant of decay of the Ca2+ transient because, the higher the buffer power, the smaller the change of free [Ca2+] resulting from a given rate of Ca2+ pumping 43. Adding exogenous buffer also decreases the rate of spontaneous beating of sinoatrial node cells, presumably by decreasing the changes of [Ca2+]i that contribute to pacemaker activity 44. In recent years, much work has been done using transgenic animals that express calcium indicators. In principle, the additional buffering could be a concern but it has been demonstrated that, at the concentrations expressed, this is not an issue 45.
The effect of increased buffering also depends on the kinetics of the added buffer. While fast buffers simply slow the Ca2+ transient, slower buffers produce a biphasic decay. The initial, fast phase reflects the time taken for cytoplasmic Ca2+ to bind to the buffer with the slower depending on the kinetics of Ca2+ removal from the cytoplasm 43.
It should be noted that, in the steady-state, averaged over the cardiac cycle, Ca2+ efflux must equal influx. This efflux is determined by [Ca2+]i. If one assumes that Ca2+ efflux is proportional to [Ca2+]i then, in the steady state, the decrease of amplitude of the Ca2+ transient resulting from increased buffering must exactly balance the slowing of decay of the transient and increased diastolic level such that the average level of [Ca2+]i is unaffected 46, 47. Increasing stimulation rate will load cytoplasmic Ca2+ buffers (Fig 2C). An interruption of beating will result in this extra Ca2+ being taken up by the SR and then being available for release 23. This may affect contractility and (see below) contribute to Ca-dependent arrhythmias. A more complicated question is what is the effect of increased buffering on SR Ca2+ content in the steady state? Experimental studies have found that adding exogenous cytoplasmic buffers decreases SR Ca2+ 43, 48. One explanation is that SERCA activity depends in a cooperative manner on [Ca2+]i 49 whereas NCX has a linear dependence 50. The decreased amplitude of the systolic Ca2+ transient may therefore decrease SERCA activity more than NCX leading to a net loss of SR Ca2+. Further studies are required to see if the decrease of SR content with increased buffering is a general phenomenon.
Factors that alter Ca2+ buffering
Diastolic [Ca2+]i
For a simple buffer, total ([CaT]) and free ([Ca2+]) are related by:
| (1) |
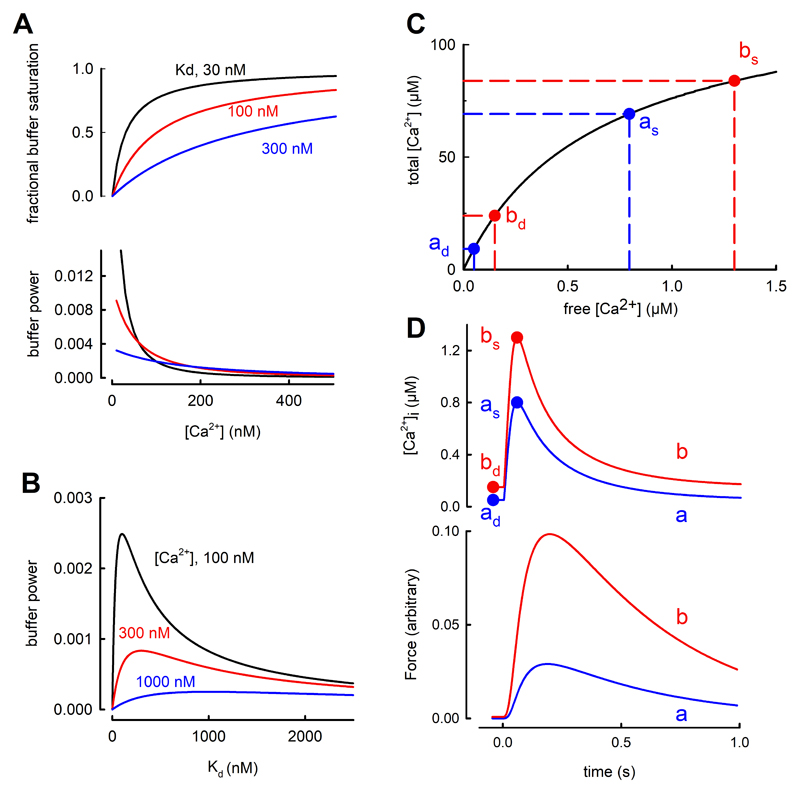
where Bmax is the total buffer concentration and Kd the concentration of Ca2+ at which 50% of the buffer has Ca2+ bound. The upper panel of Fig 4A shows such relationships for three values of Kd.
Fig. 4.
Effects of [Ca2+]i and buffer Kd on buffer power. A. Effects of [Ca2+]. The top graph shows fractional saturation of a single buffer as a function of [Ca2+]. Curves are shown for three different values of Kd. The lower graph shows calculated buffer power (change of total Ca2+/change of [Ca2+]i) as a function of [Ca2+] for these values of Kd. Colours correspond to those above. B. Dependence of buffer power on Kd at the three values of [Ca2+] indicated. C. The effects of a small increase of diastolic [Ca2+]i on the change of systolic [Ca2+]i produced by the addition of a fixed amount of total [Ca2+]. The buffer curve (fast buffers only) shows two levels of diastolic [Ca2+]i indicated as ad (50 nM) and bd (150 nM). The systolic levels (respectively as and bs) are obtained by adding 60 µM total [Ca2+] resulting in a larger increase of systolic [Ca2+]i in b compared to a. D. Simulation of the effect of adding and removing 60 µM total Ca2+ with kinetics designed to represent a Ca2+ transient. Labels correspond to points in C. Upper graph, simulated Ca2+ transients; lower, predicted force responses calculated using a published model53.
Buffer power (β) is defined as the change of total Ca2+ divided by that of free Ca
| (2) |
The individual contributions of the major individual buffers to the total buffer power are shown in Fig 2A(ii). At [Ca2+]i around 100 nM, the Mg2+ sites on TnC make the largest contribution whereas, above 200 nM, these are tending to saturation and the regulatory site and SERCA contribute most. The lower panel of Fig 4A shows that buffer power has its highest value (equal to Bmax/Kd) at low [Ca2+] and decreases as [Ca2+] increases. When [Ca2+] = Kd the buffer power is 30% of the value at 0.1Kd and, at 2Kd, it is only 13% of this level. Consequently, the greater the diastolic level of [Ca2+]i, the larger will be the increase of [Ca2+]i produced by a given release of total Ca from the SR 51, 52. Some appreciation of the importance of this effect is provided by the buffer curve of Fig. 4C. Due to the flattening of the buffer curve at elevated [Ca2+]i, an increase of 60 µM total [Ca2+] produces a larger increase of free [Ca2+] when applied from a higher diastolic [Ca2+]i than from a lower. This is clear in the simulated transients of the upper part of Fig. 4D. An increase of diastolic [Ca2+]i of only 100 nM (from 50 to 150) increases systolic [Ca2+]i by 500 nM. Therefore an increase of diastolic [Ca2+]i alone, can lead to an increase of systolic, which is predicted (see lower panel Fig 4D) to result in a large increase of developed force with little change of resting force. Finally, the decrease of buffer power at elevated [Ca2+]i has also been suggested to account for a rapid initial rate of decay of the Ca2+ transient 54.
This consequence of changes of diastolic [Ca2+]i will add to the inotropic effects of manoeuvres such as the addition of cardiac glycosides 55 or β-adrenergic stimulation56 which can increase diastolic [Ca2+]i. It is also a possible explanation for changes of the amplitude of the Ca2+ transient and force under the many conditions where there are no measurements of diastolic [Ca2+]i. Testing this will require obtaining and comparing absolute measurements of [Ca2+]i between cells or tissues from different animals or patients. There is a dearth of such measurements in the literature 57 as it is much easier to measure changes of fluorescence of a Ca2+-sensitive indicator than absolute levels of [Ca2+]i. Properly calibrated measurements, ideally using ratiometric indicators, are required.
Stimulation Rate
Repetitive stimulation will load slower Ca buffers (Fig 2C). This will decrease buffer power and might therefore increase the rise of [Ca2+]i produced by a given increase of total cytoplasmic Ca2+, contributing to the inotropic effects of increased rate 52. This effect is analogous to that discussed above for elevated diastolic [Ca2+]i but the slow kinetics result in a “memory” so that, following a change of rate, the effects on systolic [Ca2+]i and thence on the action potential duration may outlast those of diastolic [Ca2+]i. Such effects may also contribute to the slow effects of changes of rate on parameters such as action potential duration 58.
Buffer Kd
Equation 2 (Fig 4A) shows that, at lower values of [Ca2+]i, buffer power is greater the lower the value of Kd as this results in stronger Ca2+ binding. In contrast, at higher [Ca2+]i, the lower the Kd, the less the buffer power as the buffers become saturated. (See 23 for experimental demonstration). Fig 4B shows the biphasic dependence of buffer power on Kd with the maximum being reached when Kd = [Ca2+]i. Therefore increasing buffer affinity will increase buffering at diastolic levels of [Ca2+]i but decrease it at peak systolic ones.
One issue that has received no attention is whether Ca2+ buffering is the same in all cells in the ventricle. Given the regional differences of expression of other proteins including pumps 59 and channels 60, heterogeneity of buffering would not be surprising. Likewise, possible variations of Ca2+ buffering between individuals, because of mutations and polymorphisms does not appear to have been considered.
Physiological modulation of buffering
Beta-adrenergic stimulation
The two major Ca2+ buffers, TnC and SERCA are regulated by phosphorylation of, respectively, Troponin-I and phospholamban resulting in increased affinity of Ca2+ for SERCA 61 and decreased for TnC 62. One might therefore expect that beta-adrenergic stimulation would alter the buffer power. Experimental measurements, however, found no such effect 40 possibly due to two opposing factors: phosphorylation increases the affinity of Ca2+ binding to SERCA but lowers it for troponin. If the Kd values are above the range of [Ca2+]i considered, these effects will respectively increase and decrease buffer power (Fig 4A). Subsequent experiments, performed on transgenic mice in which either troponin could not be phosphorylated or lacking phospholamban found the expected increase and decrease respectively of buffer power on phosphorylation. Further work is required to investigate the possibility that, in other species, the balance is less exact and therefore phosphorylation may have a net effect on buffer power. As mentioned above, it should also be noted that the effects of a change of Ca2+ affinity on buffer power will depend on the range of [Ca2+]i under investigation.
Effects of changes of pH on buffering
Many Ca2+ buffers can bind protons as an alternative to Ca2+ ions. Direct measurements have shown that acidification decreases Ca2+ binding to troponin 21. Therefore acidification will decrease the affinity for Ca2+ with a decrease of Ca2+ buffering power predicted at values of [Ca2+]i below the Kd (Fig 4B). Surprisingly, intracellular acidification had no effect on Ca2+ buffering 63. We suggest that this may occur because, although a decrease of Ca2+ affinity of low affinity buffers will decrease buffer power, decreased affinity of very high affinity buffers will increase their contribution to buffering. Acidification has been shown to increase resting [Ca2+]i in rat ventricular myocytes, an effect attributed to displacement of Ca2+ from buffers 15. It is not clear, however, why such displacement should produce the observed maintained increase of [Ca2+]i; one would expect a transient increase which decays back to baseline as Ca2+ is pumped out of the cell. It may result from inhibition of Ca2+ efflux on NCX by acidification 64. If this is the case then the maintained effect on [Ca2+]i is presumably a consequence of the NCX effect and not of altered buffering. This question could be resolved by directly measuring the effects of pH on NCX activity.
Cardiac dysfunction and Ca2+ buffering
Atrial buffering, fibrillation and failure
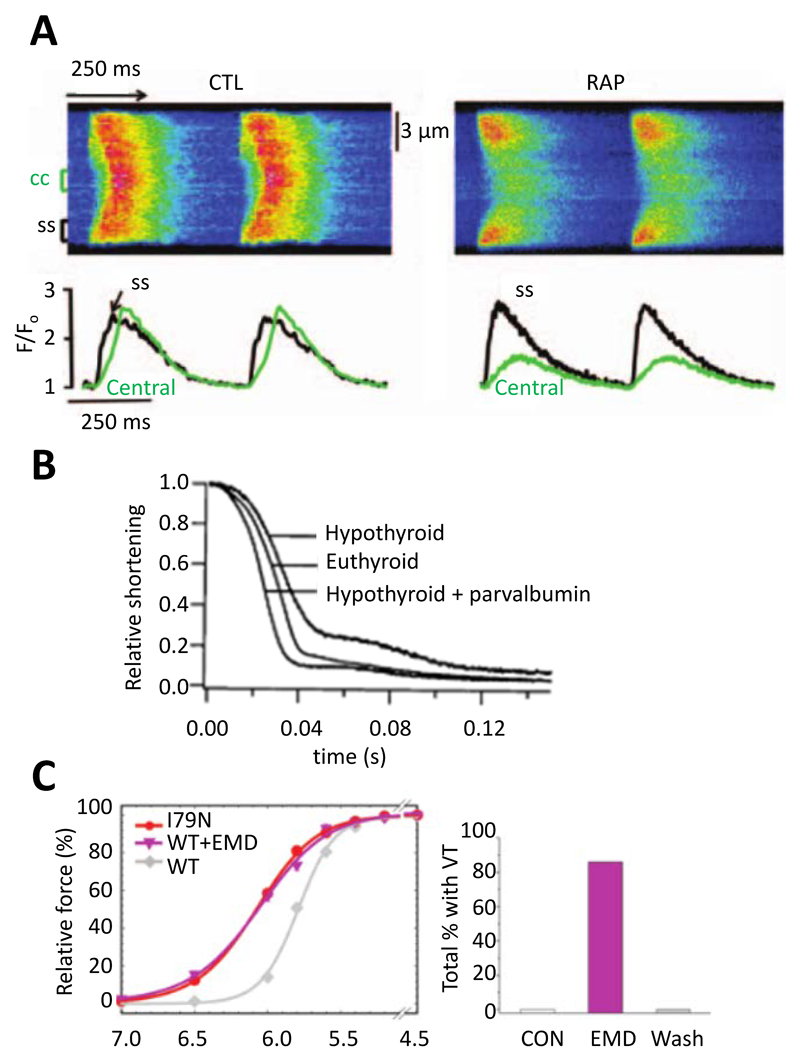
The total concentration of Ca2+ buffers in rat atrial myocytes has been reported to be about three times greater than in ventricular with no difference in apparent Kd 65, possibly due to higher SERCA expression in the atrium than the ventricle. Changes in Ca2+ buffering have been suggested to be important in both normal and abnormal atrial function. For example, in sheep atria, buffer power increases with age due to an increase of Ca2+ affinity of the buffers 66. This decreased both the amplitude and rate of decay of the systolic Ca2+ transient. Atrial myocytes from many species, including rabbit and cat have few or no t-tubules (67 for review) and the systolic Ca2+ transient begins at the periphery of the cell and then propagates towards the centre 68, 69. Increasing Ca2+ buffering by incorporation of EGTA can prevent this propagation 70. A modelling study also predicted this inhibitory effect of high buffer concentrations but pointed out that lower concentrations of mobile buffers such as ATP facilitate propagation 30. Greiser et al 71 investigated the effects of rapid atrial pacing in rabbits in order to mimic the effects of atrial fibrillation (AF). This resulted in a two to threefold increase of buffering power due, at least in part, to decreased phosphorylation of TnI which was accompanied by (Fig 5A) decreased centripetal propagation. Evidence for a causal link between increased buffering and decreased propagation was provided by showing that incorporation of BAPTA to increase buffering mimicked the effect on propagation. The effects of rapid pacing to induce heart failure have also been studied on sheep atrial myocytes where a decrease of buffer power was observed 74. This was accompanied by a decrease of the amplitude of the central calcium transient attributed to the loss of transverse tubules rather than a change of buffering 75. A similar decrease of calcium buffering in the sheep has been observed during atrial fibrillation where it was suggested to lead to arrhythmogenic Ca2+ waves thereby contributing to AF 76. More work is required on changes of atrial Ca2+ buffering and their importance in atrial function. Finally, it is worth noting that the atrial studies reviewed above could not exclude effects of small changes of end-diastolic [Ca2+]i on the measured buffer power.
Fig 5.
Effects of altered buffer power. A. Abolition of centripetal propagation of the Ca2+ transient by rapid atrial pacing (RAP). Upper traces are linescan images. Lower records show [Ca2+]i measured at surface of cell (black) and in centre (green). Left hand records from control and right hand after rapid stimulation. Reproduced from 71. B. Relaxation of contraction in isolated rat myocytes. Records show control (euthyroid), hypothyroid, and hypothyroid with parvalbumin expressed by gene transfer. Reproduced from 72. C. Left, comparison of the effects of the I79N mutation of troponin T with that of the Ca2+ sensitizing agent EMD 57033 on the relationship between pCa and force. Right, effects of EMD 57033 on occurrence of ventricular tachycardia (VT). Reproduced from 73.
Ca2+ buffering and heart failure
Ca2+ buffering is unaffected by pacing-induced heart failure in both dogs 77 and sheep 78. In contrast, in samples from human ventricle, the Ca2+ sensitivity of contraction was increased in dilated cardiomyopathy, possibly due to decreased phosphorylation of TnI 79. Increased Ca2+ sensitivity was also found in a canine dilated cardiomyopathy 80, and mouse infarct models 81, 82. As mentioned earlier, changes of Ca2+ sensitivity of contraction do not necessarily indicate altered Ca2+ binding and buffering; direct measurements of Ca2+ binding are therefore required. Increased myofilament Ca-sensitivity by itself will decrease cardiac relaxation and thereby contribute to diastolic heart failure. In addition, any consequential increase of Ca2+ buffering will slow the decay of [Ca2+]i, worsening relaxation. In contrast to the data discussed above, either the induction in rats of pressure overload-induced left ventricular hypertrophy or heart failure following myocardial ischemia resulted in a decreased Ca2+ sensitivity for activation of contraction, an effect attributed to alterations in troponin 83. Some of the controversies in this area have been reviewed 84. As far as myocardial ischemia is concerned, it is well known that troponin is lost from the heart and, indeed, the appearance of TnI and TnT in plasma is diagnostic of cardiac damage. Troponin release has also been detected from myocardium in conditions that not associated with obvious cellular degeneration but this, however, only represents a small fraction (~3%) of the total troponin 85 and will not therefore significantly affect cellular buffering.
Finally, it should be noted that many studies of heart failure find a decrease of SERCA expression 86. We speculate that the consequent decrease of Ca2+ buffering would compensate in those situations where an increase of myofilament buffering is expected and worsen where there is a decrease. Again, it will be important to repeat these studies of heart failure while measuring buffering directly.
It is also important to reemphasize the potential effects (Fig 4C) of changes of diastolic [Ca2+]i and thence of buffering power on the amplitude of the calcium transient. A major problem here is the paucity of measurements of diastolic [Ca2+]i. It is essential that studies on heart failure ask the simple question: how accurately has diastolic [Ca2+]i been measured and can it be excluded that changes (for example between animals or in disease) account for the observed changes of systolic [Ca2+]i?
Work from Metzger and colleagues has demonstrated that changes of Ca2+ buffering may not simply be involved in the development and consequences of heart failure, but may also be used to treat it. They suggested that impaired relaxation in heart failure could be ameliorated by adding intracellular buffers. They noted that “fast” Ca2+ buffers slow both the rise and fall of [Ca2+]i and decrease the amplitude of the Ca2+ transient and instead advocated the use of parvalbumin, a skeletal muscle Ca2+ buffer. This has the important property that it binds Ca2+ slowly because Mg2+ has to dissociate first and therefore there is little attenuation of the peak Ca2+ transient. It will, however, bind Ca2+ during diastole, thereby improving diastolic performance. Incorporation of alpha-parvalbumin was shown to accelerate the decay of [Ca2+]i with no effect on peak [Ca2+]i and (see Fig 5B) also reversed the slowing of relaxation produced by experimental hypothyroidism 72 and in the Dahl salt-sensitive rat model of diastolic dysfunction 87. Subsequent work has turned to altering the structure and thence the relative Ca2+ and Mg2+ affinities of parvalbumin analogs to improve the effects 88, 89. More generally, these effects of parvalbumin highlight the potential importance of endogenous slow buffers such as the Mg2+ site of TnC and myosin.
Ca2+ buffering and hypertrophic cardiomyopathy
Several studies have examined the molecular basis of familial hypertrophic cardiomyopathy (FHC). Much of this work involves the effects of mutations in thin filament proteins such as troponin and tropomyosin, which are amongst the causes of familial hypertrophic cardiomyopathy (FHC). Robinson et al 22 showed that mutations causing hypertrophic cardiomyopathy increased the binding affinity of Ca2+ to myofilaments (as assessed with a fluorescent troponin) and presumably therefore Ca2+ buffering. They proposed that alterations of buffering might lead to pathological changes of the Ca2+ transient. Troponin mutations were subsequently investigated in a mouse model of the related condition of restrictive cardiomyopathy and the predicted decreased amplitude and slowed decay of the Ca2+ transient observed 23, 90. In addition, myofilament Ca2+ sensitization with EMD 57033 mimicked the effects of troponin T mutations on Ca2+ buffering and the Ca2+ transient 23. A recent study used adenovirus to infect isolated myocytes with troponin or tropomyosin mutations and, again, found an increase of diastolic [Ca2+]i 24. Although the above results would be expected from an increase of buffering power, it has been reported that there is a decrease of SERCA expression which may also contribute91. This study also found that the late Na+ current inhibitor ranolazine abolished the slowing of decay of the Ca2+ current. Although no data are available, it seems unlikely that ranolazine would affect Ca2+ buffering. It may therefore be that some of the effects of thin filament mutations are directly due to Ca2+ buffering and others a secondary consequence of the resulting heart failure, possibly due to decreased SERCA.
As mentioned in an earlier section, the Mg2+ sites on troponin are important contributors to buffering at low [Ca2+]i. It is therefore interesting that one of the mutations associated with FHC (D145E) greatly decreases the affinity of Ca2+ binding to these sites 92. At first sight, this might appear to contrast with the association between FHC and increased affinity reviewed above. These observations may be reconciled by noting that a decrease in affinity of the very high affinity, Mg2+ TnC sites will actually increase Ca2+ buffering power in the systolic range of [Ca2+]i.
Ca2+ buffering and arrhythmias
Ventricular arrhythmias constitute a major cause of death in FHC 93. The Knollmann group has investigated the underlying mechanisms in transgenic mice. Incorporation of mutations in troponin T or tropomyosin led to ventricular tachycardia. These mutations also sensitized the contractile machinery to activation by Ca2+ (Fig 5C) with those which produced the greatest incidence of ventricular tachycardias and arrhythmias having the greatest Ca-sensitizing effect 73. A causal link between Ca-sensitization and arrhythmogenesis was provided by showing both that EMD 57033 caused arrhythmias and the contractile uncoupler blebbistatin decreased both Ca-sensitivity of the contractile machinery and arrhythmia susceptibility. These arrhythmias were accompanied by a shortening and triangulation of the action potential as well as electrical repolarization alternans (see below). Subsequent work, using myocytes derived from human-induced pluripotent stem cells, reproduced these effects of increased Ca2+ buffering by myofilaments on action potential shape and suggested that the shortened, triangulated action potential could be due to increased buffering decreasing the amplitude of the systolic Ca2+ transient and thereby the inward (depolarizing) NCX current 94. While this is an attractive explanation, it is also worth noting that (see above), as well as decreasing the amplitude of the Ca2+ transient, increased buffering slows decay, making it harder to predict the net effect of increased buffering on NCX current. Another paper showed that, when regular pacing was terminated by a pause, the next Ca2+ transient was larger than control and this effect was more prominent in troponin T mutations which sensitize to activation by Ca2+ 23. This effect was attributed to a higher cell Ca2+ content in the mutant during stimulation with the excess Ca2+ being taken up by the SR such that release after a pause results in a prolonged action potential increasing the probability of an arrhythmogenic early afterdepolarization. Any increase of diastolic [Ca2+]i and consequent decrease of buffer power may also increase the rise of [Ca2+]i due to release from the SR. In the work reviewed above, the increase of buffering was a consequence of genetic changes. Similar increases of myofilament Ca2+ sensitivity have been reported following myocardial infarction 81 where manoeuvres that decrease Ca2+ sensitivity were found to abolish ventricular tachycardia following pauses of stimulation. As reviewed above, an increase of Ca2+ buffering is pro-arrhythmogenic. It has, however, also been shown that addition of the buffer EGTA can prevent the propagation of arrhythmogenic Ca2+ waves 95 and therefore the net effect may be more complicated.
The induction of alternans of the action potential duration by increased Ca2+ buffering due to thin filament mutations73 may be a consequence of the slowed decay of the Ca2+ transient resulting in incomplete recovery at the time of the next stimulus at increased rates. This would be analogous to the idea that the increased propensity of endo- compared to epicardium to alternans is associated with a more slowly decaying Ca2+ transient due to lower SERCA expression 59. In contrast, a modelling study has predicted that increasing cytoplasmic Ca2+ buffering should decrease the probability of alternans occurring by decreasing the probability that Ca2+ released from the SR induces further Ca2+ release from neighbouring release sites 96. This is consistent with the experimental demonstration, in whole mouse hearts, that addition of the buffer EGTA decreased the occurrence of alternans48 and may be related to the experimental observation that increasing cytoplasmic Ca2+ buffering decreases the frequency of propagating Ca2+ waves 97 and makes Ca2+ sparks terminate earlier 98. Clearly further work is required in understanding the relationship between Ca2+ buffering and alternans.
Why do cells have Ca2+ buffers?
A high level of Ca2+ buffering is not unique to cardiac myocytes. For example, about 99% of the Ca2+ entering chromaffin cells binds to cytoplasmic buffers 99 The presence of Ca2+ buffers means that much larger movements of total Ca2+ are required to produce a given change of [Ca2+]i. Given that calcium movements account for up to 30 % of the total energy consumption of the heart 100, one might wonder why evolution has resulted in such strong buffering? There are several explanations. (1) It may be an inescapable consequence of the fact that using Ca2+ as a second messenger requires high concentrations of Ca2+ binding proteins, for example to activate contraction. (2) A high Ca2+ buffering may stabilize Ca2+ signalling by stopping an abnormal increase of [Ca2+]i in one part of a cell propagating throughout the cell. In this context, it is worth noting that (Fig 2Aii), the dependence of buffer power on [Ca2+]i means that the buffer power is much lower in systole than diastole. This may help Ca2+ release during systole while protecting against it in diastole. (3) The need for buffering may relate to the low intracellular concentration of calcium. A diastolic concentration of 100 nM equates to 6.1016 ions per litre corresponding to a mean distance between ions of 0.25 µm. Soeller and Cannell 8 have modelled Ca2+ fluxes into the space between the transverse tubule and sarcoplasmic reticulum (dyad). They calculated that, at a concentration of 100 nM, each dyad would contain between 0.007 and 0.028 Ca2+ ions. At 10 µM, there will be between 0.7 and 2.8 ions. This would make it impossible to control [Ca2+]i in a stable manner as a single Ca2+ transported into or out of the space would result in an enormous fractional change of [Ca2+]I. As pointed out previously 35, at such low concentrations, chance will determine whether a transporter interacts with an ion. In contrast, if total Ca2+ is 100 times the free then there will be between 70 and 280 ions per cleft. (4). If troponin was the only buffer then virtually all the total Ca2+ would be bound to troponin irrespective of its Kd. This would make it impossible to change force by altering Kd since this requires other buffers to take up a fraction of the total Ca.
Conclusions
The concentration of buffered calcium in cytoplasm is two orders of magnitude greater than that of the free concentration and therefore the buffers have an enormous effect on calcium signalling. There is a need for more work investigating whether changes of buffer properties, either directly or secondary to changes of diastolic [Ca2+]i contribute to alterations of calcium handling and contractility. The limited human data reviewed above and extrapolation from animal models argues that changes of Ca2+ buffering are important in determining both inotropy and pro-arrhythmic status in conditions such as cardiomyopathies (DCM and HCM) and ischemic heart failure in both health and disease. Clarification will require more work on human tissue.
Acknowledgements
We are indebted to Francis Burton and Susan Wray for comments on an earlier version of the manuscript and to Quentin Lachaud for the design of Fig 1.
Sources of Funding
Work from the authors’ laboratories is supported by grants from the British Heart Foundation (PG/17/12/32847 to G.L. Smith and CH/2000004/12801 to D.A. Eisner)
Footnotes
Disclosures
None
References
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Eisner DA, Caldwell JL, Kistamás K, Trafford AW. Calcium and Excitation-Contraction Coupling in the Heart. Circulation Research. 2017;121:181–195. doi: 10.1161/circresaha.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 4.Williams RJP. The evolution of calcium biochemistry. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2006;1763:1139–1146. doi: 10.1016/j.bbamcr.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 5.Carafoli E, Krebs J. Why Calcium? How Calcium Became the Best Communicator. J Biol Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kretsinger RH, Nockolds CE. Carp Muscle Calcium-binding Protein: II. Structure determination and general description. Journal of Biological Chemistry. 1973;248:3313–3326. [PubMed] [Google Scholar]
- 7.Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys J. 2008;95:4597–4612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soeller C, Cannell MB. Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophys J. 1997;73:97–111. doi: 10.1016/S0006-3495(97)78051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson SP, Johnson JD, Potter JD. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+ Biophys J. 1981;34:559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao WD, Backx PH, Azan-Backx M, Marban E. Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle. Circ Res. 1994;74:408–415. doi: 10.1161/01.res.74.3.408. [DOI] [PubMed] [Google Scholar]
- 11.Shannon TR, Ginsburg KS, Bers DM. Reverse mode of the sarcoplasmic reticulum calcium pump and load-dependent cytosolic calcium decline in voltage-clamped cardiac ventricular myocytes. Biophys J. 2000;78:322–333. doi: 10.1016/S0006-3495(00)76595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Post JA, Langer GA. Sarcolemmal calcium binding sites in heart: I. Molecular origin in "gas-dissected" sarcolemma. J Membr Biol. 1992;129:49–57. doi: 10.1007/BF00232054. [DOI] [PubMed] [Google Scholar]
- 13.Pan BS, Solaro RJ. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J Biol Chem. 1987;262:7839–7849. [PubMed] [Google Scholar]
- 14.Picht E, Zima AV, Shannon TR, Duncan AM, Blatter LA, Bers DM. Dynamic calcium movement inside cardiac sarcoplasmic reticulum during release. Circ Res. 2011;108:847–856. doi: 10.1161/CIRCRESAHA.111.240234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swietach P, Youm J-B, Saegusa N, Leem C-H, Spitzer KW, Vaughan-Jones RD. Coupled Ca2+/H+ transport by cytoplasmic buffers regulates local Ca2+ and H+ ion signaling. Proc Natl Acad Sci U S A. 2013;110:E2064–2073. doi: 10.1073/pnas.1222433110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baran EJ. Metal complexes of carnosine. Biochemistry (Mosc) 2000;65:789–797. [PubMed] [Google Scholar]
- 17.Naraghi M. T-jump study of calcium binding kinetics of calcium chelators. Cell Calcium. 1997;22:255–268. doi: 10.1016/S0143-4160(97)90064-6. [DOI] [PubMed] [Google Scholar]
- 18.Smith PD, Liesegang GW, Berger RL, Czerlinski G, Podolsky RJ. A stopped-flow investigation of calcium ion binding by ethylene glycol bis(beta-aminoethyl ether)-N,N'-tetraacetic acid. Anal Biochem. 1984;143:188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki H, Kretsinger RH. Calcium-binding proteins. 1: EF-hands. Protein Profile. 1994;1:343–517. [PubMed] [Google Scholar]
- 20.Sipido KR, Wier WG. Flux of Ca2+ across the sarcoplasmic reticulum of guinea-pig cardiac cells during excitation-contraction coupling. J Physiol. 1991;435:605–630. doi: 10.1113/jphysiol.1991.sp018528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard EM, Solaro RJ. Inhibition of the activation and troponin calcium binding of dog cardiac myofibrils by acidic pH. Circ Res. 1984;55:382–391. doi: 10.1161/01.res.55.3.382. [DOI] [PubMed] [Google Scholar]
- 22.Robinson P, Griffiths PJ, Watkins H, Redwood CS. Dilated and hypertrophic cardiomyopathy mutations in troponin and alpha-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res. 2007;101:1266–1273. doi: 10.1161/CIRCRESAHA.107.156380. [DOI] [PubMed] [Google Scholar]
- 23.Schober T, Huke S, Venkataraman R, Gryshchenko O, Kryshtal D, Hwang HS, Baudenbacher FJ, Knollmann BC. Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ Res. 2012;111:170–179. doi: 10.1161/CIRCRESAHA.112.270041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson P, Liu X, Sparrow A, Patel S, Zhang Y-H, Casadei B, Watkins H, Redwood C. Hypertrophic cardiomyopathy mutations increase myofilament Ca2+ buffering, alter intracellular Ca2+ handling, and stimulate Ca2+-dependent signaling. Journal of Biological Chemistry. 2018;293:10487–10499. doi: 10.1074/jbc.RA118.002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqui JK, Tikunova SB, Walton SD, Liu B, Meyer M, de Tombe PP, Neilson N, Kekenes-Huskey PM, Salhi HE, Janssen PML, Biesiadecki BJ, et al. Myofilament Calcium Sensitivity: Consequences of the Effective Concentration of Troponin I. Front Physiol. 2016;7:632. doi: 10.3389/fphys.2016.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers FM, Solaro RJ. Caffeine alters cardiac myofilament activity and regulation independently of Ca2+ binding to troponin C. Am J Physiol. 1995;268:C1348–1353. doi: 10.1152/ajpcell.1995.268.6.C1348. [DOI] [PubMed] [Google Scholar]
- 27.Negele JC, Dotson DG, Liu W, Sweeney HL, Putkey JA. Mutation of the high affinity calcium binding sites in cardiac troponin C. J Biol Chem. 1992;267:825–831. [PubMed] [Google Scholar]
- 28.Higgins ER, Cannell MB, Sneyd J. A buffering SERCA pump in models of calcium dynamics. Biophys J. 2006;91:151–163. doi: 10.1529/biophysj.105.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J. 2004;87:3351–3371. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michailova A, DelPrincipe F, Egger M, Niggli E. Spatiotemporal features of Ca2+ buffering and diffusion in atrial cardiac myocytes with inhibited sarcoplasmic reticulum. Biophys J. 2002;83:3134–3151. doi: 10.1016/S0006-3495(02)75317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solaro RJ, Wise RM, Shiner JS, Briggs FN. Calcium requirements for cardiac myofibrillar activation. Circ Res. 1974;34:525–530. doi: 10.1161/01.res.34.4.525. [DOI] [PubMed] [Google Scholar]
- 32.Pierce GN, Philipson KD, Langer GA. Passive calcium-buffering capacity of a rabbit ventricular homogenate preparation. Am J Physiol. 1985;249:C248–255. doi: 10.1152/ajpcell.1985.249.3.C248. [DOI] [PubMed] [Google Scholar]
- 33.Hove-Madsen L, Bers DM. Passive Ca buffering and SR Ca uptake in permeabilized rabbit ventricular myocytes. Am J Physiol. 1993;264:C677–686. doi: 10.1152/ajpcell.1993.264.3.C677. [DOI] [PubMed] [Google Scholar]
- 34.Berlin JR, Bassani JW, Bers DM. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys J. 1994;67:1775–1787. doi: 10.1016/S0006-3495(94)80652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bers DM. Excitation-contraction coupling and cardiac contractile force. 2nd ed. Dordrecht. London: Kluwer Academic; 2001. [Google Scholar]
- 36.Trafford AW, Díaz ME, Eisner DA. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflugers Arch. 1999;437:501–503. doi: 10.1007/s004240050808. [DOI] [PubMed] [Google Scholar]
- 37.Kuratomi S, Matsuoka S, Sarai N, Powell T, Noma A. Involvement of Ca2+ buffering and Na+/Ca2+ exchange in the positive staircase of contraction in guinea-pig ventricular myocytes. Pflügers Archiv. 2003;446:347–355. doi: 10.1007/s00424-003-1023-1. [DOI] [PubMed] [Google Scholar]
- 38.Negretti N, O'Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc Res. 1993;27:1826–1830. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- 39.Trafford AW, Díaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000;522:259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briston SJ, Dibb KM, Solaro RJ, Eisner DA, Trafford AW. Balanced changes in Ca buffering by SERCA and troponin contribute to Ca handling during β-adrenergic stimulation in cardiac myocytes. Cardiovasc Res. 2014;104:347–354. doi: 10.1093/cvr/cvu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steenbergen C, Murphy E, Levy L, London RE. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ Res. 1987;60:700–707. doi: 10.1161/01.res.60.5.700. [DOI] [PubMed] [Google Scholar]
- 42.Harding DP, Smith GA, Metcalfe JC, Morris PG, Kirschenlohr HL. Resting and end-diastolic [Ca2+]i measurements in the Langendorff-perfused ferret heart loaded with a 19F NMR indicator. Magn Reson Med. 1993;29:605–615. doi: 10.1002/mrm.1910290505. [DOI] [PubMed] [Google Scholar]
- 43.Díaz ME, Trafford AW, Eisner DA. The effects of exogenous calcium buffers on the systolic calcium transient in rat ventricular myocytes. Biophys J. 2001;80:1915–1925. doi: 10.1016/S0006-3495(01)76161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yaniv Y, Stern Michael D, Lakatta Edward G, Maltsev Victor A. Mechanisms of Beat-to-Beat Regulation of Cardiac Pacemaker Cell Function by Ca2+ Cycling Dynamics. Biophys J. 2013;105:1551–1561. doi: 10.1016/j.bpj.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaestner L, Scholz A, Tian Q, Ruppenthal S, Tabellion W, Wiesen K, Katus HA, Muller OJ, Kotlikoff MI, Lipp P. Genetically encoded Ca2+ indicators in cardiac myocytes. Circ Res. 2014;114:1623–1639. doi: 10.1161/CIRCRESAHA.114.303475. [DOI] [PubMed] [Google Scholar]
- 46.Neher E. Usefulness and limitations of linear approximations to the understanding of Ca++ signals. Cell Calcium. 1998;24:345–357. doi: 10.1016/S0143-4160(98)90058-6. [DOI] [PubMed] [Google Scholar]
- 47.Sankaranarayanan R, Kistamas K, Greensmith DJ, Venetucci LA, Eisner DA. Systolic [Ca2+]i regulates diastolic levels in rat ventricular myocytes. J Physiol. 2017;595:5545–5555. doi: 10.1113/JP274366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kornyeyev D, Reyes M, Escobar AL. Luminal Ca2+ content regulates intracellular Ca2+ release in subepicardial myocytes of intact beating mouse hearts: effect of exogenous buffers. Am J Physiol Heart Circ Physiol. 2010;298:H2138–2153. doi: 10.1152/ajpheart.00885.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992;267:14483–14489. [PubMed] [Google Scholar]
- 50.Barcenas-Ruiz L, Beuckelmann DJ, Wier WG. Sodium-calcium exchange in heart: membrane currents and changes in [Ca2+]i. Science. 1987;238:1720–1722. doi: 10.1126/science.3686010. [DOI] [PubMed] [Google Scholar]
- 51.MacQuaide N, Dempster J, Smith GL. Measurement and modeling of Ca2+ waves in isolated rabbit ventricular cardiomyocytes. Biophys J. 2007;93:2581–2595. doi: 10.1529/biophysj.106.102293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gattoni S, Røe ÅT, Frisk M, Louch WE, Niederer SA, Smith NP. The calcium–frequency response in the rat ventricular myocyte: an experimental and modelling study. J Physiol. 2016;594:4193–4224. doi: 10.1113/JP272011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hunter PJ, McCulloch AD, ter Keurs HE. Modelling the mechanical properties of cardiac muscle. Prog Biophys Mol Biol. 1998;69:289–331. doi: 10.1016/s0079-6107(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 54.Díaz ME, Trafford AW, Eisner DA. The role of intracellular Ca buffers in determining the shape of the systolic Ca transient in cardiac ventricular myocytes. Pflugers Arch. 2001;442:96–100. doi: 10.1007/s004240000509. [DOI] [PubMed] [Google Scholar]
- 55.Wier WG, Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gen Physiol. 1984;83:395–415. doi: 10.1085/jgp.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sankaranarayanan R, Li Y, Greensmith DJ, Eisner DA, Venetucci L. Biphasic decay of the Ca transient results from increased sarcoplasmic reticulum Ca leak. J Physiol. 2016;594:611–623. doi: 10.1113/JP271473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eisner DA. Ups and downs of calcium in the heart. J Physiol. 2018;596:19–30. doi: 10.1113/JP275130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eisner DA, Dibb KM, Trafford AW. The mechanism and significance of the slow changes of ventricular action potential duration following a change of heart rate. Exp Physiol. 2009;94:520–528. doi: 10.1113/expphysiol.2008.044008. [DOI] [PubMed] [Google Scholar]
- 59.Laurita KR, Katra R, Wible B, Wan X, Koo MH. Transmural Heterogeneity of Calcium Handling in Canine. Circulation Research. 2003;92:668–675. doi: 10.1161/01.res.0000062468.25308.27. [DOI] [PubMed] [Google Scholar]
- 60.Soltysinska E, Olesen SP, Christ T, Wettwer E, Varro A, Grunnet M, Jespersen T. Transmural expression of ion channels and transporters in human nondiseased and end-stage failing hearts. Pflugers Arch. 2009;459:11–23. doi: 10.1007/s00424-009-0718-3. [DOI] [PubMed] [Google Scholar]
- 61.Kirchberber MA, Tada M, Katz AM. Phospholamban: a regulatory protein of the cardiac sarcoplasmic reticulum. Recent Adv Stud Cardiac Struct Metab. 1975;5:103–115. [PubMed] [Google Scholar]
- 62.Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982;257:260–263. [PubMed] [Google Scholar]
- 63.Choi HS, Trafford AW, Orchard CH, Eisner DA. The effect of acidosis on systolic Ca2+ and sarcoplasmic reticulum calcium content in isolated rat ventricular myocytes. J Physiol. 2000;529:661–668. doi: 10.1111/j.1469-7793.2000.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyman L, Hagen BM, Giladi M, Hiller R, Lederer WJ, Khananshvili D. Proton-sensing Ca2+ binding domains regulate the cardiac Na+/Ca2+ exchanger. J Biol Chem. 2011;286:28811–28820. doi: 10.1074/jbc.M110.214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol. 2009;46:463–473. doi: 10.1016/j.yjmcc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Clarke JD, Caldwell JL, Pearman CM, Eisner DA, Trafford AW, Dibb KM. Increased Ca buffering underpins remodelling of Ca2+ handling in old sheep atrial myocytes. J Physiol. 2017;595:6263–6279. doi: 10.1113/JP274053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trafford AW, Clarke JD, Richards MA, Eisner DA, Dibb KM. Calcium signalling microdomains and the t-tubular system in atrial mycoytes: Potential roles in cardiac disease and arrhythmias. Cardiovasc Res. 2013;98:192–203. doi: 10.1093/cvr/cvt018. [DOI] [PubMed] [Google Scholar]
- 68.Blatter LA, Kockskämper J, Sheehan KA, Zima AV, Hüser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol. 2003;546:19–31. doi: 10.1113/jphysiol.2002.025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bootman MD, Higazi DR, Coombes S, Roderick HL. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J Cell Sci. 2006;119:3915–3925. doi: 10.1242/jcs.03223. [DOI] [PubMed] [Google Scholar]
- 70.Sheehan KA, Blatter LA. Regulation of junctional and non-junctional sarcoplasmic reticulum calcium release in excitation-contraction coupling in cat atrial myocytes. J Physiol. 2003;546:119–135. doi: 10.1113/jphysiol.2002.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greiser M, Kerfant BG, Williams GS, Voigt N, Harks E, Dibb KM, Giese A, Meszaros J, Verheule S, Ravens U, Allessie MA, et al. Tachycardia-induced silencing of subcellular Ca2+ signaling in atrial myocytes. J Clin Invest. 2014;124:4759–4772. doi: 10.1172/JCI70102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wahr PA, Michele DE, Metzger JM. Parvalbumin gene transfer corrects diastolic dysfunction in diseased cardiac myocytes. Proc Natl Acad Sci U S A. 1999;96:11982–11985. doi: 10.1073/pnas.96.21.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008;118:3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clarke JD, Caldwell JL, Horn MA, Bode EF, Richards MA, Hall MCS, Graham HK, Briston SJ, Greensmith DJ, Eisner DA, Dibb KM, et al. Perturbed atrial calcium handling in an ovine model of heart failure: Potential roles for reductions in the L-type calcium current. J Mol Cell Cardiol. 2015;79:169–179. doi: 10.1016/j.yjmcc.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, Trafford AW. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail. 2009;2:482–489. doi: 10.1161/CIRCHEARTFAILURE.109.852228. [DOI] [PubMed] [Google Scholar]
- 76.Macquaide N, Tuan HT, Hotta J, Sempels W, Lenaerts I, Holemans P, Hofkens J, Jafri MS, Willems R, Sipido KR. Ryanodine receptor cluster fragmentation and redistribution in persistent atrial fibrillation enhance calcium release. Cardiovasc Res. 2015;108:387–398. doi: 10.1093/cvr/cvv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hobai IA, O'Rourke B. Enhanced Ca2+-activated Na+-Ca2+ exchange activity in canine pacing-induced heart failure. Circ Res. 2000;87:690–698. doi: 10.1161/01.res.87.8.690. [DOI] [PubMed] [Google Scholar]
- 78.Briston SJ, Caldwell JL, Horn MA, Clarke JD, Richards MA, Greensmith DJ, Graham HK, Hall MCS, Eisner DA, Dibb KM, Trafford AW. Impaired β-adrenergic responsiveness accentuates dysfunctional excitation-contraction coupling in an ovine model of tachypacing-induced heart failure. J Physiol. 2011;589:1367–1382. doi: 10.1113/jphysiol.2010.203984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest. 1996;98:167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolff MR, Whitesell LF, Moss RL. Calcium sensitivity of isometric tension is increased in canine experimental heart failure. Circ Res. 1995;76:781–789. doi: 10.1161/01.res.76.5.781. [DOI] [PubMed] [Google Scholar]
- 81.Venkataraman R, Baldo MP, Hwang HS, Veltri T, Pinto JR, Baudenbacher FJ, Knollmann BC. Myofilament calcium de-sensitization and contractile uncoupling prevent pause-triggered ventricular tachycardia in mouse hearts with chronic myocardial infarction. J Mol Cell Cardiol. 2013;60:8–15. doi: 10.1016/j.yjmcc.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Waard MC, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje NM, Dekkers DH, Schoonderwoerd K, Schuurbiers HC, de Crom R, Stienen GJ, et al. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res. 2007;100:1079–1088. doi: 10.1161/01.RES.0000262655.16373.37. [DOI] [PubMed] [Google Scholar]
- 83.Belin RJ, Sumandea MP, Kobayashi T, Walker LA, Rundell VL, Urboniene D, Yuzhakova M, Ruch SH, Geenen DL, Solaro RJ, de Tombe PP. Left ventricular myofilament dysfunction in rat experimental hypertrophy and congestive heart failure. Am J Physiol Heart Circ Physiol. 2006;291:H2344–2353. doi: 10.1152/ajpheart.00541.2006. [DOI] [PubMed] [Google Scholar]
- 84.Solaro RJ, van der Velden J. Why does troponin I have so many phosphorylation sites? Fact and fancy. J Mol Cell Cardiol. 2010;48:810–816. doi: 10.1016/j.yjmcc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marjot J, Kaier TE, Martin ED, Reji SS, Copeland ON, Iqbal M, Goodson B, Hamren S, Harding SE, Marber MS. Quantifying the Release of Biomarkers of Myocardial Necrosis from Cardiac Myocytes and Intact Myocardium. Clinical Chem. 2017;63:990–996. doi: 10.1373/clinchem.2016.264648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–784. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- 87.Rodenbaugh DW, Wang W, Davis J, Edwards T, Potter JD, Metzger JM. Parvalbumin isoforms differentially accelerate cardiac myocyte relaxation kinetics in an animal model of diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2007;293:H1705–1713. doi: 10.1152/ajpheart.00232.2007. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J, Shettigar V, Kindell D, Liu X, Lopez J, Yerrimuni V, Davis G, Davis J. Engineering Parvalbumin for the Heart: Optimizing the Mg2+ Binding Properties of Rat β-Parvalbumin. Front Physiol. 2011;2:77. doi: 10.3389/fphys.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asp ML, Sjaastad FV, Siddiqui JK, Davis JP, Metzger JM. Effects of Modified Parvalbumin EF-Hand Motifs on Cardiac Myocyte Contractile Function. Biophys J. 2016;110:2094–2105. doi: 10.1016/j.bpj.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Zhang L, Jean-Charles PY, Nan C, Chen G, Tian J, Jin JP, Gelb IJ, Huang X. Dose-dependent diastolic dysfunction and early death in a mouse model with cardiac troponin mutations. J Mol Cell Cardiol. 2013;62:227–236. doi: 10.1016/j.yjmcc.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coppini R, Mazzoni L, Ferrantini C, Gentile F, Pioner JM, Laurino A, Santini L, Bargelli V, Rotellini M, Bartolucci G, Crocini C, et al. Ranolazine Prevents Phenotype Development in a Mouse Model of Hypertrophic Cardiomyopathy. Circ Heart Fail. 2017;10 doi: 10.10.1161/CIRCHEARTFAILURE.116.003565. pii: e003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swindle N, Tikunova SB. Hypertrophic cardiomyopathy-linked mutation D145E drastically alters calcium binding by the C-domain of cardiac troponin C. Biochemistry. 2010;49:4813–4820. doi: 10.1021/bi100400h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Kryshtal DO, Kim K, Parikh S, Cadar AG, Bersell KR, He H, Pinto JR, Knollmann BC. Myofilament Calcium-Buffering Dependent Action Potential Triangulation in Human-Induced Pluripotent Stem Cell Model of Hypertrophic Cardiomyopathy. J Am Coll Cardiol. 2017;70:2600–2602. doi: 10.1016/j.jacc.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacQuaide N, Ramay HR, Sobie EA, Smith GL. Differential sensitivity of Ca2+ wave and Ca2+ spark events to ruthenium red in isolated permeabilised rabbit cardiomyocytes. J Physiol. 2010;588:4731–4742. doi: 10.1113/jphysiol.2010.193375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nivala M, Qu Z. Calcium alternans in a couplon network model of ventricular myocytes: role of sarcoplasmic reticulum load. Am J Physiol Heart Circ Physiol. 2012;303:H341–H352. doi: 10.1152/ajpheart.00302.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nivala M, Ko Christopher Y, Nivala M, Weiss James N, Qu Z. Criticality in Intracellular Calcium Signaling in Cardiac Myocytes. Biophys J. 2012;102:2433–2442. doi: 10.1016/j.bpj.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bovo E, Mazurek SR, Fill M, Zima AV. Cytosolic Ca2+ buffering determines the intra-SR Ca2+ concentration at which cardiac Ca2+ sparks terminate. Cell Calcium. 2015;58:246–253. doi: 10.1016/j.ceca.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Neher E, Augustine GJ. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gibbs CL, Loiselle DS, Wendt IR. Activation heat in rabbit cardiac muscle. J Physiol. 1988;395:115–130. doi: 10.1113/jphysiol.1988.sp016911. [DOI] [PMC free article] [PubMed] [Google Scholar]