Abstract
Aims/hypothesis
Season of birth as a surrogate for potential environmental exposure during fetal development and early postnatal life has shown inconsistent association with adult type 2 diabetes in white populations living in high latitude regions. The present study aimed to examine the association between birth seasonality and the risk of adult type 2 diabetes in Chinese living across wide regions of low-latitude and lower- to middle-latitude.
Methods
Participants from the China Kadoorie Biobank were enrolled during 2004-2008 and followed up until 31 December 2013. After excluding participants with cancer, heart disease, stroke, and diabetes at baseline, the present study included 189,153 men and 272,058 women aged 30-79 years. We used multivariable Cox proportional hazards model to estimate the HR and 95% CI.
Results
During a median follow-up of 7.2 years (3.3 million person-years), we documented 8,784 incident cases of type 2 diabetes. In the whole cohort, compared with Summer-born participants, the adjusted HRs (95% CIs) were 1.09 (1.02, 1.16), 1.08 (1.02, 1.15), and 1.09 (1.02, 1.15) for those who were born in Spring, Autumn, and Winter respectively. The association was consistent in both men and women and across subgroups defined by residence and lifestyle factors later in life.
Conclusions/interpretation
In this large prospective study, participants born in Summer had a lower risk of adult type 2 diabetes compared with other seasons of birth, suggesting exposures in early life with some degree of seasonal variation might influence the risk of adult diabetes.
Keywords: Fetal development, Seasons, Type 2 diabetes
Introduction
There are 387 million people living with diabetes and 4.9 million attributable deaths in 2014 around the world [1]. In China, the rapid increasing prevalence of diabetes reached 9.7% in 2010 [2]. Of all cases of diabetes, type 2 diabetes represents about 85-95% [1]. There is a growing interest in the contribution of prenatal environmental exposure to the risk of type 2 diabetes in adulthood. It has been suggested that adverse environmental influences during critical periods of prenatal growth could permanently change the structure and function of organs and tissues, leading to increased susceptibility to the development of type 2 diabetes in later life [3].
Besides special famine events, the season or month of birth often serves as a surrogate for potential environmental exposure during perinatal life. Factors exhibiting seasonal variation include but are not limited to sunlight exposure, food availability and eating habits, and outdoor physical activity [4–8]. Only a few studies conducted in the white populations living in relatively high latitude regions have examined the association between the season or month of birth and type 2 diabetes in adulthood and got mixed results [9–11].
In the present study, we prospectively examined the association between season of birth and the risk of type 2 diabetes in adulthood in the China Kadoorie Biobank (CKB) study of 0.5 million adults, a population living across wide regions of low-latitude and lower- to middle-latitude. We additionally assessed whether several lifestyle factors later in life might modify the association between early life exposures and later risk of type 2 diabetes.
Methods
Study population
Further details of the CKB have been described elsewhere [12,13]. Briefly, a total of 512,891 participants aged 30-79 years were recruited in 2004-08 from ten geographically diverse survey sites across China, five urban sites (Harbin, Qingdao, Suzhou, Liuzhou, and Haikou) and five rural sites (Henan, Gansu, Sichuan, Zhejiang, and Hunan). The enrolment rate of population was comparable across latitudes and between urban or rural sites, with almost 1 in 3 (33% in rural areas and 27% in urban areas) responded. The study protocol was approved by the Ethics Review Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (UK). All participants provided written informed consent before taking part in the study.
In the present analysis, participants with major chronic diseases at baseline were excluded due to potential changes in lifestyle and subsequent risk of diabetes. We excluded participants who reported prior medical histories of heart disease (n=15,472), stroke (n=8,884), or cancer (n=2,577), had prevalent diabetes (n=30,300) based on self-reported or glucose testing at baseline (fasting glucose of 7.0 mmol/l or more, or random glucose of 11.1 mmol/l or more). Such exclusion was conducted to prevent incidence-prevalence bias and rule out the possibility of reverse causation for adjusted covariates including lifestyle and adiposity measures, and diabetes. We also excluded three people who were lost to follow-up shortly after baseline and two people with missing BMI. After these exclusions, 461,211 participants remained for the final analyses.
Assessment of exposure and covariates
All participants reported their date of birth at baseline. We categorized the month of birth into Spring (March, April, and May), Summer (June, July, and August), Autumn (September, October, and November), and Winter (December, January, and February).
Covariate information was obtained from baseline questionnaire, including socio-demographic status (age, sex, education, and marital status), lifestyle behaviors (tobacco smoking, alcohol consumption, physical activity, and intakes of fresh vegetables, fruits, and red meat), body weight at 25 years, family history of diabetes, and women’s menopausal status.
Trained staff measured body weight, standing height, sitting height, and waist circumference (WC) using standard protocol and calibrated instruments. BMI was calculated as weight in kilograms divided by the square of the standing height in meters. Weight change since 25 years of age was calculated as the difference between measured weight at baseline and weight at 25 years old. The length of the leg was calculated as the difference between standing and sitting height.
Ascertainment of incident type 2 diabetes
Incident cases of type 2 diabetes were identified by means of linkage with local disease and death registries, with the recently established national health insurance (HI) system, and by active follow-up (i.e., visiting local communities or directly contacting participants). The electronic linkage with the HI claim databases is one of the most important means of ascertaining incident cases of diabetes and has been achieved for 95% of the participants in 2013. Both urban and rural participants and different latitude of areas had similar proportions of successful linkage to HI databases. For the present analysis, we included diabetes cases coded by the ICD-10 as E11 and E14. Other cases clearly defined as non-type 2 diabetes were excluded. Although misclassification from other types of diabetes may exist, the number of any non-type 2 diabetes is small as the majority of our participants were aged over 40 years. The diagnosis validity of incident diabetes was adjudicated in a random sample of 831 reported cases from all ten survey sites, with review of hospital medical records. Overall, 98.6% diagnoses of diabetes were confirmed.
Statistical analysis
Participants contributed person-time from baseline until the date of diagnosis of diabetes, death, loss to follow-up, or December 31, 2013, whichever came first. We used multivariable Cox proportional hazards model to estimate the HR and 95% CI, with age as the underlying time scale, and stratified by 5-year age groups (age at baseline) and ten survey sites.
Multivariable models for association between season or month of birth and the risk of diabetes in adulthood were adjusted for age (years); sex (male or female, for whole cohort); level of education (no formal school, primary school, middle school, high school, college, or university or higher); marital status (married, widowed, divorced or separated, or never married); alcohol consumption (not weekly drinker, weekly but not daily drinker, daily drinker with <15, 15 to 29, 30 to 59, or ⩾25 grams per day); smoking status (never or occasional smoker, former smoker having quit smoking ⩾5, or <5 years, or current daily smoker with <15, 15 to 24, 30 to 59, or ⩾25 cigarettes per day); physical activity (metabolic equivalent of task- h/day)(MET-h/day); intake frequencies of red meat, fresh fruits, and vegetables (daily, 4 to 6 days/week, 1 to 3 days/week, monthly, or rarely or never); family history of diabetes (presence, absence, or unknown); and menopausal status (premenopausal, perimenopausal, or postmenopausal; for women only). We further explored whether the association between season of birth and the risk of diabetes in adulthood was confounded or mediated by BMI at baseline. We additionally adjusted for weight gain since 25 years as a sensitivity analysis. To control for potential confounding effects of early life factors, we also additionally adjusted for leg length, a biomarker of early life conditions [14,15]. The risk estimates did not change materially (data not shown).
We also conducted analyses stratified according to prespecified baseline subgroups: residence (urban, or rural sites), latitude (seven sites in middle latitudes, or three sites in low latitudes, with 30 degrees north laitude as the dividing line), age at baseline (<50, 50 to 59, or ≥60 years), smoking status (daily smoker, or not), alcohol consumption (weekly drinker, or not), level of physical activity (categorized using tertile cutoffs), BMI (<24.0, 24.0 to 27.9, or ≥28.0 kg/m2), WC (central obesity with men ≥ 85 and women ≥80 cm, or not), and weight gain since 25 years (<2.5, 2.5 to 9.9, or ≥10.0 kg).
We used Stata version 14.0 (StataCorp, Texas, USA) to analyze data. Statistical significance was set at two-tailed p<0.05.
Results
Of all participants, 41.0% (n=189,153) were men, and 57.8% (n=266,352) resided in rural areas. Table 1 presents the baseline characteristics of the participants according to the season of birth. Participants who were born in Spring and Summer had higher measures of adiposity and a larger increase in weight since 25 years.
Table 1. Baseline characteristics according to the season of birth among 461,211 participants.
| Characteristics | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| No. of participants | 105,779 | 112,042 | 128,548 | 114,842 |
| Age (years) | 50.4 (10.4) | 50.7 (10.4)a | 50.8 (10.5)a | 51.0 (10.6) |
| Rural area (%) | 58.7a | 57.7 | 56.5 | 58.3a |
| Married (%) | 91.1a | 91.2a | 91.2a | 90.9a |
| Middle school and higher (%) | 49.6abc | 48.9 | 49.3ab | 49.7ac |
| Daily smoker (%) | 26.9a | 27.1a | 27.1a | 26.9a |
| Weekly drinker (%) | 15.2a | 15.3a | 15.3a | 15.0a |
| Physical activity (MET-h/day) Weekly consumptiond |
21.9 (13.9)a | 21.9 (14.0)a | 21.8 (14.0)b | 21.8 (13.9)ab |
| Red meat (day) | 3.69 (2.54)abc | 3.69 (2.53)ab | 3.71 (2.52)abc | 3.71 (2.51)ac |
| Fresh vegetables (day) | 6.83 (0.83)a | 6.84 (0.78)a | 6.84 (0.77)a | 6.84 (0.75)a |
| Fresh fruits (day) | 2.56 (2.48)a | 2.55 (2.46)a | 2.56 (2.48)a | 2.56 (2.47)a |
| Postmenopausal (%)e | 48.8 | 49.2a | 49.4a | 49.2a |
| Family history of diabetes (%) | 9.2a | 9.1a | 9.0a | 9.2a |
| Body mass index (kg·m-2) | 23.58 (3.35)a | 23.55 (3.35)a | 23.50 (3.30)b | 23.48 (3.31)b |
| Waist circumference (cm) | 80.0 (9.6) | 79.8 (9.6) | 79.6 (9.5)a | 79.6 (9.6)a |
| Weight change since 25 years (kg)f | 4.8 (8.9)a | 4.8 (8.9)a | 4.6 (8.8)b | 4.5 (8.9)b |
Values are mean (standard deviation) unless otherwise stated. All variables were adjusted for age, sex, and survey sites, as appropriate.
Percentages or means sharing the same letter was not significantly different at Bonferroni-corrected level of significance. Bonferroni adjusted significance threshold was 0.008, based on six tests (pairwise comparison between four birth seasons).
Weekly consumptions of red meat, fresh vegetables, and fruits were calculated by assigning participants to the midpoint of their consumption category
Among 272,059 female participants
N=386,753
MET, metabolic equivalent of task
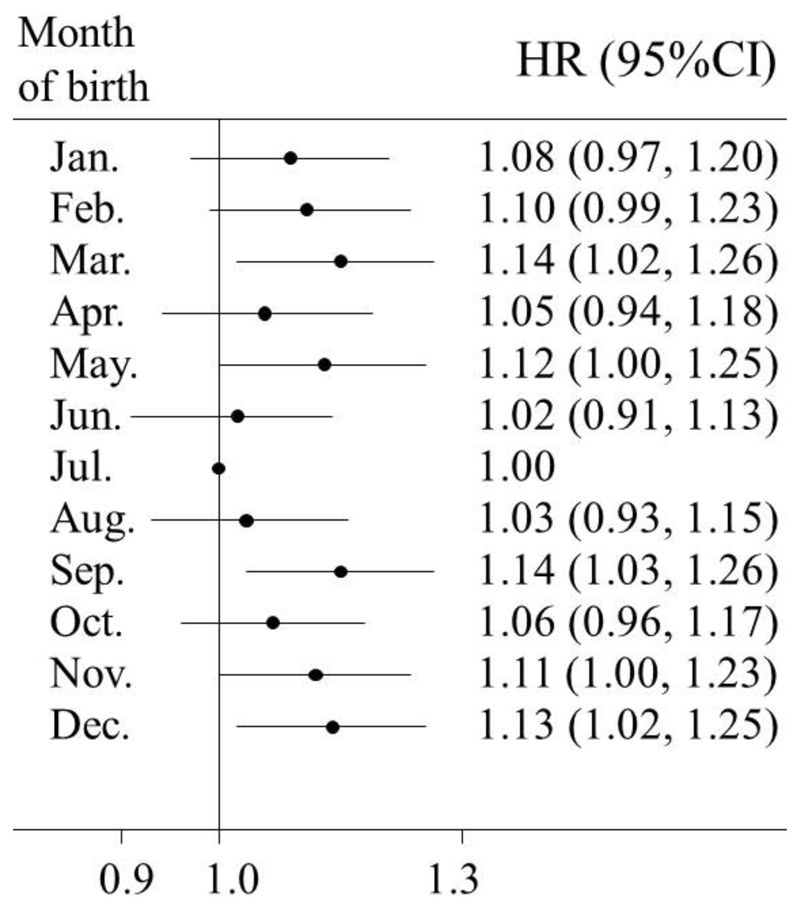
During a median of 7.2 years (interquartile range 1.88 years; 3.3 million person-years) of follow-up, we documented 3,259 incident cases of type 2 diabetes among men and 5,525 among women. In multivariable-adjusted models, the season of birth was significantly associated with the risk of incident type 2 diabetes. Further adjustment for BMI at baseline did not change the association materially. In all eligible participants, compared with participants who were born in Summer, the adjusted HRs (95% CIs) for the risk of type 2 diabetes in adulthood were 1.09 (1.02,1.16), 1.08 (1.02, 1.15), and 1.09 (1.02, 1.15) for those who were born in Spring, Autumn, and Winter respectively (Table 2). The combined HR (95% CI) for seasons of birth other than Summer was 1.09 (1.03, 1.14); the respective HRs (95% CIs) were 1.11 (1.02, 1.20) among men and 1.07 (1.01, 1.14) among women. No statistically significant difference between men and women was observed in the association between the season of birth and the risk of type 2 diabetes (p=0.670 for interaction between four seasons of birth and sex; p=0.704 for interaction between two groups of seasons of birth and sex). Fig. 1 further presents the association between month of birth and the risk of diabetes. Despite small fluctuation in some individual months, we observed a similar seasonal variation with participants born in Summer having the lowest risk of diabetes in adulthood.
Table 2. HR (95% CI) for incident type 2 diabetes by the season of birth among 461,211 participants.
| Spring | Summer | Autumn | Winter | Combined category of Spring, Autumn, and Wintera | |
|---|---|---|---|---|---|
| Whole cohort | |||||
| No. of person-years | 753,382 | 800,360 | 918,565 | 819,588 | 2,491,535 |
| No. of cases | 1,971 | 1,996 | 2,523 | 2,294 | 6,788 |
| Age adjusted | 1.09 (1.02, 1.16) | 1.00 | 1.07 (1.01, 1.14) | 1.07 (1.01, 1.14) | 1.08 (1.02, 1.13) |
| Multivariable adjustedb | 1.09 (1.02, 1.16) | 1.00 | 1.07 (1.01, 1.14) | 1.07 (1.01, 1.14) | 1.08 (1.02, 1.13) |
| +BMI at baseline | 1.09 (1.02, 1.16) | 1.00 | 1.08 (1.02, 1.15) | 1.09 (1.02, 1.15) | 1.09 (1.03, 1.14) |
| Men | |||||
| No. of person-years | 301,255 | 323,106 | 375,124 | 337,313 | 1,013,691 |
| No. of cases | 727 | 721 | 966 | 845 | 2,538 |
| Age adjusted | 1.11 (1.00, 1.23) | 1.00 | 1.12 (1.02, 1.24) | 1.07 (0.97, 1.18) | 1.10 (1.01, 1.19) |
| Multivariable adjustedb | 1.10 (0.99, 1.22) | 1.00 | 1.12 (1.02, 1.23) | 1.06 (0.96, 1.18) | 1.10 (1.01, 1.19) |
| +BMI at baseline | 1.10 (0.99, 1.22) | 1.00 | 1.13 (1.03, 1.25) | 1.08 (0.98, 1.20) | 1.11 (1.02, 1.20) |
| Women | |||||
| No. of person-years | 452,127 | 477,255 | 543,441 | 482,275 | 1,477,844 |
| No. of cases | 1,244 | 1,275 | 1,557 | 1,449 | 4,250 |
| Age adjusted | 1.08 (1.00, 1.17) | 1.00 | 1.05 (0.97, 1.13) | 1.07 (0.99, 1.16) | 1.07 (1.00, 1.13) |
| Multivariable adjustedb | 1.08 (1.00, 1.17) | 1.00 | 1.05 (0.98, 1.13) | 1.07 (1.00, 1.16) | 1.07 (1.00, 1.14) |
| +BMI at baseline | 1.08 (1.00, 1.17) | 1.00 | 1.06 (0.98, 1.14) | 1.09 (1.01, 1.17) | 1.07 (1.01, 1.14) |
Reference group: Summer-born participants.
Multivariable model was adjusted for: age (years); sex (male or female, for whole cohort); level of education (no formal school, primary school, middle school, high school, college, or university or higher); marital status (married, widowed, divorced or separated, or never married); alcohol consumption (not weekly drinker, weekly but not daily drinker, daily drinker with <15, 15 to 29, 30 to 59, or ≥25 grams per day); smoking status (never or occasional smoker, former smoker having quit smoking ≥5, or <5 years, or current daily smoker with <15, 15 to 24, 30 to 59, or ≥25 cigarettes per day); physical activity (MET-hr/day); intake frequencies of red meat, fresh fruits, and vegetables (daily, 4 to 6 days/week, 1 to 3 days/week, monthly, or rarely or never); family history of diabetes (presence, absence, or unknown); and menopausal status (premenopausal, perimenopausal, or postmenopausal; for women only).
Fig. 1.
HRs and 95% CIs for incident type 2 diabetes in adulthood according to the month of birth. Horizontal lines represent 95% CIs. July is the reference month, thus CI was not presented. Multivariable model was adjusted for: age; sex; level of education; marital status; alcohol consumption; smoking status; physical activity; intake frequencies of red meat, fresh fruits, and vegetables; family history of diabetes; and BMI at baseline.
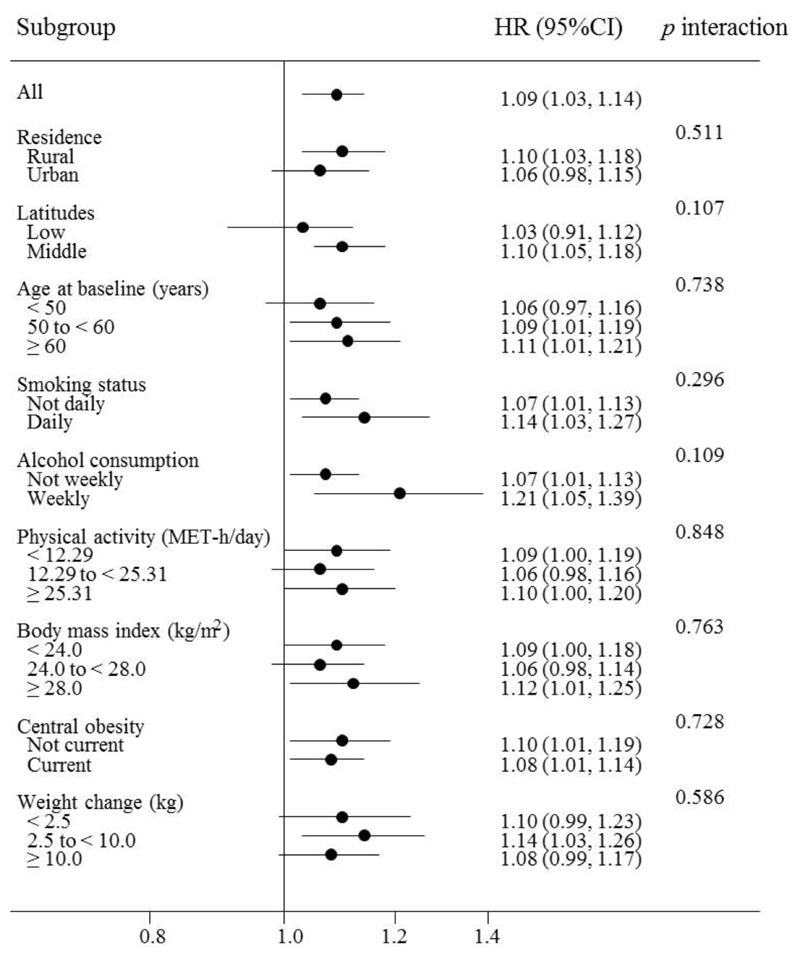
In subgroup analyses, we further examined the consistency of the association between season of birth and the risk of diabetes among different subpopulations defined by multiple baseline characteristics of the participants. The positive association was generally similar across subgroups stratified according to the rural/urban residence, residence in different latitudes, age, smoking status, alcohol consumption, the level of physical activity, BMI, WC, and weight gain since 25 years (all p values >0.05 for interaction) (Fig. 2 and ESM Table 1).
Fig. 2.
Subgroup analysis of the association between season of birth and the risk of type 2 diabetes in adulthood among the whole cohort according to potential baseline risk factors. Horizontal lines represent 95% CIs. HRs and 95%CIs are for comparison of combined category of Spring-, Autumn-, and Winter-born participants with Summer-born. Multivariable model was adjusted for: age; sex; level of education; marital status; alcohol consumption; smoking status; physical activity; intake frequencies of red meat, fresh fruits, and vegetables; family history of diabetes; and BMI at baseline. The tests for interaction were performed using likelihood ratio tests, which involved comparing models with and without cross-product terms between the baseline stratifying variable and the season of birth. Subgroup analysis according to weight change since 25 years: N=386,753.
Discussion
In this large prospective cohort of the Chinese adults, we found that seasons of birth other than Summer were associated with an increased risk of type 2 diabetes in adulthood. Compared with Summer-born participants, those who born in other seasons had a 9% higher risk of diabetes. The association was consistent in both men and women and across subgroups defined by residence and lifestyle factors later in life.
To our knowledge, only three previous studies conducted in European populations have examined the association between birth seasonality and the risk of type 2 diabetes in adulthood and showed inconsistent results. Findings from a Dutch hospital-based series study consisting 282 type 2 diabetes patients aged 30-90 years showed an excess of diabetes births in the first quarter of the year and a deficiency of them during the last one when comparing the month-of-birth with the standard birth curve [9]. In another study conducted in three Ukraine regions, a variation of the season of birth in 52,214 type 2 diabetes cases born before 1960 was observed with a peak in April and a nadir between November and December, compared with the month-of-birth patterns in the general population [10]. The only prospective study conducted in a Danish population-based cohort of 223,099 adults born between 1930 and 1989 showed no association between birth seasonality and the risk of type 2 diabetes [11]. The present study has found different results from those reported above, especially from that of Danish study with comparable study design. The explanation for this difference in results is not clear and might include the differences in population characteristics, geographic location, and adjustment for potential confounding factors. For example, the Danish population resides at a relatively high latitude and experiences less seasonal variations, thus fewer variations in environmental factors, than in our population.
The relation between season of birth and type 2 diabetes is inconsistent with our previous findings in the same population that showed Spring and early Summer peak and Winter trough for BMI and WC [16]. Low exposure to ultraviolet B and subsequently reduced level of vitamin D during the late second and early third trimesters have been suggested to explain the association between season of birth and adult adiposity measures. In addition, in the present study the risk estimates of type 2 diabetes did not change materially after adjusting for BMI at baseline or weight gain since 25 years, suggesting that adult adiposity did not mediate the effects of season of birth on the diabetes risk and other mechanisms might work.
Impaired fetal nutrition in late gestation has been linked to insulin resistance and type 2 diabetes in adulthood through permanent changes in the function of pancreatic beta cells or in the sensitivity of tissues to insulin [17–19]. Although the nutritional challenge due to seasonal variation is not as extreme as the one led by famine events, the variations in food variety, the nutritional value of food, and eating habits across seasons were still evident a few decades ago, especially in rural regions of China. It is possible that Summer-born participants experienced a richer nutritional environment in their late prenatal period and showed a lower risk of type 2 diabetes in adulthood than those born in other seasons. The season or month of birth might also be indicative of some other factors across a time span from periconception to early postnatal period. For example, mothers’ breastfeeding behavior may vary in babies who are born in different seasons [20] and has also be linked to the risk of type 2 diabetes in offspring [21]. However, it cannot explain what we observed in the CKB population. Further studies are warranted to explore the underlying mechanisms that link the season of birth and type 2 diabetes in adulthood.
To the best of our knowledge, this is by far the largest prospective study assessing the association between season of birth and the risk of type 2 diabetes in adulthood. The present study was conducted across wide regions of low-latitude and lower- to middle-latitude. This study acknowledges some limitations. We did not collect information about participants’ exposures and medical conditions during prenatal and postnatal periods due to lack of documented perinatal health records for this generation of the Chinese population and potential recall bias for self-reported information. Confounding by unmeasured factors, like birth weight, maternal nutrition during pregnancy, parental socioeconomic status, and breastfeeding was still possible. In addition, the incident cases of diabetes in this study were mainly identified using linkage with the health insurance system. Thus underestimation of the incidence of type 2 diabetes might have occurred because some asymptomatic cases of diabetes were missed. However, the nondifferential outcome misclassification on the season of birth might result in attenuation of the effect estimates. Covariate information such as lifestyle behaviors and body weight at 25 years was self-reported. Measurement error in potential confounders might result in residual confounding. We excluded prevalent cases of diabetes at baseline to prevent incidence-prevalence bias and rule out the possibility of reverse causation. However, we acknowledge that such exclusion might limit the generalizability of the results to early onset type 2 diabetes.
Conclusion
In this large prospective study of Chinese adults, we found that participants born in Summer had a lower risk of type 2 diabetes in adulthood compared with other seasons of birth. The results provide clues that exposures in early life with some degree of seasonal variation might influence the risk of diabetes in adulthood. Further studies would be essential to validate our findings and clarify the underlying mechanisms.
Acknowledgments
The most important acknowledgment is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford and the 10 regional centres.
Funding This work was supported by grants (81373082, 81390544) from the National Natural Science Foundation of China. The CKB baseline survey was supported by a grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up is supported by grants from the UK Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z).
Abbreviations
- CKB
The China Kadoorie Biobank
- MET
Metabolic equivalent of task
- WC
Waist circumference
Footnotes
For a list of members of the China Kadoorie Biobank Collaborative Group, please see the ESM.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement JL, LL, and ZC obtained funding. JL and LL conceived and designed the study. YG, ZB, LY, YC, HS, BY, JC, and ZC acquired the data. JS, XL, and CY analyzed the data. JS and CY drafted the manuscript. JL, LL, ZC, YG, ZB, LY, YC, HS, BY, JC, and XL contributed to the interpretation of the results and revised the manuscript critically for important intellectual content. All authors read and approved the final version of the manuscript to be published. LL and JL are the guarantors of this work, and, as such, had full access to all the data in the study and take responsbility for the integrity of the data and the accuracy of the data analysis.
Data availability Details of how to access China Kadoorie Biobank data and details of the data release schedule are available from www.ckbiobank.org/site/Data+Access.
References
- 1.International Diabetes Federation. IDF diabetes atlas update 2012. [accessed 10 April 2016];2012 Available from www.idf/org/diabetesatlas/update-2014.
- 2.Yang W, Lu J, Weng J, et al. Prevalence of Diabetes among Men and Women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 3.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Int J Epidemiol. 2013;42:1215–1222. doi: 10.1093/ije/dyt133. [DOI] [PubMed] [Google Scholar]
- 4.Reffelmann T, Ittermann T, Empen K, Dörr M, Felix SB. Is cardiovascular mortality related to the season of birth?: evidence from more than 6 million cardiovascular deaths between 1992 and 2007. J Am Coll Cardiol. 2011;57:887–888. doi: 10.1016/j.jacc.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Muñoz-Tudurí M, García-Moro C. Season of birth affects short- and long-term survival. Am J Phys Anthropol. 2008;135:462–468. doi: 10.1002/ajpa.20770. [DOI] [PubMed] [Google Scholar]
- 6.Krenz-Niedbała M, Puch EA, Kościński K. Season of birth and subsequent body size: the potential role of prenatal vitamin D. Am J Hum Biol. 2011;23:190–200. doi: 10.1002/ajhb.21101. [DOI] [PubMed] [Google Scholar]
- 7.Murray LJ, O’Reilly DP, Betts N, Patterson CC, Davey Smith G, Evans AE. Season and outdoor ambient temperature: effects on birth weight. Obstet Gynecol. 2000;96:689–695. doi: 10.1016/s0029-7844(00)01022-x. [DOI] [PubMed] [Google Scholar]
- 8.Waldie KE, Poulton R, Kirk IJ, Silva PA. The effects of pre- and post-natal sunlight exposure on human growth: evidence from the Southern Hemisphere. Early Hum Dev. 2000;60:35–42. doi: 10.1016/s0378-3782(00)00102-x. [DOI] [PubMed] [Google Scholar]
- 9.Jongbloet PH, van Soestbergen M, van der Veen EA. Month-of-birth distribution of diabetics and ovopathy: a new aetiological view. Diabetes Res. 1988;9:51–58. [PubMed] [Google Scholar]
- 10.Vaiserman AM, Khalangot MD, Carstensen B, et al. Seasonality of birth in adult type 2 diabetic patients in three Ukrainian regions. Diabetologia. 2009;52:2665–2667. doi: 10.1007/s00125-009-1519-0. [DOI] [PubMed] [Google Scholar]
- 11.Jensen CB, Zimmermann E, Gamborg M, et al. No evidence of seasonality of birth in adult type 2 diabetes in Denmark. Diabetologia. 2015;58:2045–2050. doi: 10.1007/s00125-015-3661-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Chen J, Collins R, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Lee L, Chen J, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 14.Bogin B, Varela-Silva MI. Leg length, body proportion, and health: a review with a note on beauty. Int J Environ Res Public Health. 2010;7:1047–1075. doi: 10.3390/ijerph7031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang N, Zhang X, Xiang YB, et al. Associations of adult height and its components with mortality: a report from cohort studies of 135,000 Chinese women and men. Int J Epidemiol. 2011;40:1715–1726. doi: 10.1093/ije/dyr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv J, Yu C, Guo Y, et al. The associations of month of birth with body mass index, waist circumference, and leg length: findings from the China Kadoorie Biobank of 0.5 million adults. J Epidemiol. 2015;25:221–230. doi: 10.2188/jea.JE20140154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hélène D, World Health Organization . Programming of chronic disease by impaired fetal nutrition--Evidence and implications for policy and intervention strategies. Geneva: World Health Organization; 2002. [Google Scholar]
- 18.Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet Lond Engl. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 19.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427:333–347. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 20.Samuelsson U, Ludvigsson J. Seasonal variation of birth month and breastfeeding in children with diabetes mellitus. J Pediatr Endocrinol Metab. 2001;14:43–46. doi: 10.1515/jpem.2001.14.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr Oslo Nor. 2015;104:30–37. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]