Abstract
Rab GTPases are compartment-specific molecular switches that regulate intracellular vesicular transport in eukaryotes. GDP/GTP exchange factors (GEFs) control Rab activation, and current models propose that localised and regulated GEF activity is important in targeting Rabs to specific membranes. Here we investigated the mechanism of GEF function using the Rab27a-GEF, Rab3GEP, in melanocytes as a model. We show that Rab3GEP deficient melanocytes (melan-R3GKO) manifest partial disruption of melanosome dispersion, a read-out of Rab27a activation and targeting. Using rescue of melanosome dispersion in melan-R3GKO cells and effector pull-down approaches we show that the DENN domain of Rab3GEP (conserved among RabGEFs) is necessary, but insufficient, for its cellular function and GEF activity. Finally using a mitochondrial re-targeting strategy we show that Rab3GEP can target Rab27a to specific membranes in a GEF-dependent manner. We conclude that Rab3GEP facilitates the activation and targeting of Rab27a to specific membranes, but that it differs from other DENN containing RabGEFs in requiring DENN and non-DENN elements for both of these activities and by lacking compartment-specific localisation.
Keywords: organelle transport, guanine nucleotide exchange factor, Rab27a, Rab3GEP, melanocyte
Introduction
Rab proteins are a family (>60 in humans) of small GTPases that regulate vesicle trafficking in eukaryotic cells (Stenmark, 2009, Hutagalung and Novick, 2011). Compartment specific localisation is vital for Rab function, but the mechanism(s) regulating this remain debatable (Barr, 2013, Pfeffer, 2017). Recent studies indicate that localised GDP/GTP exchange factors (GEFs) contribute to activation and targeting of Rabs (Allaire et al., 2010, Yoshimura et al., 2010, Ingmundson et al., 2007, Machner and Isberg, 2006, Murata et al., 2006, Tarafder et al., 2011, Zhang et al., 2006). Two studies have directly investigated this by showing that substrate Rabs follow artificially targeting GEFs to the outer mitochondrial membrane (Blumer et al., 2013, Gerondopoulos et al., 2012). Thus current Rab targeting models suggest that cytosolic Rab-GDP/GDI (Rab-GDP dissociation inhibitor) complexes continuously and reversibly deliver Rab-GDP to membranes where Rab is activated by specifically localised GEFs. Thus blocking their re-extraction by GDI, and promoting accumulation of Rab-GTP in the GEF associated membrane (Barr, 2013). Related to this the ‘GEF cascade’ model suggests that the activity of different Rabs in trafficking pathways are linked by sequential recruitment of GEFs. According to this model active upstream Rabs recruit GEFs for downstream Rabs to membranes as effectors, thereby regulating the recruitment and activation of downstream Rabs (Ortiz et al., 2002, Rink et al., 2005, Poteryaev et al., 2010, Wang and Ferro-Novick, 2002, Knodler et al., 2010, Novick, 2016).
Rab27 regulates the transport and exocytosis of dense cored secretory granules and lysosomal related organelles in many cell types, e.g. pancreatic β cell, cytotoxic T-cells, and platelets (Fukuda, 2013). In melanocytes Rab27a targets to the membrane of pigmented melanosomes where active Rab27a-GTP recruits the motor protein myosin-Va via direct interaction with effector melanophilin (Mlph) (Hammer and Sellers, 2012, Hume and Seabra, 2011). This allows actin-dependent dispersion of melanosomes into peripheral dendrites and pigment transfer to neighbouring keratinocytes. Thus providing pigmentation and photo-protection in mammals (Wu and Hammer, 2014).
Rab3GEP (aka DENN(differentially expressed in neoplastic versus normal)/MADD (mitogen-activating death domain protein) and IG20 (insulinoma-glucagonoma clone 20)) is a GEF for Rab3 whose function is linked to regulated exocytosis and protection against TNFR1/MAPK driven apoptosis (Wada et al., 1997, Tanaka et al., 2001, Yamaguchi et al., 2002, Imai et al., 2013, Li et al., 2014, Del Villar and Miller, 2004, Kurada et al., 2009). In melanocytes Rab3GEP promotes melanosome dispersion by acting as Rab27a GEF and melanosome targeting factor (Figueiredo et al., 2008, Tarafder et al., 2011). Here we further investigated Rab3GEP function in Rab27a activation and targeting in melanocytes. Our findings indicate that; the DENN domain alone of Rab3GEP is insufficient to activate and target Rab27a to melanosomes, GEF activity is essential for the Rab27a targeting activity of Rab3GEP, Rab3GEP is important, but not alone sufficient, for activation and targeting of Rab27a to melanosomes and that Rab3GEP is unlikely to stably associate with melanosomes. Based on this we suggest that Rab3GEP differs from other DENN containing RabGEFs in the mechanism by which it activates Rabs, and that other factors work alongside Rab3GEP to activate and target Rab27a.
Results and Discussion
Melanosome dispersion is partially disrupted in melan-R3GKO melanocytes
To investigate Rab3GEP function in Rab27a-dependent organelle transport we generated Rab3GEP-deficient immortal melanocyte lines, melan-R3GKO1-3 (Lavado et al., 2005, Tanaka et al., 2001) (see Material and methods). Immunoblotting confirmed that melan-R3GKO1-3 cells lacked detectable levels of Rab3GEP compared with wild-type melanocytes (melan-a) (Figure 1A). However, in contrast to previous Rab3GEP siRNA knockdown experiments, we found that melan-R3GKO1-3 cultures contained a mixture of cells in which melanosomes were either a) dispersed throughout the cytoplasm (as seen in melan-a; hereafter ‘dispersed-type’), or b) clustered in the perinuclear cytoplasm (as seen in melanash (Rab27a-/-)) melanocytes; hereafter ‘clustered-type’) (percentage of clustered-type cells 72h after plating; melan-R3GKO1-3 = 50.05 +/- 7.79, 66.22 +/- 2.10, 94.84 +/- 3.62, and melan-a =7.406 +/- 6.196; Figure 1B-C) (Figueiredo et al., 2008, Tarafder et al., 2011). This indicates that a Rab3GEP-independent mechanism(s) of melanosome dispersion exists in the long-term absence of Rab3GEP. Interestingly, we observed that as melan-R3GKO cells started to proliferate, 48-72 hours after plating, the proportion of clustered-type cells in cultures increased compare with 24 hours after plating (Figure S1). This suggests that the rate of the Rab3GEP-independent melanosome dispersion pathway(s) does not match the cellular requirement for melanosome dispersion in proliferating cells. Consistent with this by titration of the essential melanocyte proliferation factor phorbol 12-myristate 13-acetate (PMA) in the culture medium we found that there was a positive correlation between proliferation-rate and the proportion of clustered-type melan-R3GKO cells in cultures (Figure S2). A similar, although smaller, effect was seen in melan-a cells (Figure S2).
Figure 1. Melanosomes are dispersed in a sub-set of melan-R3GKO cells.
(A) Western blots comparing Rab3GEP and calnexin expression (loading control) in lysates from melan-R3GKO1-3 and melan-a. (B) Phase contrast images showing the distribution of melanosomes in melan-a, melan-ash (Rab27a -/-) and melan-R3GKO3 cells. Scale bar = 25μm. (C) A scatter plot showing the percentage of clustered-type melanocytes in melan-R3GKO1-3 and melan-a cultures 72h after plating. Data are from 3/4 independent experiments each performed in triplicate. Plotted points represent the average data in each experiment. Significance indicator are defined in materials and methods.
Melanosome dispersion in melan-R3GKO cells is dependent upon Rab27a
Next we tested whether melanosome dispersion in melan-R3GKO cells was Rab27a-dependent. Firstly, we examined the expression of Rab27a and Mlph (whose expression is Rab27a-dependent), in melan-R3GKO and melan-a cells (Hume et al., 2007, Wu et al., 2002). Immunoblotting indicated that expression of both proteins was reduced in melan-R3GKO compared with melan-a (Figure 2A). Secondly, we examined the effect of siRNA depletion of Rab27a on melanosome distribution in melan-R3GKO cells. Immunoblotting and bright-field imaging confirmed that depletion of Rab27a expression in melan-R3GKO cells in resulted in a significantly increase in the proportion of clustered-type cells (Figure 2B-D; siRNA; NT = 22.9 +/- 3.4%, mock = 11.2 +/- 4.7, Rab27a = 90.8 +/- 2.1%). Thirdly, expression of GFP-Rab27a, efficiently rescued melanosome transport defects in melan-R3GKO cells (Figure S3). Fourthly, we used confocal microscopy to show that Mlph, a proxy for active Rab27a, was more highly expressed and localised to melanosomes in dispersed- versus clustered-type melan-R3GKO cells (mean Mlph fluorescence intensity (arbitrary units AU)/cell ; melan-a = 11.93 +/- 0.96 melan-R3GKO = 3.31 +/- 0.51, melan-ln = 0.55 +/- 0.41; Figure 2E-G). These data support a role for active Rab27a in transporting melanosomes in dispersed-type melan-R3GKO cells, and indicate that Rab27a and Mlph levels are Rab3GEP-dependent.
Figure 2. Rab27a and Mlph disperse melanosomes in melan-R3GKO cells.
(A) Coomassie stained gel and western blots of cell lysates showing protein loading and Rab27a/Mlph expression. (B-D) melan-R3GKO cells were transfected with Rab27a specific and non-targeted (NT) control siRNA and the effect on protein expression and melanosome distribution investigated. (B) A western blot showing Rab27a and GAPDH expression (loading control) in lysates of melan-R3GKO non-transfected (mock) or siRNA transfected. (C) Phase contrast images showing the melanosome distribution in transfected melan-R3GKO cells. Boxes in left panels indicate the area shown in higher magnification on the right. (D) A scatter plot showing the percentage of clustered-type cells in melan-R3GKO cultures 72h after transfection. Data are from 4 independent experiments each performed in triplicate. Plotted points represent the average data from each experiment. (E-G) Melanocytes were fixed, stained for immunofluorescence and the distribution of Mlph and melanosomes recorded using a confocal microscope. (E) Fluorescent (left), phase contrast (centre) and merged images showing the distribution of Mlph, melanosomes and their overlap. White boxes indicate the regions shown in high magnification images below. White arrows indicate co-localisation of melanosomes and Mlph. (F-G) Scatter plots showing the average anti-Mlph fluorescence intensity/cell for different cell types (F), and clustered- and dispersed-type melan-R3GKO cells (G). Results presented are representative of 3 independent experiments. Scale bars = 20μm.
The DENN domain is not sufficient for the GEF activity and cellular function of Rab3GEP
We then used the melan-R3GKO cells to dissect the role of Rab3GEP domains in Rab27a targeting and activation. To test whether the DENN domain of R3G is sufficient for GEF activity, as seen in other DENN containing RabGEFs, we generated a model of Rab3GEP-DENN based on the structure of the DENND1B:Rab35 complex (Allaire et al., 2010, Wu et al., 2011, Ioannou et al., 2015) (Figure S4A; see Material and methods). Thus we identified residues in Rab3GEP-DENN that could interact with Rab27a and generated vectors expressing mutants expected to disrupt this interaction in melanocytes (Rab binding site I: I353D and L366K, and site II: T371R/P372R) (Figure 3A, S4B). [To help quantify Rab3GEP function in melanosome dispersion we standardised melanocyte shape by growing the cells on fibronectin micro-patterns (Evans et al., 2014).] In melan-R3GKO we found that Rab3GEPI353D and other mutants dispersed melanosomes to an intermediate level compared with GFP and Rab3GEP-WT (Pigment dispersion distance (PDD); Rab3GEPI353D = 14.47 +/- 2.156 μm, Rab3GEPL366K = 14.87 +/- 2.121 μm, Rab3GEPT371R/P372R = 15.23 +/- 1.36 μm; Rab3GEP-WT = 16.89 +/- 0.5225 μm and GFP = 12.63 +/- 2.648 μm; Figure 3B-C). In contrast the Rab3GEPR514A mutant, in which the altered residue is outside the predicted Rab binding sites (Figure S4B), rescued melanosome dispersion in melan-R3GKO cells with similar efficiency to Rab3GEP-WT (PDD = 16.71 +/- 1.138 μm; Figure 3A-C). These data support the importance of the Rab3GEP-DENN, and suggest that the interaction mechanism of DENN containing GEFs with Rabs is conserved.
Figure 3. Rab3GEP-DENN is necessary, but insufficient, for melanosome dispersal in melan-R3GKO cells and Rab27a activation.
melan-R3GKO cells were infected with adenoviruses expressing Rab3GEP-WT and mutants, or GFP, plated onto micro-patterned cover-slips, fixed and processed for immunofluorescence. (A) A schematic representation of the domain organisation of Rab3GEP (block diagram) showing the position of point mutations. Line diagram indicates the regions included in each of the truncations used here. (B) Representative images of melanosome (phase) and GFP (centre) distribution in individual cells expressing the indicated proteins and pigment probability maps (right) for each population of cells (n of each indicated in brackets). White circles indicate the shape of the micro-pattern (diameter = 46μm). Scale bar = 10μm. (C) Scatter plot showing the pigment dispersion distance (PDD) for each cell in each population. The significance of differences in PDD values for each mutant compared with the GFP and RabGEP-WT was calculated and are displayed below and above each scatter, respectively. (D) Scatter plot showing the ability of Rab3GEP-WT and mutant variants, and GFP alone to activate Rab27a as reported by effector pull-down in vitro. Activity is expressed relative to Rab3GEP-WT and GFP (normalised to = 1 and 0). Results are from 3 independent experiments. The significance of differences in active Rab27a values for each mutant and GFP compared with RabGEP-WT are above each scatter. Significance indicator are defined in materials and methods.
To better understand how DENN mutants reduced cellular Rab3GEP function we tested their effect on Rab3GEP GEF activity using an effector pull-down assay that reports Rab27a-GTP levels (see Material and methods; (Figueiredo et al., 2008)). In accord with the results of the melanosome dispersion assay we saw that the Rab27a-GTP levels in Rab3GEPI353D and Rab3GEPR514A were similar to those seen in GFP and Rab3GEP expressing cells, indicating that GEF activity is essential for Rab3GEP function in melanosome transport and that the DENN domain plays an important role in both of these activities (Figure 3D).
We next used a DENN alone truncation to test whether Rab3GEP-DENN catalysed Rab27a-specific GEF activity and Rab27a-dependent melanosome dispersion. Using both the cell and pull-down assays we found that Rab3GEPDENN functioned with comparable efficiency to GFP and significantly less efficiently than Rab3GEP-WT (PDD = 11.94 +/- 1.859 μm; Figure 3B-D). Similar results were obtained using the Rab3GEPΔDD mutant that lacks the C-terminus death domain (DD) (PDD = 14.12 +/- 1.922 μm; Figure 3A-C). These observations, with others using N-terminus Rab3GEP truncation mutants, indicate that while the DENN is important, other parts of the protein, including the DD and central region, are also required for for Rab3GEP function (PDD = 14.71 +/- 1.369 μm and 14.13 +/- 2.257 μm for ΔDENN and DD; Figure 3A-C). As previously seen, Rab3GEP and variants were distributed throughout the melanocyte cytoplasm (even at very low expression levels) and not enriched near melanosomes (Figure 3B, S4C) (Figueiredo et al., 2008). This indicates that Rab3GEP is unlikely to stably associate with melanosomes. The exception to this was the Rab3GEPDD mutant which distributed in cytoplasmic punctae, consistent with the ability of DDs to oligomerise (Feinstein et al., 1995) (Figure 3B, S4C). Results were similar for Rab3GEP and mutants in non-pattern grown cells (Figure S4C).
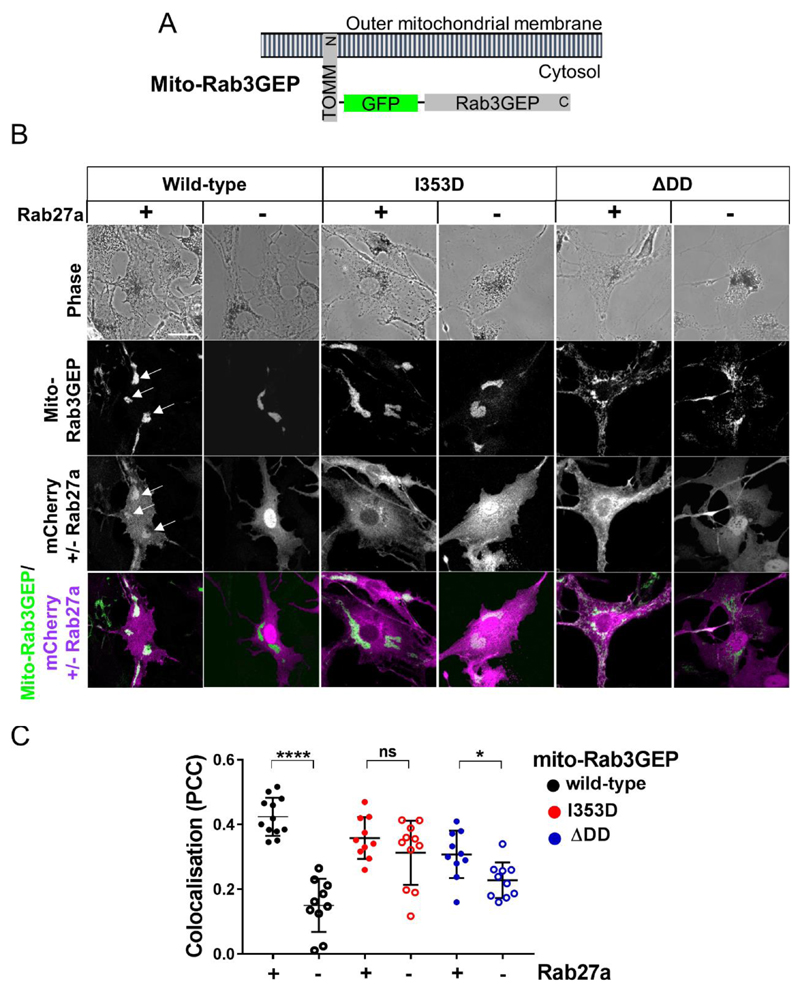
Intact mitochondria localised Rab3GEP targets Rab27a to mitochondria
Finally we investigated the role of Rab3GEP in targeting Rab27a to organelle membranes. For this we generated a fusion protein that targeted Rab3GEP-WT to the outer mitochondrial membrane (mito-Rab3GEP) and tested its ability to target mCherry-Rab27a to mitochondria in melan-R3GKO cells (Figure 4A). Mito-Rab3GEP localised to mitotracker labelled mitochondria but did not disperse melanosomes (Figure 4B Figure S3). In most cells we saw that mito-Rab3GEP positive mitochondria aggregated close to the nucleus, in contrast to their normal dispersed cytoplasmic distribution, suggesting that mito-Rab3GEP causes their aggregation. We also saw that mCherry-Rab27a co-localised with mito-Rab3GEP to a significantly greater extent than mCherry alone. (Figure 4B-C; PCC mito-Rab3GEP/mcherry-Rab27a = 0.424+/-0.016 versus mito-Rab3GEP/mCherry 0.149+/-0.026). This indicates that Rab3GEP influences Rab27a localisation and that tethering Rab3GEP to mitochondria reduces its ability to activate and target Rab27a to melanosomes.
Figure 4. Mito-Rab3GEP can re-target Rab27a to mitochondria by a GEF-dependent mechanism.
melan-R3GKO cells were co-infected with adenoviruses expressing 1) mito-Rab3GEP or mutants (I353D and ΔDD), and 2) mCherry-Rab27a or mCherry, fixed and protein distribution recorded by confocal microscopy. Co-localisation of GFP and mCherry was determined using Pearson correlation analysis. (A) Schematic representation of mito-Rab3GEP showing the arrangement of proteins within the fusion and the topology of its association with the outer mitochondrial membrane. (B) Confocal images showing the distribution of melanosomes (phase), mito-Rab3GEP and mutants, mCherry +/- Rab27a, and their co-localisation. Scale bar = 20μm. (C) Scatter plot showing the extent of colocalisation of 1) mito-Rab3GEP and mutant variants and 2) mCherry +/- Rab27a. The significance of differences between Pearson correlation co-efficient values for mCherry versus mCherry-Rab27a expressing populations for each mito-Rab3GEP protein were calculated using an unpaired student’s t-test. ****, * and ns indicate p = <0.0001, <0.05 and >0.05.
We then examined the importance of the GEF activity of Rab3GEP in Rab27a targeting. We found that GEF deficient mito-Rab3GEPI353D and mito-Rab3GEPΔDD mutants localised to mitochondria, but recruited mCherry-Rab27a to a significantly lower extent than wild-type mito-Rab3GEP (Figure 4B-C; PCC mito-Rab3GEPI353D/mcherry-Rab27a = 0.358+/-0.020; mito-Rab3GEPI353D/mCherry 0.312+/-0.029; mito-Rab3GEPΔDD/mcherry-Rab27a = 0.307+/-0.023; mito-Rab3GEPΔDD/mCherry 0.227+/-0.017). This indicates that the GEF activity of Rab3GEP is required for Rab27a targeting. We observed that mitochondria in mito-Rab3GEPΔDD expressing cells, but not other mito-Rab3GEPs, were dispersed throughout the cytoplasm indicating that DD promotes mitochondrial aggregation possibly through oligomerisation (Feinstein et al., 1995).
Here we investigated how Rab3GEP regulates Rab27a activation/targeting using melanocytes as a model and present several novel findings. Firstly, Rab27a can undergo Rab3GEP-independent activation/targeting and that although Rab3GEP enhances these activities it is not absolutely required. One possible mechanism for this is that other GEFs compensate for the loss of Rab3GEP e.g. DENND4B and GRAB/Rab3IL (Figueiredo et al., 2008, Yoshimura et al., 2010). However, siRNA depletion of these targets did not augment the proportion of clustered-type cells seen in melan-R3GKO cells indicating that these GEFs do not contribute to Rab27a activation (Figure S4A-B). Another possible mechanism is that the intrinsic nucleotide exchange activity of Rab27a disperses melanosomes, in slow-growing cells. Supporting this in vitro studies of GTP loading of purified Rabs indicate that Rab27a has a higher intrinsic nucleotide exchange activity compared with Rab1 and Rab5a that reaches ~35% of the level achieved in the presence of purified Rab3GEP (Figure S4C). This coupled with the low rate of intrinsic Rab27a GTPase activity (~30-folder slower than Rab5a) could explain the existence of a pool of active Rab27a in melan-R3GKO cells (Larijani et al., 2003). Interestingly yeast Rab7 mutant (Ypt7K127E), that has enhanced nucleotide exchange and reduced nucleotide affinity, targeted to vacuolar membranes in the absence of its GEF Mon1-Ccz1 (Cabrera and Ungermann, 2013). These data underline that RabGEFs are enhancers rather than absolute determinants of Rab activation/targeting. Thus it is likely that the severe phenotypic alterations seen in Rab3GEP deficient mice and worms result from reduced function of their multiple Rab substrates (Iwasaki et al., 1997, Tanaka et al., 2001). Secondly, Rab3GEP-DENN is necessary, but insufficient, to catalyse Rab27a activation/targeting. This contradicts studies showing that DENN-only truncations of connecdenn/DENND1B and DENND2B maintained GEF activities comparable with their intact counterparts (Allaire et al., 2010, Wu et al., 2011, Ioannou et al., 2015). In contrast our data indicate that elements throughout Rab3GEP make significant contributions to its activity. Studies using Rab3a as a Rab3GEP substrate reached similar conclusions (Oishi et al., 1998, Coppola et al., 2002). Meanwhile sequence comparison indicates that among there is significant conservation of DENN and non-DENN elements of Rab3GEP throughout evolution (Iwasaki et al., 1997, Mahoney et al., 2006). Thus Rab3GEP likely activates/targets Rab27a and Rab3a via a similar mechanism, but this may differ from that of other DENN containing RabGEFs e.g. connecdenn/DENND1A and DENND2B. Thirdly, the GEF activity of Rab3GEP is absolutely required for its function in targeting Rab27a to membranes. These observations are consistent with previous work showing that the membrane targeting activity of Rabex-5, DrrA and Rabin8 was dependent upon their GEF activity (Blumer et al., 2013). Fourthly, we show that the cytoplasmic localisation of Rab3GEP is important for its function in Rab27a targeting/activation.
In conclusion our data are broadly consistent with models suggesting that RabGEFs serve as important targeting factors by locally activating Rabs and stabilising their association with membranes by preventing their extraction by Rab-GDI. Nevertheless our findings here and before indicate that Rab3GEP is unlikely to be the sole Rab27a targeting factor (Tarafder et al., 2011). Future work should aim to identify and characterise these factors.
Methods
Derivation and maintenance of immortal melanocytes
Cultures of immortal Rab3GEP-deficient melanocytes (melan-RG3KO) were derived essentially as described previously (Lavado et al., 2005). In brief mice heterozygous for a previously generated Rab3GEP loss of function allele were crossed with Ink4a-Arf mutant mice in order to generate embryos homozygous for the Rab3GEP mutant allele and heterozygous for Ink4a-Arf mutant allele. Genotyping of the embryos was as previously described (Lavado et al., 2005, Tanaka et al., 2001). Melanocytes were then derived from the dorsal skin of mutant embryos as previously described (Bennett et al., 1989). melan-R3GKO1-3 melanocytes were derived from 3 different embryos from the same litter. Cultures of immortal melan-RG3KO, melan-ash and melan-a melanocytes were maintained, infected with adenovirus expression vectors, transfected with siRNA oligonucleotides and tested for contamination as described previously (Hume et al., 2006, Hume et al., 2007). The melanocyte cell lines described here are available from the Wellcome Trust Functional Genomics Cell Bank http://www.sgul.ac.uk/depts/anatomy/pages/WTFGCB.htm.
Immunoblotting
Immunoblotting was performed as described previously (Hume et al., 2007) using rabbit anti-Rab3GEP (diluted 1:1000) goat anti-melanophilin (Everest Biotech EB05444; 1:1000), goat anti-GAPDH (Sicgen Ab0049-200; 1:5000), goat anti-calnexin (Sicgen Ab3741-200; 1:1000) and goat anti-Rab27 (Sicgen Ab1023-200; 1:1000) primary antibodies, and IRDye 800CW conjugated secondary antibodies (Odyssey 926-32214; 1:10000). Signal was detected using a Li-Cor infrared scanner (Odyssey).
Plasmid and virus constructs
Generation of virus vectors allowing expression of full-length human Rab3GEP as a fusion to the C-terminus of EGFP was previously described (Figueiredo et al., 2008). Rab3GEPI353D, Rab3GEPR514A, Rab3GEPL366K, Rab3GEPT371R/P372R, Rab3GEPΔDD, Rab3GEPΔDENN, Rab3GEPDENN and Rab3GEPDD expressing adenoviruses were all generated using quick-change site-directed mutagenesis, using pENTR-GFP-Rab3GEP as template (the sequences of primers used for this are available on request). To generate mito-Rab3GEP expressing adenoviruses we PCR amplified a 240bp fragment of DNA corresponding to the N-terminus mitochondrial targeting sequence of murine TOMM70a (accession number AAI39422.1) this was then ligated into an engineered HindIII site located upstream of the 5’ end of the EGFP coding sequence of pENTR-GFP-Rab3GEP. Mutants variants of this were generated by site-directed mutagenesis as described above.
Microscopy and image analysis
Cells for immunofluorescence were cultured on 13mm coverslips (1.5 thickness; Scientific Laboratory Supplies UK 6422-307164), paraformaldehyde fixed, stained and fluorescence and transmitted light images of melanocytes were then collected using a Zeiss LSM710 confocal microscope fitted with a 63x 1.4NA oil immersion Apochromat lens or a Zeiss Axiovert 100S inverted microscope fitted with a 10x objective and an Axiocam MR3 CCD camera. All images presented are single sections in the z-plane. Antibodies and stains were used as indicated; mouse monoclonal anti-GFP (Roche 11814460001; 1:200) rabbit anti-Mlph (antigen mouse Mlph 150-400aa (Strom et al 2001); 1:100) goat anti-rabbit and goat anti-mouse IgG secondary antibodies both Alexa568 labelled (Invitrogen A-11001 and A-11011; both 1:500). For live cell experiments confirming the targeting of mito-Rab3GEP to mitochondria cells were plated in 35mm diameter glass bottomed petri dishes (Matek P35G-1.5-20-C) (1x104 cells/dish). 24 hours later cells were infected with adenoviruses expressing mito-Rab3GEP or GFP alone, and after a further 48 hours the mitochondria were labelled by incubation for 30 minutes in 200nM MitoTracker Red FM (Thermo-Fisher product M7512). After washing twice in medium (L-15 supplemented with 10% fetal calf serum, 100 U/ml penicillin G, and 100 mg/ml streptomycin) without mito-tracker cells were transferred to the environmental chamber (37°C) surrounding the stage of the Zeiss LSM710 confocal microscope and images of the distribution of GFP, mito-tracker labelled mitochondria and melanosomes were acquired using the 488nm Ar and 568nm HeNe laser lines, respectively. Analysis of melanosome clustering in siRNA transfected cells and melanosome distribution in micro-pattern grown cells was as previously described (Evans et al., 2014, Robinson et al., 2017).
Effector pull-down assay for detection of Rab27a-GTP
HEK293a cells were plated in 10cm dishes (~5x106 cells/dish) and 24 hours later co-infected with adenoviruses expressing mCherry-Rab27a and either GFP, Rab3GEP-WT or mutant variants of Rab3GEP. After expression for 24 hours cells were washed twice with ice-cold PBS and lysed using buffer A (50mM Tris pH7.5, 150mM NaCl, 5mM MgCl2, 1 mM dithiothreitol, 1% CHAPS, and protease inhibitor cocktail (Complete ULTRA Tablets; Roche) for 30 min on ice. Cell contents were harvested using a scraper and lysates were clarified by centrifugation at 12,000 × RPM for 15 min at 4°C using Eppendorf centrifuge 5415R. Supernatant was collected and proteins quantification was performed using a Bradford protein assay (Bio-Rad; UK). Two mg of the total protein was pre-cleared by incubation with 20µl of glutathione-sepharose 4B Fast Flow beads (GE Healthcare, 17-5132-01) for 2 hours. After pelleting the beads by centrifugation at 4000 RPM at 4°C the supernatant was incubated with 200 pmol of GST or 200 pmol of GST-Slp1 (1-200 amino acid fragment) and 25µl of glutathione-sepharose 4B beads for 16 hours at 4°C. Beads were pelleted as above and washed 3 times in buffer A 10min. Bound proteins were released from the beads by incubation in Laemmli buffer (100mM Tris-Cl (pH6.8), 4% (w/v) SDS, 0.2% (w/v) bromophenol blue, 20% (v/v) glycerol and 200mM β-mercaptoethanol) for 5 min at 95°C. Bound proteins were then resolved by SDS-PAGE and mCherry-Rab27a was detected by immunoblotting and signal intensity quantified using ImageJ.
Modelling of the structure of the DENN domain of Rab3GEP
The structural model of Rab3GEP has been produced using the HHpred server in conjunction with MODELLER (Alva et al., 2016). In brief, the amino acid sequence of the GEF-domain of Rab3GEP has been submitted to HHpred first. The server identified the GEF-domain of DENND1A as the closest structural homologue. Consequently, the generated amino acid sequence alignment between Rab3GEP and DENND1A has been used as a basis for producing a structural model of Rab3GEP by using MODELLER by directly forwarding the result produced through HHpred.
Satitstical analysis of date
Unless otherwise indicated Statistical analysis of data was carried out with GraphPad Prism 7 software using the one-way ANOVA test and Bonferroni’s multiple comparisons post-test facility within the software and assuming nonparametric distribution of data. ****, ***, **, * and ns indicate p = <0.0001, <0.001, <0.01, <0.05 and >0.05, respectively.
Quantitative real-time PCR
Primers and probes for Q-RT-PCR targets (from Sigma Genosys, Cambridge, UK) were designed using Primer Express software (Life Technologies). Probes were labelled at the 5’- and 3’- ends with fluorophore 6-FAM (6-carboxyfluorescein) and quencher TAMRA (tetramethylrhodamine), respectively. For GRAB and DENND4B the primers were 5’-CAGCCTGTTTGAGGAAGCTC-3’ (sense strand) and 5’-TGGTGTGGATGTGATGACCA-3’ (reverse strand), and 5’-TGCGCCACGTCGGACTCAAC-3’ (sense strand) and 5’-TCCTTGCCCATGCTGCTGGC-3’ (reverse strand) and the probes were 5’- CGCCTGCTTCATGTTGGCTTCCCG -3’ and 5’-GAGACGCTAGGGCCCCCTCC -3’. For GAPDH the primers were 5’-GTGTCCGTCGTGGATCTGA-3’ (sense strand) and 5’- CCTGCTTCACCACCTTCTTGA-3’ (reverse strand) and the probe was 5’- CCGCCTGGAGAAACCTGCCAAGTATG-3’. To generate mRNA samples pools melan-R3GKO cells grown in 6-well plates (1x105/well) were transfected with siRNA in triplicate as described previously (Hume et al., 2007). 72 hours later cells were harvested and mRNA extracted using the RNeasy Mini RNA extraction kit (Qiagen). cDNA was generated using Moloney Murine Leukemia Virus M-MLV reverse transcriptase (Promega) using random primers. To generate a standard curve of signal:template concentration for each Q-RT-PCR assay a pool containing 5% of each of the cDNA samples analysed was generated. This pool was serially diluted in DEPC water (1:4, 1:16, 1:64, 1:256) and these were used as template in Rab1a and GAPDH Q-RT-PCR assays. [The shape of the standard curve indicates the relationship between signal and template concentration. For both assays standard curves gave straight lines with R2 >0.99 indicating that there is a linear relationship between signal and template]. To measure the expression of targets in siRNA transfected cells each neat cDNA was diluted 1:32 in DEPC water and the following reagents were added per well of a 96-well plate: 6.5μl TaqMan Fast 2X PCR Master Mix (Life Technologies); 0.4μl forward primer (10μM); 0.4μl reverse primer (10μM); 0.25μl probe (10μM); 3μl cDNA; 2.45μl DEPC water. For each sample, 3 technical repeats were performed. Reaction plates were sealed with optically clear adhesive film, centrifuged, and qRT-PCR performed using a StepOnePlus Real-Time PCR system (Applied Biosystems) using the ’fast’ mode. CT values for each reaction were determined by the StepOne software. The slope (S), intercept (I) and R2 values were calculated for the standard curve of each Q-RT-PCR assay. CT values from siRNA transfected samples were then processed to generate a ‘quantity value’ for each CT value as follows; 1) (CT-I)/S=LQ, 2) 10LQ=Q, 3) Qx(1/MNT)= GOIP (where MNT= mean non-targeted quantity value) and 4) GOIP/GP = normalised expression of target relative to GAPDH (where GP is the normalised quantity value for the GAPDH primer).
Supplementary Material
Summary.
We show that Rab3GEP plays an important, but not exclusive, role in organelle targeting of Rab27a and that the Rab3GEP DENN domain alone is insufficient to activate and target Rab27a.
Acknowlegements
We thanks Dr Yoshimi Takai (Department of Molecular Biology and Biochemistry, Osaka University Graduate School of Medicine/Faculty of Medicine, Osaka 565-0871, Japan) for Rab3GEP deficient mice and Rab3GEP-specific antibodies, Tim Self (confocal microscopy), Chris Gell (Image analysis), Sue Cooper and Carol Sculthorpe (general) (all University of Nottingham, UK) for technical assistance, Antonia Booth and Noor Mohd-Naim (both Imperial College London, UK) for assistance in the production of Rab3GEP expressing adenoviruses.
Funding.
This work was supported by a Medical Research Council New Investigator Award to ANH (grant reference G1100063), Wellcome Trust Project Grant Awards (grant references 091346/Z/10/Z to MCS and ANH and 108429/Z/15/Z to EVS) and a University of Nottingham Vice-Chancellor's PhD Scholarship for Research Excellence (European Union) awarded to PS. LM is funded through the Spanish Ministry of Economy. Industry and Competitiveness (MINECO) [BIO2015-70978-R]. A.I. is grateful for funding by the DFG/ANR grant IT 91/2-1.
Footnotes
Competing interests.
The authors declare no competing or financial interests.
Author contribution
PS, RDE, DAB, and ANH conducted the experiments; MC, LM, SP and EVS generated the immortal melan-R3GKO melanocytes; AI carried out the structural modelling of the DENN domain of Rab3GE; PS, MCS, and ANH designed the studies and wrote the paper.
References
- Allaire PD, Marat AL, Dall'armi C, Di Paolo G, Mcpherson PS, Ritter B. The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes. Mol Cell. 2010;37:370–82. doi: 10.1016/j.molcel.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alva V, Nam SZ, Soding J, Lupas AN. The MPI bioinformatics Toolkit as an integrative platform for advanced protein sequence and structure analysis. Nucleic Acids Res. 2016;44:W410–5. doi: 10.1093/nar/gkw348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr FA. Review series: Rab GTPases and membrane identity: causal or inconsequential? J Cell Biol. 2013;202:191–9. doi: 10.1083/jcb.201306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DC, Cooper PJ, Dexter TJ, Devlin LM, Heasman J, Nester B. Cloned mouse melanocyte lines carrying the germline mutations albino and brown: complementation in culture. Development. 1989;105:379–85. doi: 10.1242/dev.105.2.379. [DOI] [PubMed] [Google Scholar]
- Blumer J, Rey J, Dehmelt L, Mazel T, Wu YW, Bastiaens P, Goody RS, Itzen A. RabGEFs are a major determinant for specific Rab membrane targeting. J Cell Biol. 2013;200:287–300. doi: 10.1083/jcb.201209113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Ungermann C. Guanine nucleotide exchange factors (GEFs) have a critical but not exclusive role in organelle localization of Rab GTPases. J Biol Chem. 2013;288:28704–12. doi: 10.1074/jbc.M113.488213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola T, Perret-Menoud V, Gattesco S, Magnin S, Pombo I, Blank U, Regazzi R. The death domain of Rab3 guanine nucleotide exchange protein in GDP/GTP exchange activity in living cells. Biochem J. 2002;362:273–9. doi: 10.1042/0264-6021:3620273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Villar K, Miller CA. Down-regulation of DENN/MADD, a TNF receptor binding protein, correlates with neuronal cell death in Alzheimer's disease brain and hippocampal neurons. Proc Natl Acad Sci U S A. 2004;101:4210–5. doi: 10.1073/pnas.0307349101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RD, Robinson C, Briggs DA, Tooth DJ, Ramalho JS, Cantero M, Montoliu L, Patel S, Sviderskaya EV, Hume AN. Myosin-Va and dynamic actin oppose microtubules to drive long-range organelle transport. Curr Biol. 2014;24:1743–50. doi: 10.1016/j.cub.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein E, Kimchi A, Wallach D, Boldin M, Varfolomeev E. The death domain: a module shared by proteins with diverse cellular functions. Trends Biochem Sci. 1995;20:342–4. doi: 10.1016/s0968-0004(00)89070-2. [DOI] [PubMed] [Google Scholar]
- Figueiredo AC, Wasmeier C, Tarafder AK, Ramalho JS, Baron RA, Seabra MC. Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes. J Biol Chem. 2008;283:23209–16. doi: 10.1074/jbc.M804134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14:949–63. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- Gerondopoulos A, Langemeyer L, Liang JR, Linford A, Barr FA. BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr Biol. 2012;22:2135–9. doi: 10.1016/j.cub.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer JA, 3rd, Sellers JR. Walking to work: roles for class V myosins as cargo transporters. Nat Rev Mol Cell Biol. 2012;13:13–26. doi: 10.1038/nrm3248. [DOI] [PubMed] [Google Scholar]
- Hume AN, Seabra MC. Melanosomes on the move: a model to understand organelle dynamics. Biochem Soc Trans. 2011;39:1191–6. doi: 10.1042/BST0391191. [DOI] [PubMed] [Google Scholar]
- Hume AN, Tarafder AK, Ramalho JS, Sviderskaya EV, Seabra MC. A coiled-coil domain of melanophilin is essential for Myosin Va recruitment and melanosome transport in melanocytes. Mol Biol Cell. 2006;17:4720–35. doi: 10.1091/mbc.E06-05-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume AN, Ushakov DS, Tarafder AK, Ferenczi MA, Seabra MC. Rab27a and MyoVa are the primary Mlph interactors regulating melanosome transport in melanocytes. J Cell Sci. 2007;120:3111–22. doi: 10.1242/jcs.010207. [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–49. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai A, Ishida M, Fukuda M, Nashida T, Shimomura H. MADD/DENN/Rab3GEP functions as a guanine nucleotide exchange factor for Rab27 during granule exocytosis of rat parotid acinar cells. Arch Biochem Biophys. 2013;536:31–7. doi: 10.1016/j.abb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Ingmundson A, Delprato A, Lambright DG, Roy CR. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature. 2007;450:365–9. doi: 10.1038/nature06336. [DOI] [PubMed] [Google Scholar]
- Ioannou MS, Bell ES, Girard M, Chaineau M, Hamlin JN, Daubaras M, Monast A, Park M, Hodgson L, Mcpherson PS. DENND2B activates Rab13 at the leading edge of migrating cells and promotes metastatic behavior. J Cell Biol. 2015;208:629–48. doi: 10.1083/jcb.201407068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K, Staunton J, Saifee O, Nonet M, Thomas JH. aex-3 encodes a novel regulator of presynaptic activity in C. elegans. Neuron. 1997;18:613–22. doi: 10.1016/s0896-6273(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346–51. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurada BR, Li LC, Mulherkar N, Subramanian M, Prasad KV, Prabhakar BS. MADD, a splice variant of IG20, is indispensable for MAPK activation and protection against apoptosis upon tumor necrosis factor-alpha treatment. J Biol Chem. 2009;284:13533–41. doi: 10.1074/jbc.M808554200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larijani B, Hume AN, Tarafder AK, Seabra MC. Multiple factors contribute to inefficient prenylation of Rab27a in Rab prenylation diseases. J Biol Chem. 2003;278:46798–804. doi: 10.1074/jbc.M307799200. [DOI] [PubMed] [Google Scholar]
- Lavado A, Matheu A, Serrano M, Montoliu L. A strategy to study tyrosinase transgenes in mouse melanocytes. BMC Cell Biol. 2005;6:18. doi: 10.1186/1471-2121-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LC, Wang Y, Carr R, Haddad CS, Li Z, Qian L, Oberholzer J, Maker AV, Wang Q, Prabhakar BS. IG20/MADD plays a critical role in glucose-induced insulin secretion. Diabetes. 2014;63:1612–23. doi: 10.2337/db13-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell. 2006;11:47–56. doi: 10.1016/j.devcel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Mahoney TR, Liu Q, Itoh T, Luo S, Hadwiger G, Vincent R, Wang ZW, Fukuda M, Nonet ML. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell. 2006;17:2617–25. doi: 10.1091/mbc.E05-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol. 2006;8:971–7. doi: 10.1038/ncb1463. [DOI] [PubMed] [Google Scholar]
- Novick P. Regulation of membrane traffic by Rab GEF and GAP cascades. Small GTPases. 2016;7:252–256. doi: 10.1080/21541248.2016.1213781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi H, Sasaki T, Nagano F, Ikeda W, Ohya T, Wada M, Ide N, Nakanishi H, Takai Y. Localization of the Rab3 small G protein regulators in nerve terminals and their involvement in Ca2+-dependent exocytosis. J Biol Chem. 1998;273:34580–5. doi: 10.1074/jbc.273.51.34580. [DOI] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–15. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol Biol Cell. 2017;28:712–715. doi: 10.1091/mbc.E16-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–49. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Robinson CL, Evans RD, Briggs DA, Ramalho JS, Hume AN. Inefficient recruitment of kinesin-1 to melanosomes precludes it from facilitating their transport. J Cell Sci. 2017;130:2056–2065. doi: 10.1242/jcs.186064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Miyoshi J, Ishizaki H, Togawa A, Ohnishi K, Endo K, Matsubara K, Mizoguchi A, Nagano T, Sato M, Sasaki T, Takai Y. Role of Rab3 GDP/GTP exchange protein in synaptic vesicle trafficking at the mouse neuromuscular junction. Mol Biol Cell. 2001;12:1421–30. doi: 10.1091/mbc.12.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarafder AK, Wasmeier C, Figueiredo AC, Booth AE, Orihara A, Ramalho JS, Hume AN, Seabra MC. Rab27a targeting to melanosomes requires nucleotide exchange but not effector binding. Traffic. 2011;12:1056–66. doi: 10.1111/j.1600-0854.2011.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Nakanishi H, Satoh A, Hirano H, Obaishi H, Matsuura Y, Takai Y. Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily small G proteins. J Biol Chem. 1997;272:3875–8. doi: 10.1074/jbc.272.7.3875. [DOI] [PubMed] [Google Scholar]
- Wang W, Ferro-Novick S. A Ypt32p exchange factor is a putative effector of Ypt1p. Mol Biol Cell. 2002;13:3336–43. doi: 10.1091/mbc.01-12-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bradley MJ, Cai Y, Kummel D, De La Cruz EM, Barr FA, Reinisch KM. Insights regarding guanine nucleotide exchange from the structure of a DENN-domain protein complexed with its Rab GTPase substrate. Proc Natl Acad Sci U S A. 2011;108:18672–7. doi: 10.1073/pnas.1110415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hammer JA. Melanosome transfer: it is best to give and receive. Curr Opin Cell Biol. 2014;29:1–7. doi: 10.1016/j.ceb.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA., 3rd Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4:271–8. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Tanaka M, Mizoguchi A, Hirata Y, Ishizaki H, Kaneko K, Miyoshi J, Takai Y. A GDP/GTP exchange protein for the Rab3 small G protein family up-regulates a postdocking step of synaptic exocytosis in central synapses. Proc Natl Acad Sci U S A. 2002;99:14536–41. doi: 10.1073/pnas.212511399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Gerondopoulos A, Linford A, Rigden DJ, Barr FA. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J Cell Biol. 2010;191:367–81. doi: 10.1083/jcb.201008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, He X, Fu XY, Chang Z. Varp is a Rab21 guanine nucleotide exchange factor and regulates endosome dynamics. J Cell Sci. 2006;119:1053–62. doi: 10.1242/jcs.02810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.