Abstract
Humans live in a 24-hour environment, in which light and darkness follow a diurnal pattern. Our circadian pacemaker, the suprachiasmatic nuclei (SCN) in the hypothalamus, is entrained to the 24-hour solar day via a pathway from the retina and synchronises our internal biological rhythms. Rhythmic variations in ambient illumination impact behaviours such as rest during sleep and activity during wakefulness as well as their underlying biological processes. Rather recently, the availability of artificial light has substantially changed the light environment, especially during evening and night hours. This may increase the risk of developing circadian rhythm sleep–wake disorders (CRSWD), which are often caused by a misalignment of endogenous circadian rhythms and external light–dark cycles. While the exact relationship between the availability of artificial light and CRSWD remains to be established, nocturnal light has been shown to alter circadian rhythms and sleep in humans. On the other hand, light can also be used as an effective and noninvasive therapeutic option with little to no side effects, to improve sleep,mood and general well-being. This article reviews our current state of knowledge regarding the effects of light on circadian rhythms, sleep, and mood.
Keywords: Circadian rhythms, Natural light, Artificial light, Depression, Light therapy
Anatomical architecture of the circadian system
The central master-clock in mammalian species, including humans, is the suprachiasmatic nuclei (SCN), a paired structure in the hypothalamus with a volume just about 0.25 mm3 per nucleus (e.g. [45, 57, 84]). Within the mammalian SCN, a molecular oscillator keeps the clock oscillating at its normal pace. The basis of this oscillator is two interconnected molecular feedback loops of clock gene expression, a detailed description of which is beyond the scope of this review though (see [12] for a detailed explanation).
Successful interaction between body and environment however needs more than just a central clock; it also requires input pathways relaying information about the environment and the body to the SCN to achieve adequate entrainment as well as output pathways communicating timing information to the body to synchronise bodily processes with the circadian phase (Fig. 1).
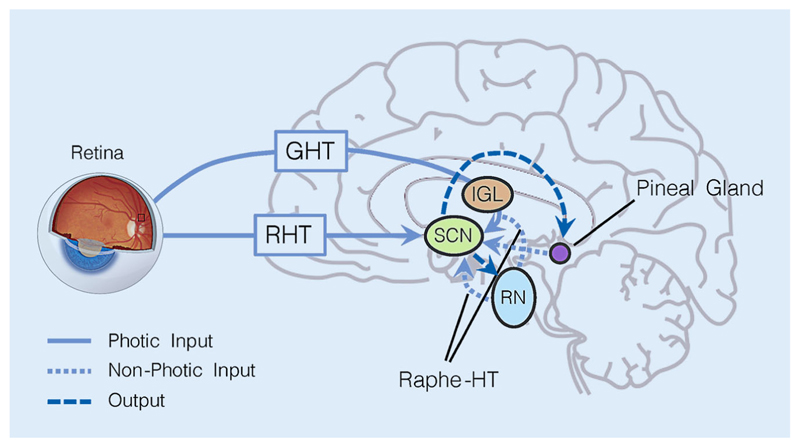
Fig. 1.
Input and output pathways to/from the suprachiasmatic nuclei (SCN). The photic input pathways that relay information about the intensity and spectral composition of ambient light are the retinohypothalamic tract (RHT) and the geniculohypothalamic tract (GHT), which connects retina and SCN via the intergeniculate leaflet (IGL) in the thalamus. Additionally, the SCN also receive non-photic information from the raphe nuclei (RN) via the raphe-hypothalamic tract (raphe-HT) and from the pineal gland. The main output is from the SCN to the serotonergic raphe nuclei (RN, receive information about the phase of the circadian clock and regulate vigilance state of the body) and the pineal gland, where melatonin is produced. Input and output pathways form reciprocal loops
The most important zeitgeber (from German, something that “gives time”) reaching the SCN is ambient light in the environment. In addition to processing visual stimuli in the environment, allowing us to see, the retina carries this photic information via the retinohypothalamic tract (RHT) to the SCN. The SCN also receive non-photic information from within the body. Here, the involved pathways comprise the geniculohypothalamic tract (GHT), which communicates both non-photic and photic information (via the intergeniculate leaflet; IGL), and the raphe-hypothalamic tract (raphe-HT). Additionally, SCN activity is also modulated by non-photic information via neurotransmitters and hormones such as serotonin [54] and melatonin [23], and from peripheral clocks in other tissues (see [55] for an overview).
SCN neurons adjust their circadian phase (of neural activity) according to the input of ambient light levels and its spectral composition and communicate this information via humoral and autonomic nervous system signals to the rest of the body. These output pathways are also reciprocal and thus feed information back to the SCN: The SCN-serotonin-producing raphe nuclei(RN)-SCN loop as well as the SCN-melatonin-producing pineal gland-SCN loop (Fig. 1). More specifically, the RN can alter vigilance levels in accordance with circadian phase via serotonergic wakefulness-promoting projections to the hypothalamus and the cortex [30, 56].
The SCN also projects to the pineal gland, where the sleep-facilitating hormone melatonin is produced during the biological night, thereby modulating the diurnal variations between wakefulness and sleep [23]. In addition to the pathway between retina and SCN, there is recent evidence from animal studies showing that also the habenula in the thalamus is innervated by retinal projections [38, 110] which may specifically mediate mood-related non-visual effects of light.
Fundamentals of light
To understand the effects of light on the human physiology, it is important to understand light. Briefly, light is radiation in a specific range of the electromagnetic spectrum. It is best and most completely described by its spectral distribution, which quantifies the amount of energy (or the number of photons) as a function of wavelength (with visible light in the wavelength range between 380 and 780 nm).
During the day, light intensities outside can reach illuminances up to 100,000 lx in direct sunlight and 25,000 lx in full daylight. Light intensities in closed rooms are considerably lower and standard office lighting is only ~500 lx, often lower [37, 81]. The spectrum of daylight, which is light from the sun filtered by the atmosphere is relatively broadband in its distribution (Fig. 2a). The availability of daylight depends on geographical location and season. In the timeframe of human evolution, it is a rather recent development that light can be available during all times of day through artificial light. Artificial light allows for illuminating indoor and outdoor spaces. It comes in many forms, e.g. incandescent, fluorescent, or light-emitting diode (LED) lighting. While light generated by these technologies may all appear “white”, the underlying spectra are rather different (Fig. 2b). The reason why many different types of spectra might have the same appearance lies in the retina. Critically, different spectra, even if they create the same visual impression, may vary in their chronobiological effects on the circadian clock.
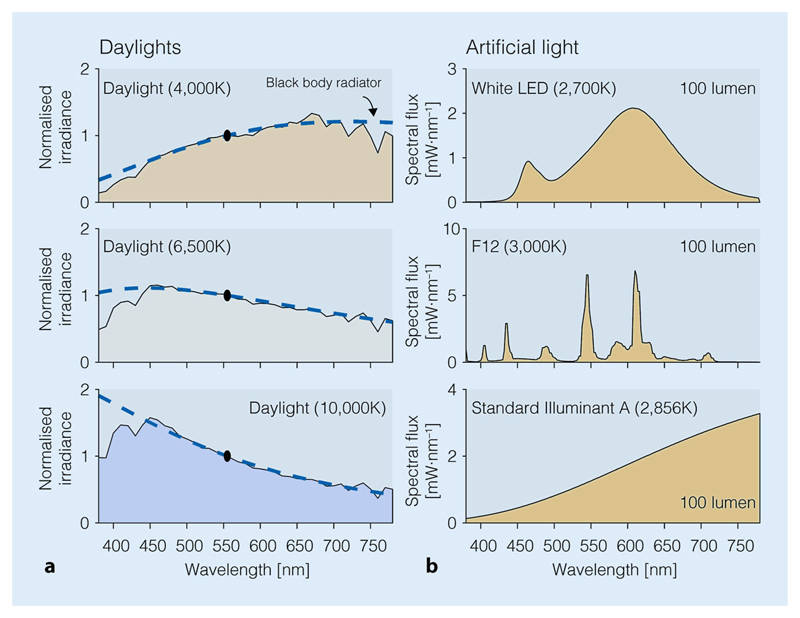
Fig. 2.
Spectral power distributions of common light sources in our environment. a Spectral power distributions of daylights at different correlated colour temperatures (CCT; 4000 K; 6500 K; 10,000 K). Spectra are normalised to 555 nm. b Spectral power distributions of a white LED (top), a fluorescent source at 3000 K (middle), and an incandescent source (tungsten-filament; 2856 K, bottom). All three artificial sources have the same luminous flux (normalised to 100 lm), and approximately the same colour temperature (2700–3000 K), but the spectra are very different in shape and scale (see y axis)
It is important to keep in mind that there are multiple ways how light is quantified and reported in the literature in particular when focussing on its repercussions on human physiology. For example, while the absolute spectral distribution of a light is the most complete description, many investigators report the illuminance (in lux [lx]), or the correlated colour temperature, which is the temperature of a hypothetical black-body radiator with the same colour as the light source in question. Unfortunately, until recently, there have been no standard quantities that experimenters were asked to report, and therefore, summarising the chronobiological and somnological literature on the effects of light remains a challenge. Recently, the Commission International de l’Eclairage (CIE), the international standard body for quantities related to light, issued a new standard containing a reference framework for quantifying the effects of light on non-visual functions [31]. In practice, experimenters employing light as an intervention should report, at a minimum, the spectral power distribution of the light, as seen from the participant’s point of view. Detailed minimum guidelines are given in [83].
Photoreceptors in the retina
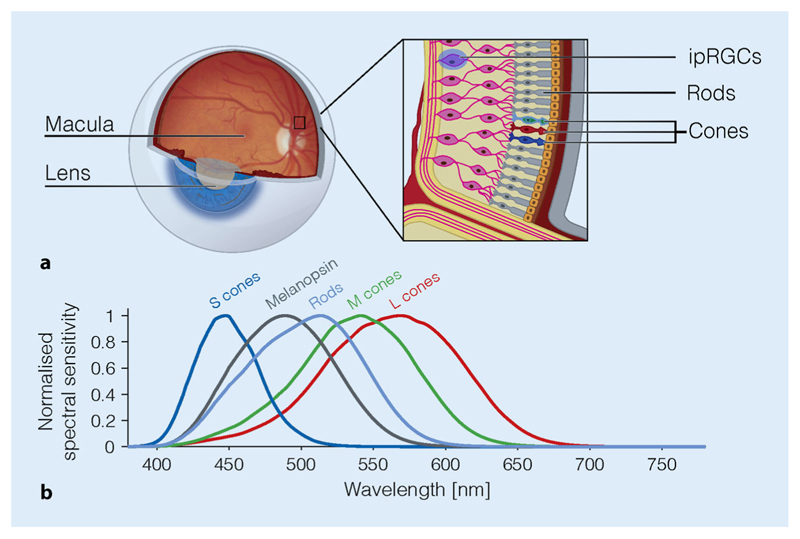
In humans, the known effects of light on circadian rhythms and sleep are all, without exception, mediated by the retina. The retina is a fine layer of nerve tissue at the back of our eyes, containing specialised photoreceptors (Fig. 3a). The so-called cones exist in the highest density in the centre of the retina—the fovea. There are three types of cones, differing in their preference for light at specific wavelengths (Fig. 3b): The long-wavelength-sensitive cones (L cones), the medium-wavelength-sensitive cone (M cones) and the short-wavelength-sensitive cones (S cones). Cones allow us to see colour, spatial detail and motion at light levels typical for daytime. Rods, by contrast, are suppressed at daytime light levels and only signal at light levels typical for twilight and darker. Rods are absent in the fovea, cannot distinguish between different colours and only allow for rudimentary vision.
Fig. 3.
Overview of the retina photoreceptors. a Schematic view of the eye with the retina at the back of the eye (the fundus), containing cones, rods and the intrinsically photosensitive retinal ganglion cells (ipRGCs) expressing the photopigment melanopsin. b Spectral sensitivities of the photoreceptors in the human eye
Cones and rods are not the only photoreceptors in the retina. A small fraction of secondary neurons in the retina—the retinal ganglion cells (RGCs), which integrate information and send it to the brain via the optic nerve—express the photopigment melanopsin [62]. Melanopsin is a short-wavelength-sensitive pigment with a peak spectral sensitivity near around 480 nm [4], rendering some RGCs intrinsically photosensitive [79]. These intrinsically photosensitive retinal ganglion cells (ipRGCs) are thought to mediate most effects of light on the circadian clock. However, ipRGCs are not independent of rod and cone input. Rather, they also receive information from these receptors, suggesting that ipRGCs indeed act as “integrators of information” regarding the light environment across a wide range of wavelengths and light levels. Surprisingly, the input from the S cones into the ipRGCs has a negative sign [32]. In humans, this has the paradoxical consequence that increases in S cone activation lead to a dilation of the pupil [80, 100], which is also controlled by the ipRGCs.
It has long been thought that cones and rods mediate what is typically considered “vision” (seeing colour, motion, spatial detail), and that melanopsin mediates the “other”, non-visual effects of light, i.e. melatonin suppression, circadian phase shifting, and alertness. However, at second sight, this dichotomy breaks down. There is now converging evidence that melanopsin signals reach the primary visual cortex (V1) [82], where they may contribute to and modulate our visual perception [20, 25, 109].
It is important to keep in mind that the retinal photoreceptors experience an altered version of the light relative to the cornea, the front surface of the eye. This is because the eye itself contains filters. In the centre of the retina, this includes the macular pigment, which is present in the fovea but drops off in the peripheral retina. More importantly for ipRGCs, the crystalline lens and ocular media filter out short-wavelength light. This natural “blue-blocking” filter increases density with increasing age, with less and less short-wavelength light reaching the retina.
While the field of vision science has a long history (>150 years) in examining how different types of light stimuli are encoded, processed and perceived, we still remain largely in the dark about many aspects of the effects of light on the circadian clock. The discovery that the production of melatonin is suppressed in humans in response to light dates back to only 1980 [51]. Teasing apart how the different elements in the retina contribute to the effects of light on circadian rhythms, sleep and mood remains an important challenge.
Effects of light on the circadian clock
Two effects of light have been interrogated extensively in human circadian and sleep research: (1) the acute suppression of melatonin in response to light exposure and (2) the ability of light exposure to shift circadian phase. However, these two effects are not arising from a unitary pathway resulting in a direct relationship between melatonin suppression and phase shifts. There is now accruing evidence that they may be indeed separable [63]. As a consequence, one should not be used as a proxy for the other [106].
The system mediating melatonin suppression has a spectral sensitivity that is broadly consistent with the spectral sensitivity of melanopsin [17, 60, 88]. Similarly, the spectral sensitivity of circadian phase shifting shows its maximal effect near the peak spectral sensitivity of melanopsin [101]. However, this does not imply that cones and rods may not participate in these non-visual effects of light. Indeed, there is evidence that cones do contribute, though at a different time scale than the ipRGCs [42].
The effects of light on the phase of the circadian clock depend on the timing of light exposure. This is formally summarised in the phase response curve (PRC), which describes the amount of phase shift (in minutes and hours) achieved by exposure of light at a given circadian phase. Roughly speaking, the effect of morning light is that it advances the clock, while evening and night light delays the clock. The human circadian system integrates across multiple light exposures as short as five minutes [48], even intermittent bright light exposure can shift the circadian phase [43, 66]. It has been shown that under certain circumstances, a train of very brief flashes light flashes on the millisecond scale can cause circadian phase shifts which are larger than those caused by continuous light [59, 108].
Both melatonin suppression and circadian phase shifts are modulated by the “photic history”, i.e. the amount of light seen during the day [27, 44, 77]. The long-term adaptive influences of the “spectral diet” in the real world remain an important area of investigation [93].
Effects of light on sleep
The human sleep–wake cycle, that is periods of sleep during the night and wakefulness during the day, is one of the most prominent examples of a circadian behavioural pattern. It results from the interaction between two factors: the circadian drive for wakefulness and the homeostatic sleep pressure. The interaction between this circadian “process C” and the homeostatic “process S” has been conceptualised in the widely known “two-process model of sleep” [13, 15], which accounts for the timing and intensity of sleep in many experimental settings. Indeed, in well-controlled studies the circadian pacemaker in the SCN and the sleep homeostat have been shown to interact in a fashion designed to allow for consolidated periods of wakefulness and sleep during day and night, respectively (e.g. [35]). Specifically, the activity of the circadian pacemaker is aligned to counteract the increasing sleep pressure resulting from sustained wakefulness during day-time. Likewise, the nocturnal increase in circadian sleep tendency counteracts the decrease in sleep propensity resulting from accumulated sleep thereby supporting a consolidated phase of nocturnal sleep.
As outlined above, light is the key zeitgeber in the circadian system and interacts with the master clock in the SCN via non-image-forming pathways connecting retina and SCN. Unsurprisingly, light therefore also affects sleep. Natural daylight at high intensities as experienced outside buildings has previously been shown to (1) advance the timing of sleep to earlier hours, (2) affect the duration of sleep, and (3) improve sleep quality. More precisely, the phase-advancing effects of daylight have for example been reported by Roenneberg and colleagues [67] who, using questionnaire data, found that each additional hour spent outdoors advanced sleep by ~30 min. Despite light being the strongest zeitgeber, this phase-advance could also result from physical exercise during daytime [102, 105], which is often confounded with time spent outdoors. The relative contributions of light and physical activity remain to be determined. Moreover, light exposure during the day has also been shown to affect sleep duration. Here, shorter daylight exposure and longer nights are associated with a longer biological night as indexed by the duration of melatonin secretion, and thus longer sleep duration [85, 94, 95], which may also reflect a seasonality effect [104]. Likewise, exposure to daylight has been shown to increase sleep duration, possibly by advancing sleep timing [16]. Beyond this, sleep quality is also related to light exposure during the day. Several studies report that daytime exposure to white light enriched in short-wavelength content was associated with increased evening fatigue [91], and sleep quality [16, 39, 91], decreased sleep-on-set latency [39], and increased slow-wave sleep accumulation [92], which is related to the dissipation of the homeostatic sleep pressure [1, 14, 34]. However, also the timing of light exposure seems to matter for sleep. In this context, Wams and colleagues [92] report that participants with later exposure to light >10lx had more nocturnal awakenings and less slow-wave sleep. In sum, research seems to agree that daylight (at high intensities) is beneficial for sleep.
Exposure to artificial lighting, smartphones and visual display units
In addition to natural daylight, humans are nowadays also exposed to a considerable amount of artificial light. This is particularly the case in the evening hours, i.e. when the circadian system is most sensitive to light-induced phase delays. Thereby, artificial light can delay the timing of the circadian clock and thus sleep [102]. Indeed, light from LED screens has repeatedly been suggested to interfere with sleep and the physiological processes involved (e.g., melatonin secretion [24]). Chang and colleagues [26] for example found that reading a book from an e-reader for four hours before sleep increased sleep onset latency, reduced evening sleepiness, melatonin secretion as well as next-morning alertness, and delayed the timing of the biological clock, which is also in line with other findings [72, 107]. It should be noted though that exposure to the “circadian-active” light source was very long in these studies (4–6.5 h) and it is unclear whether the same results can be expected for shorter exposures.
Evaluating sleep objectively with electroencephalography (EEG), Münch and colleagues [58] found that exposure to short-wavelength light for two hours starting 3 h before habitual bedtime first lead to decreased slow wave activity (SWA) and thus shallower sleep. From this, the authors concluded that the alerting effects of short-wavelength light persist into sleep, which is in line with findings by Chellappa and colleagues [28], who reported a decrease in homeostatic sleep pressure following short-wavelength light exposure in the evening. However, short-wavelength light exposure in the evening was also associated with increased SWA later during the night, suggesting a possible compensatory mechanism [58].
Also, the effects of evening light exposure do not seem to be independent from exposure during the preceding day. More specifically, Rångtell and colleagues [64] examined the effects of reading a novel on a tablet computer (~102 ± 41 lx, 7718 K) vs. in a physical book (~67 ± 50 lx, 2674 K) for two hours following prolonged (6.5 h) exposure to bright light (~569 lx, 3149 K) between 2:30 pm and 9 pm. Contrasting other findings, the light from the tablet did not suppress melatonin or alter subjective and objective sleep parameters. Note though that also exposure was shorter than in studies that reported significant effects [26, 72, 107].
Several studies have reported that smartphone ownership and use before bedtime may be associated with more self-reported sleeping problems [74], decreased sleep efficiency, longer sleep onset latency and poor sleep quality [29], and delays sleep thereby also shortening sleep duration [29, 50, 74]. Modern smartphones contain a “night shift” feature changing the colour balance in the evening hours (Infobox 1 for details). How much of the reported detrimental effect of smartphone use on sleep is due to light per se, or to some other feature (e.g. psychological engagement), is currently not known.
Infobox 1. Smartphones and sleep.
Smartphone use may delay sleep onset. One factor is the light emitted by their screens, but another may also be its entertaining character or related psychological effects, or both. Using the “night shift”mode of modern smartphones, the colour balance of the screen can be shifted to “warmer” and orangeish colours depleted in short-wavelength light. On a recent iPhone 7, this amounts to a reduction of melanopsin activation by 67% at full display brightness. This might seem like a large reduction at first, though by simply dimming the smartphone to its minimum level, the melanopsin activation can be reduced to less than 1% of the activation at maximum display brightness. Whether or not the “night shift mode” has an appreciable effect on the circadian system and how it interacts with other properties of smartphone use is currently not known. Recent research using so-called metameric displays, which do not differ in their appearance but only differ in the amount that they stimulate melanopsin, show that the non-visual properties of light can be modulated independently of visual appearance [3, 78].
Effects of light on mood
Mood variations have been shown to be influenced by a complex and non-additive interaction between circadian phase and the duration of prior wakefulness. Specifically, relatively moderate changes in the timing of the sleep–wake cycle can significantly modulate mood [11].
Light can affect mood in several ways: by directly modulating the availability of neurotransmitters such as serotonin, which is involved in mood regulation, and by entraining and stabilising circadian rhythms, thereby addressing circadian desynchronisation and sleep disorders, which are rather common in people suffering from mental disorders. Therefore, in the last decades, light as an intervention—light therapy—has found an increasingly widespread use for treating mood and other psychiatric disorders [73, 97].
The precise mechanisms by which light exerts a positive influence on mood are currently not known though. In addition to the circadian effects of light mediated via the SCN, a pathway from the retina to the habenula has recently been found to be involved in mediating effects of light on mood in animal models [38, 110]. This pathway, connecting some ipRGCs with the habenula and bypassing the SCN altogether, has been suggested to specifically mediate light-induced alterations in mood [38]. Although it is unclear to what extent these findings can be applied to humans, imaging studies at least suggest that the human habenula is also sensitive to modulations of ambient light [46]. More research is needed to identify the mechanisms underlying light therapy.
In the following, we will provide an overview of the major clinical applications of light therapy and a brief guide to its use in daily clinical practice.
Light therapy as an intervention in psychiatric conditions
Bright light therapy (BLT) for mood disorders was first introduced for the treatment of Seasonal Affective Disorder (SAD) in 1984 [68]. SAD is a subtype of depression characterised by strong seasonal variations in mood states. BLT is nowadays established as first-line treatment for SAD [61, 75] leading to an amelioration of symptoms after a few days of treatment. Light therapy is also effective as second-line treatment for non-seasonal depression, although it usually takes longer (2–5 weeks) than in SAD to achieve a therapeutic effect [2, 75, 87]. BLT, especially in combination with selective serotonin reuptake inhibitors (SSRIs), can accelerate the clinical improvement and lead to significantly fewer residual symptoms [7, 53]. In patients with chronic depression, BLT has been shown to lead to remarkable remission rates compared to placebo [41] and represents a valid therapeutic option also in gender-related mood disorders, such as premenstrual dysphoric disorder and perinatal depression [47, 96].
BLT can be delivered by special, commercially available therapy lamps, which operate at illuminance levels between 7000 and 10,000 lx, but natural daylight during a regular one-hour morning walk has been shown to be similarly effective [99]. In populations who suffer from depressive mood resulting from of a lack of exposure to natural daylight due to, for example, working duties in shift workers, patients with altered sleep–wake rhythms (e.g. delayed sleep–wake phase disorder), or social withdrawal (patients with psychiatric disorders, elderly people), BLT provides an effective treatment and valid alternative to pharmacological approaches [98].
Not only “active” chronotherapeutic approaches, but also an adequate architectural design of the light environment may have relevant clinical implications for psychiatric patients. The availability of light in hospital rooms has been shown to decrease the length of stay of depressed patients in a clinic [6]. Moreover, retrospective analyses revealed a three-day shorter hospitalisation in bipolar depressed inpatients exposed to natural light in sunny hospital rooms compared to those in darker rooms [8].
Light therapy as an intervention in other medical conditions
In recent years, light therapy has been increasingly implemented as an adjunctive therapy for several other medical conditions. In patients with anorexia or bulimia nervosa, light not only improves mood but also helps to better control specific disease-related symptoms (for a review see [5, 49]). Well-controlled longitudinal studies have demonstrated that light not only has antidepressant effects in age-related depression, but can also slow down the progressive cognitive decline in dementia [52, 65]. More generally, due to its rhythm-synchronising properties and its enhancing effects on sleep quality and wakefulness, BLT is becoming an important tool in geriatric care, to treat sleep–wake disturbances and reduce general listlessness [76]. The stabilising effects of light also make BLT a useful additional treatment in adult attention deficit hyperactivity disorder (ADHD) [69], border-line personality disorder [19], and other conditions characterised by sleep–wake disruption, such as schizophrenia [18] or neurodegenerative diseases [103]. New applications are also emerging in internal medicine, e.g. in intensive care units, where day and night differences in lighting are often severely attenuated, which may result in patients developing a fragmented sleep–wake cycle with a negative impact on their recovery [36]. Studies have also demonstrated beneficial effects of BLT in patients with sleep–wake abnormalities after renal transplantation [21] or in cirrhotic patients [33], as well as in severely brain-injured patients in post-comatose states [9, 10], and Parkinson’s disease [90]. Finally, one of the most common applications of light, often in combination with exogenous melatonin, is found in sleep medicine [70, 71] for the treatment of specific circadian rhythm sleep–wake disorders (CRSWD), including advanced and delayed sleep–wake phase disorder, jet lag, shift work, sighted non-24 and irregular sleep–wake phase disorder (for diagnostic criteria see [89]).
Light therapy in practice
Timing, frequency and duration of light therapy sessions
The antidepressant effect of light is most pronounced when it is administered in the early morning hours [86, 97]. For CRSWD, the timing of therapeutic light exposure depends on the type of circadian disturbance and the direction of phase shift (advance or delay) to be pursued in order to achieve circadian resynchronisation. Therefore, a reliable marker of circadian phase should be first assessed to identify the phase position and then determine the timing of light treatment. The gold standard for measuring circadian phase is obtained by quantifying the so-called dim light melatonin onset (DLMO), i.e. the time at which melatonin levels rise above baseline, indicating that melatonin secretion has started. However, implementing DLMO assessment in the clinical practice remains difficult due to the limited availability of equipped centres that perform melatonin analyses and the costs of this diagnostic procedure, which are currently not reimbursed by health insurances in most European countries.
BLT is particularly effective when exposure to light occurs regularly, i.e. on a daily basis, for at least 30–60 min. Therefore, it is commonly performed in a domestic setting, which facilitates the required compliance, especially regarding timing, frequency, and duration of the treatment sessions. Disease relapses due to lacking therapeutic adherence depend on the underlying pathological condition: while SAD may rapidly reappear after a short therapy break, isolated days without light therapy are unlikely to have any negative consequences on circadian rhythm stabilisation in CRSWD, if regular sleep-wake schedules are maintained.
Light therapy devices
Most light therapy devices on the market are suitable for clinical use. They reach a corneal illuminance of 7000–10,000 lx at a viewing distance of 20–35 cm and are equipped with a protective screen with almost complete UV filtering. Ideally designed devices illuminate the patient diagonally from above with an irradiation angle of ~ 15°. A bevelled light surface prevents annoying glare and allows simultaneous reading, thus being better tolerated. To obtain a therapeutic effect, it is not necessary to look directly into the light source, but the eyes must be open. Available light therapy glasses, which even allow mobility during the sessions, also partially meet the required criteria of sufficient light illuminance. However, most of them have not yet been evaluated in large, randomised clinical trials. Another alternative to receive light in the early morning hours is through dawn simulators. These devices start providing a relatively weak light signal about 90 min before wake-up time, which, covering the patients’ final sleep cycle, then gradually increases in intensity from about 0.001 lx to about 300 lx. However, also for these devices, the design plays an important role, as a diffuse, wide lighting area is necessary to reach the sleeper in the different lying positions. For the same reason, other types of available miniature lighting devices are not recommended because of their small luminous field [98].
Adverse reactions
Adverse reactions to light therapy include eye irritation, blurry vision, grumpiness, headache or nausea after light exposure. However, these effects are usually rare and lessen after a few days of treatment or under reduced dosage [98]. Isolated cases of increased excitability following light therapy have been reported in patients with bipolar disorder [98]. Occurring sleep problems such as problems related to initiating sleep when light is administered in the evening, or early morning awakenings when light is administered in the morning, are mostly related to an unappropriated time of light exposure and can be quickly resolved by modifying the timing of light therapy sessions.
Contraindications
Some relative contraindications should be taken into account when considering light therapy in patients with ophthalmological diseases or taking photosensitising drugs. These are summarised in Table 1; [22, 40].
Table 1. Relative contraindications to light therapy.(Modified from [22]).
| Ophthalmological examination recommended in the following conditions |
|
| Caution needed by patients taking following photosensitizing drugs |
|
Summary
Light not only enables us to see fine detail, colour and motion, but also exerts non-visual effects on circadian rhythms, sleep and mood. Light at the wrong time may disrupt circadian rhythms and sleep, but in the form of light therapy, light exposure can be used as an intervention for psychiatric and other medical conditions.
Funding
C. Blume is supported by an Erwin Schroedinger Fellowship from the Austrian Science Fund (FWF J-4243), a grant from the Freiwillige Akademische Gesellschaft (FAG) Basel, and a grant from the Psychiatric Hospital of the University of Basel (UPK). C. Garbazza is supported by a grant of the Swiss National Science Foundation (SNSF 160250). M. Spitschan is supported by a Sir Henry Wellcome Trust Fellowship (Wellcome Trust 204686/Z/16/Z) and Junior Research Fellowship from Linacre College, University of Oxford. Open access funding provided by Austrian Science Fund (FWF).
Abbreviations
- ADHD
Attention deficit hyperactivity disorder
- BLT
Bright light therapy
- CCT
Correlated colour temperature
- CIE
Commission Internationale de l’Eclairage
- CRSWD
Circadian rhythm sleep-wake disorders
- DLMO
Dim-light melatonin onset
- EEG
Electroencephalogram
- GHT
Geniculohypothalamic tract
- IGL
Intergeniculate leaflet
- ipRGC
Intrinsically photosensitive retinal ganglion cell
- LED
Light-emitting diode
- PRC
Phase response curve
- RGC
Retinal ganglion cell
- RHT
Retinohypothalamic tract
- RN
Raphe nuclei
- SAD
Seasonal affective disorder
- SCN
Suprachiasmatic nuclei
- SSRI
Selective serotonin reuptake inhibitor
- SWA
Slow wave activity
- UV
Ultraviolet
Biographies
 Dr. Christine Blume Centre for Chronobiology, Psychiatric Hospital of the University of Basel (UPK), Basel, Switzerland
Dr. Christine Blume Centre for Chronobiology, Psychiatric Hospital of the University of Basel (UPK), Basel, Switzerland
 Dr. med. Corrado Garbazza Centre for Chronobiology, Psychiatric Hospital of the University of Basel (UPK), Basel, Switzerland
Dr. med. Corrado Garbazza Centre for Chronobiology, Psychiatric Hospital of the University of Basel (UPK), Basel, Switzerland
 Dr. Manuel Spitschan Department of Experimental Psychology, University of Oxford, Oxford, UK
Dr. Manuel Spitschan Department of Experimental Psychology, University of Oxford, Oxford, UK
Footnotes
Conflict of interest C. Blume, C. Garbazza and M. Spitschan declare that they have no relevant competing interests.
For this review article no studies with human participants or animals were performed by any of the authors.
References
- 1.Achermann P, Dijk D-J, Brunner DP, et al. A model of human sleep homeostasis based on EEG slow-wave activity: Quantitative comparison of data and simulations. Brain Res Bull. 1993;31:97–113. doi: 10.1016/0361-9230(93)90016-5. [DOI] [PubMed] [Google Scholar]
- 2.Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: meta-analysis of clinical trials. J Affect Disord. 2016;198:64–71. doi: 10.1016/j.jad.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Allen AE, Hazelhoff EM, Martial FP, et al. Exploiting metamerism to regulate the impact of a visual display on alertness and melatonin suppression independent of visual appearance. Sleep. 2018 doi: 10.1093/sleep/zsy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (lambdamax approximately 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 2013;280 doi: 10.1098/rspb.2012.2987. 20122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beauchamp MT, Lundgren JD. A systematic review of bright light therapy for eating disorders. Prim Care Companion CNS Disord. 2016 doi: 10.4088/PCC.16r02008. [DOI] [PubMed] [Google Scholar]
- 6.Beauchemin KM, Hays P. Sunny hospital rooms expedite recovery from severe and refractory depressions. J Affect Disord. 1996;40:49–51. doi: 10.1016/0165-0327(96)00040-7. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti F, Barbini B, Fulgosi MC, et al. Combined total sleep deprivation and light therapy in the treatment of drug-resistant bipolar depression: acute response and long-term remission rates. J Clin Psychiatry. 2005;66:1535–1540. doi: 10.4088/jcp.v66n1207. [DOI] [PubMed] [Google Scholar]
- 8.Benedetti F, Colombo C, Barbini B, et al. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord. 2001;62:221–223. doi: 10.1016/s0165-0327(00)00149-x. [DOI] [PubMed] [Google Scholar]
- 9.Blume C, Angerer M, Raml M, et al. Healthier rhythm, healthier brain? Integrity of circadian melatonin and temperature rhythms relates to the clinical state of brain-injured patients. Eur J Neurol. 2019 doi: 10.1111/ene.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blume C, Lechinger J, Santhi N, et al. Significance of circadian rhythms in severely brain-injured patients: a clue to consciousness? Neurology. 2017 doi: 10.1212/WNL.0000000000003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boivin DB, Czeisler CA, Dijk D-J, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 12.Bollinger T, Schibler U. Circadian rhythms—from genes to physiology and disease. Swiss Med Wkly. 2014 doi: 10.4414/smw.2014.13984. [DOI] [PubMed] [Google Scholar]
- 13.Borbély AA. A two process model of sleep regulation. Human Neurobiology. 1982;1(3):195–204. [PubMed] [Google Scholar]
- 14.Borbély AA, Achermann P, Trachsel L, et al. Sleep initiation and initial sleep intensity: interactions of homeostatic and circadian mechanisms. J Biol Rhythms. 1989;4:37–48. [PubMed] [Google Scholar]
- 15.Borbély AA, Daan S, Wirz-Justice A, et al. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25:131–143. doi: 10.1111/jsr.12371. [DOI] [PubMed] [Google Scholar]
- 16.Boubekri M, Cheung IN, Reid KJ, et al. Impact of windows and daylight exposure on overall health and sleep quality of office workers: a case-control pilot study. J Clin Sleep Med. 2014;10:603–611. doi: 10.5664/jcsm.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bromundt V, Köster M, Georgiev-Kill A, et al. Sleep–wake cycles and cognitive functioning in schizophrenia. Br J Psychiatry. 2011;198:269–276. doi: 10.1192/bjp.bp.110.078022. [DOI] [PubMed] [Google Scholar]
- 19.Bromundt V, Wirz-Justice A, Kyburz S, et al. Circadian sleep-wake cycles, well-being, and light therapy in borderline personality disorder. J Pers Disord. 2013;27:680–696. doi: 10.1521/pedi_2012_26_057. [DOI] [PubMed] [Google Scholar]
- 20.Brown TM, Tsujimura S, Allen AE, et al. Melanopsin-based brightness discrimination in mice and humans. Curr Biol. 2012;22:1134–1141. doi: 10.1016/j.cub.2012.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkhalter H, Wirz-Justice A, Denhaerynck K, et al. The effect of bright light therapy on sleep and circadian rhythms in renal transplant recipients: a pilot randomized, multicentre wait-list controlled trial. Transpl Int. 2015;28:59–70. doi: 10.1111/tri.12443. [DOI] [PubMed] [Google Scholar]
- 22.Cajochen C. Chronobiologie: Licht-und Wachtherapie bei psychiatrischen Erkrankungen. Psych Up2date. 2013;7:173–184. [Google Scholar]
- 23.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 24.Cajochen C, Munch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 25.Cao D, Chang A, Gai S. Evidence for an impact of melanopsin activation on unique white perception. J Opt Soc Am A Opt Image Sci Vis. 2018;35:B287–B291. doi: 10.1364/JOSAA.35.00B287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang A-M, Aeschbach D, Duffy JF, et al. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015;112:1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang AM, Scheer FA, Czeisler CA. The human circadian system adapts to prior photic history. J Physiol. 2011;589:1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chellappa SL, Steiner R, Oelhafen P, et al. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22:573–580. doi: 10.1111/jsr.12050. [DOI] [PubMed] [Google Scholar]
- 29.Christensen MA, Bettencourt L, Kaye L, et al. Direct measurements of smartphone screen-time: relationships with demographics and sleep. PLoS ONE. 2016;11:e165331. doi: 10.1371/journal.pone.0165331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciarleglio C, Resuehr H, Mcmahon D. Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience. 2011;197:8–16. doi: 10.1016/j.neuroscience.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 31.Commision Internationale d’Eclairage [CIE] CIE system for metrology of optical radiation for ipRGC-influenced responses to light. CIE Central Bureau; Vienna,Austria: 2018. [Google Scholar]
- 32.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 33.De Rui M, Middleton B, Sticca A, et al. Sleep and circadian rhythms in hospitalized patients with decompensated cirrhosis: effect of light therapy. Neurochem Res. 2015;40:284–292. doi: 10.1007/s11064-014-1414-z. [DOI] [PubMed] [Google Scholar]
- 34.Dijk D-J, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activityinhumans. JNeurosci. 15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dijk D-J, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 36.Engwall M, Fridh I, Johansson L, et al. Lighting, sleep and circadian rhythm: an intervention study in the intensive care unit. Intensive Crit Care Nurs. 2015;31:325–335. doi: 10.1016/j.iccn.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 37.European Union. EN12464–1:2011. 2011. European Lighting Standard. [Google Scholar]
- 38.Fernandez DC, Fogerson PM, Lazzerini Ospri L, et al. Light affects mood and learning through distinct retina-brain pathways. Cell. 2018;175:71–84.e18. doi: 10.1016/j.cell.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figueiro MG, Steverson B, Heerwagen J, et al. The impact of day time light exposures on sleep and mood in office workers. Sleep Health. 2017;3:204–215. doi: 10.1016/j.sleh.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Gallin PF, Terman M, Reme CE, et al. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthalmol. 1995;119:202–210. doi: 10.1016/s0002-9394(14)73874-7. [DOI] [PubMed] [Google Scholar]
- 41.Goel N, Terman M, Terman JS, et al. Controlled trial of bright light and negative air ions for chronic depression. Psychol Med. 2005;35:945–955. doi: 10.1017/s0033291705005027. [DOI] [PubMed] [Google Scholar]
- 42.Gooley JJ, Rajaratnam SM, Brainard GC, et al. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gronfier C, Wright KP, Jr, Kronauer RE, et al. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004 doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hebert M, Martin SK, Lee C, et al. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibuka N, Kawamura H. Loss of circadian rhythm in sleep-wakefulness cycle in the rat by suprachiasmatic nucleus lesions. Brain Res Brain Res Protoc. 1975;96:76–81. doi: 10.1016/0006-8993(75)90574-0. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser C, Kaufmann C, Leutritz T, et al. The human habenula is responsive to changes in luminance and circadian rhythm. Neuroimage. 2019;189:581–588. doi: 10.1016/j.neuroimage.2019.01.064. [DOI] [PubMed] [Google Scholar]
- 47.Krasnik C, Montori VM, Guyatt GH, et al. The effect of bright light therapy on depression associated with premenstrual dysphoric disorder. Am J Obstet Gynecol. 2005;193:658–661. doi: 10.1016/j.ajog.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 48.Kronauer RE, Forger DB, Jewett ME. Quantifyinghumancircadianpacemakerresponse to brief, extended, and repeated light stimuli over thephototopic range. J Biol Rhythms. 1999;14:501–516. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- 49.Lam RW, Goldner EM, Solyom L, et al. A controlled study of light therapy for bulimia nervosa. Am J Psychiatry. 1994;151:744. doi: 10.1176/ajp.151.5.744. [DOI] [PubMed] [Google Scholar]
- 50.Lemola S, Perkinson-Gloor N, Brand S, et al. Adolescents’ electronicmedia use at night, sleep disturbance, and depressive symptoms in the smartphoneage. J Youth Adolesc. 2015;44:405–418. doi: 10.1007/s10964-014-0176-x. [DOI] [PubMed] [Google Scholar]
- 51.Lewy AJ, Wehr TA, Goodwin FK, et al. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 52.Lieverse R, Van Someren EJ, Nielen MM, et al. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68:61–70. doi: 10.1001/archgenpsychiatry.2010.183. [DOI] [PubMed] [Google Scholar]
- 53.Martiny K. Adjunctive bright light in non-seasonal major depression. Acta Psychiatr Scand. 2004;110:7–28. doi: 10.1111/j.1600-0447.2004.00460_2.x. [DOI] [PubMed] [Google Scholar]
- 54.Meyer-Bernstein E, Morin L. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore RY. Suprachiasmatic nucleus in sleep–wake regulation. Sleep Med. 2007;8:27–33. doi: 10.1016/j.sleep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Mouret J, Coindet J, Debilly G, et al. Suprachiasmatic nuclei lessions in the rat: alterations in sleep circadian rhythms. Electroencephalogr Clin Neurophysiol. 1978;45:402–408. doi: 10.1016/0013-4694(78)90191-8. [DOI] [PubMed] [Google Scholar]
- 58.Münch M, Kobialka S, Steiner R, et al. Wavelength-dependent effects of evening light exposure on sleep architecture and sleep EEG power density in men. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1421–R1428. doi: 10.1152/ajpregu.00478.2005. [DOI] [PubMed] [Google Scholar]
- 59.Najjar RP, Zeitzer JM. Temporal integration of light flashes by the human circadian system. J Clin Invest. 2016;126:938–947. doi: 10.1172/JCI82306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nowozin C, Wahnschaffe A, Rodenbeck A, et al. Applying Melanopic lux to measure biological light effects on Melatonin suppression and subjective sleepiness. Curr Alzheimer Res. 2017;14:1042–1052. doi: 10.2174/1567205014666170523094526. [DOI] [PubMed] [Google Scholar]
- 61.Partonen T, Pandi-Perumal S. Seasonal affective disorder: practice and research. Oxford University Press; Oxford, UK: 2010. [Google Scholar]
- 62.Provencio I, Rodriguez IR, Jiang G, et al. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman SA, St Hilaire MA, Gronfier C, et al. Functional decoupling of melatonin suppression and circadian phase resetting in humans. J Physiol. 2018;596:2147–2157. doi: 10.1113/JP275501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rångtell FH, Ekstrand E, Rapp L, et al. Two hours of evening reading on a self-luminous tablet vs. reading a physical book does not alter sleep after daytime bright light exposure. Sleep Med. 2016;23:111–118. doi: 10.1016/j.sleep.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 65.Riemersma-Van Der Lek RF, Swaab DF, Twisk J, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 66.Rimmer DW, Boivin DB, Shanahan TL, et al. Dynamic resetting of the human circadian pace-maker by intermittent bright light. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1574–R1579. doi: 10.1152/ajpregu.2000.279.5.R1574. [DOI] [PubMed] [Google Scholar]
- 67.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human Chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 68.Rosenthal NE, Sack DA, Gillin JC, et al. Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 69.Rybak YE, Mcneely HE, Mackenzie BE, et al. An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67:1527–1535. doi: 10.4088/jcp.v67n1006. [DOI] [PubMed] [Google Scholar]
- 70.Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. Sleep. 2007;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sack RL, Lewy AJ, Hughes RJ. Use of melatonin for sleep and circadian rhythm disorders. Ann Med. 1998;30:115–121. doi: 10.3109/07853899808999393. [DOI] [PubMed] [Google Scholar]
- 72.Santhi N, Thorne HC, Van Der Veen DR, et al. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz RS, Olds J. The psychiatry of light. Harv Rev Psychiatry. 2015;23:188–194. doi: 10.1097/HRP.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 74.Schweizer A, Berchtold A, Barrense-Dias Y, et al. Adolescents with a smartphone sleep less than their peers. Eur J Pediatr. 2017;176:131–136. doi: 10.1007/s00431-016-2823-6. [DOI] [PubMed] [Google Scholar]
- 75.Sheaves B, Freeman D, Isham L, et al. Stabilising sleep for patients admitted at acute crisis to a psychiatric hospital (OWLS): an assessor-blind pilot randomised controlled trial. Psychol Med. 2018;48:1694–1704. doi: 10.1017/S0033291717003191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shochat T, Martin J, Marler M, et al. Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res. 2000;9:373–379. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 77.Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J Clin Endocrinol Metab. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- 78.Souman JL, Borra T, de Goijer I, et al. Spectral tuning of white light allows for strong reduction in melatonin suppression without changing illumination level or color temperature. J Biol Rhythms. 2018;33(4):420–431. doi: 10.1177/0748730418784041. [DOI] [PubMed] [Google Scholar]
- 79.Spitschan M. Melanopsin contributions to non-visual and visual function. Curr Opini Behav Sci. 2019;30:67–72. doi: 10.1016/j.cobeha.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spitschan M, Jain S, Brainard DH, et al. Opponent melanopsin and S-cone signals in the human pupillary light response. Proc Natl Acad Sci U S A. 2014;111:15568–15572. doi: 10.1073/pnas.1400942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spitschan M, Aguirre GK, Brainard DH, et al. Variation of outdoor illumination as a function of solar elevation and light pollution. Sci Rep. 2016;6 doi: 10.1038/srep26756. 26756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spitschan M, Bock AS, Ryan J, et al. The human visual cortex response to melanopsin-directed stimulation is accompanied by a distinct perceptual experience. Proc Natl Acad Sci U S A. 2017;114:12291–12296. doi: 10.1073/pnas.1711522114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spitschan M, Stefani O, Blattner P, et al. How to report light exposure in human chronobiology and sleep research experiments. Clocks & Sleep. 2019;1(3):280–289. doi: 10.3390/clockssleep1030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stephan FK, Nunez AA. Elimination of circadian rhythms in drinking, activity, sleep, and temperature by isolation of the suprachiasmatic nuclei. Behav Biol. 1977;20:1–16. doi: 10.1016/s0091-6773(77)90397-2. [DOI] [PubMed] [Google Scholar]
- 85.Stothard ER, Mchill AW, Depner CM, et al. Circadian entrainment to the natural light-dark cycle across seasons and the weekend. Curr Biol. 2017;27:508–513. doi: 10.1016/j.cub.2016.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Terman JS, Terman M, Lo E-S, et al. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 87.Terman M, Terman JS, Ross DC. A controlled trial of timed bright light and negative air ionization for treatment of winter depression. Arch Gen Psychiatry. 1998;55:875–882. doi: 10.1001/archpsyc.55.10.875. [DOI] [PubMed] [Google Scholar]
- 88.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thorpy M. International classification of sleep disorders. In: Chokroverty S, editor. Sleep disorders medicine: basic science, technical considerations and clinical aspects. Springer; New York,NY: 2017. pp. 475–484. [Google Scholar]
- 90.Videnovic A, Klerman EB, Wang W, et al. Timed light therapy for sleep and daytime sleepiness associated with parkinson disease: a randomized clinical trial. Jama Neurol. 2017;74:411–418. doi: 10.1001/jamaneurol.2016.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viola AU, James LM, Schlangen LJ, et al. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34(4):297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- 92.Wams EJ, Woelders T, Marring I, et al. Linking light exposure and subsequent sleep: a field Polysomnography study in humans. Sleep. 2017 doi: 10.1093/sleep/zsx165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Webler F, Spitschan M, Foster R, et al. What is the “spectraldiet” of humans? Curr Opini Behav Sci. 2019;30:80–86. doi: 10.1016/j.cobeha.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod) J Clin Endocrinol Metab. 1991;73:1276–1280. doi: 10.1210/jcem-73-6-1276. [DOI] [PubMed] [Google Scholar]
- 95.Wehr TA, Moul DE, Barbato G, et al. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol Regul Integr Comp Physiol. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- 96.Wirz-Justice A, Bader A, Frisch U, et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry. 2011;72:986–993. doi: 10.4088/JCP.10m06188blu. [DOI] [PubMed] [Google Scholar]
- 97.Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders: a clinician’s manual for light and wake therapy. 2nd edn. Karger Medical and Scientific Publishers; Basel, Switzerland: 2013. [Google Scholar]
- 98.Wirz-Justice A, Bromundt V. Lichttherapie. Schlaf. 2013;2:20–29. [Google Scholar]
- 99.Wirz-Justice A, Graw P, Kräuchi K, et al. ‘Natural’light treatment of seasonal affective disorder. J Affect Disord. 1996;37:109–120. doi: 10.1016/0165-0327(95)00081-x. [DOI] [PubMed] [Google Scholar]
- 100.Woelders T, Leenheers T, Gordijn MCM, et al. Melanopsin- and L-cone-induced pupil constriction is inhibited by S- and M-cones in humans. Proc Natl Acad Sci U S A. 2018;115:792–797. doi: 10.1073/pnas.1716281115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wright HR, Lack LC. Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int. 2001;18:801–808. doi: 10.1081/cbi-100107515. [DOI] [PubMed] [Google Scholar]
- 102.Wright KP, Mchill AW, Birks BR, et al. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wulff K, Gatti S, Wettstein JG, et al. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 104.Yetish G, et al. Natural sleep and its seasonal variations in three pre-industrial societies. Current Biology. 2015;25(21):2862–2868. doi: 10.1016/j.cub.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Youngstedt SD, Elliott JA, Kripke DF. Human circadian phase—response curves for exercise. J Physiol. 2019;597:2253–2268. doi: 10.1113/JP276943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zeitzer JM. When is a proxy not a proxy? The foibles of studying non-image forming light. J Physiol. 2018;596:2029–2030. doi: 10.1113/JP276076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeitzer JM, Dijk D-J, Kronauer RE, et al. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeitzer JM, Fisicaro RA, Ruby NF, et al. Millisecond flashes of light phase delay the human circadian clock during sleep. J Biol Rhythms. 2014;29:370–376. doi: 10.1177/0748730414546532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zele AJ, Adhikari P, Feigl B, et al. Cone and melanopsin contributions to human brightness estimation. J Opt Soc Am A Opt Image Sci Vis. 2018;35:B19–B25. doi: 10.1364/JOSAA.35.000B19. [DOI] [PubMed] [Google Scholar]
- 110.Zhang B-B, Yao Y-Y, Zhang H-F, et al. Left Habenula mediates light-preference behavior in Zebrafish via an asymmetrical visual pathway. Neuron. 2017;93:914–928.e914. doi: 10.1016/j.neuron.2017.01.011. [DOI] [PubMed] [Google Scholar]