Abstract
Background and Purpose
Research into memory deficits associated with hypoxic ischemic encephalopathy (HIE) has typically focused on the hippocampus but there is emerging evidence that the medial diencephalon may also be compromised. We hypothesized that mammillary body (MB) damage occurs in perinatal asphyxia, potentially resulting in MB atrophy and subsequent memory impairment.
Methods
We retrospectively reviewed brain MRIs of 235 clinically confirmed full-term HIE patients acquired at a single-center during the period 2004-2017. MRIs were performed within 10 days of birth (median 6, IQR 2). Two radiologists independently assessed the MBs for abnormal signal on T2-weighted and DWI sequences. Follow-up MRIs were available for 9 patients; these were examined for evidence of MB and hippocampal atrophy.
Results
In 31 neonates (13.2%) abnormal high MB signal was seen on T2-weighted sequences, 4 with mild, 25 with moderate, and two with severe HIE. In addition, restricted diffusion was seen in 6 neonates who had an MRI between day 5 and 7. For these 31 neonates, the most common MRI pattern (41.9%) was abnormal signal restricted to the MBs with the rest of the brain appearing normal. Follow-up MRIs were available for 9 patients: 8 acquired between 3 and 19 months and one acquired at 7.5years. There was MB atrophy in 8 of the 9 follow-up MRIs.
Conclusion
Approximately 13% of full-term infants with HIE showed abnormal high MB signal on T2-weighted images during the acute phase, which progressed to MB atrophy in all but one of the infants who had a follow-up MRI. This MB involvement does not appear to be related to the severity of encephalopathy, MRI patterns of HIE, or pathology elsewhere in the brain.
Keywords: Mammillary body, neonate, hypoxic ischemic encephalopathy (HIE), MRI
Introduction
The mammillary bodies (MBs) are a pair of small projections situated at the posterior margin of the hypothalamus at the base of the brain, named ‘mammillary’ because of their shape. They have repeatedly been implicated in mnemonic processes and are particularly important for episodic (i.e., event) memory1–3. MB damage is typically associated with conditions linked to thiamine-deficiency, in particular Korsakoff syndrome4, however, it appears that the MBs may be implicated in a greater number of neurological conditions than previously appreciated5–7.
In Infants, brain areas that are typically vulnerable to hypoxic-ischemic encephalopathy (HIE) include deep gray nuclei, cortex and white matter, depending on the severity and duration of the insult8. In addition, the limbic system is involved in some patients, showing diffusion restriction on diffusion weighted imaging (DWI), particularly in the hippocampal region9, 10. Full-term neonates with perinatal asphyxia can exhibit hippocampal volume loss from childhood onwards as a consequence of possible cytotoxic injury at the time of hypoxia-ischemia11. Developmental amnesia, a disorder primarily affecting episodic memory, typically occurs as a result of early-onset hypoxia-induced damage to the hippocampus12. However, the extent of hippocampal pathology does not always predict the severity of subsequent memory impairment13. Consistent with this, a recent study by Dzieciol et al. examined MR images of 18 developmental amnesia patients (age 11-35 years) and uncovered a marked degree of atrophy in the MBs in two-thirds of the patients14. However, as the MR images were acquired a number of years following the hypoxic-ischemic event, the authors were unable to conclude whether the MB atrophy was directly related to the hypoxic-ischemic episode or due to subsequent degeneration14, 15.
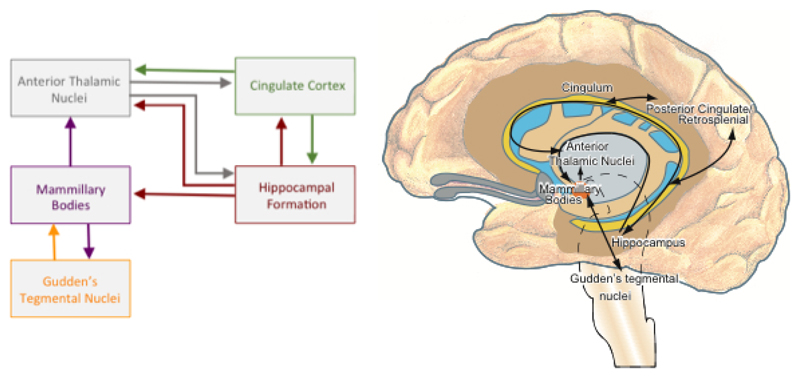
The aim of the present study, therefore, was to examine whether the MBs are affected in infants with HIE. Given previous reports of MB sensitivity to hypoxia in adults16, 17, we hypothesized that the MBs would be directly compromised in HIE in neonates and that this could be detected on MRIs acquired during the neonatal period. We assessed MRI signal change in the MBs of infants with perinatal asphyxia. The sample-group included infants treated with therapeutic hypothermia and those who did not undergo hypothermia treatment. In infants with abnormal MB signal, the wider Papez circuit (e.g., hippocampal formation, fornix, cingulate gyri and the anterior thalamic nuclei; Figure1) was also examined for abnormalities. Where possible, follow-up MR images were evaluated to determine whether abnormal MB signal during the acute phase was associated with subsequent MB atrophy.
Figure 1.
Schematic of the extended Papez system highlighting the principal connections of the mammillary bodies.
Materials and Methods
Patients
In this retrospective observational single-center study, a total of 235 full-term neonates were enrolled, meeting the previously described clinical criteria of HIE18, and having undergone a brain MRI at the University Medical Center Utrecht, Wilhelmina Children’s Hospital during the period 2004-2017. The patients have been included in previous studies8, 9, 19–22. A waiver for informed consent was obtained, according to European regulations, as the present study involved the analysis of anonymized data.
The total group included two subsets. The first subset comprised 70 neonates, born between 2004 and January 2008, who did not receive therapeutic hypothermia. The second subset comprised 165 neonates, born between February 2008 and 2017, who were treated with whole body hypothermia. Clinical data were retrieved from the patients’ records.
MRI Acquisition and Assessment
Cerebral MRI was performed within 3-10 days of birth (median 6, IQR 2), on day 3-9 (normothermia infants) and day 5-10 (hypothermia infants)20, 23. MRI protocols have been described previously19. Briefly, MRI was performed on a 1.5 Tesla or 3.0 Tesla MR System (Philips Medical Systems, Best, The Netherlands). The scanning protocol included sagittal T1 prior to 2013 and sagittal T2-weighted imaging after 2013 (sagittal T2-weighted images were available 64 patients). This was followed by axial T1-weighted and T2-weighted sequences with 2 mm slice thickness. In addition, an echo planar imaging technique was used for diffusion-weighted imaging, axial with 4 mm slice thickness, 0 mm section gap and b-values of 0 and 1000 s/mm2 (1.5 T) or b-values 0 and 800 s/mm2 (3.0 T).
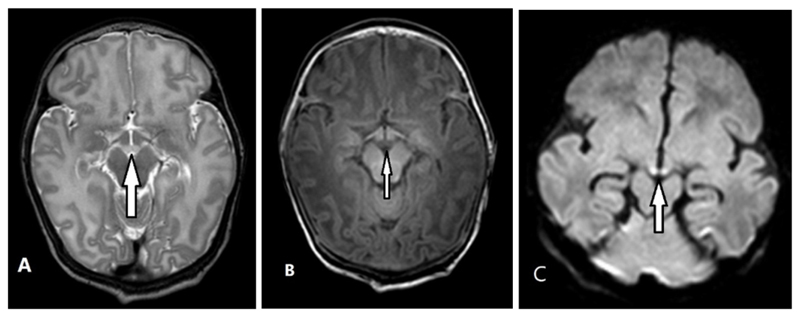
All MRIs were retrospectively screened, independently, by two radiologists (ML and MM; with experience of reporting neonatal MRIs for 20 years and 3 years, respectively). The MBs were assessed for abnormal high signal on the T2-weighted images and/or abnormal signal on the DWI images (high signal on the trace maps and low signal on the ADC maps). Given the size and location of the MBs, it was important to reduce the likelihood of identifying artifactual signal due to partial volume effects. Therefore, an increased signal intensity had to be clearly visible over two consecutive T2-weighted slices. This increased signal intensity was then confirmed by subjectively assessing swelling in MBs and/or observing decreased signal intensity on T1-weighted images. All available sections and directions were evaluated. DWI images were considered to be positive when signal changes (high signal on trace maps and low signal on ADC maps) were present over two consecutive slices. The slice thickness in DWI sequences was 4mm, and therefore more prone to artifactual signals, as such, only infants with obvious signal changes on T2-weighted images were included (Figure 2). In addition to examining the MBs, images were examined for abnormal signal intensity in other constituents of the extended Papez circuit24, 25 (see Figure 1): hippocampi (HC), fornixes (F), anterior nuclei of the thalamus (AT) and cingulate gyri (CG). The tegmental nuclei in the brainstem were too small to assess due to the slice thickness of 2mm (T2) or more (DWI).
Figure 2.
Arrows indicate: abnormally high signal MBs on axial T2W image (A); abnormally low signal MBs on axial T1W image (B); abnormally high signal MBs on DWI, B value 1000, accompanied by low ADC (not shown) (C).
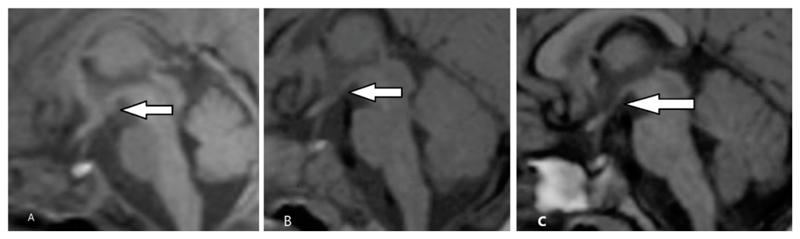
Nine patients underwent a follow-up MRI: eight at 3-19 months and one at 7.5 years. Two radiologists (ML & MM) independently assessed the scans for evidence of subjectively-judged MB atrophy. The MBs were considered atrophic if there was a lack of protrusion clearly seen on sagittal sections (Figure 3). The hippocampi were also examined for signs of atrophy.
Figure 3.
Sagittal T1-weighted MRI. The arrows indicate abnormal low signal in MBs at 6 days (A) and MB atrophy at 3 months (B) and 19 months (C).
Neurodevelopmental follow up
Clinical follow-up data was reviewed, where available, which focused on IQ, motor and neurological functions. The assessments provide a general evaluation of development but do not specifically measure memory. The tests administered were Bayley Scales of Infant Development (BSID), Griffiths Mental Development Scales (GMDS), Wechsler Preschool and Primary Scales of Intelligence (WPPSI) and the Movement ABC test. Patients were tested on the BSID and GMDS at 24 months and on the WPPSI and Movement ABC test at 5 years.
Descriptive statistics were calculated using SPSS V.21 (SPSS-IBM, Chicago, Illinois, USA).
Results
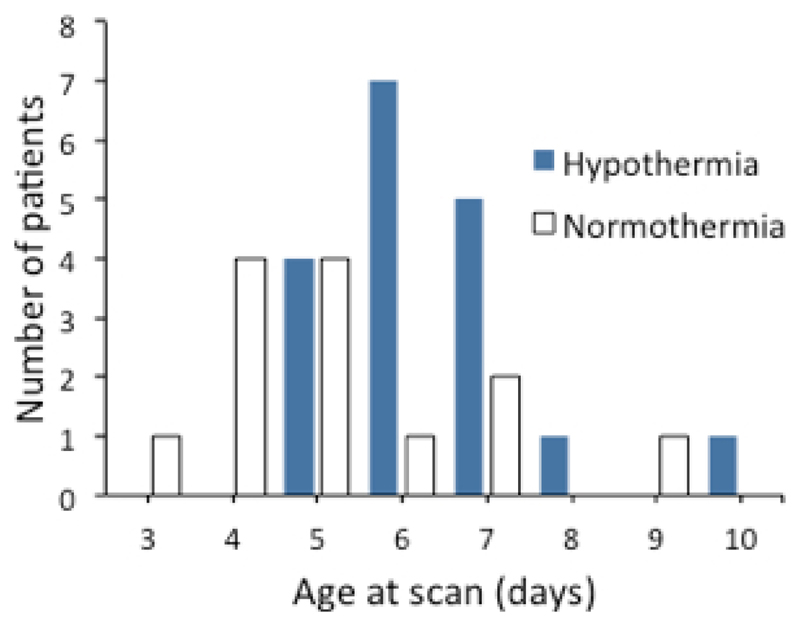
An increased T2-weighted signal was noted in the MBs in 31 of the 235 infants (13.2%) (Figure2). Details of these patients are presented in Table 1. T2-weighted hyperintensity was observed in the MBs of 18 infants (10.9%) with therapeutic hypothermia, and 13 infants (18.6%) without therapeutic hypothermia. Concomitant diffusion restriction was found in 6 of these infants (4 with therapeutic hypothermia and 2 without). The day of MRI acquisition is shown in Figure 4; abnormal MB signal could be identified across imaging days 3-10.
Table 1. general demographic and clinical findings of neonates with high MB signal on T2W images.
| T2W high signal MB cases(31) | |
|---|---|
| Gender male/female | 15/16 |
| Gestational Age (mean ± SD) | 40 ± 2.0 |
| Birth weight (mean ± SD) | 3113 ± 556 |
| Apgar 1 min (median, IQR) | 2 (3) |
| Apgar 5 min (median, IQR) | 5 (4) |
| Age at first MRI in days after birth (median, IQR) | 6 (2) |
| Field strength of MRI (1.5 T/3.0 T) | 17/14 |
| Grade of encephalopathy (Sarnat I/II/III) | 4/25/2 |
| Hypothermia (%) | 18 (58) |
| Died (%) | 4 (13) |
| Normal clinical follow up (%) | 21 (68) |
| Abnormal clinical follow up (%) | 5(16) |
| Severe hyponatremia (<130 mEq/L)(%) | 8 (26) |
| Systemic infection (%) | 3 (10) |
Figure 4.
Day (post-birth) of initial MRI scan in patients with hypothermia-treatment or without (normothermia). The day of scanning was typically day 5-7 for the hypothermia-treated patients whereas there was a greater range of scanning days for normothermia patients.
In 13 (41.9%) of these 31 patients, the MRI appeared otherwise normal. The occurrence rates of common pathology patterns associated with hypoxia are summarized in Table 2. When additional damage was present, the watershed predominant pattern of damage occurred most commonly (in 29% of infants). Involvement of other structures within the Papez circuit is shown in Table 3; hippocampal involvement was the most common presentation occurring in approximately one-third of the infants.
Table 2. MRI pattern of hypoxic-ischemic lesion.
| NL | 13(41.9%) |
| BGT | 5(16.1%) |
| WS | 9(29%) |
| NT | 3(9.7%) |
| Large subdural hematoma | 1(3.2%) |
| Total | 31 |
NL: normal, BGT: basal ganglia and thalamus, WS: watershed, NT: near total
Table 3. Involvement of additional structures within the Papez circuit.
| F | 1 |
| HC | 4 |
| AT | 0 |
| CG | 1 |
| AT/F | 1 |
| HC/F | 7 |
| CG | 0 |
| More than two involved | 4 |
| None | 13(41.9%) |
| Total | 31 |
F: fornix, HC: hippocampus, AT: anterior thalamus, CG: cingulate gyrus
Of the 31 patients, 8 patients had at least one follow-up MRI ranging between 3 and 19 months after the first MRI and one patient had follow-up MRI at 7.5 years. Clinical findings for patients with available follow-up MRIs are provided in Table 4. MB atrophy was observed in 8 out of 9 infants with a follow-up MRI (Table 4); hippocampal atrophy was present in 6 of these patients.
Table 4. Clinical and MRI findings for patients with follow-up MRI.
| # | GA | BW | APGAR 1-5 | MRI | DWI | T2W | other Papez inv. | Sys. Infec. | Severe Hypo Na+ | Therapeutic hypothermia | follow up MRI (month later after the first MRI) | Sarnat grade | clinical FU data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | 2150 | 9-10 | WS | n | y | HC/F | - | yes | + | MB VL(19) | II | 5.7 y: normal |
| 2 | 39 | 3175 | 9-10 | NT | n | y | N | y | no | + | MB & HC VL(19) | II | 5.2 y cerebral visual impairment; Mov-ABC p4; TIQ 94; PIQ 102; VIQ 93 |
| 3 | 39 | 2800 | 4-6 | NT | n | y | F/AT | - | no | + | MB VL (19) | II | CP, PIQ 52; hearing loss; concentration problems |
| 4 | 40 | 3900 | 1-3 | NL | n | y | HC | - | - | + | Normal (7.5 years) | 7y: TIQ 78; VIQ 85; PIQ 76 | |
| 5 | 41 | 3105 | 5-7 | WS | n | y | F | - | + | MB & HC VL (3) | II | 5.5 y: normal | |
| 6 | 38 | 2740 | 1-4 | WS | n | y | N | - | + | MB & HC VL (3) | II | 5.6 y: low IQ, no formal tests | |
| 7 | 39 | 2620 | 1-3 | WS | n/y | y | HC | yes | - | MB & HC VL (3) | II | 2 y: normal | |
| 8 | 36 | 2630 | 2-4 | WS | y | y | HC/F | yes | - | MB & HC VL (4) | II | 9 months: normal | |
| 9 | 36 | 3118 | 1-3 | NL | y | y | HC/F | no | - | MB & HC VL (3) | II | 2y: normal |
MB: mammillary bodies, HC: hippocampus, VL: volume loss, F: fornix, WS: watershed, NL: normal, NT: near total Mov-ABC: Movement-ABC, TIQ: total intelligence quotient, PIQ: performance intelligence quotient, VIQ: verbal intelligence quotient
Clinical follow up results
Of the 31 identified neonates with abnormal MB signal, four died before discharge due to redirection of care because of severe brain injury or associated multi-organ failure. The majority (21) was assessed as “normal” at early follow-up. Four patients had an abnormal outcome. Three of these patients had a total IQ on the WPPSI < 85 at 5 years. In one of these patients, this low-IQ was associated with hearing loss and concentration problems. The fourth patient had a cerebral visual impairment, although normal performance on IQ tests. One patient showed some behavioral problems but was otherwise normal. The final patient was under 12 months, so too young to be assessed.
Discussion
Improvements in medical care have resulted in an increasing number of children surviving perinatal asphyxia. Therefore, there is a growing need to identify the sites of neuropathology following hypoxic-ischemic (HI) episodes in neonates, and how this may contribute to subsequent cognitive impairments.
The present study focused on the mammillary bodies (MBs) as atrophy in this region has been reported in an older group of patients who had suffered a HI episode in childhood14. However, the suggestion from this earlier study was that the MB atrophy was a result of degeneration following primary damage to the hippocampus or limbic cortex14. Contrary to this explanation, we found evidence of MRI signal change in the MBs of neonates with hypoxic-ischemic encephalopathy (HIE) with T2 signal rise in 13% of HIE patients. In 13/31 infants the signal intensity abnormalities were only seen in the MBs making them almost certain to be the site of primary injury. It is, therefore, possible to conclude that the MBs can be directly affected in HIE. Where signal change was observed in multiple regions, it is possible that the MB involvement is secondary. However, if this were the case, on the basis of known connectivity the most likely site of primary pathology would be the hippocampal formation1. A study in rhesus monkeys found no abnormal MB signal 6 days following experimental hippocampal lesions26, again suggesting that in the timescale of the current study, the MB involvement is likely to be a primary effect. Together, the implication is that while secondary degeneration may cause additional pathology within the MBs at longer time periods, this structure is nonetheless sensitive to direct effects of HI episodes in neonates.
To the best of our knowledge, this is the first study to assess MB MRI signal changes in neonates with HIE. Therefore the current findings add to the increasing body of literature from adult patients where there is ante-mortem and post-mortem evidence of MB necrosis following severe HI17, 27. Reduced MB volume has also been reported in patients with obstructive sleep apnea6 and patients with heart failure28, again conditions linked to both acute and chronic hypoxia. The findings from both neonatal and adult patients further highlight the need to assess MB status in conditions with both acute and chronic low brain oxygen levels.
MB pathology is most commonly associated with, or exaggerated by, thiamine deficiency4, 29–31. Thiamine is essential for intracellular metabolism and acts as a neuroprotective agent against oxidative stress. Reduced thiamine may exacerbate the effects of hypoxia on MBs and has been highlighted as a possible contributing factor in a number of studies where adult MB atrophy has been found following hypoxic-ischemic episodes6, 17, 27, 28. But thiamine deficiency is not a prerequisite for hypoxia-related MB atrophy in adults16. Furthermore, all patients in the present study were administered thiamine following birth, therefore thiamine deficiency would not be a contributing factor to the increased signal intensity observed in the current cohort.
We also checked for signs of cerebritis, which may be a possible cause of MB signal rise and DWI restriction. No evidence of cerebral infection was found either on MR images or clinically, except in three patients with signs of a systemic infection. None of the 31 patients underwent a lumbar puncture, so a CNS infection cannot be ruled out completely, but it seems unlikely looking at the clinical outcome and, in some patients, follow-up MRI findings.
Surprisingly, we noticed MB involvement not only in severe HIE patients but also in milder HIE. On the other hand, it was of interest that some infants with severe HIE did not show any MB abnormality on T2 or DWI series. These preliminary findings would suggest that MB involvement does not directly correspond to HIE severity. Furthermore, in some patients the MBs were the only structure that showed a signal change again suggesting that this region has a different sensitivity to hypoxia than other brain structures. The duration and severity of the HI episode may be key determinants of the MBs sensitivity. For example, a short acute moment of hypoperfusion of the brain due to a HIE triggered cardiac malfunctioning may be sufficient to cause damage to the MBs. This could explain those patients with abnormal MB signal but an otherwise normal MRI. The lack of MB pathology in some severely asphyxiated patients would, however, suggest some degree of neuroprotection in these patients, which needs to be investigated further.
Given the importance of the MBs for episodic memory32, it is likely that MB pathology in HIE patients contributes to the memory impairments that can be associated with this patient group. However, the concomitant hippocampal damage makes it difficult to attribute specific impairments to the MBs. In the current study, there were 13 patients (42%) where the initial scan showed MB signal rise without any visible signal changes of other structures within the Papez circuit. These patients would be of particular interest in terms of neuropsychological follow-up. While there are reports of localized MB infarcts in adults causing amnesia33, 34, it is not known whether developmentally acquired MB damage would produce similar neuropsychological outcomes.
In one case we noted an acute T2W signal change in the MBs but there was no observable atrophy on follow-up MRI. Unfortunately long-term clinical follow up was not available in this case. It is possible that the MBs were dysfunctional despite no obvious cell loss, alternatively it is possible that there was some degree of neuroprotection against subsequent cell loss. Again, this highlights the importance of assessing individual differences in response to HIE as this could prove critical in generating treatments that provide additional neuroprotection to those populations most at risk.
While 13% of examined cases were found to have signal change in the MBs, this is likely to be an underestimation of total occurrence. Given the size of the MBs, particularly in neonates, a 4 mm slice thickness results in the MBs being poorly visualized35. Furthermore, the location of the MBs at the base of the brain can produce artifact, which obscures imaging details. This problem was exacerbated further with the ADC maps but, in some patients, we noted low ADC signal at the MBs suggesting ischemia. This emphasizes the need to focus on the T2 sequence, because in all 6 patients where the DWI was positive, the T2 images were also positive, but the reverse was not found to be true.
In summary, this study has shown that abnormal MB signal can be found in the acute period following HIE in neonates and this abnormal signal appears an effective predictor of subsequent MB atrophy. The sensitivity for damage to the MBs does not seem to be directly related to severity of hypoxia, as indicated by standard measures, and does not appear to be affected by treatment involving whole-body cooling. Given the importance of the MBs for memory, this further highlights the need to more closely examine this structure in both HIE patient groups and other conditions associated with reduced brain oxygen levels.
Future
This study highlights the need to carefully assess the MBs in neonates with HIE. For the most accurate assessment of the MBs high-resolution images are needed using the thinnest slices possible (≤2 mm for the T2-weighted sequence). Furthermore, the use of a dedicated thin-slice DWI sequence, adjusted for the skull base, would improve the accuracy of identifying MB involvement in this patient group. Repeated scanning over a range of days after birth could also be informative for identifying the time-window most sensitive to MB signal change. With detailed clinical assessment and subsequent MRI and neurocognitive follow-up at school age, it may be possible to determine the consequences of early-onset MB pathology and identify those patients most at risk during the acute phase.
Acknowledgements
SDV is funded by a Welcome Trust Senior Research Fellowship in Biomedical Sciences (WT 212273/Z/18/Z).
Abbreviations
- MB
Mammillary body
- HIE
Hypoxic-ischemic encephalopathy
- DWI
Diffusion weighted imaging
References
- 1.Vann SD. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia. 2010;48:2316–2327. doi: 10.1016/j.neuropsychologia.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Sziklas V, Petrides M. Memory and the region of the mammillary bodies. Prog Neurobiol. 1998;54:55–70. doi: 10.1016/s0301-0082(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 3.Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- 4.Kopelman MD. The Korsakoff syndrome. Brit J Psychiatry. 1995;166:154–173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- 5.Tsivilis D, Vann SD, Denby C, et al. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- 6.Kumar R, Birrer BVX, Macey PM, et al. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett. 2008;438:330–334. doi: 10.1016/j.neulet.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein HG, Krause S, Krell D, et al. Strongly reduced number of parvalbumin-immunoreactive projection neurons in the mammillary bodies in schizophrenia: further evidence for limbic neuropathology. Ann N Y Acad Sci. 2007;1096:120–127. doi: 10.1196/annals.1397.077. [DOI] [PubMed] [Google Scholar]
- 8.de Vries LS, Groenendaal F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology. 2010;52:555–566. doi: 10.1007/s00234-010-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alderliesten T, Nikkels PG, Benders MJ, et al. Antemortem cranial MRI compared with postmortem histopathologic examination of the brain in term infants with neonatal encephalopathy following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2013;98:F304–309. doi: 10.1136/archdischild-2012-301768. [DOI] [PubMed] [Google Scholar]
- 10.Kasdorf E, Engel M, Heier L, et al. Therapeutic hypothermia in neonates and selective hippocampal injury on diffusion-weighted magnetic resonance imaging. Pediatr Neurol. 2014;51:104–108. doi: 10.1016/j.pediatrneurol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Maneru C, Serra-Grabulosa JM, Junque C, et al. Residual hippocampal atrophy in asphyxiated term neonates. J Neuroimaging. 2003;13:68–74. [PubMed] [Google Scholar]
- 12.Gadian DG, Aicardi J, Watkins KE, et al. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123(Pt 3):499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JM, Gadian DG, Jentschke S, et al. Neonatal hypoxia, hippocampal atrophy, and memory impairment: evidence of a causal sequence. Cereb Cortex. 2015;25:1469–1476. doi: 10.1093/cercor/bht332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzieciol AM, Bachevalier J, Saleem KS, et al. Hippocampal and diencephalic pathology in developmental amnesia. Cortex. 2017;86:33–44. doi: 10.1016/j.cortex.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loftus M, Knight RT, Amaral DG. An analysis of atrophy in the medial mammillary nucleus following hippocampal and fornix lesions in humans and nonhuman primates. Exp Neurol. 2000;163:180–190. doi: 10.1006/exnr.2000.7361. [DOI] [PubMed] [Google Scholar]
- 16.Johkura K, Naito M. Wernicke's encephalopathy-like lesions in global cerebral hypoxia. J Clin Neurosci. 2008;15:318–319. doi: 10.1016/j.jocn.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Schmidtke K. Wernicke-Korsakoff syndrome following attempted hanging. Rev Neurol (Paris) 1993;149:213–216. [PubMed] [Google Scholar]
- 18.Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–742. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 19.Alderliesten T, de Vries LS, Benders MJ, et al. MR imaging and outcome of term neonates with perinatal asphyxia: value of diffusion-weighted MR imaging and 1H MR spectroscopy. Radiology. 2011;261:235–242. doi: 10.1148/radiol.11110213. [DOI] [PubMed] [Google Scholar]
- 20.Alderliesten T, de Vries LS, Staats L, et al. MRI and spectroscopy in (near) term neonates with perinatal asphyxia and therapeutic hypothermia. Arch Dis Child Fetal Neonatal Ed. 2017;102:F147–F152. doi: 10.1136/archdischild-2016-310514. [DOI] [PubMed] [Google Scholar]
- 21.Alderliesten T, de Vries LS, Khalil Y, et al. Therapeutic hypothermia modifies perinatal asphyxia-induced changes of the corpus callosum and outcome in neonates. PLoS One. 2015;10:e0123230. doi: 10.1371/journal.pone.0123230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weeke LC, Groenendaal F, Mudigonda K, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatrics. 2018;192:33–40 e32. doi: 10.1016/j.jpeds.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bednarek N, Mathur A, Inder T, et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology. 2012;78:1420–1427. doi: 10.1212/WNL.0b013e318253d589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–743. [Google Scholar]
- 25.Vann SD, Nelson AJ. The mammillary bodies and memory: more than a hippocampal relay. Prog Brain Res. 2015;219:163–185. doi: 10.1016/bs.pbr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Froudist-Walsh S, Browning PGF, Croxson PL, et al. The Rhesus Monkey Hippocampus Critically Contributes to Scene Memory Retrieval, But Not New Learning. J Neurosci. 2018;38:7800–7808. doi: 10.1523/JNEUROSCI.0832-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vortmeyer AO, Hagel C, Laas R. Hypoxia-ischemia and thiamine deficiency. Clin Neuropathol. 1993;12:184–190. [PubMed] [Google Scholar]
- 28.Kumar R, Woo MA, Birrer BVX, et al. Mammillary bodies and fornix fibers are injured in heart failure. Neurobiol Dis. 2009;33:236–242. doi: 10.1016/j.nbd.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beh SC, Frohman TC, Frohman EM. Isolated mammillary body involvement on MRI in Wernicke's encephalopathy. J Neurol Sci. 2013;334:172–175. doi: 10.1016/j.jns.2013.07.2516. [DOI] [PubMed] [Google Scholar]
- 30.Kornreich L, Bron-Harlev E, Hoffmann C, et al. Thiamine deficiency in infants: MR findings in the brain. AJNR American journal of neuroradiology. 2005;26:1668–1674. [PMC free article] [PubMed] [Google Scholar]
- 31.Zuccoli G, Siddiqui N, Bailey A, et al. Neuroimaging findings in pediatric Wernicke encephalopathy: a review. Neuroradiology. 2010;52:523–529. doi: 10.1007/s00234-009-0604-x. [DOI] [PubMed] [Google Scholar]
- 32.Dillingham CM, Milczarek MM, Perry JC, et al. Mammillothalamic disconnection alters hippocampo-cortical oscillatory activity and microstructure: Implications for diencephalic amnesia. J Neurosci. 2019 doi: 10.1523/JNEUROSCI.0827-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amuluru K, Filippi CG, Lignelli A. Acute Amnesia due to Isolated Mammillary Body Infarct. J Stroke Cerebrovasc Dis. 2015;24:e303–305. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Male S, Zand R. Isolated Mammillary Body Infarct Causing Global Amnesia: A Case Report. J Stroke Cerebrovasc Dis. 2017;26:e50–e52. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.115. [DOI] [PubMed] [Google Scholar]
- 35.Denby CE, Vann SD, Tsivilis D, et al. The frequency and extent of mammillary body atrophy associated with surgical removal of a colloid cyst. AJNR American journal of neuroradiology. 2009;30:736–743. doi: 10.3174/ajnr.A1424. [DOI] [PMC free article] [PubMed] [Google Scholar]