CKD and structural heart disease are closely associated
Chronic kidney disease (CKD) and heart failure (HF) frequently co-exist and both are associated with high morbidity and mortality [1, 2]. Numerous studies have shown that there is an inverse association between kidney function and cardiovascular risk [3, 4]. Structural heart disease, which may manifest clinically as HF, is a leading cause of cardiovascular disease in CKD patients and its prevalence increases with declining kidney function [2, 5]. A cross-sectional echocardiographic observational study reported an increasing prevalence of left ventricular hypertrophy (LVH) with decreasing eGFR (from 32% among patients with eGFR ≥60 mL/min/1.73m2 to 75% among patients with eGFR <30 mL/min/1.73m2) [6, 7]. Studies using cardiac magnetic resonance imaging with gadolinium enhancement have found that diffuse late gadolinium enhancement (LGE) is associated with the degree of LVH [8] and indicates myocyte disarray and interstitial fibrosis histologically [9]. Although overt systolic dysfunction is not common (affecting only 8% of patients in the above cross-sectional echocardiographic study) and not clearly associated with kidney function [7], more subtle disturbances in ventricular function (such as reduced left ventricular deformation, early myocardial relaxation velocity or reduction in global longitudinal strain which may contribute to diastolic dysfunction) are more common and are present even in the early stages of CKD [10, 11]. These abnormalities provide the anatomical substrate for the excess risk of symptomatic HF, arrhythmia and sudden cardiac death observed among patients with advanced CKD. Conversely, in large heart failure registries, 20-68% of patients with HF have moderate to severe kidney disease [1]. The presence of CKD is associated with poor prognosis in HF and can be used to stratify risk of patients with HF [6, 12, 13].
Pathophysiology of HF in CKD
The pathophysiological relationship between the heart and the kidneys involves many different pathways. CKD may disturb homeostasis in ways which may be directly damaging to the cardiovascular system (ie, “direct” risk factors such as high blood pressure or vascular calcification), or the kidneys and circulation may both be subject to “indirect” risk factors (eg, diabetes mellitus, smoking). In addition, HF may worsen CKD by decreasing renal perfusion, causing renal venous congestion and activation of the sympathetic nervous system and renin–angiotensin–aldosterone system (RAS, which may in turn cause inflammation and oxidative stress). Treatment for HF in CKD can be considered in two broad types: (i) treatments that intervene on pathophysiological links between CKD and HF to prevent HF; and (ii) treatments known to improve prognosis in established HF among people without CKD.
Treatment to prevent HF in CKD
CKD is commonly associated with high blood pressure (BP), due to salt and water retention, activation of sympathetic nervous and other neuro-hormonal systems and accumulation of endogenous vasopressors [14]. Studies of living kidney donors suggest that reducing GFR by 10 mL/min as a consequence of donor nephrectomy leads to a 5 mmHg increase in systolic BP [15]. BP is positively associated with the risk of death from heart failure [16] and randomized trials have demonstrated that this association is causal [17]. Meta-analysis of all the major BP-lowering trials has shown that a 10 mmHg reduction in systolic BP lowers the risk of heart failure by 28% (95% CI 22-33%) [18]. Most classes of antihypertensive treatments have similar effects with the exception of calcium-channel blockers (which may have a smaller benefit) and diuretics (which may have a larger benefit) [18]. A subgroup analysis within this meta-analysis (which included 13 trials involving nearly 38,000 participants of whom 6000 had CKD) suggested that the effect of BP-lowering on HF was larger among patients without CKD (RR 0.48; 95% CI 0.38-0.62) than among patients with CKD (RR 0.95; 95% CI 0.70-1.04; p for interaction <0.001) [18]. Nevertheless, the benefits of lowering BP on other cardiovascular outcomes remain clear even among patients with CKD.
Anaemia is a well-recognised complication of CKD and has been proposed as a direct cause of HF in patients with CKD following observational and non-randomized interventional studies which suggested that anaemia was associated with LVH and correcting the anaemia reversed the LVH [19, 20]. However, randomized trials have shown that full or partial correction of anaemia with erythropoiesis-stimulating agents (ESA) does not reduce left ventricular mass nor the risk of heart failure and may even increase the risk of other cardiovascular outcomes such as stroke [21].
Reducing parathyroid hormone (PTH) concentrations with calcimimetic therapy might reduce the risk of non-atherosclerotic cardiovascular events (such as HF) among haemodialysis patients [22, 23]. Such treatment also reduces fibroblast growth factor 23 (see below). Unfortunately the randomized data on other interventions that target CKD-specific mechanisms of HF are much less robust. For example, although there is evidence that hyperphosphataemia (a) can cause vascular smooth muscle cells to adopt an osteoblastic phenotype and cause vascular calcification (which in turn increases cardiac afterload) [24], and (b) is associated with LVH [25], no sufficiently large trials of phosphate reduction have been conducted to elucidate whether these associations are causal. Although FGF23 has been found to induce LVH after direct intracardiac injection in mice [26], the totality of the observational evidence does not suggest that FGF23 is a cause of cardiovascular disease (and no trials of FGF23 reduction in CKD exist) [27].
Treatment to improve prognosis in established HF in the general population
The main objectives of HF therapy in CKD (as well as in non-CKD) patients are to (i) decrease the preload and afterload and to reduce LVH; (ii) treat myocardial ischaemia; and (iii) inhibit neuro-humoral hyperactivity, especially the sympathetic nervous system and RAS [28]. However, the optimum treatment of HF in patients with CKD remains unclear, as there is little direct evidence to support any recommendations. Most of the pivotal randomized trials that guide the management of heart failure define CKD as a baseline eGFR <60 mL/min/1.73m2 but have excluded patients with more advanced stages of chronic kidney disease (ie, eGFR <30 mL/min/1.73m2).
Many pharmacological and device treatments are recommended for heart failure with reduced ejection fraction (HFrEF) [29]. The mainstay of such treatment is angiotensin converting enzyme inhibition (ACEi) and beta-blockade. The largest trial of ACEi in HFrEF was SOLVD-TREATMENT which compared enalapril 10 mg twice daily with placebo among 2569 patients with HFrEF and demonstrated a 16% (95% CI 5-26%) reduction in mortality (primary outcome) [30]. This effect was similar in patients with and without CKD [31]. Similarly, in the four large trials of beta-blockers in HFrEF, there was no good evidence that the benefits of beta-blocker therapy were modified by baseline kidney function. The results of these trials (and their published effects by baseline kidney function) are summarised in Table 1.
Table 1. Effect of kidney function on the efficacy of established treatments for chronic heart failure with reduced ejection fraction.
Data extracted from large trials where subgroup analysis by kidney function is available.
| Trial (ref) | Intervention (sample size) | Main eligibility criteria | Follow-up (y) |
Primary outcome | Overall treatment effect (95% CI) | CKD subgroups (eGFR, mL/min/1.73m2) | Treatment effect in CKD | p for treatment x CKD interaction |
|---|---|---|---|---|---|---|---|---|
| ACEi | ||||||||
| SOLVD-TREATMENT [31] | Enalapril vs placebo (n=2569) | LVEF ≤35%; NYHA I-IV; creatinine <177 μmol/L |
3.5 | All-cause mortality | 0.84 (0.74-0.95) | ≥60 (n=1466) | 0.82 (0.69-0.98) | 0.62 |
| <60 (n=1036) | 0.88 (0.73-1.06) | |||||||
| Beta-blocker | ||||||||
| CIBIS-II [56] | Bisoprolol vs placebo (n=2647) |
LVEF ≤35%; NYHA III-IV; creatinine <300 μmol/L |
1.3 | All-cause mortality | 0.66 (0.54-0.81) | <45 (n=450) | 0.71 (0.48-1.05) | 0.81 |
| ≥45<60 (n=669) | 0.69 (0.46-1.04) | |||||||
| ≥60<75 (n=640) | 0.53 (0.34-0.82) | |||||||
| >75 (n=863) | 0.64 (0.42-0.99) | |||||||
| MERIT-HF [57, 58] | Metoprolol vs placebo (n=3991) |
LVEF≤40%; NYHA II-IV; “significant” kidney disease |
1 | All-cause mortality | 0.66 (0.53-0.81) | <45 (n=493) | 0.41 (0.25-0.68) | 0.095 |
| ≥45≤60 (n=976) | 0.68 (0.45-1.02) | |||||||
| >60 (n=2496) | 0.71 (0.54-0.95) | |||||||
| SENIORS [59, 60] | Nebivolol vs placebo (n=2128) |
LVEF <35% or hospitilisation for decompensated HF; NYHA II-IV; creatinine <250 μmol/L |
1.75 | All-cause mortality or CV hospital admission | 0.86 (0.74-0.99) | <55.5 (n=704) | 0.84 (0.67-1.07) | 0.442 |
| 55.5-72.8 (n=704) | 0.79 (0.60-1.04) | |||||||
| >72.8 (n=704) | 0.86 (0.65-1.14) | |||||||
| Mineralocorticoid receptor antagonist | ||||||||
| RALES [37, 61] | Spironolactone vs placebo (n=1663) |
LVEF<35%; NYHA III-IV; creatinine ≤221 μmol/L; |
2 | All-cause mortality | 0.70 (0.60-0.82) | <60 (n=792) | 0.68 (0.56-0.84) | N/A |
| ≥60 (n=866) | 0.71 (0.57-0.90) | |||||||
| EMPHASIS-HF [62] | Eplerenone vs placebo (n=2737) |
LVEF ≤35%; NYHA II; eGFR ≥30 mL/min/1.73m2 |
1.75 | CV death or hospitalisation for HF | 0.63 (0.54-0.74) | <60 (n=912) | N/A | 0.50 |
| ≥60 (n=1821) | N/A | |||||||
| Angiotensin receptor neprilysin inhibitor | ||||||||
| PARADIGM-HF [43] | Sacubitril/Valsartan vs enalapril (n=8442) |
LVEF ≤40%; NYHA II-IV; eGFR ≥30 mL/min/1.73m2 |
2.25 | CV death or hospitalization for HF | 0.80 (0.73-0.87) | <60 (n=3061) | N/A | 0.91 |
| ≥60 (n=5338) | N/A | |||||||
| Implantable defibrillator (ICD) | ||||||||
| MADIT II [63] | Prophylactic ICD vs conventional medical therapy (n=1232) |
LVEF ≤30%; NYHA III; eGFR ≥ 15mL/min/1.73m2 |
2.67 | All-cause mortality | 0.69 (0.51-0.93) | <35 (n=80) | 1.09 (0.49-2.43) | 0.29 |
| 35-59 (n=387) | 0.74 (0.48-1.15) | |||||||
| ≥60 (n=756) | 0.66 (0.43-1.02) | |||||||
| Cardiac resynchronization therapy (CRT) | ||||||||
| CARE-HF [64] | CRT vs conventional medical therapy (n=813) |
LVEF ≤35%; NYHA III-IV; |
1.5 | Death from any cause or unplanned hospitalization for a major CV event | 0.63 (0.51-0.77) | <60 (n=369) | 0.67 (0.50-0.89) | N/A |
| ≥60 (n=370) | 0.57 (0.40-0.80) | |||||||
ACEi: angiotensin converting enzyme inhibitor; CRT: cardiac resynchronisation therapy; ICD: implantable cardiac defibrillator; HF: heart failure; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; CV: cardiovascular; N/A: not available
For patients with HFrEF (with a left ventricular ejection fraction [LVEF] <35%) who remain symptomatic after optimisation of ACEi and beta-blocker therapy, guidelines recommend a mineralcorticoid receptor antagonist (MRA). This recommendation follows two large trials (see Table 1). Again, the effect of treatment on the primary outcome was not modified by baseline kidney function. However, these trials highlight the importance of safety as a consideration in the treatment of patients with CKD. Patients with CKD are at higher risk of hyperkalaemia (due to the reduced ability of their kidneys to excrete potassium) which is associated with an increased risk of hospitalization and death [32]. The trials had stringent monitoring of serum potassium and developed criteria for reducing the dose or stopping the MRA, such that there was no excess of death due to hyperkalaemia in the trials. The importance of such monitoring is highlighted by population-based studies which demonstrate increased rates of hospitalization for hyperkalaemia since the publication of these trials [33]. Device therapies (implantable cardioverter defibrillators [ICD] and cardiac resynchronisation therapy [CRT]) also improve prognosis in selected patients with HFrEF). A meta-analysis of the trials of ICDs has raised the hypothesis that worse kidney function might attenuate the benefit of these devices [34], but this is not the case for CRT devices. Intravenous iron has been shown to improve functional capacity among patients with HFrEF and results of clinical outcomes trials are needed [35]. Indeed, the PIVOTAL trial among haemodialysis patients suggests that intravenous iron may reduce cardiovascular morbidity in this population [36]. This finding may alter the interpretation of the placebo-controlled ESA trials in which participants allocated placebo received more iron.
However, as noted above, few patients with CKD have HFrEF whereas structural substrates for diastolic dysfunction are common among patients with CKD. By contrast with HFrEF, no treatment has yet demonstrated convincing benefit (in terms of morbidity and mortality) in patients with heart failure with moderately reduced ejection fraction (HFmrEF: LVEF ≥40 <50%) or heart failure with preserved ejection fraction (HFpEF: LVEF ≥50%). The TOPCAT trial tested spironolactone (15-45 mg daily) versus placebo in 3445 patients with LVEF ≥45% and observed a non-significant 11% (95% CI -4 to 23%) reduction in the primary outcome of cardiovascular death, aborted cardiac arrest or hospitalization for heart failure [37]. There was again no modification of the treatment effect by baseline kidney function. However, post hoc analyses have suggested that patients recruited from certain geographic regions had significantly worse adherence to treatment (when measured biochemically) which may have made the overall result a “false negative” [38].
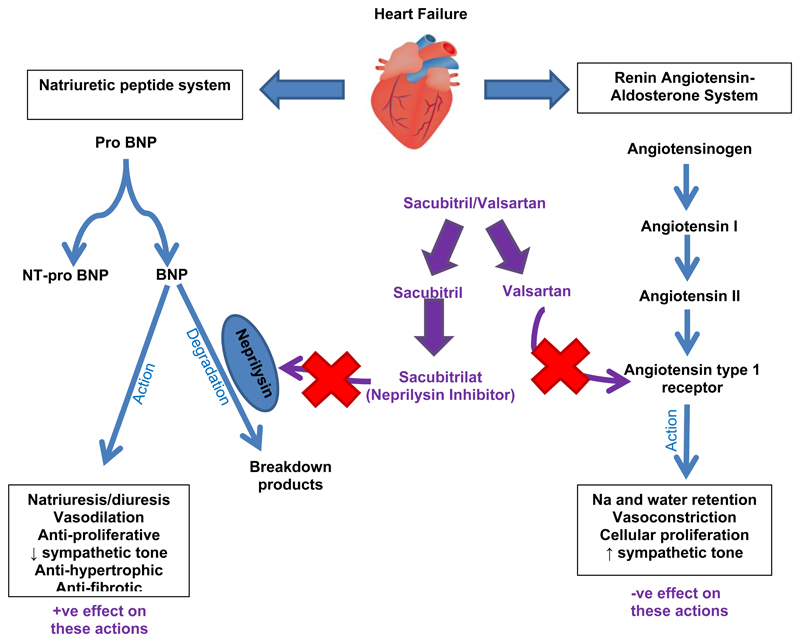
Neprilysin inhibition
Neprilysin (also known as neutral endopeptidase, NEP) degrades natriuretic and other vasoactive peptides (including bradykinin, substance P, endothelin and angiotensin II) and therefore neprilysin inhibition (NEPi) enhances the activity of the natriuretic peptide system leading to natriuresis, diuresis, blood pressure reduction and inhibition of RAS and sympathetic nervous system [39]. Isolated NEPi causes reflex activation of the RAS so development of NEPi has always been combined with ACEi or angiotensin receptor blockade (ARB). The potential of NEPi in HFrEF was suggested in the OVERTURE trial which compared omapatrilat (a combined ACEi and NEPi) to enalapril in 5770 patients with HF and found a non-significant 6% (95% CI -3 to 14%) reduction in the primary outcome of all-cause mortality or hospitalization for heart failure [40]. However, development of omapatrilat was stopped when the OCTAVE trial (in 25,302 patients with hypertension) found an excess risk of angioedema compared to enalapril (2.17% versus 0.68%; p<0.005) [41]. This was thought to be due to excessive bradykinin concentrations (as both ACE and NEP degrade bradykinin) and led to the development of a new class of drug called an angiotensin receptor neprilysin inhibitor (ARNI), which combines NEPi with an ARB.
Sacubitril/valsartan is a first-in-class ARNI that is rapidly metabolized after ingestion to the NEPi pro-drug sacubitril and the ARB valsartan. Sacubitril/valsartan reduces BP more than equivalent doses of valsartan alone [42]. The PARADIGM-HF trial randomized 8442 participants with HFrEF to treatment with sacubitril/valsartan or enalapril and was terminated earlier than planned based on the recommendation by the Data Monitoring Committee after interim efficacy analysis showed overwhelming evidence of benefit at a median follow-up duration of 27 months. Compared to those assigned to enalapril, participants assigned to sacubitril/valsartan in PARADIGM-HF experienced a 20% (95% CI 13-27%) reduction in the primary composite endpoint of cardiovascular death or HF hospitalization. This effect was again similar among participants with and without CKD. Sacubitril/valsartan is now recommended in the European Society of Cardiology guidelines as a replacement for ACEi (or ARB) in patients who have symptomatic heart failure with a reduced left ventricular ejection fraction of ≤ 35% and who remain symptomatic despite maximum-tolerated evidence-based treatment [29, 43].
Sacubitril/valsartan has also been tested among patients with HFpEF. The PARAMOUNT trial compared sacubitril/valsartan with valsartan in 301 patients with change in NT-proBNP as the primary outcome [44]. At 12 weeks, among participants assigned sacubitril/valsartan NT-proBNP was 23% (95% CI 8-36%) lower compared to participants assigned valsartan. The PARAGON-HF trial has recruited 4822 participants with HFpEF to compare sacubitril/valsartan with valsartan and is scheduled to complete in mid-2019 [45]. The primary outcome is the composite of cardiovascular death and total (first and recurrent) hospitalizations for heart failure.
In addition to its known benefits in HFrEF (and potential for benefit in HFpEF), neprilysin inhibition might also have beneficial effects on the kidney. Experiments using 5/6 nephrectomy models suggested that neprilysin inhibition reduces proteinuria and histological markers of kidney damage more than ACE inhibition alone [46, 47]. In addition, sacubitril/valsartan appeared to slow the deterioration of kidney function in PARADIGM-HF [48] and PARAMOUNT [49]. However, it also modestly increased albuminuria in both trials (although baseline levels were very low in these heart failure populations) [50].
The UK HARP-III trial was designed to investigate the short- to medium-term effects of sacubitril/valsartan 97/103 mg twice daily versus irbesartan 300 mg once daily on kidney function among patients with established CKD [51]. Patients were eligible for UK HARP-III if either (i) their eGFR was ≥20 <45 mL/min/1.73m2; or (ii) their eGFR was ≥45 <60 mL/min/1.73m2 and urine albumin:creatinine ratio >20 mg/mmol. Other pre-specified outcomes included albuminuria, blood pressure and cardiac biomarkers. 414 participants were randomized and the average estimated glomerular filtration rate (GFR) was 35 mL/min/1.73m2 and median urine albumin:creatinine ratio was 54 mg/mmol. Only 4% and 13% reported heart failure and coronary heart disease respectively at baseline.
The primary outcome of measured GFR at 12 months did not differ between the two groups: the difference in means was -0.1 (SE 0.7) mL/min/1.73m2 [52]. Albuminuria was not significantly reduced (9% [95% CI -1 to 18%] among those assigned sacubitril/valsartan) despite an additional 5.4/2.1 (both p<0.001) mmHg reduction in blood pressure. Despite the apparent lack of an effect on short-to medium-term kidney function, allocation to sacubitril/valsartan did reduce both NT-proBNP and troponin I compared to allocation to irbesartan. Study average concentations of NT-proBNP and troponin I were 18% (95% CI 11-25%) and 16% (95% CI 8-23%) lower respectively.
Although the effects on kidney function are not encouraging, they do not exclude a benefit on long-term progression of CKD (although any effect would not be large). However, the effects on BP and cardiac biomarkers support the hypothesis that sacubitril/valsartan might reduce the risk of cardiovascular events (and in particular those related to heart failure) among patients with CKD, irrespective of whether they have known cardiac disease. The neutral effects on tolerability and safety outcomes in UK HARP-III would also support further investigation of this hypothesis.
Conclusion
The burden of HF among patients with CKD is considerable and contributes significantly to the excess of cardiovascular morbidity and mortality observed in this growing population. The anatomical substrates of HF develop early in the progression of CKD and strategies to prevent it have not been rigorously tested in the CKD population. Furthermore, trials among patients with known HF have usually excluded patients with moderate or advanced CKD so the efficacy and – importantly – safety of these treatments in the CKD population are uncertain. Neprilysin inhibition looks promising as a treatment that could reduce the risk of HF safely among patients with CKD, but clinical outcome trials are required. Newer treatments for HF, such as sodium glucose co-transporter-2 inhibitors, are being tested in large trials in both HF and CKD populations [53–55] and may be the first treatments which have proven efficacy for HF among patients with a wide-spectrum of kidney disease. Nevertheless, further trials of established and future interventions are required that allow doctors to confidently reduce the excess risk of cardiovascular disease in CKD.
Figure 1.
Effects of sacubitril/valsartan on vasoactive peptides (DRAFT – to be redrawn by NDT artist)
Footnotes
Conflict of interest
The UK HARP-III trial was funded by a grant to the University of Oxford from Novartis Pharma. The trial was conducted, analysed and published independently of the funder. See www.ctsu.ox.ac.uk.
References
- 1.Ahmed A, Campbell RC. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin. 2008;4(4):387–399. doi: 10.1016/j.hfc.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segall L, Nistor I, Covic A. Heart failure in patients with chronic kidney disease: a systematic integrative review. Biomed Res Int. 2014;2014 doi: 10.1155/2014/937398. 937398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mafham M, Emberson J, Landray MJ, Wen CP, Baigent C. Estimated glomerular filtration rate and the risk of major vascular events and all-cause mortality: a meta-analysis. PLoS One. 2011;6(10):e25920. doi: 10.1371/journal.pone.0025920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chronic Kidney Disease Prognosis C. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagshaw SM, Cruz DN, Aspromonte N, Daliento L, Ronco F, Sheinfeld G, Anker SD, Anand I, Bellomo R, Berl T, et al. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25(5):1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 6.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, Committee ASA, Investigators High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13(6):422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. Journal of the American Society of Nephrology : JASN. 2012;23(10):1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, Steedman T, Dargie HJ, Jardine AG. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69(10):1839–1845. doi: 10.1038/sj.ki.5000249. [DOI] [PubMed] [Google Scholar]
- 9.Aoki J, Ikari Y, Nakajima H, Mori M, Sugimoto T, Hatori M, Tanimoto S, Amiya E, Hara K. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney International. 2005;67(1):333–340. doi: 10.1111/j.1523-1755.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- 10.Rakhit DJ, Zhang XH, Leano R, Armstrong KA, Isbel NM, Marwick TH. Prognostic role of subclinical left ventricular abnormalities and impact of transplantation in chronic kidney disease. Am Heart J. 2007;153(4):656–664. doi: 10.1016/j.ahj.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Edwards NC, Hirth A, Ferro CJ, Townend JN, Steeds RP. Subclinical abnormalities of left ventricular myocardial deformation in early-stage chronic kidney disease: the precursor of uremic cardiomyopathy? J Am Soc Echocardiogr. 2008;21(12):1293–1298. doi: 10.1016/j.echo.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Campbell RC, Sui X, Filippatos G, Love TE, Wahle C, Sanders PW, Ahmed A. Association of chronic kidney disease with outcomes in chronic heart failure: a propensity-matched study. Nephrol Dial Transplant. 2009;24(1):186–193. doi: 10.1093/ndt/gfn445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Ide T, Takeshita A, Tsutsui H, Investigators J-C Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) Circ J. 2009;73(8):1442–1447. doi: 10.1253/circj.cj-09-0062. [DOI] [PubMed] [Google Scholar]
- 14.Edmunds ME, R G. Hypertension in renal failure. In: JD S, editor. Textbook of hypertension. Oxford: Blackwell Scientific Publications; 1994. [Google Scholar]
- 15.Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen-Philbrook H, Yang RC, Rosas-Arellano MP, Housawi A, Garg AX, Donor Nephrectomy Outcomes Research N Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145(3):185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 17.Turnbull F, Blood Pressure Lowering Treatment Trialists C Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362(9395):1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 18.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 19.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34(1):125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 20.Macdougall IC, Lewis NP, Saunders MJ, Cochlin DL, Davies ME, Hutton RD, Fox KA, Coles GA, Williams JD. Long-term cardiorespiratory effects of amelioration of renal anaemia by erythropoietin. Lancet. 1990;335(8688):489–493. doi: 10.1016/0140-6736(90)90733-l. [DOI] [PubMed] [Google Scholar]
- 21.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, Pellegrini F, Ravani P, Jardine M, Perkovic V, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Annals of internal medicine. 2010;153(1):23–33. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 22.Investigators ET. Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler DC, London GM, Parfrey PS, Block GA, Correa-Rotter R, Dehmel B, Drueke TB, Floege J, Kubo Y, Mahaffey KW, et al. Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: the EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J Am Heart Assoc. 2014;3(6):e001363. doi: 10.1161/JAHA.114.001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circulation research. 2000;87(7):E10–17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 25.Chue CD, Edwards NC, Moody WE, Steeds RP, Townend JN, Ferro CJ. Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: a cardiac magnetic resonance study. Heart. 2012;98(3):219–224. doi: 10.1136/heartjnl-2011-300570. [DOI] [PubMed] [Google Scholar]
- 26.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, et al. FGF23 induces left ventricular hypertrophy. The Journal of clinical investigation. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marthi A, Donovan K, Haynes R, Wheeler DC, Baigent C, Rooney CM, Landray MJ, Moe SM, Yang J, Holland L, et al. Fibroblast Growth Factor-23 and Risks of Cardiovascular and Noncardiovascular Diseases: A Meta-Analysis. Journal of the American Society of Nephrology : JASN. 2018;29(7):2015. doi: 10.1681/ASN.2017121334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang AY, Sanderson JE. Treatment of heart failure in long-term dialysis patients: a reappraisal. Am J Kidney Dis. 2011;57(5):760–772. doi: 10.1053/j.ajkd.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 30.Investigators S. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 31.Bowling CB, Sanders PW, Allman RM, Rogers WJ, Patel K, Aban IB, Rich MW, Pitt B, White M, Bakris GC, et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: insights from the SOLVD Treatment trial. Int J Cardiol. 2013;167(1):151–156. doi: 10.1016/j.ijcard.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomsen RW, Nicolaisen SK, Hasvold P, Sanchez RG, Pedersen L, Adelborg K, Egstrup K, Egfjord M, Sorensen HT. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes-a Danish population-based cohort study. Nephrol Dial Transplant. 2017 doi: 10.1093/ndt/gfx312. [DOI] [PubMed] [Google Scholar]
- 33.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 34.Pun PH, Al-Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, et al. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in CKD: a meta-analysis of patient-level data from 3 randomized trials. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(1):32–39. doi: 10.1053/j.ajkd.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Luscher TF, Bart B, Banasiak W, Niegowska J, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. The New England journal of medicine. 2009;361(25):2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 36.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, McMurray JJV, Murray H, Tomson CRV, Wheeler DC, et al. Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. The New England journal of medicine. 2019;380(5):447–458. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 37.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 38.de Denus S, O'Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, et al. Spironolactone Metabolites in TOPCAT - New Insights into Regional Variation. N Engl J Med. 2017;376(17):1690–1692. doi: 10.1056/NEJMc1612601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Judge P, Haynes R, Landray MJ, Baigent C. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant. 2015;30(5):738–743. doi: 10.1093/ndt/gfu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106(8):920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 41.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17(2):103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375(9722):1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 43.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England journal of medicine. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 44.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 45.Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, et al. Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial. JACC Heart Fail. 2017;5(7):471–482. doi: 10.1016/j.jchf.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Taal MW, Nenov VD, Wong W, Satyal SR, Sakharova O, Choi JH, Troy JL, Brenner BM. Vasopeptidase inhibition affords greater renoprotection than angiotensin-converting enzyme inhibition alone. J Am Soc Nephrol. 2001;12(10):2051–2059. doi: 10.1681/ASN.V12102051. [DOI] [PubMed] [Google Scholar]
- 47.Cao Z, Burrell LM, Tikkanen I, Bonnet F, Cooper ME, Gilbert RE. Vasopeptidase inhibition attenuates the progression of renal injury in subtotal nephrectomized rats. Kidney Int. 2001;60(2):715–721. doi: 10.1046/j.1523-1755.2001.060002715.x. [DOI] [PubMed] [Google Scholar]
- 48.Packer M, Claggett B, Lefkowitz MP, McMurray JJV, Rouleau JL, Solomon SD, Zile MR. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018;6(7):547–554. doi: 10.1016/S2213-8587(18)30100-1. [DOI] [PubMed] [Google Scholar]
- 49.Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2015;17(5):510–517. doi: 10.1002/ejhf.232. [DOI] [PubMed] [Google Scholar]
- 50.Ruggenenti P, Remuzzi G. Combined neprilysin and RAS inhibition for the failing heart: straining the kidney to help the heart? Eur J Heart Fail. 2015;17(5):468–471. doi: 10.1002/ejhf.267. [DOI] [PubMed] [Google Scholar]
- 51.Judge PK, Haynes R, Herrington WG, Storey BC, Staplin N, Bethel A, Bowman L, Brunskill N, Cockwell P, Dayanandan R, et al. Randomized multicentre pilot study of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease: United Kingdom Heart and Renal Protection (HARP)-III-rationale, trial design and baseline data. Nephrol Dial Transpl. 2017;32(12):2043–2051. doi: 10.1093/ndt/gfw321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, et al. Effects of Sacubitril/Valsartan Versus Irbesartan in Patients With Chronic Kidney Disease. Circulation. 2018;138(15):1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818. [DOI] [PubMed] [Google Scholar]
- 53.Zannad FF, Filippatos G, Butler J, Salsali A, Kimura K, Schnee J, Zeller C, Pocock S, George J, Brueckmann M, et al. Design and rationale of the EMPagliflozin outcome trial in patients with chronic heart failure (EMPEROR-Reduced) European Journal of Heart Failure. 2018;20:441–441. [Google Scholar]
- 54.Butler J, Packer M, Filippatos G, Zannad F, Salsali A, Kimura K, Schnee J, Zeller C, Pocock S, George J, et al. Design and rationale of the EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure (EMPEROR-Preserved) European Journal of Heart Failure. 2018;20:232–232. [Google Scholar]
- 55.Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, George JT, Green JB, Landray MJ, Baigent C, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11(6):749–761. doi: 10.1093/ckj/sfy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castagno D, Jhund PS, McMurray JJ, Lewsey JD, Erdmann E, Zannad F, Remme WJ, Lopez-Sendon JL, Lechat P, Follath F, et al. Improved survival with bisoprolol in patients with heart failure and renal impairment: an analysis of the cardiac insufficiency bisoprolol study II (CIBIS-II) trial. Eur J Heart Fail. 2010;12(6):607–616. doi: 10.1093/eurjhf/hfq038. [DOI] [PubMed] [Google Scholar]
- 57.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–2007. [PubMed] [Google Scholar]
- 58.Ghali JK, Wikstrand J, Van Veldhuisen DJ, Fagerberg B, Goldstein S, Hjalmarson A, Johansson P, Kjekshus J, Ohlsson L, Samuelsson O, et al. The influence of renal function on clinical outcome and response to beta-blockade in systolic heart failure: insights from Metoprolol CR/XL Randomized Intervention Trial in Chronic HF (MERIT-HF) J Card Fail. 2009;15(4):310–318. doi: 10.1016/j.cardfail.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Cohen-Solal A, Kotecha D, van Veldhuisen DJ, Babalis D, Bohm M, Coats AJ, Roughton M, Poole-Wilson P, Tavazzi L, Flather M, et al. Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail. 2009;11(9):872–880. doi: 10.1093/eurjhf/hfp104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26(3):215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 61.Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, Solomon SD, Investigators R Influence of baseline and worsening renal function on efficacy of spironolactone in patients With severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study) J Am Coll Cardiol. 2012;60(20):2082–2089. doi: 10.1016/j.jacc.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 62.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group E-HS Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 63.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, Greenberg H, Case RB, Multicenter Automatic Defibrillator Implantation Trial III Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98(4):485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L, Cardiac Resynchronization-Heart Failure Study I The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]