Abstract
A considerable limitation of current small animal positron emission tomography/computed tomography (PET/CT) imaging is the low throughput of image acquisitions. Subsequently, to design sufficiently-powered studies, high costs accumulate. Together with Mediso Medical Imaging Systems, a four-bed mouse ‘hotel’ was developed to simultaneously image up to four mice, thereby reducing the cost and maximising radiotracer usage when compared to scans performed with a single mouse bed.
Methods
For physiological evaluation of the four-bed mouse hotel, temperature and anaesthesia were tested for uniformity, followed by 18F-2-fluoro-2-deoxyglucose (18F-FDG) PET/CT imaging of ‘mini’ image quality (IQ) phantoms specifically designed to fit the new imaging system. Post-reconstruction, National Electrical Manufacturers Association (NEMA) NU-4 tests examined uniformity, recovery coefficients (RCs) and spill-over ratios (SORs). To evaluate the bed under standard in vivo imaging conditions, four mice were simultaneously scanned by dynamic 18F-FDG PET/CT over 60 minutes using the four-bed mouse hotel, with quantified images compared to those acquired using a single mouse bed.
Results
The bed maintained a constant temperature of 36.8°C ± 0.4°C (n = 4), with anaesthesia distributed evenly to each nose cone (2.9 ± 0.1 L/min, n = 4). The NEMA tests performed on reconstructed mini IQ phantom images acquired using the four-bed mouse hotel revealed values within the tolerable limits for uniformity, RC values in >2mm rods, and SORs in the non-radioactive water- and air-filled chambers. There was low variability in radiotracer uptake in all major organs of mice scanned using the four-animal bed versus those imaged using a single bed imaging platform.
Conclusion
Analysis of images acquired using the four-bed mouse hotel confirmed its utility to increase the throughput of small animal PET imaging without considerable loss of image quality and quantitative precision. In comparison to a single mouse bed, the cost and time associated with each scan were substantially reduced.
Keywords: High-throughput, mouse hotel, micro PET/CT, 18F-FDG, economic
Introduction
As a non-invasive imaging tool, positron emission tomography (PET) is used in preclinical research across multidisciplinary areas of work for whole-body, dynamic examination of biochemical processes under normal and pathophysiological conditions (1–4). As an important translational tool, preclinical PET has enabled the development of a multitude of different radiotracers that are currently in clinical use. For example, the development of radiotracers targeting PSMA for human prostate cancer imaging, theranostic approaches, and the ability to track therapeutic cells in vivo all stem from their thorough evaluation in rodent models (5–7).
A considerable limitation of current small-animal PET-computed tomography (PET/CT) imaging is the low throughput of image acquisitions. Single animal imaging becomes particularly restrictive when radioactive isotopes with short half-lives, such as carbon-11 and fluorine-18, or complex dynamic imaging studies are employed. Subsequently, to design sufficiently-powered studies, high costs accumulate. For many research groups, these high imaging costs present a barrier for wide-spread preclinical adoption of PET and may restrict the frequency of radioactive preparations available to others that have invested in this imaging modality. A potential solution to this economic problem is to scan multiple animals simultaneously. A number of commercially-available preclinical scanners possess adequate axial length and diameter to achieve multi-animal imaging, and as a result, many user-designed multi-animal holders have entered into routine use (8–10). Often, however, these beds lack the thorough characterization required for the production of reproducible and quantifiable PET data, with animal heating and monitoring capabilities frequently omitted from the bed design. It is well recognised that temperature and anaesthesia can greatly impact the biodistribution of injected radiotracers and so maintaining control of these variables is essential to maintain reproducibility across subjects and between sites (11). It is also important to consider the impact on image quality and quantitative accuracy when multiple animals are scanned in the same field of view. The presence of more than one concentrated source of radioactivity may negatively affect attenuation, increase the singles and randoms rates, the number of scatter events, and the detector and system deadtime, with reductions in resolution and sensitivity resulting as subjects are placed away from the centre field of view.
To examine and overcome the low throughput of conventional PET imaging, a four-bed mouse ‘hotel’ was developed and validated. The animal hotel was designed to deliver an even distribution of anaesthesia to each nose cone and maintain animals at 37°C. Heat and temperature tests were performed and the National Electrical Manufacturers Association (NEMA) NU-4 2008 standard protocol for small animal PET scans was used to assess the image quality of phantom PET scans. We then investigated whether the mouse hotel negatively impacted the quality of in vivo dynamic PET images simultaneously acquired with four mice. Following injection of 18F-2-fluoro-2-deoxyglucose (18F-FDG) into female balb/c mice, images were subsequently compared to those acquired using a single animal bed.
Materials and Methods
Design and Development of the Four-Bed Mouse Hotel
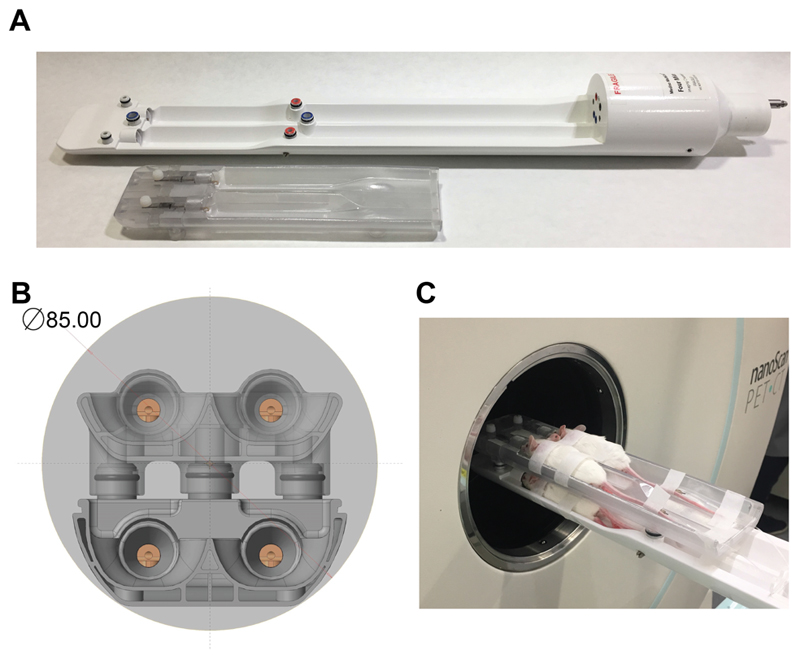
We designed the four-bed mouse hotel for use in a Mediso nanoScan PET/CT (Fig. 1A). The hotel was designed using high temperature-resistant plastic and consisted of four holding chambers with individual nose cones for anaesthesia delivery (Fig. 1B). The bed contained chambers to allow the flow of heated air to all four beds within the animal hotel, with each mouse placed equidistant from the centre field of view and designed to allow the simultaneous imaging of up to four mice within the same single field of view (Fig. 1C). The modular design allowed the removal of the top bed layer when only 1-2 mice were required for imaging, e.g. when increasing the group size to five or more animals.
Figure 1.
Design of the four-bed mouse hotel. (A) The modular design with removable top bed allowed between one and four 50 g mice to be simultaneously imaged. (B) Cross-section of the bed within the PET field of view (light grey). The four individual nose cones are visible, with the air-filled chamber located under each animal bed also in view. (C) Photograph of four female balb/c mice within the four-bed animal hotel.
Temperature and Anaesthesia Tests
The air flow temperature delivered to the bed was set at 38°C and allowed to plateau for 5 min before temperature readings were taken using a thermal camera (FLIR systems AB-E60). Each animal holding bed within the hotel was measured individually at multiple positions. To measure anaesthesia delivery, a flowmeter (Dwyer RMA-26-68V) was attached to each individual anaesthesia nose cone.
NEMA Mini Image Quality Phantoms Studies
Cylindrical mouse-sized mini image quality phantoms were designed specifically for use in the four-bed mouse hotel by Mediso Medical Imaging Systems. Each phantom, made from Plexiglas, consisted of three parts: a large uniform compartment; a solid region with 5 fillable rods of 1 mm, 2 mm, 3 mm, 4 mm and 5 mm in diameter; and a non-radioactive region containing water- and air-filled chambers, designed to replicate the conditions of an in vivo PET/CT scan. In comparison to the standard NEMA image quality phantom, the diameter of the mini image quality phantom was reduced from 30 mm to 20 mm, which reduced the phantom volume from 20 mL to 10 mL. In addition, the spill-over-ratio chambers were reduced to a diameter of 5.5 mm in the mini image quality phantom versus 8 mm in the NEMA image quality phantom. The image quality program used to determine scanner performance (Mediso) was modified based on NEMA conversions for these changes to meet the NEMA standards.
Following the guidelines suggested by NEMA NU-4, each phantom was filled with 3.7 MBq of 18F-FDG in 10 mL of PBS (Thermo Scientific), decay-corrected to the start of acquisition. Clinical-grade 18F-FDG was obtained from PETNET solutions. All phantoms were thoroughly mixed and bubbles carefully removed before being placed onto the imaging bed. The phantoms were examined using different arrangements within the bed. For multiple phantom scans, four phantoms were placed in the animal hotel at the same time (configuration 1). For single phantom scans, one phantom was placed into each bed location within the hotel and imaged individually (configuration 2). Additionally, a single phantom was imaged using a single mouse bed for comparison (configuration 3).
NEMA Mini Image Quality Phantoms Studies
Four phantoms were imaged on the four-animal bed following the exact specification suggested by the PET manufacturer for single mouse imaging. Dynamic PET acquisition was performed on a Mediso nanoScan PET/CT over 20 min followed by CT (480 projections; 50kVp tube voltage; 600 μA; 300ms exposure time; 1:4 binning; helical acquisition). Whole body Tera-Tomo 3D reconstruction with 4 iterations and 6 subsets was performed (1-5 coincidence mode) using an isotropic voxel size of 0.3 mm3. Images were corrected for attenuation, scatter and decay. A gaussian filter of 0.7 was added to the post reconstructed PET images using VivoQuant software (v.2.5; Invicro Ltd.).
To optimise the manufacturer’s suggested scanner and reconstruction parameters, the tube voltage for CT imaging was increased to 70kVp, reducing the current to 310 μA. Additionally, whole body Tera-Tomo 3D reconstruction with 10 iterations and 6 subsets was performed (1-5 coincidence mode) using an isotropic voxel size of 0.3 mm3 following an iterative process to quantitatively improve bed performance. Images were corrected for attenuation, scatter and decay. A gaussian filter of 0.7 was added to the post reconstructed PET images using VivoQuant software (v.2.5; Invicro Ltd.).
NEMA NU-4 2008 Tests
Post reconstruction, NEMA NU-4 2008 tests were performed to evaluate the effect of multiple subjects on scanner performance. Image noise was expressed as percentage standard deviation (%STD) by selecting a large field of view (75% of the active diameter) in the centre of the fillable region of the phantom. Activity recovery coefficients (RCs) in the five fillable rods were calculated from the maximum detected activity in each rod, divided by the mean total phantom activity concentration. To evaluate scatter correction, spill-over ratios in the non-radioactive water- (SORwater) and air-filled (SORair) chambers were measured as the activity detected in these regions, divided by the mean total phantom activity concentration. Data were exported and analysed in Graphpad Prism (v.8.0).
PET/CT Animal Imaging Studies
All animal experiments were performed in accordance with the United Kingdom Home Office Animal (scientific procedures) Act 1986. PET acquisition was performed on a Mediso nanoScan PET/CT system. Female Balb/c mice aged 9-12 weeks (Charles River Laboratories) were fasted for 24 h prior to image acquisition. Mice were anaesthetized with 2.5% isoflurane in oxygen and a tail vein cannula inserted. A bolus of 3.7 MBq of 18F-FDG was injected in approximately 100 μL of PBS and dynamic images were acquired immediately over a period of 60 minutes. For attenuation correction and anatomical reference, CT images were acquired following PET imaging (480 projections; 70kVp tube voltage; 300 ms exposure time; 1:4 binning; helical acquisition;). Animals received 2.5% isoflurane in oxygen throughout the scan and were maintained at 37°C by the air flow heated bed. Breathing rate and body temperature were visually monitored for all animals throughout the imaging procedure.
The acquired data were sorted into 19 time frames of 4 × 15 seconds, 4 × 60 seconds, and 11 × 300 seconds for image reconstruction (whole body Tera-Tomo 3D reconstruction with 10 iterations and 6 subsets; 400-600 keV; 0.4 mm3 voxel size). VivoQuant software (v.2.5, Invicro Ltd.) was used to analyse reconstructed images. Regions of interest (ROIs) were drawn manually using CT images and 30-60 minute summed dynamic PET images as reference. Time versus radioactivity curves (TACs) were generated using normalised count densities to the injected activity and the area under the time versus radioactivity curve (AUC) generated.
Statistics
All data were expressed as the mean ± one standard deviation (SD). Statistical significance was determined using either a two-tailed t-test or analysis of variance (ANOVA) followed by t-tests multiple comparison correction (Tukey method; GraphPad Prism v.8.0).
Results
Physiological Regulation
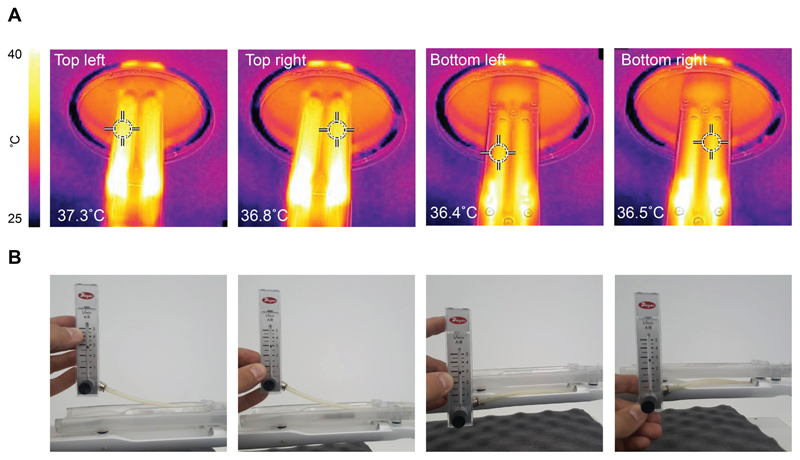
The animal bed was designed to circulate heated air evenly across the four animal chambers. This design facilitated a constant and even temperature distribution to all four animal holding beds (36.8 ± 0.4°C; n = 4; Fig. 2A). As a design consideration, air inlets, where temperatures over 37°C were observed, were placed away from the region of the bed where the mice were positioned. Flow meter readings showed anaesthesia was distributed evenly to each nose cone (2.8 ± 0.1 L/min; from the four nose cones; Fig. 2B).
Figure 2.
Temperature and anaesthesia flow rate uniformity testing in the four-animal bed. (A) Temperature measurements were acquired using a thermal camera following heating of the four-bed mouse hotel. The bed temperature for the bottom bed layer was assessed following removal of the top layer. Elevated temperature was evident by the air inlets that were positioned away from the mice towards the back of the bed. (B) Flowmeter values representing anaesthesia output from each nose cone.
Phantom Studies
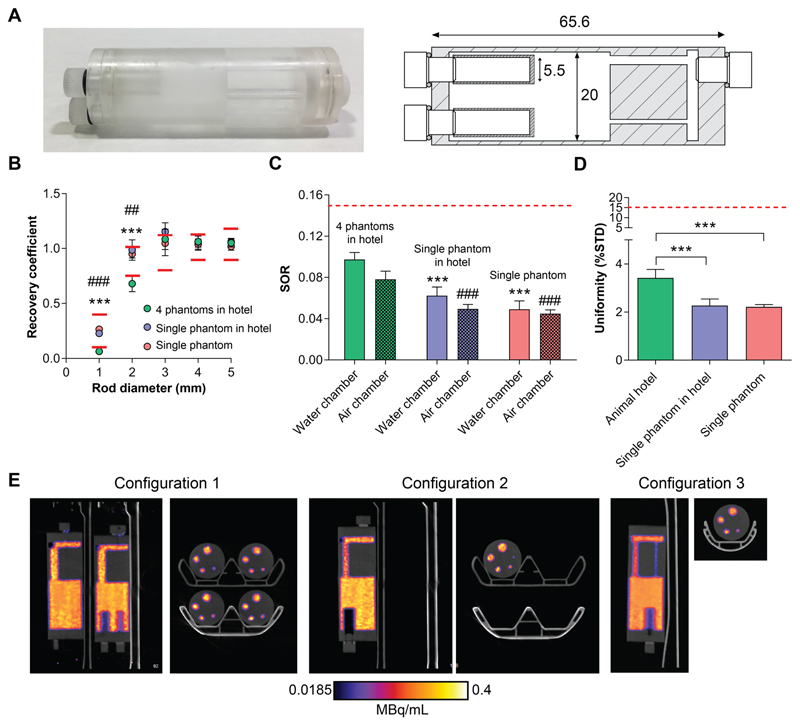
The bed’s performance was evaluated using mini image quality NEMA NU-4 phantoms specifically designed for their use in the four-bed mouse hotel (Fig. 3A). Each phantom contained three discrete regions, holding a total volume of 10 mL. Quantitative analysis was performed using NEMA NU- 4 tests following reconstruction, with the tolerable limits set by NEMA shown in Supplemental Table 1. Initially, phantom images were reconstructed using those parameters recommended by the manufacturer for standard scans on a single animal. The NEMA NU-4 test results using these standard parameters are shown in Supplemental Figure 1. Subsequently, these reconstruction parameters were optimised to improve image quality. In addition, the x-ray tube voltage was increase from 50 kVP to 70 kVP, which had minimal effect on absorbed dose (CT dosage index of 3.8 cGy vs. 3.7 cGy for a 100 mm length field of view, respectively). The RC values measured in the five fillable rods with diameters of 1-5 mm were used to evaluate spatial resolution of images acquired using the animal hotel and were compared to a single animal bed. Reduced spatial resolution was evident in phantoms imaged using the four-bed mouse hotel when examining the RCs of the 1 mm and 2 mm rods, with values falling outside the tolerable limits suggested by the manufacturer’s guidelines (0.06 ± 0.01 and 0.68 ± 0.07, respectively; n = 4). Spatial resolution, however, was not affected in the larger rods (1.08 ± 0.09, 1.06 ± 0.05, 1.05 ± 0.04, for 3 mm, 4 mm and 5 mm rod, respectively; n = 4), with values similar to those acquired using a single mouse bed (1.05 ± 0.11, 1.04 ± 0.05, 1.02 ± 0.04; n = 4; Fig. 3B). Interestingly, when imaging a single phantom in the four bed mouse hotel, with the exception of the 3 mm rod, which was placed furthest away from the centre field of view, the RC values were all within the tolerable limits at all rod diameters tested, presumably due to the presence of a single point-source of radioactivity (0.23 ± 0.02, 0.99 ± 0.09, 1.2 ± 0.08, 1.1 ± 0.08 and 1.1 ± 0.03 for 1 mm, 2 mm, 3 mm, 4 mm and 5 mm rods, respectively; n = 4).
Figure 3.
Evaluation of image quality using mini IQ NEMA NU-4 phantoms. Mini IQ phantoms were designed specifically for the evaluation of the four-bed mouse hotel (A). Dimensions are shown in mm. Mini IQ phantoms were filled with ~3.7MBq of 18F-FDG and imaged over 20 mins. Following reconstruction, NEMA NU-4 tests were performed. The RC values of 1-5 mm rods (B), SORs (C) and uniformity values (D) of phantoms imaged using the four-bed mouse hotel and a single mouse bed were acquired and compared. (E). Representative sagittal and axial PET images of 0-20 min summed activity of imaging configurations 1, 2 and 3, reconstructed using 10 iterations and 6 subsets. Red lines represent the tolerable limits set by NEMA NU-4. For (B), *** = P < 0.001 for configuration 1 vs configuration 2; ### = P < 0.001 for configuration 1 vs configuration 3; for (C), *** = P < 0.001 for configuration 1 SORwater vs configuration 2 and 3; ### = P < 0.001 for configuration 1 SORair vs configuration 2 and 3.
The SORs for both the water- and air-filled chambers were within the tolerable limit of 15 % for all imaging configurations, suggesting an appropriate scatter correction was applied. SORs for the four-bed mouse hotel were significantly higher than those acquired using a single mouse bed, however. For configuration 1, 2 and 3, the SORwater was 9.7% ± 0.7%, 6.2% ± 0.8%, 4.9% ± 0.8%, respectively (P = 0.0003 and P < 0.0001, for configuration 1 vs. configuration 2 and configuration 1 vs. configuration 3, respectively). The SORair was 7.8% ± 0.08%, 4.9% ± 0.4%, 4.5% ± 0.4%, for configuration 1, 2 and 3, respectively (P = 0.0001 and P < 0.0001, for configuration 1 vs. configuration 2 and configuration 1 vs. configuration 3, respectively; n = 4; Fig. 3C). The variation in activity concentration, as measured by the % STD in the uniform region of the phantom, was higher when four phantoms were imaged simultaneously compared to the single phantom configurations, representing elevated image noise (3.4% ± 0.35%, 2.3% ± 0.27% and 2.2% ± 0.1% for configuration 1, 2 and 3, respectively; P = 0.003 for configuration 1 vs. 2; P = 0.004 for configuration 1 vs. 3; n = 4; Fig. 3D). All values, however, were well below the tolerable limit of 15%. Representative single-slice 18F-FDG PET/CT images (0 – 20 min summed activity) of the mini IQ phantoms are displayed in Fig. 3E, comparing the three imaging configurations.
Animal Studies
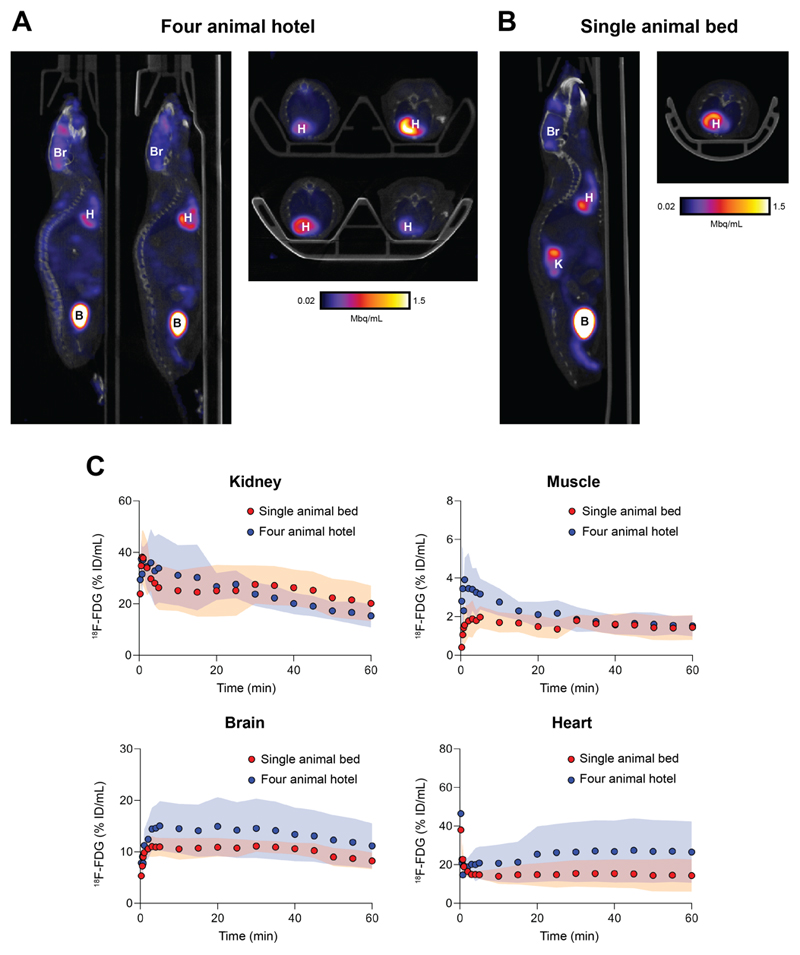
To evaluate the bed under standard in vivo imaging conditions, four healthy mice were simultaneously imaged with 18F-FDG PET dynamically over 60 min in the four-bed mouse hotel (Fig. 4A). An additional four mice were imaged in a single mouse bed for comparison (Fig. 4B). Following reconstruction, TACs revealed low levels of variability in 18F-FDG uptake for all major organs for animals imaged in the four-bed animal hotel (Fig. 4C). There was no significant difference in the area under the TAC for all major organs (Supplemental Fig. 2). When compared to the single animal scans, however, the variation in radiotracer tissue uptake between subjects increased with the mouse hotel. Representative maximum intensity projections following the manual removal of the bed are shown in Supplemental Fig. 3.
Figure 4.
In vivo validation of the four-bed mouse hotel and comparison to a single mouse holder. Representative sagittal and axial PET images of 30-60 min post injection of mice imaged using (A) the four-bed mouse hotel and (B) a single mouse bed. Br, brain; H, heart; K, kidney; B, bladder. (C). Time vs. radioactivity curves (TACs) of major organs of interest normalised to the percentage injected activity. Shaded regions represent one s.d. from the mean value (n = 4 animals).
Discussion
Here, we sought to validate the performance of a commercially-designed four-bed mouse hotel for use in a Mediso nanoScan PET/CT imaging system. Utilizing a four-bed mouse hotel for PET/CT small animal imaging has a major benefit over conventional single animal imaging, saving investigators’ time and reducing overall costs. Additionally, by increasing the number of animals that can be scanned for the same cost and time, the statistical power and corresponding confidence in the imaging data can be substantially increased.
The feasibility of imaging multiple animals in the same small animal PET/CT imaging system has been reported in multiple studies (8–10,12), facilitated through the development of new small animal PET/CT imaging platforms which offer sensitive and reproducible imaging over both a large axial and transaxial field of view. These large axial field of views enable several animals to be imaged simultaneously. To the best of our knowledge, however, physiological regulation and monitoring has not been incorporated into the user-developed animal holders currently in use. Unlike previous unheated animal bed holders which can only be used for a short duration (12), dynamic imaging over multiple hours is achievable on this four bed mouse hotel as a result of fine temperature regulation. Changes in physiological conditions can affect mammalian physiology, including disruption to thermoregulation, respiration and cardiac output (13). Maintaining a constant bed temperature will therefore limit variations in radiotracer pharmacokinetics, whilst minimizing any potential pain, suffering, distress or lasting harm that may be caused to the animal.
To evaluate the performance of the bed, phantom tests were performed under the conditions of the NEMA NU-4 2008 standard protocol for small-animal PET systems. The NEMA NU-4 test results acquired using the suggested reconstruction parameters set by the manufacturer’s guidelines are provided in Supplemental Fig. 1. To improve upon the data acquired using the manufacturer’s suggested parameters for a conventional scan using the single-mouse bed, the reconstruction parameters were altered, with the number of iterations increased from four to ten. Following completion of the NEMA tests on the newly reconstructed images, low-level radioactivity was detected in the water and air chambers within the NEMA phantoms due to the scattering of photons. The SOR in both the air and water chambers was increased in phantoms imaged using the four-bed mouse hotel compared to the single-bed images, which can be attributed to the increased number of subjects in the field of view, leading to elevated photon scattering. The amount of scatter detected in the non-radioactive chambers of phantoms imaging using the four-bed mouse hotel, however, was below the maximum tolerable limit recommended by the manufacturer, suggesting an acceptable loss in image quality.
To assess the expected impact on spatial resolution in the mouse hotel, RCs values for the 1-5 mm rods in the mini IQ phantom were calculated. For the 1 mm and 2 mm rods, the RC values were decreased by 76% and 26%, respectively, compared to phantoms scanned on a single mouse bed. For the larger diameter rods, all values fell within the tolerable limits. A reduction in spatial resolution is expected with the four-mouse bed as each imaging chamber is placed away from the centre field of view. Matching previous findings (14), increasing the number of iterations from four to ten during reconstruction improved the RCs of all rods, however, this amplified image noise and subsequently reduced observable image quality. During small animal PET imaging of tumor-bearing mice, lowered spatial resolution is unlikely to alter quantitative information as most tumors imaged are typically larger than 50 mm3; further optimising the number of iterations during reconstruction therefore may not be beneficial. Care, however, should be taken when evaluating radiotracer uptake in small tissues, such as lymph nodes or small metastatic lesions when using the four-animal bed.
Systematic bias may be introduced through possible differences in scanner performance at each of the four positions of the mouse ‘hotel’. To address this potential confound, we assessed quantitative accuracy and precision using a single mini IQ phantom and corresponding NEMA tests at each of the four bed positions (Figures 3B-E). Minimal differences in recovery coefficients, spill-over ratios and uniformity were measured between all four bed positions, as shown by the small standard deviation of the observed measurements. This was encouraging as variation in performance at the different bed positions was taken into consideration at the design stage, whereby all positions were set to be equidistant from the centre field of view (Fig. 1B). In vivo, 18F-FDG radiotracer uptake in the major organs of healthy mice scanned simultaneously versus those imaged individually produced similar quantitative values, despite the expected biological variation in both radiotracer retention and excretion. To ensure maximum image quality, applying scatter and attenuation correction to all in vivo imaging studies using the four-bed mouse hotel is highly recommended.
Whilst we have demonstrated the feasibility of simultaneous imaging of up to four mice whilst maintaining quantitative precision, there are a number of improvements in bed design that should be incorporated prior to commercialization. At the time of data acquisition, both breathing and heart rate monitoring was available for only one of the four mice using this prototype bed. An updated bed that incorporates physiological monitoring for all four mice has subsequently been developed. An additional consideration is the potential degradation of image quality when four animals are imaged simultaneously. Here, phantom imaging studies revealed potential issues with image reconstruction, leading to loss of data quality. Suboptimal image reconstruction and lowered image resolution was initially seen through the loss of detected radioactivity in the small 1 mm and 2 mm rods of phantoms scanned in the four-bed mouse hotel. Furthermore, in our in vivo studies, increased variation in 18F-FDG uptake in both the brain and heart were observed in the TACs of animals scanned using the four bed mouse hotels versus a single mouse imaging bed (Fig. 4C). Whilst this variation might be biological in-nature, due to alterations in cardiac output and metabolism between subjects, for example, caution should be applied when analysing image-derived outputs using small numbers of animals. Future work will assess bed performance in higher-powered studies with a range of different radiotracers.
Despite some minor drawbacks, a considerable benefit of using the four-bed mouse hotel during PET imaging are the large cost savings that can be made compared to a conventional single animal scanner. A clinical dose of ~370 MBq 18F-FDG on average costs ~£300 in the UK. Considering the time required to position the animal on the bed, time taken to prepare the radioactive dose radioactive dose and a typical 10 min CT scan, with a constantly-decaying radiotracer, typically each clinical dose will allow for approximately five 60 min dynamic scans. For novel radiotracer development and discovery PET imaging where the radioactivity concentration received may be substantially less than a clinical dose of 18F-FDG, the number of consecutive scans that can be achieved is even fewer. For sufficiently powered studies, a sample group size of eight or more animals are typically required to identify moderate changes in radioactive concentration with statistical confidence. Performing 60-minute dynamic 18F-FDG PET/CT on a conventional single mouse bed would require a minimum of two radioactive preparations to achieve this statistical power. Additionally, imaging institutes may charge ~£200 per hour for use of the scanner. To image eight mice using a single animal bed, a total of 12 hours split over two days would be required. However, imaging eight mice using the four-bed mouse hotel would only require a maximum of four hours in one day. In total, by using the four-bed mouse hotel, we estimate scanning/radiotracer costs could be reduced by approximately 60% using commercially-available 18F-FDG, with far higher savings potentially achieved with low-yielding novel radiotracers.
Conclusion
A four-bed mouse hotel was designed to aid imaging scientists conduct their research in a more time-efficient, cost-effective manner by quadrupling the number of mice that can be imaged in a single session. In particular, when performing experiments using short-lived isotopes, multiple animals can be scanned using a single synthesis, which would otherwise not be possible. The design of the four-bed mouse hotel allowed for uniform control over temperature and anaesthesia, with phantoms studies and in vivo imaging of mice confirming its utility to increase the throughput of small animal PET imaging without considerable loss of image quality and quantitative precision. In comparison to a single mouse bed, cost and time associated with each scan were substantially reduced.
Supplementary Material
Key points.
Question
Can a four-bed mouse ‘hotel’, which was developed to simultaneously image up to four mice, reduce costs and maximise radiotracer usage when compared to scans performed with a single mouse bed?
Pertinent findings
Analysis of images acquired using the four-bed mouse hotel confirmed its utility to increase the throughput of small animal PET imaging without considerable loss of image quality and quantitative precision, with the cost and time associated with each scan substantially reduced.
Implications for patient care
Small animal imaging experiments are vital for the successful development of novel radiotracers and are required for the biological validation of these tracers prior to their translation to the clinic. By increasing the throughput of preclinical imaging fourfold, high-powered studies can be completed faster and cheaper compared to conventional scanning using a single animal bed.
Acknowledgements
The authors would like to thank Mike Bewick, Max Capel and Gabor Nemeth for insightful scientific discussions and Tammy Kelber for help with in vivo experiments.
Financial Support: This study was funded through a Wellcome Trust and Royal Society Sir Henry Dale Fellowship (107610/Z/15/Z) and a CRUK UCL Centre Non-Clinical Training Award (A23233) to Timothy H. Witney.
Footnotes
Disclosure: Authors Gábor Mócsai, Zoltán Nyitrai and Sandor Hobor are employed by Mediso Medical Imaging Ltd, but do not own any shares or have any additional financial benefits in addition to general compensation for employment. They do not hold any patents related to the topic of the publication. None of the other authors have a conflict of interest to declare. This study was funded through a Wellcome Trust and Royal Society Sir Henry Dale Fellowship (107610/Z/15/Z) and a CRUK UCL Centre Non-Clinical Training Award (A23233) to Timothy H. Witney.
References
- 1.Hargreaves RJ. The role of molecular imaging in drug discovery and development. Clin Pharmacol Ther. 2009;86:221–221. doi: 10.1038/sj.clpt.6100467. [DOI] [PubMed] [Google Scholar]
- 2.Michalski MH, Chen XY. Molecular imaging in cancer treatment. Eur J Nucl Med Mol Imaging. 2011;38:358–377. doi: 10.1007/s00259-010-1569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witney TH, James ML, Shen B, et al. PET imaging of tumor glycolysis downstream of hexokinase through noninvasive measurement of pyruvate kinase M2. Sci Transl Med. 2015;7:310ra169. doi: 10.1126/scitranslmed.aac6117. [DOI] [PubMed] [Google Scholar]
- 4.Mccormick PN, Greenwood HE, Glaser M, et al. Assessment of tumor redox status through (S)-4-(3-[18F]fluoropropyl)-L-glutamic acid positron emission tomography imaging of system xc-activity. Cancer Res. 2019;79:853–863. doi: 10.1158/0008-5472.CAN-18-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pen tanedioic acid, [18F]dcfpyl, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–7653. doi: 10.1158/1078-0432.CCR-11-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keu KV, Witney TH, Yaghoubi S, et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baum RP, Kulkarni HR. From molecular imaging using ga-68 labeled tracers and pet/ct to personalized radionuclide therapy - the bad berka experience. Theranostics. 2012;2:437–447. doi: 10.7150/thno.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng TE, Yoder KK, Normandin MD, et al. A rat head holder for simultaneous scanning of two rats in small animal PET scanners: Design, construction, feasibility testing and kinetic validation. J Neurosci Methods. 2009;176:24–33. doi: 10.1016/j.jneumeth.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yagi M, Arentsen L, Shanley RM, Hui SK. High-throughput multiple-mouse imaging with micro-PET/CT for whole-skeleton assessment. Physica medica. 2014;30:849–853. doi: 10.1016/j.ejmp.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aide N, Desmonts C, Beauregard JM, et al. High throughput static and dynamic small animal imaging using clinical PET/CT: potential preclinical applications. Eur J Nucl Med Mol Imaging. 2010;37:991–1001. doi: 10.1007/s00259-009-1352-1. [DOI] [PubMed] [Google Scholar]
- 11.Fueger BJ, Czernin J, Hildebrandt I, et al. Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med. 2006;47:999–1006. [PubMed] [Google Scholar]
- 12.Habte F, Ren G, Doyle TC, Liu H, Cheng Z, Paik DS. Impact of a multiple mice holder on quantitation of high-throughput micropet imaging with and without Ct attenuation correction. Mol Imaging Biol. 2013;15:569–575. doi: 10.1007/s11307-012-0602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tremoleda JL, Kerton A, Gsell W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: considerations on experimental outcomes and animal welfare. EJNMMI Research. 2012;2:44–44. doi: 10.1186/2191-219X-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harteveld AA, Meeuwis AP, Disselhorst JA, et al. Using the NEMA NU 4 PET image quality phantom in multipinhole small-animal SPECT. J Nucl Med. 2011;52:1646–1653. doi: 10.2967/jnumed.110.087114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.