Abstract
Purpose
We sought to complete the baseline trachoma map of the Solomon Islands by establishing prevalences of active trachoma and trichiasis in the provinces of Choiseul, Western, Rennell-Bellona, and Temotu.
Methods
Using the standardized methodology developed for the Global Trachoma Mapping Project, we conducted cross-sectional community-based surveys from September to November 2013. Choiseul and Western provinces were each mapped as separate evaluation units (EUs); Rennell-Bellona and Temotu were combined to form a third EU.
Results
A total of 9819 individuals were sampled for inclusion, with 9224 (93.3%) consenting to examination, of whom 4587 (46.3%) were female. Survey teams visited 82 villages, and surveyed 2448 households. Two EUs had prevalences of trachomatous inflammation – follicular (TF) in 1–9-year-olds over the 10% threshold at which WHO recommends mass distribution of azithromycin for at least 3 years (Western 20.4%, 95% confidence interval, CI 15.6–26.3%; Rennell-Bellona/Temotu 22.0%, 95% CI 18.5–26.0%). Choiseul had a TF prevalence of 6.1% (95% CI 4.1–8.6%), and met the criterion for a single round of mass antibiotic distribution before re-survey. The adjusted prevalences of trichiasis in those aged 15+ years were 0.0% (95% CI 0.0–0.2%) in Choiseul, 0.16% (95% CI 0.0–0.5%) in Western, and 0.10% (95% CI 0–0.3%) in Rennell-Bellona/Temotu provinces. All three EUs require implementation of the facial cleanliness and environmental improvement components of the trachoma elimination strategy.
Conclusion
Active trachoma is prevalent in the Solomon Islands. However, there is little evidence of the blinding complications of trachoma being a public health problem there. Further research into the explanation for this phenomenon is warranted.
Keywords: Chlamydia trachomatis, Pacific, prevalence, Solomon Islands, survey, trachoma
Introduction
The World Health Organization (WHO) estimates that more than 50 countries are endemic for trachoma, the most common infectious cause of blindness worldwide, responsible for visual impairment in an estimated 1.9 million people.1 In endemic communities, Chlamydia trachomatis serotypes A–C circulate among young children causing self-limiting conjunctival infections that decrease in duration with age.2 Trachomatous inflammation – follicular (TF) and trachomatous inflammation – intense (TI) are clinical manifestations of infection. Repeat inflammatory episodes may lead to accumulation of scar tissue, which can distort the eyelids and cause eyelashes to abrade the eye; when this occurs, it is known as trachomatous trichiasis (TT). This may lead to corneal opacification and blindness in severe cases.3 Several systems have been used to describe this complex, multistage process, however, a simplified grading system is the public health tool of choice for describing trachoma phenotypes.4
To eliminate trachoma, the WHO have endorsed the SAFE strategy (surgery, mass antibiotic treatment, promotion of facial cleanliness and environmental improvement), implemented in a stratified manner based on the population-level prevalence of clinical signs of disease. Trachoma is considered to be a public health problem if the prevalence of unmanaged TT is >0.2% of the population aged 15 years and older, or if the prevalence of TF is ≥5% in those aged 1–9 years.5,6
The Solomon Islands is an archipelago of almost 1000 volcanic islands and coral atolls, with a population of approximately 520,000 inhabitants, spread over 28,400km2 of the South Pacific Ocean and the Solomon Sea.7 A number of small, school- or hospital-based studies in the early 20th century indicated that cases of trachoma were present across the archipelago,8 however, no systematic investigation was carried out until a trachoma rapid assessment in 2007. Cases of TF, trachomatous scarring and TT were found in a targeted assessment of high-risk communities in three provinces of the country.9 In 2012, the International Agency for the Prevention of Blindness commissioned population-based prevalence surveys in five provinces, the results of which indicated a high prevalence of TF in those aged 1–9 years (mean prevalence 18.7%, range 12.0–24.3%) and some cases of TT in those aged 15 years and older (mean prevalence across three provinces 0.1%, range 0.0–0.3%). Data also indicate trachoma endemicity in neighboring Kiribati, Fiji, and Vanuatu.10–11 Prior to the present study, no prevalence data existed for Choiseul, Western, Temotu or Rennell and Bellona provinces of the Solomon Islands, whose combined population comprises approximately a quarter of the nation’s population.7 Complete geographical survey coverage of the country was needed to understand the burden of trachoma, and the level of priority for intervention.12
The Global Trachoma Mapping Project (GTMP)12 aimed to complete the baseline map of trachoma globally in over 30 countries using standardized methodologies, allowing interventions to be directed to those in need. We conducted GTMP-supported surveys to complete the national map of trachoma in the remaining four unmapped provinces of the Solomon Islands.
Materials and methods
The surveys used the GTMP methodology described elsewhere.12 The four provinces were mapped as three evaluation units (EUs): Choiseul (26,732 inhabitants), Western (76,649), and Rennell and Bellona plus Temotu (24,403).7 Based on the latest census data,7 we estimated a mean of 1.47 children aged 1–9 years per household. To see 30 households per village, and achieve a sample size of 1222 children, 1222/(30×1.47) = 27.7 villages should be surveyed. Therefore, we planned to survey 28 clusters per EU. Fieldwork was undertaken from 6 September to 21 November 2013.
For each island within an EU, the number of clusters sampled was assigned in proportion to the population of the island. Thereafter, villages were sampled from a standard census list with a probability proportion to size methodology, providing self-weighting of results. On the day of the survey, village leaders were engaged to create a comprehensive list of households, from which 30 households were randomly sampled by drawing lots. All inhabitants of these households who were at least 1 year of age were considered eligible for inclusion. Demographic data were collected from study participants and a standardized examination was conducted by GTMP-certified graders12 to look for trichiasis, TF and TI. Verbal consent was obtained from the head of the household, from each participating adult, and from parents or guardians on behalf of the children in their care. In addition, verbal assent was obtained from those under 18 years of age. The study was approved by the Solomon Islands National Health Research Ethics Committee and the Ethics Committee of the London School of Hygiene & Tropical Medicine (6319).
Water, sanitation and hygiene (WASH) variables
Recorders were trained to collect household-level WASH variables linked to WHO/UNICEF Joint Monitoring Programme outcomes.13 Data were collected by carrying out a focused interview with the head of the household using a standard questionnaire and direct observation.12 Separate questions were asked about sources of water drinking and washing faces; many Pacific Islands households use seawater for personal hygiene purposes.
Statistical analysis
Adjustment of prevalence results was carried out using R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria14). The overall EU-level adjusted TF prevalence was calculated by adjusting the proportion of children with TF in each cluster in 1-year age groups using 2009 Solomon Islands census data,7 then determining the mean of all such cluster-level proportions. The same method was used to calculate the overall adjusted trichiasis prevalence, but with adjustment for both sex and age in 5-year age groups.12 Confidence intervals (CIs) around the prevalence estimates were calculated by bootstrapping the cluster means over 10,000 iterations and taking the 2.5th and 97.5th centiles of all ordered results.15 Risk factor analysis was carried out in Stata 10.2 (Stata Corp, College Station, TX, USA). A 2-level hierarchical model was used with adjustment for clustering at village and household levels. Univariable associations were considered for inclusion in the multivariable model if p ≤ 0.05 (Wald’s test).
Results
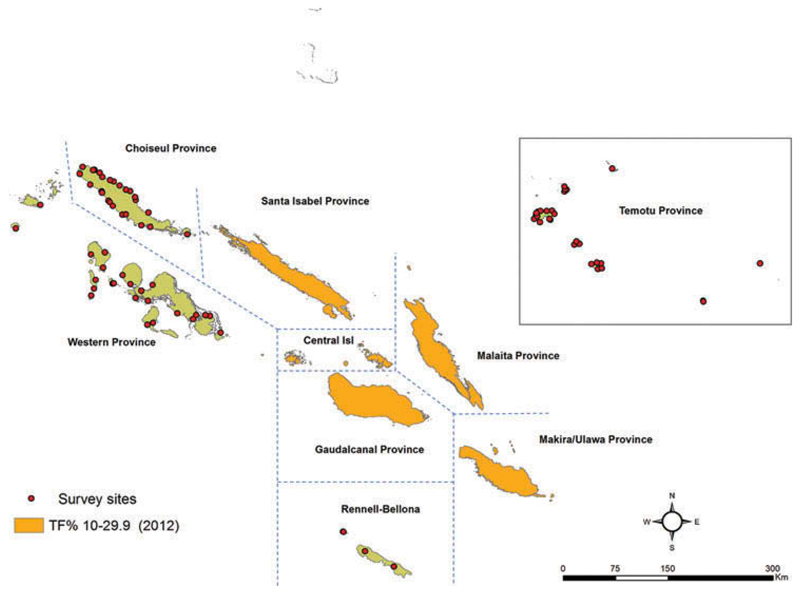
A total of 82 villages were surveyed over three EUs. The coordinates of sampled villages are shown in Figure 1. A total of 2448 households were visited, 9891 inhabitants were eligible to participate in the study, of whom 9224 individuals (93.3%) consented to examination and were examined. Overall, 25 villages were sampled in Choiseul, 25 in Western, and 32 in Rennell-Bellona/Temotu. The number of villages was increased in Rennell-Bellona/Temotu due to low numbers of children found in the villages first sampled. The demographics of those sampled are shown in Table 1.
Figure 1.
Coordinates of villages sampled in the three evaluation units surveyed, Global Trachoma Mapping Project, Solomon Islands, September–November 2013, and trachomatous inflammation – follicular (TF) prevalence estimates in five other provinces.10 Shapefile source: Global administrative areas (gadm.org).
Table 1.
Sampled population, Global Trachoma Mapping Project, Solomon Islands, September–November 2013.
| Province |
||||
|---|---|---|---|---|
| Choiseul | Western | Rennell-Bellona/Temotu | Total | |
| Sampled, n | 2709 | 3129 | 4053 | 9891 |
| Examined, n (%) | 2634 (97.2) | 2914 (93.1) | 3676 (90.7) | 9224 (93.3) |
| Absent, n (%) | 56 (2.1) | 213 (6.8) | 340 (8.4) | 609 (6.2) |
| Refused, n (%) | 19 (0.7) | 2 (0.1) | 36 (0.9) | 57 (0.6) |
| Age, median years (range) | 17 (1–99) | 17 (1–95) | 19 (1–100) | 18 (1–100) |
| Female, n (%) | 1165 (43.0) | 1458 (46.6) | 1964 (48.5) | 4587 (46.3) |
| Households, n | 742 | 751 | 955 | 2448 |
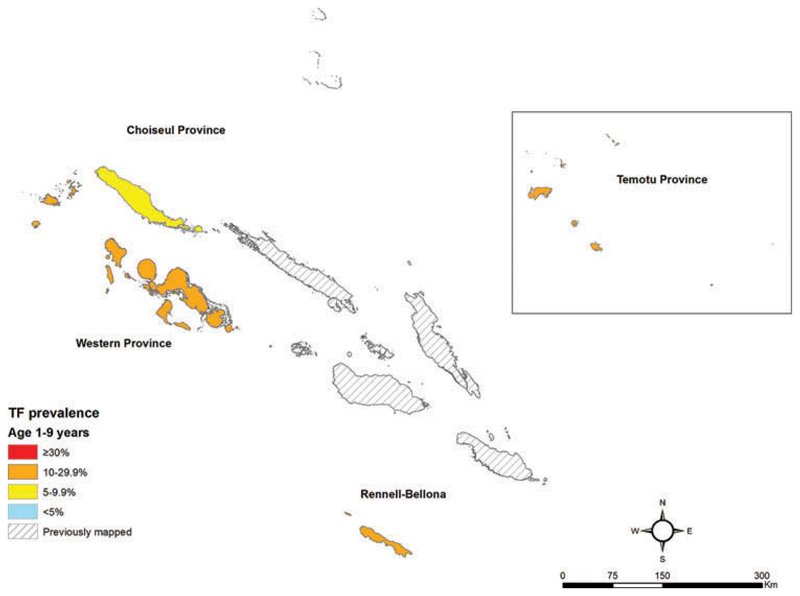
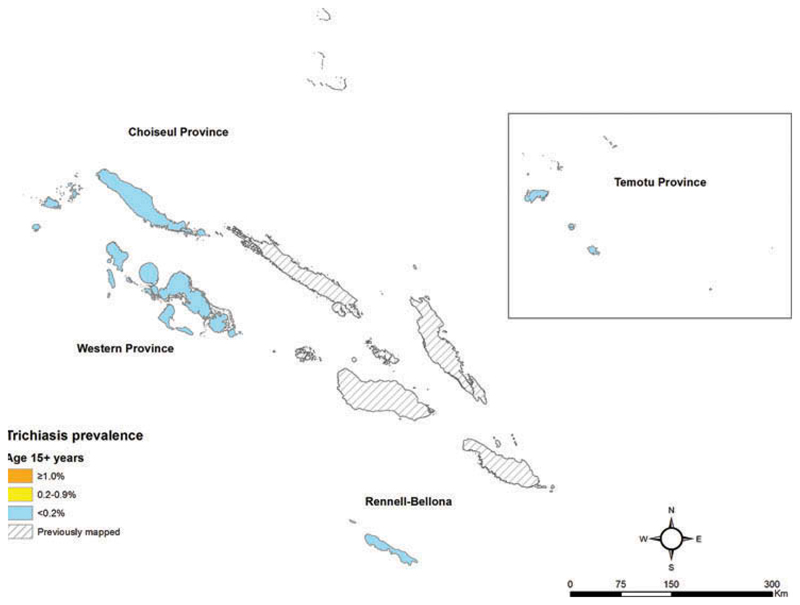
Prevalences of TF in children aged 1–9 years (Figure 2, Table 2) and prevalences of trichiasis in adults (Figure 3, Table 3) are shown by EU. A total of eight cases of trichiasis were found in the survey; six of these cases were found in Western province, and two in Rennell-Bellona/Temotu. The age- and sex-adjusted trichiasis prevalences in those aged 15+ years were 0.0% in Choiseul, 0.16% in Western, and 0.1% in Rennell-Bellona/Temotu.
Figure 2.
Trachomatous inflammation – follicular (TF) prevalence in children aged 1–9 years, Global Trachoma Mapping Project, Solomon Islands, September–November 2013. Shapefile source: Global administrative areas (gadm.org).
Table 2.
Prevalence of trachomatous inflammation – follicular (TF) in children aged 1–9 years, Global Trachoma Mapping Project, Solomon Islands, September–November 2013.
| Evaluation unit | Examined, n | TF, n (%) | Adjusteda TF, % (95% CI) |
|---|---|---|---|
| Choiseul | 881 | 66 (7.5) | 6.1 (4.1–8.6) |
| Western | 996 | 226 (22.7) | 20.4 (15.6–26.3) |
| Rennell-Bellona/Temotu | 1136 | 296 (26.1) | 22.0 (18.5–26.0) |
Adjusted by age in 1-year bands.
CI, confidence interval.
Figure 3.
Trichiasis prevalence in those aged 15 years and older, Global Trachoma Mapping Project, Solomon Islands, September–November 2013. Shapefile source: Global administrative areas (gadm.org).
Table 3.
Prevalence of trichiasis in those aged 15 years or older, Global Trachoma Mapping Project, Solomon Islands, September–November 2013.
| Examined, n (%) |
Trichiasis, n (%) |
||||
|---|---|---|---|---|---|
| Evaluation unit | Male | Female | Male | Female | Adjusteda trichiasis, % (95% CI) |
| Choiseul | 533 (36.9) | 913 (63.1) | 0 (0.0) | 0 (0.0) | 0.0 (0.0–0.2) |
| Western | 712 (42.4) | 967 (57.6) | 3 (0.4) | 3 (0.3) | 0.16 (0.0–0.5) |
| Rennell-Bellona/Temotu | 1064 (45.5) | 1275 (54.5) | 0 (0.0) | 2 (0.2) | 0.04 (0.0–0.3) |
Adjusted for sex and age in 5-year bands.
CI, confidence interval.
WASH data
WASH data were obtained from 2448 households. Practicing open defecation was reported by adults in 1443 households (58.9%), with the most common site of defecation being in the sea or on the beach. Only 1006 households (41.1%) reported using some kind of latrine: 302 houses (12.3%) had an improved latrine, of which 260 were private latrines, and 704 (28.8%) households had an unimproved latrine. Overall, 1443 houses (58.9%) had access to improved washing water and 1595 households (65.1%) had improved drinking water. In the univariate logistic regression analysis, no household level WASH variables were associated with the presence of TF in 1–9-year-old children. The full results are shown in Table 4.
Table 4.
Multilevel univariable random effects logistic regression of factors against the outcome of trachomatous inflammation – follicular (TF) in children aged 1–9 years, Global Trachoma Mapping Project, Solomon Islands, September–November 2013.
| WASH variable | Odds ratio (95% CI) | p-value |
|---|---|---|
| Use of improved drinking source | 1.31 (0.96–1.80) | 0.084 |
| Use of improved washing source | 1.01 (0.69–1.47) | 0.972 |
| Household private latrine | 0.87 (0.54–1.41) | 0.576 |
| Household improved latrine | 0.79 (0.50–1.25) | 0.306 |
| Overall latrine category (open defecation vs unimproved vs improved latrine) | 0.29 | |
| Open defecation | 1.0 (reference) | |
| Unimproved latrine | 0.89 (0.71–1.12) | |
| Improved latrine | 0.76 (0.53–1.11) | |
| Time to get to washing water | 0.13 | |
| All face washing done at water source | 1.0 (reference) | |
| Water source in the yard | 0.73 (0.37–1.43) | |
| Less than 30 minutes | 1.21 (0.62–2.32) | |
| Between 30 minutes and 1 hour | 0.98 (0.47–2.03) | |
| More than 1 hour | 1.19 (0.44–3.17) | |
| Time to get to drinking water | 0.579 | |
| Water source in the yard | 1.0 (reference) | |
| Less than 30 minutes | 1.28 (0.86–1.90) | |
| Between 30 minutes and 1 hour | 1.18 (0.70–2.00) | |
| More than 1 hour | 1.48 (0.77–2.84) | |
CI, confidence interval.
Discussion
The prevalence of active trachoma was above 5% in each EU, and above 10% in two of the three EUs (three of four provinces) studied. WHO guidelines recommend implementation of the A, F and E components of SAFE, including mass administration of azithromycin, in Western and Rennell-Bellona/Temotu provinces, for at least 3 years before impact surveys are done. However, the prevalence of trichiasis in each province was low, and below the level established by WHO as being indicative of a public health problem in all three EUs. This is consistent with the experience of ophthalmologists in the Solomon Islands, who see cases of trichiasis in their clinics very rarely.9,16 This raises the question of whether trachoma can truly be thought to be a significant public health problem in these populations.
Trachoma surveys from endemic countries of Sub-Saharan Africa, such as Ethiopia or Nigeria, report much higher prevalences of trichiasis in districts with prevalent TF. It is therefore unclear why, even in the presence of a high prevalence of active trachoma in children, the blinding effects of trachoma are rarely seen in adults in the Solomon Islands. A similar question is raised by corresponding GTMP data from other countries in the South Pacific.17 The anomaly could be attributable to a different set of C. trachomatis genotypes in the region, a different bacterium altogether being responsible for the active trachoma phenotype, or a more muted scarring response of those infected.18–20
Recent studies on causes of blindness in the Solomon Islands are scarce. Reports from the Fred Hollows Foundation eye health outreach services describe 10 cases of trichiasis identified in the Solomon Islands between 2011 and 2013.16 These reports suggest cataract is the dominant cause of blindness in the country, and do not suggest a major burden of trachoma. While the clinical snapshot obtained from the current surveys indicates a low prevalence of trachoma’s blinding sequelae, it is not currently clear whether prevalence is changing over time; trachoma could be in a period of increase, stasis or decline.
Trachoma has been linked with low levels of access to sanitation.21 The availability of sanitation found in this study was very low. Adults in a high proportion of households (58.9%) practiced open defecation, yet children from these homes were no more likely to have TF than those from households with the best sanitation access. None of the WASH variables collected were associated with TF in children. This is not too surprising; although the disease is almost exclusively associated with rural areas of poverty and social deprivation, there is some inconsistency in WASH associations and no universal risk factors for trachoma. For example, it has previously been associated with poor access to water,22–26 but clear exceptions exist.27–29
The inhabitants of this diverse group of islands are separated from each other not just geographically, but also culturally and ethnically. It is possible that particular behaviors associated with these cultures could in part explain the differences in trachoma prevalence between EUs, and help to inform appropriate control strategies, but these considerations were not examined in this set of surveys.
Based on our results, current WHO guidelines would indicate that azithromycin mass drug administration is needed in all three EUs. Azithromycin distribution would have the added benefit of treating yaws30 and may also treat urogenital C. trachomatis,31 both of which are highly prevalent locally.32,33 However, given the paucity of data showing blinding complications of trachoma here, there is also an obligation to undertake intensive basic and operational research into the etiology of, and the effect of SAFE implementation on, the active trachoma phenotype in these populations.
Funding
This study was principally funded by the Global Trachoma Mapping Project (GTMP) grant from the United Kingdom’s Department for International Development (ARIES: 203145) to Sightsavers, which led a consortium of non-governmental organizations and academic institutions to support ministries of health to complete baseline trachoma mapping worldwide. The GTMP was also funded by the United States Agency for International Development (USAID), through the ENVISION project implemented by RTI International under cooperative agreement number AID-OAA-A-11-00048, and the END in Asia project implemented by FHI360 under cooperative agreement number OAA-A-10-00051. A committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially funded by a grant from Pfizer to the International Trachoma Initiative. MM was a Wellcome Trust Clinical Research Fellow (102807) and AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) at the London School of Hygiene & Tropical Medicine. RB was funded by the Wellcome Trust (098521/B/12/Z). None of the funders had any role in project design, in project implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer-reviewed press, or in preparation of the manuscript.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.World Health Organization. [accessed 30 July 2016];Trachoma: fact sheet. updated July 2016. http://www.who.int/mediacentre/factsheets/fs382/en/
- 2.Bailey R, Duong T, Carpenter R, et al. The duration of human ocular Chlamydia trachomatis infection is age dependent. [Accessed January 14, 2014];Epidemiol Infect. 1999 123:479–486. doi: 10.1017/s0950268899003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon AW, Peeling RW, Foster A, et al. Diagnosis and assessment of trachoma. Clin Microbiol Rev. 2004;17:982–1011. doi: 10.1128/CMR.17.4.982-1011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thylefors B, Dawson CR, Jones BR, et al. A simple system for the assessment of trachoma and its complications. [Accessed January 2, 2014];Bull World Health Organ. 1987 65:477–483. [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Report of the 3rd global scientific meeting on trachoma. Johns Hopkins University; Baltimore, MD: 2010. Jul 19–20, [Google Scholar]
- 6.World Health Organization. Validation of elimination of trachoma as a public health problem (WHO/HTM/NTD/2016.8) Geneva: WHO; 2016. [Google Scholar]
- 7.Solomon Island Government. Report on 2009 population and housing census. 2011.
- 8.Maccallan AF. Trachoma in the British Colonial Empire – its relation to blindness, the existing means of relief, means of prophylaxis. [Accessed January 2, 2014];Br J Ophthalmol. 1934 18:625–645. doi: 10.1136/bjo.18.11.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew AA, Keeffe JE, Le Mesurier RT, et al. Trachoma in the Pacific Islands: evidence from Trachoma Rapid Assessment. Br J Ophthalmol. 2009;93:866–870. doi: 10.1136/bjo.2008.151720. [DOI] [PubMed] [Google Scholar]
- 10.International Association for the Prevention of Blindness. Trachoma Mapping in the Pacific. 2013.
- 11.Kama M, Cama A, Rawalai K, et al. Active ocular trachoma in Fiji – A population based prevalence survey. Fiji J Public Health. 2013;2:11–17. [Google Scholar]
- 12.Solomon AW, Pavluck AL, Courtright P, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22:214–225. doi: 10.3109/09286586.2015.1037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization (WHO)/UNICEF. Core questions on drinking water and sanitation for household surveys. 2006 Available at: http://www.who.int/water_sanitation_health/monitoring/oms_brochure_ core_questionsfinal24608.pdf.
- 14.R Core Team. R: a language and environment for statistical computing. R Found Stat Comput; 2014. Available at: http://www.r-project.org. [Google Scholar]
- 15.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–1164. doi: 10.1002/(sici)1097-0258(20000515)19:9<1141::aid-sim479>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.Lees J, McCool J, Woodward A. Eye health outreach services in the Pacific Islands region: an updated profile. [Accessed November 13, 2015];N Z Med J. 2015 128(1420):25–33. [PubMed] [Google Scholar]
- 17.Ko R, Macleod C, Pahau D, et al. Population-Based Trachoma Mapping in Six Evaluation Units of Papua New Guinea. Ophthalmic Epidemiol. 2016 doi: 10.1080/09286586.2016.1235715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean D, Rothschild J, Ruettger A, et al. Zoonotic Chlamydiaceae species associated with trachoma, Nepal. Emerg Infect Dis. 2013;19:1948–1955. doi: 10.3201/eid1912.130656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton MJ, Hu VH, Massae P, et al. What is causing active trachoma? The role of nonchlamydial bacterial pathogens in a low prevalence setting. Invest Ophthalmol Vis Sci. 2011;52:6012–6017. doi: 10.1167/iovs.11-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natividad-Sancho A, Holland MJ, Mabey DCW, et al. Host genetic studies in human ocular Chlamydial infection. [Accessed August 9, 2013];Drugs Today (Barc) 2009 45(Suppl.B):61–66. [PubMed] [Google Scholar]
- 21.Stocks ME, Ogden S, Haddad D, et al. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. Hunter PR, editor. PLoS Med. 2014;11(2):e1001605. doi: 10.1371/journal.pmed.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emerson PM, Burton M, Solomon AW, et al. The SAFE strategy for trachoma control: Using operational research for policy, planning and implementation. [Accessed January 19, 2015];Bull World Health Organ. 2006 84:613–619. doi: 10.2471/blt.05.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prost A, Negrel AD. Water, trachoma and conjunctivitis. Bull World Health Organ. 1989;67:9–18. Available at: //WOS:A1989U282600002. [PMC free article] [PubMed] [Google Scholar]
- 24.Golovaty I, Jones L, Gelaye B, et al. Access to water source, latrine facilities and other risk factors of active trachoma in Ankober, Ethiopia. PLoS One. 2009;4(8):e6702. doi: 10.1371/journal.pone.0006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Last AR, Burr SE, Weiss HA, et al. Risk factors for active trachoma and ocular Chlamydia trachomatis infection in treatment-naïve trachoma-hyperendemic communities of the Bijagós Archipelago, Guinea Bissau. PLoS Negl Trop Dis. 2014;8(6):e2900. doi: 10.1371/journal.pntd.0002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalua K, Chirwa T, Kalilani L, et al. Prevalence and risk factors for trachoma in Central and Southern Malawi. PLoS One. 2010;5(2):e9067. doi: 10.1371/journal.pone.0009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller H, Gallego G, Rodriguez G. [Clinical evidence of trachoma in Colombian Amerindians of the Vaupes Province] Biomedica. 2010;30:432–439. [PubMed] [Google Scholar]
- 28.PINES N. [Incidence of trachoma in London] Rev Int Trach. 1954;31:153–156. [PubMed] [Google Scholar]
- 29.Meighan SS. Trachoma in Glasgow: Discussion at the International Conference on Trachoma, held in London on April 3, 1935. Br J Ophthalmol. 1935;19:326. doi: 10.1136/bjo.19.6.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marks M, Vahi V, Sokana O, et al. Impact of community mass treatment with azithromycin for trachoma elimination on the prevalence of yaws. PLoS Negl Trop Dis. 2015;9(8):e0003988. doi: 10.1371/journal.pntd.0003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks M, Bottomley C, Tome H, et al. Mass drug administration of azithromycin for trachoma reduces the prevalence of genital Chlamydia trachomatis infection in the Solomon Islands. Sex Transm Infect. 2016;92:261–265. doi: 10.1136/sextrans-2015-052439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks M, Vahi V, Sokana O, et al. Mapping the epidemiology of yaws in the Solomon Islands: a cluster randomized survey. Am J Trop Med Hyg. 2015;92:129–133. doi: 10.4269/ajtmh.14-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks M, Kako H, Butcher R, et al. Prevalence of sexually transmitted infections in female clinic attendees in Honiara, Solomon Islands. BMJ Open. 2015;5:e007276. doi: 10.1136/bmjopen-2014007276. [DOI] [PMC free article] [PubMed] [Google Scholar]