Abstract
Several genetic discoveries robustly implicate five single nucleotide variants in the progression of non-alcoholic fatty liver disease to non-alcoholic steatohepatitis and fibrosis (NASH-fibrosis), including a recently identified variant in MTARC1. To better understand these variants as potential therapeutic targets, we aimed to characterize their impact on metabolism using comprehensive metabolomics data from two population-based studies. 9,135 participants from the Fenland Study and 9,902 participants from the EPIC-Norfolk cohort were included in the study. We identified individuals with risk alleles associated with NASH-fibrosis: rs738409C>G in PNPLA3, rs58542926C>T in TM6SF2, rs641738C>T near MBOAT7, rs72613567TA>T in HSD17B13, and rs2642438A>G in MTARC1. Circulating levels of 1449 metabolites were measured using targeted and untargeted metabolomics. Associations between NASH-fibrosis variants and metabolites were assessed using linear regression. The specificity of variant-metabolite associations was compared to metabolite associations with: ultrasound-defined steatosis, gene variants linked to liver fat (in GCKR, PPP1R3B, and LYPLAL1), and gene variants linked to cirrhosis (in HFE and SERPINA1). Each NASH-fibrosis variant demonstrated a specific metabolite profile with little overlap (8/97 metabolites) comprising diverse aspects of lipid metabolism. Risk alleles in PNPLA3 and HSD17B13 were both associated with higher 3-methylglutarylcarnitine and three variants were associated with lower lysophosphatidylcholine C14:0. The risk allele in MTARC1 was associated with higher levels of sphingomyelins. There was no overlap with metabolites that associated with HFE or SERPINA1 variants. Our results suggest a link between the NASH-protective variant in MTARC1 to the metabolism of sphingomyelins and identify distinct molecular patterns associated with each of the NASH-fibrosis variants under investigation.
Introduction
Non-alcoholic steatohepatitis (NASH) is a common, multifactorial condition that may progress to cirrhosis, liver failure, and hepatocellular carcinoma(1). NASH affects approximately 20% of individuals with non-alcoholic fatty liver disease (NAFLD), which is strongly associated with obesity and insulin resistance(2). Certain single nucleotide polymorphisms (SNPs) have been linked with disease progression through development of NASH and fibrosis, apparently independent of insulin resistance(3).
A combination of exome-wide and genome-wide association studies (GWAS) have led to the identification of five loci (rs738409 in PNPLA3(4), rs58542926 near TM6SF2(5), rs72613567 in HSD17B13(6), rs641738 near TMC4-MBOAT7(7–9), and rs2642438 in MTARC1(10)) linked to non-alcoholic steatohepatitis (NASH)(11), fibrosis(12), and hepatocellular carcinoma (HCC)(13) in patients with NAFLD. Following in vitro and in vivo experiments, rs738409C>G (p.Ile148Met) in PNPLA3 has been identified as a regulator of hepatic lipolysis (14,15). However, the potential pathophysiological consequence of the other variants are less understood or entirely unknown, with respect to the MTARC1 p.Thr165Ala variant (10).
Metabolite association studies are an established technique for exploring the role of gene variants(16–18). Serum metabolite and plasma lipid profiling has been used to investigate some of these NASH-fibrosis gene variants and to identify differences between healthy subjects and those with NAFLD (for example, higher alanine in patients with NAFLD)(19,20). Metabolomics and lipidomics have also been used to differentiate NAFL from NASH (for example, higher phosphatidylcholine C32:0 in patients with NASH) in a selected cohort of adults who underwent liver biopsy (21).
We hypothesised that variants associated with NASH-fibrosis would be associated with perturbation of metabolic pathways among adults from population-based cohort studies. To study this, we examined the serum metabolite and lipid profile of five variants associated with NASH-fibrosis in two population-based cohorts. We aimed to discover novel gene-metabolite associations that may be involved in the pathogenesis of NASH.
Results
Baseline participant characteristics
9,135 participants from the Fenland cohort study were included, where 2301 (25%) had ultrasound evidence of hepatic fat accumulation (Supplementary Table 2). Steatosis was associated with male sex, higher waist-to-hip ratio, lower HDL cholesterol, and higher fasting insulin (Supplementary Table 2).
Metabolite profile of hepatic steatosis
We first aimed to describe how hepatic steatosis may have influenced circulating metabolites. The serum metabolite profile of steatosis showed increased branched chain amino acids and increased unsaturated short chain triglyceride species, similar to the pattern observed for insulin resistance and dyslipidaemia. (Supplementary figure 1 & Supplementary table 3). Adjusting for fasting insulin partially attenuated these changes (Supplementary figure 1).
NASH-fibrosis SNPs have specific metabolite profiles
We then proceeded to study the characteristics of individuals with risk alleles from the five NASH-fibrosis variants. None of the risk alleles for each SNP was associated with classical markers of insulin resistance (Supplementary Tables 4-8). For example, neither rs738409 C>G in PNPLA3 nor rs58542926 C>T near TM6SF2 (risk alleles for type 2 diabetes (22)) were significantly associated with differences in fasting insulin, glucose, or HbA1c (Supplementary Tables 4 & 5). Two variants did, however, associate with differences in lipoproteins that would classically be associated with lower risk of atherosclerosis: lower LDL-cholesterol and total TG with rs58542926 C>T near TM6SF2 (Supplementary Table 5) & 5), and higher HDL-cholesterol with rs2642438 A>G in MTARC1 (Supplementary Table 8).
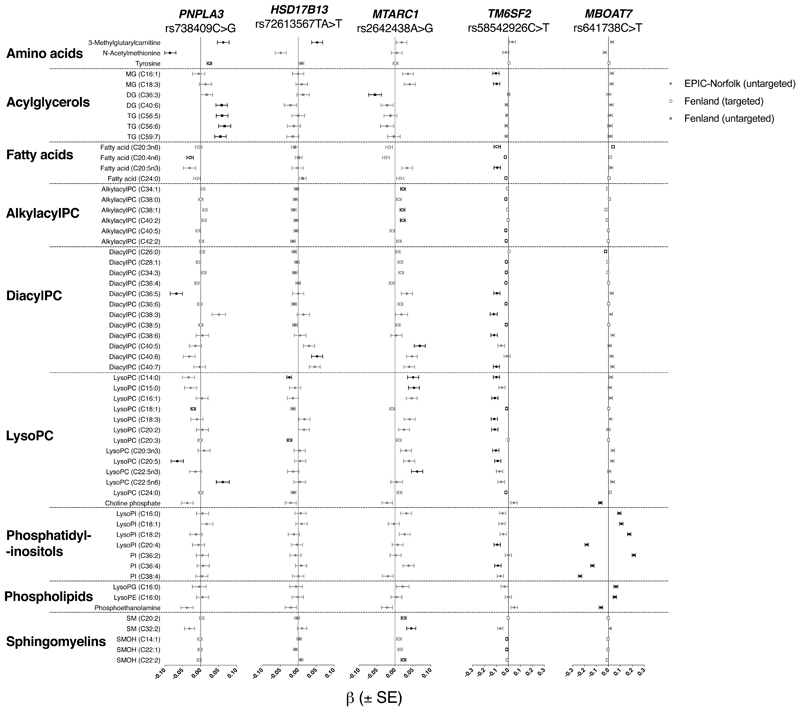
Next, we used targeted metabolite profiling (in 9,135 participants) and untargeted lipidomics (in 1,356 participants) from the Fenland cohort to test for SNP-metabolite associations. This analysis was complemented by a separate cohort of 9,902 participants from the EPIC-Norfolk cohort(23) with untargeted metabolite profiling using a different platform for metabolomics measurements (Figure 1). Each NASH-fibrosis SNP was found to have a specific metabolite profile (Table 1 and Supplementary Table 3).
Figure 1.
Associations of metabolites with NASH-fibrosis variants in the Fenland and EPIC-Norfolk cohort. Metabolites are arranged by class and annotated whether they were obtained by untargeted or targeted methods. β ±standard error (SE) per allele from linear regression models are presented, adjusted for age, sex and the first ten genetic principal components and after standardization of each metabolite. Only metabolites significantly associated with at least one variant of interest (Q<0.05) are presented and marked in black. MG, monoglyceride; PC, phosphatidylcholine; PI, phosphatidylinositol; SM, sphingomyelin.
Table 1.
Top associations between metabolites and NASH-fibrosis variants in the EPIC-Norfolk and Fenland cohorts. Beta represents the change in 1 normalised standard deviation of each metabolite per effect allele (Supplementary Table 8 for a full list of significant associations, Q<0.05). DG, diacylglycerol; GPC, glycerophosphatidylcholine; GPI, glycerophosphatidylinositol; PC, phosphatidylcholine; Q, Q-value (false discovery rate-corrected P-value); SM, sphingomyelin; SMOH, sphingomyelin hydroxide; TG, triacylglycerol.
| Group | Metabolite | Beta (SE) | p | q | n | Cohort | Method |
|---|---|---|---|---|---|---|---|
| rs72613567TA>T in HSD17B13 | |||||||
| Amino acid | 3-methylglutarylcarnitine | .05 (.02) | 5.3E-04 | 0.024 | 9756 | EPIC | Untargeted |
| Phosphatidylcholines | LysoPC (C14:0) | -.02 (.01) | 1.3E-04 | 0.006 | 9317 | Fenland | Targeted |
| Phosphatidylcholines | LysoPC (C20:3) | -.02 (.01) | 2.9E-04 | 0.012 | 9317 | Fenland | Targeted |
| Phosphatidylcholines | DiacylPC (C40:6) | .05 (.02) | 6.3E-04 | 0.028 | 9902 | EPIC | Untargeted |
| rs641738C>T near MBOAT7 | |||||||
| Fatty acid | Fatty acid (C20:3n6) | .04 (.01) | 2.5E-04 | 0.010 | 4279 | Fenland | Targeted |
| Lysolipid | LysoPI (C20:4) | -.18 (.01) | 9.3E-38 | 3.0E-34 | 9902 | EPIC | Untargeted |
| Lysolipid | LysoPI (C18:2) | .17 (.01) | 2.1E-34 | 5.2E-31 | 9902 | EPIC | Untargeted |
| Phosphatidylcholines | DiacylPC (C26:0) | -.02 (.01) | 0.001 | 0.037 | 9317 | Fenland | Targeted |
| Phosphatidylinositol | PI (C38:4) | -.24 (.01) | 1.8E-62 | 1.7E-58 | 9902 | EPIC | Untargeted |
| Phosphatidylinositol | PI (C36:2) | .21 (.01) | 1.1E-50 | 5.4E-47 | 9902 | EPIC | Untargeted |
| rs2642438A>G in MTARC1 | |||||||
| Lysolipid | LysoPC (C22:5) | .06 (.02) | 6.5E-05 | 0.005 | 9858 | EPIC | Untargeted |
| Lysolipid | LysoPC (C15:0) | .05 (.02) | 6.1E-04 | 0.028 | 9901 | EPIC | Untargeted |
| Lysolipid | LysoPC (C14:0) | .05 (.02) | 0.001 | 0.039 | 9901 | EPIC | Untargeted |
| Phosphatidylcholines | DiacylPC (C40:5) | .07 (.02) | 7.5E-06 | 8.6E-04 | 9901 | EPIC | Untargeted |
| Phosphatidylcholines | Alkylacyl PC (C34:1) | .02 (.01) | 8.6E-04 | 0.026 | 9317 | Fenland | Targeted |
| Sphingomyelin | SM (C20:2) | .02 (.01) | 3.9E-04 | 0.015 | 9317 | Fenland | Targeted |
| Sphingomyelin | SMOH (C22:2) | .02 (.01) | 5.4E-04 | 0.019 | 9317 | Fenland | Targeted |
| Sphingomyelin | SM (C32:2) | .05 (.01) | 3.9E-04 | 0.019 | 9901 | EPIC | Untargeted |
| rs738409C>G in PNPLA3 | |||||||
| Amino acid | N-acetylmethionine | -.09 (.02) | 3.6E-07 | 5.8E-05 | 9893 | EPIC | Untargeted |
| Amino acid | Tyrosine | .02 (.01) | 1.3E-04 | 0.006 | 9317 | Fenland | Targeted |
| Amino acid | 3-methylglutarylcarnitine | .06 (.02) | 1.2E-04 | 0.008 | 9756 | EPIC | Untargeted |
| Fatty acid | Fatty acid (C20:4n6) | -.03 (.01) | 0.001 | 0.037 | 4279 | Fenland | Targeted |
| Triacylglyceride | TG (C56:6) | .07 (.02) | 7.3E-05 | 0.004 | 1356 | Fenland | Untargeted |
| Triacylglyceride | TG (C56:5) | .06 (.02) | 2.6E-04 | 0.011 | 1356 | Fenland | Untargeted |
| Triacylglyceride | TG (C59:7) | .06 (.02) | 5.6E-04 | 0.019 | 1356 | Fenland | Untargeted |
| rs58542926C>T in TM6SF2 | |||||||
| Fatty acid | Fatty acid (C20:4n6) | -.03 (.01) | 1.9E-05 | 0.001 | 4279 | Fenland | Targeted |
| Fatty acid | Fatty acid (C20:3n6) | -.09 (.03) | 3.7E-04 | 0.019 | 9891 | EPIC | Untargeted |
| Lysolipid | LysoPC (C14:0) | -.1 (.03) | 1.7E-04 | 0.010 | 9901 | EPIC | Untargeted |
| Phosphatidylcholines | DiacylPC (C34:4) | -.03 (.004) | 2.0E-10 | 7.8E-08 | 9317 | Fenland | Targeted |
| Phosphatidylcholines | DiacylPC (C34:4) | -.15 (.03) | 1.0E-08 | 2.5E-06 | 9900 | EPIC | Untargeted |
| Phosphatidylcholines | Alkylacyl PC (C40:5) | -.02 (.004) | 1.0E-07 | 2.3E-05 | 9317 | Fenland | Targeted |
| Phosphatidylcholines | DiacylPC (C34:4) | -.15 (.03) | 1.7E-07 | 3.0E-05 | 7991 | EPIC | Untargeted |
| Phosphatidylcholines | Alkylacyl PC (C38:0) | -.02 (.004) | 1.8E-07 | 3.2E-05 | 9317 | Fenland | Targeted |
| Sphingomyelin | SMOH (C22:1) | -.01 (.004) | 7.3E-04 | 0.023 | 9317 | Fenland | Targeted |
| Sphingomyelin | SMOH (C14:1) | -.01 (.004) | 0.001 | 0.038 | 9317 | Fenland | Targeted |
The strongest association for the rs738409 C>G in PNPLA3 variant was observed with N-acetylmethionine (β= -.09, Q=5.7x10-5). The variant was further positively associated with plasma levels of TG and DG species carrying long-chain polyunsaturated fatty acids (Supplementary Figure 2), which was replicated in external datasets (Supplementary Table 9). It was also positively associated with levels of 3-methylglutarylcarnitine (Figure 1).
rs72613567 TA>T in HSD17B13 was positively associated with long-chain diacylPC and negatively associated with short chain lysoPC (Supplementary Figure 3 & Supplementary Table 9). It was also positively associated with 3-methylglutarylcarnitine and pyroglutamine (Supplementary Table 3).
rs58542926C>T near TM6SF2 was inversely associated with plasma levels of several lipid classes, including: diacylPC, alkylacylPC, fatty acids, lysoPC, and sphingomyelins (Supplementary Figure 4). This was consistent across both cohorts and in external datasets (Supplementary Tables 3 & 9). The magnitude of these associations were attenuated when adjusting for total cholesterol and triglycerides (Supplementary Figure 5).
There was a trend towards lower TG and DG (in line with the main serum biochemistry results), though no individual species reached significance after adjusting for multiple testing (Supplementary Figure 4E & 4F). A similar trend in inverse associations with TG species was found in publicly available datasets (Supplementary Figure 6 & Supplementary Table 9).
The rs2642438 A>G risk variant in MTARC1 was associated with higher sphingomyelins (Supplementary Figure 7), for example C20:2, (β= .02, Q=0.015). This finding was replicated across several sphingomyelin species in both cohorts and in external datasets (Supplementary Figures 3 & 6, and Supplementary Table 9). This variant was also associated with higher diacylPC (18:0/22:5), higher lysoPC (C14:0 and C15:0), and higher alkylacyl PC (C34:1 and C40:2, for example, Supplementary Figure 7). The magnitude of these associations were minimally affected by adjusting for HDL cholesterol (Supplementary Figure 5).
Finally, rs641738 C>T near MBOAT7 was found to have a strong association with phosphatidylinositols (PI), which was not replicated in any other NASH-fibrosis SNP (for example, PI C18:0/C20:4: β -0.2, Q=1.7x10-58, Figure 1). The variant was associated heterogeneously with species carrying omega-6 polyunsaturated fatty acids: for example, lower phosphatidylinositols (PI) with stearic acid and omega-6 polyunsaturated fatty acids (e.g. PI(18:0/20:4), β= -.24, Q=1.7x10-58); higher PI with stearic acid and linoleic acid (e.g. PI(18:0/18:2), β= .21, Q=1.1x10-50); and higher dihomo-gamma-linolenic acid (DHGL, Supplementary Figure 8). These findings were replicated in external datasets (Supplementary Table 9).
Overlap between metabolite profiles
We compared SNP-metabolite associations to assess whether there were clear trends that reflected steatosis, NASH, or fibrosis. Out of the 97 different metabolites significantly associated with at least one of the five NASH-fibrosis variants, only 8.2% (8/97) were associated with two or more variants (Supplementary Table 3). For all eight metabolites, the directions of the associations were consistent across SNPs effect (Figure 1). For example, risk alleles near or in TM6SF2, HSD17B13, and MTARC1 were all associated with lower plasma levels of lysoPC (C14:0, Figure 1). Similarly, higher levels of 3-methylglutarylcarnitine were associated with risk alleles in PNPLA3 and HSD17B13 (Figure 1).
Next, we assessed which of the five variants contributed most to the overall variation in these metabolites. In the Fenland cohort, rs738409 C>G in PNPLA3 accounted for the most variability (adjusted R2 5.8%) in the 41 significantly associated metabolites. Compared to 3.4% for rs72613567 TA>T in HSD17B13, 3.2% for rs58542926C>T near TM6SF2, 1.1% for rs641738 C>T near MBOAT7, and 0.0% for rs2642438 A>G in MTARC1.
Comparison with HFE and SERPINA1
To determine whether these results were specific to NASH-fibrosis or were more generally reflective of hepatocyte dysfunction, we compared associations from the five NASH-fibrosis variants with SNP-metabolite associations for HFE and SERPINA1 variants. 37 metabolites were associated with rs1800562 G>A in HFE (p.Cys282Tyr) or rs28929474 C>T in SERPINA1 (p.Glu366Lys), variants linked to metabolic cirrhosis (Supplementary Table 3). There was no overlap between these 37 metabolites and the 97 associated with NASH-fibrosis variants (Supplementary Table 3).
Comparison with steatosis and insulin resistance
Finally, we aimed to test whether the SNP-metabolite associations were indicative of steatosis or insulin resistance or both. In the Fenland cohort, out of 41 different metabolites associated with the five different NASH-fibrosis variants, 78% (32/41) were also significantly altered in steatosis (Supplementary Table 3). The majority (69%, 22/32) were PC species though the direction of change was not consistent (Supplementary Table 3). For example, several NASH-fibrosis variants were associated with lower levels of lysoPC (C14:0), whereas steatosis was associated with higher levels (β= .07, Q=7.7x10-8, Supplementary Figure 1 & Supplementary table 3).
Liver-fat related variants in GCKR, PPP1R3B, and LYPLAL1 were associated with the plasma levels of 281 metabolites. 52% (50/97) of metabolites associated with NASH-fibrosis variants were also associated with liver fat variants, again the majority (86%, 43/50) were PC species due to similarity between GCKR and TM6SF2 metabolite associations (Supplementary Table 3).
In order to determine whether these SNP-metabolite associations were indicative of insulin resistance or body composition we compared the metabolite associations of published genetic risk scores with those from NASH-fibrosis SNPs. There was some overlap between metabolites associated with NASH-fibrosis variants and genetic risk scores for BMI, BMI-adjusted waist-to-hip ratio, body fat percentage, and insulin resistance. 24 out of 97 different metabolites (24.7%) were also associated with one of the four genetic risk scores (Supplementary table 3), most of which (54%, 13/24) were PC species, pointing to shared pathways between higher genetic susceptibility to adverse body composition or insulin resistance and NASH.
Discussion
There is strong human genetic evidence for five common variants in the pathogenesis of fibrotic NASH. To improve our understanding about functional consequences of these SNPs we used blood metabolite profiling in two large population cohorts. Metabolite profiles were found to be highly specific with only few metabolites significantly associated with more than one variant, such as 3-methylglutarylcarntine (PNPLA3 and HSD17B13) and lysoPC C14:0 (TM6SF2, HSD17B13, and MTARC1) that were not replicated in other variants associated with liver fat but not fibrosis (e.g. LYPLAL1) or fibrosis without steatosis (e.g. SERPINA1). The specific (lipid) profiles of each variant might be interpreted as distinct hits on lipid metabolism resulting in the same consequence, accelerating progression from NAFLD to NASH.
rs2642438 A>G in MTARC1 (p.Thr165Ala) has very recently been identified as a risk variant in NAFLD and all-cause cirrhosis(10). MTARC1 encodes for an outer mitochondrial membrane-bound molybdenum enzyme recognised to be involved in drug metabolism and has capacity for nitrite reduction. Its function in liver disease is largely unknown(24–27). We found that this MTARC1 variant was associated with higher levels of a range of sphingomyelin species across all studied cohorts and our results are supported by previous studies(28,29). Sphingomyelins are primarily plasma membrane components that are synthesised from ceramides. Higher sphingomyelins have been implicated in the development of cirrhosis(30,31) as well as insulin resistance(32,33). Several members of the ceramide-sphingomyelin pathway have been linked to lipotoxicity and it is likely that perturbation of the balance in this metabolic pathway is harmful in liver disease from multiple aetiologies(34). How this variant relates to the sphingomyelin pathway is unclear and requires further investigation, particularly given that there are many variants that affect sphingomyelin levels and have no identified impact on NAFLD, for example variants in sphingosine-1-phosphate phosphatase 1 (SGPP1) (35).
Along with rs58542926 near TM6SF2, rs2642438 A>G in MTARC1 was found to be associated with changes in major classes of serum lipoparticles. Metabolite associations for rs2642438 A>G remained after adjusting for HDL cholesterol levels, however further refined studies (such as lipidomics separated by lipoparticles) are needed to give biological insight into these observations. Mechanistic studies, similar to those performed for TM6SF2(36), would help to identify the role MTARC1 plays in lipoparticle export and/or lipidation.
rs738409 C>G (p.Ile148Met) in PNPLA3 is the genetic variant most strongly associated with hepatic outcomes in NAFLD. Three recent studies have provided compelling evidence that PNPLA3 binds to ABHD5 (abhydrolase domain containing 5) and regulates ATGL (adipose triglyceride lipase)-mediated hydrolysis from lipid droplets(14,15,37). We found this variant to be associated with higher TG with long-chain polyunsaturated fatty acids, consistent with mouse data and other human studies(38–40). These have been classed as ‘healthy lipids’(41) and are not associated with insulin resistance(42,43), despite this variant being positively associated with T2DM(22). These data suggest that length and saturation of TG fatty acids may influence or depend on the PNPLA3-ABHD5-ATGL interaction.
The TA-duplication allele of rs72613567 in HSD17B13 reduces the risk of NAFLD-cirrhosis and mitigates the risk conferred by carriage of the G-allele in PNPLA3(44). Abul-Husn et al. found that the encoded enzyme 17β-Hydroxysteroid dehydrogenase type 13 to have activity on several steroids and bioactive lipids and did not influence total hepatocyte TG in vitro, however, a mouse knockout did have increased hepatic steatosis(45). It has been recently reported to act as a hepatic retinol dehydrogenase(46), but we did not find an effect on plasma retinol levels. We also found lower circulating lysoPC species, which is consistent with the recent findings of Luukkonen et al. who analysed the hepatic lipid profile of NAFLD patients carrying the protective variant (rs72613567T>TA) and identified higher hepatic phospholipids(47).
One metabolite of interest is 3-methylglutarylcarnitine, where lower levels were associated with HSD17B13 (protective) and higher levels were associated with PNPLA3 (harmful). This metabolite is typically elevated in 3-hydroxy-3-methylglutaryl-coenzyme A lyase deficiency(48), a Reye syndrome-like inborn error of metabolism that presents with microvesicular steatosis and liver failure. This enzyme is also necessary for metabolising β-hydroxy β-methylglutaryl-CoA towards ketogenesis and away from cholesterol synthesis(49). The consistent direction of association across two NASH-fibrosis variants with this pathway warrants further investigation. However, it should be noted that we were unable to validate this finding in any external cohort, therefore further replication is also needed.
rs641738C>T near TMC4-MBOAT7 was initially identified at genome-wide significance for alcoholic liver disease but a recent meta-analysis has demonstrated it to be associated with NASH, fibrosis, and HCC(9). Its function remains unclear as the variant lies within TMC4, a putative transmembrane transporter with no known role in the liver. It also lies close to MBOAT7, an acyltransferase that incorporates (very) long-chain polyunsaturated fatty acids into lysophosphatidylinositols(7,29,50–52) and rs641738C>T is reported to be associated with reduced liver expression of MBOAT7(7,53). We found this variant to be very strongly associated with greater DHGL or higher or lower phosphatidylinositols with different omega-6 fatty acids. These data suggest that rs641738C>T near TMC4-MBOAT7 results in reduced MBOAT7 activity, which lead to differential degrees of acylation of different omega-6 polyunsaturated fatty acids. Therefore, these metabolomics data provide evidence for MBOAT7 as the causal gene in this locus. Its underlying mechanism and clinical significance are unclear, but still implicative of its relevance to type 2 diabetes epidemics, in addition to NASH, because we previously found heterogeneous associations of omega-6 fatty acids with type 2 diabetes incidence(54). Further mechanistic work is warranted to understand how this variant influences the development of NASH.
We found metabolite changes to be relatively specific to each variant, rather than common and reflect NASH or fibrogenesis. There was only 8% overlap in metabolites between the five NASH-fibrosis metabolites though where overlap did occur, the direction of association was consistent between SNP-metabolite pairs. No metabolite associations were shared with HFE or SERPINA1 variants, which suggests levels of serum metabolites reflect specific metabolic pathway perturbations, rather than a generic response to hepatocyte dysfunction. PC species were the most ubiquitously altered metabolites and accounted for the majority of overlap between steatosis and NASH-fibrosis variants. One might speculate that PC species are surrogate markers of lipoprotein metabolism reflecting the final effect of impaired liver function in the circulation due to intrahepatic deteriorations in lipid metabolism accumulating during lifetime in risk allele carriers. Other studies have shown that NAFLD is associated with non-specific changes in amino acids and phospholipids, which have also been linked to insulin resistance(55–63). These findings suggest common genetic variants influence the perturbation of different metabolic pathways, which may result in NASH being a metabolically heterogeneous disease.
There are four broad processes that can affect liver fat: de novo lipogenesis, import of lipids, export of lipids, and metabolic breakdown of lipids. It is likely that each of these plays some role in the development and progression of NAFLD. Each will also contribute to the serum metabolite profile in different ways and this may be reflected in our results. From this perspective it may not be surprising that there is minimal overlap in the serum metabolite profile associated with each variant.
Two studied variants (rs738409C>G in PNPLA3 and rs58542926 near TM6SF2) are positively associated with type 2 diabetes through large GWAS(22) yet we found them not to be associated with fasting insulin or glucose. Detailed studies (including use of hyperinsulinaemic clamps) in smaller groups of individuals have yielded similar results(40,64,65). This observation could be explained if the development of hepatic steatosis in response to these variants was the intermediate causal step. Both of these variants are strongly associated with higher liver fat and steatosis itself may affect systemic insulin resistance(66,67). Experimentally dissecting these mechanisms is challenging as it would require sensitive measurements in individuals matched for adiposity and liver fat, yet stratified by genotype with sufficient power to demonstrate effects.
The main strength of this study is that it is based on two well-characterised, population-based cohorts. Our study evaluated 19,037 in total whereas previous relevant work has analysed data from up to 1810 adults from population-based cohorts(20) or 695 biopsied NAFLD patients(68). For many lipid classes we were able to validate our results across multiple metabolomics platforms and independent cohorts, thought difference in the platforms may mean that the beta-regression coefficients are not directly comparable. This is also the first study to report characterisation of the MTARC1 variant in a population-based cohort. In addition, our conclusions should be free from potential selection bias of previous NAFLD or NASH cohorts or case-control studies (21,68,77–81,69–76).
A potential limitation of our study is the use of ultrasound for definition of steatosis, rather than quantitative magnetic resonance spectroscopy or proton density fat fraction. Ultrasound identification of hepatic steatosis was not available in the EPIC-Norfolk cohort. Furthermore, we did not have complete overlap of metabolomics between Fenland and EPIC-Norfolk cohorts, which reduced the power for identifying associations in those analyses. This meant we were unable to explore some specific steatosis-metabolite associations, for example phosphatidylinositols, which were only covered by untargeted profiling in the EPIC-Norfolk cohort. In addition, we did not have detailed data on liver related outcomes (for example, progression of fibrosis or development of NASH) or aspartate aminotransferase, therefore were unable to calculate non-invasive scores of hepatic fibrosis. Because of our cross-sectional design, we could not determine the causality whether metabolites affected hepatic steatosis or vice versa, whereas gene-metabolite associations should be free from the concern. As population-based cohorts comprised of healthy individuals, this study did not have liver biopsy data. Therefore, we were unable to correlate metabolite changes to histological stage of disease or assess for effect on the hepatic metabolite profile. These would be important future studies to validate these findings.
It should be noted that other variants in (or near) the five studied genes show strong variant-metabolite associations, though have not been associated with liver disease to date. For example, Draisma et al. found rs2576452C>T near MBOAT7 was negatively associated with 1-arachidonoylglycerophosphoinositol, p=8.8x10-18 (28). We selected these five variants for inclusion based on their clear and replicated associations with NASH-fibrosis however studying other variant-metabolite associations in PNPLA3, TM6SF2, MBOAT7, MTARC1, and HSD17B13 may reveal additional insights into the function of these genes.
There are some limitations of the techniques used for metabolite profiling in this study. There is a risk of misidentification of species from untargeted metabolomics, which is most relevant for some metabolites where we were unable to validate trends using targeted metabolomics from the Fenland (or an external) cohort, for example 3-methylglutarylcarnitine. In addition, the platform used with the EPIC-Norfolk cohort has a preference towards hydrophilic metabolites and may be less accurate with more hydrophobic metabolites. Data on triglycerides and diglycerides were only available from the Fenland cohort and in a small number of individuals (1356), which limited power for detecting associations and performing replication, particularly for TM6SF2. Though reassuringly, our results for rs738409C>G in PNPLA3 are consistent with previous reports(40,64,82).
In conclusion, metabolite profiling from two large cohort studies demonstrates a specific signature of pathway perturbation associated with five NASH-fibrosis variants. For example, MTARC1 p.Thr165Ala is associated with higher sphingomyelin species. These findings suggest that common genetic variants may influence different pathophysiologic pathways in the development of NAFLD.
Materials and Methods
Fenland study cohort
The Fenland study is a population-based cohort of 12,435 individuals recruited in 2005-2015 from general practice lists from Cambridgeshire, England. The study and its methods were described in detail previously(83). Briefly, the study aimed to examine genetic, metabolic, lifestyle, and societal determinants for the development of type 2 diabetes and related metabolic disorders. Therefore, all the participants were eligible if they were free from type 2 diabetes prior to the first study visit. Participants underwent detailed metabolic phenotyping, genome-wide genotyping, and serum metabolomic profiling. For inclusion in the current analysis, participants must have had an abdominal ultrasound scan (US) for determination of hepatic steatosis, densely imputed genotype data for the SNPs of interest (as described below), and body composition analysed by either dual energy X-ray absorptiometry (DXA) or bioelectrical Impedance analysis (BIA). Furthermore, participants were excluded if: no genotype data were available, related individuals, genetic ancestry outliers. Applying those criteria left 9,135 participants to be included in statistical analyses. The study was approved by the Cambridge Local Research Ethics Committee (ref: 04/Q0108/19) and all participants provided written informed consent to participate in the study. Sociodemographic factors (e.g. ethnicity, sex) and alcohol use were extracted from the Fenland General Questionnaire.
Measures of body composition – Fenland cohort
Anthropometric measures and DXA were described in detail elsewhere(83). In brief, participants’ height, weight, waist- and hip- circumferences were measured. Body mass index (BMI) was calculated by weight (kg) divided by squared height (m2). DXA (Lunar Prodigy Advanced fan beam scanner, GE Healthcare, Hatfield, UK) estimated lean and fat mass with their relative distributions using the GE software (version no. 14; GE Healthcare). BIA was performed using TANITA BC-418 MA body fat monitor (Tanita, Tokyo, Japan) to estimate total body fat percentage. Body fat percentage was used from DXA where available and from BIA if unavailable. All measurements were performed by trained operators.
Abdominal ultrasound scan (US) – Fenland cohort
Liver US images were taken for the Fenland cohort as described elsewhere(84). The images were recorded and scored retrospectively by two operators who were blinded to all other study measures. The ultrasound images were acquired using the LOGIQ Book and Logic GE Healthcare ultrasound systems with 3C MHz-RS and 2-5 MHz 3C-RC curved array transducers respectively, and were qualitatively scored according to standardised criteria(85–88). The hepatic steatosis scoring criteria were: Criterion 1, increased echo reflectivity of the liver parenchyma (bright liver in comparison with the kidney); Criterion 2, decreased visualisation of the intra-hepatic vasculature; Criterion 3, attenuation of ultrasound beam. Each criterion was scored on a 4-point scale (i.e. as 1, 2, 3 or 4) and summed, resulting in cumulative liver fat score (range: 3 to 12). A score of ≤4 was classified as normal liver and ≥5 was classified as steatosis.
EPIC-Norfolk study cohort
The EPIC-Norfolk study is a prospective population cohort of over 25,000 individuals aged 40-79 years at recruitment living in Norfolk, England (baseline years=1993-1997) (89), nested within the European Prospective Investigation into Cancer and Nutrition (EPIC). The study was approved by the Norfolk Research Ethics Committee (ref. 05/Q0101/191) and all participants gave their written consent on entering the study. Metabolomics, genotyping, and clinical outcome data were available for 9,902 participants. No liver imaging (for identification of steatosis) was available in this cohort.
Genotyping data – Fenland and EPIC-Norfolk Cohorts
Participants were genotyped using Affymetrix Axiom UKBiobank, Affymetrix 500K Array Set, and Illumina Infinium Core Exome 24v1 arrays. Results were imputed to the HRC and UK10k panels, followed by combination of imputation results.
Genotype was extracted for five SNPs associated with NASH-cirrhosis: rs738409C>G in PNPLA3 (NC_000022.11:g.43928847C>G / NP_079501.2:p.Ile148Met), rs58542926C>T near TM6SF2 (NC_000019.10:g.19268740C>T / NP_001001524.2:p.Glu167Lys), rs641738C>T near TMC4-MBOAT7 (NC_000019.10:g.54173068T>C / NP_001138775.2:p.Glu17Gly, referred to as ‘MBOAT7’), rs72613567TA>T in HSD17B13 (NC_000004.12:g.87310241dup), and rs2642438A>G in MTARC1 (NC_000001.11:g.220796686A>G / NP_073583.3:p.Thr165Ala). Call rate was >98% for all variants.
In addition, genotype was extracted for three variants at genome-wide significance for liver fat (rs780094C>T in GCKR (NC_000002.12:g.27518370C>T), rs4240624G>A near PPP1R3B (NC_000008.11:g.9326721G>A), rs12137855T>C in LYPLAL1 (NC_000001.11:g.219275036C>T)) and variants associated with haemochromatosis (rs1800562G>A in HFE (NC_000006.12:g.26092913G>A / NP_000401.1:p.Cys282Tyr)) and alpha-1-antitrypsin deficiency (rs28929474C>T in SERPINA1 (NC_000014.9:g.94378610C>G / NP_001002236.1:p.Glu366Lys). These three variants in or near GCKR, PPP1R3B, and LYPLAL1 are well established determinants of liver fat but do not appear to associated with cirrhosis, therefore were included for comparison against the NASH-fibrosis variants(10,90) as ‘steatosis only’ variants. Whilst the variants in HFE and SERPINA1 are associated with cirrhosis but not liver fat by reasonably well-established metabolic pathways. These were included for comparison against NASH-fibrosis variants to assess whether metabolite associations were variant-specific or whether they non-specifically reflected hepatic fibrosis. A summary of the evidence for inclusion of variants in the analysis is presented in Supplementary Table 1. Finally, genotyping data were used to calculate previously published SNP scores for metabolic traits: BMI(91), BMI-adjusted waist-to-hip ratio(92), body fat percentage(93), and insulin resistance(94).
Metabolomics profiling and gas chromatography of fatty acids – Fenland cohort
Fasting serum was used for targeted metabolomics using AbsoluteIDQ p180 kit (BIOCRATES Life Sciences AG, Innsbruck, Austria), which includes: 24 amino acids, 10 amines, 40 carinitines, 14 lysophosphatidylcholines (lyso-PC), 37 diacylphosphatidylcholines (diacyl-PC), 37 alkylacylphosphatidylcholines (alkylacyl-PC), 11 sphingomyelins (SM), and the sum of hexoses(95). The panel was measured using ABSciex 5500 Qtrap with a Waters Acquity UPLC as described elsewhere(63,95). In addition, 37 fatty acids were measured using an automated, high-throughput gas chromatography method in a subset (n=4266), as described previously(96). In short, the plasma phospholipid fraction was obtained using solid phase extraction and hydrolysed. Isolated fatty acids were then methylated, yielding fatty acid methyl esters (FAME), and separated by gas chromatography (J&W HP-88, 30 m length) equipped with flame ionisation detection (7890N GC Agilent Technologies, United States). Samples were processed in a random order, and laboratory staff was blinded to any participant characteristics. Fatty acids were identified by their retention times compared with those of commercial standards and expressed as percent of total phospholipid fatty acids (mol%). All assays were performed according to the manufacturers’ instructions.
Lipidomics – Fenland cohort
Untargeted lipidomic measurement was performed on fasting serum for a subset of participants (n=1356), as previously described(77). Briefly, samples were diluted with 100 μl of MilliQ H2O in a well of a glass-coated 2.4 ml deep well plate (Plate+TM, Esslab, Hadleigh, UK), then 250 μl of MeOH was added. Lipids were partitioned into 500 μl of Methyl-tertiary-butyl ether. After centrifugation, the organic layer was concentrated and used for lipid analysis. Samples were infused into a Thermo Exactive benchtop orbitrap (Hemel Hampstead, England), using an Advion Triversa Nanomate (Ithaca, United States) and data acquired in both positive (+1.2 kV) and negative (−1.5 kV) mode voltages. All experiments were run with blank controls and two different quality control samples. In total, 218 lipid signals were detected and annotated as described previously(77) based on the identification at level 2 of the Metabolomics Standards Initiative. Identified lipid species included: 8 alkenylphosphatidylcholines (alkenyl-PC), 11 alkylacylphosphatidylcholines (alkylacyl-PC), 10 cholesterol esters (CE), 32 diacylphosphatidylcholines (diacyl-PC), 9 lysophosphatidylcholines (lyso-PC), 10 phosphoethanolamines (PE), 19 sphingomyelins (SM), 32 diacylglycerols (DG), and 41 triacylglycerols (TG).
Untargeted metabolomics – EPIC-Norfolk cohort
Untargeted metabolomics were measured using the DiscoveryHD4® platform(97) (Metabolon, Inc., Durham, USA), which uses a Hydrophilic Interaction Liquid Chromatographic (HILIC) method in non-fasted citrated plasma samples, in two quasi-randomly selected substudies. Metabolite levels were median-normalized across rundays and no imputation of missing values was performed. All the analyses were performed for each dataset separately. The reported analyses included 9,902 individuals with full covariate information and plasma levels of 977 metabolites. Fixed-effects meta-analysis was used to combine the metabolite results from the two datasets.
Statistics
In both cohorts, anthropometry, body composition measurements, metabolomics, and lipidomics variables were transformed logarithmically. Metabolite levels were further winsorised (to 5 sd) and standardised (μ=0, sd=1) using statistics specific to each dataset.
In the Fenland study cohort, characteristics of participants with and without hepatic steatosis were compared using linear regression for continuous variables and logistic regression for categorical variables, adjusting for age, sex, and BMI. NAFLD-associated genotypes were assessed for associations with anthropometric and metabolic traits using linear regression (coding effect allele dosage as 0, 1, and 2) adjusting for age, sex, and the first ten principal components from a principal component analyses on the genetic data to account for population stratification, as has been used previously by our group(63,98,99).
Targeted metabolomic and untargeted lipidomic profiles were compared between participants with and without hepatic steatosis. The semi-quantitative score of the degree of steatosis was analysed as an ordinal variable in secondary analysis. Associations between metabolites and steatosis were first assessed using logistic regression corrected for age, sex, and population stratification. Each metabolite was used as a dependent variable and either steatosis status (yes/no) or steatosis score (3 to 12 points) was used as the independent variables. To illustrate the effect of insulin resistance on these associations, the analysis was repeated additionally correcting for BMI and fasting insulin.
Associations of metabolite levels with SNP effect alleles were assessed using linear regression adjusted for age, sex, and population stratification, for all SNPs. Each metabolite was used as a dependent variable and number of risk alleles (0, 1, or 2) was used as the independent variable.
Variants in rs58542926C>T near TM6SF2 and rs2642438A>G in MTARC1 were found to influence total serum lipids and lipoprotein levels. Therefore, regression analyses were repeated for these variants additionally adjusting for: total cholesterol and total triglycerides for rs58542926C>T near TM6SF2; and, HDL cholesterol for rs2642438A>G in MTARC1.
To provide further confidence of the validity of our results, we tested metabolite-variant associations in random subgroups of the cohorts. The Fenland cohort was randomly divided into two groups (g1 & g2) and regression analyses were repeated. The EPIC-Norfolk cohort was already comprised of two substudies (g1 & g2). Metabolite-variant associations were considered significant where: overall Q-value<0.05 and there was a directionally consistent beta-regression co-efficient across g1, g2, and the overall cohort. Next, we examined classes of metabolites (e.g. triglycerides, diacylphosphatidylcholines (diacylPC)) for consistent trends in variant-metabolite associations across different analysis platforms and cohorts. Beta-regression co-efficients were plotted against number of carbons and/or double-bounds in lipid chains. Simple linear regression was performed on these plots, as a form of meta-regression, separately for each cohort (and metabolomics platform).
For external validation of our results, we compared our results against publicly available datasets, where possible(17,28,29,100–103). These were examined for directionally consistent associations across classes of metabolites.
In order to determine which of the five NASH-fibrosis variants was most informative in the variation of relevant metabolites we performed multiple linear regression in the Fenland cohort. Each of the 41 metabolites associated with at least one of the variants were included as dependent variables and number of risk alleles (0, 1, or 2) was used as the independent variable. Adjusted R2 was recorded as a measure of relative contribution to the variation in the included metabolites.
Benjamini-Hochberg correction for multiple testing was used throughout with Q-value<0.05 considered significant.
Statistical analyses were conducted using Stata v14.1 (StataCorp), GraphPad Prism (v8.0 for Mac, GraphPad Software, La Jolla California, USA) and R 3.5.1.
Supplementary Material
Acknowledgements
This study has been conducting using data from the Fenland study. The authors gratefully acknowledge the help of the MRC Epidemiology Unit Support Teams, including Field, Laboratory and Data Management Teams.
Funding
This work was supported by the Medical Research Council (Lipid Profiling and Signalling, MC UP A90 1006 & Lipid Dynamics and Regulation, MC PC 13030) and grants to MRC Epidemiology Units (MC UU12015/1, MC UU 12015/5, MC_PC_13046, and MR/L00002/1) and the Innovative Medicines Initiative Joint Undertaking under EMIF grant agreement no. 115372, resources of which are composed of financial contributions from the European Union's Seventh Framework Programme (FP7/2007-2013).
JPM is supported by a Wellcome Trust fellowship (216329/Z/19/Z) and a European Society for Paediatric Research Young Investigator Award. MP is supported by a fellowship from the German Research Foundation (DFG PI 1446/2-1).
Role of Sponsor
The funding bodies had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript or the decision to submit the manuscript for publication.
Abbreviations
- BIA
bioelectrical Impedance analysis
- BMI
body mass index
- DG
diacylglycerol
- DHGL
dihomo-gamma-linolenic acid
- DXA
dual energy X-ray absorptiometry
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- SNP
single nucleotide polymorphism
- TG
triacylglycerol
- US
ultrasound
- VLDL
very-low density lipoprotein
Footnotes
Author contributions: JPM, MP, LBW, EDLR, NK, FI, NGF, LL, JLG, AK, NKW, and CL were all involved in the design and concept, data collection, data analysis, manuscript drafting, and review of the final manuscript. MEA and EF was involved in the design and concept, manuscript drafting, and review of the final manuscript.
Conflict of Interest statement
The authors report no conflict of interest relative to this study.
References
- 1.Diehl AM, Day C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 2.Samuel VT, Shulman GI. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018;27:22–41. doi: 10.1016/j.cmet.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio La, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Heather H, Tybjærg-hansen A, Vogt TF, Hobbs HH, Jonathan C. Exome-wide association study identifies TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pirola CJ, Garaycoechea M, Flichman D, Arrese M, San J, Gazzi C, Castaño GO, Sookoian S. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. J Lipid Res. 2019;60:176–185. doi: 10.1194/jlr.P089953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancina RM, Dongiovanni P, Petta S, Pingitore P, Meroni M, Rametta R, Boren J, Montalcini T, Pujia A, Wiklund O, et al. The MBOAT7-TMC4 Variant rs641738 Increases Risk of Nonalcoholic Fatty Liver Disease in Individuals of European Descent. Gastroenterology. 2016;150:1219–1230e6. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buch S, Stickel F, Trépo E, Way M, Herrmann A, Nischalke HD, Brosch M, Rosendahl J, Berg T, Ridinger M, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. 2015;47:1443–1448. doi: 10.1038/ng.3417. [DOI] [PubMed] [Google Scholar]
- 9.Teo K, Abeysekera KW, Adams L, Aigner E, Banerjee R, Basu P, Berg T, Bhatnagar P, Buch S, Canbay A, et al. rs641738C>T near MBOAT7 promotes steatosis, NASH, fibrosis and hepatocellular carcinoma in non-alcoholic fatty liver disease: a meta-analysis. medRxiv. 2019 doi: 10.1101/19013623. [DOI] [Google Scholar]
- 10.Emdin CA, Haas M, Khera AV, Aragam K, Chaffin M, Klarin D, Hindy G, Jian L, Wei W-Q, Feng Q, et al. A missense variant in Mitochondrial Amidoxime Reducing Component 1 gene and protection against liver disease. PLoS Genet. 2019;16:e1008629. doi: 10.1371/journal.pgen.1008629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanyal AJ. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2019;16:377–386. doi: 10.1038/s41575-019-0144-8. [DOI] [PubMed] [Google Scholar]
- 12.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol. 2019;16:411–428. doi: 10.1038/s41575-019-0145-7. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Kory N, BasuRay S, Cohen JC, Hobbs HH. PNPLA3, CGI-58, and Inhibition of Hepatic Triglyceride Hydrolysis in Mice. Hepatology. 2019;16:2427–2441. doi: 10.1002/hep.30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BasuRay S, Wang Y, Smagris E, Cohen JC, Hobbs HH. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc Natl Acad Sci; 2019. pp. 9521–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartiala JA, Wilson Tang WH, Wang Z, Crow AL, Stewart AFR, Roberts R, McPherson R, Erdmann J, Willenborg C, Hazen SL, et al. Genome-wide association study and targeted metabolomics identifies sex-specific association of CPS1 with coronary artery disease. Nat Commun. 2016;7:10558. doi: 10.1038/ncomms10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kettunen J, Demirkan A, Würtz P, Draisma HHM, Haller T, Rawal R, Vaarhorst A, Kangas AJ, Lyytikäinen LP, Pirinen M, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7:11122. doi: 10.1038/ncomms11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers JC, Zhang W, Sehmi J, Li X, Wass MN, Van Der Harst P, Holm H, Sanna S, Kavousi M, Baumeister SE, et al. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet. 2011;43:1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musso G, Cassader M, Paschetta E, Gambino R. Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:282–302.e8. doi: 10.1053/j.gastro.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Sliz E, Sebert S, Würtz P, Kangas AJ, Soininen P, Lehtimäki T, Kähönen M, Viikari J, Männikkö M, Ala-Korpela M, et al. NAFLD risk alleles in PNPLA3, TM6SF2, GCKR and LYPLAL1 show divergent metabolic effects. Hum Mol Genet. 2018;27:2214–2223. doi: 10.1093/hmg/ddy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S, Min J, Spann NJ, McDonald JG, Kelly SL, et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, Payne AJ, Steinthorsdottir V, Scott RA, Grarup N, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50:1505–1513. doi: 10.1038/s41588-018-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slimani N, Kaaks R, Ferrari P, Casagrande C, Clavel-Chapelon F, Lotze G, Kroke A, Trichopoulos D, Trichopoulou A, Lauria C, et al. European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr. 2002;5:1125–1145. doi: 10.1079/PHN2002395. [DOI] [PubMed] [Google Scholar]
- 24.Kubitza C, Bittner F, Ginsel C, Havemeyer A, Clement B, Scheidig AJ. Crystal structure of human mARC1 reveals its exceptional position among eukaryotic molybdenum enzymes. Proc Natl Acad Sci; 2018. pp. 11958–11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein JM, Busch JD, Pottings C, Baker MJ, Langers T, Schwarz G. The mitochondrial amidoxime-reducing component (mARC1) is a novel signal-anchored protein of the outer mitochondrial membrane. J Biol Chem. 2012;287:42795–42803. doi: 10.1074/jbc.M112.419424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparacino-Watkins CE, Tejero J, Sun B, Gauthier MC, Thomas J, Ragireddy V, Merchan BA, Wang J, Azarov I, Basu P, et al. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J Biol Chem. 2014;289:10345–10358. doi: 10.1074/jbc.M114.555177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luukkonen PK, Juuti A, Sammalkorpi H, Penttilä A, Orešic M, Hyötyläinen T, Arola J, Orho-Melander M, Yki-Järvinen H. MARC1 variant rs2642438 increases hepatic phosphatidylcholines and decreases severity of non-alcoholic fatty liver disease in humans. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Draisma HHM, Pool R, Kobl M, Jansen R, Petersen AK, Vaarhorst AAM, Yet I, Haller T, Demirkan A, Esko T, et al. Genome-wide association study identifies novel genetic variants contributing to variation in blood metabolite levels. Nat Commun. 2015;6:7208. doi: 10.1038/ncomms8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabassum R, Ramo J, Ripatti P, Koskela J, Kurki M, Karjalainen J, Palta P, Hassan S, Nunez-Fontarnau J, Kiiskinen T, et al. Genetic architecture of human plasma lipidome and its link to cardiovascular disease. Nat Commun. 2019;10:4329. doi: 10.1038/s41467-019-11954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S, Min J, Spann NJ, McDonald JG, Kelly SL, et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grammatikos G, Ferreiros N, Waidmann O, Bon D, Schroeter S, Koch A, Herrmann E, Zeuzem S, Kronenberger B, Pfeilschifter J. Serum sphingolipid variations associate with hepatic decompensation and survival in patients with cirrhosis. PLoS One. 2015;10:e0138130. doi: 10.1371/journal.pone.0138130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longato L, Tong M, Wands JR, de la Monte SM. High fat diet induced hepatic steatosis and insulin resistance: Role of dysregulated ceramide metabolism. Hepatol Res. 2012;42:412–427. doi: 10.1111/j.1872-034X.2011.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanamatsu H, Ohnishi S, Sakai S, Yuyama K, Mitsutake S, Takeda H, Hashino S, Igarashi Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr Diabetes. 2014;4:e141–7. doi: 10.1038/nutd.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Régnier M, Polizzi A, Guillou H, Loiseau N. Sphingolipid metabolism in non-alcoholic fatty liver diseases. Biochimie. 2019;159:9–22. doi: 10.1016/j.biochi.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 35.Hicks AA, Pramstaller PP, Johansson Å, Vitart V, Rudan I, Ugocsai P, Aulchenko Y, Franklin CS, Liebisch G, Erdmann J, et al. Genetic determinants of circulating sphingolipid concentrations in European populations. PLoS Genet. 2009;5:e1000672. doi: 10.1371/journal.pgen.1000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smagris E, Gilyard S, BasuRay S, Cohen JC, Hobbs HH. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. J Biol Chem. 2016;291:10659–10676. doi: 10.1074/jbc.M116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang A, Mottillo E, Mladenovic-Lucas L, Zhou L, Granneman JG. Dynamic interactions of ABHD5 with PNPLA3 regulate triacylglycerol metabolism in brown adipocytes. Nat Metab. 2019;1:560–569. doi: 10.1038/s42255-019-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y, He S, Li JZ, Seo Y-K, Osborne TF, Cohen JC, Hobbs HH. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci U S A; 2010. pp. 7892–7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luukkonen PK, Ikonen E, Yki-järvinen H, Luukkonen PK, Nick A, Hölttä-vuori M, Thiele C, Isokuortti E, Lallukka-brück S, Zhou Y, et al. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight. 2019;4:e127902. doi: 10.1172/jci.insight.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jha P, McDevitt MT, Halilbasic E, Williams EG, Quiros PM, Gariani K, Sleiman MB, Gupta R, Ulbrich A, Jochem A, et al. Genetic Regulation of Plasma Lipid Species and Their Association with Metabolic Phenotypes. Cell Syst. 2018;6:709–721.e6. doi: 10.1016/j.cels.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Speliotes EK, Butler JL, Palmer CD, Voight BF, GIANT Consortium, MIGen Consortium, NASH Clinical Research Network. Hirschhorn JN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904–912. doi: 10.1002/hep.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121:1402–1411. doi: 10.1172/JCI44442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, Liu Y, Kozlitina J, Stender S, Wood GC, et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N Engl J Med. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adam M, Heikelä H, Sobolewski C, Portius D, Mäki-Jouppila J, Mehmood A, Adhikari P, Esposito I, Elo LL, Zhang F-P, et al. Hydroxysteroid (17β) dehydrogenase 13 deficiency triggers hepatic steatosis and inflammation in mice. FASEB J. 2018;32:3434–3447. doi: 10.1096/fj.201700914R. [DOI] [PubMed] [Google Scholar]
- 46.Ma Y, Belyaeva OV, Brown PM, Fujita K, Valles K, Karki S, de Boer YS, Koh C, Chen Y, Du X, et al. 17-Beta Hydroxysteroid Dehydrogenase 13 Is a Hepatic Retinol Dehydrogenase Associated With Histological Features of Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luukkonen PK, Tukiainen T, Juuti A, Sammalkorpi H, Haridas PAN, Niemela O, Arola J, Orho-Melander M, Hakkarainen A, Kovanen P, et al. Hydroxysteroid 17-β dehydrogenase 13 variant increases phospholipids and protects against fibrosis in nonalcoholic fatty liver disease. JCI Insight. 2020;5:e132158. doi: 10.1172/jci.insight.132158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roe CR, Millington DS, Maltby DA. Identification of 3-methylglutarylcarnitine. A new diagnostic metabolite of 3-hydroxy-3-methylglutaryl-coenzyme A lyase deficiency. J Clin Invest. 1986;77:1391–1394. doi: 10.1172/JCI112446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pié J, López-Viñas E, Puisac B, Menao S, Pié A, Casale C, Ramos FJ, Hegardt FG, Gómez-Puertas P, Casals N. Molecular genetics of HMG-CoA lyase deficiency. Mol Genet Metab. 2007;92:198–209. doi: 10.1016/j.ymgme.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 50.Luukkonen PK, Zhou Y, Hyötyläinen T, Leivonen M, Arola J, Orho-Melander M, Orešic M, Yki-Järvinen H. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J Hepatol. 2016;65:1263–1265. doi: 10.1016/j.jhep.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 51.Donati B, Dongiovanni P, Romeo S, Meroni M, McCain M, Miele L, Petta S, Maier S, Rosso C, De Luca L, et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7:4492. doi: 10.1038/s41598-017-04991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee HC, Inoue T, Imae R, Kono N, Shirae S, Matsuda S, Gengyo-Ando K, Mitani S, Arai H. Caenorhabditis elegans mboa-7, a member of the MBOAT family, is required for selective incorporation of polyunsaturated fatty acids into phosphatidylinositol. Mol Biol Cell. 2008;19:1174–1184. doi: 10.1091/mbc.E07-09-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, Ye Z, Sluijs I, Guevara M, Huerta JM, et al. Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study. PLoS Med. 2016;13:e1002094. doi: 10.1371/journal.pmed.1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sookoian S, Pirola CJ. The nonalcoholic steatohepatitis metabotype: Imbalance of circulating amino acids and transamination reactions reflect impaired mitochondrial function. Hepatology. 2018;67:1177–1178. doi: 10.1002/hep.29705. [DOI] [PubMed] [Google Scholar]
- 56.Gaggini M, Carli F, Rosso C, Buzzigoli E, Marietti M, Della Latta V, Ciociaro D, Abate ML, Gambino R, Cassader M, et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology. 2018;67:145–158. doi: 10.1002/hep.29465. [DOI] [PubMed] [Google Scholar]
- 57.Pietzner M, Budde K, Homuth G, Kastenmüller G, Henning A-K, Artati A, Krumsiek J, Völzke H, Adamski J, Lerch MM, et al. Hepatic steatosis associates with adverse molecular signatures in subjects without diabetes. J Clin Endocrinol Metab. 2018;103:3856–3868. doi: 10.1210/jc.2018-00999. [DOI] [PubMed] [Google Scholar]
- 58.Chang AY, Lalia AZ, Jenkins GD, Dutta T, Carter RE, Singh RJ, Nair KS. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metabolism. 2017;71:52–63. doi: 10.1016/j.metabol.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koch M, Freitag-Wolf S, Schlesinger S, Borggrefe J, Hov JR, Jensen MK, Pick J, Markus MRP, Höpfner T, Jacobs G, et al. Serum metabolomic profiling highlights pathways associated with liver fat content in a general population sample. Eur J Clin Nutr. 2017;71:995–1001. doi: 10.1038/ejcn.2017.43. [DOI] [PubMed] [Google Scholar]
- 60.Kaikkonen JE, Würtz P, Suomela E, Lehtovirta M, Kangas AJ, Jula A, Mikkilä V, Viikari JSA, Juonala M, Rönnemaa T, et al. Metabolic profiling of fatty liver in young and middle-aged adults: Cross-sectional and prospective analyses of the Young Finns Study. Hepatology. 2017;65:491–500. doi: 10.1002/hep.28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng JS, Sharp SJ, Imamura F, Koulman A, Schulze MB, Ye Z, Griffin J, Guevara M, Huerta JM, Kröger J, et al. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: A cross-sectional analysis in the EPIC-InterAct study. BMC Med. 2017;15:203. doi: 10.1186/s12916-017-0968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tiwari-Heckler S, Gan-Schreier H, Stremmel W, Chamulitrat W, Pathil A. Circulating phospholipid patterns in NAFLD patients associated with a combination of metabolic risk factors. Nutrients. 2018;10:649. doi: 10.3390/nu10050649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, Schmidt AF, Imamura F, Stewart ID, Perry JRB, et al. Genetic Predisposition to an Impaired Metabolism of the Branched-Chain Amino Acids and Risk of Type 2 Diabetes: A Mendelian Randomisation Analysis. PLoS Med. 2016;13:e1002179. doi: 10.1371/journal.pmed.1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y, Llauradó G, Orešic M, Hyötyläinen T, Orho-Melander M, Yki-Järvinen H. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. J Hepatol. 2015;62:657–663. doi: 10.1016/j.jhep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 65.Luukkonen PK, Zhou Y, Sädevirta S, Leivonen M, Arola J, Orešič M, Hyötyläinen T, Yki-Järvinen H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith GI, Polidori DC, Yoshino M, Kearney ML, Patterson BW, Mittendorfer B, Klein S. Influence of adiposity, insulin resistance, and intrahepatic triglyceride content on insulin kinetics. J Clin Invest. 2020;130:3305–3314. doi: 10.1172/JCI136756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mayo R, Crespo J, Martínez-Arranz I, Banales JM, Arias M, Mincholé I, Aller de la Fuente R, Jimenez-Agüero R, Alonso C, de Luis DA, et al. Metabolomic-based noninvasive serum test to diagnose nonalcoholic steatohepatitis: Results from discovery and validation cohorts. Hepatol Commun. 2018;2:807–820. doi: 10.1002/hep4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anjani K, Lhomme M, Sokolovska N, Poitou C, Aron-Wisnewsky J, Bouillot JL, Lesnik P, Bedossa P, Kontush A, Clement K, et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J Hepatol. 2015;62:905–912. doi: 10.1016/j.jhep.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Kimberly WT, O’Sullivan JF, Nath AK, Keyes M, Shi X, Larson MG, Yang Q, Long MT, Vasan R, Peterson RT, et al. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight. 2017;2:e92989. doi: 10.1172/jci.insight.92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qi S, Xu D, Li Q, Xie N, Xia J, Huo Q, Li P, Chen Q, Huang S. Metabonomics screening of serum identifies pyroglutamate as a diagnostic biomarker for nonalcoholic steatohepatitis. Clin Chim Acta. 2017;473:89–95. doi: 10.1016/j.cca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 72.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min HK, Contos MJ, Sterling RK, Fuchs M, Zhou H, et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiappini F, Coilly A, Kadar H, Gual P, Tran A, Desterke C, Samuel D, Duclos-Vallee J-C, Touboul D, Bertrand-Michel J, et al. Metabolism dysregulation induces a specific lipid signature of nonalcoholic steatohepatitis in patients. Sci Rep. 2017;7:46658. doi: 10.1038/srep46658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo J-J, Cao H-X, Yang R-X, Zhang R-N, Pan Q. PNPLA3 rs139051 is associated with phospholipid metabolite profile and hepatic inflammation in nonalcoholic fatty liver disease. World J Clin Cases. 2018;6:355–364. doi: 10.12998/wjcc.v6.i10.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papandreou C, Bullò M, Tinahones FJ, Martínez-González MÁ, Corella D, Fragkiadakis GA, López-Miranda J, Estruch R, Fitó M, Salas-Salvadó J. Serum metabolites in non-alcoholic fatty-liver disease development or reversion; A targeted metabolomic approach within the PREDIMED trial. Nutr Metab. 2017;14:58. doi: 10.1186/s12986-017-0213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 77.Sanders FWB, Acharjee A, Walker C, Marney L, Roberts LD, Imamura F, Jenkins B, Case J, Ray S, Virtue S, et al. Hepatic steatosis risk is partly driven by increased de novo lipogenesis following carbohydrate consumption. Genome Biol. 2018;19:79. doi: 10.1186/s13059-018-1439-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tamura S, Shimomura I. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. Contribution of adipose tissue and de novo lipogenesis to nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1139–1142. doi: 10.1172/JCI24930. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merino J, Leong A, Liu CT, Porneala B, Walford GA, von Grotthuss M, Wang TJ, Flannick J, Dupuis J, Levy D, et al. Metabolomics insights into early type 2 diabetes pathogenesis and detection in individuals with normal fasting glucose. Diabetologia. 2018;61:1315–1324. doi: 10.1007/s00125-018-4599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou Y, Orešic M, Leivonen M, Gopalacharyulu P, Hyysalo J, Arola J, Verrijken A, Francque S, Van Gaal L, Hyötyläinen T, et al. Non-invasive Detection of Non-alcoholic Steatohepatitis Using Clinical Markers and Circulating Levels of Lipids and Metabolites. Clin Gastroenterol Hepatol. 2016;14:1463–1472.e6. doi: 10.1016/j.cgh.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 81.Peng K-Y, Watt MJ, Rensen S, Greve JW, Huynh K, Jayawardana KS, Meikle PJ, Meex RCR. Mitochondrial dysfunction-related lipid changes occur in non-alcoholic fatty liver disease progression. J Lipid Res. 2018;59:1977–1986. doi: 10.1194/jlr.M085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luukkonen PK, Zhou Y, Nidhina Haridas PA, Dwivedi OP, Hyötyläinen T, Ali A, Juuti A, Leivonen M, Tukiainen T, Ahonen L, et al. Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. J Hepatol. 2017;67:128–136. doi: 10.1016/j.jhep.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 83.Rolfe E, Loos RJ, Druet C, Stolk RP, Ekelund U, Griffin SJ, Forouhi NG, Wareham NJ, Ong KK. Association between birth weight and visceral fat in adults. Am J Clin Nutr. 2010;92:347–352. doi: 10.3945/ajcn.2010.29247. [DOI] [PubMed] [Google Scholar]
- 84.De Lucia Rolfe E, Brage S, Sleigh A, Finucane F, Griffin SJ, Wareham NJ, Ong KK, Forouhi NG. Validity of ultrasonography to assess hepatic steatosis compared to magnetic resonance spectroscopy as a criterion method in older adults. PLoS One. 2018;13:e0207923. doi: 10.1371/journal.pone.0207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saverymuttu SH, Joseph AEA, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–15. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Needleman L, Kurtz AB, Rifkin MD, Cooper HS, Pasto ME, Goldberg BB. Sonography of diffuse benign liver disease: Accuracy of pattern recognition and grading. Am J Roentgenol. 1986;146:1011–1015. doi: 10.2214/ajr.146.5.1011. [DOI] [PubMed] [Google Scholar]
- 87.Mendler MH, Bouillet P, Le Sidaner A, Lavoine E, Labrousse F, Sautereau D, Pillegand B. Dual-energy CT in the diagnosis and quantification of fatty liver: Limited clinical value in comparison to ultrasound scan and single-energy CT, with special reference to iron overload. J Hepatol. 1998;28:785–794. doi: 10.1016/s0168-8278(98)80228-6. [DOI] [PubMed] [Google Scholar]
- 88.Joseph AEA, Saverymuttu SH, Al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol. 1991;43:26–31. doi: 10.1016/s0009-9260(05)80350-2. [DOI] [PubMed] [Google Scholar]
- 89.Day N, Oakes S, Luben R, Khaw K-T, Bingham S, Welch A, Wareham NJ. EPIC-Norfolk: Study design and characteristics of the cohort. Br J Cancer. 1999;80:95–103. [PubMed] [Google Scholar]
- 90.Speliotes EK, Yerges-armstrong LM, Wu J, Hernaez R, Lauren J, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJ, et al. Genome-Wide Association Analysis Identifies Variants Associated with Nonalcoholic Fatty Liver Disease That Have Distinct Effects on Metabolic Traits. Plos Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, Frayling TM, Hirschhorn J, Yang J, Visscher PM. Meta-analysis of genome-wide association studies for height and body mass index in ~700 000 individuals of European ancestry. Hum Mol Genet. 2018;27:3641–3649. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, Yengo L, Ferreira T, Marouli E, Ji Y, et al. Meta-Analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28:166–174. doi: 10.1093/hmg/ddy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu Y, Day FR, Gustafsson S, Buchkovich ML, Na J, Bataille V, Cousminer DL, Dastani Z, Drong AW, Esko T, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7:10495. doi: 10.1038/ncomms10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, MäGi R, Strawbridge RJ, Rehnberg E, Gustafsson S, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44:991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siskos AP, Jain P, Römisch-Margl W, Bennett M, Achaintre D, Asad Y, Marney L, Richardson L, Koulman A, Griffin JL, et al. Interlaboratory Reproducibility of a Targeted Metabolomics Platform for Analysis of Human Serum and Plasma. Anal Chem. 2017;89:656–665. doi: 10.1021/acs.analchem.6b02930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang LY, Summerhill K, Rodriguez-Canas C, Mather I, Patel P, Eiden M, Young S, Forouhi NG, Koulman A. Development and validation of a robust automated analysis of plasma phospholipid fatty acids for metabolic phenotyping of large epidemiological studies. Genome Med. 2013;5:39. doi: 10.1186/gm443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Evans A, Bridgewater B, Liu Q, Mitchell M, Robinson R, Dai H, Stewart S, DeHaven C, Miller L. High Resolution Mass Spectrometry Improves Data Quantity and Quality as Compared to Unit Mass Resolution Mass Spectrometry in High-Throughput Profiling Metabolomics. Metabolomics. 2014;4:132. [Google Scholar]
- 98.Mardinoglu A, Stancáková A, Lotta LA, Kuusisto J, Boren J, Blüher M, Wareham NJ, Ferrannini E, Groop PH, Laakso M, et al. Plasma Mannose Levels Are Associated with Incident Type 2 Diabetes and Cardiovascular Disease. Cell Metab. 2017;26:281–283. doi: 10.1016/j.cmet.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Lotta LA, Pietzner M, Stewart ID, Wittemans LBL, Li C, Raffler J, Biggs EK, Oliver-williams C, Auyeung VPW, Surendran P, et al. Cross-platform genetic discovery of small molecule products of metabolism and application to clinical outcomes. bioRxiv. 2020 doi: 10.1101/2020.02.03.932541. [DOI] [Google Scholar]
- 100.Suhre K, Shin SY, Petersen AK, Mohney RP, Meredith D, Wägele B, Altmaier E, Deloukas P, Erdmann J, Grundberg E, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–62. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin S-Y, Fauman EB, Petersen A-K, Krumsiek J, Santos R, Huang J, Arnold M, Erte I, Forgetta V, Yang T-P, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46:543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Long T, Hicks M, Yu H-C, Biggs WH, Kirkness EF, Menni C, Zierer J, Small KS, Mangino M, Messier H, et al. Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet. 2017;49:568–578. doi: 10.1038/ng.3809. [DOI] [PubMed] [Google Scholar]
- 103.Staley JR, Blackshaw J, Kamat MA, Ellis S, Surendran P, Sun BB, Paul DS, Freitag D, Burgess S, Danesh J, et al. PhenoScanner: A database of human genotype-phenotype associations. Bioinformatics. 2016;32:3207–3209. doi: 10.1093/bioinformatics/btw373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.