Abstract
During complex tasks, patterns of functional connectivity differ from those in the resting state. However, what accounts for such differences remains unclear. Brain activity during a task reflects an unknown mixture of spontaneous and task-evoked activities. The difference in functional connectivity between a task state and the resting state may reflect not only task-evoked functional connectivity, but also changes in spontaneously emerging networks. Here, we characterized the differences in apparent functional connectivity between the resting state and when human subjects were watching a naturalistic movie. Such differences were marginally explained by the task-evoked functional connectivity involved in processing the movie content. Instead, they were mostly attributable to changes in spontaneous networks driven by ongoing activity during the task. The execution of the task reduced the correlations in ongoing activity among different cortical networks, especially between the visual and non-visual sensory or motor cortices. Our results suggest that task-evoked activity is not independent from spontaneous activity, and that engaging in a task may suppress spontaneous activity and its inter-regional correlation.
Keywords: task evoked functional connectivity, natural vision, spontaneous activity, task–rest interaction
1 |. INTRODUCTION
Functional connectivity (FC) captures the correlation of different networks or regions in the brain. Its structure and dynamics have been useful in characterizing the brain’s functional organization. Patterns of FC are similar across distinct states of consciousness (Horovitz et al., 2008; Vincent et al., 2007), and they are also largely conserved during the performance of various tasks (Arfanakis et al., 2000; Cole, Bassett, Power, Braver, & Petersen, 2014; Fair et al., 2007; Gratton, Laumann, Gordan, Adeyemo, & Petersen, 2016; Harrison et al., 2008; Krienen, Yeo, Buckner, & Buckner, 2014). However, increasing evidence suggests that FC is altered within and between brain states (Buckner, Krienen, & Yeo, 2013; Chang & Glover, 2010; Di & Biswal, 2018; Hutchison et al., 2013; Mennes, Kelly, Colcombe, Xavier Castellanos, & Milham, 2013; Rehme, Eickhoff, & Grefkes, 2013; Sepulcre et al., 2010; Tomasi & Volkow, 2018; Van Dijk et al., 2010; Wong, Olafsson, Tal, & Liu, 2013; Wen & Liu, 2016). Such evidence lends support to the use of FC as a network signature of how the brain engages itself in various behavioral or cognitive tasks, for example, watching a movie. In fact, FC signatures have been used to accurately classify a multitude of brain states (Gonzalez-Castillo et al., 2015), leveraging this notion.
During a task, brain activity measurements reflect a mixture of spontaneous and evoked activities. Disentangling their differential contributions to the pattern of apparent FC is necessary for proper interpretation of any FC difference between a task and the resting state or between different tasks. If the task-dependent FC is due to the task-evoked activity, its pattern reflects the network interactions directly involved in information processing for task execution. If the task-dependent FC is attributed to ongoing activity, its pattern is driven by the brain’s functional re-organization or adaption to facilitate the task. Alternatively, evoked activity may interact with spontaneous activity. As such, the task-dependent FC should reflect the changes in both task-evoked networks and spontaneously emerging networks.
There is a lack of consensus on the relationship between taskevoked and ongoing activities. Some electrophysiology studies suggest that task-evoked activity is independent from spontaneous activity (Arieli, Sterkin, Grinvald, & Aertsen, 1996; Mäkinen, Tiitinen, & May, 2005; Tsodyks, Kenet, Grinvald, & Arieli, 1999). Initial evidence has led to the notion that spontaneous and evoked processes linearly sum to yield the activity observed during a task, both using neural recordings (Arieli et al., 1996; Azouz & Gray, 1999) and fMRI or other hemodynamic measures (Becker, Reinacher, Freyer, Villringer, & Ritter, 2011; Fox, Snyder, Zacks, & Raichle, 2006; Saka, Berwick, & Jones, 2010). There are, however, other reports to the contrary. Using electrophysiology, several groups have shown a reduction in neural variability following the onset of a stimulus, suggesting that the task suppresses ongoing activity during the task (Borg-Graham, Monier, & Fregnac, 1998; Churchland et al., 2010; Finn, Priebe, & Ferster, 2007; Oram, 2011; Ponce-Alvarez, Thiele, Albright, Stoner, & Deco, 2013). Using fMRI, He et al. (2013) also found a negative interaction between spontaneous activity and task-evoked activity during a visual attention task. However, how (and whether) such an interaction may occur with respect to functional connectivity has not been fully investigated.
Prior studies have established some valuable analysis methods to address this question. Simony et al. proposed the use of inter-subject functional connectivity (ISFC) during sustained and naturalistic stimulation to extract task-evoked functional connectivity without contributions from ongoing activity or non-neuronal noise (Simony et al., 2016). For any given pair of regions, the cross-correlation between one subject’s fMRI time-series signal in one region and the average fMRI signals from all other subjects in the other region was only attributable to task-evoked activity. This technique builds off of the Hasson, Nir, Levy, Fuhrmann, and Malach (2004) study, which showed that natural stimulation gave rise to reliable responses reproducible across individuals. Like ISFC, a similar strategy is to assess the inter-regional correlation across different sessions of the same stimuli for the same subject (Wilf et al., 2017; Zhang, et al., 2017), while further discounting the variation across subjects.
Using this strategy, we sought to examine whether task-evoked functional connectivity was additive to spontaneous functional connectivity, and if it was able to explain the changes in FC during movie watching relative to the resting state (or the “task-rest FC difference” for simplicity). To address these questions, we began with examining the seed-based correlations for exploratory analysis, and subsequently performed systematic analysis of functional connectivity among brain parcels.
2 |. MATERIALS AND METHODS
2.1 |. Subjects
Thirteen healthy volunteers (20–31 years old, 8 females, 12 righthanded, normal or corrected to normal vision) participated in this study in accordance with a protocol approved by the Institutional Review Board at Purdue University. As in our prior studies (Lu et al., 2016; Marussich, Lu, Wen, & Liu, 2017), three subjects were excluded because they either self-reported to fall asleep or had excessive head motion (>1.5 mm of translational motion in any direction or >0.035 radians of rotation along any direction) based on motion correction through rigid registration to the first fMRI volume within each session.
2.2 |. Experimental design
Each of the remaining 10 subjects (20–31 years old, 6 females, 9 right-handed) underwent four fMRI sessions. Two of the four sessions were obtained in the eyes-closed resting state, and the other two sessions occurred during free-viewing of an identical movie clip (The Good, the Bad, and the Ugly, 1966, from 162:54 to 168:33 min in the film), as used in prior studies (Hasson et al., 2004; Lu et al., 2016). The visual stimulus was presented using the MATLAB Psychophysics Toolbox (Brainard, 1997; Pelli, 1997); it was delivered to the subjects through a binocular goggle system (NordicNeuroLab, Norway) mounted on the head coil. The display resolution was 800 × 600; through the goggle system, the visual field covered by the movie was about 26.9° × 20.3°. No sound was presented during the movie to isolate visual processing with respect to other sensory systems. Cross-modal interactions during otherwise audiovisual movie presentation are complex and may complicate the initial characterization of task–rest interaction, which was the focus of this study. Each movie-stimulation session began with a blank gray screen presented for 42 s, followed by the movie presented for 5 min and 37 s, and ended with the blank screen again for 30 s. The resting-state sessions had the same duration as the movie-stimulation sessions. The session order was randomized and counterbalanced across subjects. For simplicity, hereafter the resting-state and movie-stimulation sessions were referred to as the “rest” and “task” conditions, following the general notions in a broader context (Cole et al., 2014).
2.3 |. Data acquisition
Whole-brain structural and functional MRI images were acquired using a 3-Tesla Signa HDx MRI system (General Electric Health Care, Milwaukee). As described previously (Lu, Hung, Wen, Marussich, & Liu, 2016; Marussich et al., 2017), the fMRI data were acquired using a single-shot, gradient-recalled (GRE) echo-planar imaging (EPI) sequence (38 interleaved axial slices with 3.5 mm thickness and 3.5 × 3.5 mm2 in-plane resolution, TR = 2,000 ms, TE = 35 ms, flip angle = 78°, field of view = 22 × 22 cm2). T1-weighted anatomical images covering the whole head were acquired with a spoiled gradient recalled acquisition (SPGR) sequence (1 × 1 × 1 mm3 voxel size, TR/TE = 5.7/2 ms, flip angle = 12°). A 16-channel receive-only phase array coil (NOVA Medical, Wilmington) was used for image acquisition.
2.4 |. Pre-processing
Pre-processing of the fMRI images was carried out with a combination of AFNI (v17.0.01) (Cox, 1996), FSL (v5.0.8) (Smith et al., 2004), and MATLAB 2017A (Mathworks, Natick, MA). T1-weighted anatomical images were nonlinearly registered to the Montreal Neurological Institute (MNI) brain template using a combination of flirt and fnirt in FSL (Smith et al., 2004). T2*-weighted functional image time series were corrected for time differences between slices using slicetimer in FSL, co-registered to the first volume within each series to correct for head motion using mcflirt in FSL, restricted to within-brain tissues, aligned to the T1-weighted structural MRI using FSL’s Boundary Based-Registration (BBR) function (Greve & Fischl, 2009), and registered to the MNI space with 3-mm isotropic voxels using applywarp in FSL. The first six volumes in the fMRI data were discarded to avoid any pre-steady-state longitudinal magnetization.
The remaining pre-processing steps were conducted in MATLAB using in-house code. For the task sessions, we only analyzed the fMRI data during the movie while excluding any transient fMRI response during the first 8 s from the start of the movie. After they watched the movie for the first time, subjects might anticipate the ending of the movie when it was presented for the second time. As this anticipatory effect was not reproducible across the two repeated movie sessions, we also excluded the last 14 s of the movie.
For each session and each voxel, we first corrected for the effects of head motion by regressing out six head motion parameters, an approach used in previous studies (Van Dijk, Sabuncu, & Buckner, 2012; Satterthwaite et al., 2012). Then, the voxel time series was detrended by regressing out a third-order polynomial function that modeled any slow trend; the detrended signal was band-pass filtered (0.0001–0.1 Hz) using a pair of forward and inverse Fourier transforms (Cong et al., 2014). Spatial smoothing was applied by using a Gaussian kernel (FWHM = 6 mm). At each voxel, the signal mean was subtracted and the signal was then normalized to unit variance. These pre-processing steps were performed prior to each of the analyses described in the subsequent sections.
2.5 |. Seed-based voxel-level functional connectivity in rest and task states
We first explored the difference in seed-based correlation patterns between the resting state and the task state. For this purpose, seed voxels were selected from the primary visual cortex (V1), the middle temporal visual area (V5), precuneus (PCu), and primary motor cortex (M1); each of these regions of interest was defined using an atlas from an independent study (Shirer, Ryali, Rykhlevskaia, Menon, & Greicius, 2012). The MNI coordinates of these seed regions were (0, −54, 30) for PCu, (0, −87, 9) for V1, (48, −78, 0) for V5, and (39, −18, 57) for M1. These seed locations were chosen because they are representative of major functional systems activated by visual (V1 and V5) or motor tasks (M1), or deactivated by cognitive tasks (PCu as a part of the default-mode network).
Separately for the rest or task condition, the correlation between the seed voxel’s time series and every other voxel’s time series was calculated for each session, and the correlation coefficient was converted to a z-score using the Fisher’s transform. The voxel-wise z-score was then averaged within each subject. The significance of the average z-score (against zero) was evaluated by applying one-sample t-test (df = 9) to the voxel-wise results obtained from individual subjects.
To determine the task-rest FC difference, the z-scores of the movie sessions were then compared with those of the resting-state sessions by applying a paired t-test to the subject-wise z-score at each voxel. To correct for multiple comparisons, we used a cluster-level significance level of 0.05 with a minimal cluster size of 173 mm3, as implemented by 3dClustSim in AFNI (Cox, Chen, Glen, Reynolds, & Taylor, 2017).
2.6 |. Task-evoked inter-subject functional connectivity
To determine the task-evoked FC, the fMRI data from repeated sessions of movie viewing were averaged within each subject. This helped increase the SNR given the high intra-subject reproducibility of movie-evoked responses (Lu et al., 2016). Then, for each pair of subjects (45 pairs given 10 subjects), the inter-subject functional connectivity (ISFC) was evaluated as the z-transformed correlation between the time series from a seed voxel in one subject and the time series from every voxel in the other subject. Note that for each pair of subjects (namely subject A and subject B), this analysis yielded two sets of distinct results, corresponding to the correlations between the seed voxel in subject A and every voxel in subject B and the correlations between the seed voxel in subject B and every voxel in subject A. The two results were averaged for each pair of subjects. To evaluate the significance of ISFC, we applied a parametric test based on linear mixed-effects (LME) modeling as described previously (Chen, Taylor, Shin, Reynolds, & Cox, 2017) and implemented in AFNI to account for the dependences among pairs of subjects. Using 3dClustSim (Cox et al., 2017), the same cluster-level threshold (p < .05, cluster size: 173 mm3) was used as aforementioned.
2.7 |. Whole-brain inter-regional functional connectivity
The above seed-based analysis depended on the subjective choice of the seed location. For more systematic characterization, we evaluated functional connectivity between regions of interest (ROIs) defined in two levels of granularity based on a 17-network atlas (Yeo et al., 2011) and a 246-region functional atlas (Fan et al., 2016).
The fMRI signal was averaged across voxels within each ROI. For each pair of distinct ROIs, their z-transformed correlations were calculated for each session. Separately for the rest and task conditions, the ROI-based correlation was then averaged over repeated sessions within each subject. At the group level, the inter-ROI FC were averaged across subjects and tested for significance by applying one-sample t-test to subject-wise FC in either the task or rest condition. The difference between the task and rest conditions was further evaluated with paired t-test. The significance level was corrected at false discovery rate q < 0.05.
Task-evoked FC between ROIs were evaluated in a similar way as the seed-based voxel-level analyses. Specifically, the voxel-wise fMRI signal was averaged across repeated sessions of movie viewing within each subject, and then averaged across voxels within each ROI. For each pair of subjects, ISFC was evaluated as the z-transformed correlation between the ROI time series from different subjects. ISFC was also tested for significance by using the LME-based parametric test as described in (Chen et al., 2017) and used in our earlier study (Zhang, Chen, et al., 2017). To further correct for multiple comparison, we used the false discovery rate (q < 0.05).
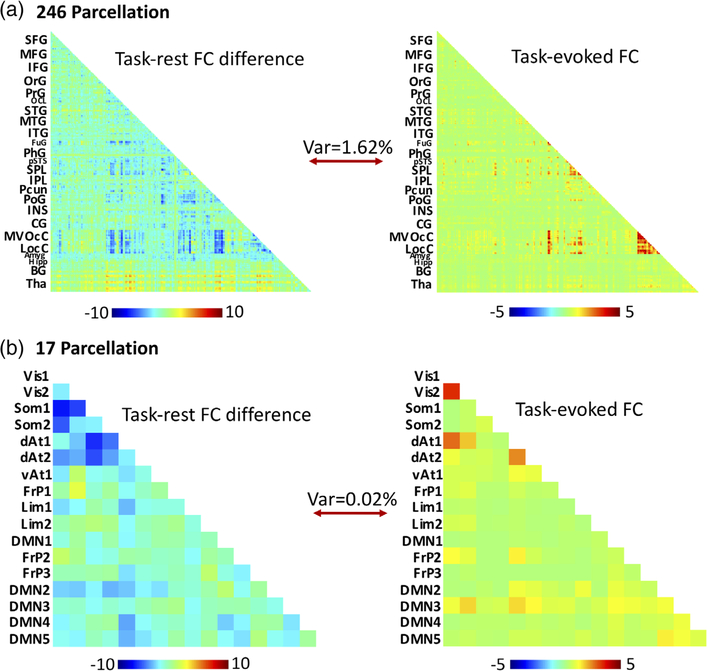
To determine the extent to which the task-evoked FC explains the task-rest FC difference, we used the group-averaged, non-thresholded task-evoked FC as a regressor for the group-averaged, non-thresholded task-rest FC difference (Figure 3). The lower-triangular elements in the task-evoked FC matrix and the task-rest FC difference matrix were both vectorized. The former was used as the regressor to predict the latter through a linear regression model. The model parameters were estimated by using least-squares estimation. The percentage of the model-predicted variance was then calculated.
FIGURE 3.
To what extent does task-evoked FC explain the difference in FC between task and rest conditions? The task-evoked FC was used as a regressor for the task-rest FC difference, using the 246-region Brainnetome atlas (a, top) and the Yeo et al. 17-network atlas (b, bottom). The percent variance explained by the task-evoked connectivity for the 17-network atlas was 0.02% and 1.62% for the 246-region atlas. The color bar indicates z-transformed correlation coefficients [Color figure can be viewed at wileyonlinelibrary.com]
3 |. RESULTS
3.1 |. Seed-based FC in rest versus task
The questions of interest to this study were whether functional connectivity (i.e., temporal correlations between voxels or regions) shows different patterns in the resting state versus during free viewing of a natural movie (herein we referred to it as “task” for simplicity). If yes, we wanted to investigate whether this task-versus-rest difference could be explained by functional connectivity involved in processing the movie, or alternatively reflected changes in spontaneously emerging networks.
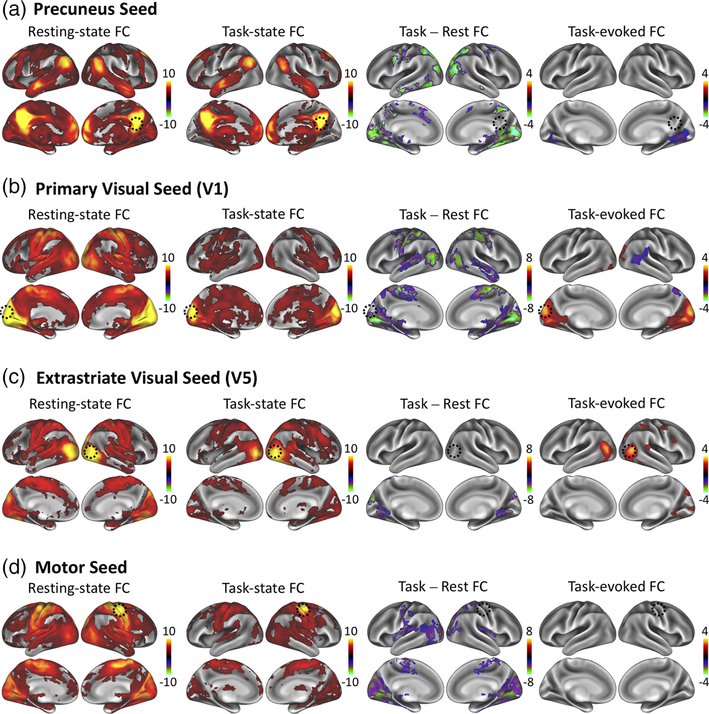
To address these questions, we began with exploratory seedbased correlation analyses in the resting and task states. Four seed voxels were explored, including PCu, V1, V5, and M1 as representative cortical locations in task-negative networks, early visual areas, higher-order visual areas, and non-visual sensorimotor areas presumably unrelated to the movie stimuli. These seeds were used to assess voxel-wise FC at rest, voxel-wise FC during the movie task, their difference, and the task-evoked FC. Results are projected onto the cortical surface for the sake of illustration (see Figure 1).
FIGURE 1.
Seed-based functional connectivity findings. Seed-based functional connectivity using a seed in (a) PCu, (b) V1, (c) V5, and (d) M1. Each panel shows the result for within-session FC during eyes-closed resting-state (left), within-session FC during the movie task (left middle), the within-session FC difference during the movie relative to rest (right middle), and the task-evoked FC computed using inter-subject correlations (right). Cluster-level significance thresholds were set at a p < 0.05 corrected for multiple comparisons. The color bar indicates z-values [Color figure can be viewed at wileyonlinelibrary.com]
For each of the seed locations, the FC patterns in the restingstate and during the movie were mostly similar; however, statistically significant differences were found as shown in Figure 1. The PCu-seeded FC exhibited a more widespread positive distribution in the resting-state than during the task (Figure 1a, far left and left middle columns). Similarly, in the resting-state, the V1 seed was more coupled not only to higher visual areas located more laterally, but also to the superior/medial motor cortex; during the movie task, the correlation was weaker and more confined. In addition, the V5 seed was more correlated to more medial visual areas (e.g., fusiform gyri) during the resting state (Figure 1c, far left and left middle columns) than the task state. Finally, the M1 seed elicited stronger FC with visual regions at rest, but such correlations were much weaker during the movie task. Overall, seed-based FC appeared to be stronger and more extended in the resting state than in the task state.
3.2 |. Task-evoked FC
We evaluated ISFC or inter-regional correlations in the fMRI signal from different subjects viewing the same movie (Kim, Kay, Shulman, & Corbetta, 2018; Simony et al., 2016; Wilf et al., 2017). As spontaneous activity from different subjects is not expected to be correlated in time, ISFC was regarded as the task-evoked FC. Using the same seed locations (PCu, V1, V5, M1), the task-evoked FC was mapped as the far-right column of Figure 1. When the seed was placed at the left V1, the task-evoked FC covered all early visual areas in both hemispheres. When the seed was placed at the right V5, the task-evoked FC highlighted the homologous area in the left V5, as well as the intrahemispherical correlation with dorsal-stream visual areas, including intra-parietal sulcus and frontal eye fields. Given the visual nature of the movie viewing task (without sound), it was not surprising that the task-evoked networks included visual areas. No significant task-evoked FC was found when the seed was placed at the right M1, indicating the absence of explicit involvement of motor cortex in processing any information in the movie. Interestingly, when the seed was placed at PCu, task-evoked FC showed negative correlations with bilateral ventral visual areas. This anti-correlated pattern was not observed in the apparent FC in either the resting or task state without performing global signal regression. In addition to this obvious difference, the task-evoked FC was generally weaker and more confined to the visual system than the resting-state FC.
3.3 |. Task-rest FC difference versus task-evoked FC
We further asked whether the task-evoked FC explained the FC difference between the task and resting states. If the task does not alter the FC of spontaneous activity during the task (i.e. the task-evoked FC is simply an independent and additive component), then the task-versus-rest FC difference is expected to be attributable to task-evoked FC. However, to our surprise, the task-rest FC difference was largely different from the task-evoked FC during the movie-viewing task.
In the seed-based analysis, the task-rest FC difference was mostly negative regardless of whether the seed was at task-related locations (e.g., V1 and V5), or task-unrelated locations (e.g., M1 and PCu) (Figure 1, the right-middle column). In other words, the task suppressed the apparent FC or the correlation in the fMRI signal during task performance. The suppression of FC in the visual cortex during movie viewing was most noticeable when the seed was placed at V1, and it was also observable when the seed was at V5 despite a lesser extent. Beyond the visual system, the suppression of FC was also observed between V1 and the somatosensory, motor, and auditory areas. In addition, the suppression of FC to PCu was found to be widespread, covering both dorsal and ventral visual streams, as well as the dorsal attention network.
None of these patterns of seed-based task-rest FC difference appeared similar to those of task-evoked FC. Specifically, two major distinctions were noticeable. The task-rest FC difference was more widespread than the task-evoked FC. The former was mostly negative, whereas the latter was mostly positive. Despite the polarity difference, the spatial patterns of task-rest FC difference and task-evoked FC overlapped either partially (when the seed was at V1 or PCu) or not at all (when the seed was at M1 or V5).
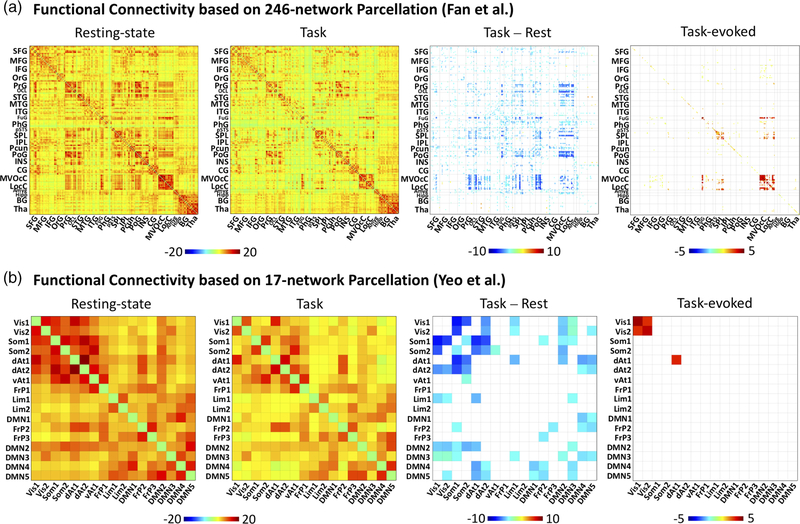
Extending the seed-based analysis, we evaluated the FC between every pair of regions defined more coarsely in a 17-network atlas (Yeo et al., 2011) or more finely in a 246-region functional atlas (the Brainnetome Atlas) (Fan et al., 2016). This analysis generated the matrices of resting-state FC, task-state FC, task-rest FC difference, and task-evoked FC, as shown in Figure 2. Given finely defined ROIs, although the FC matrices for the resting and task states were similar, FC appeared to be weaker and sparser in the task state than in the resting state. The task-rest FC difference was significantly negative for many pairs of regions, including regions within the visual cortex as well as between visual areas and regions in other functional systems. Fewer pairs of regions showed higher FC in the task state than in the resting state. In contrast, the task-evoked FC was mostly positive and confined to visual areas (e.g. lateral occipital complex, fusiform gyrus) with few exceptions of weakly negative FC between areas in visual cortex and those in the default-mode network (e.g., inferior and medial frontal gyrus). Similar results were observed given coarsely defined ROIs. Task-rest FC difference was mostly negative, especially between visual cortex and non-visual sensory cortices as well as between visual areas and default-mode networks, whereas task-evoked FC was confined to visual cortex. More quantitatively, we used (non-thresholded) task-evoked FC as a regressor to model the (non-thresholded) task-rest FC difference. It turned out that such a model only explained less than 2% of variance, suggesting that patterns of task-rest FC difference and task-evoked FC were largely uncorrelated (Figure 3).
FIGURE 2.
Whole-brain inter-regional functional connectivity with two levels of granularity. Cross-correlation matrices corresponding to the FC during the resting state (far left) and the movie task (left middle), the FC difference during the movie relative to rest (right middle), and the task-evoked FC computed using the inter-subject approach (far right). FC matrices were calculated using Fan et al. Brainnetome atlas 246-region functional parcellation (A, top) and the Yeo et al. 17-network parcellation (B, bottom). The color bar indicates the average z-transformed cross correlation values; only significant correlations (q < 0.05) are displayed. Abbreviations for the Yeo parcellation: Vis1, Visual network 1; Vis2, Visual network 2; Som1, Somatomotor network 1; Som2, Somatomotor network 2; dAt1, Dorsal attention network 1; dAt2, Dorsal attention network 2; vAt1, Ventral attention network 1; FrP1, Frontoparietal network 1; Lim1, Limbic network 1; Lim2, Limbic network 2; DMN1, Default mode network 1; FrP2, Frontoparietal network 2; FrP3, Frontoparietal network 3; DMN2, Default mode network 2; DMN3, Default mode network 3; DMN4, Default mode network 4; DMN5, Default mode network 5. The Brainnetome atlas abbreviations are provided in Fan et al. (2016) [Color figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
We have shown that the difference between FC at rest and during a movie-viewing task cannot be explained by the task-evoked FC involved in processing the information in the movie. Specifically, resting-state FC was mostly conserved during free viewing of a naturalistic movie; however, FC between visual and other sensory areas tended to be stronger in the resting state than during the movie. The FC difference between the resting and task states was negative and widespread beyond the visual cortex, whereas the task-evoked FC was positive and restricted to the visual cortex. There was no or little overlap between the task-rest FC difference and the task-evoked FC, with the former being only explainable by the latter by less than 2%. Implications of these findings are discussed as below.
4.1 |. ISFC as a surrogate measure of task-evoked FC
Naturalistic movie stimuli provide a condition similar to the resting state in the sense that the fMRI signal fluctuates irregularly while its correlation reveals patterns of functional networks. Unlike the taskfree resting state, naturalistic stimuli carry rich information to engage subjects in a more behaviorally relevant context while inducing highly reliable responses within and across subjects (Hasson et al., 2004; Hasson, Malach, & Heeger, 2010; Jääskeläinen et al., 2008; McMahon, Russ, Elnaiem, Kurnikova, & Leopold, 2015; Mukamel et al., 2005; Lu et al., 2016; Simony et al., 2016; Wilf et al., 2017). Such response reproducibility offers the basis for mapping the regional activation (Hasson et al., 2004) and inter-regional interaction (Simony et al., 2016) driven by naturalistic stimuli.
Using inter-subject functional connectivity (Simony et al., 2016; Chen et al., 2017), we found that passive viewing of a silent movie evoked functional connectivity mostly restricted to the visual system (including higher-order ventral and dorsal areas). This finding might seem odd if compared to the pattern of FC evoked by a naturalistic auditory narrative, which has been shown to extend beyond the auditory cortex and involve default-mode network (Simony et al., 2016). Note that naturalistic speech stimuli involve auditory cortex for acoustic processing, semantic system for word-level processing, and language system for higher-level comprehension, and beyond. Hence, it is not surprising that speech stimuli evoke a broader extent of FC than a silent movie stimulus. In addition, default-mode network partially overlaps with the semantic system (Binder, Desai, Graves, & Conant, 2009) but is not directly involved in visual processing.
Although it is a reasonable measure of task-evoked FC, ISFC is expected to under-estimate task-evoked FC. Task-evoked responses cannot be entirely identical across sessions or subjects. The same subject watching the movie for the second time likely remembers or recalls from the experience of watching the movie for the first time, but not vice versa. As such, evoked responses may differ between repeated sessions. Further variation is expected to exist between subjects, including individual variations in functional activity and connectivity as well as cortical anatomy, which may cause uncertainties in cross-subject registration.
4.2 |. FC varies from the rest to a task
Consistent with several prior studies (Cole et al., 2014; Gratton et al., 2016; Krienen et al., 2014), we also identified a relatively high degree of similarity between the apparent FC during resting state and the task state. This is likely due to the presence of spontaneous and ongoing sources that dominate functional connectivity in both conditions. Despite this similarity, we observed more widespread and stronger connectivity in the resting state than during the movie-viewing state. The difference was most apparent between different visual areas, between visual and non-visual sensory or motor areas, and with respect to default-mode and attention networks. Prior studies have also reported task-dependent changes of FC relative to the resting state and have shown that the common changes across various tasks occur at flexible “hubs” in the frontal parietal network, default-mode network, and attention network (Bray, Arnold, Levy, & Iaria, 2015; Cole et al., 2013; Dixon et al., 2017; Gilson et al., 2017). Although our observation partly agrees with these findings, we cannot either confirm or reject the “flexible hub” hypothesis as we only use one type of task (natural movie viewing) in this study.
What causes the observed negative and widespread FC differences between the task and resting states might be multiple. In the task-free resting state, some sources of the fMRI signal are hardly controllable, including arousal fluctuations (Chang et al., 2016), head motion (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012), and respiratory and cardiac rate fluctuations (Birn, Diamond, Smith, & Bandettini, 2006; Chang, Cunningham, & Glover, 2009; Shmueli et al., 2007). When subjects are viewing a Hollywood movie, the brain is in a higher arousal condition with less head motion (Vanderwal et al., 2017), thereby receiving reduced systematic fluctuations that might affect widespread areas. Other than such non-neural or modulatory fluctuations, neural-activity-related fluctuations and their correlations might also be affected by the task.
4.3 |. FC differences between task and rest are not explained by task-evoked connectivity
The neuronal effect of movie viewing on apparent FC can be further attributed to task-evoked FC and/or spontaneous FC during the task. A major conclusion of this study is that task-evoked FC contributes little to the FC difference between the task and resting states. As it is evaluated between sessions, task-evoked FC is not affected by any sources of activity fluctuation or correlation that are not temporally synchronized with the movie’s spatiotemporal features (Lu et al., 2016; Shi, Wen, Zhang, Han, & Liu, 2018; Wen et al., 2017). The fact that task-evoked FC does not explain the task-rest FC difference suggests that ongoing activity that occurs during the movie but not in synchronization with movie has altered its correlational patterns and/or strengths compared with those in the resting state. Since data acquisitions were relatively short and the number of participants was relatively small, further investigation with a larger sample size would help strengthen the conclusion.
Also note that task-evoked FC is mostly positive whereas the task-rest FC difference is mostly negative. Ongoing activity during the task has to reduce its correlations to an extent that overrides the positive correlation in task-evoked activity. The reduction may in part result from reduced head motion or arousal fluctuation (Vanderwal et al., 2017), or may also plausibly reflect task-induced suppression of spontaneous neuronal activity and its correlation between regions.
4.4 |. Negative task–rest interaction
We speculate that the reduction of FC in ongoing activity during a movie-viewing task results from a negative “task–rest” interaction. In other words, task engagement suppresses spontaneous activity and its correlations.
He (2013) and several others (Bianciardi et al., 2009; Churchland et al., 2010; Monier, Chavane, Baudot, Graham, & Frégnac, 2003; Ponce-Alvarez et al., 2013) suggest a notion of negative task–rest interaction. In this notion, the variance of the unknown mixture of the task-evoked and spontaneous signals is less than the sum of the variance of the spontaneous signal and the variance of the task-evoked signal. This negative interaction may help facilitate the task execution (Boly et al., 2007; Deneux & Grinvald, 2017; Hesselmann, Kell, Eger, & Kleinschmidt, 2008) [see Northoff, Qin, and Nakao (2010) and Ferezou and Deneux (2017) for review], and may increase with task difficulty (Garrett, McIntosh, & Grady, 2014; Szostakiwskyj, Willatt, Cortese, & Protzner, 2017). If a task reduces the magnitude of spontaneous activity, it is expected to reduce its correlation as well simply due to the reduction in the signal to noise ratio.
The negative task–rest interaction may or may not hold true for all tasks. Passive versus active task engagement may not equally affect spontaneous signals (Broday-Divir, Grossman, Furman-Haran, & Malach, 2017; Ferezou, Bolea, & Petersen, 2006; Otazu, Tai, Yang, & Zador, 2009). Crochet and Petersen (2006) found that active and conscious engagement in a task gave rise to more desynchronization of ongoing activity than passive or less conscious states (e.g., in the anesthetized states). In our natural vision task, subjects actively engaged in the movie with free eye movement. Speculatively, cognitively engaging in the task itself, rather than simply having a visual experience, may explain the negative interaction between spontaneous and task-evoked functional connectivity. However, this hypothesis remains to be tested.
In addition, a task may desynchronize ongoing activity across regions, independent of the effect on the signal amplitude. In EEG, alpha band oscillations are postulated to stem from the rhythmic fluctuations of inhibitory neurons. Task engagement may alleviate or reduce co-fluctuating inhibitory signals, e.g. in terms of alpha desynchronization at task-related regions [see Klimesch, Sauseng, & Hanslmayr (2007) for review]. Moreover, inhibitory neurotransmitter concentrations, such as GABA (Muthukumaraswamy, Edden, Jones, Swettenham, & Singh, 2009; Northoff et al., 2007) may undergo task-dependent changes. Here, we cannot disentangle whether suppression of FC in ongoing activity is due to reduction of regional activity or inter-regional synchronization. We speculate both effects play a role, because the reduction of FC is neither confined to task-evoked regions nor always accompanied with reduced magnitude of activity.
ACKNOWLEDGMENTS
We thank Dr. Gang Chen from National Institute of Mental Health for technical assistance. The research was supported in part by NIH R01MH104402 (Z. Liu, PI) and a predoctoral fellowship awarded to Lauren Lynch as supported by TL1 TR001107 and UL1 TR001108 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Andrew J. Saykin acknowledges support from NIH grants P30 AG010133 and R01 AG019771.
Funding information
National Institutes of Health, Grant/Award Numbers: UL1 TR001108, TL1 TR001107; NIH, Grant/Award Numbers: R01 AG019771, P30 AG010133, R01MH104402; National Institute of Mental Health
REFERENCES
- Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, & Meyerand ME (2000). Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magnetic Resonance Imaging, 18, 921–930. [DOI] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, & Aertsen A (1996). Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science, 273, 1868–1871. [DOI] [PubMed] [Google Scholar]
- Azouz R, & Gray CM (1999). Cellular mechanisms contributing to response variability of cortical neurons in vivo. The Journal of Neuroscience, 19, 2209–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Reinacher M, Freyer F, Villringer A, & Ritter P (2011). How ongoing neuronal oscillations account for evoked fMRI variability. Journal of Neuroscience, 31, 11016–11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, Gelderen PV, Horovitz SG, De JA, & Duyn JH (2009). Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. NeuroImage, 45, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant LL (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, & Bandettini PA (2006). Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage, 31(4), 1536–1548. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, … Peigneux P (2007). Baseline brain activity fluctuations predict somatosensory perception in humans. Proceedings of the National Academy of Sciences of the United States of America, 104, 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Graham LJ, Monier C, & Fregnac Y (1998). Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature, 393, 369–373. [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Bray S, Arnold AE, Levy RM, & Iaria G (2015). Spatial and temporal functional connectivity changes between resting and attentive states. Human Brain Mapping, 36, 549–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broday-Divir R, Grossman S, Furman-Haran E, & Malach R (2017). Quenching of spontaneous fluctuations by attention in human visual cortex. Neuroimage, 171, 84–98. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, & Yeo TBT (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nature Reviews Neuroscience, 16, 832–837. [DOI] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, & Glover GH (2009). Influence of heart rate on the BOLD signal: The cardiac response function. NeuroImage, 44(3), 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, & Glover G (2010). Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage, 50, 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Leopold DA, Scholvinck ML, Mandelkow H, Picchioni D, Liu X, … Duyn JH (2016). Tracking brain arousal fluctuations with fMRI. PNAS, 113(16), 4518–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Taylor PA, Shin YW, Reynolds RC, & Cox RW (2017). Untangling the relatedness among correlations, part II: Inter-subject correlation group analysis through linear mixed-effects modeling. Neuroimage, 147, 825–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, … Shenoy KV (2010). Stimulus onset quenches neural variability: A widespread cortical phenomenon. Nature Neuroscience, 13, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, & Petersen SE (2014). Intrinsic and task-evoked network architectures of the human brain. Neuron, 83, 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, & Braver TS (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16, 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Puolivali T, Alluri V, Sipola T, Burunat I, Toiviainen P, … Ristaniemi T (2014). Key issues in decomposing fMRI during naturalistic and continuous music experience with independent component analysis. Journal of Neuroscience Methods, 223, 74–84. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance Neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds R, & Taylor P (2017). FMRI clustering in AFNI: False positive rates redux. Brain Connectivity, 7, 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, & Petersen CC (2006). Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nature Neuroscience, 9, 608–610. [DOI] [PubMed] [Google Scholar]
- Deneux T, & Grinvald A (2017). Milliseconds of sensory input abruptly modulate the dynamics of cortical states for seconds. Cerebral Cortex, 27, 4549–4563. [DOI] [PubMed] [Google Scholar]
- Di X, & Biswal BB (2018) Toward task connectomics: examining whole-brain task modulated connectivity in different task domains. Cerebral Cortex, bhy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Andrews-Hanna JR, Spreng RN, Irving ZC, Mills C, Girn M, & Christoff K (2017). Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage, 147, 632–649. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, … Petersen SE (2007). A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage, 35, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, … Jiang T (2016). The human Brainnetome atlas: A new brain atlas based on connectional architecture. Cerebral Cortex, 26, 3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, & Petersen CC (2006). Visualizing the cortical representation of whisker touch: Voltage-sensitive dye imaging in freely moving mice. Neuron, 50, 617–629. [DOI] [PubMed] [Google Scholar]
- Ferezou I, & Deneux T (2017). Review: How do spontaneous and sensory-evoked activities interact? Neurophotonics, 4, 031221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn IM, Priebe NJ, & Ferster D (2007). The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. Neuron, 54, 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, & Raichle ME (2006). Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nature Neuroscience, 9, 23–25. [DOI] [PubMed] [Google Scholar]
- Garrett DD, McIntosh AR, & Grady CL (2014). Brain signal variability is parametrically modifiable. Cerebral Cortex, 24, 2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson M, Deco G, Friston KJ, Hagmann P, Mantini D, Betti V, … Corbetta M (2017). Effective connectivity inferred from fMRI transition dynamics during movie viewing points to a balanced reconfiguration of cortical interactions. Neuroimage, 1–13, 10.1016/j.neuroimage.2017.09.061 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Hoy CW, Handwerker DA, Robinson ME, Buchanan LC, Saad ZS, & Bandettini PA (2015). Tracking ongoing cognition in individuals using brief, whole-brain functional connectivity patterns. Proceedings of the National Academy of Sciences of the United States of America, 112, 8762–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Laumann TO, Gordan EM, Adeyemo B, & Petersen SE (2016). Evidence for two independent factors that modify brain networks to meet task goals. Cell Report, 16, 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, & Fischl B (2009). Accurate and robust brain image alignment using boundary-based registration. Neuroimage, 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, López-Solà M, Hernández-Ribas R, Deus J, Ortiz H, … Cardoner N (2008). Consistency and functional specialization in the default mode brain network. Proceedings of the National Academy of Sciences of the United States of America, 105, 9781–9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Malach R, & Heeger DJ (2010). Reliability of cortical activity during natural stimulation. Trends in Cognitive Sciences, 14, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, & Malach R (2004). Intersubject synchronization of cortical activity during natural vision. Science, 303, 1634–1640. [DOI] [PubMed] [Google Scholar]
- He BJ (2013). Spontaneous and task-evoked brain activity negatively interact. Journal of Neuroscience, 33, 4672–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann G, Kell CA, Eger E, & Kleinschmidt A (2008). Spontaneous local variations in ongoing neural activity bias perceptual decisions. Proceedings of the National Academy of Sciences of the United States of America, 105, 10984–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, De Zwart JA, Van Gelderen P, Fulton SC, Balkin TJ, & Duyn JH (2008). Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Human Brain Mapping, 29, 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, … Chang C (2013). Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage, 80, 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen IP, Koskentalo K, Balk MH, Autti T, Kauramäki J, Pomren C, & Sams M (2008). Inter-subject synchronization of prefrontal cortex hemodynamic activity during natural viewing. The Open Neuroimaging Journal, 2, 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kay K, Shulman GL, & Corbetta M (2018). A new modular brain organization of the BOLD signal during natural vision. Cerebral Cortex, 28, 3065–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, & Hanslmayr S (2007). EEG alpha oscillations: The inhibition-timing hypothesis. Brain Research Review, 53, 63–88. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Yeo BTT, Buckner RL, & Buckner RL (2014). Reconfigurable task-dependent functional coupling modes cluster around a core functional architecture. Philosophical transactions of the Royal Society B, 5, 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KH, Hung SC, Wen H, Marussich L, & Liu Z (2016). Influences of high-level features, gaze, and scene transitions on the reliability of BOLD responses to natural movie stimuli. PLoS One, 11, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen V, Tiitinen H, & May P (2005). Auditory event-related responses are generated independently of ongoing brain activity. NeuroImage, 24, 961–968. [DOI] [PubMed] [Google Scholar]
- Marussich L, Lu K-H, Wen H, & Liu Z (2017). Mapping white-matter functional Organization at Rest and during naturalistic visual perception. NeuroImage, 146, 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DBT, Russ BE, Elnaiem HD, Kurnikova AI, & Leopold DA (2015). Single-unit activity during natural vision: Diversity, consistency, and spatial sensitivity among AF face patch neurons. Journal of Neuroscience, 35, 5537–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Colcombe S, Xavier Castellanos F, & Milham MP (2013). The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cerebral Cortex, 23, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, & Frégnac Y (2003). Orientation and direction selectivity of synaptic inputs in visual cortical neurons: A diversity of combinations produces spike tuning. Neuron, 37, 663–680. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, & Malach R (2005). Coupling between neuronal firing, field potentials, and fMRI in human auditory cortex. Science, 309, 951–954. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, & Singh KD (2009). Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proceedings of the National Academy of Sciences of the United States of America, 106, 8356–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, & Nakao T (2010). Rest-stimulus interaction in the brain: A review. Trends Neurosci, 33, 277–284. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, … Boesiger P (2007). GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nature Neuroscience, 10, 1515–1517. [DOI] [PubMed] [Google Scholar]
- Oram MW (2011). Visual stimulation Decorrelates neuronal activity. Journal of Neurophysiology, 105, 942–957. [DOI] [PubMed] [Google Scholar]
- Otazu GH, Tai LH, Yang Y, & Zador AM (2009). Engaging in an auditory task suppresses responses in auditory cortex. Nature Neuroscience, 12, 646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442. [PubMed] [Google Scholar]
- Ponce-Alvarez A, Thiele A, Albright TD, Stoner GR, & Deco G (2013). Stimulus-dependent variability and noise correlations in cortical MT neurons. Proceedings of the National Academy of Sciences of the United States of America, 110, 13162–13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, & Grefkes C (2013). State-dependent differences between functional and effective connectivity of the human cortical motor system. NeuroImage, 67, 237–246. [DOI] [PubMed] [Google Scholar]
- Saka M, Berwick J, & Jones M (2010). Linear superposition of sensory-evoked and ongoing cortical hemodynamics. Frontiers in neuroenergetics, 2, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, … Gur RE (2012). Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. NeuroImage, 60, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Liu H, Talukdar T, Martincorena II, Thomas Yeo BT, & Buckner RL (2010). The organization of local and distant functional connectivity in the human brain. PLoS Computational Biology, 6, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wen H, Zhang Y, Han K, & Liu Z (2018). Deep recurrent neural network reveals a hierarchy of process memory during dynamic natural vision. Human Brain Mapping, 39(5), 2269–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, & Greicius MD (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral Cortex, 22, 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma MJ, & Duyn JH (2007). Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. NeuroImage, 38(2), 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simony E, Honey CJ, Chen J, Lositsky O, Yeshurun Y, Wiesel A, & Hasson U (2016). Dynamical reconfiguration of the default mode network during narrative comprehension. Nature Communications, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, 208–219. [DOI] [PubMed] [Google Scholar]
- Szostakiwskyj JMH, Willatt SE, Cortese F, & Protzner AB (2017). The modulation of EEG variability between internally- and externally-driven cognitive states varies with maturation and task performance. PLoS One, 12, e0181894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, & Volkow ND (2018) Association Between Brain Activation and Functional Connectivity. Cerebral Cortex, bhy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, & Arieli A (1999). Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science, 286, 1943–1946. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, & Buckner RL (2010). Intrinsic functional connectivity as a tool for human Connectomics: Theory, properties, and optimization. Journal of Neurophysiology, 103, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, & Buckner RL (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T, Eilbott J, Finn ES, Craddock RC, Turnbull A, & Castellanos FX (2017). Individual differences in functional connectivity during naturalistic viewing conditions. Neuroimage, 157, 521–530. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, … Raichle ME (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 447, 83–86. [DOI] [PubMed] [Google Scholar]
- Wen H, & Liu Z (2016). Broadband electrophysiological dynamics contribute to global resting-state fMRI signal. Journal of Neuroscience, 36, 6030–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Shi J, Zhang Y, Lu K-H, Cao J, & Liu Z (2017). Neural encoding and decoding with deep learning for dynamic natural vision. Cerebral Cortex. 1–25, 10.1093/cercor/bhx268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilf M, Strappini F, Golan T, Hahamy A, Harel M, & Malach R (2017). Spontaneously emerging patterns in human visual cortex reflect responses to naturalistic sensory stimuli. Cerebral Cortex, 27, 750–763. [DOI] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, & Liu TT (2013). The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage, 83, 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang H, Chen X, Wang Q, Yap PT, & Shen D (2017). Learning-based structurally-guided construction of resting-state functional correlation tensors. Magnetic Resonance Imaging, 43, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen G, Wen H, Lu KH, & Liu Z (2017). Musical imagery involves Wernicke’s area in bilateral and anti-correlated network interactions in musicians. Science Reports, 7, 17066. [DOI] [PMC free article] [PubMed] [Google Scholar]